Cohort profile: the Swiss Cerebral Palsy Registry (Swiss-CP-Reg) cohort study
DOI: https://doi.org/10.4414/SMW.2022.w30139
Fabiën N.
Belleab, Sandra
Hunzikera, Joël
Flussc, Sebastian
Gruntd, Stephanie
Juenemanne, Christoph
Kuenzlef, Andreas
Meyer-Heimg, Christopher J.
Newmanhi, Gian Paolo
Ramellij, Peter
Webere, Claudia E.
Kuehniak, Anne
Tschertera
aInstitute of Social and Preventive Medicine, University of Bern, Bern, Switzerland
bCentre for Primary Care and Public Health (Unisanté), University of Lausanne, Lausanne, Switzerland
cPaediatric Neurology Unit, University Children's Hospital Geneva, Geneva, Switzerland
dDivision of Neuropaediatrics, Development and Rehabilitation, Department of Paediatrics, Inselspital Bern, University Hospital, University of Bern, Switzerland
eDivision of Neuropaediatric and Developmental Medicine, University Children's Hospital of Basel (UKBB), University of Basel, Basel, Switzerland
fChildren’s Hospital of Eastern Switzerland, St Gallen, Switzerland
gSwiss Children’s Rehab, University Children’s Hospital Zurich, Affoltern am Albis, Switzerland
hPaediatric Neurology and Neurorehabilitation Unit, Lausanne University Hospital (CHUV), Lausanne, Switzerland
iUniversity of Lausanne (UNIL), Lausanne, Switzerland
jNeuropaediatric Unit, Paediatric Institute of Southern Switzerland, Ospedale San Giovanni, Bellinzona, Switzerland
kChildren's University Hospital, Inselspital, University of Bern, Bern, Switzerland
Summary
BACKGROUND: Cerebral Palsy (CP) is a group of permanent disorders of movement and posture that follow injuries to the developing brain. It results in motor dysfunction and a wide variety of comorbidities like epilepsy; pain; speech, hearing and vision disorders; cognitive dysfunction; and eating and digestive difficulties. Central data collection is essential to the study of the epidemiology, clinical presentations, care, and quality of life of patients affected by CP. CP specialists founded the Swiss Cerebral Palsy Registry (Swiss-CP-Reg) in 2017. This paper describes the design, structure, aims and achievements of Swiss-CP-Reg and presents its first results.
METHODS: Swiss-CP-Reg records patients of any age diagnosed with CP who are born, are treated, or live in Switzerland. It collects data from medical records and reports, from questionnaires answered by patients and their families, and from data linkage with routine statistics and other registries. The registry contains information on diagnosis, clinical presentation, comorbidities, therapies, personal information, family history, and quality of life.
RESULTS: From August 2017 to August 2021, 546 participants (55% male, mean age at registration 8 years [interquartile range IQR: 5–12]), were enrolled in Swiss-CP-Reg. Most had been born at term (56%), were less than two years old at diagnosis (73%, median 18 months, IQR: 9–25), and were diagnosed with spastic CP (76%). Most (59%) live with a mild motor impairment (Gross Motor Function Classification System [GMFCS] level I or II), 12% with a moderate motor impairment (GMFCS level III), and 29% with a severe motor impairment (GMFCS level IV or V). In a subset of 170 participants, we measured intelligence quotient (IQ) and saw lower IQs with increasing GMFCS level. Swiss-CP-Reg has a strong interest in research, with four nested projects running currently, and many more planned.
CONCLUSIONS: Swiss-CP-Reg collects and exchanges national data on people living with CP to answer clinically relevant questions. Its structure enables retrospective and prospective data collection and knowledge exchange between experts to optimise and standardise treatment and to improve the health and quality of life of those diagnosed with CP in Switzerland.
ClinicalTrials.gov identifier: NCT04992871
Abbreviations
- CFCS
-
Communication Function Classification System
- CP
-
Cerebral palsy
- FSO
-
Federal Statistics Office
- GMFCS
-
Gross Motor Function Classification System
- ISPM
-
Institute of Social and Preventive Medicine
- IQ
-
Intelligence quotient
- MACS
-
Manual Ability Classification System
- REDCap
-
Research Electronic Data Capture
- SACD
-
Swiss Academy of Childhood Disability
- SCPE
-
Surveillance of Cerebral Palsy in Europe
- Swiss-CP-Reg
-
Swiss Cerebral Palsy Registry
- SwissNeoNet
-
Swiss Neonatal Network and Follow-up Group
- SwissPedNet
-
Swiss Research Network of Clinical Paediatric Hubs
- SwissPedReg
-
Swiss Research Platform for Paediatric Registries
Introduction
Cerebral palsy (CP) is a group of permanent disorders of movement, posture, and motor function which cause activity limitation. With a prevalence of 1.5 to 2.5 per 1,000 live-born children [1], it is the most common cause of physical disability in children. It is estimated, based on international data and extrapolation of data from the eastern part of the country (St. Gallen), that approximately 3,000 children and adolescents and 12,000 adults in Switzerland live with CP [2]. CP results from a non-progressive lesion or brain malformation that occurs during the prenatal, perinatal, or postnatal period, e.g., ischemic lesions of the neonatal brain or genetic predispositions leading to brain malformation [3]. CP shows large variability in the type and distribution of movement abnormalities and the degree of functional impairment. Besides motor dysfunction, individuals with CP suffer from many comorbidities, including epilepsy; speech, hearing or vision disorders; cognitive dysfunction; eating and digestive difficulties; behavioural disorders; and secondary musculoskeletal problems including associated pain [1, 3–5]. These comorbidities can restrict individuals with CP in various dimensions of social life such as education, play and sporting activities, and work participation [6–10]. In Switzerland, only 20% of adults with CP are in paid employment in the primary labour market [11]. Besides these restrictions for individuals with CP, their family’s everyday life is also influenced by their disability. A child’s CP can impact the mental and physical health and well-being of affected parents [12–18]. Furthermore, it can limit the activities of siblings [19, 20]. Currently, there is no cure for CP and patients often need lifelong support from an interdisciplinary team of specialists.
The involvement of an interdisciplinary team containing many different specialists makes it difficult for researchers to obtain the full spectrum of patient data. A centralised data collection, where all information on people with CP can be linked, is therefore essential to optimise and standardise care, improve clinical surveillance, develop preventive strategies for comorbidities, and study the quality of life of those affected. Other countries have built up regional [5, 21, 22] or national [23–30] registries, or participate in networks [31, 32]. In Switzerland, knowledge about CP and its supportive treatment is sparse. Previous research on CP in Switzerland includes regional projects that focused on specific questions, such as epidemiological research [33–35] and interventions to improve physical and mental well-being [36–47]. Data have also been contributed to international studies [39, 40, 48, 49]. To collect national data and promote CP research in Switzerland, CP specialists founded the Swiss Cerebral Palsy Registry (Swiss-CP-Reg) in 2017. Swiss-CP-Reg aims to optimise the treatment and improve the health and quality of life of children living with CP by establishing a platform for research and knowledge exchange. This article describes the design, structure, aims, and achievements of Swiss-CP-Reg and presents the baseline characteristics of its participants.
Materials and methods
Study design
Swiss-CP-Reg (www.swiss-cp-reg.ch) is a national patient registry (ClinicalTrials.gov registration number: NCT04992871) that collects medical and socio-demographic data on people with CP. Individuals living with CP who give their consent are registered in Swiss-CP-Reg, and their data are currently collected at regular intervals at the paediatric clinics in Basel, Bellinzona, Bern, Geneva, Lausanne, St. Gallen, and Zurich. In the coming years, data collection will extend to include smaller hospitals and medical practices, and adults will be invited to participate so that Swiss-CP-Reg will become fully population-based.
Aims of Swiss-CP-Reg
The aims of the registry are:
- To identify all children, adolescents, and adults living with CP across the country; to characterise their phenotypes; and to determine CP incidence, prevalence, time trends and regional differences.
- To document diagnostic evaluations, treatments, quality of life, morbidity, mortality, and risk factors.
- To establish a research platform for clinical, epidemiological, and basic research; to support participant recruitment for population-based and interventional studies; and to answer questions on topics like health, healthcare, education, social aspects, and quality of life.
- To establish a platform for knowledge exchange between clinics, researchers, therapists, national and cantonal health authorities, and international parties.
Study population and recruitment procedure
Swiss-CP-Reg registers all children, adolescents, and adults diagnosed with CP who were born, are treated, or live in Switzerland. The diagnosis is made based on the Surveillance of Cerebral Palsy in Europe (SCPE) decision tree [31]: the core inclusion criterion is whether a child has a disorder of movement or posture of central origin. All progressive conditions are excluded. To ensure that only children who meet the inclusion criteria are included, registration of participants is restricted to paediatricians specialised in neurology, rehabilitation, or development, and a confirmation of the diagnosis is required at the age of five years in cases of children who are enrolled at an earlier age. We exclude participants with pure muscular hypotonia, neurometabolic diseases (e.g., neuronal storage diseases, leukodystrophies), and other progressive neurological diseases (e.g., spinocerebellar ataxias, hereditary spastic paraplegia, Rett syndrome, epileptic encephalopathy).
Physicians in clinics or practices identify eligible participants according to their clinical diagnosis and recruit them during routine medical consultations (figure 1). Physicians inform eligible participants and caregivers and collect informed consent during their consultations. Study information and consent forms are available in four languages: French, German, Italian, and English. Those who provide informed consent are registered in Swiss-CP-Reg. For those who do not consent, the registry collects an anonymised, minimal data set consisting of year of birth, year of death (if applicable), sex, gestational age, birth weight, and CP type (table 1).
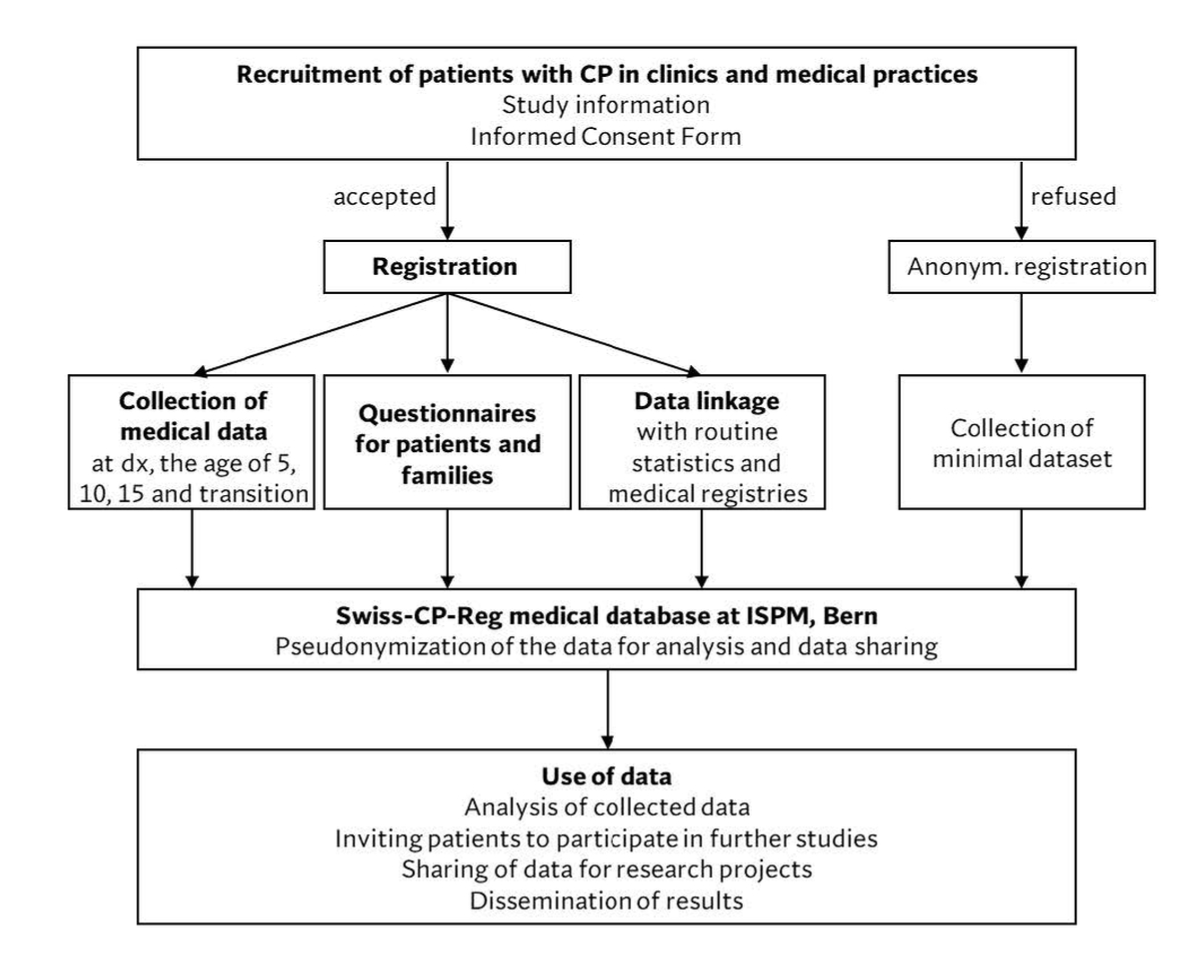
Figure 1 Schematic chart of patient recruitment, data collection, and data use by the Swiss Cerebral Palsy Registry.
CP, cerebral palsy; dx, diagnosis; ISPM, Institute of Social and Preventive Medicine; Swiss-CP-Reg, Swiss Cerebral Palsy Registry; transition, transition to adult care
Table 1Description of the data collected in the Swiss Cerebral Palsy Registry and the time points of assessment.
|
Dx
|
Follow-up
**
|
| Patient information*
|
| Name, address, medical centre, sex***, date/year*** of birth, cause of death, date/year*** of death, parents’ education, and occupation |
X |
X |
| Medical data****
|
| Date of examination/last consultation |
X |
X |
| Confirmation of CP diagnosis |
|
X |
| Anthropometric data |
Height, weight, head circumference |
X |
X |
| CP diagnosis |
Age at diagnosis, post-neonatal CP (cause, time of insult, ICD code) |
X |
|
| CP classification |
CP type***, GMFCS, MACS |
X |
X |
| Syndromes and anomalies |
Genetic analysis, diagnosed syndromes (ICD code), cardiac malformation (ICD code), additional congenital anomalies, hydrocephalus |
X |
X |
| Imaging |
Type (US, MRI, CT), date, classification of results, side |
X |
X |
| Perinatal information |
Maternal place of residence at birth, maternal infection during pregnancy (trimester, pathogen), place of birth, gestational age (weeks and days)***, delivery mode, birth weight***, birth length, head circumference, multiple birth, Apgar score at 5 min, admission to neonatal care unit, convulsions, therapeutic hypothermia (active/passive) |
X |
|
| Cognition |
Neurodevelopmental and cognitive assessments (date, type, result) |
|
X |
| Comorbidities |
Communication (VSS, CFCS, AAC), nutrition (EDACS), visual and hearing impairments (severity), epilepsy (diagnosis, onset, drugs), pain (chronicity, description) |
|
X |
| Musculoskeletal system |
Pathological fractures, hip dislocation (date, side, MI), scoliosis (date, Cobb angle) |
|
X |
| Treatments |
Therapies (physiotherapy, psychotherapy; occupational, equine-assisted, speech, and language therapy), surgeries, medical management, postural management |
|
|
Data collection and data sources
Swiss-CP-Reg collects data from three main sources: (1) medical records from clinics and private practices; (2) questionnaires sent to patients and families; and (3) re-use of data from routine statistics and other registries.
Medical data
Baseline medical data are collected in the participating clinics with the support of the registry team at the Institute of Social and Preventive Medicine (ISPM) in Bern (figure 1, figure 2). The list of variables collected is based on the SCPE data set [21] and includes additional information on specific topics such as therapies, scoliosis, hip surveillance, and pain (table 1). Personal information collected from the medical records includes the patient’s name, contact information, and date of birth, and the contact information of the treating paediatrician. This is necessary to ensure long-term follow-up of the patients over decades and to enable patients and families to be invited to participate in surveys and clinical studies. The data set contains a detailed description of people living with CP, their clinical condition, diagnosis, measures of severity, and care. CP is diagnosed according to the SCPE decision tree and the SCPE hierarchical classification tree of cerebral palsy sub-types [31]. Severity and comorbidities are assessed using standardised tools such as the Gross Motor Function Classification System (GMFCS) [50, 51], the Manual Ability Classification System (MACS) [52], or the Communication Function Classification System (CFCS) [53]. The use of these instruments allows changes in the condition over time to be recorded and comparisons between centres. Medical data are collected at regular intervals (figure 2, table 1). The main time periods of data collection are: at diagnosis (independent of age), at the ages of 5, 10, and 15 years, and at the time of transition to adult care. Follow-up during adulthood is foreseen. When a patient is included in Swiss-CP-Reg, available data up to the time point of enrolment are collected retrospectively. From then onwards, follow-up data are collected prospectively until death or loss to follow-up. Centres currently collect all available medical data from birth onwards for young children born after 2009, but because of limited resources, for adolescents born before 2010 we currently only collect CP classification and severity data (GMFCS and intelligence quotient [IQ]). Retrospective data collection from birth until enrolment will follow for adolescents as soon as resources allow.
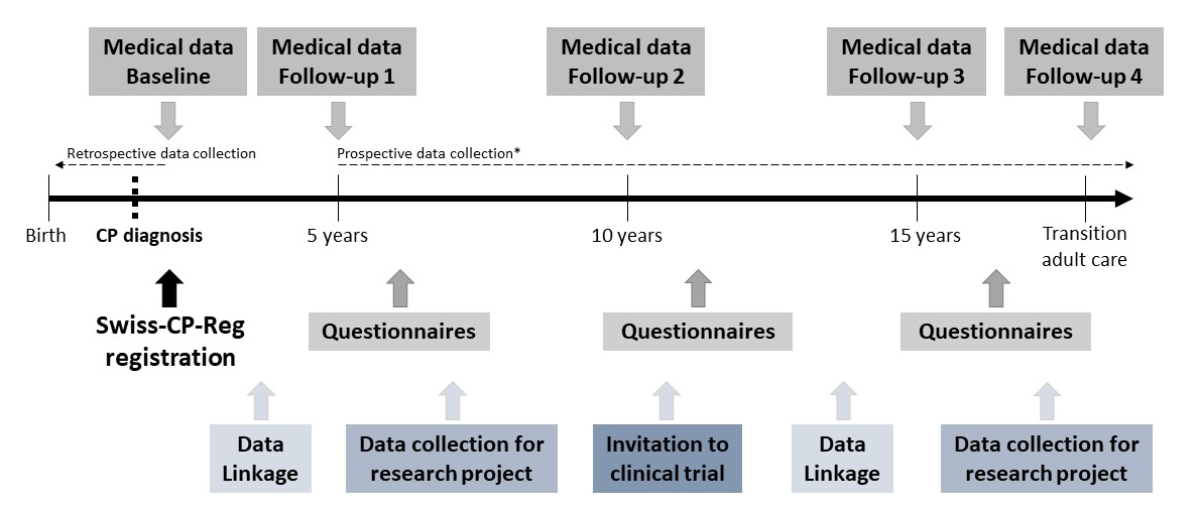
Figure 2 Schematic flow chart of the Swiss Cerebral Palsy Registry; data collection management and timelines.
CP, cerebral palsy; Swiss-CP-Reg, Swiss Cerebral Palsy Registry
* Medical follow-up data can be collected retrospectively if a participant is registered after the age of 5 years.
Questionnaire data
Questionnaires are sent to people with CP in the registry and their families at regular intervals. Participation is voluntary and can be accepted or declined for each survey. The questionnaires cover different topics, including healthcare, nutrition, sleep, pain, use of medical equipment, use of ancillary services, academic information including early childhood education level, school and professional integration, family history, health behaviour, quality of life, daily life participation, and the needs and concerns of patients and families.
Re-use of data from routine statistics and other registries
Data from routine statistics and other registries can be linked to Swiss-CP-Reg to answer specific research questions. For example, the Federal Statistics Office (FSO) Live Birth Registry can provide information on gestational age, birth weight, birth length, and parental age; the FSO Cause of Death and Stillbirth Statistics can contribute data on causes of death; Hospital Episode Statistics can provide information on the length of hospital stay; the Swiss Neonatal Network and Follow-up Group (SwissNeoNet) has information on the perinatal period and follow-up data from high-risk infants; and the Swiss Neuropediatric Stroke Registry includes infarct data. Authorisation for data linkage is needed from the data providers, e.g., from FSO or SwissNeoNet, for each linkage.
Database management and data flows
The Swiss-CP-Reg database is built and managed using Research Electronic Data Capture (REDCap; Nashville, TN, USA) [54, 55] hosted at ISPM. REDCap is a secure, web-based software platform supporting data capture for research studies. Captured personal data are strictly confidential. Access to the ISPM server and the Swiss-CP-Reg database is only given to authorised personnel and data are handled with the uttermost discretion. Daily, weekly, and monthly back-up of the database is performed and the back-ups are securely stored on the ISPM servers.
Most data are entered into the REDCap database directly by local healthcare professionals or by members of the Swiss-CP-Reg team (figure 2). The Swiss-CP-Reg team enters additional information that is sent to the ISPM via post or a secure e-mail address. Paper forms are stored securely at ISPM and can only be accessed by Swiss-CP-Reg team members. All paper forms, except for completed questionnaires, consent forms, and withdrawals, are destroyed after digitisation and storage in the REDCap database.
Data quality, analyses, and data sharing
Patient data are checked manually by the Swiss-CP-Reg team and by REDCap for completeness, plausibility, and consistency. Ambiguities are resolved in collaboration with the treating healthcare professionals at the time of data entry or after the manual quality check. We perform random control checks to detect systematic mistakes during data entry. In addition, selected data points are cross-checked for plausibility with previously entered data. Appropriate data analyses are selected based on the research question and are performed using dedicated programs (Stata, R). In this data presentation, we report continuous data as medians with interquartile ranges (IQR) and categorical data as numbers with percentages. We used chi-square statistics for between-group comparisons and we considered a two-sided p-value <0.05 as significant. We used Stata (version 16, Stata Corporation, Austin, Texas) for our analyses.
Pseudonymised data can be made available to other research projects when legal requirements are met. This includes data usage by regional, national, or international research projects. Researchers interested in collaborative work can contact the Swiss-CP-Reg team (swiss-cp-reg[at]ispm.unibe.ch) to discuss planned projects or to analyse existing data. Decisions on collaboration are made by the steering board of Swiss-CP-Reg.
Governance, organisational structure, and funding
Organisational structure
In 2014, the setting up of a national registry for CP in Switzerland was initiated by Dr. med. Christoph Kuenzle (Ostschweizer Kinderspital, St. Gallen). The setting up was divided into three phases. In the preparation phase we defined the stakeholders, organisational structure, fundraising strategy, objectives, data set, inclusion and exclusion criteria, and participant recruitment and data collection methodologies. During the following build-up phase, we applied for ethical approval; developed data transfer and use agreements and data collection guidelines; set up a REDCap database and dissemination tools; and requested funding. During the third phase, we started to recruit participants and to collect data. We also developed data quality checks and revised the initial data set where needed.
We developed an inclusive and broad organisational structure, which comprises several specialised bodies. The steering board includes specialised paediatricians from all seven large Swiss children’s hospitals. The involvement of the steering board led to a harmonised national data collection and a more complete record of each child's clinical picture. The operational management is located at ISPM Bern and supports the data providers in recruitment and data collection. It hosts and maintains the database, takes care of the legal aspects and public relations, and promotes research. The expert groups support the registry and include specialists from different fields, such as geneticists, paediatric orthopaedic surgeons, physiotherapists, speech therapists, and nutritionists, and parents of children or adolescents with CP. The general assembly includes representatives of clinical centres, national patient organisations, medical societies, and expert groups. They meet once per year to discuss ongoing research and promote study participation. The clinical centres provide support for the data collection.
Swiss-CP-Reg collaborates closely with the Swiss Research Platform for Paediatric Registries (SwissPedReg; www.swisspedregistry.ch). This platform provides advice to registries in a build-up phase on, for example, the ethics application process, data sets and information collection, and organisational structure. Swiss-CP-Reg collaborates closely with national stakeholders, including patients and their families, patient organisations, clinics, medical societies, experts from various disciplines and regions, researchers, and international networks. All national stakeholders are included in the organisational structure of the registry. Swiss-CP-Reg also exchanges information with the collaborative network of CP registries across Europe (SCPE) and will apply for membership as soon as it reaches the prerequisites set by the SCPE, e.g., the recruitment of patients extended to small clinics and private practices.
Funding
Swiss-CP-Reg (salaries, consumables, equipment) is financed by several funding bodies, including the "Schweizerische Stiftung für das cerebral gelähmte Kind (Stiftung Cerebral)", the Anna Mueller Grocholski Foundation, the Swiss Academy of Childhood Disability (SACD), "Hand in Hand Anstalt", "Ostschweizer Kinderspital", the ACCENTUS Charitable Foundation (Walter Muggli Fund), Ebnet-Stiftung and the Children’s Research Centre (University Children’s Hospital Zurich). Stiftung Cerebral was the main sponsor during the build-up phase of the registry.
Ethical approval
In 2017, Swiss-CP-Reg obtained authorisation from the Cantonal Ethics Committee of Bern (2017-00873, risk category A, observational study) to collect national medical data from hospitals, clinics, and private practices; to collect self-reported data from patients and families; to link data; and to retrospectively register deceased patients and patients lost to follow-up.
Results
Status of patient registration and data collection
Active patient enrolment started in August 2017 in St. Gallen, followed in 2018 by Basel, Bellinzona, and Bern; in 2019 by Zurich and Geneva; and in 2020 by Lausanne. The COVID-19 pandemic slowed down recruitment in 2020 and 2021. By the end of August 2021, Swiss-CP-Reg included data from 515 children and adolescents (0–17 years), representing an estimated 17% of all eligible children with CP in Switzerland, and 31 adults (≥18 years). Among the eligible participants who were identified and approached, only 30 (5%) refused participation and 4 (1%) were non-responders. For these we collected an anonymised, minimal data set (table 2). The informed consent is pending for about 80 patients, and therefore these patients have not yet been registered. By August 2021, we had collected baseline data at time of diagnosis for 400 participants, 5-year follow-up data for 266, 10-year follow-up data for 162, 15-year follow-up data for 31, and follow-up data at the time point of transition to adult care for two participants.
Table 2Characteristics of study participants in the Swiss Cerebral Palsy Registry (n = 546, status at 31.08.2021).
|
Characteristics
|
n*
|
Registered individuals; n (%)
|
| Informed consent |
546 |
|
| – Signed |
|
512 (94) |
| – Refused |
|
30 (5) |
| – Non-responder |
|
4 (1) |
| Sex |
546 |
|
| – Female |
|
248 (45) |
| – Male |
|
298 (55) |
| Age at registration, years, median (IQR) |
546 |
8 (5-12) |
| – <5 |
|
144 (26) |
| – 5–9.99 |
|
191 (35) |
| – 10–14.99 |
|
169 (31) |
| – ≥15 |
|
42 (8) |
| Language region of Switzerland |
546 |
|
| – German-speaking |
|
448 (82) |
| – French-speaking**
|
|
74 (14) |
| – Italian-speaking |
|
24 (4) |
| Year of birth |
546 |
|
| – 1996–2000 |
|
9 (2) |
| – 2001–2005 |
|
58 (11) |
| – 2006–2010 |
|
195 (36) |
| – 2011–2015 |
|
189 (35) |
| – 2016–2020 |
|
95 (17) |
| Gestational age, weeks |
362 |
|
| – <28 |
|
39 (11) |
| – 28–31 |
|
60 (17) |
| – 32–36 |
|
62 (17) |
| – ≥37 |
|
201 (56) |
| Age at diagnosis, months, median (IQR) |
283 |
18 (9–25) |
| – ≤24 |
|
208 (73) |
| – 25–48 |
|
57 (20) |
| – 49–72 |
|
10 (4) |
| – >72 |
|
8(3) |
| CP type***
|
432 |
|
| – Spastic, all |
|
329 (76) |
| – Spastic, bilateral |
|
172 (40) |
| – Spastic, unilateral |
|
155 (36) |
| – Spastic, unknown |
|
2 (<1) |
| – Dyskinetic |
|
48 (11) |
| – Ataxic |
|
55 (13) |
| GMFCS***
|
402 |
|
| – I Walks without limitations |
|
150 (37) |
| – II Walks with limitations |
|
87 (22) |
| – III Walks using a hand-held mobility device |
|
49 (12) |
| – IV Self-mobility with limitations; may use powered mobility |
|
64 (16) |
| – V Transport in a manual wheelchair |
|
52 (13) |
Characteristics of the study population
Table 2 summarises the basic socio-demographic and medical characteristics of the 546 registered participants. Slightly more than half were male (55%) and the median age at registration was 8 years (IQR: 5–12 years). Most lived in the German-speaking language region (82%); were born during the last 10 years (years of birth 2011–2020, 52%) and at term (gestational age ≥37 weeks, 56%); and were younger than two years at diagnosis (73%, median age 18 months, IQR: 9-25). The most prevalent type of diagnosis was spastic CP (76%), with 52% of those with spastic CP having bilateral and 47% having unilateral spastic CP. Most (59%) live with a mild motor impairment (GMFCS level I, walks without limitations, or II, walks with limitations). A moderate motor impairment was diagnosed in 12% (GMFCS level III, walks using a hand-held mobility device) and 29% have a severe motor impairment (GMFCS level IV, self-mobility with limitations; may use powered mobility, or V, transport in a manual wheelchair). We collected information on IQ in a subset of 170 children and adolescents. There was a clear decrease in IQ with increasing level of GMFCS (figure 3).
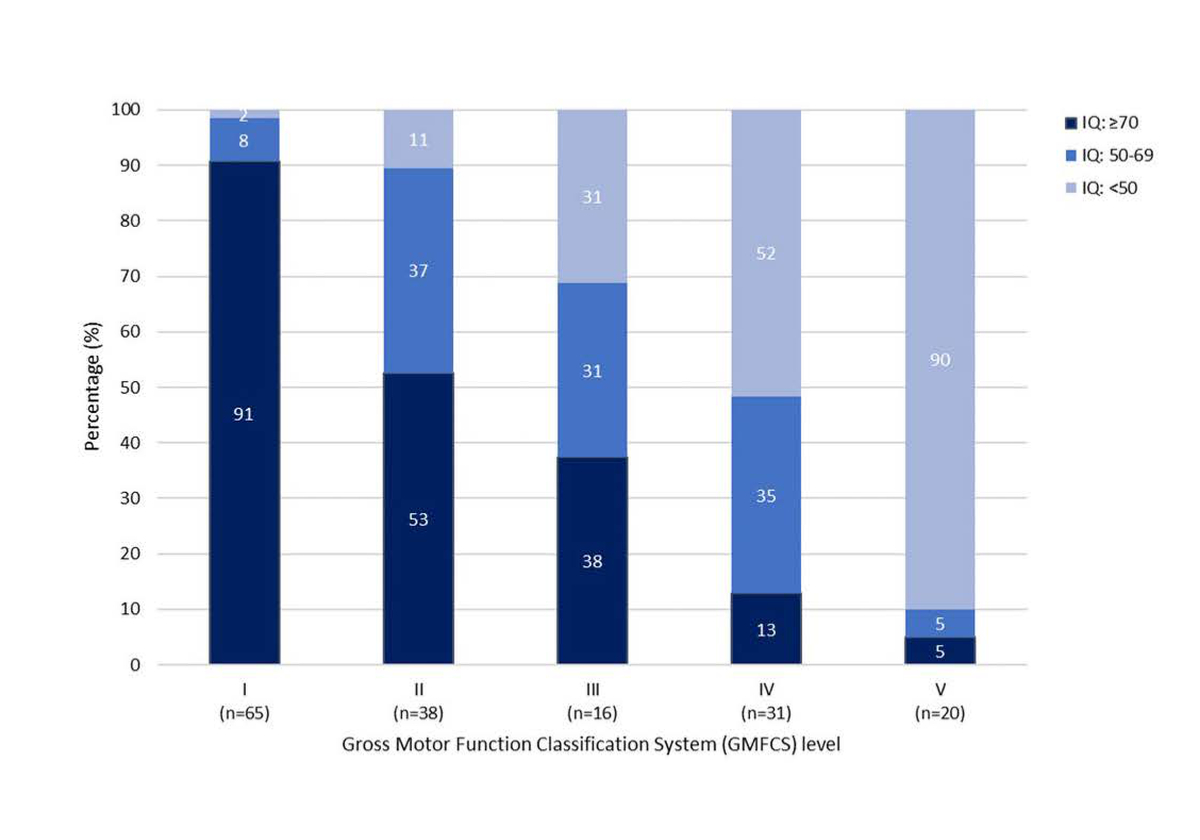
Figure 3 Intelligence quotient (IQ)1 distribution by Gross Motor Function Classification System2 in a subset of 170 children and adolescents included in the Swiss Cerebral Palsy Registry.
The p-value calculated from chi-square statistics comparing GMFCS levels (two-sided) is <0.001.
1 Intelligence quotient (IQ) is not assessed in every child/adolescent; the decision lies with the treating physician. Tools used to assess IQ are the Kaufman Assessment Battery for Children (K-ABC or K-ABC II), Wechsler Preschool and Primary Scale of Intelligence (WPPSI-III or WPPSI-IV), Wechsler Intelligence Scale for Children (WISC-IV or WISC-V), Snijders-Oomen Nonverbal Intelligence Test (SON-R 2.5–7, SON-R 2–8 or SON-R 6–40), or Test of Nonverbal Intelligence (Toni-4). In half of the children/adolescents, IQ was assessed by the treating physician as a clinical estimate and collected as a range.
2 The Gross Motor Function Classification System (GMFCS level) is subdivided into level I, walks without limitations; level II, walks with limitations; level III, walks using a hand-held mobility device; level IV, self-mobility with limitations; may use powered mobility; and level V, transported in a manual wheelchair.
Research projects
Swiss-CP-Reg collaborates with experts from various fields to realise research projects that aim to standardise and improve CP therapies. It supports the monitoring of and research into a wide range of outcomes, as well as data exchange via linkages with national and regional projects led by interested investigators. Ongoing projects address the following broad range of topics: needs and wishes of patients and families, hip surveillance in a variety of Swiss paediatric clinics, risk factors for and outcomes of unilateral CP, and data linkage with SwissNeoNet (table 3). In addition, master’s students can conduct projects within the scope of Swiss-CP-Reg. Three master’s theses have been carried out, addressing medical cannabinoids [56], the epidemiology of epilepsy [57], and magnetic resonance imaging classification [58]. Current theses address the relationship between neuroimaging patterns and upper limb function in unilateral spastic CP; the timing, type, and treatment of epileptic seizures; nutrition (all S. Grunt); and pain (C. Kuenzle). Liliane Raess evaluated, in her doctoral thesis (under supervision of A. Meyer-Heim and H. van Hedel), the perinatal history, imaging, outcomes, and treatment of the Zurich cohort of children with CP and compared this cohort to other registries [59]. A second aim of her thesis was to investigate the differences in early history and diagnostic process between the rare and common neurologic subtypes of CP in the Zurich cohort.
Table 3Ongoing research projects and collaborations of the Swiss Cerebral Palsy Registry (September 2021).
|
Principal investigator, place
|
Project
|
| PD Dr. A. Tscherter, Bern |
Needs and wishes of patients with CP and their families: Data on needs and wishes of children with CP and their families living in Switzerland are collected via questionnaires. The aim of this study is to uncover sources of concern for patients and families (such as quality of life, nutrition, communication, use of aids).
|
| Prof. Dr. med. T. Dreher, Zurich |
Hip surveillance: Monitoring the hips of patients with CP is important to detect and treat luxation at an early stage. Several countries have implemented hip surveillance programmes [66]. The aim of this project is to establish such a monitoring programme in Switzerland. Currently, the data set and the data collection procedure are being validated in Zurich. |
| Dr. M. Adams, PD and Dr. A. Tscherter, Zurich, Bern |
Swiss Neonatal Network & Follow-up Group (SwissNeoNet): Partial pseudonymised data from SwissNeoNet and Swiss-CP-Reg are regularly linked to identify missing children and to facilitate collaborative research. In the first data linkage in August 2021, we identified 186 preterm children not yet included in Swiss-CP-Reg. |
| Dr. A. Girardet, PD and Dr. J. Fluss, Geneva |
Risk factors and outcomes of unilateral CP: Unilateral CP is the most common form of CP, affecting up to 40% of patients [61]. This project assesses different motor and cognitive patterns in Swiss children with unilateral CP. The results are compared with imaging, clinical, and socio-demographic data. |
We pursue participation in international research projects, in particular with the SCPE. C. Kuenzle, a member of the Swiss-CP-Reg steering board, is part of the SCPE. He founded a CP registry in the canton of St. Gallen in 2013. He recruited most of the children and adolescents with CP born between 1995 and 2011 and collected their data [2]. Swiss-CP-Reg participates in SCPE projects using the data from St. Gallen [60].
Discussion
Swiss-CP-Reg has standardised and harmonised the collection of data on people with CP throughout Switzerland; facilitates data comparisons between patients, regions, and countries; and has become a successful communication platform and a powerful tool for answering clinically relevant questions in CP. By August 2021, 546 patients had been enrolled.
Comparison with other registries
The aims of Swiss-CP-Reg are comparable to those of other CP registries [61], but in addition include the creation of a research platform and a network for knowledge exchange. Like most registries (64%), Swiss-CP-Reg uses the SCPE definition of CP [61], which facilitates comparisons. Many (46%) registries require a minimum age of survival (44%) or a minimum severity criterion (46%) for inclusion [61]. We do not restrict inclusion according to these criteria to ensure that severely affected patients who died at a young age and mild cases are all included. This reduces selection bias. We collect a complete data set after consent. Other registries use permission from government bodies or opt-out options [62], which make recruitment easier. Most CP registries are government-funded (72%); others, including Swiss-CP-Reg, are supported by non-profit organisations/charities (12%) [61].
Preliminary results show that CP classification, severity, and prevalence of comorbidities are in line with other European countries [5,63-65]. Most participants are male, are diagnosed with a spastic bilateral CP subtype, and have mild motor impairment (GMFCS levels I or II), in line with expectations [5,63,64].
Strengths and limitations
The successful launch of Swiss-CP-Reg is in large part indebted to its steering board. It has been a bottom-up approach, with all large Swiss clinics being represented. We strived to reach a consensus on a fully standardised and complete data collection. The board also set up a thorough methodology for patient identification and registration, individualised for each clinic. The continuous motivation of local medical staff and the optimisation of recruitment and data collection by steering board members is important because recruitment and data collection are time-consuming. All steering board members are dedicated and have broad national and international connections, which facilitate funding and strengthen our relationships with all stakeholders. This, together with the dissemination activity of the registry, creates a platform for interdisciplinary and interregional communication and knowledge exchange, one of the key goals of the registry. Another strength of Swiss-CP-Reg is its role as a research platform. Several other factors contribute to its success. First, we developed our data set based on the data set established by SCPE, which facilitates comparisons with other registries. Secondly, Swiss-CP-Reg simplifies research projects because it obtains broad ethical approval via a single informed consent to collect data from multiple sources, conduct surveys, and link data. We can also contact participants and their families directly to invite them to participate in nested research. Finally, the registry can share partial coded data with research projects when they meet all legal requirements.
A current limitation of Swiss-CP-Reg is that it is not population-based. At present, it misses patients who are diagnosed and treated in smaller clinics and private practices, as well as adult patients. This might have biased the distribution of severity reported in this study, as it is possible that university hospitals treat more severe cases than smaller clinics and private practices. Efforts are being made to become population-based by including all eligible patients. This will allow us to make reliable statements about CP in Switzerland in the future and will enable comparisons with registries which apply different inclusion criteria, e.g., minimal severity of CP or exclusion of severely affected patients who died at a young age. Secondly, the registry is currently limited by the retrospective data collection at the time of recruitment. This will improve as soon as all patients already in follow-up have been included, so that recruitment can focus on incident cases, where data can be collected prospectively.
Future developments
In the coming years patient recruitment will be expanded to include more clinics and private practices. We will also expand recruitment to adult participants. We plan to conduct in-depth analyses of topics causing patients with CP and their families concern. Data will be collected via questionnaires. The topics studied will depend on the outcome of our current project on the needs and wishes of patients with CP and their families. The aim of these surveys will be to support CP patients and their families by uncovering possible intervention strategies for each topic.
An important focus of our research is the participation of people with CP in daily activities. Children and adolescents with CP participate less in social life than their peers [9]. Participation can be increased through therapeutic measures, but data on the situation in Switzerland is lacking. Therefore, we are currently setting up a study to investigate barriers and facilitators of the social participation of children with CP. This study will assess how children with CP and their families evaluate their participation in social life. In addition, future research projects of Swiss-CP-Reg aim to understand the aetiology of CP; uncover prognostic factors of CP during the perinatal phase; evaluate the occurrence of associated syndromes and anomalies; determine comorbidities; evaluate and improve treatments and therapies; detect regional differences in medical coverage; evaluate the impact of socio-economic factors on diagnosis, prognosis, and quality of life; develop strategies to enhance quality of life; uncover the needs and concerns of patients and families; and evaluate the early education and integration of CP patients within the school system and the working environment. Furthermore, this data can be used to develop the diagnostic and therapeutic knowledge and skills of young medical staff, as part of their education, to ensure improvement in the care of patients living with CP in the future.
Conclusions
Swiss-CP-Reg is now established in Switzerland. It generates rich information on people living with CP and has facilitated the development of national and international research projects in various fields. Swiss-CP-Reg serves as an instrument for medical care, clinical and epidemiological research, the assessment of healthcare structures, and the evaluation of medical procedures, thus enabling us to increase our understanding of CP, explore the causes of CP and associated conditions, and improve clinical practice.
Acknowledgments
The authors express their gratitude to Dr. Christoph Kuenzle, who initiated Swiss-CP-Reg, and all local hubs of the Swiss Research Network of Clinical Paediatric Hubs (SwissPedNet) for their support in patient recruitment and data collection. We thank all patients included in Swiss-CP-Reg and their families for taking part. We thank all the physicians and local study teams who helped to identify and register patients and worked closely with us throughout the building of Swiss-CP-Reg. We are grateful to Prof. Dr. Thomas Dreher, Dr. Anne Girardet, Prof. Dr. Hubertus van Hedel, and Prof. Dr. Christina Schulze for their collaboration on various projects and to the SACD for hosting the general assembly of Swiss-CP-Reg during their annual conference. We are thankful to our sponsors (see ‘Funding’), without whom this work would not be possible. SwissPedReg, a member of SwissPedNet, supports Swiss-CP-Reg with its research infrastructure.
Anne Tscherter, PD Dr. phil. nat., Dr. sci. med.
Institute of Social and Preventive Medicine
University of Bern
Mittelstrasse 43
CH-3012 Bern
anne.tscherter[at]ispm.unibe.ch
References
1.
Graham HK
,
Rosenbaum P
,
Paneth N
,
Dan B
,
Lin JP
,
Damiano DL
, et al.
Cerebral palsy. Nat Rev Dis Primers. 2016 Jan;2(1):15082. https://doi.org/10.1038/nrdp.2015.82
2.
Kuenzle C
,
Stojicevic V
,
Forni R
,
van Son C
,
Maier O
. Epidemiology of cerebral palsy in eastern switzerland (st. Gallen). Dev Med Child Neurol. 2015;57:49–49. https://doi.org/10.1111/dmcn.12780_66
3.
Rosenbaum P
,
Paneth N
,
Leviton A
,
Goldstein M
,
Bax M
,
Damiano D
, et al.
A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007 Feb;109:8–14.
4.
Hollung SJ
,
Bakken IJ
,
Vik T
,
Lydersen S
,
Wiik R
,
Aaberg KM
, et al.
Comorbidities in cerebral palsy: a patient registry study. Dev Med Child Neurol. 2020 Jan;62(1):97–103. https://doi.org/10.1111/dmcn.14307
5.
Jonsson U
,
Eek MN
,
Sunnerhagen KS
,
Himmelmann K
. Cerebral palsy prevalence, subtypes, and associated impairments: a population-based comparison study of adults and children. Dev Med Child Neurol. 2019 Oct;61(10):1162–7. https://doi.org/10.1111/dmcn.14229
6.
Fauconnier J
,
Dickinson HO
,
Beckung E
,
Marcelli M
,
McManus V
,
Michelsen SI
, et al.
Participation in life situations of 8-12 year old children with cerebral palsy: cross sectional European study. BMJ. 2009 Apr;338 apr23 2:b1458. https://doi.org/10.1136/bmj.b1458
7.
Michelsen, S.I.
;
Flachs, E.M.
;
Uldall, P.
;
Eriksen, E.L.
;
McManus, V.
;
Parkes, J.
;
Parkinson, K.N.
;
Thyen, U.
;
Arnaud, C.
;
Beckung, E.
, et al.
Frequency of participation of 8-12-year-old children with cerebral palsy: A multi-centre cross-sectional european study. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society 2009, 13, 165-177.
8.
Colver A
,
Thyen U
,
Arnaud C
,
Beckung E
,
Fauconnier J
,
Marcelli M
, et al.
Association between participation in life situations of children with cerebral palsy and their physical, social, and attitudinal environment: a cross-sectional multicenter European study. Arch Phys Med Rehabil. 2012 Dec;93(12):2154–64. https://doi.org/10.1016/j.apmr.2012.07.011
9.
Michelsen SI
,
Flachs EM
,
Damsgaard MT
,
Parkes J
,
Parkinson K
,
Rapp M
, et al.
European study of frequency of participation of adolescents with and without cerebral palsy. Eur J Paediatr Neurol. 2014 May;18(3):282–94. https://doi.org/10.1016/j.ejpn.2013.12.003
10.
Colver A
,
Rapp M
,
Eisemann N
,
Ehlinger V
,
Thyen U
,
Dickinson HO
, et al.
Self-reported quality of life of adolescents with cerebral palsy: a cross-sectional and longitudinal analysis. Lancet. 2015 Feb;385(9969):705–16. https://doi.org/10.1016/S0140-6736(14)61229-0
11.
Bundesamt für Sozialversicherungen BSV
. Schweizerische eidgenossenschaft. Die medizinischen massnahmen in der invaliden- und krankenversicherung. https://www.parlament.ch/centers/documents/de/med-massnahmen-iv-kv-2013-03-15-d.pdf (14.07.2021)
12.
Brehaut JC
,
Kohen DE
,
Raina P
,
Walter SD
,
Russell DJ
,
Swinton M
, et al.
The health of primary caregivers of children with cerebral palsy: how does it compare with that of other Canadian caregivers? Pediatrics. 2004 Aug;114(2):e182–91. https://doi.org/10.1542/peds.114.2.e182
13.
Raina P
,
O’Donnell M
,
Rosenbaum P
,
Brehaut J
,
Walter SD
,
Russell D
, et al.
The health and well-being of caregivers of children with cerebral palsy. Pediatrics. 2005 Jun;115(6):e626–36. https://doi.org/10.1542/peds.2004-1689
14.
Yamaoka Y
,
Tamiya N
,
Moriyama Y
,
Sandoval Garrido FA
,
Sumazaki R
,
Noguchi H
. Mental health of parents as caregivers of children with disabilities: based on japanese nationwide survey. PLoS One. 2015 Dec;10(12):e0145200. https://doi.org/10.1371/journal.pone.0145200
15.
Lach LM
,
Kohen DE
,
Garner RE
,
Brehaut JC
,
Miller AR
,
Klassen AF
, et al.
The health and psychosocial functioning of caregivers of children with neurodevelopmental disorders. Disabil Rehabil. 2009;31(9):741–52. https://doi.org/10.1080/08916930802354948
16.
Sipal RF
,
Schuengel C
,
Voorman JM
,
Van Eck M
,
Becher JG
. Course of behaviour problems of children with cerebral palsy: the role of parental stress and support. Child Care Health Dev. 2010 Jan;36(1):74–84. https://doi.org/10.1111/j.1365-2214.2009.01004.x
17.
Majnemer A
,
Shevell M
,
Law M
,
Poulin C
,
Rosenbaum P
. Indicators of distress in families of children with cerebral palsy. Disabil Rehabil. 2012;34(14):1202–7. https://doi.org/10.3109/09638288.2011.638035
18.
Park MS
,
Chung CY
,
Lee KM
,
Sung KH
,
Choi IH
,
Kim TW
. Parenting stress in parents of children with cerebral palsy and its association with physical function. J Pediatr Orthop B. 2012 Sep;21(5):452–6. https://doi.org/10.1097/BPB.0b013e32835470c0
19.
Tseng MH
,
Chen KL
,
Shieh JY
,
Lu L
,
Huang CY
,
Simeonsson RJ
. Child characteristics, caregiver characteristics, and environmental factors affecting the quality of life of caregivers of children with cerebral palsy. Disabil Rehabil. 2016 Dec;38(24):2374–82. https://doi.org/10.3109/09638288.2015.1129451
20.
Woodgate RL
,
Edwards M
,
Ripat JD
,
Rempel G
,
Johnson SF
. Siblings of children with complex care needs: their perspectives and experiences of participating in everyday life. Child Care Health Dev. 2016 Jul;42(4):504–12. https://doi.org/10.1111/cch.12345
21.
Surveillance of Cerebral Palsy in Europe
. Surveillance of cerebral palsy in europe (scpe): Scientific report 1998-2018. Available from: https://eu-rd-platform.jrc.ec.europa.eu/scpe_en
22.
Cans C
,
Guillem P
,
Arnaud C
,
Baille F
,
Chalmers J
,
McManus V
, et al.
Prevalence and characteristics of children with cerebral palsy in Europe. Dev Med Child Neurol. 2002 Sep;44(9):633–40.
23.
Australian Cerebral Palsy Register
. G. Australia and the australian cerebral palsy register for the birth cohort 1993 to 2006. Dev Med Child Neurol. 2016;58 Suppl 2:3–4. https://doi.org/10.1111/dmcn.13002
24.
Shevell MI
,
Dagenais L
,
Hall N
,
Consortium R
; REPACQ Consortium
. Comorbidities in cerebral palsy and their relationship to neurologic subtype and GMFCS level. Neurology. 2009 Jun;72(24):2090–6. https://doi.org/10.1212/WNL.0b013e3181aa537b
25.
Alriksson-Schmidt A
,
Rimstedt AB
,
Hagglund G
. Cpup- a multidisciplinary secondary prevention program for individuals with cerebral palsy. Int J Integr Care. 2019;19(4):19. https://doi.org/10.5334/ijic.s3380
26.
Uldall P
,
Michelsen SI
,
Topp M
,
Madsen M
. The Danish Cerebral Palsy Registry. A registry on a specific impairment. Dan Med Bull. 2001 Aug;48(3):161–3.
27.
Yim SY
,
Yang CY
,
Park JH
,
Kim MY
,
Shin YB
,
Kang EY
, et al.; Society of Pediatric Rehabilitation and Developmental Medicine, Korea.
Korean database of cerebral palsy: A report on characteristics of cerebral palsy in south korea. Ann Rehabil Med. 2017 Aug;41(4):638–49. https://doi.org/10.5535/arm.2017.41.4.638
28.
Badawi N
,
Honan I
,
Finch-Edmondson M
,
Hogan A
,
Fitzgerald J
,
Imms C
. The australian & new zealand cerebral palsy strategy. Dev Med Child Neurol. 2020 Aug;62(8):885. https://doi.org/10.1111/dmcn.14554
29.
Gincota Bufteac E
,
Andersen GL
,
Torstein V
,
Jahnsen R
. Cerebral palsy in Moldova: subtypes, severity and associated impairments. BMC Pediatr. 2018 Oct;18(1):332. https://doi.org/10.1186/s12887-018-1305-6
30.
Almasri NA
,
Saleh M
,
Abu-Dahab S
,
Malkawi SH
,
Nordmark E
. Development of a cerebral palsy follow-up registry in jordan (cpup-jordan). Child Care Health Dev. 2018 Jan;44(1):131–9. https://doi.org/10.1111/cch.12527
31.
Surveillance of Cerebral Palsy in Europe
. Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Dev Med Child Neurol. 2000 Dec;42(12):816–24.
32.
Hurvitz EA
,
Gross PH
,
Gannotti ME
,
Bailes AF
,
Horn SD
. Registry-based research in cerebral palsy: the cerebral palsy research network. Phys Med Rehabil Clin N Am. 2020 Feb;31(1):185–94. https://doi.org/10.1016/j.pmr.2019.09.005
33.
Wiedemann, A.
;
Pastore-Wapp, M.
;
Slavova, N.
;
Steiner, L.
;
Weisstanner, C.
;
Regényi, M.
;
Steinlin, M.
;
Grunt, S.
Impact of stroke volume on motor outcome in neonatal arterial ischemic stroke. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society 2020, 25, 97-105.
34.
Caspar-Teuscher, M.
;
Studer, M.
;
Regényi, M.
;
Steinlin, M.
;
Grunt, S.
Health related quality of life and manual ability 5 years after neonatal ischemic stroke. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society 2019, 23, 716-722.
35.
Grunt S
,
Mazenauer L
,
Buerki SE
,
Boltshauser E
,
Mori AC
,
Datta AN
, et al.
Incidence and outcomes of symptomatic neonatal arterial ischemic stroke. Pediatrics. 2015 May;135(5):e1220–8. https://doi.org/10.1542/peds.2014-1520
36.
Grunt S
,
Newman CJ
,
Saxer S
,
Steinlin M
,
Weisstanner C
,
Kaelin-Lang A
. The mirror illusion increases motor cortex excitability in children with and without hemiparesis. Neurorehabil Neural Repair. 2017 Mar;31(3):280–9. https://doi.org/10.1177/1545968316680483
37.
Bruchez R
,
Jequier Gygax M
,
Roches S
,
Fluss J
,
Jacquier D
,
Ballabeni P
, et al.
Mirror therapy in children with hemiparesis: a randomized observer-blinded trial. Dev Med Child Neurol. 2016 Sep;58(9):970–8. https://doi.org/10.1111/dmcn.13117
38.
Weisstanner C
,
Saxer S
,
Wiest R
,
Kaelin-Lang A
,
Newman CJ
,
Steinlin M
, et al.
The neuronal correlates of mirror illusion in children with spastic hemiparesis: a study with functional magnetic resonance imaging. Swiss Med Wkly. 2017 Feb;147:w14415.
39.
Datta AN
,
Furrer MA
,
Bernhardt I
,
Hüppi PS
,
Borradori-Tolsa C
,
Bucher HU
, et al.; GM Group
. Fidgety movements in infants born very preterm: predictive value for cerebral palsy in a clinical multicentre setting. Dev Med Child Neurol. 2017 Jun;59(6):618–24. https://doi.org/10.1111/dmcn.13386
40.
van Hedel HJ
,
Severini G
,
Scarton A
,
O’Brien A
,
Reed T
,
Gaebler-Spira D
, et al.; ARTIC network
. Advanced Robotic Therapy Integrated Centers (ARTIC): an international collaboration facilitating the application of rehabilitation technologies. J Neuroeng Rehabil. 2018 Apr;15(1):30. https://doi.org/10.1186/s12984-018-0366-y
41.
Meyer-Heim A
,
Ammann-Reiffer C
,
Schmartz A
,
Schäfer J
,
Sennhauser FH
,
Heinen F
, et al.
Improvement of walking abilities after robotic-assisted locomotion training in children with cerebral palsy. Arch Dis Child. 2009 Aug;94(8):615–20. https://doi.org/10.1136/adc.2008.145458
42.
Ammann-Reiffer C
,
Bastiaenen CH
,
Meyer-Heim AD
,
van Hedel HJ
. Effectiveness of robot-assisted gait training in children with cerebral palsy: a bicenter, pragmatic, randomized, cross-over trial (PeLoGAIT). BMC Pediatr. 2017 Mar;17(1):64. https://doi.org/10.1186/s12887-017-0815-y
43.
Rutz E
,
Vavken P
,
Camathias C
,
Haase C
,
Jünemann S
,
Brunner R
. Long-term results and outcome predictors in one-stage hip reconstruction in children with cerebral palsy. J Bone Joint Surg Am. 2015 Mar;97(6):500–6. https://doi.org/10.2106/JBJS.N.00676
44.
Brégou Bourgeois A
,
Mariani B
,
Aminian K
,
Zambelli PY
,
Newman CJ
. Spatio-temporal gait analysis in children with cerebral palsy using, foot-worn inertial sensors. Gait Posture. 2014;39(1):436–42. https://doi.org/10.1016/j.gaitpost.2013.08.029
45.
Bonnefoy-Mazure A
,
De Coulon G
,
Armand S
. Self-perceived gait quality in young adults with cerebral palsy. Dev Med Child Neurol. 2020 Jul;62(7):868–73. https://doi.org/10.1111/dmcn.14504
46.
Brunner AL
,
Rutz E
,
Juenemann S
,
Brunner R
. Continuous vs. blocks of physiotherapy for motor development in children with cerebral palsy and similar syndromes: A prospective randomized study. Dev Neurorehabil. 2014 Dec;17(6):426–32. https://doi.org/10.3109/17518423.2014.923057
47.
Weber, P.
;
Bolli, P.
;
Heimgartner, N.
;
Merlo, P.
;
Zehnder, T.
;
Kätterer, C.
Behavioral and emotional problems in children and adults with cerebral palsy. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society 2016, 20, 270-274.
48.
Araneda R
,
Sizonenko SV
,
Newman CJ
,
Dinomais M
,
Le Gal G
,
Ebner-Karestinos D
, et al.
Protocol of changes induced by early Hand-Arm Bimanual Intensive Therapy Including Lower Extremities (e-HABIT-ILE) in pre-school children with bilateral cerebral palsy: a multisite randomized controlled trial. BMC Neurol. 2020 Jun;20(1):243. https://doi.org/10.1186/s12883-020-01820-2
49.
Aurich-Schuler T
,
Warken B
,
Graser JV
,
Ulrich T
,
Borggraefe I
,
Heinen F
, et al.
Practical recommendations for robot-assisted treadmill therapy (lokomat) in children with cerebral palsy: Indications, goal setting, and clinical implementation within the who-icf framework. Neuropediatrics. 2015 Aug;46(4):248–60. https://doi.org/10.1055/s-0035-1550150
50.
Palisano R
,
Rosenbaum P
,
Walter S
,
Russell D
,
Wood E
,
Galuppi B
. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997 Apr;39(4):214–23. https://doi.org/10.1111/j.1469-8749.1997.tb07414.x
51.
Palisano RJ
,
Rosenbaum P
,
Bartlett D
,
Livingston MH
. Content validity of the expanded and revised gross motor function classification system. Dev Med Child Neurol. 2008 Oct;50(10):744–50. https://doi.org/10.1111/j.1469-8749.2008.03089.x
52.
Eliasson AC
,
Krumlinde-Sundholm L
,
Rösblad B
,
Beckung E
,
Arner M
,
Ohrvall AM
, et al.
The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol. 2006 Jul;48(7):549–54. https://doi.org/10.1017/S0012162206001162
53.
Hidecker MJ
,
Paneth N
,
Rosenbaum PL
,
Kent RD
,
Lillie J
,
Eulenberg JB
, et al.
Developing and validating the Communication Function Classification System for individuals with cerebral palsy. Dev Med Child Neurol. 2011 Aug;53(8):704–10. https://doi.org/10.1111/j.1469-8749.2011.03996.x
54.
Harris PA
,
Taylor R
,
Minor BL
,
Elliott V
,
Fernandez M
,
O’Neal L
, et al.; REDCap Consortium
. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019 Jul;95:103208. https://doi.org/10.1016/j.jbi.2019.103208
55.
Harris PA
,
Taylor R
,
Thielke R
,
Payne J
,
Gonzalez N
,
Conde JG
. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–81. https://doi.org/10.1016/j.jbi.2008.08.010
56.
Morosoli F
,
Hunziker S
,
Zuercher K
,
Tscherter A
,
Grunt S
; Swiss Cerebral Palsy Registry Group
. Prescription practices of medical cannabinoids in children with cerebral palsy - a survey of the swiss cerebral palsy registry. medRxiv 2021, 2021.2011.2018.21266388.
57.
Graf L
. Cerebralparese und Epilepsie bei Kindern – Daten aus dem Schweizer CP-Register aus dem Kinder-Universitätsspital Bern. Master Thesis, University of Bern, 2021.
58.
Jaeger O
. Cerebralparese und Motorik bei Kindern – Daten aus Bern aus dem Schweizer Cerebralparese Register. Master Thesis, University of Bern, 2021.
59.
Raess LF
. Report on the zurich cohort of the swiss cerebral palsy registry and evaluation of predictors of age at diagnosis. Doctoral Thesis, University of Zurich, 2021.
60.
Sellier E
,
Goldsmith S
,
McIntyre S
,
Perra O
,
Rackauskaite G
,
Badawi N
, et al.; Surveillance Of Cerebral Palsy Europe Group And The Australian Cerebral Palsy Register Group
. Cerebral palsy in twins and higher multiple births: a Europe-Australia population-based study. Dev Med Child Neurol. 2021 Jun;63(6):712–20. https://doi.org/10.1111/dmcn.14827
61.
Ashwal S
,
Russman BS
,
Blasco PA
,
Miller G
,
Sandler A
,
Shevell M
, et al.; Quality Standards Subcommittee of the American Academy of Neurology; Practice Committee of the Child Neurology Society
. Practice parameter: diagnostic assessment of the child with cerebral palsy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2004 Mar;62(6):851–63. https://doi.org/10.1212/01.WNL.0000117981.35364.1B
62.
Goldsmith S
,
McIntyre S
,
Smithers-Sheedy H
,
Blair E
,
Cans C
,
Watson L
, et al.; Australian Cerebral Palsy Register Group
. An international survey of cerebral palsy registers and surveillance systems. Dev Med Child Neurol. 2016 Feb;58 Suppl 2:11–7. https://doi.org/10.1111/dmcn.12999
63.
Surveillance of Cerebral Palsy in Europe (SCPE)
. Prevalence and characteristics of children with cerebral palsy in Europe. Dev Med Child Neurol. 2002 Sep;44(9):633–40.
64.
Andersen, G.L.
;
Irgens, L.M.
;
Haagaas, I.
;
Skranes, J.S.
;
Meberg, A.E.
;
Vik, T.
Cerebral palsy in norway: Prevalence, subtypes and severity. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society 2008, 12, 4-13.
65.
Oskoui M
,
Coutinho F
,
Dykeman J
,
Jetté N
,
Pringsheim T
. An update on the prevalence of cerebral palsy: a systematic review and meta-analysis. Dev Med Child Neurol. 2013 Jun;55(6):509–19. https://doi.org/10.1111/dmcn.12080
66.
Robb JE
,
Hägglund G
. Hip surveillance and management of the displaced hip in cerebral palsy. J Child Orthop. 2013 Nov;7(5):407–13. https://doi.org/10.1007/s11832-013-0515-6


