
Figure 1 Timeline of the milestones and major developments in the evolution of coronary stents for interventional cardiology.
DOI: https://doi.org/10.4414/SMW.2022.w30130
In 1986, Ulrich Sigwart and Jacques Puel implanted the first coronary stents at the Division of Cardiology, CHUV Lausanne and the University Hospital Toulouse, thereby overcoming the limitations of earlier coronary angioplasty, namely acute vessel closure or restenosis due to thrombosis, dissection, elastic recoil, or vascular remodelling [1]. While this innovation improved the success rate of coronary interventions and eliminated the need for surgical standby [2], first-generation bare-metal stents resulted in repeated revascularisation in up to 17% of patients within the first year due to restenosis caused by neointimal hyperplasia [3]. It was not until the development of drug-eluting stents (DES) that the interventional approach proved to be non-inferior to bypass surgery for left main coronary artery disease (CAD) and low-to-intermediate complex multi-vessel CAD [4]. Key to this development were constant refinements in the material, design and drug coating, which led to a broad armamentarium of coronary stents (figure 1 illustrates the milestones in the development of coronary stents). In particular, initial DES types suffered from high rates of late stent thrombosis, which were attributed to delayed endothelialisation caused by the anti-restenotic drugs. This problem was largely solved with the introduction of custom-made anti-proliferative agents and polymers in the second generation of DES. Simultaneously, increasing economic constraints forced healthcare providers [5] to optimise purchase of supplies, including stents, leading to a reduction in available stent types at some hospitals and consequently limiting the possibility of individualised stent therapy. While many lesions can be successfully treated irrespective of the chosen stent, there are situations in which specific stent properties are essential for a favourable outcome. In this short review, we sought to highlight the four most important areas of individualisation of current stent techniques that should be available at every catheterisation laboratory.

Figure 1 Timeline of the milestones and major developments in the evolution of coronary stents for interventional cardiology.
Heavily calcified coronary arteries represent one of the most challenging scenarios in which stent deployment is particularly difficult. Not only that extensive lesion calcification increases the risk of target lesion failure, percutaneous coronary intervention (PCI) of calcified lesions is also associated with higher mortality, myocardial infarction and repeated revascularisation when compared with lesions without calcification [6]. The first step to successful intervention of a severely calcified vessel is thorough lesion preparation and pre-dilatation. Common preparation techniques, such as rotational and orbital atherectomy, as well as the recently introduced intravascular coronary lithotripsy, act predominantly by debulking or by modifying plaque composition, with the aim of producing calcium cracks that are associated with improved stent expansion [7, 8]#_ENREF_7. If stent delivery is still impaired, the use of guide-extension catheters, which are smaller catheters that can be advanced into the coronary artery, can provide additional backup and facilitate stent placement. In these situations, a high radial strength of the stents is essential to resist the recoil forces of these rigid lesions. Radial strength is determined by three factors: the choice of metal alloy, strut thickness and stent architecture. With the majority of stents being made out of cobalt-chrome alloy, it is a combination of strut thickness and stent design that determines the radial strength. Another important stent characteristic for calcified lesions is radio-opacity, which allows precise positioning and post-dilatation of stents. To increase radio-opacity while simultaneously providing a high radial strength, some stents are equipped with a radio dense platinum-iridium core. Figure 2 shows a stent implantation in a patient with severely calcified in-stent restenosis. Particularly in cases of complex or challenging stent implantations, the information provided by coronary angiography may be insufficient and intracoronary imaging is needed. Figure 2D shows an example of intracoronary imaging by optical coherence tomography (OCT), which is one of the two standard methods together with intravascular ultrasound (IVUS). IVUS is a well-established method based on conventional ultrasound and provides a good penetration depth that usually allows to display outer vessel wall structures also. OCT, in contrast, is based on the reflection of infrared light with lower penetration depth and requires the administration of a contrast agent during imaging. In return, it provides an exceptionally high spatial resolution, which allows a detailed analysis of plaque composition or the delineation of individual stent struts.
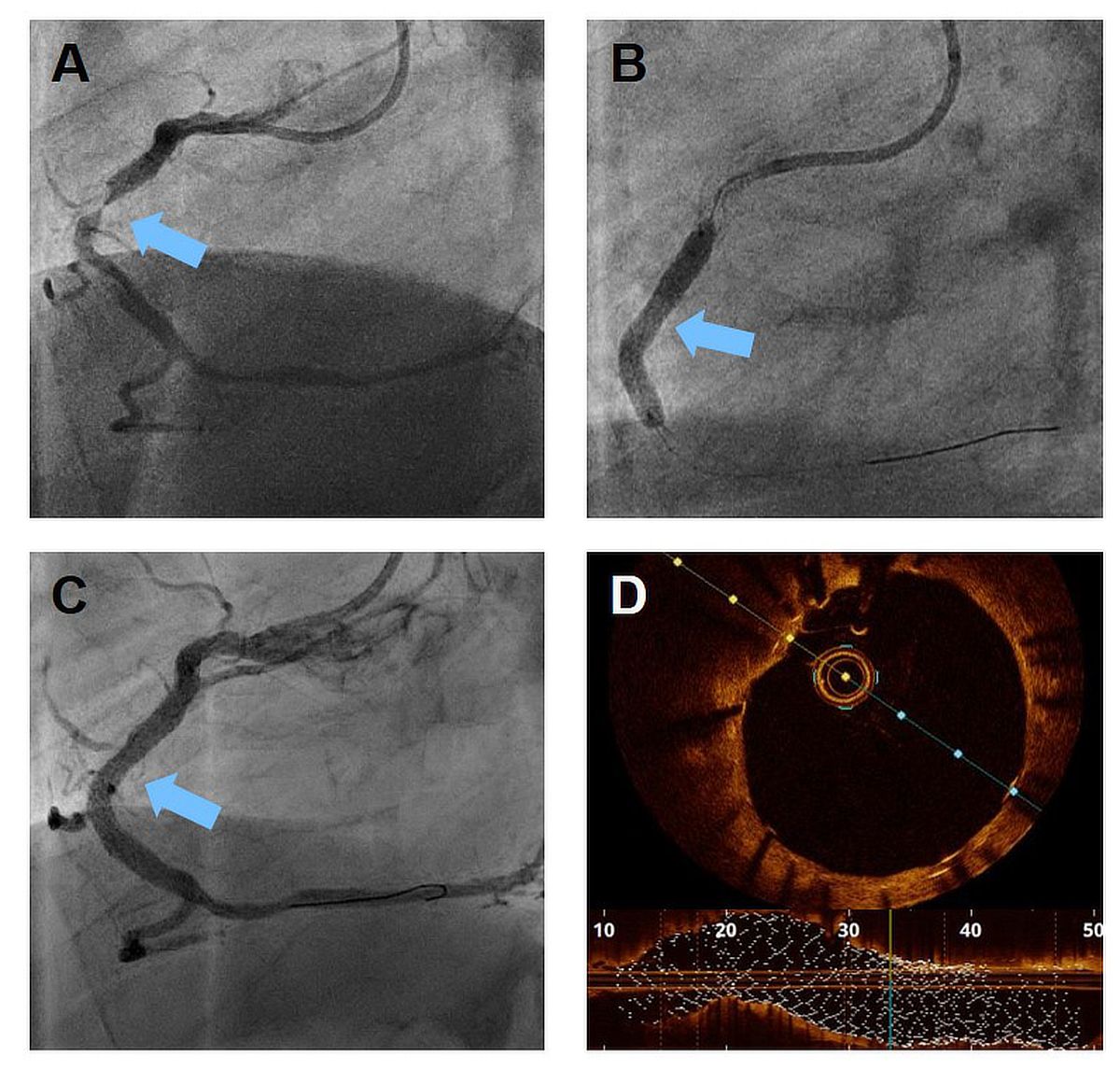
Figure 2 Representative patient with a severely calcified, subtotal in-stent restenosis of the right coronary artery (A, blue arrow).
After extensive lesion preparation including intravascular coronary lithotripsy and the use of high pressure balloons, a stent with superb radial force can be deployed (B). The control coronary angiography shows a good result without residual stenosis (C). With the use of intravascular imaging such as optical coherence tomography (D) an excellent stent expansion and good strut apposition can be confirmed.
Besides calcification, aneurysmal and ectatic transformation is another type of coronary artery degeneration that is prone to thrombotic occlusion and represents a significant challenge for interventional cardiologists. Since stents are usually sized to the often smaller distal landing zone, achieving good stent apposition over the entire length of an ectatic vessel usually requires placement of multiple stents of different diameters, which in turn is associated with an increased risk of stent thrombosis for every overlap with two or more stent layers. An alternative solution is a stent with a high maximum expansion capacity. The overexpansion limit is the maximum size that a stent can be post-dilated to without harming its mechanical integrity. Stents are often produced in a limited number of designs, with each design having a different overexpansion limit. A variety of stent sizes is then achieved by using different sized stent balloons in the same stent design. Thus, knowledge of the different designs and their maximum achievable diameter is critical for stents implanted in segments with marked disparities in diameter [9]. In stents with a large expansion capacity, post-dilatation to ≥2 mm above the nominal size can be safely achieved, allowing optimal lesion coverage and good stent apposition with only one or two stents per lesion. Another situation in which the overexpansion limit is of critical importance are bifurcation lesions in which one stent reaches from the distal side branch into a much larger main vessel. As well as an expansion capacity that can bridge this increment in diameter, it is important that stents allow easy access to the overstented side branch. Therefore, a cell design with large, open cells facilitates crossing of struts into the side branch with a guidewire and placement of subsequent balloons or additional stents.
Different stent properties are required for interventional treatment of small coronary arteries. Often, advancing the stent to the distal lesion can be challenging, in particular through tortuous or calcified vessels. Thus, good stent deliverability is of paramount importance. A small crossing profile of the crimped stent and a good transmission of force from the body of the stent to the tip facilitate stent placement in this situation. When successfully implanted, the size of the small vessel is a predictor of early stent thrombosis and the haemodynamic effects as well as acute vessel injury caused by stent struts are of increasing significance. Therefore, stent-specific properties that counteract stent thrombosis such as thinner struts and a small implantation footprint are particularly desirable [10].
An increasingly senior population presenting with acute coronary syndrome equates to a higher burden of comorbidities, which often result in an increased bleeding risk. In such patients, limiting the duration of dual antiplatelet therapy (DAPT) reduces bleeding events. Since DAPT is required to prevent stent thrombosis until the stent is fully endothelialised, any stent properties that enhance endothelialisation also enable a safe reduction in DAPT duration. A major contribution to delayed endothelialisation comes from antiproliferative drugs in the coating of modern DES. While initial stent designs were entirely covered with the antiproliferative drug to prevent restenosis, recent stent designs have shown that focal distribution of smaller amounts of coating are equally able to prevent restenosis but simultaneously allow quicker endothelialisation. Another important way to limit DAPT induced bleeding risks is to avoid unnecessary stent implantation. To this end, hemodynamic measurements such as fractional flow reserve or the instantaneous wave-free ratio can be used to guide stent implantation in angiographically ambiguous cases.
Since 1986, the rapid technological development of coronary stents has revolutionised cardiac care of patients with myocardial infarction and coronary artery disease. Today’s stents are mostly designed as all-rounders, but knowledge about specific stent features is essential for a tailored approach to treating challenging coronary lesions (figure 3). Future improvements in coronary angioplasty may be achieved by a stent design that is optimised for lesion-specific characteristics to create purpose-built stents for the next level of individualized stent therapy.
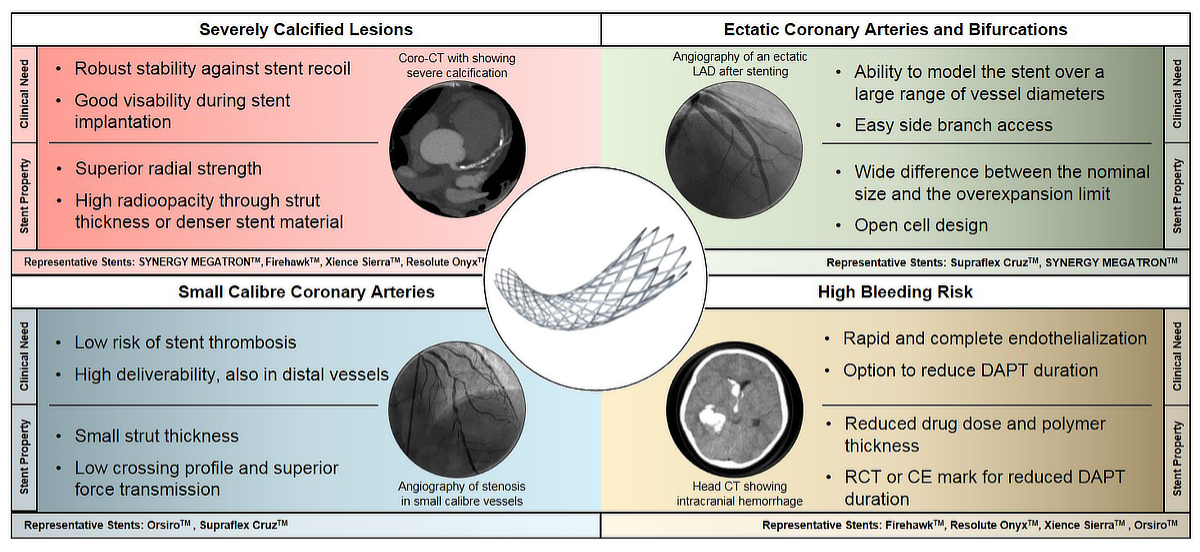
Figure 3 Comparison of clinical needs and desired stents properties in four different clinical scenarios of challenging coronary artery lesions.
A selection of representative stents for the individual clinical scenario is given in the last line of each panel.
Both authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. AG declares no conflict of interest. CT received institutional grants from Abbott Vascular, Medtronic and SMT as well as advisory and consulting grants from Boston Scientific, Biotronik, Microport, Schnell Medical and Schockwave Medical.
1. Sigwart U , Puel J , Mirkovitch V , Joffre F , Kappenberger L . Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med. 1987 Mar;316(12):701–6. https://doi.org/10.1056/NEJM198703193161201
2. Serruys PW , de Jaegere P , Kiemeneij F , Macaya C , Rutsch W , Heyndrickx G , et al.; Benestent Study Group . A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. N Engl J Med. 1994 Aug;331(8):489–95. https://doi.org/10.1056/NEJM199408253310801
3. Serruys PW , Unger F , Sousa JE , Jatene A , Bonnier HJ , Schönberger JP , et al.; Arterial Revascularization Therapies Study Group . Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel disease. N Engl J Med. 2001 Apr;344(15):1117–24. https://doi.org/10.1056/NEJM200104123441502
4. Stone GW , Sabik JF , Serruys PW , Simonton CA , Généreux P , Puskas J , et al.; EXCEL Trial Investigators . Everolimus-Eluting Stents or Bypass Surgery for Left Main Coronary Artery Disease. N Engl J Med. 2016 Dec;375(23):2223–35. https://doi.org/10.1056/NEJMoa1610227
5. Lüscher TF . Ist die Medizin ein Business? Kardiovaskuläre Medizin. 2007;10:351–5.
6. Bourantas CV , Zhang YJ , Garg S , Iqbal J , Valgimigli M , Windecker S , et al. Prognostic implications of coronary calcification in patients with obstructive coronary artery disease treated by percutaneous coronary intervention: a patient-level pooled analysis of 7 contemporary stent trials. Heart. 2014 Aug;100(15):1158–64. https://doi.org/10.1136/heartjnl-2013-305180
7. Okamoto N , Ueda H , Bhatheja S , Vengrenyuk Y , Aquino M , Rabiei S , et al. Procedural and one-year outcomes of patients treated with orbital and rotational atherectomy with mechanistic insights from optical coherence tomography. EuroIntervention. 2019 Apr;14(17):1760–7. https://doi.org/10.4244/EIJ-D-17-01060
8. Patrascu A , Cieslik M , Templin C . [Treatment of Heavily Calcified Coronary Lesions]. Ther Umsch. 2021 Feb;78(1):16–22. https://doi.org/10.1024/0040-5930/a001232
9. Foin N , Sen S , Allegria E , Petraco R , Nijjer S , Francis DP , et al. Maximal expansion capacity with current DES platforms: a critical factor for stent selection in the treatment of left main bifurcations? EuroIntervention. 2013 Mar;8(11):1315–25. https://doi.org/10.4244/EIJV8I11A200
10. Byrne RA , Joner M , Kastrati A . Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Grüntzig Lecture ESC 2014. Eur Heart J. 2015 Dec;36(47):3320–31. https://doi.org/10.1093/eurheartj/ehv511