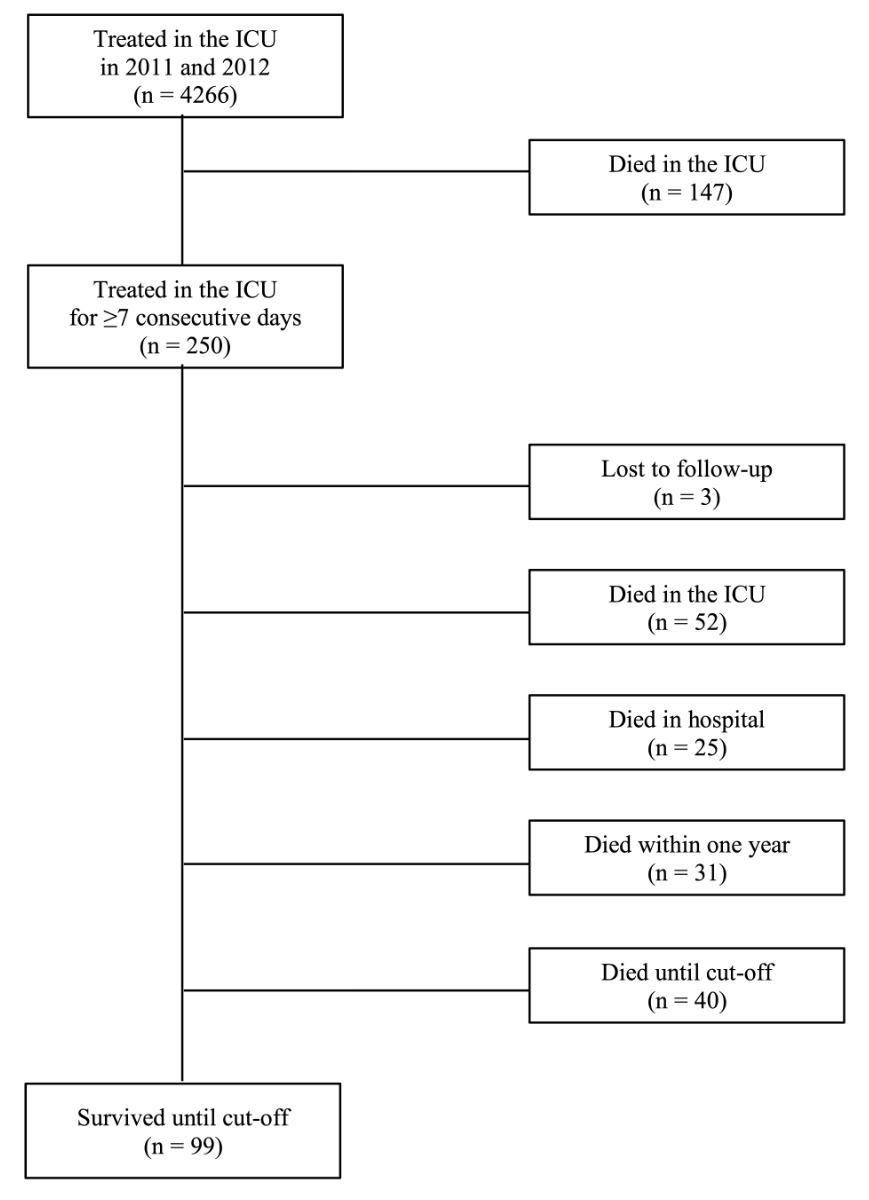
Figure 1 Flow chart of included patients. ICU: intensive care unit
DOI: https://doi.org/10.4414/SMW.2022.w30144
“An intensive care unit is an organized system for the provision of care to critically ill patients that provides intensive and specialized medical and nursing care, an enhanced capacity for monitoring, and multiple modalities of physiologic organ support to sustain life during a period of acute organ system insufficiency” is the definition of an intensive care unit (ICU) according to the Task Force of the World Federation of Societies of Intensive and Critical Care Medicine [1]. This definition clearly states the tasks of intensive care medicine and ICUs including the monitoring, maintenance and, if necessary, replacement of vital functions. Usually, patients are treated in an ICU for only a few days. However, a small proportion requires a longer treatment period, with 5–10% of all patients developing a persistent or chronic critical illness [2–5]. Reasons for prolonged intensive care treatment may include: (1) absence of efficacious therapy for an underlying long-term disease, which is not immediately lethal; (2) complications during the ICU stay (e.g., persistent single-organ failure, cascade of multiorgan failure, prolonged mechanical ventilation due to muscle weakness or persistent delirium); and (3) system factors (e.g., patients cannot be transferred to the ward, patients with unrealistic expectations, lack of palliative care facilities) [5].
Long-term treatment in an ICU confers a substantial physical, psychological and social burden on patients and their relatives [6]. The treatment team can also reach its limits, and the costs of long-term intensive care treatment are considerable [7–9]. Stricker et al. showed that 11% of patients (583 out of 4898) who were treated >7 days in a mixed ICU consumed over 50% of the available resources [10]. A study from the USA found that patients (142 out of 2618, 5.4%) who received intensive care after heart surgery for 10 or more days accounted for 50% of the treatment days and caused 48% of the direct ICU costs [11]. Given these high costs in addition to the considerable burden on the patients receiving long-term intensive treatment, their families and caregivers, it is essential to know the outcome and to establish factors that possibly can predict mortality.
This study examined the mortality of patients who were treated continuously for 7 or more consecutive days in the surgical ICU of the University Hospital Basel and how survivors differ from the deceased in terms of various parameters. Only a few Swiss studies address this issue previously [10, 12, 13].
This retrospective observational study was performed in the 22-bed ICU of the University Hospital of Basel, a Swiss tertiary academic medical care centre, which treated approximately 2500 mainly surgical and trauma patients per year at the time of study. As there is no generally accepted definition of prolonged ICU stay [8, 14] (supplementary table S1 in the appendix), all patients who were treated in the surgical ICU for at least 7 consecutive days between 1 January 2011 and 31 December 2012 were included. The Ethics Committee of Northwest and Central Switzerland (EKNZ) approved this study (2018-00184). The objectives of this study were to assess mortality in the ICU and the nursing department in the year following the beginning of ICU treatment, and until the cut-off-date (30 September 2018) (fig. 1). We sought to identify factors associated with 1-year mortality.

Figure 1 Flow chart of included patients. ICU: intensive care unit
Patient data, including patient age and sex, main surgical and secondary diagnoses, admission status, severity of illness using the simplified acute physiology score (SAPS II) [15], measurement of the nursing workload according to the nine equivalents of nursing manpower use score (NEMS) [16], ICU complications, ICU treatments including the duration, and hospital outcome, were collected from the patients’ charts. Survival after discharge was determined by hospital records or by contact with registration office or the family doctor. Follow-up was closed on 30 September 2018.
ICU complications were defined as follows: delirium as a transient, usually reversible, global cognitive dysfunction (screening for delirium was made using the Intensive Care Delirium Screening Checklist (ICDSC) every 8 hours); stroke as new ischaemic or haemorrhagic cerebral event evidenced on computed tomography (CT); tracheobronchitis as an inflammation of the airways between the larynx and the bronchioles; pneumonia as an inflammation of the lung tissue accompanied by infiltration of alveoli; pleural empyema as a collection of pus in the pleural cavity; acute respiratory distress syndrome (ARDS) as acute onset of respiratory failure, bilateral infiltrates on chest radiograph, and hypoxaemia; septic shock as persisting hypotension requiring vasopressors, and a serum lactate level >2 mmol/l (18 mg/dl) despite adequate volume resuscitation; acute renal failure as an acute increase of creatinine of >26.4 μmol/l (0.3 mg/dl), a percentage increase creatinine >50%, or a reduction of urine output; acute on chronic renal insufficiency as increased serum creatinine by 50% or more from index serum creatinine prior to this acute illness, critical illness polyneuropathy as a peripheral neuropathy involving both motor and sensory fibres accompanied by limb and respiratory muscle weakness.
Variables were summarised by comparing patients who survived less than one year with patients who survived longer than one year. We compared between these groups using the chi-square test for nominal variables. Continuous variables were compared using t-tests or, if strongly skewed, using the non-parametric Kruskal-Wallis test. No correction for multiple testing (i.e., alpha inflation) was performed. For normally distributed numerical variables, mean and standard deviation (SD) were calculated. For non-normal numerical data, median and interquartile range (IQR) were computed. Categorical data are summarised in percent. Kaplan-Meier curves including a table for number at risk were fit to compare survival of groups concerning age, with and without comorbidities, complications, and treatment. All statistical analyses were performed using R Statistical Language and Environment.
In the years 2011 and 2012, a total of 4926 patients were treated in the examined ICU. Of the 4676 patients (94.9%) requiring short-term care, 147 (3.2%) died during their ICU stay. A total of 250 patients (5.1%) needed a treatment of ≥7 consecutive days, three of whom were lost to follow up. Therefore, further evaluation included 247 patients (fig. 1).
Fifty-two long-term patients (21.1%) died in the ICU, 25 (10.1%) after transfer to the normal ward (table 1). Thirty-one patients (12.5%) died within 1 year after the beginning of intensive care treatmen. Altogether, the 1-year mortality was 43.7% (108 patients) (table 2). At the end of follow-up after 6 to 7 years, 99 patients (40.1%) were still alive (table 1).
Table 1Patient characteristics and outcomes.
| Died in ICU (n = 52) | Died in hospital (n = 25) | Died after discharge (n = 71) | Survived to follow-up (n = 99) | All patients (n = 247) | ||
| Age (years), median (IQR) | 69 (62.5–75) | 71 (62–79) | 71 (61–76) | 59 (42–67) | 66 (56–75) | |
| Sex, n (%) | Male | 35 (67.3) | 15 (60.0) | 43 (60.6) | 70 (70.7) | 163 (66.0) |
| Female | 17 (32.7) | 10 (40.0) | 28 (39.4) | 29 (29.3) | 84 (34.0) | |
| Charlson comorbidity index, mean (SD) | 2.0 (1.8) | 2.4 (1.8) | 2.3 (1.9) | 0.9 (1.4) | 1.7 (1.8) | |
| Emergency admission, n (%) | 29 (55.8) | 18 (72.0) | 49 (69.0) | 75 (75.8) | 171 (69.2) | |
| Length of stay (days), median (IQR) | 16.0 (10.4–23.3) | 12.1 (9.0–20.1) | 11.0 (8.9–18.9) | 11.5 (8.6–16.9) | 12.7 (9.0–18.6) | |
| Main diagnosis, n (%) | Traumatic brain injury | 1 (9.1) | 1 (9.1) | 2 (18.2) | 7 (63.6) | 11 (4.5) |
| Polytrauma | 1 (3.2) | 1 (3.2) | 1 (3.2) | 28 (90.3) | 31 (12.6) | |
| Internal disease | 1 (20.0) | 2 (40.0) | 0 | 2 (40.0) | 5 (2.0) | |
| Type of surgery, n (%) | Cardiac | 24 (24.2) | 7 (7.1) | 31 (31.3) | 37 (37.4) | 99 (40.1) |
| Neurological | 2 (13.3) | 4 (26.7) | 3 (20.0) | 6 (40.0) | 15 (6.1) | |
| Pulmonary | 6 (40.0) | 2 (13.3) | 7 (46.7) | 0 | 15 (6.1) | |
| Abdominal | 10 (25.6) | 5 (12.8) | 13 (33.3) | 11 (28.2) | 39 (15.8) | |
| Vascular | 2 (11.8) | 2 (11.8) | 8 (47.1) | 5 (29.4) | 17 (6.9) | |
| Maxillofacial | 2 (40.0) | 0 | 2 (40.0) | 1 (20.0) | 5 (2.0) | |
| Orthopaedic | 1 (14.3) | 1 (14.3) | 3 (42.9) | 2 (28.6) | 7 (2.8) | |
| Urological | 2 (66.7) | 0 | 1 (33.3) | 0 | 3 (1.2) | |
| SAPS-II, mean (SD) | 53.2 (15.7) | 52.2 (14.4) | 51.9 (18.0) | 43.2 (16.9) | 48.7 (17.3) | |
| NEMS, mean (SD) | 2061.0 (1432.1) | 1442.2 (1244.1) | 1338.2 (868.8) | 1254.5 (873.5) | 1467.4 (1092.1) | |
| ICU complications, n (%) | Delirium | 22 (42.3) | 11 (44.0) | 47 (66.2) | 60 (60.6) | 140 (56.7) |
| Stroke | 2 (3.8) | 2 (8.0) | 7 (9.9) | 7 (7.1) | 18 (7.3) | |
| Tracheobronchitis | 11 (21.2) | 6 (24.0) | 14 (19.7) | 31 (31.3) | 62 (25.1) | |
| Pneumonia | 31 (59.6) | 9 (36.0) | 28 (39.4) | 28 (28.3) | 96 (38.9) | |
| Pleural empyema | 9 (17.3) | 1 (4.0) | 5 (7.0) | 3 (3.0) | 18 (7.3) | |
| ARDS | 9 (17.3) | 2 (8.0) | 2 (2.8) | 9 (9.1) | 22 (8.9) | |
| Septic shock | 29 (55.8) | 13 (52.0) | 23 (32.4) | 19 (19.2) | 84 (34.0) | |
| Acute renal insufficiency | 22 (42.3) | 9 (36.0) | 21 (29.6) | 22 (22.2) | 74 (30.0) | |
| Acute on chronic renal insufficiency | 15 (28.8) | 7 (28.0) | 21 (29.6) | 10 (10.1) | 53 (21.5) | |
| Critical illness polyneuropathy | 11 (21.2) | 2 (8.0) | 8 (11.3) | 1 (1.0) | 22 (8.9) | |
| Number of complications, mean (SD) | 3.0 (1.6) | 2.4 (1.7) | 2.5 (1.4) | 1.9 (1.3) | 2.4 (1.5) | |
| Requiring mechanical ventilation n (%) | 51 (98.1) | 23 (92.0) | 65 (91.5) | 93 (93.9) | 232 (93.9) | |
| Days of ventilation, median (IQR) | 10 (6–13) | 5 (3–10) | 6 (4–11) | 7 (4–11) | 7 (4–11) | |
| Requiring tracheostomy, n (%) | 17 (32.7) | 5 (20.0) | 24 (33.8) | 27 (27.3) | 73 (29.2) | |
| Requiring RRT, n (%) | 22 (42.3) | 5 (20.0) | 12 (16.9) | 15 (15.2) | 54 (21.9) | |
| Days of RRT, median (IQR) | 6.5 (3–14) | 5 (2–6) | 6.5 (4.5–16) | 7 (4–13) | 6 (3.75–14) | |
| Requiring IABP, n (%) | 7 (13.5) | 0 | 7 (9.9) | 11 (11.1) | 25 (10.1) | |
| Days of IABP, median (IQR) | 8 (5–9) | 0 | 3 (1–5) | 2 (2–4) | 3 (2–5.5) | |
| Requiring ECMO, n (%) | 4 (7.7) | 0 | 2 (2.8) | 3 (3.0) | 9 (3.6) | |
| Days of ECMO, median (IQR) | 8.5 (8–11.5) | 0 | 4.5 (2–7) | 4 (2–5) | 7 (3–8.5) | |
| Requiring ICP monitoring, n (%) | 1 (1.9) | 4 (16.0) | 4 (5.6) | 19 (19.2) | 28 (11.3) | |
| Days of ICP monitoring, median (IQR) | 8 | 7 (3.5–19.5) | 9 (7–10) | 7 (4–10) | 8 (4.25–10) | |
| Number of ICU treatments, mean (SD) | 1.6 (1.5) | 1.7 (1.2) | 1.8 (1.2) | 1.3 (0.6) | 1.56 (1.1) | |
ARDS: acute respiratory distress syndrome; ECMO: extracorporeal membrane oxygenation; IABP: intra-aortic balloon pump; ICP: intracranial pressure; IQR: interquartile range; NEMS: nine equivalents of nursing manpower score; ICU: intensive care unit; RRT: renal replacement therapy; SAPS: simplified acute physiological score; SD: standard deviation
The median age of all long-term patients was 66 years (IQR 56–75) and two thirds were men. Almost 70% of them were admitted to the hospital as emergency patients. The median length of stay of was 12.7 days (IQR 9.0–18.6), the mean SAPS-II was 48.7 points (SD 17.3) and the mean NEMS 1467.4 points (SD 1092.1). Eighty-four patients (34%) were treated more than once in the ICU. Ninety-nine patients (40.1%) underwent heart surgery; 39 (15.8%) were post-abdominal surgery patients. A total of 42 patients suffered a polytrauma (31 patients, 12.6%) or isolated traumatic brain injury (11 patients, 4.5%). All other diagnosis groups included less than 10% of patients for each group. More than 90% of patients with polytrauma survived until the cut-off date. Among cardiac surgery patients, 37 of 99 patients (37.4%), and after abdominal surgery, 11 of 39 patients (28.2%) survived. All 15 patients who underwent lung surgery died.
Delirium occurred in more than half of the patients (140 patients, 56.7%). Eighty-four patients (34.0%) suffered from septic shock, 62 (25.1%) from tracheobronchitis, 96 (38.9%) from pneumonia, and 74 (30.0%) from acute renal failure. On average, 2.4 complications occurred per patient. Almost all patients (232 patients, 93.9%) were transferred intubated to the ICU or were intubated at least once during the course of treatment. Median ventilation time was 7 days (IQR 4–11). Seventy-three patients (29.2%) had to be tracheotomised. Renal replacement therapy (RRT) was necessary in 54 patients (21.9%), an intra-aortic balloon pump (IABP) in 25 (10.1%, corresponding to 25% of the cardiac surgery patients), an extracorporeal membrane oxygenation (ECMO) device in 9 (3.6%), and an intracranial pressure (ICP) measurement in 28 (11.3%) (table 1).
Patients who died within one year of beginning treatment in the ICU were significantly older (median 71 vs 63 years, p <0.001), had a higher Charlson comorbidity index (CCI) (mean 2.3 vs 1.2, p <0.001), a longer ICU stay (median 13.9 vs 10.6 days, p = 0.001), a higher SAPS-II (mean 52.7 vs 45.6, p = 0.001), a higher NEMS (mean 1772.4 vs 1230.4, p <0.001) and more complications (mean 2.9 vs 2.0, p <0.001) compared with those surviving at least one year. Moreover, the deceased more frequently developed pneumonia (50.9% vs 29.5%, p = 0.001), pleural empyema (13.0% vs 2.9%, p = 0.005), septic shock (51.9% vs 20.1%, p <0.001), or critical illness polyneuropathy (16.7% vs 2.9%, p <0.001). They also more frequently required RRT (30.6% vs 15.1%, p = 0.006) (table 2).
Table 2Patient characteristics and survival.
| Died within 1 year (n = 108) | 1-year survivor (n=139) | p-Value | ||
| Age (years), median (IQR) | 71 (63–71) | 63 (47–71) | <0.001 | |
| Sex, n (%) | Male | 71 (65.7) | 92 (66.2) | 1.000 |
| Female | 37 (34.3) | 47 (33.8) | ||
| Charlson comorbidity index, mean (SD) | 2.3 (1.8) | 1.2 (1.6) | <0.001 | |
| Emergency admission, n (%) | 65 (60.2) | 106 (76.3) | 0.010 | |
| Length of stay (days), median (IQR) | 13.9 (10.2–21.2) | 10.6 (8.5–17.1) | 0.001 | |
| Main surgical diagnosis, n (%) | Traumatic brain injury | 3 (27.3) | 8 (72.7) | |
| Polytrauma | 2 (6.5) | 29 (93.5) | ||
| Internal disease | 3 (60.0) | 2 (40.0) | ||
| Type of surgery, n (%) | Cardiac | 42 (42.4) | 57 (57.6) | |
| Neurological | 7 (46.7) | 8 (53.3) | ||
| Pulmonary | 13 (86.7) | 2 (13.3) | ||
| Abdominal | 20 (51.3) | 19 (48.7) | ||
| Vascular | 9 (52.9) | 8 (47.1) | ||
| Maxillofacial | 4 (80.0) | 1 (20.0) | ||
| Orthopaedic | 3 (42.9) | 4 (57.1) | ||
| Urological | 2 (66.7) | 1 (33.3) | ||
| SAPS-II, mean (SD) | 52.7 (16.0) | 45.6 (17.6) | 0.001 | |
| NEMS, mean (SD) | 1772.4 (1275.7) | 1230.4 (857.3) | <0.001 | |
| ICU complications, n (%) | Delirium | 51 (47.2) | 89 (64.0) | 0.012 |
| Stroke | 8 (7.4) | 10 (7.2) | 1.000 | |
| Tracheobronchitis | 26 (24.1) | 36 (25.9) | 0.857 | |
| Pneumonia | 55 (50.9) | 41 (29.5) | 0.001 | |
| Pleural empyema | 14 (13.0) | 4 (2.9) | 0.005 | |
| ARDS | 12 (11.1) | 10 (7.2) | 0.397 | |
| Septic shock | 56 (51.9) | 28 (20.1) | <0.001 | |
| Acute renal insufficiency | 41 (38.0) | 33 (23.7) | 0.023 | |
| Acute on chronic renal insufficiency | 32 (29.6) | 21 (15.1) | 0.009 | |
| Critical illness polyneuropathy | 18 (16.7) | 4 (2.9) | <0.001 | |
| Number of complications, mean (SD) | 2.9 (1.6) | 2.0 (1.2) | <0.001 | |
| Requiring mechanical ventilation n (%) | 102 (94.4) | 130 (93.5) | 0.026 | |
| Days of ventilation, median (IQR) | 8 (5–12) | 7 (4–11) | 0.038 | |
| Requiring tracheostomy, n (%) | 36 (33.3) | 37 (26.6) | 0.314 | |
| Requiring RRT, n (%) | 33 (30.6) | 21 (15.1) | 0.006 | |
| Days of RRT, median (IQR) | 6 (3–14) | 5 (4–11) | 1.000 | |
| Requiring IABP, n (%) | 9 (8.3) | 16 (11.5) | 0.543 | |
| Days of IABP, median (IQR) | 6 (5–8) | 2 (2–3.2) | <0.001 | |
| Requiring ECMO, n (%) | 4 (3.7) | 5 (3.6) | 1.000 | |
| Days of ECMO, median (IQR) | 8.5 (8–10.2) | 4 (2–5) | 0.014 | |
| Requiring ICP monitoring, n (%) | 6 (5.6) | 22 (15.8) | 0.020 | |
| Days of ICP monitoring, median (IQR) | 8.5 (5–9.8) | 7.5 (5–9.8) | 0.866 | |
| Number of ICU treatments, mean (SD) | 1.7 (1.5) | 1.4 (0.7) | 0.026 | |
ARDS: acute respiratory distress syndrome; ECMO: extracorporeal membrane oxygenation; IABP: intra-aortic balloon pump; ICP: intracranial pressure; IQR: interquartile range; NEMS: nine equivalents of nursing manpower score; ICU: intensive care unit; RRT: renal replacement therapy; SAPS: simplified acute physiological score; SD: standard deviation
Supplementary table S1 (appendix) lists patients with different treatment times. Ninety-two patients (37.2%) received intensive care for 7–9 days, 60 patients (24.3%) for 10–14 days, 49 patients (19.8%) for 15–20 days, and 46 patients (18.6%) for ≥21 days. The mortality among the four groups in the ICU or after transfer to the normal ward was 18.5%, 32.7%, 40.8% and 43.4% and increased until follow-up to 53.3%, 60%, 59.2% and 73.9%, respectively.
The four Kaplan-Meier curves comparing patient mortality in age groups >65 years and <66 years, patients with a CCI ≤3 or >4, patients with or without septic shock, and patients who received ≥2 treatments compared with just one are shown in figure 2. Short vertical lines crossing the curves indicate censoring (i.e., right censoring indicates end of follow-up). Tables below each Kaplan-Meier curve indicate the number of patients “at risk”, which corresponds to the number of patients still alive at this time point. The numbers at the 7-year time point are fragmentary, as not all patients were observed for 7 years in total.
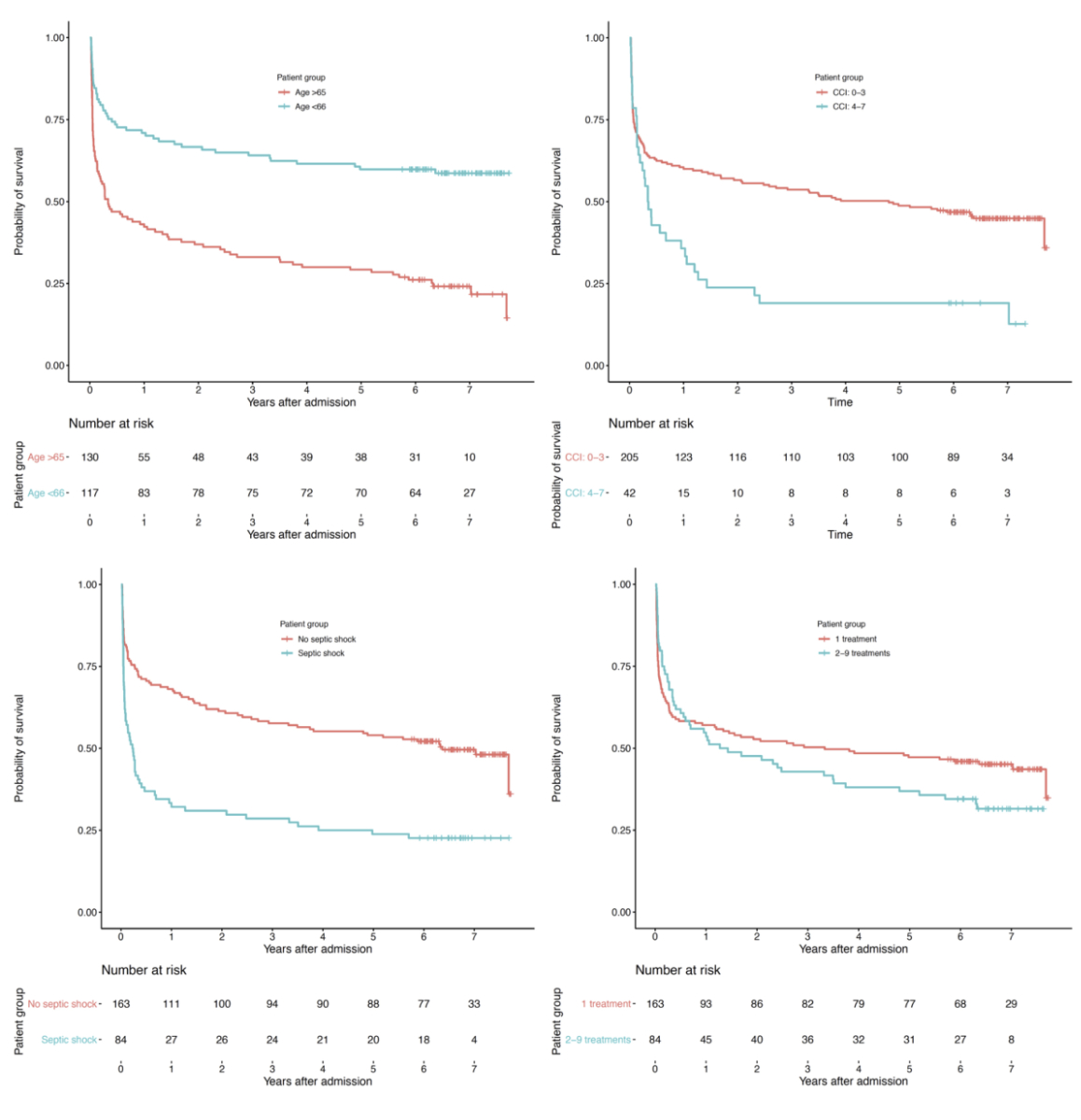
Figure 2 Kaplan-Meier curves for age, Charlson comorbidity index, septic shock, and number of treatments.
This study examines mortality after an ICU treatment of 7 or more consecutive days. Seventy-six of the 247 (30.8%) patients died in hospital, and another 32 (12.9%) within one year. At the end of follow-up, at least 6.6 years after the beginning of intensive care treatment, 99 patients (40.1%) were still alive. One-year mortality was significantly higher with increased age, higher CCI, longer length of ICU treatment, higher SAPS-II and NEMS score, the occurrence of pneumonia, sepsis or critical illness polyneuropathy, the need for RRT, and a higher number of complications.
As there is no generally accepted definition of prolonged ICU stay (PrLOS) [8, 14], we selected 7 days as the cut-off for long-term ICU treatment in this study. If the disease is more severe or if complications occur, ICU treatment can be prolonged, sometimes massively. The acute critical illness then turns into a persistent or chronic critical illness. Iwashyna et al. [17] defined a persistent critical illness as “when a patient's reason for being in ICU is now more related to their ongoing critical illness than their original reason for admission to the ICU.” In a single-centre cohort study in Australia, Williams et al. found the long-term mortality to reach a plateau after 10 days of ICU treatment [18]. Baghsaw et al. determined this transition point to be approximately 9 days after ICU admission in a cohort of about 18,000 patients in 12 Canadian ICUs [19]. This point can show a wide range. Iwashyna and colleagues found it between day 7 and day 22 across diagnosis-based subgroups and between day 6 and day 15 across risk-of-death-based subgroups [8]. A retrospective observational cohort study of three large Anglo-American intensive care databases showed that the probability of future survival does not decrease further after 5 to 10 days, except for patients older than 75 years [14]. A cut-off of 7 days, therefore, seems to be an adequate threshold for PrLOS.
Obviously, this is not the first study examining the outcome of patients with a PrLOS. Supplementary table S2 summarises 32 of these studies. Only studies that also investigated factors associated with short- or long-term mortality were included. This includes 11 studies from the USA [9, 11, 20–28], six from Germany [29–34], three from Canada [35–37], two from Sweden [38, 39], and one each from Scotland [40], Switzerland [12], Italy [41], Israel [42], France [43], Austria [44], Belgium [45], New Zealand [46] and Taiwan [47]. Nine studies examined a mixed ICU [20, 34, 36, 40, 43–46, 48], three a surgical ICU [30, 32, 47] and five studies examined the outcome after trauma [9, 23, 24, 26, 28]. Most studies investigated patients after heart surgery [11, 12, 21, 22, 25, 27, 29, 33, 35, 37–39, 41, 42]. The definition of a PrLOS shows a wide variation. The number of days defined as cut-off varies from 3 to 90 days. A stay of at least 7 days [22, 29, 33, 41, 46] and more than 30 days were chosen most often [9, 26, 36, 40, 44]. Eight studies compared different groups of days [21, 23, 25, 27, 28, 31, 34, 45]. Despite a rather wide variation in hospital mortality, there is a trend towards increasing mortality with longer treatment time. If the length of stay is <10 days, hospital mortality is most often less than 18%. Exceptions include very old patients (hospital mortality 23.8%) [35] and patients on mechanical ventilation for longer than 7 days (hospital mortality) [41]. All but seven studies had a follow-up after 6 months to 5 years. Except for a few studies, total mortality at follow-up exceeded 50%, sometimes considerably. Trauma patients represent an exception, having a good short- and long-term prognosis, with a survival rate of usually over 90% [23, 28].
Many factors were shown to be associated with short or long-term mortality. Age [9, 20, 23, 24, 26, 28–30, 32, 34, 36, 39–42, 46, 48] and acute renal failure with or without renal replacement therapy [12, 20–23, 26, 27, 30, 31, 33, 36, 37, 39, 42–47] were found most frequently. Length of mechanical ventilation was associated with mortality in 10 studies [12, 22, 29, 32, 33, 36, 37, 41, 43, 45]. Length of the ICU or hospital stay was shown to be associated with short- or long-term mortality five times [33–35, 37, 42], and APACHE-II score [32, 35, 46, 48] and perioperative stroke four times [22, 23, 25, 42]. Other associated factors such as perioperative infarction [23, 29, 42], or the CCI [31] were reported less often.
These findings correlate well with the present study. Age, acute renal failure and the length of ICU stay were dominant in our data as well as in other studies, and the mortality of our patients – short- and long-term – was comparable. It is not surprising that age is associated with 1-year mortality. Some studies focussed particularly on outcome of older patients. Octogenarians after cardiac surgery and an ICU stay of at least 5 days have higher hospital mortality than those with a shorter stay (23.8% vs 5.5%) and a significantly lower 1- and 5-year functional survival [35]. In a review article, Conti et al. examined the outcome of elderly patients after intensive care treatment [49]. The 22 included studies had very different definitions to describe “elderly”, ranging from 65 to 90 years. Long-term mortality increased with older age. If mortality is adjusted according to disease severity and comorbidities, the difference diminishes. In recent years, the concept of frailty (i.e., the combination of decreased mobility, weakness, reduced muscle mass, poor nutritional status and diminished cognitive function) has received increasing attention and has been shown to have a prognostic value [50]. In a 2017 systematic review by Muscedere et al., both hospital and long-term mortality of frail patients was higher and discharge home less likely in 10 observational studies examining the outcome of frail patients after intensive care treatment [51]. Acute renal failure – particularly if RRT is needed – is the second important factor that can indicate a bad outcome after PrLOS [52]. It is often the result of septic shock and triggers a cascade of systemic effects by release of cytokines and inflammatory mediators [53]. In recent years several strategies to prevent acute renal failure have been proposed that have the potential to improve outcome [54, 55]. Surprisingly, delirium was more often observed in the group of 1-year survivors. In a systematic review of 42 studies, Salluh et al. found increased hospital mortality in ICU patients with delirium [56]. One possible explanation for our contradictory results is the longer period of mechanical ventilation in those patients who died in the ICU compared with those who survived at least one year (median 10 vs 7 days). A delirium could have been masked by intubation and sedation.
Efforts were made to identify patients at risk for PrLOS or increased mortality. Wesch et al. presented a risk score assessed at day 7 for predicting a PrLOS for more than 20 days. This score includes the factors mechanical ventilation for >14 hours (98 points), need for parenteral nutrition on day 7 (36 points), lowest albumin <20 g/l (28 points), and a CCI >2 (23 points) with a cut-off at 100 points (sensitivity 88%, specificity 75%) [57]. The ProVent risk model uses the variables age >65 years (2 points), age 50–64 years (1 point), platelet count <150,000 /ml (1 point), vasopressors (1 point), haemodialysis (1 point) for patients requiring at least 21 days of mechanical ventilation [20]. A score >2 identifies patients who are at high risk of death within one year. For patients at risk for PrLOS, multimodal care management can optimise care and treatment [3, 57]. An evidence-based assessment and treatment plan includes early mobilisation, adapted nutrition, neurorehabilitation, scheduling daily activities and maintaining a diary. Ethical case discussions support the critical care team in end-of-life decision making for patients at high risk of death in hospital or soon after discharge [58, 59].
This study has some limitations. As a retrospective observational study involving one institution, the results cannot be generalised. It is possible that factors specific to this ICU will not be found in other ICUs. Our study focused on mortality, but this is only one important outcome. In recent years, quality of life after prolonged treatment in ICU has gained increasing attention.
Sixty percent of the patients who are treated for 7 or more consecutive days on a surgical ICU die within 7 years. One-year mortality (43.7%) is associated with age, CCI, length of ICU treatment, SAPS-II and NEMS scores, the occurrence of pneumonia, sepsis, critical illness polyneuropathy, the need for RRT and the number of complications. Scores combined with factors shown to be associated with increased short- and long-term mortality can help to identify patients at risk to die within one year after ICU treatment.
The authors thank Allison Dwileski, BS (Scientific Secretary, Department for Anesthesia, Intermediate Care, Prehospital Emergency Medicine and Pain Therapy, University Hospital Basel, Basel, Switzerland) for providing editorial assistance.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.
This study was funded solely from departmental sources.
1. Marshall JC , Bosco L , Adhikari NK , Connolly B , Diaz JV , Dorman T , et al. What is an intensive care unit? A report of the task force of the World Federation of Societies of Intensive and Critical Care Medicine. J Crit Care. 2017 Feb;37:270–6. https://doi.org/10.1016/j.jcrc.2016.07.015
2. Carson SS . Definitions and epidemiology of the chronically critically ill. Respir Care. 2012 Jun;57(6):848–56. https://doi.org/10.4187/respcare.01736
3. Desarmenien M , Blanchard-Courtois AL , Ricou B . The chronic critical illness: a new disease in intensive care. Swiss Med Wkly. 2016 Oct;146:w14336. https://doi.org/10.4414/smw.2016.14336
4. Marchioni A , Fantini R , Antenora F , Clini E , Fabbri L . Chronic critical illness: the price of survival. Eur J Clin Invest. 2015 Dec;45(12):1341–9. https://doi.org/10.1111/eci.12547
5. Iwashyna TJ , Viglianti EM . Patient and population-level approaches to persistent critical illness and prolonged intensive care unit stays. Crit Care Clin. 2018 Oct;34(4):493–500. https://doi.org/10.1016/j.ccc.2018.06.001
6. Long AC , Kross EK , Davydow DS , Curtis JR . Posttraumatic stress disorder among survivors of critical illness: creation of a conceptual model addressing identification, prevention, and management. Intensive Care Med. 2014 Jun;40(6):820–9. https://doi.org/10.1007/s00134-014-3306-8
7. Heyland DK , Konopad E , Noseworthy TW , Johnston R , Gafni A . Is it ‘worthwhile’ to continue treating patients with a prolonged stay (>14 days) in the ICU? An economic evaluation. Chest. 1998 Jul;114(1):192–8. https://doi.org/10.1378/chest.114.1.192
8. Iwashyna TJ , Hodgson CL , Pilcher D , Bailey M , van Lint A , Chavan S , et al. Timing of onset and burden of persistent critical illness in Australia and New Zealand: a retrospective, population-based, observational study. Lancet Respir Med. 2016 Jul;4(7):566–73. https://doi.org/10.1016/S2213-2600(16)30098-4
9. Trottier V , McKenney MG , Beninati M , Manning R , Schulman CI . Survival after prolonged length of stay in a trauma intensive care unit. J Trauma. 2007 Jan;62(1):147–50. https://doi.org/10.1097/01.ta.0000250496.99127.4a
10. Stricker K , Rothen HU , Takala J . Resource use in the ICU: short- vs. long-term patients. Acta Anaesthesiol Scand. 2003 May;47(5):508–15. https://doi.org/10.1034/j.1399-6576.2003.00083.x
11. Bashour CA , Yared JP , Ryan TA , Rady MY , Mascha E , Leventhal MJ , et al. Long-term survival and functional capacity in cardiac surgery patients after prolonged intensive care. Crit Care Med. 2000 Dec;28(12):3847–53. https://doi.org/10.1097/00003246-200012000-00018
12. Gersbach P , Tevaearai H , Revelly JP , Bize P , Chioléro R , von Segesser LK . Are there accurate predictors of long-term vital and functional outcomes in cardiac surgical patients requiring prolonged intensive care? Eur J Cardiothorac Surg. 2006 Apr;29(4):466–72. https://doi.org/10.1016/j.ejcts.2005.12.040
13. Merlani P , Chenaud C , Mariotti N , Ricou B . Long-term outcome of elderly patients requiring intensive care admission for abdominal pathologies: survival and quality of life. Acta Anaesthesiol Scand. 2007 May;51(5):530–7. https://doi.org/10.1111/j.1399-6576.2007.01273.x
14. Marshall DC , Hatch RA , Gerry S , Young JD , Watkinson P . Conditional survival with increasing duration of ICU admission: an observational study of three intensive care databases. Crit Care Med. 2020 Jan;48(1):91–7. https://doi.org/10.1097/CCM.0000000000004082
15. Le Gall JR , Lemeshow S , Saulnier F . A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993 Dec;270(24):2957–63. https://doi.org/10.1001/jama.270.24.2957 https://doi.org/10.1001/jama.1993.03510240069035
16. Reis Miranda D , Moreno R , Iapichino G . Nine equivalents of nursing manpower use score (NEMS). Intensive Care Med. 1997 Jul;23(7):760–5. https://doi.org/10.1007/s001340050406
17. Iwashyna TJ , Hodgson CL , Pilcher D , Bailey M , Bellomo R . Persistent critical illness characterised by Australian and New Zealand ICU clinicians. Crit Care Resusc. 2015 Sep;17(3):153–8.
18. Williams TA , Ho KM , Dobb GJ , Finn JC , Knuiman M , Webb SA ; Royal Perth Hospital ICU Data Linkage Group . Effect of length of stay in intensive care unit on hospital and long-term mortality of critically ill adult patients. Br J Anaesth. 2010 Apr;104(4):459–64. https://doi.org/10.1093/bja/aeq025
19. Bagshaw SM , Stelfox HT , Iwashyna TJ , Bellomo R , Zuege D , Wang X . Timing of onset of persistent critical illness: a multi-centre retrospective cohort study. Intensive Care Med. 2018 Dec;44(12):2134–44. https://doi.org/10.1007/s00134-018-5440-1
20. Carson SS , Kahn JM , Hough CL , Seeley EJ , White DB , Douglas IS , et al.; ProVent Investigators . A multicenter mortality prediction model for patients receiving prolonged mechanical ventilation. Crit Care Med. 2012 Apr;40(4):1171–6. https://doi.org/10.1097/CCM.0b013e3182387d43
21. Elfstrom KM , Hatefi D , Kilgo PD , Puskas JD , Thourani VH , Guyton RA , et al. What happens after discharge? An analysis of long-term survival in cardiac surgical patients requiring prolonged intensive care. J Card Surg. 2012 Jan;27(1):13–9. https://doi.org/10.1111/j.1540-8191.2011.01341.x
22. Engoren M , Buderer NF , Zacharias A . Long-term survival and health status after prolonged mechanical ventilation after cardiac surgery. Crit Care Med. 2000 Aug;28(8):2742–9. https://doi.org/10.1097/00003246-200008000-00010
23. Kisat MT , Latif A , Zogg CK , Haut ER , Zafar SN , Hashmi ZG , et al. Survival outcomes after prolonged intensive care unit length of stay among trauma patients: the evidence for never giving up. Surgery. 2016 Sep;160(3):771–80. https://doi.org/10.1016/j.surg.2016.04.024
24. Miller RS , Patton M , Graham RM , Hollins D . Outcomes of trauma patients who survive prolonged lengths of stay in the intensive care unit. J Trauma. 2000 Feb;48(2):229–34. https://doi.org/10.1097/00005373-200002000-00006
25. Yu PJ , Cassiere HA , Fishbein J , Esposito RA , Hartman AR . Outcomes of patients with prolonged intensive care unit length of stay after cardiac surgery. J Cardiothorac Vasc Anesth. 2016 Dec;30(6):1550–4. https://doi.org/10.1053/j.jvca.2016.03.145
26. Ong AW , Omert LA , Vido D , Goodman BM , Protetch J , Rodriguez A , et al. Characteristics and outcomes of trauma patients with ICU lengths of stay 30 days and greater: a seven-year retrospective study. Crit Care. 2009;13(5):R154. https://doi.org/10.1186/cc8054
27. Ryan TA , Rady MY , Bashour CA , Leventhal M , Lytle B , Starr NJ . Predictors of outcome in cardiac surgical patients with prolonged intensive care stay. Chest. 1997 Oct;112(4):1035–42. https://doi.org/10.1378/chest.112.4.1035
28. Chaudhary MA , Schoenfeld AJ , Koehlmoos TP , Cooper Z , Haider AH . Prolonged ICU stay and its association with 1-year trauma mortality: an analysis of 19,000 American patients. Am J Surg. 2019 Jul;218(1):21–6. https://doi.org/10.1016/j.amjsurg.2019.01.025
29. Grothusen C , Attmann T , Friedrich C , Freitag-Wolf S , Haake N , Cremer J , et al. Predictors for long-term outcome and quality of life of patients after cardiac surgery with prolonged intensive care unit stay. Interv Med Appl Sci. 2013 Mar;5(1):3–9. https://doi.org/10.1556/IMAS.5.2013.1.1 https://doi.org/10.1556/imas.5.2013.1.1
30. Martini V , Lederer AK , Laessle C , Makowiec F , Utzolino S , Fichtner-Feigl S , et al. Clinical characteristics and outcomes of surgical patients with intensive care unit lengths of stay of 90 days and greater. Crit Care Res Pract. 2017;2017:9852017. https://doi.org/10.1155/2017/9852017
31. Eschbach D , Bliemel C , Oberkircher L , Aigner R , Hack J , Bockmann B , et al. One-year outcome of geriatric hip-fracture patients following prolonged ICU treatment. BioMed Res Int. 2016;2016:8431213. https://doi.org/10.1155/2016/8431213
32. Hartl WH , Wolf H , Schneider CP , Küchenhoff H , Jauch KW . Acute and long-term survival in chronically critically ill surgical patients: a retrospective observational study. Crit Care. 2007;11(3):R55. https://doi.org/10.1186/cc5915
33. Schöttler J , Hagemann A , Grothusen C , Stohn S , Pleger D , von der Brelie M , et al. [Mid-term outcome of cardiac surgery patients with prolonged postoperative intensive care treatment]. Med Klin Intensivmed Notf Med. 2011 Sep;106(1):41–7. https://doi.org/10.1007/s00063-011-0025-6
34. Weiler N , Waldmann J , Bartsch DK , Rolfes C , Fendrich V . Outcome in patients with long-term treatment in a surgical intensive care unit. Langenbecks Arch Surg. 2012 Aug;397(6):995–9. https://doi.org/10.1007/s00423-012-0966-0
35. Arora RC , Manji RA , Singal RK , Hiebert B , Menkis AH . Outcomes of octogenarians discharged from the hospital after prolonged intensive care unit length of stay after cardiac surgery. J Thorac Cardiovasc Surg. 2017 Nov;154(5):1668–1678.e2. https://doi.org/10.1016/j.jtcvs.2017.04.083
36. Friedrich JO , Wilson G , Chant C . Long-term outcomes and clinical predictors of hospital mortality in very long stay intensive care unit patients: a cohort study. Crit Care. 2006;10(2):R59. https://doi.org/10.1186/cc4888
37. Manji RA , Arora RC , Singal RK , Hiebert B , Moon MC , Freed DH , et al. Long-term outcome and predictors of noninstitutionalized survival subsequent to prolonged intensive care unit stay after cardiac surgical procedures. Ann Thorac Surg. 2016 Jan;101(1):56–63. https://doi.org/10.1016/j.athoracsur.2015.07.004
38. Hellgren L , Ståhle E . Quality of life after heart valve surgery with prolonged intensive care. Ann Thorac Surg. 2005 Nov;80(5):1693–8. https://doi.org/10.1016/j.athoracsur.2005.04.042
39. Lagercrantz E , Lindblom D , Sartipy U . Survival and quality of life in cardiac surgery patients with prolonged intensive care. Ann Thorac Surg. 2010 Feb;89(2):490–5. https://doi.org/10.1016/j.athoracsur.2009.09.073
40. Hughes M , MacKirdy FN , Norrie J , Grant IS . Outcome of long-stay intensive care patients. Intensive Care Med. 2001 Apr;27(4):779–82. https://doi.org/10.1007/s001340100896
41. Pappalardo F , Franco A , Landoni G , Cardano P , Zangrillo A , Alfieri O . Long-term outcome and quality of life of patients requiring prolonged mechanical ventilation after cardiac surgery. Eur J Cardiothorac Surg. 2004 Apr;25(4):548–52. https://doi.org/10.1016/j.ejcts.2003.11.034
42. Silberman S , Bitran D , Fink D , Tauber R , Merin O . Very prolonged stay in the intensive care unit after cardiac operations: early results and late survival. Ann Thorac Surg. 2013 Jul;96(1):15–21. https://doi.org/10.1016/j.athoracsur.2013.01.103
43. Combes A , Costa MA , Trouillet JL , Baudot J , Mokhtari M , Gibert C , et al. Morbidity, mortality, and quality-of-life outcomes of patients requiring >or=14 days of mechanical ventilation. Crit Care Med. 2003 May;31(5):1373–81. https://doi.org/10.1097/01.CCM.0000065188.87029.C3
44. Delle Karth G , Meyer B , Bauer S , Nikfardjam M , Heinz G . Outcome and functional capacity after prolonged intensive care unit stay. Wien Klin Wochenschr. 2006 Jul;118(13-14):390–6. https://doi.org/10.1007/s00508-006-0616-z
45. Hermans G , Van Aerde N , Meersseman P , Van Mechelen H , Debaveye Y , Wilmer A , et al. Five-year mortality and morbidity impact of prolonged versus brief ICU stay: a propensity score matched cohort study. Thorax. 2019 Nov;74(11):1037–45. https://doi.org/10.1136/thoraxjnl-2018-213020
46. Carden GP , Graham JW , McLennan S , Celi LA . Long-term outcome of long stay ICU and HDU patients in a new zealand hospital. Crit Care Shock. 2008 Mar;11(1):26–34.
47. Huang YC , Huang SJ , Tsauo JY , Ko WJ . Definition, risk factors and outcome of prolonged surgical intensive care unit stay. Anaesth Intensive Care. 2010 May;38(3):500–5. https://doi.org/10.1177/0310057X1003800314
48. Steenbergen S , Rijkenberg S , Adonis T , Kroeze G , van Stijn I , Endeman H . Long-term treated intensive care patients outcomes: the one-year mortality rate, quality of life, health care use and long-term complications as reported by general practitioners. BMC Anesthesiol. 2015 Oct;15(1):142. https://doi.org/10.1186/s12871-015-0121-x
49. Conti M , Merlani P , Ricou B . Prognosis and quality of life of elderly patients after intensive care. Swiss Med Wkly. 2012 Sep;142:w13671. https://doi.org/10.4414/smw.2012.13671
50. Clegg A , Young J , Iliffe S , Rikkert MO , Rockwood K . Frailty in elderly people. Lancet. 2013 Mar;381(9868):752–62. https://doi.org/10.1016/S0140-6736(12)62167-9
51. Muscedere J , Waters B , Varambally A , Bagshaw SM , Boyd JG , Maslove D , et al. The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis. Intensive Care Med. 2017 Aug;43(8):1105–22. https://doi.org/10.1007/s00134-017-4867-0
52. Hoste EA , Bagshaw SM , Bellomo R , Cely CM , Colman R , Cruz DN , et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015 Aug;41(8):1411–23. https://doi.org/10.1007/s00134-015-3934-7
53. Druml W . Systemic consequences of acute kidney injury. Curr Opin Crit Care. 2014 Dec;20(6):613–9. https://doi.org/10.1097/MCC.0000000000000150
54. Joannidis M , Druml W , Forni LG , Groeneveld AB , Honore P , Oudemans-van Straaten HM , et al.; Critical Care Nephrology Working Group of the European Society of Intensive Care Medicine . Prevention of acute kidney injury and protection of renal function in the intensive care unit. Expert opinion of the Working Group for Nephrology, ESICM. Intensive Care Med. 2010 Mar;36(3):392–411. https://doi.org/10.1007/s00134-009-1678-y
55. Joannidis M , Druml W , Forni LG , Groeneveld AB , Honore PM , Hoste E , et al. Prevention of acute kidney injury and protection of renal function in the intensive care unit: update 2017 : Expert opinion of the Working Group on Prevention, AKI section, European Society of Intensive Care Medicine. Intensive Care Med. 2017 Jun;43(6):730–49. https://doi.org/10.1007/s00134-017-4832-y
56. Salluh JI , Wang H , Schneider EB , Nagaraja N , Yenokyan G , Damluji A , et al. Outcome of delirium in critically ill patients: systematic review and meta-analysis. BMJ. 2015 Jun;350(may19 3):h2538. https://doi.org/10.1136/bmj.h2538
57. Wesch C , Denhaerynck K , Barandun Schaefer U , Siegemund M , Wehrli M , Pargger H , et al. Development and validation of a multivariable risk score for prolonged length of stay in the surgical intensive care unit. Swiss Med Wkly. 2019 Sep;149:w20122. https://doi.org/10.4414/smw.2019.20122
58. Meyer-Zehnder B , Barandun Schäfer U , Albisser Schleger H , Reiter-Theil S , Pargger H . Ethische Fallbesprechungen auf der Intensivstation : Vom Versuch zur Routine. Anaesthesist. 2014 Jun;63(6):477–87. https://doi.org/10.1007/s00101-014-2331-x
59. Meyer-Zehnder B , Barandun Schäfer U , Wesch C , Reiter-Theil S , Pargger H. Weekly internal ethical case discussions in an ICU - Results based on 9 years of experience with a highly structured approach. Crit Care Explor. 2021;3(3):e0352. doi: https://doi.org/10.1097/cce.0000000000000352. PubMed PMID: 02107256-202103000-00019. https://doi.org/10.1097/CCE.0000000000000352
Table S1Patient characteristics and outcome of different LOS-groups.
| 7–9 days (n = 92) | 10–14 days (n = 60) | 15–20 days (n = 49) | ≥21 days (n = 46) | All patients (n = 247) | ||
| Outcome, n (%) | Died in ICU | 10 (10.9) | 14 (23.3) | 14 (28.6) | 14 (30.4) | 52 (21.1) |
| Died in hospital | 7 (7.6) | 10 (16.7) | 2 (4.1) | 6 (13.0) | 25 (10.1) | |
| Died after discharge | 32 (34.8) | 12 (20.0) | 13 (26.5) | 14 (30.4) | 71 (28.7) | |
| Survived at least 1 year | 66 (71.7) | 29 (48.3) | 25 (51.0) | 19 (41.3) | 139 (56.3) | |
| Survived to follow-up | 43 (46.7) | 24 (40.0) | 20 (40.8) | 12 (26.1) | 99 (40.1) | |
| Age (years), median (IQR) | 67 (48–74) | 66.5 (56.5–73.5) | 72 (60–76) | 63 (56–70) | 66 (56–75) | |
| Sex, n (%) | Male | 58 (63.0) | 45 (75.0) | 30 (61.2) | 30 (65.2) | 163 (66.0) |
| Female | 34 (37.0) | 15 (25.0) | 19 (38.8) | 16 (34.8) | 84 (34.0) | |
| Charlson comorbidity index, mean (SD) | 1.5 (1.8) | 1.7 (1.7) | 1.8 (2.0) | 2.0 (1.7) | 1.7 (1.8) | |
| Emergency admission, n (%) | 62 (67.4) | 50 (83.3) | 32 (65.3) | 27 (58.7) | 171 (69.2) | |
| Length of stay (days), median (IQR) | 8.4 (7.7–9.2) | 12.7 (10.8–13.6) | 17.6 (16.7–18.9) | 32.2 (25.2–40.5) | 12.7 (9.0–18.6) | |
| Main diagnosis, n (%) | Traumatic brain injury | 7 (63.6) | 3 (27.3) | 1 (9.1) | 0 | 11 (4.5) |
| Polytrauma | 10 (32.3) | 10 (32.3) | 7 (22.6) | 4 (12.9) | 31 (12.6) | |
| Internal disease | 1 (20.0) | 2 (40.0) | 2 (40.0) | 0 | 5 (2.0) | |
| Type of surgery, n (%) | Cardiac | 39 (39.4) | 25 (25.3) | 15 (15.2) | 20 (20.2) | 99 (40.1) |
| Neurological | 5 (33.3) | 5 (26.7) | 2 (13.3) | 3 (20.0) | 15 (6.1) | |
| Pulmonary | 4 (26.7) | 2 (13.3) | 2 (13.3) | 7 (46.7) | 15 (6.1) | |
| Abdominal | 18 (46.2) | 1 (2.6) | 10 (25.6) | 10 (25.6) | 39 (15.8) | |
| Vascular | 1 (5.9) | 9 (52.9) | 5 (29.4) | 2 (11.8) | 17 (6.9) | |
| Maxillofacial | 1 (20.0) | 1 (20.0) | 3 (60.0) | 0 | 5 (2.0) | |
| Orthopaedic | 5 (71.4) | 1 (14.3) | 1 (14.3) | 0 | 7 (2.8) | |
| Urological | 1 (33.3) | 1 (33.3) | 1 (33.3) | 0 | 3 (1.2) | |
| SAPS-II, mean (SD) | 44.3 (17.0) | 50.9 (15.0) | 49.6 (15.1) | 53.9 (20.8) | 48.7 (17.3) | |
| NEMS, mean (SD) | 735.1 (155.5) | 1134.1 (208.7) | 1645.1 (314.2) | 3177.5 (1428.2) | 1467.4 (1092.1) | |
| ICU complications, n (%) | Delirium | 52 (56.5) | 34 (56.7) | 28 (57.1) | 26 (56.5) | 140 (56.7) |
| Stroke | 7 (7.6) | 3 (5.0) | 4 (8.2) | 4 (8.7) | 18 (7.3) | |
| Tracheobronchitis | 15 (16.3) | 20 (33.3) | 13 (26.5) | 14 (30.4) | 62 (25.1) | |
| Pneumonia | 21 (22.8) | 23 (38.3) | 32 (44.9) | 30 (65.2) | 96 (38.9) | |
| Pleural empyema | 1 (1.1) | 3 (5.0) | 3 (6.1) | 11 (23.9) | 18 (7.3) | |
| ARDS | 4 (4.3) | 2 (3.3) | 7 (14.3) | 9 (19.6) | 22 (8.9) | |
| Septic shock | 22 (23.9) | 18 (30.0) | 18 (36.7) | 26 (56.5) | 84 (34.0) | |
| Acute renal insufficiency | 16 (17.4) | 14 (23.3) | 17 (35.7) | 27 (58.7) | 74 (30.0) | |
| Acute on chronic renal insufficiency | 19 (20.7) | 15 (25.0) | 10 (20.4) | 9 (19.6) | 53 (21.5) | |
| Critical illness polyneuropathy | 0 | 3 (5.0) | 1 (2.0) | 18 (39.1) | 22 (8.9) | |
| Number of complications, mean (SD) | 1.7 (1.0) | 2.3 (1.5) | 2.5 (1.3) | 3.7 (1.4) | 2.4 (1.5) | |
| Requiring mechanical ventilation, n (%) | 80 (87.0) | 57 (95.0) | 49 (100) | 46 (100) | 232 (93.9) | |
| Days of ventilation, median (IQR) | 4 (2–6) | 8 (4–11) | 10 (8–13) | 13.5 (10–21) | 7 (4–11) | |
| Requiring tracheostomy, n (%) | 14 (15.2) | 12 (20.0) | 19 (38.8) | 28 (60.9) | 73 (29.2) | |
| Requiring RRT, n (%) | 9 (9.8) | 12 (20.0) | 13 (26.5) | 20 (43.5) | 54 (21.9) | |
| Days of RRT, median (IQR) | 4 (3–5) | 4 (3–11.5) | 6 (4–10) | 13.5 (7–18) | 6 (3.75–14) | |
| Requiring IABP, n (%) | 5 (5.4) | 7 (11.7) | 6 (12.2) | 7 (15.2) | 25 (10.1) | |
| Days of IABP, median (IQR) | 6 (4–8) | 4 (3–5) | 2 (2–5) | 2 (1–5) | 3 (2–5.5) | |
| Requiring ECMO, n (%) | 4 (4.3) | 2 (3.3) | 2 (4.1) | 1 (2.2) | 9 (3.6) | |
| Days of ECMO, median (IQR) | 5 (2–8.5) | 11 (8–14) | 4.5 (4–5) | 7 | 7 (3–8.5) | |
| Requiring ICP monitoring, n (%) | 10 (10.9) | 11 (18.3) | 5 (10.2) | 2 (4.3) | 28 (11.3) | |
| Days of ICP monitoring, median (IQR) | 6.5 (5–10) | 6 (4–9) | 12 (9–15) | 20 (11–29) | 8 (4.25–10) | |
| Number of ICU treatments, mean (SD) | 1.3 (0.6) | 1.6 (1.1) | 1.5 (1.1) | 2.1 (1.7) | 1.56 (1.1) | |
ARDS: acute respiratory distress syndrome; ECMO: extracorporeal membrane oxygenation; IABP: intra-aortic balloon pump; ICP: intracranial pressure; IQR: interquartile range; NEMS: nine equivalents of nursing manpower score; ICU: intensive care unit; RRT: renal replacement therapy; SAPS: simplified acute physiological score; SD: standard deviation
Table S2Studies examining the outcome after prolonged ICU length of stay and associated factors.
| Reference, year, country | Type pat./ICU | Definition of PrLOS | Patients (n) | Follow-up period | Mortality | Factors associated with short- or long-term mortality |
| Arora et al. [35], 2017, Canada | Cardiac surgery | Age >80 y and >5 d | 105 | 1 and 5 y | 25 (23.8%) in-hospital; another 11 (10.5%) within 1 y; another 38 (36.2%) within 5 y | Diabetes / APACHE II ≥20 / Days in hospital / More physician visits within 30 d |
| Bashour et al. [11], 2000, USA | Cardiac surgery | >10 d | 142 | 16-37 mo | 47 (33%) in-hospital; another 44 (31%) until follow-up | Previous myocardial infarction / Preoperative hypalbuminaemia / History of congestive heart failure |
| Elfstrom et al. [21], 2012, USA | Cardiac surgery | (a) <3 d; (b) 3–7 d; (c) 7–14 d; (d) >14 d | (a) 8666; (b) 1625; (c) 405; (d) 388 | 1 y | One-year: (a) 259 (3%); (b) 144 (8.9%); (c) 49 (12.1%); (d) 123 (31.7%) | Postoperative renal failure / Reintubation |
| Engoren et al. [22], 2000, USA | Cardiac surgery | Mechanical ventilation ≥7 d | 123 | 5 y | 19 (15%) in-hospital; Another 51 (41%) until follow-up | Aortic valve surgery / Pre- or postoperative stroke / Admitting creatinine / Postoperative renal failure / Days of mechanical ventilation / Discharge on tube feeding |
| Gersbach et al. [12], 2006, Switzerland | Cardiac surgery | >5 d | 194 | 1 and 3 y | 17 (8.7%) in-hospital; Another 19 (9.8%) until follow-up | Mechanical ventilation >5 d / Renal replacement therapy |
| Grothusen et al. [29], 2013, Germany | Cardiac surgery | >7 d | 230 | 5 y | 28 (12%) in-hospital; Another 91 (39.5%) until follow-up | Age >70 y / Preoperative atrial fibrillation / Myocardial infarction / Mechanical ventilation >8 d |
| Hellgren et al. [38], 2005, Sweden | Cardiac surgery | >8 d | 225 | 1-5 y | 27 (12%) in-hospital; Another 44 (19.5%) until follow-up | NYHA ≥III |
| Lagercrantz et al. [39], 2010, Sweden | Cardiac surgery | >10 d | 141 | 1, 3 and 5 y | 46 (33%) in-hospital; Another 19 (14%) until follow-up; | Renal replacement therapy / Age |
| Manji et al. [37], 2016, Canada | Cardiac surgery | >5 d | 728 | 1 and 5 y | 132 (18%) in-hospital; Another 300 (41%) until follow-up | Age >80 y / Preoperative dialysis / Cerebrovascular disease / Peripheral vascular disease / Preoperative infection / ECMO/VAD / Days in ICU / Ventilator days / Renal replacement therapy |
| Pappalardo et al. [41], 2004, Italy | Cardiac surgery | Mechanical ventilation >7 d | 148 | 36±12 mo | 67 (45.3%) in-hospital; Another 11 (7.4%) until follow-up | Age / Diabetes / Mechanical ventilation >21 d |
| Ryan et al. [27], 1997, USA | Cardiac surgery | (a) >14 d; (b) >28 d | (a) 324; (b) 166 | None | In-hospital: (a) 141 (43.5%); (b) 74 (45%) | Reduced GCS / Vasopressors / Renal replacement therapy / Lower platelet count / Lower serum albumin |
| Schöttler et al. [33], 2011, Germany | Cardiac surgery | >7 d | 223 | 1 y | 28 (12.6%) in-hospital; Another 60 (26.9%) until follow-up | Preoperative atrial fibrillation / Preoperative pulmonary hypertension / Days in ICU / Days with vasopressors / Days with mechanical ventilation / Tracheostomy / Pneumonia / Renal replacement therapy |
| Silberman et al. [42], 2013, Israel | Cardiac surgery | >14 d | 331 | 5 y | 131 (40%) in-hospital; Another 96 (29%) until follow-up | Days in ICU / Age / Female sex / COPD / Sepsis / Stroke / Perioperative myocardial infarction / Renal deterioration |
| Yu et al. [25], 2016, USA | Cardiac surgery | (a) 1–2 wk; (b) 2–4 wk; (c) >4 wk | (a) 162; (b) 79; (c) 142 | 2 y | In-hospital: (a) 18 (11.1%); (b) 21 (26.6%); (c) 44 (31%); Total mortality after 2 y: (a) 24.7% ; b) 25.9% ; c) 58.9% | Preoperative low haematocrit / Postoperative stroke / Peripheral vascular disease |
| Carden et al. [46], 2008, New Zealand | Mixed | >7d | 207 | 1 y | 60 (28%) in-hospital; Another 24 (12%) within 1 y | Age / APACHE II score / Sepsis / Renal replacement therapy / Cardiac arrest in ICU |
| Carson et al. [20], 2012, USA | Mixed | Mechanical ventilation >21 d | 260 | 1 y | 72 (28%) in-hospital; Another 52 (20%) until follow-up | Age / Platelet count >150 / Vasopressors / Renal replacement therapy |
| Combes et al. [43], 2003, France | Mixed | Mechanical ventilation >14 d | 347 | 3 y | 150 (43%) ICU; Another 58 (29%) until follow-up | Age >65 y / NYHA ≥III / Preadmission immunocompromised status / Septic shock and nosocomial septicaemia / Renal replacement therapy / Mechanical ventilation >35 d |
| Delle Karth et al. [44], 2006, Austria | Mixed | >30 d | 135 | 1-4 y | 46 (34%) in-hospital; Another 46 (34%) until follow-up | Renal replacement therapy |
| Friedrich et al. [36], 2006, Canada | Mixed | >30 d | 182 | 6 mo | 76 (42%) in-hospital; Another 15 (8%) until follow-up | Age / Immunosuppression / Mechanical ventilation >90 d / Vasopressor for >3 d / Renal replacement therapy |
| Hermans et al. [45], 2019, Belgium | Mixed | (a) <8 d; (b) >8 d | (a) 3410; (b) 1209 | 5 y | In-hospital: (a) 146 (4.3%); (b) 158 (13.1%); Another until follow-up: (a) 673 (19.7%); (b) 449 (37.2%) | Hypoglycaemia in ICU / Infection in ICU / Mechanical ventilation >2 d / Renal replacement therapy |
| Hughes et al. [40], 2001, Scotland | Mixed | >30 d | 322 | None | 129 (40.1%) in-hospital | Age >70 y |
| Steenbergen et al. [48], 2015, Netherland | Mixed | >72 h | 740 | 1 y | 191 (26%) in-hospital; Another 175 (28%) until follow-up; | Age / APACHE-IV PM Score / Comorbidity / ICU readmission |
| Weiler et al. [34], 2012, Germany | Mixed | (a) 5–19 d; (b) >20 d | (a) 87; (b) 67 | 1 y | In-hospital: (a) 38 (43.7%); (b) 29 (43.2%); Total mortality after 1 y: (a) 49 (56.3%); (b) 41 (61.2%) | Age / Days in ICU / SAPS II |
| Hartl et al. [32], 2007, Germany | Surgical | >28 d | 390 | 1, 3 and 5 y | 186 (47.7%) in-hospital; Survival of discharged patients:1 y 61.8%; 3 y 44.7%; 5 y 37.0% | Age / APACHE II score / Thoracic surgery / Palliative surgery / Mechanical ventilation >50 d / Number of surgical revisions / Pneumonia |
| Huang et al. [47], 2010, Taiwan | Surgical | >16 d | 377 | 1 y | 132 (35%) in-hospital; Another 66 (14.4%) until follow-up | Renal replacement therapy |
| Martini et al. [30], 2017, Germany | Surgical | >90 d | 19 | 1 y | 10 (52%) in-hospital; Another 4 (21%) until follow-up | Age / Renal replacement therapy |
| Chaudhary et al. [28], 2019 , USA | Trauma | (a) 2–9 d; (b) >9 d | (a) 4346; (b) 1043 | 1 y | Until follow-up: (a) 154 (3.5%); (b) 50 (4.8%) | Age |
| Eschbach et al. [31], 2016, Germany | Hip-fracture, Age >60 y | (a) <3 d; (b) >3 d | (a) 275; (b) 61 | 1 y | In-hospital: (a) 9 (3.2%); (b) 16 (26.2%); Another until follow-up: (a) 60 (21.8%) ; (b) 33 (54.1%) | ASA class / Charlson Comorbidity Index / Renal replacement therapy |
| Kisat et al. [23], 2016, USA | Trauma | (a) 1 d; (b) 2–9 d; (c) 10–40 d; (d) >40 d | (a) 168,604; (b) 324,419; (c) 8,334; (d) 4,794 | None | In-hospital: (a) 9.9%; (b) 4.9%; (c) 6.6%; (d) 9.8% | Age >35 y / Higher ISS / Lower GCS / Myocardial infarction / Stroke / Renal failure |
| Miller et al. [24], 2000, USA | Trauma | >3 wk | 115 | NA | In-hospital: 25 (22%); After discharge another: 11 (9.6%) | Age |
| Ong et al. [26], 2009, USA | Trauma | >30 d | 205 | None | 25 (12%) in-hospital | Age >64 y / Preexisting cardiac or renal disease / ARDS / Renal replacement therapy |
| Trottier et al. [9], 2007, USA | Trauma | >30 d | 339 | None | 45 (13.3%) in-hospital | Age >50 y |
APACHE II: acute physiology and chronic health evaluation; ARDS: acute respiratory distress syndrome; ASA = American Society of Anesthesiologists: COPD: chronic obstructive pulmonary disease; d: day, ECMO: extracorporeal membrane oxygenation; GCS: Glasgow Coma Scale; ICU: intensive care unit; ISS: injury severity score; mo: month; NYHA: New York Heart Association; PM: predicted mortality; PrLOS: prolonged ICU stay; SAPS II: simplified acute physiological score; VAD: ventricular assist device; wk: week; y: year