Young adults after arterial switch operation for transposition of the great arteries in Switzerland: a growing population
DOI: https://doi.org/10.4414/SMW.2022.w30114
Results from the Prospective Cohort of Adult Congenital Heart Disease Patients (SACHER)
Francisco Javier
Ruperti-Repiladoab*, Jan
Affolterb, Judith
Bouchardycd, Harald
Gabriele, Simon F.
Stämpflif, Reto
Engelg, Markus
Schwerzmanna, Matthias
Greutmannh, Daniel
Toblerb
aCentre for Congenital Heart Disease, Cardiology, University Hospital Inselspital, University of Bern, Switzerland
bDivision of Cardiology, University Hospital of Basel, Basel, Switzerland
cDepartment of Cardiology and Cardiac Surgery, University Hospital Lausanne, Lausanne, Switzerland
dDivision of Cardiology, University Hospital Geneva, Geneva, Switzerland
eMedical University of Vienna, Department of Cardiology, Adult Congenital Heart Disease Programme, Vienna, Austria
fHeart Centre Lucerne, Luzerner Kantonsspital, Lucerne, Switzerland
gDepartment of Cardiology, Kantonsspital St Gallen, St Gallen, Switzerland
hUniversity Heart Centre, Department of Cardiology, University of Zurich, Switzerland
*These authors contributed equally to the study design, data interpretation and manuscript preparation and are, therefore, joint first authors.
Summary
BACKGROUND: Adults with transposition of the great arteries (d-TGA) after the arterial switch operation (ASO) are an evolving cohort in adult cardiology. We aimed to analyze cardiac function and cardiac events after transition to the adult clinic in Switzerland.
MATERIALS AND METHODS: Adults with prior ASO enrolled in the Swiss Adult Congenital HEart disease Registry (SACHER) were included. We analyzed initial cardiac anatomy, surgical history, residual lesions and cardiac function at the time of inclusion, as well as cardiac events during follow-up. Patients were classified as complex (with ventricular septal defect) or simple (with intact interventricular septum) d-TGA.
RESULTS: The cohort included 149 patients (99 simple d-TGA, 50 complex d-TGA; age 21±3 years; 71% male, follow-up 27 [15–46] months). Prior to inclusion, patients with complex d-TGA had undergone more interventions related to the left ventricular outflow tract (16% vs. 3%, p = 0.01). Functional and cardiovascular status were similar between the groups. Eleven patients (7%) had a total of 19 cardiac events (5 complications and 14 re-interventions) during follow-up. Patients with complex d-TGA had more cardiac-related complications compared to those with simple d-TGA (8% vs. 1%, p = 0.03). The frequency of re-interventions was not statistically different between the two groups (12% vs. 4%, p = 0.07). During follow-up, an increase in QRS duration was observed. Other parameters of cardiac function remained unchanged.
CONCLUSION: The majority of adult ASO patients have normal functional class and cardiac function. Complex anatomy and residual lesions play a key role when regarding the occurrence of cardiac-related complications during follow-up. The role of QRS prolongation over time needs to be investigated further.
Introduction
Dextro-transposition of the great arteries (d-TGA) is the second most common cyanotic heart defect in newborns and accounts for 3% of all congenital heart lesions [1, 2]. Without surgical intervention, survival beyond the first year of life is unlikely [3]. Surgical repair has transitioned from atrial switch procedures (Senning or Mustard operations) to the arterial switch operation (ASO) since the 1980s [4]. Survival to adulthood after the ASO is excellent, with few complications reported during the first two decades after the operation [5–7]. Long-term cardiac-related complications due to haemodynamic residuae or sequelae related to the surgical repair procedure have been observed. Early studies in adult survivors have raised concerns about progressive dilatation of the neo-aortic root and malfunction of the neo-aortic valve, pulmonary artery stenosis and stenosis of the right ventricular outflow tract (RVOT), ventricular dysfunction, and coronary artery obstruction [5, 8–17].
A better understanding of the factors associated with long-term complications among ASO patients beyond childhood may be important when designing interventions to improve outcomes for these patients during adulthood. We therefore aimed to comprehensibly analyze a cohort of adults who had an ASO for d-TGA from the Swiss Adult Congenital HEart disease Registry (SACHER) in order to raise awareness among general practitioners and general cardiologists about this growing population in the current Swiss adult cardiology [18].
Methods
Setting and study population
The Swiss registry for adults with congenital heart disease (SACHER; www.sacher-registry.com; ClinicalTrials.gov Identifier NCT2258724, Ref. No. EK:180/13), established in 2013, is a prospective cohort study of adult patients with congenital heart disease (ACHD) with predefined follow-up protocols and regular assessment of adverse events. Patients with structural congenital heart defects or hereditary aortopathies, who are followed at dedicated ACHD clinics, have been asked to participate in SACHER since 2013. Data are pseudonymized and stored in an electronic, web-based database (secuTrial®). Collected data include detailed diagnosis, type of repair procedures, previous complications and adverse outcomes during follow-up. The detailed methodology of the registry has been published previously [18]. For the purpose of this study, patients who underwent ASO for d-TGA were identified from SACHER. There were no predefined time intervals between clinical visits. Clinical visits were planned according to the discretion of the treating physician. The baseline visit was defined as the closest (in time) clinical visit to the inclusion of the patient into SACHER, while the last clinical visit previous to the analysis of the data was considered to be the follow-up visit.
All patients had given their written informed consent and the study was approved by the local ethics committees.
Clinical parameters
Baseline and follow-up information including demographic characteristics, cardiac anatomy and function, surgical and medical history, cardiac imaging, cardiovascular fitness, functional status (according to the New York Heart Association [NYHA] functional classification), laboratory parameters, office blood pressure, echocardiographic and cardiac magnetic resonance imaging (cMRI) parameters and cardiac-related events during follow-up were extracted from local charts.
The following echocardiographic parameters were obtained: left ventricular ejection fraction (LVEF), left ventricular end-diastolic and end-systolic diameters (LVEDD and LVESD), severity of neo-aortic regurgitation (AR), presence of right ventricular outflow tract stenosis, presence of abnormal wall motion and dimensions of the aortic root and ascending aorta. The following parameters obtained from cMRI were collected: right ventricular ejection fraction (RVEF), dimensions of the aortic root and ascending aorta, late gadolinium enhancement, and diameters of the main, right and left pulmonary arteries.
Definitions
Cardiac morphology was classified as simple TGA (intact interventricular septum) or complex TGA (ventricular septum defect [VSD]). AR was defined and graded as mild, moderate or severe in accordance with the published guidelines [19]. Predicted (%) peak oxygen uptake (peak VO2) was obtained from cardiopulmonary exercise tests. Both the type of exercise test and the formula used to calculate the peak VO2 were centre-specific and no study protocol was applied with this regard.
Outcome definitions
Cardiac events (cardiac-related re-interventions and cardiac-related complications) occurring during follow-up were registered. The following events were considered cardiac complications: cardiac-related death, hospitalization for heart failure, sustained supraventricular and ventricular tachycardia requiring medical intervention, myocardial infarction, stroke and infective endocarditis. The following events were considered cardiac-related interventions: any form of valvar replacement, ablation procedures for the treatment of cardiac arrhythmias, percutaneous interventions and cardiac device-related interventions. The combined primary outcome was defined as the occurrence of either cardiac events or cardiac-related complications.
Obstetric complications (such as clinically relevant minor bleeding, major bleeding, preterm labour and pre-eclampsia/eclampsia) and adverse maternal cardiac events during pregnancy, age at gestation, method of delivery, birth weight, and foetal and neonatal adverse events were obtained from cardiac and obstetric charts. Adverse maternal cardiac events of interest included tachyarrhythmias and bradyarrhythmias requiring treatment, pulmonary oedema (diagnosed by chest x-ray), myocardial infarction and other thromboembolic events. When possible, foetal and neonatal adverse events were also obtained from charts. These included foetal (<20 weeks of gestation) or neonatal death (within 28 days after birth), premature birth (delivery <37 weeks of gestation), small for gestational age birth weight (<10th percentile of gestational age), intraventricular haemorrhage, respiratory distress and congenital heart disease in the newborn. The above-mentioned obstetric complications and parameters related to cardiovascular status and function (see clinical parameters) were considered as secondary outcomes.
Statistics
SPSS software (version 26.0, SPSS Inc., Chicago, Illinois) was used for data analysis. Baseline characteristics were stratified according to cardiac anatomy. The distributions of continuous variables were assessed using skewness, kurtosis and visual inspection of the histogram. Continuous variables were presented as mean (standard deviation [SD]) or median (interquartile range [IQR]) and compared using paired t-tests or Wilcoxon tests, as appropriate, for the comparison of dependent samples (baseline vs. follow-up parameters), and using unpaired t-tests or Kruskal-Wallis tests, as appropriate, for the comparison of independent samples (stratified baseline characteristics). Categorical variables were presented as counts (percentages) and compared using chi-square or McNemar tests, as appropriate. Survival analysis for the occurrence of the combined primary outcome in patients with simple d-TGA vs. complex d-TGA at 48 months was assessed using the Kaplan-Meier estimator. The statistical significance of differences between groups was assessed by means of the log-rank test. For all analyses, the null hypothesis was rejected for p-values <0.05.
Results
Baseline characteristics
Out of a total of 4,686 patients included in SACHER, 344 (7%) had d-TGA, of whom 195 (57%) were operated on with an atrial switch and have been excluded from the analysis. All patients with d-TGA who were operated with an ASO have been included in the analysis (no exclusion criteria, figure S1). Among the 149 patients with previous ASO for surgical repair of d-TGA who were included in the analysis, 71% were male. Ninety-nine (66%) had a simple cardiac anatomy. Baseline characteristics stratified by cardiac anatomy (simple vs complex d-TGA) are summarized in table 1.
Table 1Baseline characteristics.
|
All patients
|
Simple d-TGA
|
Complex d-TGA
|
p
|
| N |
149 |
99 |
50 |
|
| Demographics |
| – Male gender (%) |
105 (70) |
70 (71) |
35 (70) |
0.9 |
| – Age at repair (days) |
7 (5-12) |
7 (5-9) |
11 (5-405) |
0.001
|
| Associated cardiovascular malformations (other than VSD or DORV) |
| – Atrial septal defects (%) |
50 (37) |
31 (31) |
19 (38) |
0.4 |
| – Patent ductus arteriosus (%) |
33 (22) |
19 (19) |
14 (28) |
0.2 |
| – Related to LVOT and aorta:
|
Aortic arch hypoplasia (%) |
4 (3) |
0 (0) |
4 (8) |
0.004
|
|
Supravalvar aortic stenosis (%) |
2 (1) |
1 (1) |
1 (2) |
0.62 |
|
Subaortic stenosis (%) |
2 (1) |
0 (0) |
2 (4) |
0.05
|
| Coarctation of the aorta (%) |
7 (5) |
1 (1) |
6 (12) |
0.003
|
| – Related to RVOT: |
Congenital pulmonary valve stenosis |
3 (2) |
2 (2) |
1 (2) |
0.99 |
| Infundibular pulmonary stenosis |
5 (3) |
2 (2) |
3 (6) |
0.2 |
| – Coronary anomalies+
|
18 (12) |
12 (12) |
6 (12) |
0.99 |
| Subsequent interventions before inclusion |
63 (42) |
36 (36) |
27 (54) |
0.04
|
| – Interventions related to the LVOT and aorta |
11 (7) |
3 (3) |
8 (16) |
0.004
|
| – Interventions related to pulmonary valve and pulmonary arteries |
41 (28) |
24 (24) |
17 (35) |
0.2 |
| – Pacemaker/AICD |
7 (5) |
2 (2) |
5 (10) |
0.03
|
| – Interventions related to coronary arteries |
3 (2) |
2 (2) |
1 (2) |
0.99 |
| – Other interventions |
17 (11) |
12 (12) |
5 (10) |
0.7 |
| Valve replacement |
14 (10) |
7 (6) |
7 (19) |
0.03
|
| Pacemaker/AICD placement |
6 (4) |
1 (1) |
5 (10) |
0.03*
|
| – AICD |
2 (1) |
1 (1) |
1 (2) |
|
| – AICD-CRT |
1 (1) |
0 |
1 (2) |
|
| – Pacemaker |
3 (2) |
0 |
3 (6) |
|
In patients with complex d-TGA, ASO was performed later in life (median [IQR] age 11 [5–405] days vs. 7 [5–9] days, p = 0.001). Among patients with complex d-TGA, the ASO was performed beyond the neonatal period (>30 days of age) more often compared to in newborns with simple d-TGA (19/50 (49%) vs. 5/99 (5%), p <0.001). Patients with complex d-TGA were more often born with concomitant aortic arch hypoplasia (8% vs. 0%, p = 0.004) and aortic coarctation (12% vs. 1%, p = 0.003). Previous device implantation before inclusion (pacemaker or automated implantable cardioverter defibrillator [AICD]) was significantly more frequent among patients with complex d-TGA compared to among those with simple d-TGA (10% vs. 1%, p = 0.03). Accordingly, patients with complex d-TGA had more often interventions related to cardiac devices during childhood (10% vs. 2%, p = 0.03). Also, interventions related to the aorta were more common among patients with complex d-TGA. The frequency of and age at first cardiac-related complications until inclusion are provided in table S1 of the supplementary material.
Functional status and residual lesions at the time of inclusion in the adult cohort
Mean age at inclusion was 21±3 years. At baseline, patients had overall preserved biventricular systolic function (LVEF 59±8%, RVEF 54±8%) and most of them (95%) had a normal functional class (NYHA I). The LVEF was <45% in 6/104 (6%) patients with valid data, and of these only one patient had an LVEF <30% (due to dilated cardiomyopathy). There were no statistically significant differences concerning cardiac function and cardiovascular status between patients with simple versus complex d-TGA (table 2). However, patients with complex d-TGA had longer QRS durations compared to those with simple d-TGA (106 [90–140] ms vs. 96 [90–105] ms, p = 0.001).
Table 2Functional status and residual lesions at the time of inclusion (adult cohort).
|
All patients
|
Simple d-TGA
|
Complex d-TGA
|
p
|
| N |
149 |
99 |
50 |
|
| BSA (m2) |
1.82±0.2 |
1.81±0.2 |
1.83±0.2 |
0.5
|
| Age at inclusion (years) |
21±3 |
21±4 |
21±3 |
0.7
|
| Cardiovascular and functional status |
| BMI (kg/m2)
|
23 (20–26) |
22 (21–24) |
23 (20–25) |
0.5 |
| NYHA class ≥II (%) |
8 (5) |
5 (5) |
3 (6) |
0.8 |
| NT-pro BNP (ng/L) (n = 91) |
63 (37–121) |
55 (117–133) |
75 (41–168) |
0.1 |
|
VO2 max. (% of predicted, n=69) |
78±17 |
79±19 |
77±15 |
0.7 |
| LVEF (%) |
59±8 |
59±8 |
59±9 |
0.4 |
| RVEF (%)a
|
54±8 |
53±8 |
55±8 |
0.4 |
| QRS duration (ms)b
|
98 (90–112) |
96 (90–104) |
116 (97–142) |
0.002
|
| Sinus rhythm (%) |
147 (99) |
98 (100) |
49 (98) |
0.1 |
| Residual lesions |
| Aortic regurgitation: mild to moderate (%) |
77 (53) |
50 (52) |
27 (55) |
0.9 |
| Aortic regurgitation: severe (%)
|
3 (2) |
2 (2) |
1 (1) |
0.9 |
| Neo-aortic root diameter (mm) |
38±5 |
38±6 |
38±5 |
0.7 |
| Z-scores for the neo-aortic root diameterc
|
2.6±1.9 |
2.6±2.1 |
2.6±1.6 |
0.97 |
| Ascending aortic diameter (mm)
|
32±6 |
32±6 |
32±6 |
0.9 |
| RVOT stenosis (%)
|
27 (18) |
19 (19) |
8 (16) |
0.6 |
| Residual coarctation (%) |
3 (2) |
0 |
3 (6) |
0.01
|
The mean diameter of the neo-aortic root was 38±5 mm, with no difference between patients with simple and complex d-TGA. At baseline, the dimension of the aortic root was 40–44 mm in 22/98 (22%) patients; 45–50 mm in 11/98 (11%) patients, and >50 mm in 3/98 (3%) patients. The dimension of the ascending aorta was >40 mm in 11/98 (11%) patients. In total, 80 (55%) patients had some degree of neo-aortic valve regurgitation and 3 (2%) patients had severe neo-aortic regurgitation. Three out of seven patients with repaired coarctation had residual coarctation at inclusion. Twenty-seven (18%) patients had residual pulmonary artery or other RVOT stenosis. Myocardial perfusion imaging (mainly stress cMRI) to evaluate myocardial ischaemia was performed in 52 patients. In 41 (79%) the imaging results were reported as normal, in 9 (17%) patients there was a myocardial scar and 2 (6%) patients had a perfusion defect.
Follow-up
Median follow-up duration after inclusion in the adult cohort was 27 [15–46] months. Follow-up duration was slightly, but statistically significantly longer for patients with complex d-TGA compared to patients with simple d-TGA (34 [23–49] vs. 24 [0–43] months, p = 0.002).
Cardiac-related complications and re-interventions
Cardiac events which occurred during follow-up are outlined in table 3.
Table 3Cardiovascular-related events during follow-up.
|
Type of TGA (associated lesions)
|
Description of complication/re-intervention
|
| Complex d-TGA (subpulmonary VSD, PDA and ASD II) |
Male, 25 years, severe pulmonary arterial hypertension after intracardiac repair. |
| Died two days after bilateral lung transplantation from haemorrhagic complications and multi-organ failure |
| Complex d-TGA (VSD, PDA and ASD) |
Male, 21 years, 7 surgical and percutaneous re-interventions before inclusion. |
| At 21 years of age stenting of residual coarctation |
| At 25 years of age (twice) neo-aortic and pulmonary root replacement for infective endocarditis with complicated perioperative courses (a total of three interventions/operations needed) |
| At 30 years of age death due to multi-organ failure from infective prosthetic valve endocarditis of the neo-aortic valve prosthesis |
| Complex d-TGA (VSD) |
Male, 22 years, recurrent atrial flutter |
| Elective ablation of the cavotricuspid isthmus |
| Complex d-TGA (DORV, hypoplasia of ascending aorta and aortic arch) |
Male, 21 years, previous neo-aortic valve replacement (twice) and recurrent ablation procedures for atrial arrhythmias before inclusion |
| Elective electrophysiologic study and radio-frequency ablation for recurrent IART |
| Simple d-TGA (pulmonary artery stenosis) |
Female, 21 years, pulmonary branch stenosis |
| At age 21 years surgical reconstruction of branch pulmonary artery stenosis and bio-prosthetic pulmonary valve replacement |
| At age 22 years (12 months after pulmonary valve implantation) infective endocarditis, managed medically |
| Simple d-TGA |
Male, 22 years, elective stenting of the left pulmonary artery |
| Complex d-TGA (VSD) |
Male, 22 years, re-coarctation 22 years after surgical repair (end-to-end anastomosis) |
| Elective stenting of the aortic isthmus due to arterial hypertension and peak-to-peak gradient of 20 mmHg |
| Complex d-TGA (DORV) |
Female, 19 years, electively admitted for pacemaker box change |
| Simple d-TGA (pulmonary artery stenosis) |
Female, 28 years, with pulmonary artery stenoses |
| At age 24 years balloon angioplasty/stenting of the right pulmonary artery and balloon angioplasty of the left pulmonary artery |
| At age 25 years transcatheter pulmonary valve implantation for severe symptomatic pulmonary valve regurgitation |
| Simple d-TGA (ASD, PDA and coronary artery anomaly) |
Male, 28 years, repair of aneurysm of the neo-aortic root and mechanical neo-aortic valve replacement |
| Complex d-TGA (VSD, congenital AV-Block III) |
Male, 30 years, electively admitted for pacemaker box change and upgrade to AICD |
A cardiac event occurred in 11 (7%) patients. Five (3%) patients suffered from cardiac-related complications and 14 cardiac-related interventions among 10 patients were reported. Two patients died during follow-up. One patient with severe pulmonary hypertension died two days after bilateral lung transplantation due to multiple organ failure. The other patient died due to septic shock related to prosthetic valve endocarditis. Two patients had infective endocarditis after the implantation of a bio-prosthetic valve and two patients had recurrent supraventricular arrhythmia. Patients with complex d-TGA had more cardiac-related complications compared to patients with simple-d-TGA (8% vs. 1%, p = 0.03).
Five cardiac-related interventions were related to the left side of the heart (two stent implantations for re-coarctation and three aortic valve replacements), while three were related to the right side (stenting of pulmonary branch stenosis and patch augmentation of the pulmonary artery with concomitant pulmonary valve replacement). In two patients, transcatheter ablation techniques for the treatment of recurrent supraventricular tachycardia were performed. Although not statistically significant, cardiac-related interventions were more commonly seen among patients with a complex anatomy (12% vs. 4%, p = 0.07) (see figure 1).

Figure 1 Comparison of cardiovascular-related events during follow-up (simple vs. complex anatomy). Differences in the incidence of patients suffering from cardiac-related complications or having undergone cardiac-related interventions during follow-up between patients with simple vs. complex dextro-transposition of the great arteries (d-TGA). The comparison was assessed by means of a chi-square test.
Functional and cardiovascular status over time
Over time, LVEF, RVEF, VO2max and NT-proBNP did not change significantly and no progression in the severity of neo-aortic regurgitation was observed (Table S2 of the supplementary material). There was a non-statistically significant increase in the diameter of the neo-aortic root (38±5mm to 39±5mm, p = 0.07), while QRS duration showed a statistically significant increase (107±24 vs. 111±26, p <0.001). This increase in QRS duration during follow-up was observed in both patients with simple d-TGA and those with complex d-TGA (∆median QRS [ms] of 4, p = 0.001, and 2, p = 0.007, for simple and complex d-TGA, respectively).
Pregnancies
Among seven women, twelve pregnancies carried to term. Maternal mean age at delivery was 26±4 years. The mean gestational age at delivery was 39 weeks ± 12 days and the mean birth weight was 3,017±424 grams. There were eight (67%) vaginal deliveries and four (33%) caesarean sections. There were no cardiac events during pregnancy, peri- or post-partum. There were no adverse maternal cardiac events during pregnancy. Concerning offspring complications, there was one foetal death due to foetal thrombotic vasculopathy with subsequent intrauterine growth restriction. Two newborns from the same mother were born small for their gestational age (active smoker during both pregnancies). Besides that, there were no reported congenital heart defects or chromosomopathy among the 11 healthy newborns. See table 4 for details.
Table 4Completed pregnancies.
|
Patient
|
Age*
|
Type of d-TGA
|
Gestational age* (weeks+days)
|
Birth weight (g)
|
Delivery
|
Obstetric complications
|
Offspring complications
|
| 1 |
21 |
Simple d-TGA |
39+1 |
2,720 |
C-section |
None |
Small for gestational age |
| 1 |
22 |
Simple d-TGA |
38+6 |
2,890 |
C-section |
None |
Small for gestational age |
| 2 |
23 |
Complex d-TGA (VSD) |
37+3 |
2,500 |
Vaginal |
None |
Intrauterine foetal death |
| 2 |
25 |
Complex d-TGA (VSD) |
35+3 |
2,320 |
C-section |
Recurrent vaginal bleedings and preterm labour (26 GW) |
None |
| 3 |
24 |
Simple d-TGA |
40+3 |
3,780 |
Vaginal |
None |
None |
| 4 |
30 |
Complex d-TGA (VSD) |
40 |
2,845 |
Vaginal |
None |
None |
| 4 |
33 |
Complex d-TGA (VSD) |
40 |
2,970 |
Vaginal |
Postnatal bleeding |
None |
| 5 |
29 |
Complex d-TGA (DORV) |
38+4 |
3,070 |
Vaginal |
Pre-eclampsia, postnatal bleeding |
None |
| 5 |
31 |
Complex d-TGA (DORV) |
41 |
3,145 |
Vaginal |
None |
None |
| 6 |
21 |
Simple d-TGA |
37 |
2,950 |
C-section |
None |
None |
| 7 |
28 |
Simple d-TGA |
40+5 |
3,535 |
Vaginal |
None |
None |
| 7 |
30 |
Simple d-TGA |
41 |
3,475 |
Vaginal |
None |
None |
Survival analysis for the combined primary outcome
Survival analysis for the occurrence of the combined primary outcome for patients with simple vs. complex d-TGA was assessed using the Kaplan-Meier estimator and is depicted in figure 2.

Figure 2 Survival analysis for the occurrence of the combined primary outcome in patients with simple d-TGA vs. complex d-TGA. Survival analysis for the occurrence of the combined primary outcome in patients with simple d-TGA vs. complex d-TGA at 48 months. The statistical significance of the difference between groups was assessed by means of a log-rank test.
Even though the primary outcome-free survival time was better among patients with simple d-TGA (red), no statistically significant differences between the groups were found.
Discussion
Herein we describe a cohort of adults with prior ASO for d-TGA by analyzing patient characteristics, occurrence of cardiac events during follow-up and pregnancy outcomes. Overall, these patients predominantly had a normal functional class and presented with good cardiac function and acceptable exercise capacity. However, two out of five patients had undergone subsequent cardiac interventions after their initial ASO during childhood, and many were left with various types of residual lesions when entering adulthood (e.g. a dilated neo-aortic root was the most frequent residual lesion). Despite the short follow-up of our study (27 [15–46] months), 7% of the patients suffered from cardiac-related complications or underwent cardiac-related re-interventions. This was particularly striking in patients with complex d-TGA, where cardiac events occurred in 18% of them. Patients with complex d-TGA had more re-interventions related to left ventricular outflow tract lesions and more prosthetic valve implantations before inclusion, and were therefore at a higher risk for prosthetic valve endocarditis, valvar re-interventions and re-interventions for aortic coarctation. Together with infective endocarditis, arrhythmias were the most common cardiovascular complications. Interestingly, even though the combined primary outcome was significantly more frequent among patients with complex d-TGA, no significant differences regarding event-free follow-up were observed. However, there was a trend towards better event-free survival at 48 months among patients with simple d-TGA. Therefore, the lack of statistical significance in the survival analysis might have just been due to the overall low number of outcomes.
Both cases of infective endocarditis occurred in patients with a prosthetic valve, highlighting the high-risk situation of young patients with replaced heart valves. This is especially true after transcatheter pulmonary valve implantation (as was the case in one of our patients), where annualized incidence rates for infective endocarditis vary between 1.3% and 9.1% per patient-year [20].
One of the expected advantages of ASO over the abandoned atrial switch operation (Senning or Mustard procedure) is the reduction in the incidence of supraventricular arrhythmia in long-term follow-up, and in particular a lower incidence of scar-related/incisional intra-atrial re-entrant tachycardia. Surprisingly, despite the young median age of the patients in our cohort, recurrent supraventricular tachycardia requiring transcatheter ablation procedures were observed in two patients during follow-up. As studies have shown that the incidence rates of atrial tachycardias increase with ageing in all patients with repaired congenital heart disease, our observation shows the necessity of careful assessment of potential risk factors for (atrial) arrhythmias in adults after ASO, with the aim of reducing the occurrence of this cumbersome complication in the future [21].
In contrast to patients with complex d-TGA, those with simple d-TGA seem to have a lower risk of developing cardiac-related complications during early adulthood (only 1 out of 99 patients in our cohort). However, for the following reasons, careful long-term observation of this novel patient cohort is warranted:
a) Recent studies have shown persistent neo-aortic growth beyond childhood, with no stabilization becoming apparent over time. The growth of the neo-aortic root was associated with an increase in aortic regurgitation [15, 16]. Even though aortic dissection is not frequently seen among young patients after ASO, it is unclear how this progressive aortic dilatation will affect the risk of future aortic dissections among these patients later in life. In our study with a short follow-up duration, a trend of increasing neo-aortic root dimensions was observed, albeit it was not statistically significant (p = 0.07). More than half of the patients in our cohort had at least some degree of neo-aortic regurgitation which may progress over time. Furthermore, in one patient (male, 28 years), repair of an aneurysm of the neo-aortic root and mechanical neo-aortic valve replacement were performed during follow-up (table 2).
b) Balloon dilatation/stenting for the treatment of pulmonary branch stenosis is the most frequent intervention after ASO, both in children and in adults [14, 22, 23]. Studies on the impact of branch pulmonary artery stenosis on mid-term right ventricular function and exercise capacity in children and young adults have yielded contradictory results. Some data suggest better exercise capacity in patients with simple d-TGA and in patients without the need for any re-operation after the initial ASO compared to patients with complex d-TGA and to those who required re-interventions within the RVOT [5]. In our cohort, there were no differences in the prevalence of residual branch pulmonary artery stenoses between simple and complex d-TGA. In addition, there was no difference regarding prior interventions related to the RVOT at inclusion in the adult cohort. During follow-up, interventions for branch pulmonary stenoses were observed in patients with simple and complex d-TGA.
c) A major concern among patients after ASO is the fate of the re-implanted coronary arteries. Reduced coronary blood flow reserve and impaired coronary blood flow under stress have been observed in children after ASO [24] .Moreover, intracoronary ultrasound assessment within this population has revealed proximal eccentric intimal thickening in most of the vessels, suggesting early atherosclerosis in the re-implanted coronary arteries [25]. In adults after ASO, only partial sympathetic re-innervation of the coronary arteries was observed. This leads to impairment of the maximal dilator capacity of the coronary microvasculature [12]. In light of these pathophysiological changes after ASO, there remains a concern about premature coronary atherosclerosis and consecutive myocardial ischaemia. In line with previous studies, no coronary or cerebrovascular events occurred during follow-up in our study. Again, this might be related to the young age of our cohort and the short follow-up time.
Although functional status remained unchanged in short-term follow-up within our cohort, the increase in QRS duration was rather unexpected. This was observed in both complex and simple d-TGA patients. Information related to branch bundle block was not collected. Therefore, this factor cannot be taken into account when considering the analysis of QRS duration and its progression over time. The long-term relevance of this finding needs to be further investigated.
Reassuringly, pregnancy outcomes were favourable, as no maternal cardiac-related complications and only minor offspring problems (small for gestational age) were observed. The only foetal death was not associated with the congenital heart lesion of the mother. These results are in line with the previous observations [26].
Outlook: lifelong management of patients after ASO
As survival of newborns with d-TGA is excellent in the current era, with a survival rate of 97% into adulthood [5-7], consideration of ‘long-term’ outcomes must encompass lifelong management [27]. In this scenario, preventive measures, such as prophylaxis of infective endocarditis among patients with prosthetic valves, are crucial. Anticipatory care with regular discussions about healthy lifestyle (normal body weight, regular physical activity, no smoking, moderation in alcohol consumption and healthy diet) should be implemented to reduce the risk of premature atherosclerosis and myocardial infarction later in life. Periodic testing for measurable cardiovascular risk factors (cholesterol, diabetes) should be considered. Determining whether regular screening for coronary ischaemia in asymptomatic adults late after ASO is necessary or not requires further studies. It is not (yet) recommended in the current guidelines [28, 29]. Finally, the progression of the neo-aortic root diameter, the severity of aortic valve regurgitation and the extent of pulmonary branch stenosis need to be regularly assessed by means of cardiac imaging.
Because the long-term impact of residual lesions in adults after ASO on cardiac outcomes is still unknown, larger studies with longer follow-up periods (decades) are urgently needed. The ongoing prospective, international, multicentre EPOCH-ASO study is expected to fill some of these gaps [30].
Limitations
No core laboratory for cardiac imaging interpretation was available and interobserver variability may have been particularly relevant for the measurements of the dimensions of the aorta. However, this mirrors real-life clinical practice and should therefore not be considered a major limitation. Moreover, we analyzed only patients included in the SACHER registry. Although in Switzerland transition processes from paediatric to adult care are well organized, the possibility of patients being lost to follow-up during this process is still an issue. In addition, patients who died before their inclusion in the registry are not represented. However, because all Swiss tertiary care centres which specialize in the management of ACHD patients were included in the registry, our ASO cohort can be considered representative. Furthermore, the short follow-up duration does not reflect the magnitude of the clinical problem, particularly as some cardiac-related complications are expected to occur as this ASO cohort ages. Moreover, differences in follow-up duration between patients with simple and complex d-TGA might have had an impact on our results. Also, as mentioned before, information related to branch bundle block was not collected. Therefore, this factor cannot be taken into account when considering the analysis of QRS duration and its progression over time. Furthermore, because of our short follow-up, the retrospective nature of our analysis and the limitations related to these aspects we are not able to change any already existing recommendations for the management of these patients [31-33]. Finally, the rare occurrence of complications makes it difficult to infer the true risk to these patients, as some complications (e.g. the supra ventricular tachycardia seen in two patients) after ASO may be just a random occurrence.
Conclusion
The majority of adult patients with previous ASO have normal functional class and cardiac function. However, residual lesions present among many of them determine the occurrence of a variety of cardiac-related complications (such as infective endocarditis and supraventricular arrhythmia) and re-interventions during follow-up. This is especially true for patients with complex d-TGA. Pregnancy seems to be well tolerated and the offspring were healthy overall. The role of QRS duration, its progression over time, the presence of a hypoplastic arch and residual coarctation, residual branch pulmonary artery stenoses, and the progression of neo-aortic dilatation and neo-aortic regurgitation need to be further investigated in larger cohorts with longer follow-up durations. Adults with prior ASO need lifelong specialized follow-up and meticulous anticipatory care.
Acknowledgements
FJRR, JA, DT, MG and MS contributed to the drafting of the manuscript, the conception of the research and the critical revision of the manuscript. All other authors contributed to the patient recruitment and data collection and to the critical revision of the manuscript.
Daniel Tobler, MD
University Hospital Basel
Petersgraben 4
CH-4031 Basel
daniel.tobler[at]usb.ch
References
1.
Liu Y
,
Chen S
,
Zühlke L
,
Black GC
,
Choy MK
,
Li N
, et al.
Global birth prevalence of congenital heart defects 1970-2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol. 2019 Apr;48(2):455–63. https://doi.org/10.1093/ije/dyz009
2.
Centers for Disease Control and Prevention (CDC)
. Improved national prevalence estimates for 18 selected major birth defects—united States, 1999-2001. MMWR Morb Mortal Wkly Rep. 2006 Jan;54(51):1301–5.
3.
Liebman J
,
Cullum L
,
Belloc NB
. Natural history of transpositon of the great arteries. Anatomy and birth and death characteristics. Circulation. 1969 Aug;40(2):237–62. https://doi.org/10.1161/01.CIR.40.2.237
4.
Jatene AD
,
Fontes VF
,
Paulista PP
,
Souza LC
,
Neger F
,
Galantier M
, et al.
Anatomic correction of transposition of the great vessels. J Thorac Cardiovasc Surg. 1976 Sep;72(3):364–70. https://doi.org/10.1016/S0022-5223(19)40063-9
5.
Tobler D
,
Williams WG
,
Jegatheeswaran A
,
Van Arsdell GS
,
McCrindle BW
,
Greutmann M
, et al.
Cardiac outcomes in young adult survivors of the arterial switch operation for transposition of the great arteries. J Am Coll Cardiol. 2010 Jun;56(1):58–64. https://doi.org/10.1016/j.jacc.2010.03.031
6.
Khairy P
,
Clair M
,
Fernandes SM
,
Blume ED
,
Powell AJ
,
Newburger JW
, et al.
Cardiovascular outcomes after the arterial switch operation for D-transposition of the great arteries. Circulation. 2013 Jan;127(3):331–9. https://doi.org/10.1161/CIRCULATIONAHA.112.135046
7.
Kempny A
,
Wustmann K
,
Borgia F
,
Dimopoulos K
,
Uebing A
,
Li W
, et al.
Outcome in adult patients after arterial switch operation for transposition of the great arteries. Int J Cardiol. 2013 Sep;167(6):2588–93. https://doi.org/10.1016/j.ijcard.2012.06.066
8.
Lo Rito M
,
Fittipaldi M
,
Haththotuwa R
,
Jones TJ
,
Khan N
,
Clift P
, et al.
Long-term fate of the aortic valve after an arterial switch operation. J Thorac Cardiovasc Surg. 2015 Apr;149(4):1089–94. https://doi.org/10.1016/j.jtcvs.2014.11.075
9.
Co-Vu JG
,
Ginde S
,
Bartz PJ
,
Frommelt PC
,
Tweddell JS
,
Earing MG
. Long-term outcomes of the neoaorta after arterial switch operation for transposition of the great arteries. Ann Thorac Surg. 2013 May;95(5):1654–9. https://doi.org/10.1016/j.athoracsur.2012.10.081
10.
Delmo Walter EM
,
Miera O
,
Nasseri B
,
Huebler M
,
Alexi-Meskishvili V
,
Berger F
, et al.
Onset of pulmonary stenosis after arterial switch operation for transposition of great arteries with intact ventricular septum. HSR Proc Intensive Care Cardiovasc Anesth. 2011;3(3):177–87.
11.
Morgan CT
,
Mertens L
,
Grotenhuis H
,
Yoo SJ
,
Seed M
,
Grosse-Wortmann L
. Understanding the mechanism for branch pulmonary artery stenosis after the arterial switch operation for transposition of the great arteries. Eur Heart J Cardiovasc Imaging. 2017 Feb;18(2):180–5. https://doi.org/10.1093/ehjci/jew046
12.
Possner M
,
Buechel RR
,
Vontobel J
,
Mikulicic F
,
Gräni C
,
Benz DC
, et al.
Myocardial blood flow and cardiac sympathetic innervation in young adults late after arterial switch operation for transposition of the great arteries. Int J Cardiol. 2020 Jan;299:110–5. https://doi.org/10.1016/j.ijcard.2019.07.041
13.
Legendre A
,
Losay J
,
Touchot-Koné A
,
Serraf A
,
Belli E
,
Piot JD
, et al.
Coronary events after arterial switch operation for transposition of the great arteries. Circulation. 2003 Sep;108(10_suppl_1 Suppl 1):II186–90. https://doi.org/10.1161/01.cir.0000087902.67220.2b
14.
Nellis JR
,
Turek JW
,
Aldoss OT
,
Atkins DL
,
Ng BY
. Intervention for Supravalvar Pulmonary Stenosis After the Arterial Switch Operation. Ann Thorac Surg. 2016 Jul;102(1):154–62. https://doi.org/10.1016/j.athoracsur.2016.01.068
15.
van der Bom T
,
van der Palen RL
,
Bouma BJ
,
van Veldhuisen SL
,
Vliegen HW
,
Konings TC
, et al.
Persistent neo-aortic growth during adulthood in patients after an arterial switch operation. Heart. 2014 Sep;100(17):1360–5. https://doi.org/10.1136/heartjnl-2014-305702
16.
van der Palen RL
,
van der Bom T
,
Dekker A
,
Tsonaka R
,
van Geloven N
,
Kuipers IM
, et al.
Progression of aortic root dilatation and aortic valve regurgitation after the arterial switch operation. Heart. 2019 Nov;105(22):1732–40. https://doi.org/10.1136/heartjnl-2019-315157
17.
Bonhoeffer P
,
Bonnet D
,
Piéchaud JF
,
Stümper O
,
Aggoun Y
,
Villain E
, et al.
Coronary artery obstruction after the arterial switch operation for transposition of the great arteries in newborns. J Am Coll Cardiol. 1997 Jan;29(1):202–6. https://doi.org/10.1016/S0735-1097(96)00433-0
18.
Tobler D
,
Schwerzmann M
,
Bouchardy J
,
Engel R
,
Stambach D
,
Attenhofer Jost C
, et al.; On Behalf Of Sacher
. Swiss Adult Congenital HEart disease Registry (SACHER) - rationale, design and first results. Swiss Med Wkly. 2017 Oct;147:w14519.
19.
Nishimura RA
,
Otto CM
,
Bonow RO
,
Carabello BA
,
Erwin JP
,
Guyton RA
, et al.
2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014;63(22):e57-e185.
20.
Abdelghani M
,
Nassif M
,
Blom NA
,
Van Mourik MS
,
Straver B
,
Koolbergen DR
, et al.
Infective Endocarditis After Melody Valve Implantation in the Pulmonary Position: A Systematic Review. J Am Heart Assoc. 2018 Jun;7(13):e008163. https://doi.org/10.1161/JAHA.117.008163
21.
Arslani K
,
Roffler N
,
Zurek M
,
Greutmann M
,
Schwerzmann M
,
Bouchardy J
, et al.; SACHER Investigators
. Patterns of Incidence Rates of Cardiac Complications in Patients With Congenital Heart Disease. Can J Cardiol. 2018 Dec;34(12):1624–30. https://doi.org/10.1016/j.cjca.2018.09.010
22.
Hutter PA
,
Kreb DL
,
Mantel SF
,
Hitchcock JF
,
Meijboom EJ
,
Bennink GB
. Twenty-five years’ experience with the arterial switch operation. J Thorac Cardiovasc Surg. 2002 Oct;124(4):790–7. https://doi.org/10.1067/mtc.2002.120714
23.
Losay J
,
Touchot A
,
Serraf A
,
Litvinova A
,
Lambert V
,
Piot JD
, et al.
Late outcome after arterial switch operation for transposition of the great arteries. Circulation. 2001 Sep;104(12 Suppl 1):I121–6. https://doi.org/10.1161/hc37t1.094716
24.
Hauser M
,
Bengel FM
,
Kühn A
,
Sauer U
,
Zylla S
,
Braun SL
, et al.
Myocardial blood flow and flow reserve after coronary reimplantation in patients after arterial switch and ross operation. Circulation. 2001 Apr;103(14):1875–80. https://doi.org/10.1161/01.CIR.103.14.1875
25.
Pedra SR
,
Pedra CA
,
Abizaid AA
,
Braga SL
,
Staico R
,
Arrieta R
, et al.
Intracoronary ultrasound assessment late after the arterial switch operation for transposition of the great arteries. J Am Coll Cardiol. 2005 Jun;45(12):2061–8. https://doi.org/10.1016/j.jacc.2005.02.076
26.
Tobler D
,
Fernandes SM
,
Wald RM
,
Landzberg M
,
Salehian O
,
Siu SC
, et al.
Pregnancy outcomes in women with transposition of the great arteries and arterial switch operation. Am J Cardiol. 2010 Aug;106(3):417–20. https://doi.org/10.1016/j.amjcard.2010.03.047
27.
Villafañe J
,
Lantin-Hermoso MR
,
Bhatt AB
,
Tweddell JS
,
Geva T
,
Nathan M
, et al.; American College of Cardiology’s Adult Congenital and Pediatric Cardiology Council
. D-transposition of the great arteries: the current era of the arterial switch operation. J Am Coll Cardiol. 2014 Aug;64(5):498–511. https://doi.org/10.1016/j.jacc.2014.06.1150
28.
Stout KK
,
Daniels CJ
,
Aboulhosn JA
,
Bozkurt B
,
Broberg CS
,
Colman JM
, et al.
2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019 Apr;139(14):e637–97. https://doi.org/10.1161/CIR.0000000000000602
29.
Baumgartner H
,
Bonhoeffer P
,
De Groot NM
,
de Haan F
,
Deanfield JE
,
Galie N
, et al.; Task Force on the Management of Grown-up Congenital Heart Disease of the European Society of Cardiology (ESC); Association for European Paediatric Cardiology (AEPC); ESC Committee for Practice Guidelines (CPG)
. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J. 2010 Dec;31(23):2915–57. https://doi.org/10.1093/eurheartj/ehq249
30.
Ruperti-Repilad FJ
,
Gallego P
,
Dos L
,
Rueda Soriano J
,
Bouma B
,
Gabriel H
, et al.
Tobler10 D, Greutmann M. Comprehensive Long-Term Follow up of Adults with Arterial Switch Operation– European Collaboration for Prospective Outcome Research in Congenital Heart Disease (EPOCH-ASO)–Study Design and Protocols. Congenit Heart Dis. 2020;15(5):29.
31.
Bouchardy Judith GM
. Schwerzmann Markus, Attenhofer Jost Christine, De Pasquale Gabriella, Oxenius Angela, Rutz Tobias, Wustmann Kerstin, Balmer Christian, Tobler Daniel. Grown-up congenital heart disease: recommendations for standards of care. Cardiovasc Med. 2015;18(04):144–5.
32.
Stout KK
,
Daniels CJ
,
Aboulhosn JA
,
Bozkurt B
,
Broberg CS
,
Colman JM
, et al.
2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019 Apr;73(12):e81–192. https://doi.org/10.1016/j.jacc.2018.08.1029
33.
Baumgartner H
,
De Backer J
,
Babu-Narayan SV
,
Budts W
,
Chessa M
,
Diller GP
, et al.; ESC Scientific Document Group
. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2021 Feb;42(6):563–645. https://doi.org/10.1093/eurheartj/ehaa554
34.
Devereux RB
,
de Simone G
,
Arnett DK
,
Best LG
,
Boerwinkle E
,
Howard BV
, et al.
Normal limits in relation to age, body size and gender of two-dimensional echocardiographic aortic root dimensions in persons ≥15 years of age. Am J Cardiol. 2012 Oct;110(8):1189–94. https://doi.org/10.1016/j.amjcard.2012.05.063
Appendix
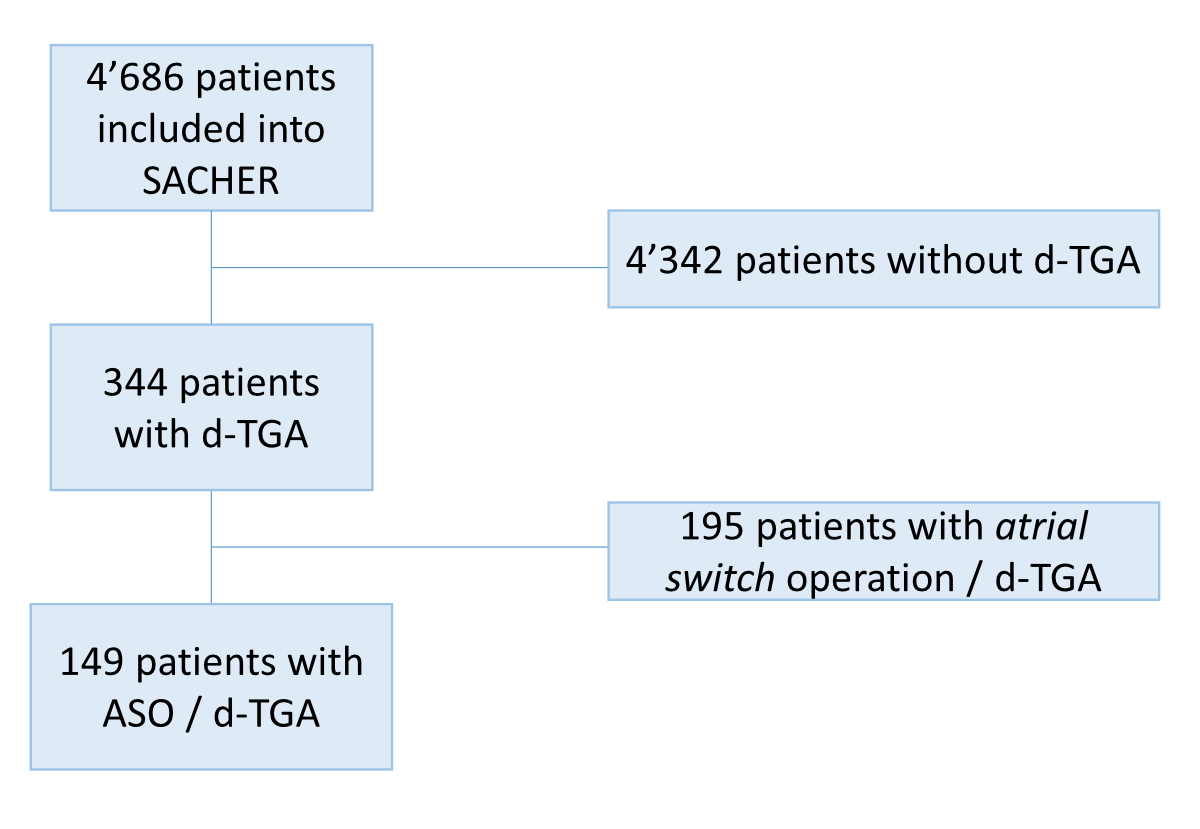
Figure S1 Flow chart. Out of a total of 4,686 patients included in SACHER, 344 (7%) had d-TGA, of whom 195 (57%) were operated on with an atrial switch and have been excluded from the analysis. All patients with d-TGA who were operated with an ASO have been included in the analysis (no exclusion criteria).
Table S1Number of previous cardiac-related complications and interventions until baseline.
|
Cases (%)
|
Age
|
| Heart failure |
2 (1) |
3 and 4 days |
| Stroke |
7 (5) |
14 (4–621) days |
| Infective endocarditis |
2 (1) |
9 and 19 years |
| Supraventricular tachycardia |
2 (1) |
5 days and 18 years |
| Atrial fibrillation |
1 (1) |
24 years |
| Ventricular tachycardia |
3 (2) |
5 days; and 10 and 25 years |
| Atrioventricular block >2° |
5 (3) |
8 and 23 days; 3 and 10 months; and 10 years |
| Myocardial infarction* |
6 (4) |
4, 5 and 7 days; and 13 and 17 years |
| Atrial flutter |
3 (2) |
15 years (all 3) |
| Arterial hypertension |
1 (1) |
8 years |
| Eisenmenger syndrome |
1 (1) |
2 years |
| Other** |
2 (1) |
– |
Table S2Functional and cardiovascular status over time.
|
Baseline
|
Follow-up
|
p
|
| Cardiovascular status |
| – Office systolic BP (mmHg) (n = 114) |
126±17 |
127±13 |
1 |
| – Office diastolic BP(mmHg) (n = 114) |
71±10 |
71±10 |
0.9 |
| – BMI (kg/m2) (n = 118) |
23±4 |
24±4 |
<0.001 |
| – VO2 max. (% of predicted ) (n = 35) |
77±17 |
80±18 |
0.1 |
| – NYHA class (>I) (n = 117) |
8 |
7 |
1 |
| Medication (n = 118) |
| – Antihypertensive |
12 (8.3) |
17 (14.7) |
– |
| – Anticoagulant |
4 (2.8) |
4 (3.4) |
– |
| – None |
95 (65.5) |
73 (62.9) |
– |
| Laboratory |
| – NT-pro BNP (ng/L) (n = 38) |
63 (37-121) |
73 (47-165) |
0.2 |
| – LDL (mmol/L)*
|
1.9±0.6 |
– |
| – HBA1c (%)*
|
5.4 (4.7-5.3) |
– |
| ECG |
| – QRS duration (ms) (n = 113)**
|
106±22 |
110±25 |
<0.001 |
| – Sinus rhythm (yes) (n = 118) |
117 (99) |
117 (99) |
1 |
| Echocardiography |
| – LVEF (%) (n = 92) |
59±8 |
58±7 |
0.8 |
| – LVEDD (mm) (n = 107) |
49±6 |
49±6 |
0.6 |
| – LVESD (mm) (n = 66) |
33±6 |
32±7 |
0.04 |
| – Ventricular wall motion abnormality (n = 85) |
10 (12) |
15 (18) |
0.06 |
| Aortic regurgitation (n = 118) |
| – mild to moderate |
77 (52) |
72 (48) |
– |
| – severe |
3 (2) |
3 (2) |
– |
| Aortic sinus diameter (mm) (n = 73) |
38±5 |
39±5 |
0.07 |
| Ascending aorta diameter (mm) (n = 49) |
32± 6 |
31±7 |
0.3 |
| RVEF (%) (n = 26) |
54±6 |
55±7 |
0.5 |


