Increasing prevalence of obesity and diabetes among patients evaluated for liver transplantation in a Swiss tertiary referral center: a 10-year retrospective analysis
DOI: https://doi.org/10.4414/SMW.2022.w30138
Sophie
Kasmia, Florent
Artrua, Joana
Vieira Barbosaa, Ansgar
Rudolf Deibelb, Lucie
Favrec, Claire
Peubled, Anne-Catherine
Saoulid, Nicolas
Goossensef, Beat
Müllhauptb, Manuel
Pascuald, Darius
Moradpoura, Julien
Vionnetad, Montserrat
Fragaa
aDivision of Gastroenterology and Hepatology, Lausanne University Hospital and University of Lausanne, Switzerland
bDivision of Gastroenterology and Hepatology, University Hospital Zürich, Switzerland
cDivision of Endocrinology, Diabetology and Metabolism, Lausanne University Hospital and University of Lausanne, Switzerland
dTransplantation Centre, Lausanne University Hospital and University of Lausanne, Switzerland
eDivision of Gastroenterology and Hepatology, University Hospital Geneva, Switzerland
fDivision of Transplantation, University Hospital Geneva, Switzerland
Summary
BACKGROUND AND AIMS: Non-alcoholic fatty liver disease (NAFLD) is now the first cause of chronic liver disease in developed countries. We aimed to assess trends in the prevalence of obesity, type 2 diabetes mellitus (T2DM) and NAFLD in patients undergoing liver transplantation evaluation and to assess whether obese patients were less likely to be listed or had an increased drop-out rate after listing.
METHODS: We conducted a retrospective study of all consecutive patients who underwent liver transplantation evaluation at a Swiss tertiary referral centre between January 2009 and March 2020.
RESULTS: A total of 242 patients were included, 83% were male. The median age was 59 years (IQR, 51–64 years). The most common causes of end-stage liver disease were viral hepatitis (28%), alcoholic liver disease (21%) and NAFLD (12%). Obesity was present in 28% of our cohort, with a significant increase over time. Prevalence of type 2 diabetes mellitus followed the same trend (p = 0.02). The proportions of non-listed and listed obese patients did not differ (21% vs. 30% respectively; p = 0.3).
CONCLUSIONS: The prevalence of obesity and type 2 diabetes mellitus significantly increased over our study period. Obese patients had similar chances of being listed. The landscape of liver transplantation indications is shifting towards NAFLD, highlighting the urgent need to prevent NAFLD progression.
Abbreviations
- ALP
-
alkaline phosphatase
- ALT
-
alanine aminotransferase
- BMI
-
body mass index
- GGT
-
gamma-glutamyl transferase
- HCV
-
hepatitis C virus
- MELD
-
model for end-stage liver disease
- NAFLD
-
non-alcoholic fatty liver disease
- NASH
-
non-alcoholic steatohepatitis
Introduction
The prevalence of obesity has increased at an alarming pace over the last four decades. Once a relatively minor public health issue, overnutrition and obesity have become a major threat, and it is estimated that at least one third of the world’s adult population is now overweight or obese [1]. It is predicted that the prevalence of severe obesity will continue to increase and that by 2030 nearly one in two adults in the United States will be obese [2].
The global epidemic of obesity is also reflected among solid organ transplant recipients. In the renal transplant population, the proportion of recipients with a body mass index (BMI) ≥30 kg/m2 has doubled every 15 years [3, 4]. Similar observations have been made in the liver transplant populations in North America and in Europe [5–7].
Obesity, defined as a BMI ≥30 kg/m2 by the World Health Organization (WHO), is the most common risk factor for the development of non-alcoholic fatty liver disease (NAFLD), followed by type 2 diabetes mellitus [8, 9]. The clinical spectrum of NAFLD ranges from simple steatosis to the more aggressive non-alcoholic steatohepatitis (NASH), which can eventually progress to advanced fibrosis and cirrhosis [10]. Whereas chronic hepatitis C classically dominated the indications for liver transplantation in Europe and North America, the advent of direct-acting antivirals has dramatically changed the landscape of liver transplantation. In the meantime, NAFLD has become the most common chronic liver disease in many developed countries [11–14].
In parallel with the development of cirrhosis in patients with NASH, obesity also contributes significantly to the burden of hepatocellular carcinoma, as recently highlighted by several large-scale epidemiological studies [15]. A worrisome feature is that hepatocellular carcinoma can even develop in individuals with NAFLD who do not have advanced liver fibrosis or cirrhosis [16, 17].
Obesity and NAFLD are also known to be associated with increased cardiovascular morbidity which, in turn, may preclude listing for liver transplantation [18]. Notably, the American Association for the Study of Liver Diseases (AASLD) and the American Society of Transplantation have proposed that a BMI >40 kg/m2 should represent a relative contraindication to liver transplantation [19]. Indeed, morbid obesity, defined as a BMI ≥40 kg/m2, was reported as an independent predictor of drop-out and death in liver transplantation candidates [20, 21].
Here, we first aimed to assess the trends in the prevalences of obesity, type 2 diabetes mellitus and NAFLD in patients undergoing liver transplantation evaluation at Lausanne University Hospital between January 2009 and March 2020. Second, we hypothesized that access to liver transplantation was impaired in obese patients, for instance because of the presence of other major comorbidities or because of the challenge of the surgical procedure in obese patients. Therefore, we assessed whether grade II (BMI ≥35 kg/m2) and grade III (BMI ≥40 kg/m2) obese patients were less likely to be listed or had an increased drop-out rate from the waiting list.
Methods
Study population and design
This is a retrospective study analyzing medical data from the Division of Gastroenterology and Hepatology of Lausanne University Hospital, a tertiary referral centre in Switzerland with more than 10,000 outpatient consultations per year. All patients who underwent a formal workup for liver transplantation at the Lausanne University Hospital between January 2009 and March 2020 were included in this study, whether they had been grafted or not at the end of the evaluation period.
After identifying all patients fulfilling our inclusion criteria, we reviewed electronic medical records and medical archives. Data extraction and coding was performed manually from September 2020 to January 2021.
In our centre, patients are referred for liver transplantation evaluation by primary care providers, as well as gastroenterologists and other specialists in private practice or regional hospitals. They are then initially evaluated at the outpatient hepatology unit. In cases of advanced cirrhosis, patients are evaluated at the inpatient service.
Formal eligibility for liver transplantation is afterwards discussed at a multidisciplinary meeting, including hepatologists, transplant surgeons, anaesthesiologists radiologists and psychiatrists, for every patient with end-stage liver disease, hepatocellular carcinoma or other rare indications, in accordance with standard and commonly applied criteria [22, 23].
In the case of a favourable evaluation by this multidisciplinary team, patients are then hospitalized for an extensive assessment to rule out any medical or psychiatric contraindication to liver transplantation. This workup is performed a few weeks prior to listing and systematically includes an extensive cardiopulmonary assessment, including an evaluation of cardiovascular risk factors, and a nutritional evaluation, including BMI calculation.
Inclusion criteria for this study were: (1) age >18 years, (2) patients with a complete liver transplantation evaluation. Exclusion criteria were: (1) patients assessed for liver transplantation in the setting of acute liver failure, (2) patients assessed for retransplantation in the setting of graft dysfunction, (3) candidates for multi-organ transplantation and (4) patients who were lost to follow-up.
Ethical approval: This study was approved by the “Commission cantonale d’éthique de la recherche sur l’être humain” (CER-VD) on November 28, 2019 (protocol number 2019-01728).
Baseline evaluation
Demographic, clinical and laboratory data were obtained from electronic medical records and medical archives. Demographic data were assessed at baseline and included sex, age and origin. Clinical data, such as BMI, and comorbidities, such as type 2 diabetes mellitus, hypertension and dyslipidaemia, were retrieved from the first inpatient evaluation for liver transplantation. Grade I, II and III obesity were defined by BMI ≥30, ≥35 and ≥40 kg/m2 respectively, according to World Health Organization definitions [9].
Subjects were considered as having metabolic syndrome if they fulfilled the diagnostic criteria defined by the American Heart Association (AHA) and the National Heart, Lung, and Blood Institute (NHLBI) [24].
Laboratory parameters were retrieved from the first visit for liver transplantation evaluation. These included sodium, alanine aminotransferase, gamma-glutamyl transferase, alkaline phosphatase, total bilirubin, albumin, creatinine, prothrombin time and international normalized ratio (INR). Liver function was also assessed and included Child-Pugh score, model for end-stage liver disease (MELD) score and MELD-Na score.
Assignment of chronic liver disease aetiology
Aetiology of chronic liver disease was assessed for each patient based on medical records and liver histology to ensure assignment to the correct group. Six groups were defined: (1) chronic viral hepatitis (chronic hepatitis B, D and C), (2) alcoholic liver disease, defined as alcohol intake >30 g/day for men and >20 g/day for women, (3) NAFLD, (4) mixed aetiologies including a NAFLD component (e.g. patient with chronic HCV and NAFLD), (5) mixed aetiologies without a NAFLD component (e.g. alcoholic liver disease combined with chronic HCV) and (6) other causes, including auto-immune liver diseases (auto-immune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis, overlap syndromes) and rare causes (e.g. Wilson’s disease, vascular liver disease, transthyretin amyloidosis).
Every patient assessed for liver transplantation in our centre underwent a transjugular liver biopsy. NAFLD was diagnosed based on the criteria defined by the European Association for the Study of the Liver’s (EASL) Clinical Practice Guidelines for the management of NAFLD [25]. Patients with cirrhosis in the presence of two or more metabolic risk factors (diabetes, obesity, dyslipidaemia and hypertension) and in the absence of other causes of chronic liver disease were assigned to the NAFLD group. Importantly, all patients in our study underwent a formal histological assessment. A NAFLD diagnosis was systematically supported by histology after exclusion of other chronic liver diseases.
Follow-up evaluation
All patients included in the present study benefited from a regular medical follow-up at our outpatient hepatology clinic. Follow-up data were retrieved from consultation files and included: (1) laboratory data, MELD and Child-Pugh scores; (2) liver-related complications, such as episodes of decompensation or hepatocellular carcinoma; and (3) non-liver-related complications, such as extrahepatic neoplasia, cardiovascular events or death. Figure 1 illustrates a patient's medical course from initial assessment to liver transplantation.
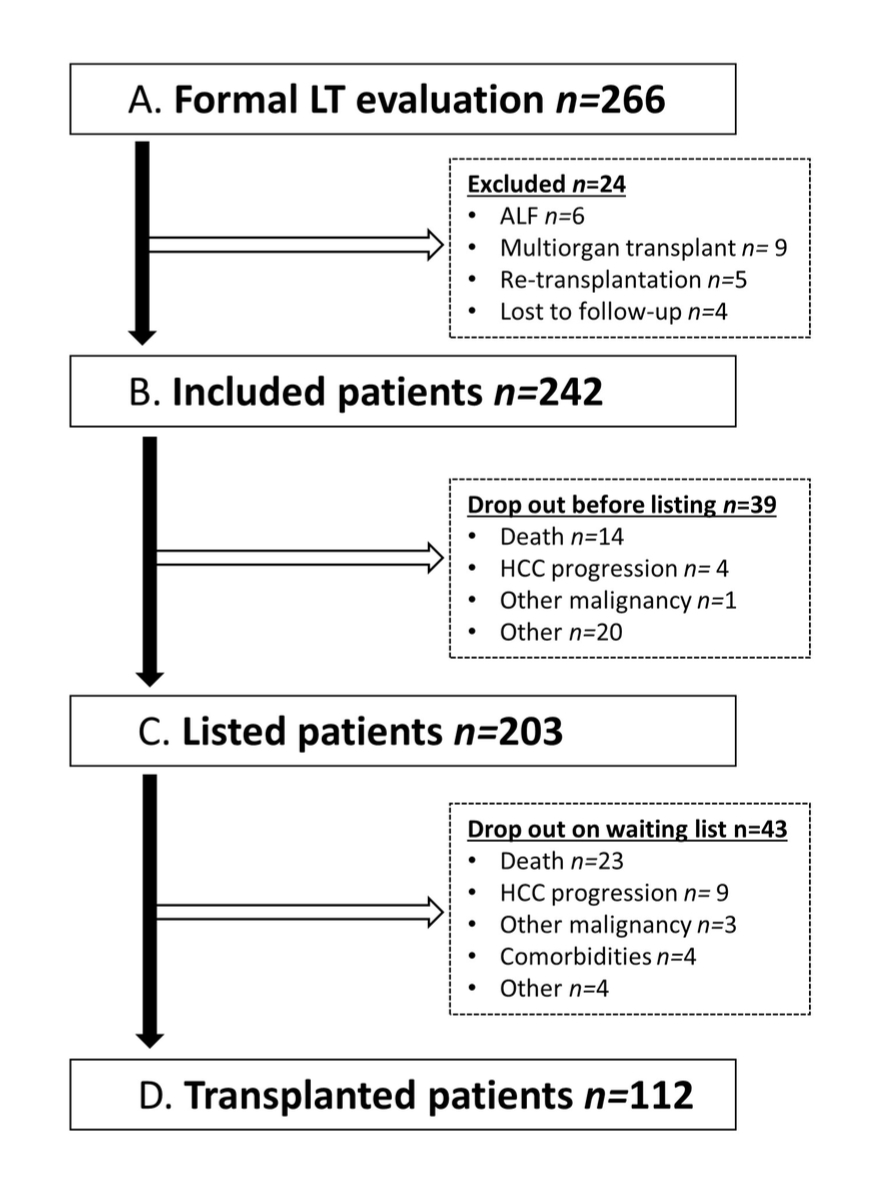
Figure 1 Patient flow chart. Illustration of a patient's medical course from initial assessment to liver transplantation. Reasons for exclusion and for drop-out preceding listing and liver transplantation are detailed. liver transplantation, liver transplantation; ALF, acute liver failure; FU, follow-up
The date of evaluation at our centre, the transplant listing date, the date and reasons for delisting (hepatocellular carcinoma progression, extrahepatic neoplasia, comorbidities and other causes, death) and the date of liver transplantation were recorded.
Statistical analyses
Continuous variables were expressed as median and interquartile range (IQR). Categorical variables were described as frequency and percentage. The distribution of patient characteristics was compared between five time periods (2009–2011, 2012–2013, 2014–2015, 2016–2017 and 2018
Continuous variables were expressed as median and interquartile range (IQR). Categorical variables were described as frequency and percentage. The distribution of patient characteristics was compared between five time periods (2009–2011, 2012–2013, 2014–2015, 2016–2017 and 2018 – March 2020) using the chi-square test. The drop-out curves at two years were estimated using the Kaplan-Meier method, calculated with a 95% confidence interval (CI), and compared across the different groups using the log-rank test. Univariate and multivariate analyses of variables associated with non-listing or drop-out after placement on the waiting list were performed using logistic regression and the results were reported as odds ratios (ORs) and 95% CIs. Covariates with p ≤0.1 in the univariate regression model and obesity were retained for a multivariable analysis. The significance level was set at 0.05 with a two-sided test. All statistical analyses were performed using NCSS 2011 software.
Results
Table 1 summarizes the demographic, clinical and laboratory characteristics of the patients included in the analysis. From January 2009 to March 2020, 266 adult patients were formally assessed for liver transplantation at Lausanne University Hospital. Twenty-four patients were excluded from the analysis for the following reasons: acute liver failure (n = 6), multiorgan transplant (n = 9), retransplantation for allograft dysfunction (n = 5) and lost to follow-up (n = 4).
Table 1Demographic and clinical characteristics of patients at liver transplantation assessment (n = 242). Obesity grades and metabolic syndrome are defined according to WHO definitions [9, 40].
| Male, n (%) |
201 (83) |
| Female, n (%) |
41 (17) |
| Age (years), median (IQR) |
59 (51–64) |
| Caucasian, n (%) |
215 (89) |
| African, n (%) |
15 (6) |
| Asian, n (%) |
8 (3) |
| Hispanic, n (%) |
4 (2) |
| Aetiology of chronic liver disease |
| – Viral hepatitis, n (%) |
67 (28) |
| – Alcoholic liver disease, n (%) |
52 (21) |
| – NAFLD, n (%) |
30 (12) |
| – Mixed aetiologies with a NAFLD component, n (%) |
23 (10) |
| – Mixed aetiologies without a NAFLD component, n (%) |
29 (12) |
| – Other, n (%) |
41 (17) |
| BMI (kg/m2), median (IQR) |
26 (24–31) |
| Obesity |
| – Obesity grade I, n (%) |
49 (20) |
| – Obesity grade II, n (%) |
15 (6) |
| – Obesity grade III, n (%) |
4 (2) |
| – Total, n (%) |
68 (28) |
| Cardiovascular risk factors |
| – Type 2 diabetes mellitus, n (%) |
82 (34) |
| – Arterial hypertension, n (%) |
91 (38) |
| – Dyslipidaemia, n (%) |
42 (18) |
| – Metabolic syndrome, n (%) |
40 (17) |
| Child-Pugh score in cirrhotic patients (n = 236) |
| – A, n (%) |
197 (84) |
| – B, n (%) |
29 (12) |
| – C, n (%) |
10 (4) |
| Hepatocellular carcinoma, n (%) |
115 (48) |
| Hepatocellular carcinoma as indication for liver transplantation, n (%) |
111 (46) |
| Laboratory parameters, median (IQR) |
| – Total bilirubin (µmol/l) |
27 (2–703) |
| – Albumin (g/l) |
36 (21–51) |
| – Creatinine (µmol/l) |
78 (40–464) |
| – Prothrombin time (%) |
65 (11–120) |
| – INR |
1 (1–3) |
| MELD score, median (IQR) |
12 (6–40) |
| MELD-Na score, median (IQR) |
14 (6–62) |
Of the remaining 242 patients, 201 (83%) were male and the median age was 59 years (IQR, 51-64 years; range, 18–75 years). The vast majority (89%) were of Caucasian origin, 6% were of African origin, 3% were of Asian origin and 2% were of Hispanic origin.
The number of patients evaluated for liver transplantation during each defined time period increased markedly, from 29 in 2009-2011 to 91 in 2018-2020 (+310%). The most common cause of end-stage liver disease in our cohort was viral hepatitis (28%), followed by NAFLD alone or combined (22%) and alcoholic liver disease (21%) (table 1). The distribution of the causes of end-stage liver disease changed significantly over the five time periods (p = 0.0006). Indeed, the proportion of patients with viral hepatitis as an indication for liver transplantation declined over time, from 32% to 14%, while the proportions of patients with alcoholic liver disease and NAFLD increased from 8% to 25% and from 8% to 38% respectively (figure 2).
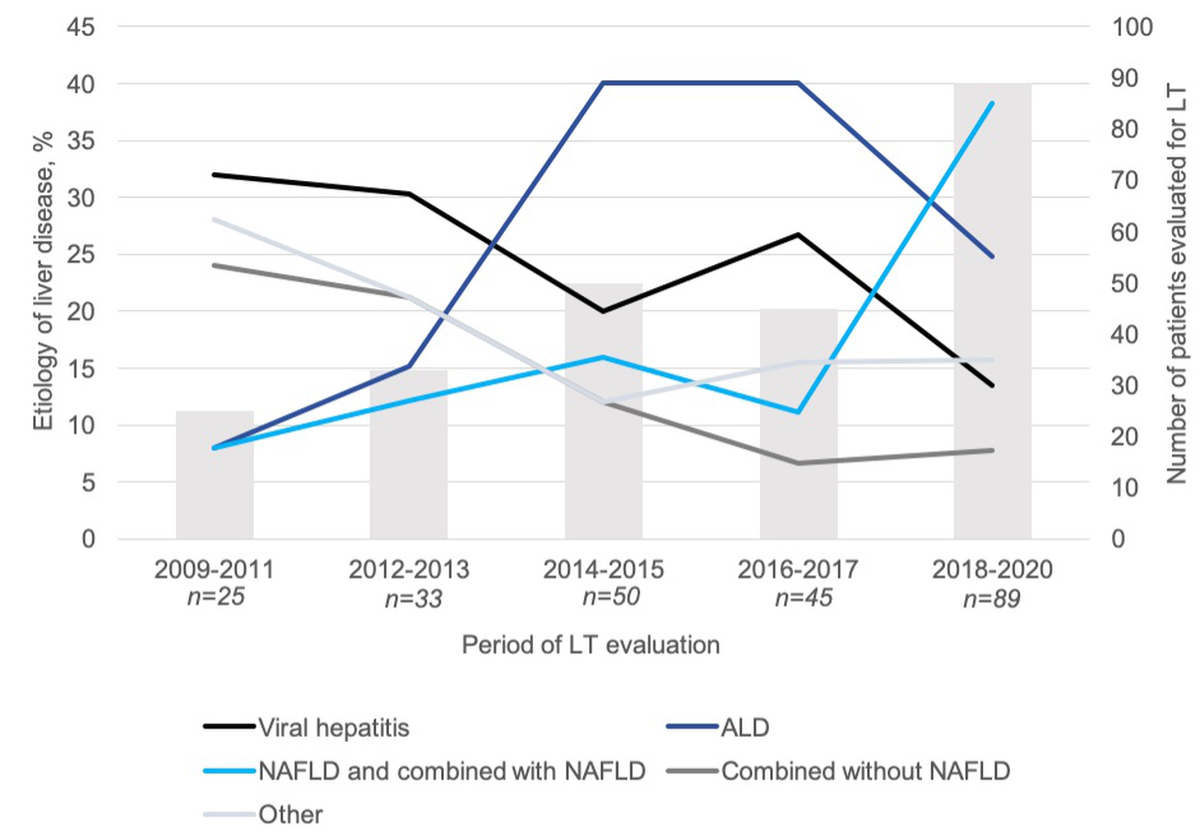
Figure 2 Aetiology of liver disease among patients evaluated for liver transplantation between January 2009 and March 2020. Frequency of liver disease causes (y axis, %) among patients evaluated for liver transplantation is represented by continuous lines over time. The numbers of patients (x axis, n) evaluated for liver transplantation for each time period are represented by columns. Frequencies of liver disease aetiologies were compared between five time periods using the chi-square test (p = 0.0006). ALD, alcoholic liver disease; NAFLD, non-alcoholic fatty liver disease
Sixty-eight patients (28% of the study population) were obese as defined by a BMI >30 kg/m2, with grade II and III obesity in 28% of those 68. The highest BMI among patients evaluated for liver transplantation was 43 kg/m2. BMI was not available for two patients. Notably, only 12% of the patients were obese in the 2009–2011 period, whereas this proportion increased to 34% in the most recent observation period, i.e. 2018-2020 (figure 3).
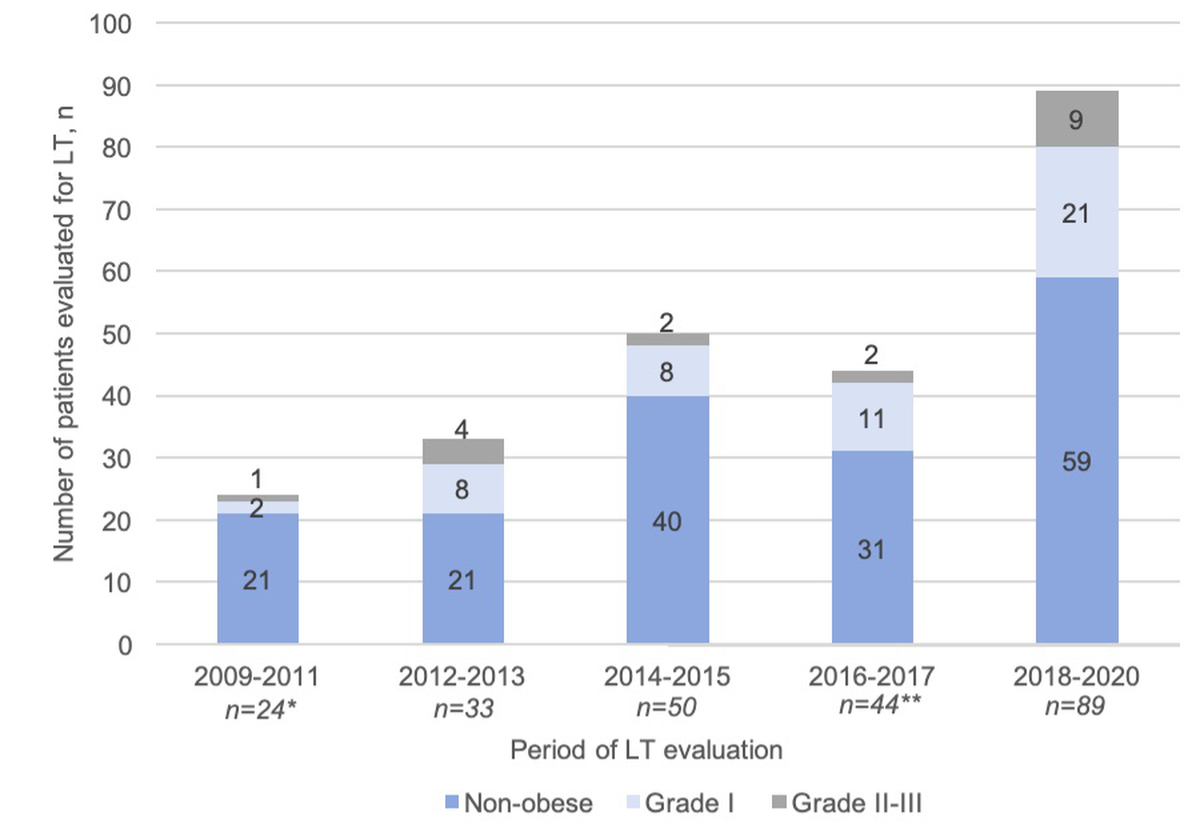
Figure 3 Evolution of BMI distribution over time in patients evaluated for liver transplantation. Distribution of body mass index (BMI) classes among patients evaluated for liver transplantation (y axis) is represented by histograms over the five time periods (x axis). Obesity was classified as Grade I (BMI ≥30 kg/m2), grade II (BMI ≥35 kg/m2) or grade III (BMI ≥40 kg/m2). Frequencies of BMI classes were compared between five time periods using the chi-square test (p = 0.4).
(*,**) two BMI values were missing.
Eighty-two patients had a diagnosis of type 2 diabetes mellitus (34%), 41 (17%) of whom were insulin-dependent. The prevalence of diabetes significantly increased over time (p = 0.02) (figure 4). Metabolic syndrome was present in 40 patients (17% of our study population).
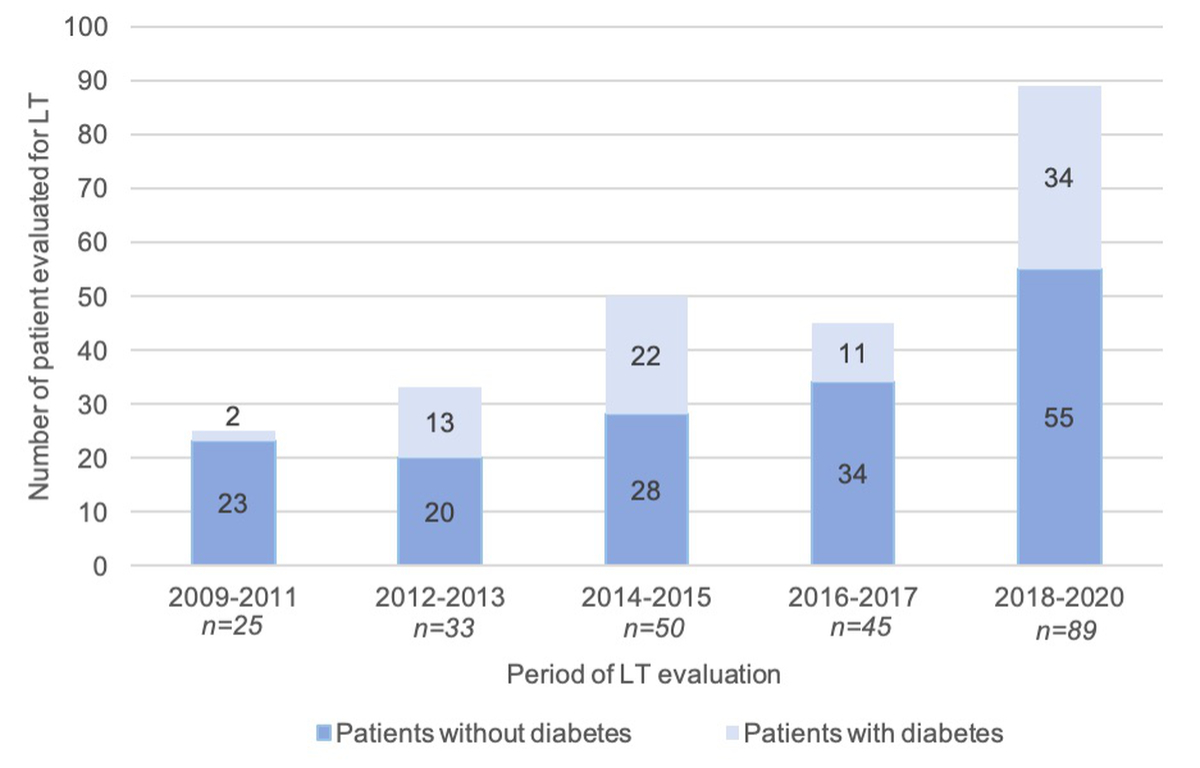
Figure 4 Evolution of type 2 diabetes mellitus prevalence over time. Frequency of type 2 diabetes mellitus among patients evaluated for liver transplantation (y axis) over time (x axis) is represented by columns and was compared using chi-square tests (p = 0.02).
Of the 242 patients assessed for liver transplantation, 203 patients [60 (30%) obese patients] were listed and 112 patients [35 (31%) obese patients] underwent liver transplantation (table 2). In the univariate analysis, none of the variables – including BMI and obesity – were associated with not being listed (supplementary table 1).
Table 2Follow-up of patients after first assessment for liver transplantation (n = 242).
| Follow-up (days), median (IQR) |
689 (238–1,656) |
| Time from assessment to listing (days), median (IQR) |
59 (31–93) |
| Time from listing to liver transplantation (days), median (IQR) |
362 (194–454) |
| Patients still on the waiting list, n (%) |
48 (20) |
| Patients transplanted, n (%) |
112 (46) |
| Patients dropped out from the transplant program, n (%) |
82 (34) |
| – Drop-out before listing, n (%) |
39 (16) |
| – Drop-out while on waiting list, n (%) |
43 (18) |
| Reason for drop-out while on waiting list |
| – Death, n (%) |
23 (53) |
| – Hepatocellular carcinoma progression, n (%) |
9 (21) |
| – Other malignancy*, n (%) |
3 (7) |
| – Comorbidities**, n (%) |
4 (9) |
| – Other, n (%) |
4 (9) |
Similarly, none of the variables included in the univariate and multivariate analyses were independently associated with drop-out after listing (Supplementary Table 2). When analyzing drop-out-free survival, there was no significant difference between obese and non-obese patients (64% vs. 68% respectively; p = 0.9) (figure 5). Reasons for drop-out are given in Table 2. The proportions of non-listed and listed obese patients was not significantly different (21% vs. 30%. p = 0.3) (figure 5).
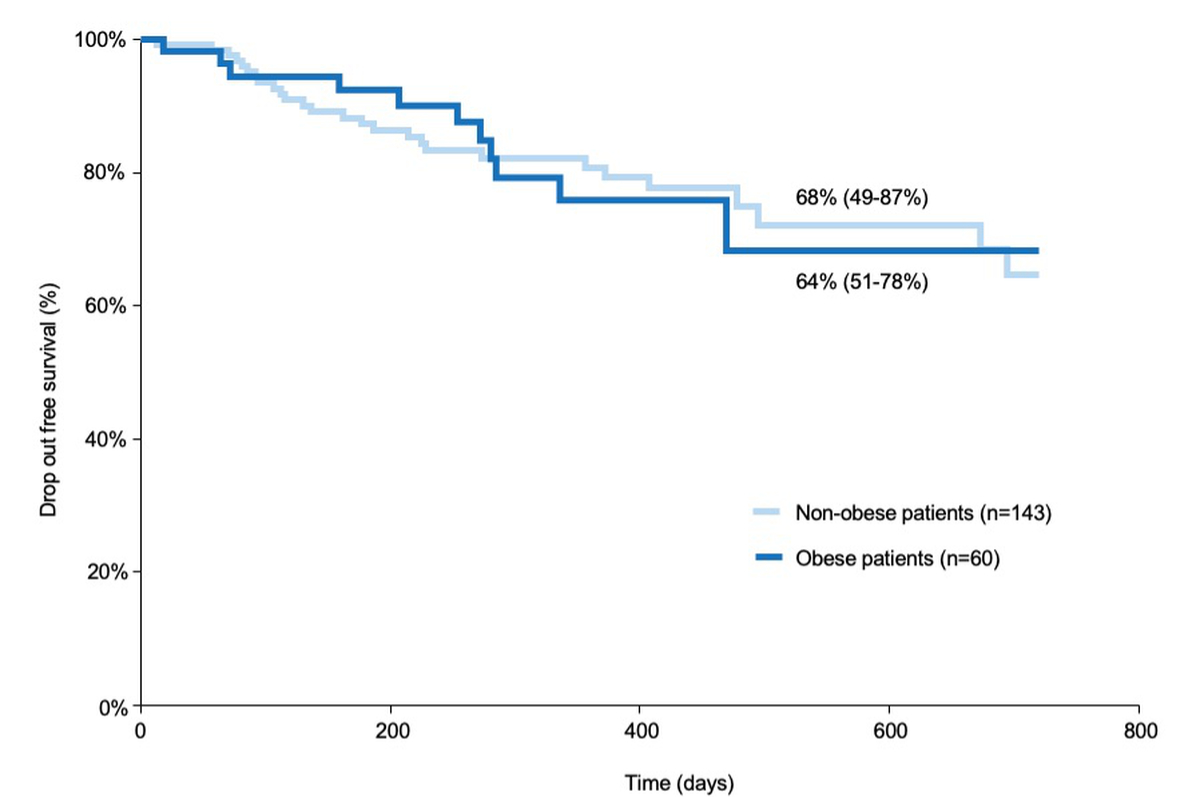
Figure 5 Drop-out curves for obese and non-obese patients over time. The drop-out rate (y axis) over time (x axis) was compared between obese and non-obese patients using the log-rank test and did not differ statistically between the two groups (p = 0.9). Results are represented by a Kaplan–Meier curve. Drop-out includes delisted patients or death while on the waiting list
Hepatocellular carcinoma represented the primary indication for liver transplantation in approximately half of our cohort (46%). This proportion remained stable throughout the study period. Importantly, underlying chronic liver disease among patients with hepatocellular carcinoma evolved significantly over time, with increasing prevalence of NAFLD among patients with hepatocellular carcinoma (p = 0.03) (figure 6).
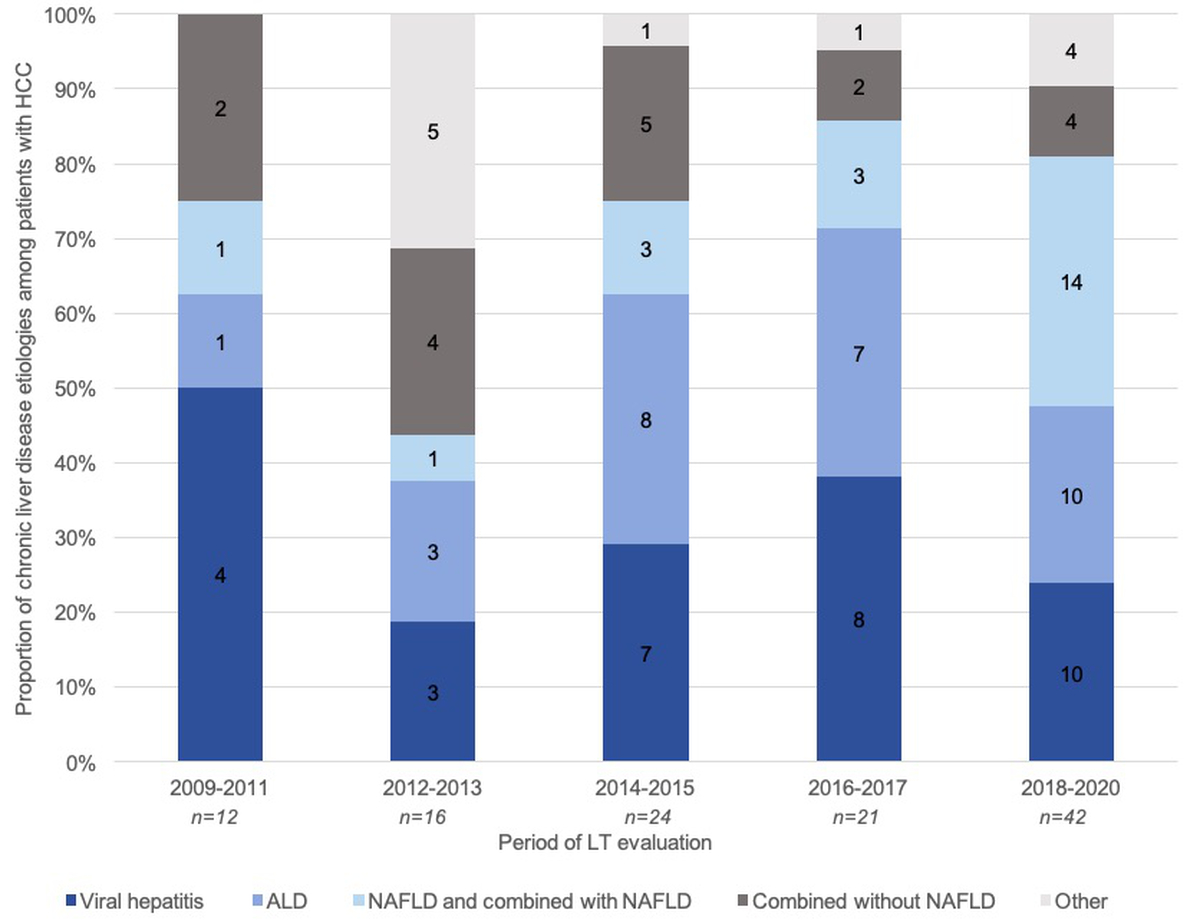
Figure 6 Causes of chronic liver disease among patients with hepatocellular carcinoma (HCC). Proportions of different chronic liver disease aetiologies among patients with hepatocellular carcinoma evaluated for liver transplantation (y axis, %) are represented by columns over five time periods (x axis, year). The distributions of different aetiologies varied significantly over time (p = 0.03)
Discussion
We retrospectively assessed indications for liver transplantation in a tertiary referral centre in Switzerland over the last decade. More specifically, we focused our analysis on the evolution of the prevalence of obesity, type 2 diabetes mellitus and NAFLD among patients referred for liver transplantation evaluation from January 2009 to March 2020.
First, our study demonstrates that the landscape of liver transplantation indications is shifting towards NAFLD in Switzerland, as predicted in a recent modelling study and described in other countries [11, 26, 24, 28]. By the end of the study period, the prevalence of NAFLD in patients evaluated for liver transplantation had surpassed the prevalence of chronic hepatitis B and C, as well as of alcoholic liver disease. Indeed, during the most recent study period, i.e. between January 2018 and March 2020, NAFLD and mixed chronic liver diseases with a NAFLD component represented nearly 40% of the patients assessed for liver transplantation. This may be explained by the fact that viral hepatitis-related indications for liver transplantation declined over time, especially following the introduction of potent direct-acting antiviral therapies to treat chronic hepatitis C, and also by the obesity and metabolic syndrome epidemics of recent decades.
Importantly, all patients evaluated for liver transplantation were included in our cohort and not only those who underwent liver transplantation. One main reason for this approach was to assess whether NAFLD is even more frequent among patients evaluated for liver transplantation than among those who were transplanted. Thus, we hypothesized that these patients could have an increased risk of not being listed or of drop-out from the liver transplantation waiting list, for instance because of increased cardiovascular or oncological risk [15, 29, 30]. Indeed, the cardiovascular and oncological risks associated with obesity are known to be further increased post-liver transplantation [31]. Moreover, morbid obesity has been reported as an independent predictor of death and drop-out among liver transplantation candidates [21]. Based on these considerations, the American Association for the Study of Liver Diseases advises against liver transplantation in patients with grade III obesity, whereas the European Association for the Study of the Liver recommends multidisciplinary evaluation in patients with grade II obesity [19, 22]. In our cohort, no patient was denied access to liver transplantation evaluation and listing based solely on their BMI. Drop-out rates in grade II to III obese patients were equivalent to those in non-obese patients in our centre, and thus in contradiction with recently published data reporting increased drop-out rates in patients with BMIs >40 kg/m2 [21].
We acknowledge that our study has some limitations, particularly regarding the comparison of drop-out rates in obese vs. non-obese patients evaluated for liver transplantation. Our cohort is relatively small, and further investigations will require a multicentre and a prospective extension of our study. It is also possible that some obese patients with criteria for liver transplantation assessment were not referred to our centre, as it is known that stigma associated with obesity negatively impacts on quality of care and outcomes [32]. Secondly, weight loss, malnutrition and sarcopenia affect up to 60% of cirrhotic patients [33]. Thus, it is possible that the obesity burden among our patients may have been underestimated.
Increased morbidity after surgery is well documented in obese patients [34]. The outcome of obese patients after liver transplantation is an important question that goes beyond the scope of our analysis and will have to be addressed in future studies. Recent data from the European Liver Transplant Registry (ELTR) suggest that survival of patients and grafts in patients with NASH is comparable to that of patients transplanted for other indications [35].
We report that the worldwide epidemic of obesity has a direct impact on the characteristics of the liver transplantation waiting list population in Switzerland. Epidemiological data in Switzerland show increasing numbers of patients requiring liver transplantation over the last decade [36]. Considering the increasing prevalence of NAFLD and obesity, this trend is likely to continue in the coming years and to further impact on organ shortages. Based on our observations, we advocate for the improvement and implementation of multidisciplinary strategies to screen for and treat NAFLD in order to prevent liver fibrosis progression.
There are currently few therapeutic alternatives for obesity, with bariatric surgery remaining one of the main options. This procedure was linked to the resolution of NASH in up to 85% of patients, with improved histological features at one year [37, 38]. It is indeed a recognized therapeutic option for obese patients with NASH who do not respond to lifestyle modifications.Bariatric surgery at the time of liver transplantation or in the postoperative course has the potential not only to improve obesity‐related conditions such as diabetes, but also to reduce the incidence of de novo NASH of the allograft [39]. In the face of the increasing prevalence of obesity among patients evaluated for liver transplantation, bariatric surgery should be considered and discussed at earlier stages in order to prevent further progression of liver disease and, in consequence, the need for liver transplantation.
Conclusion
This study confirms the changing landscape of indications for liver transplantation and highlights the many challenges that lie ahead, including the implementation of early multidisciplinary strategies to treat obese patients in order to prevent NAFLD progression and its consequences. Finally, because of a similar pre-liver transplantation drop-out rate in obese and non-obese patients, we encourage care providers to refer obese patients needing liver transplantation for a proper assessment and not to preclude them from access to liver transplantation solely based on their BMI.
Author contributions
SK, DM and MF designed the study; SK, FA, CP, ACS and MF acquired the data; FA and JV performed statistical analyses; SK, FA, DM, JV and MF wrote the manuscript; all authors revised the manuscript.
Montserrat Fraga, MD
Division of Gastroenterology and Hepatology
Lausanne University Hospital
Rue du Bugnon 44
CH-1011 Lausanne
Montserrat.Fraga[at]chuv.ch
References
1.
Seidell JC
,
Halberstadt J
. The global burden of obesity and the challenges of prevention. Ann Nutr Metab. 2015;66 Suppl 2:7–12. https://doi.org/10.1159/000375143
2.
Ward ZJ
,
Bleich SN
,
Cradock AL
,
Barrett JL
,
Giles CM
,
Flax C
, et al.
Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N Engl J Med. 2019 Dec;381(25):2440–50. https://doi.org/10.1056/NEJMsa1909301
3.
Yach D
,
Stuckler D
,
Brownell KD
. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat Med. 2006 Jan;12(1):62–6. https://doi.org/10.1038/nm0106-62
4.
Friedman AN
,
Miskulin DC
,
Rosenberg IH
,
Levey AS
. Demographics and trends in overweight and obesity in patients at time of kidney transplantation. Am J Kidney Dis. 2003 Feb;41(2):480–7. https://doi.org/10.1053/ajkd.2003.50059
5.
Parikh ND
,
Marrero WJ
,
Wang J
,
Steuer J
,
Tapper EB
,
Konerman M
, et al.
Projected increase in obesity and non-alcoholic-steatohepatitis-related liver transplantation waitlist additions in the United States. Hepatology. 2019 Aug;70(2):487–95. https://doi.org/10.1002/hep.29473
6.
Belli LS
,
Perricone G
,
Adam R
,
Cortesi PA
,
Strazzabosco M
,
Facchetti R
, et al.; all the contributing centers (www.eltr.org) and the European Liver and Intestine Transplant Association (ELITA)
. Impact of DAAs on liver transplantation: major effects on the evolution of indications and results. An ELITA study based on the ELTR registry. J Hepatol. 2018 Oct;69(4):810–7. https://doi.org/10.1016/j.jhep.2018.06.010
7.
Cholankeril G
,
Ahmed A
. Alcoholic Liver Disease Replaces Hepatitis C Virus Infection as the Leading Indication for Liver Transplantation in the United States. Clin Gastroenterol Hepatol. 2018 Aug;16(8):1356–8. https://doi.org/10.1016/j.cgh.2017.11.045
8.
Chalasani N
,
Younossi Z
,
Lavine JE
,
Charlton M
,
Cusi K
,
Rinella M
, et al.
The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018 Jan;67(1):328–57. https://doi.org/10.1002/hep.29367
9.
Alberti KG
,
Zimmet PZ
. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998 Jul;15(7):539–53. https://doi.org/10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S
10.
Angulo P
. Nonalcoholic fatty liver disease. N Engl J Med. 2002 Apr;346(16):1221–31. https://doi.org/10.1056/NEJMra011775
11.
Younossi ZM
,
Koenig AB
,
Abdelatif D
,
Fazel Y
,
Henry L
,
Wymer M
. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016 Jul;64(1):73–84. https://doi.org/10.1002/hep.28431
12.
Terrault NA
,
Pageaux GP
. A changing landscape of liver transplantation: king HCV is dethroned, ALD and NAFLD take over! J Hepatol. 2018 Oct;69(4):767–8. https://doi.org/10.1016/j.jhep.2018.07.020
13.
Axley P
,
Ahmed Z
,
Arora S
,
Haas A
,
Kuo YF
,
Kamath PS
, et al.
NASH Is the most rapidly growing etiology for acute-on-chronic liver failure-related hospitalization and disease burden in the United States: A population-based study. Liver Transpl. 2019 May;25(5):695–705. https://doi.org/10.1002/lt.25443
14.
Younossi Z
,
Stepanova M
,
Ong JP
,
Jacobson IM
,
Bugianesi E
,
Duseja A
, et al.; Global Nonalcoholic Steatohepatitis Council
. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol. 2019 Mar;17(4):748–755.e3. https://doi.org/10.1016/j.cgh.2018.05.057
15.
Calle EE
,
Rodriguez C
,
Walker-Thurmond K
,
Thun MJ
. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003 Apr;348(17):1625–38. https://doi.org/10.1056/NEJMoa021423
16.
Baffy G
,
Brunt EM
,
Caldwell SH
. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol. 2012 Jun;56(6):1384–91. https://doi.org/10.1016/j.jhep.2011.10.027
17.
Paradis V
,
Zalinski S
,
Chelbi E
,
Guedj N
,
Degos F
,
Vilgrain V
, et al.
Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009 Mar;49(3):851–9. https://doi.org/10.1002/hep.22734
18.
Targher G
,
Day CP
,
Bonora E
. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010 Sep;363(14):1341–50. https://doi.org/10.1056/NEJMra0912063
19.
Martin P
,
DiMartini A
,
Feng S
,
Brown R Jr
,
Fallon M
. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014 Mar;59(3):1144–65. https://doi.org/10.1002/hep.26972
20.
Kardashian AA
,
Dodge JL
,
Roberts J
,
Brandman D
. Weighing the risks: morbid obesity and diabetes are associated with increased risk of death on the liver transplant waiting list. Liver Int. 2018 Mar;38(3):553–63. https://doi.org/10.1111/liv.13523
21.
Kaur N
,
Emamaullee J
,
Lian T
,
Lo M
,
Ender P
,
Kahn J
, et al.
Impact of Morbid Obesity on liver transplant candidacy and outcomes: national and regional trends. Transplantation. 2021 May;105(5):1052–60. https://doi.org/10.1097/TP.0000000000003404
22.
European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu
. EASL Clinical Practice Guidelines: liver transplantation. J Hepatol. 2016 Feb;64(2):433–85. https://doi.org/10.1016/j.jhep.2015.10.006
23.
Galle PR
,
Forner A
,
Llovet JM
,
Mazzaferro V
,
Piscaglia F
,
Raoul JL
, et al.; European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver
. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018 Jul;69(1):182–236. https://doi.org/10.1016/j.jhep.2018.03.019
24.
Grundy SM
,
Cleeman JI
,
Daniels SR
,
Donato KA
,
Eckel RH
,
Franklin BA
, et al.; American Heart Association; National Heart, Lung, and Blood Institute
. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005 Oct;112(17):2735–52. https://doi.org/10.1161/CIRCULATIONAHA.105.169404
25.
European Association for the Study of the Liver (EASL)
European Association for the Study of Diabetes (EASD)
European Association for the Study of Obesity (EASO)
. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016 Jun;64(6):1388–402. https://doi.org/10.1016/j.jhep.2015.11.004
26.
Angulo P
,
Lindor KD
. Non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2002 Feb;17 Suppl:S186–90. https://doi.org/10.1046/j.1440-1746.17.s1.10.x
27.
Goossens N
,
Bellentani S
,
Cerny A
,
Dufour JF
,
Jornayvaz FR
,
Mertens J
, et al.
Nonalcoholic fatty liver disease burden - Switzerland 2018-2030. Swiss Med Wkly. 2019 Dec;149:w20152. https://doi.org/10.4414/smw.2019.20152
28.
Younossi ZM
,
Stepanova M
,
Ong J
,
Trimble G
,
AlQahtani S
,
Younossi I
, et al.
Nonalcoholic steatohepatitis is the most rapidly increasing indication for liver transplantation in the United States. Clin Gastroenterol Hepatol. 2021 Mar;19(3):580–589.e5. https://doi.org/10.1016/j.cgh.2020.05.064
29.
Eslam M
,
Newsome PN
,
Sarin SK
,
Anstee QM
,
Targher G
,
Romero-Gomez M
, et al.
A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020 Jul;73(1):202–9. https://doi.org/10.1016/j.jhep.2020.03.039
30.
Csige I
,
Ujvárosy D
,
Szabó Z
,
Lőrincz I
,
Paragh G
,
Harangi M
, et al.
The impact of obesity on the cardiovascular system. J Diabetes Res. 2018 Nov;2018:3407306. https://doi.org/10.1155/2018/3407306
31.
Lucey MR
,
Terrault N
,
Ojo L
,
Hay JE
,
Neuberger J
,
Blumberg E
, et al.
Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl. 2013 Jan;19(1):3–26. https://doi.org/10.1002/lt.23566
32.
Phelan SM
,
Burgess DJ
,
Yeazel MW
,
Hellerstedt WL
,
Griffin JM
,
van Ryn M
. Impact of weight bias and stigma on quality of care and outcomes for patients with obesity. Obes Rev. 2015 Apr;16(4):319–26. https://doi.org/10.1111/obr.12266
33.
Bunchorntavakul C
,
Reddy KR
. Review article: malnutrition/sarcopenia and frailty in patients with cirrhosis. Aliment Pharmacol Ther. 2020 Jan;51(1):64–77. https://doi.org/10.1111/apt.15571
34.
Ri M
,
Aikou S
,
Seto Y
. Obesity as a surgical risk factor. Ann Gastroenterol Surg. 2017 Oct;2(1):13–21. https://doi.org/10.1002/ags3.12049
35.
Haldar D
,
Kern B
,
Hodson J
,
Armstrong MJ
,
Adam R
,
Berlakovich G
, et al.; European Liver and Intestine Transplant Association (ELITA)
. Outcomes of liver transplantation for non-alcoholic steatohepatitis: A European Liver Transplant Registry study. J Hepatol. 2019 Aug;71(2):313–22. https://doi.org/10.1016/j.jhep.2019.04.011
36. Swisstransplant - Annual figures [Internet]. Available from: https://www.swisstransplant.org/en
37.
Lassailly G
,
Caiazzo R
,
Buob D
,
Pigeyre M
,
Verkindt H
,
Labreuche J
, et al.
Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology. 2015;149(2):379-388-16.
38.
Lassailly G
,
Caiazzo R
,
Ntandja-Wandji LC
,
Gnemmi V
,
Baud G
,
Verkindt H
, et al.
Bariatric surgery provides long-term resolution of nonalcoholic steatohepatitis and Regression of Fibrosis. Gastroenterology. 2020 Oct;159(4):1290–1301.e5. https://doi.org/10.1053/j.gastro.2020.06.006
39.
Diwan TS
,
Rice TC
,
Heimbach JK
,
Schauer DP
. Liver transplantation and bariatric surgery: timing and outcomes. Liver Transpl. 2018 Sep;24(9):1280–7. https://doi.org/10.1002/lt.25303
Appendix: supplementary tables
Supplementary table 1Univariate analysis of variables associated with non-listing.
|
|
Univariate analysis
|
|
Covariant
|
OR
|
95% CI
|
p value
|
| Male gender |
0.92 |
0.66–1.76 |
0.52 |
| Age |
0.99 |
0.96–1.03 |
0.81 |
| BMI |
1.01 |
0.95–1.09 |
0.63 |
| Obesity |
1.58 |
0.66–3.65 |
0.26 |
| Type 2 diabetes mellitus |
0.75 |
0.49–2.12 |
0.95 |
| Arterial hypertension |
1.90 |
0.89–4.21 |
0.12 |
| Metabolic syndrome |
1.38 |
0.50–3.99 |
0.52 |
| Aetiology of chronic liver disease |
| – Viral hepatitis |
1.15 |
0.92–1.43 |
0.21 |
| – Alcoholic liver disease |
0.84 |
0.60–1.18 |
0.32 |
| – NAFLD and mixed aetiologies with NAFLD |
1.21 |
0.88–1.53 |
0.44 |
| – Mixed aetiologies without a NAFLD component |
1.15 |
0.84–1.32 |
0.30 |
| – Other |
1.45 |
0.43–2.99 |
0.59 |
| Hepatocellular carcinoma as indication for liver transplantation |
1.36 |
0.67–3.74 |
0.38 |
| Child-Pugh score |
1.14 |
0.95–1.36 |
0.15 |
| MELD score |
1.04 |
0.98–1.10 |
0.12 |
| Time period |
| – 2009–2011 |
1.01 |
0.36–2.77 |
0.98 |
| – 2012–2013 |
1.90 |
0.38–9.44 |
0.42 |
| – 2014–2015 |
1.17 |
0.31–4.42 |
0.81 |
| – 2016–2017 |
2.66 |
0.54–13.0 |
0.22 |
| – 2018–2020 |
0.56 |
0.18–1.87 |
0.36 |
Supplementary table 2Univariate and multivariate analysis of variables associated with drop-out after placement on waiting list.
|
|
Univariate analysis
|
Multivariate analysis
|
|
Covariant
|
OR
|
95% CI
|
p value
|
OR
|
95% CI
|
p value
|
| Male gender |
1.31 |
0.58–2.94 |
0.51 |
|
|
|
| Age |
1.02 |
0.98–1.05 |
0.19 |
|
|
|
| BMI |
1.01 |
0.79–1.02 |
0.56 |
|
|
|
| Obesity |
0.77 |
0.36–1.64 |
0.50 |
0.82 |
0.36–1.67 |
0.58 |
| Type 2 diabetes mellitus |
1.32 |
0.67–2.57 |
0.41 |
|
|
|
| Hypertension |
0.84 |
0.43–1.66 |
0.62 |
|
|
|
| Metabolic syndrome |
1.26 |
0.55–2.88 |
0.58 |
|
|
|
| Aetiology of chronic liver disease |
| – Viral hepatitis |
0.67 |
0.32–1.40 |
0.29 |
|
|
|
| – Alcoholic liver disease |
2.01 |
0.76–5.34 |
0.15 |
|
|
|
| – NAFLD and mixed aetiologies with NAFLD |
1.49 |
0.52–4.28 |
0.45 |
|
|
|
| – Mixed aetiologies without NAFLD |
2.07 |
0.73–5.92 |
0.17 |
|
|
|
| – Other |
0.27 |
0.03–2.41 |
0.25 |
|
|
|
| Hepatocellular carcinoma as indication |
1.06 |
0.55 2.04 |
0.88 |
|
|
|
| Child-Pugh score |
1.07 |
0.97–1.24 |
0.30 |
|
|
|
| MELD score |
1.03 |
0.99–1.09 |
0.09 |
1.04 |
0.99–1.09 |
0.10 |
| Time period |
| 2009–2011 |
0.37 |
0.09–1.54 |
0.17 |
0.34 |
0.12–1.69 |
0.12 |
| 2012–2013 |
3.09 |
0.58–16.42 |
0.18 |
1.7 |
0.31–9.9 |
0.53 |
| 2014–2015 |
4.92 |
0.98–23.60 |
0.06 |
3.80 |
0.754–19.18 |
0.12 |
| 2016–2017 |
3.72 |
0.75–18.37 |
0.11 |
2.77 |
0.54–14.27 |
0.22 |
| 2018–2020 |
1.47 |
0.30–7.21 |
0.63 |
1.18 |
0.22–6.14 |
0.84 |





