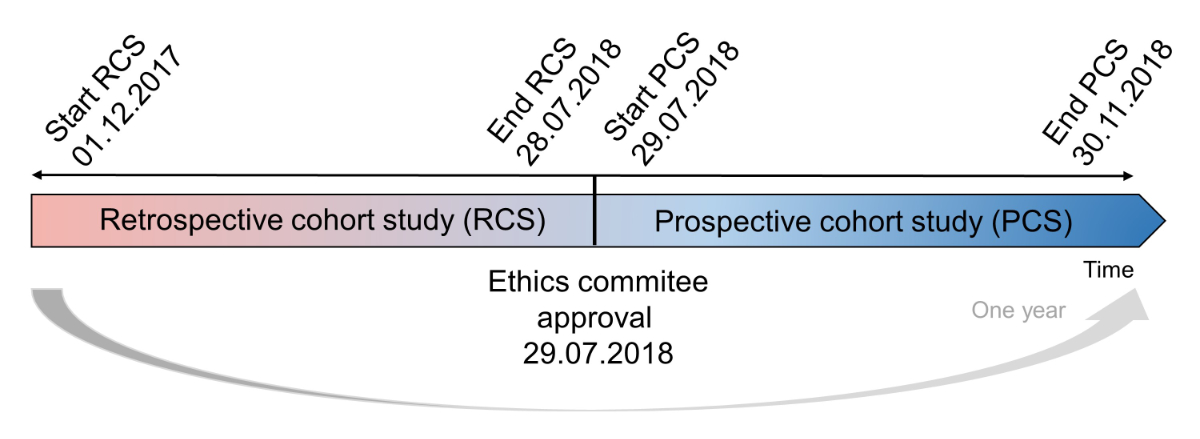
Figure 1 Study designs of the retrospective and prospective cohort studies.
DOI: https://doi.org/10.4414/SMW.2022.w30124
According to the World Health Organization (WHO), in Europe half of all mental health problems in adulthood have their onset during or before adolescence. Suicide appears to be the leading cause of death among adolescents (10–19 years old) in low- and middle-income countries and the second leading cause in high-income countries [1]. In the USA, one in six children (2–8 years old) has a mental, behavioural or developmental disorder. Three to nine percent of American children and adolescents had received a diagnosis of attention-deficit hyperactivity disorder, behaviour problems, anxiety or depression in 2016 and the rates of these conditions were increased compared with those at the beginning of the 2000s [2].
Not all psychiatric patients receive a specific treatment and treatment rates for children (3–17 years old) vary among different mental disorders, ranging from 53% for behavioural disorders to 78% for depression [2]. Psychotropic drugs are defined as substances that affect the production, the release or the mechanism of action of several neurotransmitters (i.e., dopamine, serotonin, gamma aminobutyric acid or norepinephrine) in the central nervous system (CNS) [3]. Because of the complexity of their mechanisms of action, one psychotropic drug can simultaneously have several effects in the CNS. However, they are generally considered to belong to one of six classes, driven by their main effect: antipsychotics, antidepressants, anxiolytics, hypnotics and sedatives, psychostimulants and mood stabilisers [4].
Because child psychiatrists often target specific symptoms that occur before conditions fully manifest (prodrome) and before a clear diagnosis can be identified [5, 6], paediatric patients can receive off-label psychotropic drug prescriptions, which are defined as prescriptions that do not comply with the recommendations detailed in the marketing authorisation of the manufacturer (i.e. age, indication or dosage). On the other hand, unlicensed prescriptions are defined as prescriptions that involve psychotropic drugs that do not have a marketing authorisation in the country and have to be imported from abroad [7]. The European Medicines Agency (EMA) reviewed the literature published up to the early 2000s on this phenomenon and confirmed that off-label or unlicensed drug use was often associated with more side effects and medication errors than authorised drugs [8]. Switzerland is no exception to this practice: in 2001, 49% of prescriptions analysed over a 6-month period in the department of paediatrics of a Swiss university hospital were found to be off-label or unlicensed [9]. In 2014, 68% of psychotropic drug prescriptions were off-label in the adolescent psychiatry service of the same hospital [10]. Psychotropic drugs could be prescribed off-label to young patients for months or years during their growing phase and exposure to psychotropic drugs could lead to severe adverse events, such as for the metabolic adverse events associated with the prescription of atypical antipsychotics [11–13]. The high off-label use of drugs continues to raise questions of efficacy and safety, especially when prescribing psychotropic drugs, with marketing authorisations varying across countries.
The absence of a psychiatric emergency department for children and adolescents in our hospital led paediatric patients to be hospitalised in a general paediatric ward for assessment before transfer to a specialised setting for mental conditions. However, knowledge on the first choice of psychotropic drug prescription among inpatients in paediatric wards in Switzerland remains limited. Therefore, we conducted a retrospective and a prospective cohort study to characterise the prescription of psychotropic drugs over a whole year in a paediatric service of a university hospital. Specifically, we aimed to first assess the proportion of off-label use in relation to age, indication and dosage recommendations approved in Switzerland and then compare it with the proportion of off-label use in relation to the recommendations approved in France and in the USA.
To study the off-label and clinical use of psychotropic medications over one entire year, we conducted a retrospective cohort study (RCS) and a prospective cohort study (PCS).
The PCS was observational, and involved screening patients hospitalised between 29 June 2018 and 30 November 2018. For all the patients included, the data collection continued until discharge. Owing to the summer season, we predicted a low rate of hospital admissions, so we conducted a RCS that involved screening patients hospitalised between 1 December 2017 and 28 June, 2018 (fig. 1). The local ethics committee (Cantonal Ethics Committee of Vaud 2018, Switzerland) approved both studies (project number 2018-01055).

Figure 1 Study designs of the retrospective and prospective cohort studies.
In both studies, we included all patients with at least one psychotropic drug prescription at entry, during the hospital stay or at discharge. Psychotropic drugs were defined using the Anatomical Therapeutic Chemical (ATC) codes: typical antipsychotics (N05AA-N05AD, N05AF, N05AG), atypical antipsychotics (N05AE, N05AH, N05AL, N05AX), anxiolytics (N05B), antidepressants (N06A, N06CA), hypnotics and sedatives (N05C), psychostimulants (N06B, N06CB), mood stabilisers (N05AN, N03AX09, N03AG01) and phytomedicines with psychotropic effects (N05BX05, N05CM09, N06AX25). An additional inclusion criterion in the prospective study was written consent from at least one parent or the legal authority for patients younger than 14 years old, or from the patients themselves if older and with the capacity of discernment, according to the Swiss Federal Act on Research involving Human Beings [14]. Exclusion criteria in both studies were a psychotropic drug prescription for a somatic indication (epilepsy, febrile seizure or premedication in the case of surgery) and a rehospitalisation without any significant difference in the diagnosis or in the psychotropic treatment (absence of new psychotropic drug prescription in the regular treatment and absence of a dosage change).
Participants provided informed written consent to participate and, where applicable, parents provided informed consent for their child to participate and the children assented.
All data on patients were collected from the electronic patient file or the discharge letter and manually entered into the spreadsheet program (Microsoft Excel®, 2018). A code was assigned to every patient included, in order to respect the anonymity and confidentiality requirements as per protocol. The code was available to the principal investigator only .
Data included age, sex, weight, reason for admission, length of stay and destination after discharge. All medical records regarding diagnosis, comorbidities and medical history were noted. The brand name, international non-proprietary name (INN), dosage form and strength, indication, frequency and route of administration were collected for every psychotropic drug prescription during the hospital stay and at discharge. Every change of dosage, route of administration or administration mode (as needed to regular treatment and vice-versa) was noted as a new psychotropic drug prescription. Finally, when prescribed as needed (pro re nata, PRN), the date and frequency of PD administration were recorded too.
The data collection for the PCS was the same as in the RCS. Moreover, during this observational study, a pharmacist attended medical rounds once a week and interdisciplinary meeting involving paediatricians, child psychiatrists, nurses and specialised teachers also once a week. She had access to the written patient files (psychological assessments, educational assessments, etc.).
Some additional information was collected during this prospective cohort study. All the medications prescribed during the hospital stay were screened for interactions using Lexicomp® Drug Interactions and were categorised according to the degree of gravity (1 = no action needed, 2 = therapy requires monitoring, 3 = consider therapy modification and 4 = avoid combination).
Finally, we recorded all the side effects that led to a change in psychotropic drug or to a change of the dosage and then declared them to the regional pharmacovigilance centre according to the physicians’ assessment.
All prescriptions were categorised as being off-label based on patient age, indication or dosage when they differed to those approved by the health authorities in the three selected countries: Swissmedic for Switzerland, French National Agency for Medicines and Health Products Safety (ANSM) for France and Food and Drug Administration (FDA) for the USA. The analysis was stopped at the first off-label criterion: age, indication or dosage.
Where a doubt existed, a second pharmacist was included in the analysis. Off-label prescription by dosage was defined as higher dosage than the target range mentioned by the manufacturer for specific indication and age range. In fact, lower dosages were considered appropriate as PD dosages tended to be gradually titrated.
All prescriptions of unlicensed psychotropic drugs according to every local authority were excluded from our analysis, as our focus was exclusively on off-label use.
The primary outcomes were the rate of off-label prescription of psychotropic drugs based on the marketing authorisations of the selected countries, the median number of different psychotropic drugs prescribed per patient during the hospital stay and the administration rate of PRN medications.
In the prospective study, drug-drug interactions and the adverse events caused by psychotropic drug prescriptions were added as secondary outcomes
Summary of data are presented as numbers and percentages for categorical variable and as mean ± standard deviation, median [min; max] or median (q25%; q75%) for continuous variables. Proportions of off-label prescriptions and their confidence interval (95% CI) were calculated separately for the prospective, retrospective and pooled data. A two-sample test of proportions was used to compare the proportions of off-label prescriptions between Swiss marketing authorisations vs French marketing authorisations and Swiss marketing authorisations vs American marketing authorisations. The difference in proportion was considered significant when the p-value was <0.05. Statistical analyses were performed using STATA software (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC).
The results of the two studies are presented separately because of their differences in terms of sources of data and protocol for the data collection. In the retrospective cohort study, there was no missing data influencing the demographic data and the primary or secondary outcomes.
Out of the 1060 patients hospitalised on the general paediatric ward during the RCS, 65 patients were eligible according to our inclusion and exclusion criteria. Seven patients were included twice and one was included three times (fig. 2). Of the 74 patients included, the percentage of boys (n = 39; 53%) and girls (n = 35; 47%) were similar, with a mean age of 13 ± 3 years (table 1).
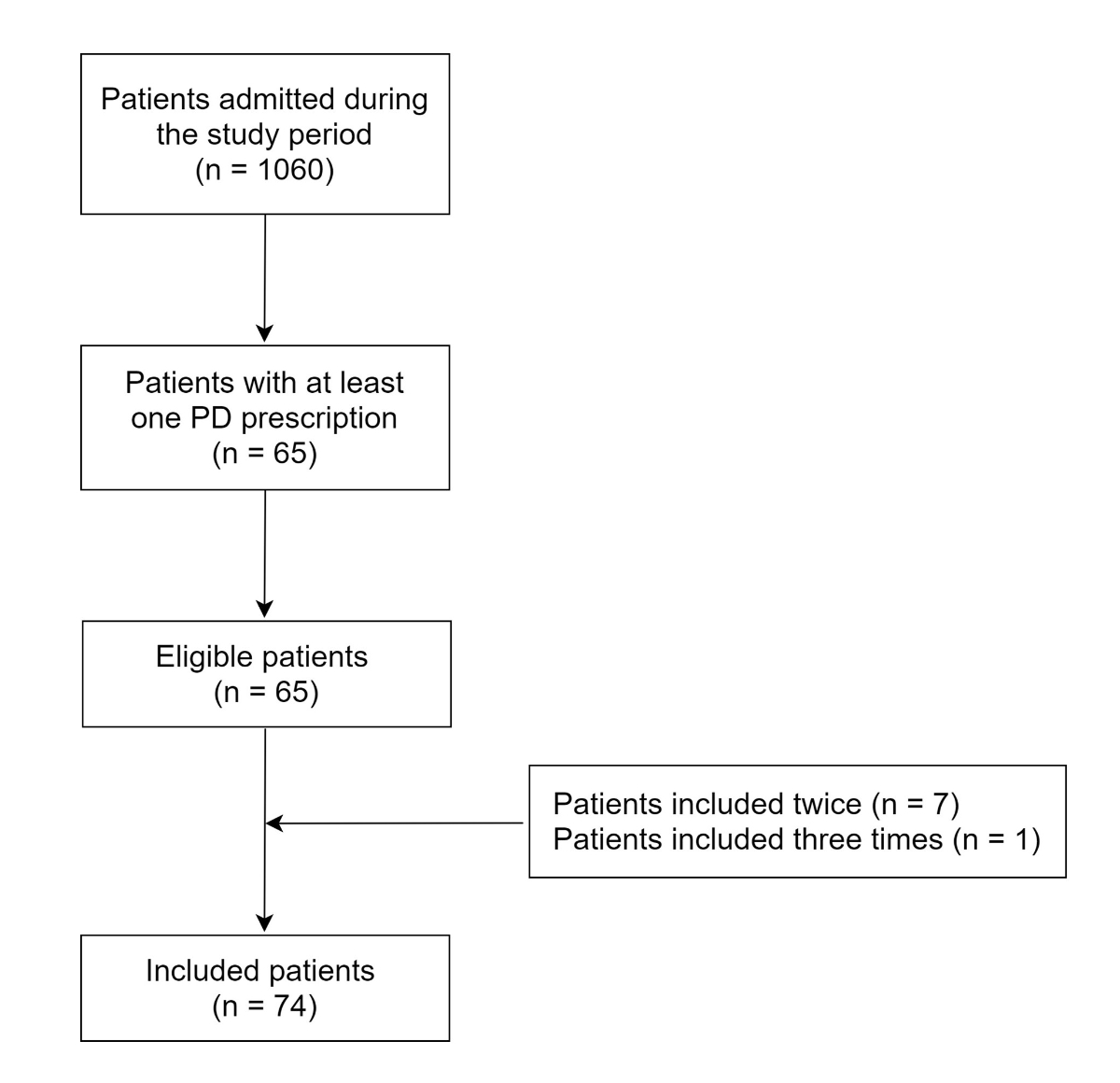
Figure 2 Flowchart of patients included in the retrospective cohort study (29 June 2018 to 30 November 2018).
Table 1Demographic information of the retrospective cohort study and the prospective cohort study populations.
| Demographic information | Retrospective data | Prospective data | |
| Included patients, n | 74 | 37 | |
| Sex | Boys, n (%) | 39 (53) | 14 (38) |
| Girls, n (%) | 35 (47) | 23 (62) | |
| Age | 0–5 years, n (%) | 3 (4) | 0 (0) |
| 6–11 years, n (%) | 19 (26) | 6 (16) | |
| 12–17 years, n (%) | 52 (70) | 31 (84) | |
| Mean ± SD, years | 13 ± 3 | 14 ± 2 | |
| Median hospital stay, days [min; max] | 7 [2; 94] | 9 [2; 78] | |
SD: standard deviation
In the PCS, only 377 patients were hospitalised during the study period: 33 patients met the inclusion and exclusion criteria and four were included twice (fig. 3). Of the 37 patients included, the percentage of girls (n = 23; 62%) was higher than the percentage of boys (n = 14; 38%), with a mean age of 14 ± 2 years (table 1).
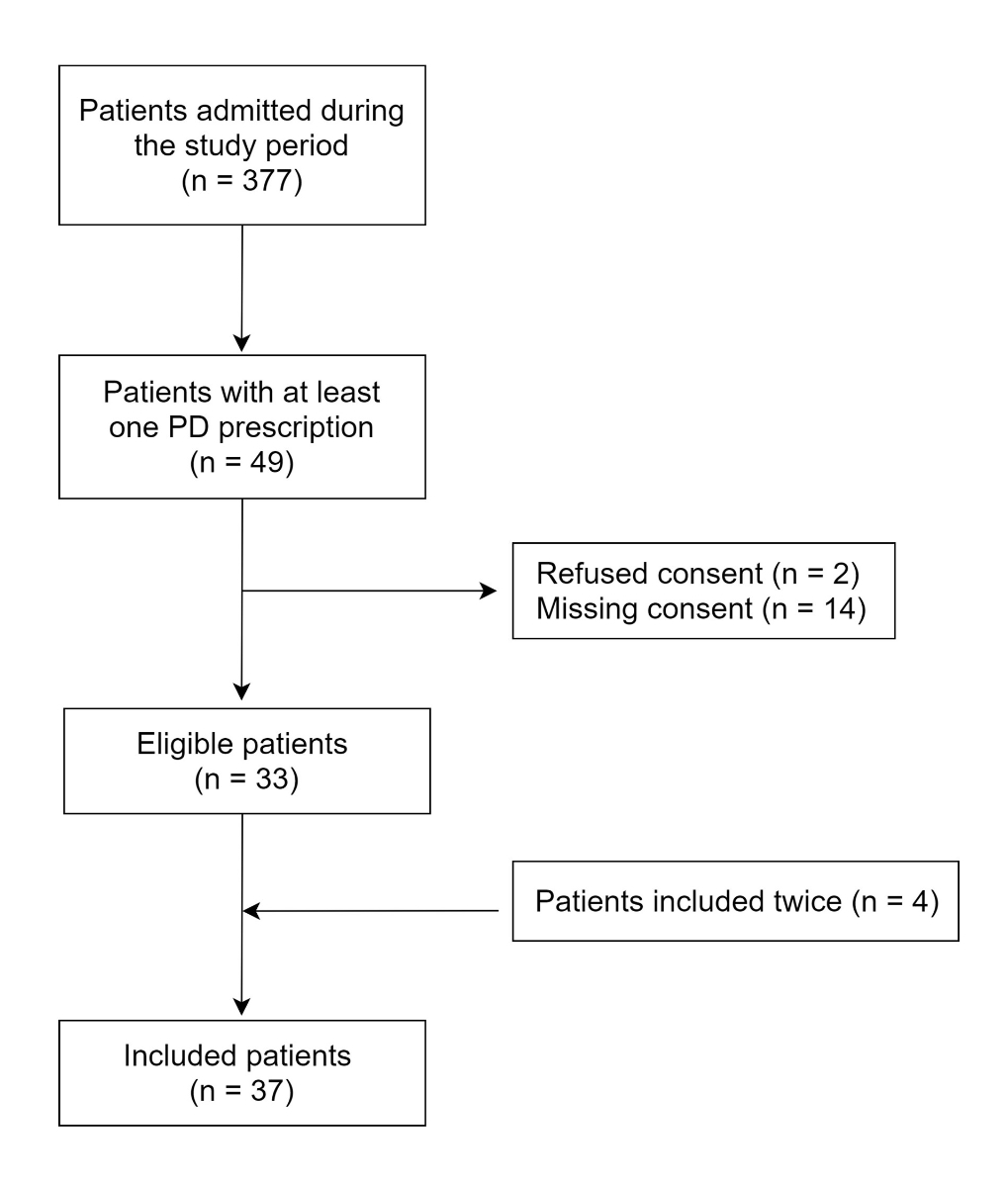
Figure 3 Flowchart of patients included in the prospective cohort study (1 December 2017 to 28 June 2018).
Suicidal thoughts (n = 14, 19%), behavioural disorders (n = 11, 15%) and suicide attempts (n = 10, 14%) were the main reasons for admission in the RCS. In the PCS, these reasons were similar: suicide attempts (n = 8, 22%), somatic indications (n = 6, 16%) and temper tantrums (n = 5, 14%).
The median length of hospitalisation was 7 days [2; 92] in the RCS and 9 days [2; 78] in the PCS (table 1).
In the RCS, three patients had no psychotropic drug prescription, despite mention of psychotropic treatment at entry; thus, overall, 71 patients received 176 psychotropic drug prescriptions. In the PCS, 37 patients had 88 psychotropic drug prescriptions.
The percentage of Swiss off-label prescriptions was 70% in the RCS and 71% in the PCS (table 2). The difference between these two results was not statistically significant (p = 0.87). In both studies, the most frequently prescribed off-label psychotropic drugs were antipsychotics and anxiolytics (fig. 4).
Table 2Off-label prescriptions of psychotropic drugs with respect to age, indication and dosage based on the national marketing authorisation (MA).
| National MA (total prescriptions) | Off-label prescription by age (n) | Off label prescription by indication (n) | Off-label prescription by dosage (n) | Total off label prescriptions/ total prescriptions, n/n (%) | |
| Retrospective data | Switzerland (n = 168) | 69 | 48 | 0 | 117/168 (70) |
| France (n = 174) | 52 | 54 | 0 | 106/174 (61) | |
| USA (n = 124) | 8 | 60 | 2 | 70/124 (56) | |
| Prospective data | Switzerland (n = 86) | 30 | 31 | 0 | 61/86 (71) |
| France (n = 84) | 23 | 33 | 0 | 56/84 (67) | |
| USA (n = 76) | 6 | 31 | 0 | 37/76 (51) | |
| Pooled data | Switzerland (n = 254) | 99 | 79 | 0 | 178/254 (70) |
| France (n = 258) | 75 | 87 | 0 | 162/258 (63) | |
| USA (n = 200) | 14 | 91 | 2 | 107/200 (54) | |
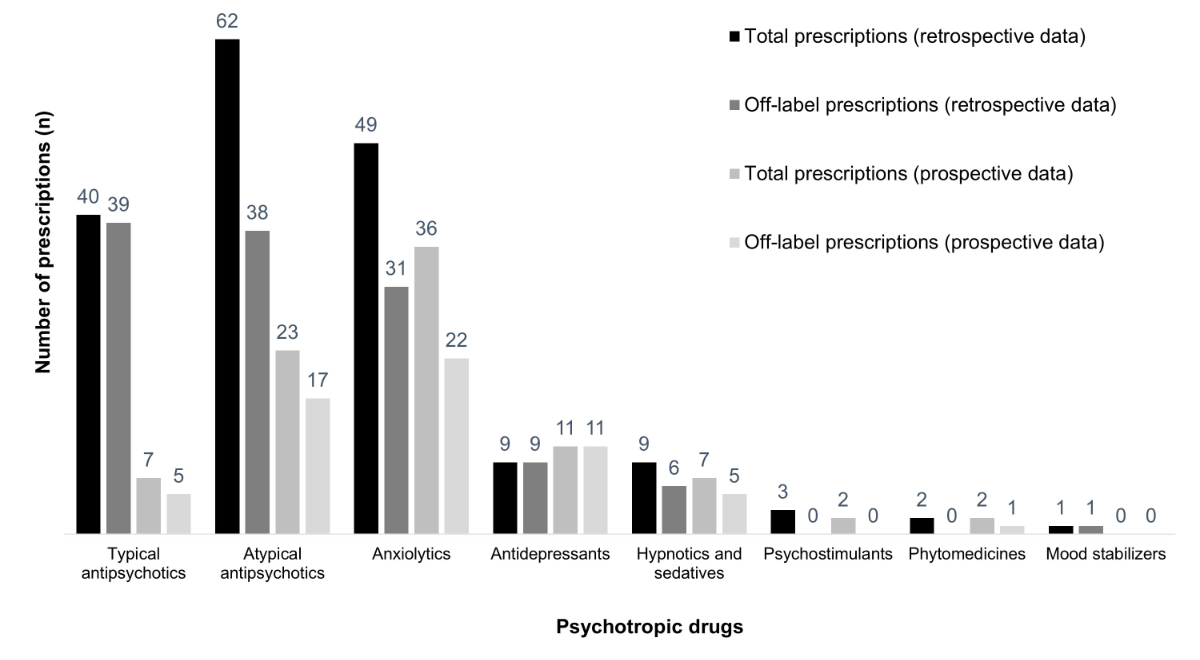
Figure 4 Total and off-label prescriptions of the studies according to Swiss marketing authorisations.
The Swiss off-label prescription rates were numerically higher than those according to French marketing authorisations, but this difference was not statistically significant (table 3). The slightly higher proportion of off-label prescriptions according to Swiss marketing authorisations was driven by off-label use by age (table 2).
Table 3Off-label prescriptions of psychotropic drugs: comparison between Swiss vs French market authorisations (marketing authorisations) and Swiss vs American marketing authorisations.
| National MA (total prescriptions) | Total off label prescriptions/ total prescriptions, n/N (%) | Binomial exact, 95% CI | Two-sample test of proportions (vs Switzerland) p-value | |
| Retrospective data | Switzerland (n = 168) | 117/168 (0.70) | 0.62–0.76] | – |
| France (n = 174) | 106/174 (0.61) | 0.53–0.68 | 0.08 | |
| USA (n = 124) | 70/124 (0.56) | 0.47–0.65 | 0.01 | |
| Prospective data | Switzerland (n = 86) | 61/86 (0.71) | 0.60–0.80 | – |
| France (n = 84) | 56/84 (0.67) | 0.56–0.77 | 0.57 | |
| USA (n = 76) | 37/76 (0.49) | 0.37–0.60 | 0.004 | |
| Pooled data | Switzerland (n = 254) | 178/254 (0.70) | 0.64–0.76 | – |
| France (n = 258) | 162/258 (0.63) | 0.57–0.69 | 0.09 | |
| USA (n = 200) | 107/200 (0.54) | 0.46–0.60 | 0.0005 | |
When assessed according to American marketing authorisations, the prescriptions were mainly off-label by indication (table 2). Overall, the off-label prescriptions according to American marketing authorisations were significantly lower than those according to Swiss marketing authorisations (table 3).
We presented the five most frequently prescribed psychotropic drugs in each cohort study in table 4, the sum of which amounted to more than 50% of total off-label prescriptions. During both studies, levomepromazine, hydroxyzine and aripiprazole were always prescribed with an off-label indication, age or dosage.
Table 4The five psychotropic drugs most frequently off-label prescribed in the retrospective cohort study and the prospective cohort study according to Swiss market authorisations.
| INN | Prescriptions, n | Off-label prescriptions, n (%) | Off-label prescriptions of the drug / total off-label prescriptions within the dataset, n/n (%) | |
| Retrospective data | Levomepromazine | 32 | 32 (100) | 32/168 (23) |
| Hydroxyzine | 25 | 25 (100) | 25/168 (15) | |
| Aripiprazole | 17 | 17 (100) | 17/168 (10) | |
| Quetiapine | 17 | 17 (100) | 17/168 (10) | |
| Lorazepam | 17 | 6 (35) | 6/168 (4) | |
| Total | 108 | 97 (90) | 97/168 (58) | |
| Prospective data | Hydroxyzine | 20 | 20 (100) | 20/86 (23) |
| Aripiprazole | 13 | 13 (100) | 13/86 (15) | |
| Levomepromazine | 5 | 5 (100) | 5/86 (6) | |
| Melatonin | 5 | 5 (100) | 5/86 (6) | |
| Sertraline | 5 | 5 (100) | 5/86 (6) | |
| Total | 48 | 48 (100) | 48/86 (56) | |
INN: international non-proprietary name
Many differences were found in the marketing authorisations for these psychotropic drugs, and their comparison is presented in table 5. This table shows how authorised ages vary dramatically depending on the indication and the selected country.
Table 5The indications and ages approved in Switzerland (CH), France (F) and USA of the five most frequently off-label prescribed psychotropic drugs in our studies.
| Psychotropic drug (route of administration) | Indications | Age (years) | ||
| CH | F | USA | ||
| Levomepromazine (oral) | Acute psychotic episode (hallucinations, maniac episode) | >18 | >18 | NA1 |
| Chronic psychotic condition (schizophrenia, bipolar disorder) | >18 | >18 | NA | |
| Aggression | >18 | >3 | NA | |
| MDD | – | >18 | NA | |
| Levomepromazine (IM) | See all the indications for the oral formulation | NA | >18 | NA |
| Aripiprazole (oral) | Schizophrenia | >13 | >15 | >13 |
| Bipolar disorder | >13 | >13 | >10 | |
| Autistic disorder | – | >18 | >6 | |
| Tourette’s disorder | – | >18 | >6 | |
| MDD | – | – | >18 | |
| Quetiapine (oral) | Schizophrenia | >13 | >18 | >13 |
| Bipolar disorder | >10 | >18 | >10 | |
| Sertraline (oral) | OCD | >6 | >6 | >6 |
| Depression | >18 | >18 | >18 | |
| PD, PTSD, SAD | >18 | >18 | >18 | |
| Hydroxyzine (oral) | Anxiety disorder | >18 | >18 | >22 |
| Pruritus/urticaria | >1 | >3 | >2 | |
| Insomnia | – | >3 | – | |
| Anaesthesia premedication | – | >3 | >2 | |
| Lorazepam (oral) | Anxiety disorder | >12 | >6 | >12 |
| Insomnia | >12 | – | >12 | |
| Anaesthesia premedication | >12 | – | – | |
| Alcohol withdrawal | >12 | >18 | – | |
| Melatonin (oral) | Insomnia | >55 | >55 | NA |
| Insomnia (ASD or SMS patients) | – | >2 | NA | |
ASD: autism spectrum disorder; MDD: major depressive disorder; OCD: obsessive compulsive disorder; PD: panic disorder; PTSD: post-traumatic stress disorder; SAD: seasonal affective disorder; SMS: Smith-Magenis syndrome
2 The manufacturer leaflet does not specify the age of the children, so we used the international definition [41]
The percentages of patients on monotherapy at admission that did not change during the hospitalisation were high in both studies: 39% (n = 28) in the RCS and 51% (n = 19) in the PCS. However, in the RCS, the median number of prescriptions per patient was 2 (1; 3) and the median number of different psychotropic drugs per patient was 1.5 (1; 2). In the PCS, fewer psychotropic drugs were prescribed as the median number of prescriptions per patient was 1 (1; 3), and the median number of different psychotropic drugs per patient was 1 (1; 2). Overall, in both studies 43% of prescriptions were PRN prescriptions. Patients with PRN prescriptions who actually received a psychotropic drug during the hospital stay were 53% (n = 26) in the RCS and 43% (n = 16) in the PCS.
At least one drug-drug interaction of second or third degree was found for 20 patients in the PCS (54%). All of the interactions involving psychotropic drugs were associated with an increased risk of depressing the CNS. There was no fourth degree interaction requiring either a dose or a drug change.
During the PCS, the dosage or psychotropic drug was changed for three patients because of adverse events. The adverse events included paradoxical reactions potentially caused by lorazepam, hyperprolactinaemia attributed to both risperidone and quetiapine treatment (during a treatment switch) and eyebrow tremors caused by sertraline. A blood level measurement was requested for the patient treated with sertraline, confirming the overexposure to this psychotropic drug and leading to a 25% dosage reduction.
Both hyperprolactinaemia and tremors were declared to the regional pharmacovigilance centre. However, for the paradoxical reactions, the physicians decided that the cause-effect relation with lorazepam was not clear enough to be declared.
At the time of our studies, only a limited number of medications had an official marketing authorisation to treat children and adolescents with a psychiatric diagnosis. The off-label prescription rate detected in our RCS based on Swiss marketing authorisations was high, and was similar to the one obtained in our PCS (70% and 71%, respectively). These findings were similar to a previous RCS in Switzerland, which reported an off-label rate of 68% for psychotropic prescriptions in an adolescent psychiatry service [10].
When compared with French marketing authorisations, our results (61% and 63% respectively) were numerically lower but not statistically different from those obtained according to Swiss marketing authorisations. These results were similar to the off-label rate of 68% in a French prospective study in a paediatric setting [15]. The reason we found a lower rate of off-label prescriptions was mainly that French physicians are authorised to prescribe several psychotropic drugs to younger children (table 2).
When compared with American marketing authorisations, we found significantly lower rates of off-label psychotropic drug prescriptions in both studies (56% and 51%). However, in the literature, we did not find a similar study on psychotropic drugs in the USA for paediatric inpatients. The larger difference between American and Swiss marketing authorisations for off-label prescription rates might be partly explained by the absence of Nozinan® (levomepromazine, also known as methotrimeprazine) in the American market (table 5). This drug was widely prescribed in both our studies for the management of agitated patients (with or without aggression), despite the fact that risperidone was supposed to be the first choice in these indications, according to local instructions on management of the paediatric patients in the emergency department [16]. No recommendations were available for levomepromazine, which is authorised only for adults in Switzerland, leading to a 100% off-label use according to the Swiss marketing authorisation (table 4) [17]. Because in France paediatricians are authorised to prescribe levomepromazine to agitated and aggressive children older than 3 years, a larger cohort study should be considered in order to better characterise levomepromazine use in Swiss paediatric wards.
Moreover, the percentage of prescriptions off-label with respect to age was strikingly different for marketing authorisations in Switzerland versus in the USA. This difference was mainly attributed to the broader range of ages and indications that are approved by the latter (table 5), especially with respect to the drug Abilify® (aripiprazole) [18]. This antipsychotic drug was often prescribed in both studies and these prescriptions were always off-label according to the Swiss marketing authorisation (table 4).
In fact, few patients had a defined diagnosis at the time of the two studies; consequently, physicians tended to prescribe psychotropic drugs to address specific symptoms. This phenomenon inevitably led to several off-label prescriptions based on indication. Swiss physicians may use off-label prescriptions in paediatric settings because the marketing authorisation holders of most of psychotropic drugs in the Swiss market do not update and extend paediatric indications, as is done in other countries. Off-label prescriptions in Switzerland are authorised as long as they are based on established studies in the published literature, the patients are informed and the physicians act by due diligence [19, 20]. However, less than 10% of parents were informed about an off-label or unlicensed medication prescription in a Swiss study conducted in a children’s hospital [21] and strong correlations between informing parents and refusal of treatment were found in other countries [22, 23]. Within this context, Swiss law recently changed. Specifically, the manufacturer must submit a Paediatric Investigation Plan (PIP) for every new medication approved for the Swiss market from 2019 onwards, including whether the drug can potentially be of use for the paediatric population [24], with some exceptions [25]. Switzerland has finally become aligned with the requirements already requested in other countries. Over the last decade, the EMA authorised over 260 new medications for paediatric use (new marketing authorisations and new indications), most of which are linked to the requirements of the Paediatric Regulation [26], and are automatically available in France. Over the last 20 years, the FDA authorised more than 600 labelling changes with paediatric-specific information in the USA [27]. These examples show how laws can directly reduce the use of off-label drugs in children and adolescents; we might see the same impact on new Swiss marketing authorisations very soon.
The gaps between the information published in the Swiss marketing authorisations and in the international literature is a common phenomenon in paediatrics [28] and could generate confusion in prescribers; do these restrictions in Swiss marketing authorisations aim to promote safer medication use or do they depend exclusively on the will of the marketing authorisation holders? To fill these gaps, since 2018, SwissPedDose (a Swiss database for dosages of medicinal products in paediatrics) provides dosage recommendations based on a consensus of experts and developed in a standardised harmonisation process throughout Switzerland. So far, 438 dosage recommendations for 128 substances are available [28], addressing paediatric somatic indications. In the future, SwissPedDose could provide dosage recommendations for psychotropic drugs too.
In paediatrics, polypharmacy is defined as the prescription of two or more different drugs [29, 30]. Our research showed that almost 50% of the patients had psychotropic polypharmacy during their hospital stay, and that the most prescribed classes were antipsychotics and anxiolytics. A recent review of the published literature demonstrated that psychotropic polypharmacy is prevalent, documented in 14% to 73% of paediatric patients in an outpatient setting, noticeably depending on the definition of polypharmacy used [31]. The median stay being approximately a week, psychotropic polypharmacy in our studies could be explained by the severe episode leading to hospitalisation. Nevertheless, there were two reassuring factors: first, psychotropic drugs were often prescribed as needed in both of our studies (53% and 43% of psychotropic drug prescriptions). Second, only half of the patients actually received one of the PRN psychotropic drugs. These findings are similar to those published in a French study in a child and adolescent mental health inpatient service [32] and those obtained in an adolescent psychiatry setting in another study [33]. In the literature, some studies claim that PRN medications in psychiatry have limited effect [34–36]. Although our research showed no excessive use of PRN medications, periodic feedback to and awareness sessions of medical staff on this topic could help decrease PRN psychotropic drug administration, as showed in 2012 in a large public-sector psychiatric hospital [37].
Concerning the safety of psychotropic drug prescriptions, we documented no level four interactions requiring changes to medication. Fifty-four percent of patients could potentially have had a pharmacodynamic interaction, increasing the depression of the CNS. This drug-drug interaction is the main pharmacodynamic interaction described for antianxiety drugs [38], defined as anxiolytics and antidepressants drugs. The low prescription rate of the latter in our studies (except for sertraline) could explain the absence of pharmacokinetic drug-drug interactions or QTc prolongation, as described in other studies [38, 39].
Other adverse events rarely occurred during the periods of our research, but when they did occur, they were severe. Tremors were an easy adverse event to detect, occurring in one patient treated with sertraline for an anxiety disorder, as they can be related to an overdose of selective serotonin reuptake inhibitor antidepressants [40], which was confirmed by measuring the blood level of sertraline. However, hyperprolactinaemia, which occurred in another patient owing to atypical antipsychotic prescription, could have gone undetected for months. This adverse event is not always symptomatic, with international guidelines on monitoring prolactin at baseline and during atypical antipsychotic treatment being subject to controversy [11, 12]. Swiss experts should provide national guidelines for monitoring paediatric patients with antipsychotics treatments, especially as they were the most prescribed psychotropic drugs in our studies.
This study had several limitations. First, in the PCS, the most severely affected patients could be transferred to a psychiatric hospital on the same day as admission; consequently, we could not obtain parental authorisation to include them. For both studies, there was a bias regarding the period of patient inclusion (fig. 1). For instance, the start and end of a school year could influence their psychiatric status so the inclusion period should have been extended over a whole year for both components. Finally, the studies were monocentric and the sampled populations were limited.
To the best of our knowledge, this study provides the first assessment of the clinical and off-label use of psychotropic drugs in a general paediatric setting in Switzerland.
Our results showed a high proportion of off-label prescriptions in a hospital setting. Compared with Swiss marketing authorisations, American marketing authorisations had broader range of ages and indications, leading to a significantly lower rate of off-label prescriptions. French marketing authorisations were similar to Swiss marketing authorisations, but still less restrictive, especially in terms of approved ages for paediatric patients.
It is difficult to justify these differences. Harmonisation of international market authorisations, an update in the Swiss law or an addition of psychotropic drugs in the national database of dosage recommendations SwissPedDose could be a step forward to improved and evidence-based use of psychotropic drugs in children and adolescents.
We thank Dr Mohamed Faouzi for the help with the statistical analysis.
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by A. Feka. The first draft of the manuscript was written by A. Feka and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.
No specific funding was received to support the conduct of these studies.
1. World Health Organization . Adolescent mental health in the European Region [Internet]. [cited 2021 Mar 15 ]. Available from: https://www.euro.who.int/en/health-topics/noncommunicable-diseases/mental-health/data-and-resources/fact-sheet-adolescent-mental-health-in-the-who-european-region
2. Centers for Disease Control and Prevention . Data and Statistics on Children's Mental Health [Internet]. [cited 2021 Mar 17 ]. Available from: https://www.cdc.gov/childrensmentalhealth/data.html
3. Elbe D , Bezchlinbnyk-Butler KZ , Virani AS , Procyshyn RM . Clinical handbook for psychotropic drugs for children and adolescents. 3rd ed. Göttingen: Hogrefe; 2014.
4. Caraci F , Enna SJ , Zohar J , Racagni G , Zalsman G , van den Brink W , et al. A new nomenclature for classifying psychotropic drugs. Br J Clin Pharmacol. 2017 Aug;83(8):1614–6. https://doi.org/10.1111/bcp.13302
5. Hauser M , Correll CU . The significance of at-risk or prodromal symptoms for bipolar I disorder in children and adolescents. Can J Psychiatry. 2013 Jan;58(1):22–31. https://doi.org/10.1177/070674371305800106
6. Kovacs M , Lopez-Duran N . Prodromal symptoms and atypical affectivity as predictors of major depression in juveniles: implications for prevention. J Child Psychol Psychiatry. 2010 Apr;51(4):472–96. https://doi.org/10.1111/j.1469-7610.2010.02230.x| https://doi.org/10.1111/j.1469-7610.2010.02230.x
7. Swissmedic. Pédiatrie: les enfants et les médicaments [Internet]. [cited 2020 Mar 27]. Available from: https://www.swissmedic.ch/swissmedic/fr/home/medicaments-a-usage-humain/besondere-arzneimittelgruppen--ham-/pediatrie.html
8. European Medicines Agency . Evidence of harm from off-label or unlicensed medicines in children. EMEA/126327/2004; (October, 2004). Available from: https://www.ema.europa.eu/en/documents/other/evidence-harm-label-unlicensed-medicines-children_en.pdf
9. Di Paolo ER , Stoetter H , Cotting J , Frey P , Gehri M , Beck-Popovic M , et al. Unlicensed and off-label drug use in a Swiss paediatric university hospital. Swiss Med Wkly. 2006 Apr;136(13-14):218–22.
10. Ansermot N , Jordanov V , Smogur M , Holzer L , Eap CB . Psychotropic drug prescription in adolescents: a retrospective study in a swiss psychiatric university hospital. J Child Adolesc Psychopharmacol. 2018 Apr;28(3):192–204. https://doi.org/10.1089/cap.2017.0054
11. American Academy of Child and Adolescent Psychiatry . Practice parameter for the use of atypical antipsychotic medications in children and adolescents [Internet]. [cited 2020 Mar 23]. Available from: https://www.aacap.org/App_Themes/AACAP/docs/practice_parameters/Atypical_antipsychotic_Medications_Web.pdf
12. Pringsheim T , Panagiotopoulos C , Davidson J , Ho J ; CAMESA guideline group . Evidence-based recommendations for monitoring safety of second generation antipsychotics in children and youth. J Can Acad Child Adolesc Psychiatry. 2011 Aug;20(3):218–33. https://doi.org/10.1093/pch/16.9.581
13. Pisano S , Catone G , Veltri S , Lanzara V , Pozzi M , Clementi E , et al. Update on the safety of second generation antipsychotics in youths: a call for collaboration among paediatricians and child psychiatrists. Ital J Pediatr. 2016 May;42(1):51. https://doi.org/10.1186/s13052-016-0259-2
14. Swissmedic. Federal Act on Research involving Human Beings. (September 30, 2011). Available from: https://www.admin.ch/opc/en/classified-compilation/20061313/202001010000/810.30.pdf
15. Winterfeld U , Le Heuzey MF , Acquaviva E , Mouren MC , Brion F , Bourdon O . [Off-label use of psychotropic medications in pediatric wards: a prospective study]. Arch Pediatr. 2009 Sep;16(9):1252–60. https://doi.org/10.1016/j.arcped.2009.06.012
16. Gehri M , Laubscher B , Di Paolo E , Roth-Kleiner M , Joseph JM , Mazouni SM . Vade-Mecum de Pédiatrie. 4th ed. Lausanne: Editions BabyGuide; 2014.
17. Sanofi-Aventis Company . Summary of Products Characteristics [Internet]. [cited 2020 Mar 23]. Available from: http://www.swissmedicinfo.ch/
18. Otsuka Pharmaceutical Company . Summary of Products Characteristics [Internet]. [cited 2020 Mar 23]. Available from: https://www.swissmedicinfo.ch/
19. Conseil Fédéral . Essais thérapeutiques. (December 11, 2015). Available from: https://www.parlament.ch/centers/eparl/curia/2011/20113001/bericht%201.1%20br%20f.pdf
20. Association des Pharmaciens Cantonaux . Recommandations de l'association des pharmaciens cantonaux concernant l'off-label use de médicaments. (June 1, 2016). Available from: https://www.ge.ch/document/label-use-medicaments/telecharger
21. Zaug C , Behringer J , Walther M , Egger R , Köhler H . P 126: Unlicensed and off-label drug prescription at discharge from a Swiss children's hospital. Annual meeting of the Swiss Society of Paediatrics. Lucerne, Switzerland; 2012.
22. Saiyed MM , Prajapati A , Shah G . Parents’ awareness and perspective on off-label medicines use in children. J Pharmacol Pharmacother. 2015 Apr-Jun;6(2):88–91. https://doi.org/10.4103/0976-500X.155485
23. Tsujii N , Saito T , Izumoto Y , Usami M , Okada T , Negoro H , et al. Experiences with Patient Refusal of Off-Label Prescribing of Psychotropic Medications to Children and Adolescents in Japan. J Child Adolesc Psychopharmacol. 2016 Sep;26(7):642–5. https://doi.org/10.1089/cap.2014.0131
24. Swissmedic. Nouvelles incitations au développement de médicaments pédiatriques dès le 1er janvier 2019 [Internet]. [cited 2020 Mar 27]. Available from: https://www.ejpd.admin.ch/content/ejpd/fr/home/aktuell/news/2018/2018-09-21.html
25. Swissmedic. Plan d'investigation pédiatrique. (October 25, 2018). Available from: https://www.swissmedic.ch/dam/swissmedic/fr/dokumente/stab/veranstaltung/hmg/paediatrischespruefkonzept.pdf.download.pdf/paediatrischespruefkonzept.pdf
26. European Commission . State of Paediatric Medicines in the EU - 10 years of the EU Paediatric Regulation. (2017). Available from: https://ec.europa.eu/health/sites/health/files/files/paediatrics/docs/2017_childrensmedicines_report_en.pdf
27. Califf RM . Best pharmaceuticals for children act and pediatric research equity act. (March 23, 2020). Available from: https://www.fda.gov/media/99184/download
28. SwissPedDose. Swiss Database for Dosing Medicinal Products in Pediatrics (SwissPedDose) [Internet]. [cited 2020 Mar 27]. Available from: https://swisspeddose.ch
29. Bakaki PM , Horace A , Dawson N , Winterstein A , Waldron J , Staley J , et al. Defining pediatric polypharmacy: A scoping review. PLoS One. 2018 Nov;13(11):e0208047. https://doi.org/10.1371/journal.pone.0208047
30. Zonfrillo MR , Penn JV , Leonard HL . Pediatric psychotropic polypharmacy. Psychiatry (Edgmont). 2005 Aug;2(8):14–9.
31. Chen H , Patel A , Sherer J , Aparasu R . The definition and prevalence of pediatric psychotropic polypharmacy. Psychiatr Serv. 2011 Dec;62(12):1450–5. https://doi.org/10.1176/appi.ps.000642011
32. Winterfeld U , Le Heuzey MF , Acquaviva E , Mouren MC , Brion F , Bourdon O . The use of prn medication in a child and adolescent mental health inpatient service in France. Int J Psychiatry Clin Pract. 2009;13(4):253–8. https://doi.org/10.3109/13651500902849987
33. Bernard P , Littlejohn R . The use of “as required” medication on an adolescent psychiatric Unit. Clin Child Psychol Psychiatry. 2000;5(2):258–66. https://doi.org/10.1177/1359104500005002011
34. Yoshida K , Suzuki T , Uchida H , Mimura M . Absence of evidence that the pro re nata regimen confers benefit: a review of the studies. Int Clin Psychopharmacol. 2013 Sep;28(5):228–37. https://doi.org/10.1097/yic.0b013e328362db99 https://doi.org/10.1097/YIC.0b013e328362db99
35. Baker M , Carlson GA . What do we really know about PRN use in agitated children with mental health conditions: a clinical review. Evid Based Ment Health. 2018 Nov;21(4):166–70. https://doi.org/10.1136/ebmental-2018-300039
36. Srivastava A . Limited evidence for the effectiveness of p.r.n. Medications among psychiatric inpatients. J Psychiatr Pract. 2009 May;15(3):193–201. https://doi.org/10.1097/01.pra.0000351879.52883.10
37. Friedman R , Nurenberg JR , Birnbaum S , Schleifer SJ . Using structured clinical feedback to encourage alternatives to use of “P.R.N.” medication in a state psychiatric hospital. J Psychiatr Pract. 2012 Sep;18(5):381–7. https://doi.org/10.1097/01.pra.0000419823.69914.c7
38. Muscatello MR , Spina E , Bandelow B , Baldwin DS . Clinically relevant drug interactions in anxiety disorders. Hum Psychopharmacol. 2012 May;27(3):239–53. Available from: http://10.0.3.234/hup.2217 https://doi.org/10.1002/hup.2217
39. Yalçın N , Özdemir N , Çak Esen HT , Çengel Kültür SE , Demirkan K . Potential drug-drug interactions with psychotropic drugs in paediatric inpatients: A cross-sectional study. Int J Clin Pract. 2021 Jun;75(6):e14107. https://doi.org/10.1111/ijcp.14107
40. Barbey JT , Roose SP . SSRI safety in overdose. J Clin Psychiatry. 1998;59 Suppl 15:42–8.
41. World Health Organization . Paediatric Age Categories to be Used in Differentiating Between Listing on a Model Essential Medicines List for Children. (April 20, 2007). Available from: https://pdf4pro.com/amp/view/position-paediatric-age-categories-to-be-used-in-16d301.html