Organ donation after circulatory death as compared with organ donation after brain death in Switzerland – an observational study
DOI: https://doi.org/10.4414/SMW.2022.w30132
Andreas
Elmera, Mara-Lisa
Rohrera, Christian
Bendenab, Nathalie
Krügela, Franziska
Beyelera, Franz F
Immera
aSwisstransplant, Bern, Switzerland
bUniversity of Zurich Faculty of Medicine, Zurich, Switzerland
*Contributed equally
Summary
AIMS OF THE STUDY: Organ donation after circulatory death (DCD) was reintroduced in Switzerland in 2011 and accounts for a third of deceased organ donors today. Controversy persists if DCD transplants are of similar quality to transplants following donation after brain death (DBD), mainly due to warm ischaemia time DCD organs are exposed to. We compared DCD with DBD in Switzerland.
METHODS: Data on deceased adults who were referred to and approved for organ donation from 1 September 2011 to 31 December 2019 were retrospectively analysed (217 DCD, 840 DBD donors). We compared DCD and DBD donor/organ characteristics, transplant rates of lungs, liver, kidneys, and pancreas, and early liver and kidney graft function in the recipient. The effect of DCD/DBD on transplant rates (organ transplanted or not) and 72-hour recipient graft function (moderate/good vs delayed graft function / organ loss) was analysed using multivariable logistic regression. Among utilised DCD donors, we analysed the effect of functional warm ischaemia time (FWIT) and donor age on 72-hour post-transplant liver and kidney graft function, also using multivariable logistic regression.
RESULTS: DCD donors were more often male (64.5% vs 56.8% p = 0.039), presented with heart disease (36.4% vs 25.5%, p <0.001), were resuscitated before hospital admission (41.9% vs 30.7%, p = 0.006), and died from anoxia (41.9% vs 23.9%). Kidney function before transplantation was comparable, lung, liver and pancreas function were poorer in DCD than DBD. Eighty-one and 91% of approved DCD and DBD donors were utilised (p <0.001). Median FWIT in DCD was 29 minutes (interquartile range 25–35). DCD transplant rates ranged from 4% (pancreas) to 73% (left kidney) and were all lower compared with DBD. Seventy-two-hour liver graft function was comparable between DCD and DBD (94.2% vs 96.6% moderate/good, p = 0.199). DCD kidney transplants showed increased risk of delayed graft function or early organ loss (odds ratios 8.32 and 5.05; 95% confidence intervals CI 5.28–13.28 and 3.22–7.95; both p <0.001, for left and right kidney transplants, respectively). No negative effect of prolonged FWIT or higher donor age was detected.
CONCLUSION: Despite less favourable donor/organ characteristics compared with donation after brain death, donation after circulatory death donors are increasingly referred and today provide an important source for scarce transplants in Switzerland. We identified a higher risk for delayed graft function or early organ loss for DCD kidney transplants, but not for DCD liver transplants. When carefully selected and allowed for other risk factors in organ allocation, prolonged functional warm ischaemia time or higher age in donation after circulatory death does not seem to be associated with impaired graft function early after transplantation.
Introduction
Organ transplantation is the treatment of choice for well-selected patients with end-stage organ failure. For some patients it is the only life-saving option, others gain improved quality of life after transplantation [1–7]. Many transplant recipients resume professional and social activities which they were unable to pursue prior to transplantation [2, 8–10].
There are two organ donation pathways from deceased persons, depending on how death occurs before the procurement of organs. First, donation after brain death (DBD), when death occurs after primary brain damage or disease, leading to irreversible loss of the functions of the brain, including the brainstem. Second, donation after circulatory death (DCD), when death occurs due to a permanent cardiac arrest after a defined stand-off period, depending on national regulation [11]. Today, the majority of transplants worldwide use organs from DBD donors. The shortage of donor organs for transplantation, however, along with technical developments leading to improved post-transplant outcomes, has resulted in an increasing number of countries promoting DCD as a supplement to DBD [12, 13].
In Switzerland, the first transplantation from a DCD donor was carried out in 1985, which makes Switzerland the second European country to introduce DCD. Until the national legislation on organ donation and transplantation [14, 15] came into force in 2007, DCD donor organs were routinely procured and transplanted at the University Hospitals of Zurich and Geneva [16]. Almost 20 years ago, Weber and colleagues from the University Hospital Zurich were the first to publish long-term outcomes of 122 DCD kidney transplants, showing no difference compared with DBD transplants [17].
Due to certain ambiguities in the wording of some articles of the law [14] and discrepancy between the law and the referred guidelines of the Swiss Academy of Medical Science (SAMS) [18], DCD programmes were discontinued in the same year the national legislation came into force, in 2007. It took almost 4 years to clarify the legal situation and until DCD programmes were re-established in Switzerland. After the law and the SAMS guidelines had been revised, the two University Hospitals of Zurich and Geneva restarted their DCD programmes on 1 September 2011 [19]. Since then, DCD was successfully introduced at many more Swiss hospitals. At the end of 2020, DCD programmes were operational at the six transplant centres (university hospitals of Basel, Bern, Geneva, Lausanne, and Zurich, and the Cantonal Hospital St Gallen), and at the cantonal hospitals of Fribourg, Sion, Chur, and Lucerne [20]. More DCD programmes at additional hospitals are currently being evaluated.
In 2020, 30% of deceased organ donors in Switzerland were DCD donors, ranking Switzerland fifth out of thirteen European countries practising DCD, after the Netherlands (60%), Belgium (43%), Spain (35%), and the United Kingdom (34%) [21]. In 2020, a total of 105 organs from DCD donors were transplanted in Switzerland, which is more than every fifth transplant [20]. Besides many other efforts, it is largely due to the reintroduction of DCD that organ donation rates in Switzerland have increased in the last decade [22].
A key issue is whether DCD transplants are of equivalent quality to DBD transplants, due to the combination of duration of warm and cold ischaemia the organs from DCD donors are exposed to. Indeed, various studies, including systematic reviews and meta-analyses, show impaired short-term graft function and patient survival for transplanted kidneys, livers, lungs, and pancreas from DCD donors when compared with DBD. Most of these studies, however, report a long-term DCD graft and patient survival similar to DBD [23–37]. Donor characteristics, utilisation and transplant rates, and early organ function in recipients of DCD, have not been investigated systematically on a national level in Switzerland to our knowledge. Thus, this study compares all Swiss DCD donors since the reintroduction of DCD with respective DBD donors (2011–2019). Additionally, we investigated whether prolonged warm ischaemia and higher donor age in DCD is associated with inferior early recipient organ function.
Materials and methods
Study population
Hospital patient data of potential solid organ donors from the national database “Swiss Organ Allocation System” (SOAS) were retrospectively analysed. SOAS data are mandatorily captured by trained hospital professionals for the purpose of legal organ allocation, using standardised online forms and under supervision of Swisstransplant, the National Foundation for Organ Donation and Transplantation. We considered all referred deceased organ donors in Switzerland since the reintroduction of DCD on 1 September 2011 until 31 December 2019, if they were approved for organ donation by Swisstransplant, with a minimum of one organ offered for transplantation (n = 1’215).
From this population we excluded 49 paediatric donors (<18 years), as indicators of organ quality differ from adult donors. We further excluded two mistaken database entries, one uncontrolled DCD donor (Maastricht Category II), and 17 donors for whom the next of kin had withdrawn consent to donation. Another 89 donors were excluded as there were no data available for body mass index (BMI) (8), and/or cause of death (16), and/or the comorbidities diabetes (32), hypertension (45), and heart disease (48). Potential DCD donors who did not die in the legal timeframe of 120 minutes were included. This led to a total of 1057 analysed patients, of which were 840 DBD donors and 217 were DCD donors. Any other missing data are indicated in table 1.
The study was approved by the Cantonal Ethics Committee Bern (Project-ID 2021-01528; categorised as further use of health-related personal data for research in absence of informed consent). Study data that underlie the results reported in this article, after de-identification, can be made available upon request. Proposals should be directed to the corresponding author.
Definitions
Deceased donation pathways
There are two pathways of deceased organ donation, depending on how death occurs. First, donation after brain death (DBD), when death occurs after primary brain damage or disease, leading to irreversible loss of the functions of the brain, including the brainstem. Death is then determined directly on the basis of neurological criteria. Second, donation after circulatory death (DCD), when death is due to permanent cardiac arrest (absence of cardiac activity), leading to cessation of the cerebral circulation [11]. In Switzerland, death is also in the case of permanent cardiac arrest, defined as irreversible loss of the functions of the brain, including the brainstem. Therefore, death must be declared also in DCD on the basis of neurological criteria. According to legally binding guidelines of the SAMS, cardiac arrest in DCD must be diagnosed by means of transthoracic echocardiography in the subxiphoid four-chamber view, or by transoesophageal echocardiography. After a defined stand-off period of at least 5 minutes without resuscitation measures, brain death must be determined by six neurological criteria, similar to DBD [18]. In 2017, the legally binding stand-off period in Switzerland was changed from 10 to 5 minutes.
Within DCD, four different categories are distinguished, called the Maastricht Categories I to IV [38]. In general, controlled DCD (cDCD) is practised in Switzerland (Maastricht Category III). cDCD usually refers to patients with a devastating brain injury in whom further treatment has been deemed futile and for whom a decision has been made in favour of withdrawal of life-sustaining therapy (WLST) [38]. When in such patients brain death is not likely to occur within a short period of time, death occurs following a planned, expected cardiac arrest after WLST, and brain death is determined after the 5-minute stand-off period. Potential cDCD donors also include patients with end-stage neurodegenerative or cardiac/respiratory diseases for whom a decision of WLST has been made, because sustaining life is no longer in the best clinical interests of the patient. We estimate that 18% of deceased ICU patients in Switzerland are potential cDCD donors, and that approximately half of these patients exhibit a severe brain damage. The so-called uncontrolled DCD (uDCD), from persons whose death has occurred following a sudden, unexpected irreversible cardiac arrest with unsuccessful attempt at resuscitation by a medical team [11], is currently not practised in Switzerland. The current law [14, 15], however, allows all types of DCD, and appropriate procedures exist in Geneva but are not applied because of limited resources.
Organ procurement techniques in DCD
Rapid cold preservation (i.e., rapid procurement) represents the traditional organ procurement technique in DCD, in which donor organs are not perfused in situ prior to the operative incision. In normothermic regional perfusion (NRP), abdominal donor organs are perfused in situ with oxygenated blood after determination of death and before the incision is made, using a device applied at normothermic temperatures. NRP can reduce the warm ischaemic damage to vulnerable organs in DCD. Recirculation is prevented by the use of vessel clamps or intravascular balloons placed at the thoraco-abdominal level of the descending aorta. If the lungs are to be retrieved, the trachea needs to be re-intubated and the lungs re-inflated after death [11, 39]. In Switzerland, NRP is currently practised only at the University Hospital of Geneva, since 2017.
Warm ischaemia times in DCD
The period during which an organ is deprived of its blood supply is called the ischaemia time. Donor warm ischaemia time (DWIT) is the time an organ remains in the donor at body temperature after its blood supply has been reduced or cut off, but before it is cooled or reconnected to a blood supply. In cDCD, the DWIT is the period from the moment of WLST (including ventilatory support) until the start of cold perfusion or, in NRP, until the start of in situ organ preservation (T0–T5 in figs 1 and 2). In the early phase after WLST, progression of circulation and oxygenation is very variable. We therefore analysed the functional warm ischaemia time (FWIT; T1–T5 in figs 1 and 2), which represents the period between the first episode of significant hypoperfusion (mean arterial pressure <50 mm Hg for >2 minutes) and the start of cold perfusion or, in NRP, until the start of in situ organ perfusion. The acceptable FWIT varies for different organs and ranges from 30 minutes (pancreas) to 120 minutes (lung and kidneys) (figures 1 and 2) [39].

Figure 1 Organ procurement steps and ischaemia times in cDCD when organs are retrieved by the rapid cold preservation technique (in short, rapid procurement). Adapted DCD Scheme of the Steering Committee of the National Committee for Organ Donation 2019.
a Heparin administration at t0, 300 IU/kg iv. If the likelihood of mortality is low and/or there is an increased risk of bleeding, the attending ICU physician may decide to administer the heparin later but no later than at t1.
b The following mean arterial pressure (MAP) values are to be used, depending on age category: 0 to 1 year: <35 mm Hg; 1 to 3 years: <40 mm Hg; 3 to 5 years: <45 mm Hg; 5 years and above: <50 mm Hg (for a minimum of 2 minutes).
c According to the Swiss Academy of Medical Sciences [18].
d Process may be stopped earlier if after 60 minutes from t0 none of the three criteria are met: pH <7.2; systolic blood pressure <70 mm Hg; SpO2 <70%.

Figure 2 Organ procurement steps and ischaemia times in cDCD when organs are retrieved by the normothermic regional perfusion technique (NRP). Adapted DCD Scheme of the Steering Committee of the National Committee for Organ Donation 2019.
a Heparin administration at t0, 300 IU/kg iv. If the likelihood of mortality is low and/or there is an increased risk of bleeding, the attending ICU physician may decide to administer the heparin later but no later than at t1.
b The following mean arterial pressure (MAP) values are to be used, depending on age category: 0 to 1 year: <35 mm Hg; 1 to 3 years: <40 mm Hg; 3 to 5 years: <45 mm Hg; 5 years and above: <50 mm Hg (for a minimum of 2 minutes).
c According to the Swiss Academy of Medical Sciences [18].
d Process may be stopped earlier if after 60 minutes from t0 none of the three criteria are met: pH <7.2; systolic blood pressure <70 mm Hg; SpO2 <70 %.
Organ characteristics
Last measured values before explantation of organ-specific clinical and laboratory indicators commonly used to assess organ function in the donor were available; for the liver, alanine transaminase (ALAT), aspartate transaminase (ASAT), gamma-glutamyltransferase (γGT), and steatosis (extent of fatty degeneration assessed by local radiologist [40]); for the lung, arterial blood oxygen partial pressure (PaO2); for the kidneys, serum creatinine level and estimated glomerular filtration rate (eGFR) [41]; for the pancreas, amylase and lipase; and lactate dehydrogenase (LDH) as multi-organ indicator. After preliminary analysis, PaO2 was excluded from further analyses as in our dataset information on the applied fraction of oxygen in the inhaled air (FiO2) was often missing.
Outcomes
The primary objective was to assess in the population of utilised donors (n = 939) the effect of the donation pathway (cDCD/DBD) on transplant rates of lungs, liver, kidneys, and pancreas (organ transplanted or not), and on early (72-hour) graft function in liver and kidney transplant recipients. Seventy-two-hour recipient graft function was analysed only for the liver and the kidneys, as for the other organs transplant numbers were considered too small for a valid analysis. Due to low case numbers in some factor levels (e.g., organ loss), the ordinal variables “72-hour graft function in recipients of liver and kidney transplants” were transformed to binary variables as follows: “good” and “moderate” organ function became “good/moderate”, and “delayed graft function” and “organ loss” became “delayed graft function / organ loss”. As a secondary objective we assessed in the subgroup of utilised cDCD donors (n = 175) the effect of FWIT and donor age on the 72-hour graft function in liver and kidney transplant recipients.
Statistical analysis
As presented in table 1, referred and approved donors (n = 1057) were divided into two groups, cDCD donors (n = 217) vs DBD donors (n = 840). Among these two groups, donor/organ characteristics and donor utilisation were compared for quantitative variables by using the t-test, or if the assumption of normality was not met, by the non-parametric Wilcoxon rank sum test. For qualitative variables, Pearson’s chi-square test was used, or Fisher’s exact test in the case of a small sample size.
As presented in table 2, utilised donors (≥1 organ transplanted, n = 939) were again grouped by cDCD (n = 175) and DBD (n = 764). Allocation outcomes for all organs (i.e., lungs, liver, kidneys, pancreas transplanted or not; no cDCD heart programme during study period) and early (72-hour) graft function in recipients of liver and kidney transplants were compared using Pearson’s chi-square test, or Fisher’s exact test in the case of a small sample size. Cold ischaemia time (CIT) of liver and kidney transplants, and organ yield (number of transplants per donor), were compared by using the t-test, or if the assumption of normality was not met, by the non-parametric Wilcoxon rank sum test. Depending on organ quality and donor-recipient size match in liver transplantation, the liver may be split into two grafts for two recipients. With this split liver procedure, mostly children receive the left lateral graft, and adults receive the right extended graft. Split livers were retrieved from 20 DBD donors in our analysed population and we counted them as one liver transplant but excluded them from recipient graft function analyses.
Among utilised donors, the possible effect of the donation pathway (DBD/cDCD) on transplant rates of lungs, liver, kidneys, and pancreas (organ transplanted or not), and on the 72-hour liver and kidney recipient graft function (moderate/good vs delayed graft function / organ loss) was analysed using multivariable logistic regression, including as regressors all donor and organ characteristics (the latter specifically by organ). In the regression models of the 72-hour liver and kidney graft function we additionally included CIT, recipient’s age and gender, and waitlist urgency status at time of transplant as a qualitative proxy for the recipient’s medical condition. In the regression analysis of 72-hour recipient liver and kidney graft function we first analysed a comprehensive model from which we manually excluded any regressor with p >0.20 (backward selection), or for which we observed multicollinearity (i.e., eGFR). We always kept donation pathway (cDCD/DBD) as our main variable of interest, donor age/gender, CIT, and recipient age/gender, independently from their significance in the comprehensive model. Results from the multivariable logistic regression of 72-hour recipient liver and kidney graft function are depicted in figure 4 (minimal models). Among utilised cDCD donors the possible effects of FWIT and donor age on the 72-hour liver and kidney graft function were analysed using the same multivariable logistic regression approach (results not depicted). All models were assessed for multicollinearity by computing variance inflation factors. We looked at potential effect modifiers by introducing interaction terms in our models. None of the assessed interaction terms were significant.
We would like to emphasise that the applied regression models were not built for the purpose of predicting the outcome, but to analyse the effect of the donation pathway (cDCD/DBD) or FWIT / donor age, while controlling the influence of as many as possible potential confounders. For all statistical analyses, the freely available software R (version 4.0.2) was used [42].
Results
Analysis of referred donors
Since the reintroduction of DCD in Switzerland in 2011, in our study population 217 cDCD donors were referred to and approved by Swisstransplant, and these numbers constantly increased over the more than 8-year study period (p <0.001). At the end of the study period, 2019, cDCD donors constituted 39.9% of the referred and approved deceased organ donors in Switzerland (table 1, fig. 3).
Table 1Donor and organ characteristics, and donor utilisation of referred and approved DBD vs cDCD donors in Switzerland from 2011–2019.
|
Study population
|
DBD
|
cDCD
|
p-value
|
| TOTAL, n (%) |
1057 (100) |
840 (79.5) |
217 (20.5) |
|
| – 2019, n (%) |
163 (100) |
98 (60.1) |
65 (39.9) |
<0.001 |
| – 2018, n (%) |
162 (100) |
122 (75.3) |
40 (24.7) |
| – 2017, n (%) |
141 (100) |
103 (73.0) |
38 (27.0) |
| – 2016, n (%) |
109 (100) |
90 (82.6) |
19 (17.4) |
| – 2015, n (%) |
139 (100) |
122 (87.8) |
17 (12.2) |
| – 2014, n (%) |
112 (100) |
94 (83.9) |
18 (16.1) |
| – 2013, n (%) |
104 (100) |
94 (90.4) |
10 (9.6) |
| – 2012, n (%) |
92 (100) |
85 (92.4) |
7 (7.6) |
| – 2011 (1 September to 31 December), n (%) |
35 (100) |
32 (91.4) |
3 (8.6) |
| Donor characteristics |
| Age, median (IQR) |
59 (47–69) |
58 (46–70) |
61 (51–68) |
0.115 |
| Gender (male), n (%) |
617 (58.4) |
477 (56.8) |
140 (64.5) |
0.039 |
| Cause of death |
| – Cerebral haemorrhage, n (%) |
474 (44.8) |
413 (49.2) |
61 (28.1) |
<0.001 |
| – Anoxia, n (%) |
292 (27.6) |
201 (23.9) |
91 (41.9) |
| – Cerebral trauma, n (%) |
200 (18.9) |
170 (20.2) |
30 (13.8) |
| – Cerebral disease, n (%) |
67 (6.3) |
46 (5.5) |
21 (9.7) |
| – Other, n (%) |
24 (2.3) |
10 (1.2) |
14 (6.5) |
| Body mass index, median (IQR) |
24.9 (22.9–27.8) |
24.8 (22.9–27.7) |
25.4 (23.1–28.3) |
0.274 |
| Comorbidities |
| – Hypertension, n (%) |
409 (38.7) |
317 (37.7) |
92 (42.4) |
0.209 |
| – Heart disease, n (%) |
293 (27.7) |
214 (25.5) |
79 (36.4) |
0.001 |
| – Diabetes, n (%) |
115 (10.9) |
90 (10.7) |
25 (11.5) |
0.734 |
| CPR before hospital admission, n (%) |
349 (33.0) |
258 (30.7) |
91 (41.9) |
0.006 |
| Organ characteristics |
| Liver-related indicators |
| – ALAT in U/l, median (IQR) |
34 (19–78) |
31 (18–77) |
50 (25–82) |
<0.001 |
| – No ALAT value available, n (%) |
1 (0.1) |
0 |
1 (0.5) |
|
| – ASAT in U/l, median (IQR) |
49 (29–97) |
47 (27–97) |
62 (36–97) |
0.005 |
| – No ASAT value available, n (%) |
1 (0.1) |
0 |
1 (0.5) |
|
| – γGT in U/l, median (IQR) |
37 (18–89) |
33 (17–75) |
67 (29–160) |
<0.001 |
| – No γGT value available, n (%) |
18 (1.7) |
12 (1.4) |
6 (2.8) |
|
| Steatosis |
|
|
|
0.348 |
| – None |
722 (68.3) |
570 (70.5) |
152 (74.9) |
|
| – Mild (<1/3 of hepatocytes) |
168 (15.9) |
134 (16.6) |
34 (16.7) |
|
| – Moderate (<2/3 of hepatocytes) |
95 (9.0) |
81 (10.0) |
14 (6.9) |
|
| – Severe (>2/3 of hepatocytes) |
26 (2.5) |
23 (2.8) |
3 (1.5) |
|
| – No data on steatosis available |
46 (4.4) |
32 (3.8) |
14 (6.5) |
|
| Kidney-related indicators |
| – Creatinine in μmol/l, median (IQR) |
75 (57–104) |
75 (57–104) |
74 (56–95) |
0.141 |
| – No creatinine value available, n (%) |
2 (0.2) |
1 (0.1) |
1 (0.5) |
|
| – eGFR in ml min-1 1.73m-2
|
88 (62 ̶ 105) |
88 (61 ̶ 104) |
90 (64 ̶ 106) |
0.150 |
| Pancreas-related indicators |
| – Pancreatic amylase in U/l, median (IQR) |
32 (17–66) |
31 (17–64) |
33 (19–70) |
0.404 |
| – No amylase value available, n (%) |
43 (4.1) |
32 (3.8) |
11 (5.1) |
|
| – Lipase in U/l, median (IQR) |
22 (14–42) |
22 (14–40) |
27 (16–51) |
0.008 |
| – No lipase value available, n (%) |
177 (16.7) |
134 (16.0) |
43 (19.8) |
|
| Multiorgan-related indicators |
| – LDH in U/l, median (IQR) |
419 (286–634) |
411 (285–620) |
462 (305–689) |
0.062 |
| – No LDH value available, n (%) |
59 (5.6) |
39 (4.7) |
20 (9.2) |
|
| Donor utilisation |
| – Utilised donors, n (%) |
939 (88.8) |
764 (91.0) |
175 (80.6) |
<0.001 |
| – Non-utilised donors, n (%) |
83 (7.9) |
48 (5.7) |
35 (16.1) |
| – Effective donors, n (%) |
35 (3.3) |
28 (3.3) |
7 (3.2) |

Figure 3 Number of referred cDCD (red dotted line) and referred DBD (blue line) donors from Switzerland from whom at least one organ was offered for transplantation by Swisstransplant from 2012–2019. cDCD: controlled donation after circulatory death; DBD: donation after brain death.
The average cDCD donor was 61 years old, hence, three years older than the average DBD donor, although this age difference was not statistically significant (p = 0.115). Of cDCD donors, 64.5% were male, which is significantly more than for DBD donors (56.8%, p = 0.039). Like DBD donors, most cDCD donors had died from a severe acute brain injury; however, the presence of the various brain injuries differed between cDCD and DBD donors (p <0.001). Among cDCD donors anoxia was the most frequent cause of death (41.9%), whereas for DBD donors it was cerebral haemorrhage (49.2%). Over the last 4 years of the study period, anoxia incidence increased, particularly among cDCD donors (from 31.6% in 2016 to 55.4% in 2019). Additionally, cDCD donors suffered more often from the comorbidities hypertension, heart disease and diabetes, when compared with DBD donors. Only for heart disease, however, was this difference statistically significant (p <0.001). Also, more cDCD donors (41.9%) than DBD donors (30.7%) had been resuscitated before they were admitted to the hospital (p = 0.006) (table 1).
Except for kidney-related indicators (creatinine, eGFR), for all other organs at least some quality indicators were significantly elevated in cDCD donors as compared with DBD donors (in a preliminary analysis paO2 as lung-related indicator was lower in cDCD donors but was not analysed further because of missing data on the applied fraction of oxygen in the inhaled air, FiO2). According to levels of ALAT, ASAT, and γGT, average liver function was poorer in cDCD than in DBD; however, steatosis was not more extensive in cDCD livers. According to levels of lipase, but not amylase, pancreas function was poorer in cDCD compared with DBD. Also LDH was, on average, more elevated in cDCD (median LDH level 462 U/l) than in DBD (median LDH level 411 U/l), although only weakly significant (p = 0.062) (table 1).
Of the 217 referred and approved cDCD donors, 175 (80.6%) were utilised, whereas of the 840 referred and approved DBD donors 764 (91.0%) were utilised. Hence, utilisation from cDCD over the study period was significantly lower than utilisation from DBD (p <0.001). In particular, cDCD resulted in three times more “non-utilised” donors (16.1% vs 5.7%), who are donors in whom no operative incision was made with the intent to remove organ(s)for transplantation (table 1). As reasons for non-utilisation we found that in 23 (66%) of the 35 non-utilised cDCD donors the cardiac arrest did not occur within the legally binding time limit after WLST, and that consequently, organs from these donors were not procured.
Analysis of utilised donors
Table 2 shows outcomes of all 764 utilised DBD donors and all 175 utilised cDCD donors. The median FWIT of cDCD donors was 29 minutes (IQR 25–35 min). Over 90% of cDCD organs were retrieved using rapid procurement. Median FWIT with NRP was similar as with rapid procurement (29 minutes for both groups, data not shown). The maximum possible number or organs transplanted per DBD donor is seven (heart, double lung, liver, two kidneys, pancreas and small bowel). The maximum possible number or organs transplanted per cDCD donor is six (double lung, liver, two kidneys, pancreas and small bowel; no heart procured during study period). Please note that organ loss as included in the early recipient graft function category “delayed graft function or organ loss” occurred very rarely (19 lost livers, 20 lost kidneys).
Table 2Circumstances of organ procurement of utilised cDCD, allocation outcome and early (72-hour) recipient graft function (liver and kidneys) of utilised DBD vs cDCD donors in Switzerland from 2011 ̶ 2019.
|
Study population
|
DBD
|
cDCD
|
p-value
|
| Total, n (%) |
939 (100) |
764 (81.4) |
175 (18.6) |
|
| Organ procurement |
| FWIT in minutes, median (IQR) |
DBD donor organs are not exposed to a warm ischaemia time. Organ recovery type applies only to cDCD. |
29 (25–35) |
NA |
| No FWIT available, n (%) |
20 (11.4) |
NA |
| Organ recovery type |
|
|
| – Rapid cold preservation, n (%) |
156 (90.2) |
NA |
| – Normothermic regional perfusion, n (%) |
17 (9.8) |
NA |
| – Unknown, n (%) |
2 (0.1) |
NA |
| Allocation outcomes |
| Heart transplanted, n (%) |
270 (28.8) |
270 (35.3) |
NA |
NA |
| Lung transplanted, n (%) |
314 (33.4) |
286 (37.4) |
28 (16.0) |
<0.001 |
| Liver transplanted, n (%) |
788 (83.9) |
667 (87.3) |
121 (69.1) |
<0.001 |
| Liver CIT in minutes, median (IQR) |
407 (341–484) |
406 (335–484) |
414 (370–485) |
0.125 |
| Kidney left transplanted, n (%) |
759 (80.8) |
631 (82.6) |
128 (73.1) |
0.004 |
| Kidney left CIT in minutes, median (IQR) |
562 (443–698) |
568 (448–716) |
544 (428 ̶ 638) |
0.017 |
| Kidney right transplanted, n (%) |
747 (79.6) |
628 (82.2) |
119 (68.0) |
<0.001 |
| Kidney right CIT in minutes, median (IQR) |
555 (437–734) |
565 (442–745) |
521 (409–648) |
0.006 |
| Pancreas transplanted, n (%) |
182 (19.4) |
175 (22.9) |
7 (4.0) |
<0.001 |
| Number of transplants per donor, mean ± SD |
3.3 ± 1.5 |
3.5 ± 1.5 |
2.3 ± 0.9 |
<0.001 |
| 72-hour recipient graft function |
| Liver transplanted, n |
768 |
647 |
121 |
|
| – Moderate or good graft function, n (%) |
738 (96.2) |
624 (96.6) |
114 (94.2) |
0.199 |
| – Delayed graft function or organ loss, n (%) |
29 (3.8) |
22 (3.4) |
7 (5.8) |
| Kidney left transplanted, n |
759 |
631 |
128 |
|
| – Moderate or good graft function, n (%) |
585 (76.3) |
525 (83.2) |
60 (46.9) |
<0.001 |
| – Delayed graft function or organ loss, n (%) |
174 (22.7) |
106 (16.8) |
68 (53.1) |
| Kidney right transplanted, n |
747 |
628 |
119 |
|
| – Moderate or good graft function, n (%) |
562 (73.3) |
502 (79.9) |
60 (50.4) |
<0.001 |
| – Delayed graft function or organ loss, n (%) |
185 (24.1) |
126 (20.1) |
59 (49.6) |
During the study period, 128 left kidneys, 119 right kidneys, 121 livers, 28 lungs, and 7 pancreases from cDCD donors were transplanted. This corresponds to cDCD transplant rates which range from 4% (pancreas) to 73% (left kidney). Transplant rates in cDCD were significantly lower when compared with DBD, also in the multivariable analysis. Consequently, organ yield in cDCD was significantly lower when compared with DBD (2.3 vs 3.5 transplants per donor, p <0.001). For liver and kidney transplants, we additionally present cold ischaemia time (CIT), which is the time an organ is cooled in the donor body after surgical incision with cold perfusion solution and sterile ice until reperfusion of the organ in the circulatory system of the recipient. The median CIT of transplanted livers was similar in cDCD and in DBD (414 vs 406 minutes, p = 0.125), the median CIT of transplanted kidneys was significantly shorter in cDCD than in DBD (544 vs 568 minutes, p = 0.017, and 521 vs 565 minutes, p = 0.006, for the left and right kidneys, respectively) (table 2).
Recipient graft function up to 72 hours after transplantation was not significantly different for cDCD livers and DBD livers (p = 0.199). Of cDCD liver transplants, 94.2% had moderate or good organ function (96.6% of DBD), and only seven (5.8 %) cDCD livers were reported with delayed graft function or loss (3.4 % of DBD). Delayed graft function or organ loss, however, occuredsignificantly more often with cDCD kidneys than with DBD kidneys (53.1% vs 16.8% for left kidneys, 49.6% vs 20.1% for right kidneys, p <0.001). Early organ loss after kidney transplantation, however, occurred very rarely, from both cDCD and DBD (i.e., 20 grafts were lost in a total of 1506 kidney transplants, which is 1.3% of grafts).
Multivariable analyses of early recipient graft function
Utilised cDCD and DBD donors
Whether the liver was retrieved through cDCD or DBD was not significantly associated with delayed graft function or early organ loss in the recipient (cDCD odds ratio 1.75, 95% CI 0.66–4.16; p = 0.230) (fig. 4A). cDCD kidneys, however, showed an increased risk of delayed graft function or early organ loss when compared with DBD kidneys (cDCD odds ratios 8.32 and 5.05; 95 % confidence intervals 5.28–13.28 and 3.22–7.95; both p <0.001, for left and right kidney transplants, respectively; figures 4B and 4C). Thus, the univariable results for 72-hour graft function in recipients of liver and kidney transplants as presented above and in table 2 were confirmed by the multivariable analysis.
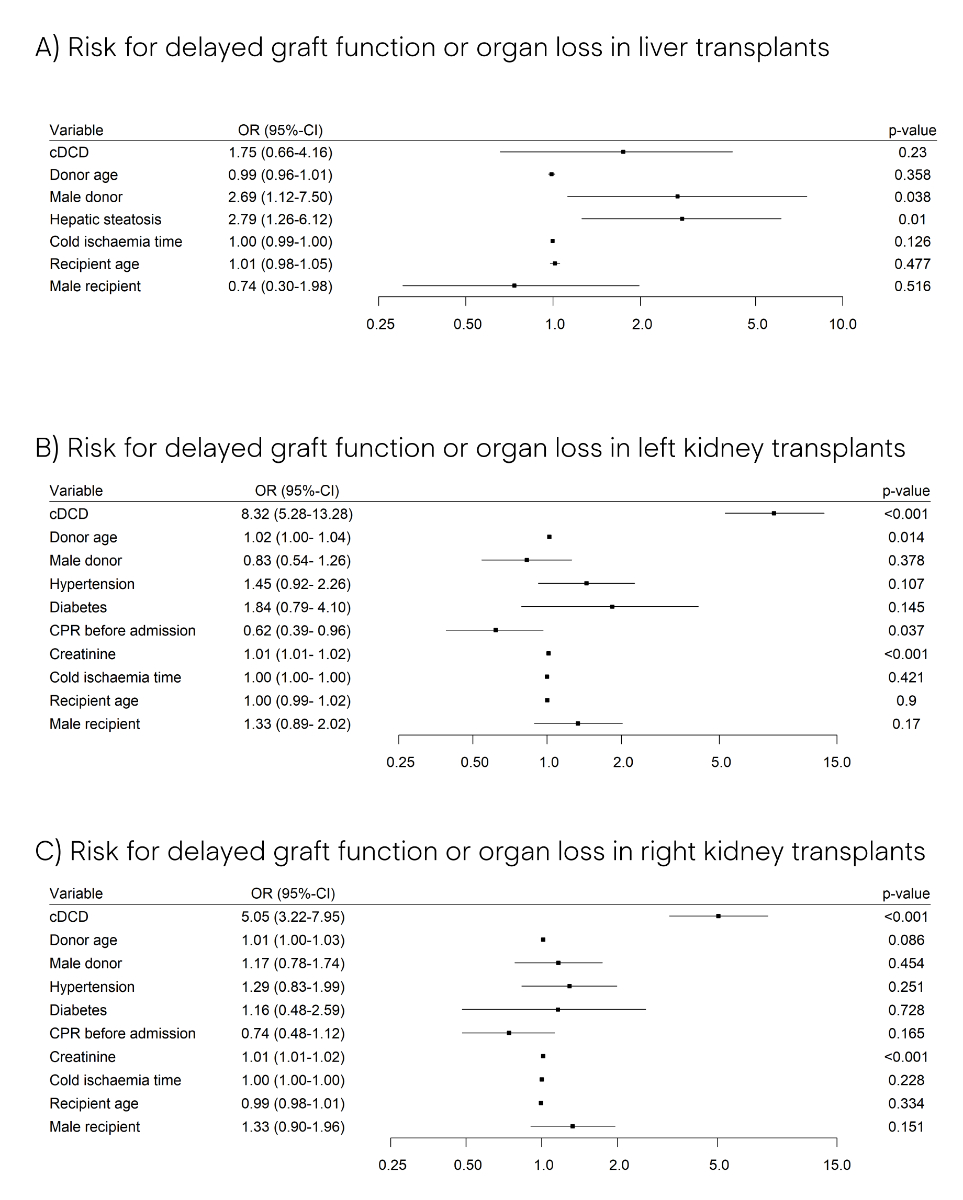
Figure 4 Risk for delayed graft function or organ loss after liver (A), left kidney (B), and right kidney (C) transplantation from 2011 to 2019. Presented are odds ratios (ORs) with 95% confidence intervals (95% CI) of multivariable logistic regression of the 72-hour post-transplant recipient graft function. In the case of the continuous variables donor age, creatinine and cold ischaemia time, ORs correspond to an increase of 1 year, 1 μmol/l, and 1 minute respectively. CPR: cardiopulmonary resuscitation; cDCD: controlled donation after circulatory death.
Utilised cDCD donors
In cDCD we were interested in the effect of FWIT and donor age on the 72-hour post-transplant liver and kidney function in recipients (table 3). Only seven cDCD liver transplants were reported to have delayed graft function or early graft loss, five in the older age group (7.8 %) and two in the younger age group (3.5 %), according to the median age of utilised cDCD donors (60 years). Four cDCD liver transplants were reported to have delayed graft function or early graft loss in the short FWIT group (7.1 %), and three cDCD livers in the long FWIT group (5.5 %), according to the median FWIT of utilised cDCD donors (29 minutes). None of these differences in liver graft function between short/long FWIT and young/old cDCD donors was statistically significant. Also using a multivariable approach, including recipient age and gender, we found no significant negative effect of FWIT or donor age on the 72-hour liver graft function. A substantial number of cDCD kidney transplants was reported to have delayed graft function or early graft loss (68 left kidneys, 59 right kidneys). We did not detect a significant negative effect of FWIT or donor age in either the univariable or multivariable analysis.
Table 3Functional warm ischaemia time (FWIT) and age distribution of transplants from cDCD donors.
|
72-hour recipient graft function
|
cDCD
|
Short FWIT
|
Long FWIT
|
p-value
|
cDCD
|
Young
|
Old
|
p-value
|
| Liver transplanted, n |
111 |
56 |
55 |
|
121 |
58 |
63 |
|
| – Moderate or good graft function, n (%) |
104 (93.7) |
52 (92.9) |
52 (94.5) |
0.752 |
114 (94.2) |
56 (96.6) |
58 (92.1) |
0.505 |
| – Delayed graft function or organ loss, n (%) |
7 (6.3) |
4 (7.1) |
3 (5.5) |
7 (5.8) |
2 (3.4) |
5 (7.9) |
| Kidney left transplanted, n |
111 |
56 |
55 |
|
128 |
77 |
51 |
|
| – Moderate or good graft function, n (%) |
49 (44.1) |
23 (41.1) |
26 (47.3) |
0.641 |
60 (46.9) |
39 (50.6) |
21 (41.2) |
0.384 |
| – Delayed graft function or organ loss, n (%) |
62 (55.9) |
33 (58.9) |
29 (52.7) |
68 (53.1) |
38 (49.4) |
30 (58.8) |
| Kidney right transplanted, n |
102 |
52 |
50 |
|
119 |
70 |
49 |
|
| – Moderate or good graft function, n (%) |
47 (46.1) |
21 (40.4) |
26 (52.0) |
0.328 |
60 (50.4) |
31 (44.3) |
29 (59.2) |
0.158 |
| – Delayed graft function or organ loss, n (%) |
55 (53.9) |
31 (59.6) |
24 (48.0) |
59 (49.6) |
39 (55.7) |
20 (40.8) |
Discussion
Organ donation from deceased persons whose death occurred after a planned, expected cardiac arrest following WLST is increasing in Switzerland. We analysed the first 217 referred and approved cDCD donors since the reintroduction of DCD in Switzerland, from 2011 to 2019, and compared them with DBD donors. We found that on average cDCD donors were slightly older, more often male, had been resuscitated more often before being admitted to hospital, and probably as a result of the latter [43], died more frequently from anoxia when compared with their DBD counterparts. It is important to note that for cDCD donors the critical illness leading to the decision in favour of WLST is reported as cause of death, and not the cause for the secondary brain death after planned cardiac arrest, which would always be anoxia. Also, cDCD donors in our study suffered more often from comorbidities, especially from heart disease. Some of these characteristics we attributed to cDCD donors are known to be associated with inferior transplant outcomes [11].
Except for kidney transplants, at least some of the routinely performed clinical tests analysed in this study indicate a lower donor organ quality in cDCD. Unsurprisingly, the utilisation rate was lower in cDCD donors compared with DBD donors. Still, from 175, or over 80% of the 217 referred and approved cDCD donors, at least one organ was transplanted. Due to the deleterious effects of warm ischaemia on organ viability, cDCD can only take place if cardiac arrest follows soon after WLST. The current legally binding time limit in Switzerland is 120 minutes, whereas in France and the UK it is 180 minutes [11, 18]. Successful kidney procurement has been reported more than 4 hours after WLST [44]. In almost two third of non-utilised cDCD donors in our study the reason was that the allowed time from WLST to cardiac arrest was exceeded. This is a rather high percentage, even when compared with countries which have the same time limit. A Dutch study found that for only 16% of referred cDCD donors were organs not procured because they did not die within 120 minutes after WLST [45]. Scoring systems to predict whether death after WLST will occur within a time period compatible with cDCD have been developed [46–48]. It remains, however, difficult to reliably identify potential cDCD donors who will die within 120 minutes after WLST [11, 23, 49, 50].
Currently, the heart is not being transplanted from DCD donors in Switzerland. DCD heart programmes, however, have been established in Australia (2014) and the United Kingdom (2015), and DCD hearts have been successfully transplanted in Austria, Belgium and the United States [51]. We found that fewer organs were transplanted from cDCD donors than from DBD donors (2.3 vs. 3.5) and it is tempting to explain this with the nonexistant Swiss DCD heart programme. However, all transplant rates were obviously lower in cDCD than in DBD, and a lower organ yield is common to all cDCD programmes, including those with established heart procurement [12]. For example, in the United Kingdom, an average of 2.7 transplantable organs are retrieved from DCD donors, compared with 3.3 from DBD donors [52]. One reason for lower DCD organ yields could be that, in general, as we were able to show, average cDCD donor and organ characteristics are not quite as favourable as those in DBD. In Switzerland it is planned to start DCD heart transplant programmes as of January 2022, using ex-vivo machine perfusion as already available for kidney and liver transplants. With increasing use of ex-vivo machine perfusion to allow for the possibility for evaluation and reconditioning of the isolated organ, and to reduce ischaemic damage during transport, it is likely that more organs per cDCD donor will be safely transplanted in the future [23].
Lower utilisation and transplant rates in cDCD, as we found, may also be explained to a certain extent by reluctance of transplant physicians to accept cDCD organs due to the warm ischaemia time these organs are exposed to after WLST, and in cases when patients have been resuscitated before admission. In the latter, renal failure is reported in the literature to occur in more than 50%, and liver failure in 24% [53, 54]. A recent publication looking at a Swiss patient cohort [43] also showed that donors who underwent cardiopulmonary resuscitation (CPR) are different from non-CPR donors with respect to age, cause of death and donation type. The latter, however, concluded that when carefully selected according to their haemodynamic condition, CPR donors are comparable to non-CPR donors in terms of utilisation and transplant rates. The median FWIT of utilised cDCD donors in our study was 29 minutes, also when NRP was applied. This is somewhat surprising, as NRP was developed to reduce FWIT, and there is evidence in the literature that early graft outcome – especially for kidney, but also for liver transplants – is slightly superior in NRP compared with rapid procurement [23, 55]. However, very few transplants in our study were procured using NRP, as NRP was introduced only in 2017 and by one procurement hospital (17 or <10% of cDCD transplants). Another possible explanation is that in Switzerland, unlike in other countries, invasive preparatory medical measures are not allowed before death has been determined. In countries where such antemortem interventions are allowed, cannulation of femoral vessels before WLST is undertaken to allow immediate initiation of NRP after death determination, and thus, minimising FWIT [11,23].
We analysed early post-transplant graft function for liver and kidney transplants by univariable and multivariable analysis, including available donor, organ, procurement, and recipient characteristics. We could show that cDCD liver transplants have outcomes similar to DBD liver transplants; over 94% of cDCD liver transplants were reported to have moderate or good early organ function. cDCD kidney transplants, however, showed an increased risk for impaired early outcomes; delayed graft function or organ loss was reported for up to more than half of cDCD, compared with only up to one fifth of DBD kidney transplants. The latter is consistent with two recent systematic reviews and meta-analyses, which also found an increased risk for primary non-function and delayed graft function of cDCD kidney transplants when compared with DBD kidney transplants [24, 25]. The same studies, however, also found long-term organ and recipient survival after kidney transplantation to be comparable between cDCD and DBD.
We further were interested in whether the extent of FWIT and donor age in cDCD would affect early liver and kidney graft function. We could not detect negative effects of prolonged FWIT or higher donor age, in either liver or in kidney transplants. In the literature there is a lack of evidence supporting acceptable times for FWIT [11], and several reports suggest that longer FWIT still yields transplantable organs, especially for kidneys [56, 57] and pancreas [58]. In liver transplantation, it has been shown that every minute of extra ischaemia decreases graft survival, with increased risk of biliary complications [59]. In our study the relatively low incidence of delayed graft function or early organ loss in utilised cDCD somewhat hampered a robust statistical analysis. To further investigate the effect of FWIT and age on early graft function in Switzerland, studies with larger cDCD donor cohorts are needed. Regarding donor age, our findings seem to support the Swiss practice, which does not apply a general age limit for liver and kidney donors.
Strengths and limitations of the study
To our knowledge, this is the first systematic and sound comparison of cDCD and DBD in Switzerland since the reintroduction of DCD in 2011. We assessed the effect of the donation pathway on transplant and early post-transplant outcomes using a multivariable approach, allowing for the different characteristics of cDCD and DBD donors, and also allowing for some recipient factors.
Our study has, however, limitations. No national consensus guidelines with respect to assessment of the early post-transplant outcome exist. If liver or kidney function is reported as good, moderate, or delayed is therefore to a certain extant biased by the subjectivity of the local treating medical team. Further, early organ function may depend on more recipient-related factors than we could include. We could not allow for more recipient-related factors as we had no access to detailed recipient data. The use of ex-vivo liver and kidney perfusion to reduce CIT has increased in Switzerland, particularly with extended criteria donors or in cDCD. Unfortunately we had no available data on the use of such ex-vivo machine perfusion. Many international transplant studies analyse mid- to long-term outcomes after transplantation, like graft function and patient survival after 1, 3, 5 or several years. It is a major limitation of our study that we could only include graft function up to 72 hours after transplantation. Unfortunately we had no access to mid- to long-term follow-up recipient data as for example collected by the Swiss Transplant Cohort Study.
Conclusion
Despite, on average, less favourable donor and organ characteristics compared with DBD donors, cDCD donors are increasingly referred and today provide an important source for scarce transplants in Switzerland, particularly for end-stage kidney disease. Consistent with the current literature, we identified a higher risk for delayed graft function or early organ loss for cDCD kidney transplants but not for cDCD liver transplants when compared with DBD. When carefully selected and allowed for other risk factors in organ allocation, extended FWIT or high age in cDCD does not seem to be associated with impaired graft function in recipients early after transplantation. A scientifically sound evaluation of cDCD in Switzerland, however, would include long-term recipient follow-up data in a multivariable analysis. We think it is our obligation to provide transplant physicians who need to accept or decline donor organs, and patients on the transplant wait list alike, with the most actual, evidence-based information on transplant outcomes and possible risk factors. However, to do so, it will be inevitable to finally link comprehensive donor, procurement, transplant and recipient data in Switzerland on a national level.
Acknowledgments
The authors would like to thank the National Committee for Organ Donation (CNDO) and the five Swiss organ donation networks for their initiative and continuous support in reintroducing and expanding DCD, and in establishing and implementing the corresponding standardised clinical procedures in Switzerland.
Franz F. Immer, MD
Swisstransplant
Effingerstrasse 1
CH-3011 Berne
franz.immer[at]swisstransplant.ch
References
1.
Bleisch B
,
Schuurmans MM
,
Klaghofer R
,
Benden C
,
Seiler A
,
Jenewein J
. Health-related quality of life and stress-related post-transplant trajectories of lung transplant recipients: a three-year follow-up of the Swiss Transplant Cohort Study. Swiss Med Wkly. 2019 Feb;149(07-08).
2.
Mazzoni D
,
Cicognani E
,
Mosconi G
,
Totti V
,
Roi GS
,
Trerotola M
, et al.
Sport activity and health-related quality of life after kidney transplantation. Transplant Proc. 2014 Sep;46(7):2231–4. https://doi.org/10.1016/j.transproceed.2014.07.049
3.
Casanovas T
,
Herdman M
,
Chandía A
,
Peña MC
,
Fabregat J
,
Vilallonga JS
. Identifying Improved and Non-improved Aspects of Health-related Quality of Life After Liver Transplantation Based on the Assessment of the Specific Questionnaire Liver Disease Quality of Life. Transplant Proc. 2016 Jan-Feb;48(1):132–7. https://doi.org/10.1016/j.transproceed.2015.11.009
4.
Singer LG
,
Chowdhury NA
,
Faughnan ME
,
Granton J
,
Keshavjee S
,
Marras TK
, et al.
Effects of Recipient Age and Diagnosis on Health-related Quality-of-Life Benefit of Lung Transplantation. Am J Respir Crit Care Med. 2015 Oct;192(8):965–73. https://doi.org/10.1164/rccm.201501-0126OC
5.
Duffy JP
,
Kao K
,
Ko CY
,
Farmer DG
,
McDiarmid SV
,
Hong JC
, et al.
Long-Term Patient Out-come and Quality of Life After Liver Transplantation: Analysis of 20-Year Survivors. Trans Meet Am Surg Assoc Am Surg Assoc. 2010;128:264–74.
6.
Kugler C
,
Gottlieb J
,
Warnecke G
,
Schwarz A
,
Weissenborn K
,
Barg-Hock H
, et al.
Health-related quality of life after solid organ transplantation: a prospective, multiorgan cohort study. Transplantation. 2013 Aug;96(3):316–23. https://doi.org/10.1097/TP.0b013e31829853eb
7.
Kostro JZ
,
Hellmann A
,
Kobiela J
,
Skóra I
,
Lichodziejewska-Niemierko M
,
Dębska-Ślizień A
, et al.
Quality of Life After Kidney Transplantation: A Prospective Study. Transplant Proc. 2016 Jan-Feb;48(1):50–4. https://doi.org/10.1016/j.transproceed.2015.10.058
8.
van Adrichem EJ
,
Siebelink MJ
,
Rottier BL
,
Dilling JM
,
Kuiken G
,
van der Schans CP
, et al.
Tol-erance of Organ Transplant Recipients to Physical Activity during a High-Altitude Expedition: Climbing Mount Kilimanjaro. Eller K, editor. PLOS ONE. 2015 Nov 25;10(11):e0142641.
9.
De Baere C
,
Delva D
,
Kloeck A
,
Remans K
,
Vanrenterghem Y
,
Verleden G
, et al.
Return to work and social participation: does type of organ transplantation matter? Transplantation. 2010 Apr;89(8):1009–15. https://doi.org/10.1097/TP.0b013e3181ce77e5
10.
Cavallini J
,
Forsberg A
,
Lennerling A
. Social function after solid organ transplantation: an integra-tive review. Nord J Nurs Res. 2015 Dec;35(4):227–34. https://doi.org/10.1177/0107408315592335
11.
EDQM
. Guide to the quality and safety of organs for transplantation [Internet]. European Direc-torate for the Quality of Medicines & HealthCare of the Council of Europe (EDQM); 2018. Available from: www.edqm.eu
12.
Lomero M
,
Gardiner D
,
Coll E
,
Haase-Kromwijk B
,
Procaccio F
,
Immer F
, et al.; European Committee on Organ Transplantation of the Council of Europe (CD-P-TO)
. Donation after circulatory death today: an updated overview of the European landscape. Transpl Int. 2020 Jan;33(1):76–88. https://doi.org/10.1111/tri.13506
13.
The World Health Organization (WHO), The Spanish Transplant Organization, Organización Nacional de Trasplantes (ONT)
. Global Observatory on Donation and Transplantation (GODT [Internet]. Number of Actual donors after circulatory death (DCD). 2019 [cited 2021 Aug 30]. Available from: http://www.transplant-observatory.org/data-charts-and-tables/chart/
14.
The Federal Assembly of the Swiss Confederation
. Federal Act on the Transplantation of Organs, Tissues and Cells [Internet]. 810.21 Oct 8, 2004. Available from: https://www.admin.ch/opc/en/classified-compilation/20010918/index.html
15.
Der Schweizerische Bundesrat
. Verordnung über die Transplantation von menschlichen Organen, Geweben und Zellen [Internet]. 810.211 Mar 16, 2007. Available from: https://www.admin.ch/opc/de/classified-compilation/20051806/index.html
16.
Wälchli-Bhend S
,
Beyeler F
,
Weiss J
,
Immer FF
. How an Existing Donation After Circulatory Death Program Was Grounded and Re-Started in Switzerland. Organs. Tissue Cell. 2011;14:25–6.
17.
Weber M
,
Dindo D
,
Demartines N
,
Ambühl PM
,
Clavien PA
. Kidney transplantation from donors without a heartbeat. N Engl J Med. 2002 Jul;347(4):248–55. https://doi.org/10.1056/NEJMoa020274
18.
Swiss Academyof Medical Sciences (SAMS)
. Determination of Death with Regard to Organ Transplantation and Preparations for Organ Removal [Internet]. 2017 p. 34. Available from: https://www.samw.ch/dam/jcr:12cfb438-d6cf-4acd-b93c-675f9efd2ff7/guidelines_sams_determination_death_organ_removal.pdf
19.
Immer FF
. Organ donation after circulatory death in Switzerland: slow but constant progress. Swiss Med Wkly. 2015 Jan;145:w14062. https://doi.org/10.4414/smw.2015.14062
20. Swisstransplant. Jahresbericht 2020 [2020 annual report] [Internet]. 2021 [cited 2021 Aug 13]. Available from: https://www.swisstransplant.org/de/swisstransplant/jahresbericht
21.
Council of Europe
. International figures on donation and transplantation 2020. 2021. (Newsletter Transplant). Report No.: 26.
22.
Weiss J
,
Elmer A
,
Béchir M
,
Brunner C
,
Eckert P
,
Endermann S
, et al.; Comité National du Don d’Organes (CNDO)
. Deceased organ donation activity and efficiency in Switzerland between 2008 and 2017: achievements and future challenges. BMC Health Serv Res. 2018 Nov;18(1):876. https://doi.org/10.1186/s12913-018-3691-8
23.
Smith M
,
Dominguez-Gil B
,
Greer DM
,
Manara AR
,
Souter MJ
. Organ donation after circulatory death: current status and future potential. Intensive Care Medicine [Internet]. 2019 Feb 6 [cited 2019 Mar 21]; Available from: http://link.springer.com/10.1007/s00134-019-05533-0
24.
Rijkse E
,
Ceuppens S
,
Qi H
,
IJzermans JN
,
Hesselink DA
,
Minnee RC
. Implementation of donation after circulatory death kidney transplantation can safely enlarge the donor pool: A systematic review and meta-analysis. Int J Surg. 2021 Aug;92:106021. https://doi.org/10.1016/j.ijsu.2021.106021
25.
Gavriilidis P
,
Inston NG
. Recipient and allograft survival following donation after circulatory death versus donation after brain death for renal transplantation: A systematic review and meta-analysis. Transplant Rev (Orlando). 2020 Oct;34(4):100563. https://doi.org/10.1016/j.trre.2020.100563
26.
de Kok MJ
,
Schaapherder AF
,
Alwayn IP
,
Bemelman FJ
,
van de Wetering J
,
van Zuilen AD
, et al.
Improving outcomes for donation after circulatory death kidney transplantation: Science of the times. Dor FJ, editor. PLoS ONE. 2020 Jul 29;15(7):e0236662.
27.
Summers DM
,
Watson CJ
,
Pettigrew GJ
,
Johnson RJ
,
Collett D
,
Neuberger JM
, et al.
Kidney donation after circulatory death (DCD): state of the art. Kidney Int. 2015 Aug;88(2):241–9. https://doi.org/10.1038/ki.2015.88
28.
Summers DM
,
Johnson RJ
,
Allen J
,
Fuggle SV
,
Collett D
,
Watson CJ
, et al.
Analysis of factors that affect outcome after transplantation of kidneys donated after cardiac death in the UK: a cohort study. Lancet. 2010 Oct;376(9749):1303–11. https://doi.org/10.1016/S0140-6736(10)60827-6
29.
Kumar S
,
Lin S
,
Schold JD
. Impact of donation after circulatory death donor allografts on outcomes following liver transplantation for fulminant hepatic failure in the United States. Am J Transplant. 2021 Jan;21(1):382–90. https://doi.org/10.1111/ajt.16286
30.
Silverstein J
,
Roll G
,
Dodge JL
,
Grab JD
,
Yao FY
,
Mehta N
. Donation After Circulatory Death Is Associated With Similar Posttransplant Survival in All but the Highest-Risk Hepatocellular Carcinoma Patients. Liver Transpl. 2020 Sep;26(9):1100–11. https://doi.org/10.1002/lt.25819
31.
Laing RW
,
Scalera I
,
Isaac J
,
Mergental H
,
Mirza DF
,
Hodson J
, et al.
Liver Transplantation Using Grafts From Donors After Circulatory Death: A Propensity Score-Matched Study From a Single Center. Am J Transplant. 2016 Jun;16(6):1795–804. https://doi.org/10.1111/ajt.13699
32.
Blok JJ
,
Detry O
,
Putter H
,
Rogiers X
,
Porte RJ
,
van Hoek B
, et al.; Eurotransplant Liver Intestine Advisory Committee
. Longterm results of liver transplantation from donation after circulatory death. Liver Transpl. 2016 Aug;22(8):1107–14. https://doi.org/10.1002/lt.24449
33.
Zhou J
,
Chen B
,
Liao H
,
Wang Z
,
Lyu M
,
Man S
, et al.
The Comparable Efficacy of Lung Donation After Circulatory Death and Brain Death: A Systematic Review and Meta-analysis. Transplantation. 2019 Dec;103(12):2624–33. https://doi.org/10.1097/TP.0000000000002888
34.
Van Raemdonck D
,
Keshavjee S
,
Levvey B
,
Cherikh WS
,
Snell G
,
Erasmus M
, et al.; International Society for Heart and Lung Transplantation
. Donation after circulatory death in lung transplantation-five-year follow-up from ISHLT Registry. J Heart Lung Transplant. 2019 Dec;38(12):1235–45. https://doi.org/10.1016/j.healun.2019.09.007
35.
Krutsinger D
,
Reed RM
,
Blevins A
,
Puri V
,
De Oliveira NC
,
Zych B
, et al.
Lung transplantation from donation after cardiocirculatory death: a systematic review and meta-analysis. J Heart Lung Transplant. 2015 May;34(5):675–84. https://doi.org/10.1016/j.healun.2014.11.009
36.
Shahrestani S
,
Webster AC
,
Lam VW
,
Yuen L
,
Ryan B
,
Pleass HC
, et al.
Outcomes From Pancreatic Transplantation in Donation After Cardiac Death: A Systematic Review and Meta-Analysis. Transplantation. 2017 Jan;101(1):122–30. https://doi.org/10.1097/TP.0000000000001084
37.
van Loo ES
,
Krikke C
,
Hofker HS
,
Berger SP
,
Leuvenink HG
,
Pol RA
. Outcome of pancreas transplantation from donation after circulatory death compared to donation after brain death. Pancreatology. 2017 Jan - Feb;17(1):13–8. https://doi.org/10.1016/j.pan.2016.11.002
38.
Thuong M
,
Ruiz A
,
Evrard P
,
Kuiper M
,
Boffa C
,
Akhtar MZ
, et al.
New classification of donation after circulatory death donors definitions and terminology. Transpl Int. 2016 Jul;29(7):749–59. https://doi.org/10.1111/tri.12776
39. Comité National du Don d’Organes (CNDO), Swisstransplant. Swiss Donation Pathway [Internet]. 2020 [cited 2021 Aug 3]. Available from: https://www.swisstransplant.org/de/fuer-fachpersonal/ausbildung/standard-titel
40.
Roeb E
,
Steffen HM
,
Bantel H
,
Baumann U
,
Canbay A
,
Demir M
, et al.
S2k-Leitlinie nicht alkoholische Fettlebererkrankungen. Z Gastroenterol. 2015 Jul;53(7):668–723. https://doi.org/10.1055/s-0035-1553193
41.
Levey AS
,
Stevens LA
,
Schmid CH
,
Zhang YL
,
Castro AF 3rd
,
Feldman HI
, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration)
. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May;150(9):604–12. https://doi.org/10.7326/0003-4819-150-9-200905050-00006
42.
R Core Team
. R: A language and environment for statistical computing. [Internet]. Vienna, Aus-tria: R Foundation for Statistical Computing; 2017. Available from: https://www.R-project.org/
43.
Gottschalk M
,
Elmer A
,
Benden C
,
Beyeler F
,
Immer F
. Impact of cardiopulmonary resuscitation on organ donation in Switzerland. Swiss Med Wkly [Internet]. 2021 Feb 5 [cited 2021 Feb 19]; Available from: https://doi.emh.ch/smw.2021.20413
44.
Reid AW
,
Harper S
,
Jackson CH
,
Wells AC
,
Summers DM
,
Gjorgjimajkoska O
, et al.
Expansion of the Kidney Donor Pool by Using Cardiac Death Donors with Prolonged Time to Cardiorespiratory Ar-rest: Expanding Pool Using DCD with Prolonged Agonal Phase. Am J Transplant. 2011 May;11(5):995–1005. https://doi.org/10.1111/j.1600-6143.2011.03474.x
45.
Leiden H
,
Haase-Kromwijk B
,
Hoitsma A
,
Jansen N
. Controlled donation after circulatory death in the Netherlands: more organs, more efforts. Neth J Med. 2016 Aug;74(7):285–91.
46.
Lewis J
,
Peltier J
,
Nelson H
,
Snyder W
,
Schneider K
,
Steinberger D
, et al.
Development of the University of Wisconsin donation After Cardiac Death Evaluation Tool. Prog Transplant. 2003 Dec;13(4):265–73. https://doi.org/10.1177/152692480301300405
47.
DeVita MA
,
Brooks MM
,
Zawistowski C
,
Rudich S
,
Daly B
,
Chaitin E
. Donors after cardiac death: validation of identification criteria (DVIC) study for predictors of rapid death. Am J Transplant. 2008 Feb;8(2):432–41. https://doi.org/10.1111/j.1600-6143.2007.02087.x
48.
Wind J
,
Snoeijs MG
,
Brugman CA
,
Vervelde J
,
Zwaveling J
,
van Mook WN
, et al.
Prediction of time of death after withdrawal of life-sustaining treatment in potential donors after cardiac death. Crit Care Med. 2012 Mar;40(3):766–9. https://doi.org/10.1097/CCM.0b013e318232e2e7
49.
Nijhoff MF
,
Pol RA
,
Volbeda M
,
Kotsopoulos AM
,
Sonneveld JP
,
Otterspoor L
, et al.
External Validation of the DCD-N Score and a Linear Prediction Model to Identify Potential Candidates for Organ Donation After Circulatory Death: A Nationwide Multicenter Cohort Study. Transplantation. 2021 Jun;105(6):1311–6. https://doi.org/10.1097/TP.0000000000003430
50.
Kotsopoulos AM
,
Böing-Messing F
,
Jansen NE
,
Vos P
,
Abdo WF
. External validation of prediction models for time to death in potential donors after circulatory death. Am J Transplant. 2018 Apr;18(4):890–6. https://doi.org/10.1111/ajt.14529
51.
Scheuer SE
,
Jansz PC
,
Macdonald PS
. Heart transplantation following donation after circulatory death: expanding the donor pool. J Heart Lung Transplant. 2021 Sep;40(9):882–9. https://doi.org/10.1016/j.healun.2021.03.011
52. NHS Blood and Transplant. Contribution of DCD to transplantation in the UK [Internet]. Donation after circulatory death. Available from: https://www.odt.nhs.uk/deceased-donation/best-practice-guidance/donation-after-circulatory-death/
53.
Sandroni C
,
Dell’anna AM
,
Tujjar O
,
Geri G
,
Cariou A
,
Taccone FS
. Acute kidney injury after cardiac arrest: a systematic review and meta-analysis of clinical studies. Minerva Anestesiol. 2016 Sep;82(9):989–99.
54.
Roedl K
,
Jarczak D
,
Blohm R
,
Winterland S
,
Müller J
,
Fuhrmann V
, et al.
Epidemiology of intensive care unit cardiac arrest: Characteristics, comorbidities, and post-cardiac arrest organ failure - A prospective observational study. Resuscitation. 2020 Nov;156:92–8. https://doi.org/10.1016/j.resuscitation.2020.09.003
55.
De Beule J
,
Vandendriessche K
,
Pengel LHM
,
Bellini MI
,
Dark JH
,
Hessheimer AJ
, et al.
A sys-tematic review and meta‐analyses of regional perfusion in donation after circulatory death solid organ transplantation. Transpl Int. 2021 Sep 27;tri.14121.
56.
Nelson HM
,
Glazier AK
,
Delmonico FL
. Changing Patterns of Organ Donation: Brain Dead Do-nors Are Not Being Lost by Donation After Circulatory Death. Transplantation. 2015;(Oct):1.
57.
Suntharalingam C
,
Sharples L
,
Dudley C
,
Bradley JA
,
Watson CJ
. Time to cardiac death after withdrawal of life-sustaining treatment in potential organ donors. Am J Transplant. 2009 Sep;9(9):2157–65. https://doi.org/10.1111/j.1600-6143.2009.02758.x
58.
Blok JJ
,
Ringers J
,
Schaapherder AF
,
Dubbeld J
,
Baranski AG
,
de Fijter JW
, et al.
Report of the first five DCDD pancreas transplants within the Eurotransplant region; excellent results with prolonged first warm ischemia times. Transpl Int. 2013 Apr;26(4):e31–3. https://doi.org/10.1111/tri.12044
59.
Taner CB
,
Bulatao IG
,
Perry DK
,
Sibulesky L
,
Willingham DL
,
Kramer DJ
, et al.
Asystole to cross-clamp period predicts development of biliary complications in liver transplantation using donation after cardiac death donors. Transpl Int. 2012 Aug;25(8):838–46. https://doi.org/10.1111/j.1432-2277.2012.01508.x



