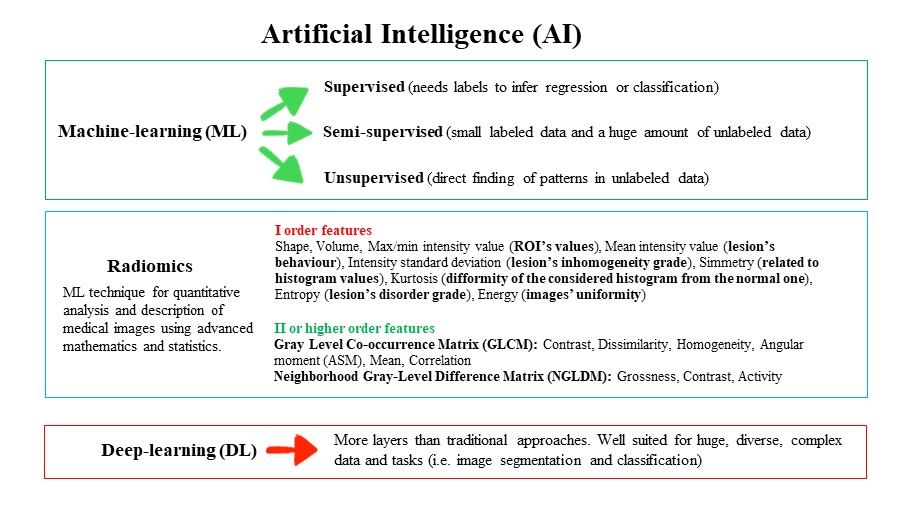
Figure 1 Schematic representation of machine and deep learning with the main I and II order radiomics features.
DOI: https://doi.org/10.4414/SMW.2022.w30123
In medical imaging, artificial intelligence (AI) is defined as the ability of a system to properly interpret (and learn from) external data, acquiring knowledge to achieve specific goals and tasks through flexible adaptation [1]. Most of the AI systems are analytical, being classified as machine- or deep-learning techniques. Machine learning is focused on studying algorithms able to learn and improve, creating models based on big datasets, and applying them to the unseen data through semi-supervised, supervised or unsupervised methods. Machine learning includes deep-learning techniques, with neural networks organised in multiple, progressive and subsequent related layers. Deep-learning approaches simultaneously learn relevant features and prediction models from input images without the need for the so-called “feature engineering” using more layers than traditional approaches, and are well suited for large, diverse, complex data and tasks (e.g, segmentation, classification). Radiomics is a machine-learning technique that aims to provide quantitative characteristics from different biomedical images that cannot be assessed by the human eye. Radiomics assumes that the smallest constituents of images may include “features” related to a patient's outcome and response to therapy, reflecting the pathophysiological process and thus potentially supporting medical decisions. We can obtain features of several orders and it is interesting how each of these may be related to a precise meaning, as we can see in I order features containing information on shape and statistics deriving from the histogram describing the distribution of grey values in the selected lesion or from II or higher orders features containing information about the relationships between adjacent pixels. In figure 1 a schematic representation of machine and deep learning is displayed, with description of the main I and II order radiomics features [1].

Figure 1 Schematic representation of machine and deep learning with the main I and II order radiomics features.
Recently, the use of AI has been extended to cardiovascular imaging techniques in order to identify novel markers able to yield improved diagnostic performance and prognostic value [2]. Among the different radiological procedures currently used in the evaluation of patients with cardiac diseases (fig. 2), positron emission tomography (PET) has attracted major interest in recent years because of its superior diagnostic performance and ability to quantify specific metabolic parameters. Also, the European Association of Nuclear Medicine (EANM) recently published a position paper on the use of AI in cardiovascular nuclear imaging [3].
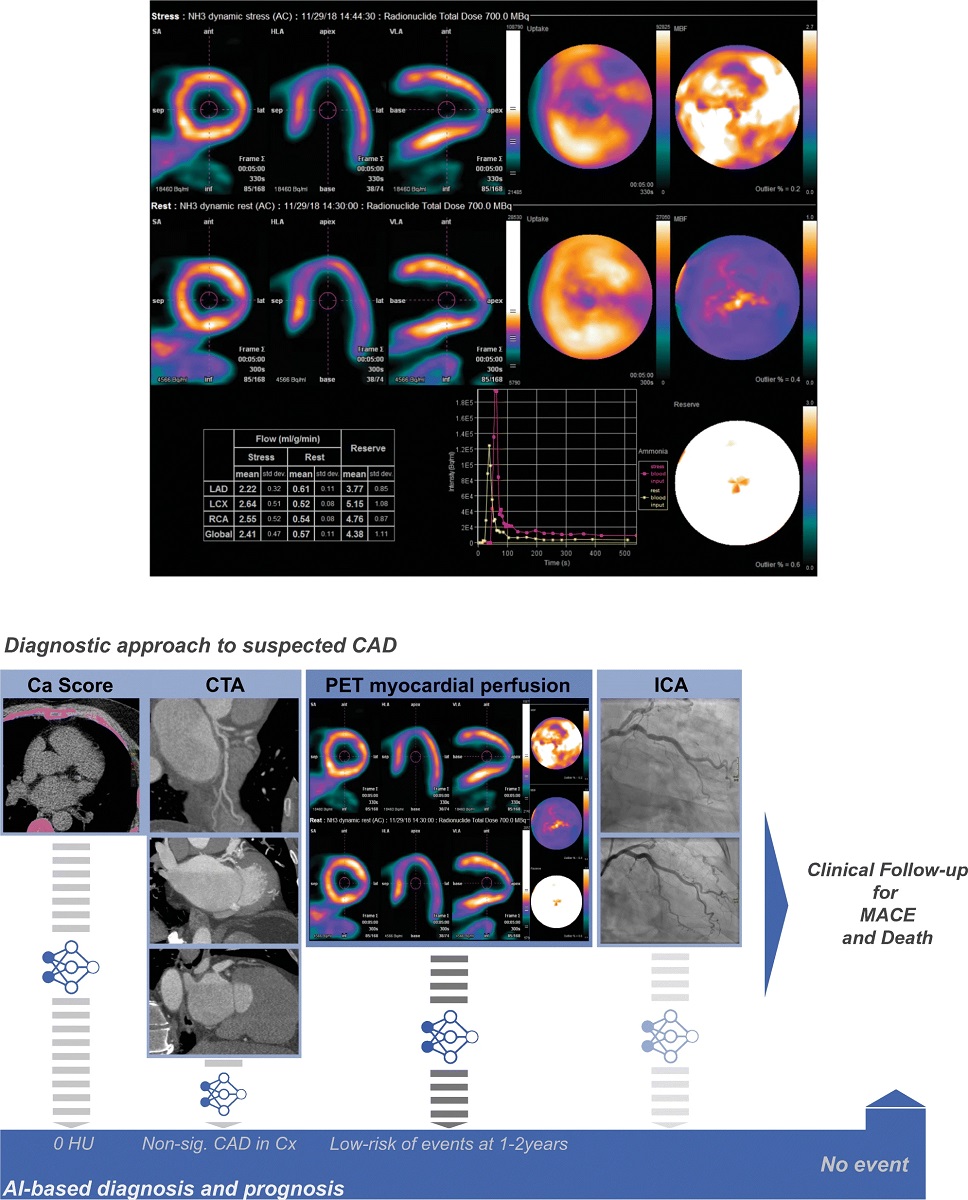
Figure 2 Potential roles of AI in cardiac imaging. Depiction of an exemplary PET/CT case. Male with non-significant atherosclerosis in the left circumflex artery and overall preserved perfusion reserve in which deep learning-based processing of PET myocardial blood flow polar maps automatically suggested a low risk of events with a 1–2-year horizon. Transparency on the workflows represents AI implementations that were not used in this particular example, namely automatic calcium score quantification, CTA (FFR) analysis and ICA analysis. AI: artificial intelligence; Ca: calcium; CAD: coronary artery disease; CTA: computed tomography angiography; ICA: invasive coronary angiography: MACE: major adverse cardiovascular events; PET: positron emission tomography. Reprinted under a Creative Commons Attribution 4.0 International License from [3]. No changes were made.
Despite the potential benefits of implementing AI in clinical practice (e.g., better clinical decisions, outcome prediction or prognosis evaluation), some concerns have been raised, mainly regarding the balance between AI and physicians’ supervision, as well as ethical and legal issues.
To date, AI in cardiac PET/computed tomography (PET/CT) imaging has shown utility in three main areas of interest: automation of image detection and segmentation, identification of patients with obstructive coronary artery disease (CAD) and risk assessment [4].
In the present review, we aim to summarise the current state of the art regarding AI models applied to cardiac PET images. In figure 3 we illustrate the main processes of AI algorithm used for cardiovascular imaging based on machine learning (A) and deep laerning (B).
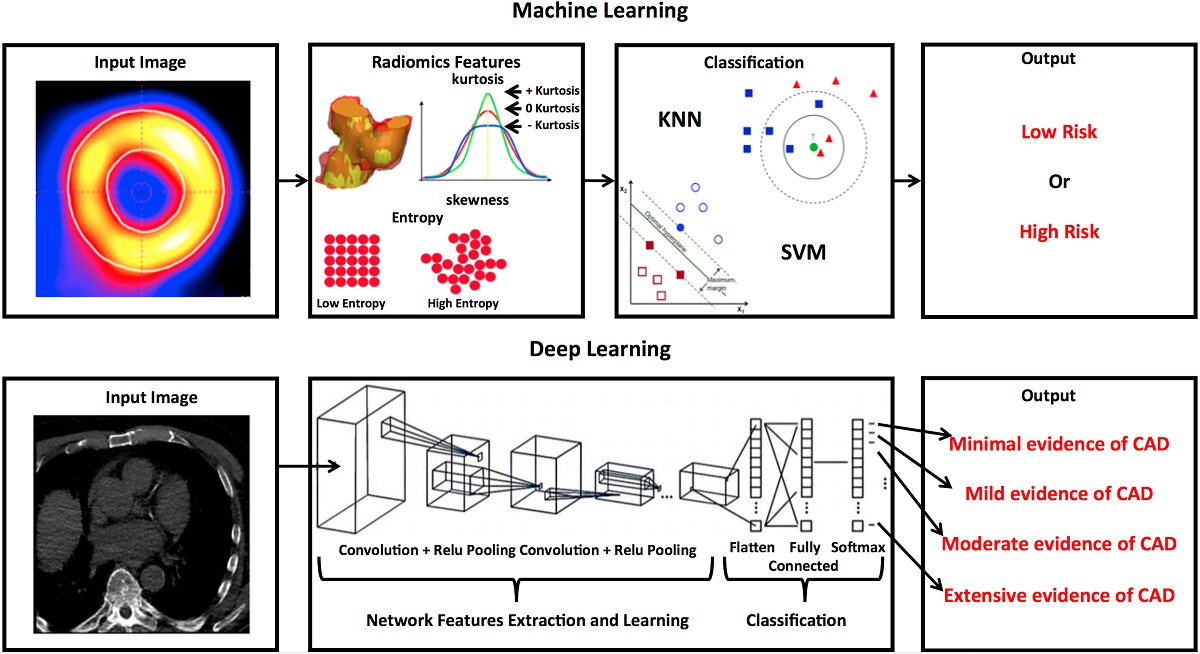
Figure 3 Exemplary summary of the main articiaL intelligence algorithms (A) machine-learning and (B) deep-learning. SVM: support vector machines; KNN: k-Nearest Neighbours, a supervised algorithm; CAD: coronary artery disease.
A comprehensive literature search strategy using PubMed database was used to look for articles on the role of AI in cardiovascular PET imaging. The string used for the search included a combination of the terms: ‘artificial intelligence” or “machine learning” or “neural network” or “deep learning” and “cardiac PET” or “cardiac positron emission tomography”. The search was updated up to August 2021, including only original papers published in English. The references of the retrieved articles were also checked so as not to miss important clinical studies. Review articles, articles not in the field of interest and commentaries were excluded. Papers on future perspectives in the field and experimental data were also considered eligible. Three researchers (CEP, RL and FC) independently reviewed the titles and the abstracts of the retrieved literature, selecting relevant articles according to the inclusion criteria mentioned above. Disagreements were resolved in a consensus meeting.
The results of the main PET studies described are displayed in table 1.
Table 1Main clinical AI applications in PET images.
| First author | Year | Tracer | Clinical scenario | Conclusion and relevance |
| Juarez-Orozco [5] | 2020 | [13N]ammonia | Identification of patients with myocardial ischaemia or high risk of MACE | ML is feasible and applicable in quantitative PET imaging for the identification of patients with an elevated risk of MACE who will present myocardial ischaemia |
| Dey D [6] | 2015 | [13N]-ammonia | Relationship of quantitative plaque features from CCTA and coronary vascular dysfunction by impaired MFR by 13N-ammonia PET | Combining data of quantitative stenosis and plaque burden as assessed by CT angiography significantly improves identification of downstream regional vascular dysfunction |
| Wang F[7] | 2020 | [13N]-ammonia and [18F]-FDG | Evaluation of the diagnostic value of both PET myocardial perfusion and metabolic imaging for vascular stenosis in patients with suspected obstructive CAD | Perfusion combined with metabolism as well as a multivariate model (SCR, MBF, and MIS) combined with a support vector machine yields improved accuracy in the diagnosis of obstructive CAD compared with perfusion information only |
| Wang X[8] | 2020 | [82Rb] | Investigation of a patch-based ANN fusion approach that integrates information from the ML and the post-smoothed ML reconstruction in improving MPI quality | The ANN fusion technique significantly improved the defect detectability of both non-transmural and transmural defects. Moreover, compared with the post-smoothed ML reconstruction, the ANN fusion improved the lesion-to-background ratio while reducing noise |
| Shi L [9] | 2021 | [82Rb] | Evaluation of an automatic (DeepMC) method for dynamic cardiac PET in improving motion correction | DeepMC showed superior performance compared with conventional registration-based methods in terms of motion estimation and MBF quantification accuracy |
| Togo R[10] | 2019 | [18F]-FDG | Determination of whether DCNN-based features can depict the difference between CS and non-CS using polar maps | The DCNN-based high-level features may be more effective than low-level features used in conventional quantitative analysis methods for CS classification |
| Ladefoged CN [11] | 2021 | [18F]-FDG | Investigate a common DL network for noise reduction in low-dose PET images and its accuracy to determine cardiac viability in patients with CAD | A significant dose reduction of 1–10% can be achieved for [18F]FDG PET/CT in the setting of cardiac viability testing, without significant loss of diagnostic accuracy when using a common DL network model for noise reduction |
| Kolossváry M [12] | 2019 | [18F]-NaF | Identification of invasive and radionuclide imaging markers of coronary plaque vulnerability using radiomics analysis of CCTA | CCTA radiomics identified invasive and radionuclide imaging markers of plaque vulnerability with good to excellent diagnostic accuracy, outperforming conventional quantitative and qualitative high-risk plaque features |
| Kwiecinski J [13] | 2021 | [18F]-NaF | Evaluation of a ML model for the prediction of myocardial infarction in patients with stable coronary disease undergoing [18F]NaF PET/CCTA | Both [18F]NaF uptake and quantitative plaque analysis measures are additive and strong predictors of outcome in patients with established CAD. Optimal risk stratification can be achieved by combining clinical data with these approaches in a ML model. |
| Santarelli MF [14] | 2021 | [18F]-florbetaben | Investigation of the potential of DL tools for identifying CA from early PET images | A deep convolutional neural network (CAclassNet) model seems very promising as an aid for the clinician in the diagnosis of CA from cardiac 18F-florbetaben PET images |
MACE: major adverse cardiovascular events; ML: machine learning; PET: positron emission tomography; CCTA: coronary computed tomography angiography; MFR: myocardial flow reserve; CAD: coronary artery disease; SCR: scar degree; MBF: myocardial blood flow; MIS: metabolism-perfusion mismatch; ANN: artificial neural network; MPI: myocardial perfusion imaging; CAC: coronary artery calcium; CVD: cardiovascular disease; CTAC: computed tomography attenuation correction; CSCT: calcium scoring CT; DCNN: deep convolutional neural network; CS: cardiac sarcoidosis; CA: cardiac amyloidosis; DL: deep learning; DeepMC: deep learning-based motion correction; 13NH: (Nitrogen-13)- ammonia; Rb: Rubidium; FDG: Fluorodeoxyglucose; NaF: Sodium Fluoride
Research in the field of myocardial perfusion imaging has focused on AI applications based on the integration of clinical and radiological data to identify patients at increased risk of cardiac events.
A first attempt to train a machine-laerning model was made by Juarez-Orozco et al. using nitrogen-13 [13N] Ammonia PET (this tracer was chosen in view of its excellent diagnostic performance in CAD). The authors investigated the prediction of myocardial ischaemia and the occurrence of major adverse cardiac events (MACE) based on global myocardial flow reserve <2.0. Sixteen variables were extracted by machine learning for model creation and showed that resting heart rate, systolic blood pressure, stress left ventricular ejection fraction and age were the most predictive variables within the analysis, consistent with similar reports based on standard statistics. Data were also tested by adding risk model variables for both PET-defined labels, as provided by the guidelines of the European Society of Cardiology (ESC). This resulted in a further improvement in diagnostic performance [5].
Similar results were reported by Dey et al. [6], who also implemented data from computed tomography-based coronary angiography (CCTA) in their machine-learning model. The aim of their study was to investigate the incremental value of quantitative features of coronary plaques on CCTA over myocardial flow reserve as measured with PET. The authors used ensemble boosting, which is expected to yield high performance of classification by combining individual classifiers, each of which has iteratively adjusted weights. As main results the authors observed a significant correlation between coronary artery non-calcified plaque burden in CCTA and impaired myocardial flow reserve of the corresponding vascular territory.
In a more recent retrospective study, 88 patients with suspected obstructive CAD were referred for [13N]-Ammonia myocardial perfusion PET/CT and [18F]-fluorodeoxyglucose myocardial metabolic PET/CT (18F-FDG PET/CT). The semi-quantitative indicator summed rest score (SRS) and five quantitative indicators, namely perfusion defect extent, total perfusion deficit, myocardial blood flow, scar degree and metabolism-perfusion mismatch were combined with seven ML algorithms to derive the optimum combination model and classification method. The authors concluded that perfusion combined with metabolism as well as a multivariate model (scar degree, myocardial blood flow and metabolism-perfusion mismatch) combined with a support vector machine reached better accuracy (area under the curve [AUC] 0.897) in the diagnosis of obstructive CAD [7].
Rubidium-82 (82Rb) is a potassium analogue widely used as a perfusion tracer in myocardial PET in those centres where a cyclotron is not available. 82Rb is eluted from a generator and is used mostlywith pharmacological stress due to its short half-life (t½ 78 seconds) requiring that the patient be scanned simultaneously with or shortly after the tracer injection. For 82Rb, there are also attempts, reported in the literature, to improve the diagnostic and prognostic value by adding additional variables extracted by AI algorithms. A new patch-based artificial neural network (ANN) fusion approach that integrates information from the machine laerning and the post-smoothed machine laerning reconstruction was proposed by Wang et al. [8] to improve myocardial perfusion PET imaging quality. Using an XCAT phantom, the authors simulated three PET imaging cases, one with normal perfusion and the other two with non-transmural and transmural regionally reduced perfusion in the left ventricle, respectively. The ANN fusion technique significantly improved the detectability of both the non-transmural and transmural defect. Moreover, the authors concluded that, compared with the post-smoothed ML reconstruction, the ANN fusion improved the lesion-to-background ratio while reducing noise, indicating its potential clinical application in PET imaging.
Besides the important role of perfusion imaging in 82Rb PET/CT, the simultaneous acquisition of CT with the calculation of the calcium score is important for the prognostic assessment in CAD. Išgum et al. [15] and Dekker et al. [16] showed that a calcium score automatically measured during 82Rb PET/CT can increase the detection rate of obstructive CAD in patients without any history of revascularisation. Also, the implementation of automatically calculated calcium score resulted in an increase of the diagnostic accuracy by 4% in CAD. [16].
Patient motion is a potential drawback in 82Rb PET imaging, as it may impair the absolute myocardial blood flow quantification. To improve the motion correction, Shi et al. [9] developed an automatic DL-based motion correction (DeepMC) method for dynamic cardiac PET featuring 1 million samples based on 65 patient scans in training and 600 samples based on 20 patient scans. The authors reported superior performance of their method over conventional registration-based methods with regard to myocardial blood flow quantification. However, the low number of patients and the lack of a realistic patient motion was a limitation of the study, hence the real clinical value still has to be confirmed in future studies.
Another possible AI application in cardiovascular molecular imaging is [18F]-FDG PET/CT, an imaging modality assessing myocardial glucose metabolism both in viability studies and for the imaging of infection and inflammation.
Specifically, the assessment of cardiac involvement in systemic sarcoidosis relies on [18F]-FDG cardiac PET/CT, which proved to be reliable both in the diagnosis and in evaluation of the response to therapy [17]. However, the interpretation of [18F]-FDG PET/CT images relies mostly on visual analysis and thus, a quantitative method based on a deep convolutional neural network would be warranted.
A pre-trained Inception-v3 network model, which is one of GoogLeNet models trained for object recognition tasks, was tested in this scenario, extracting and selecting features from polar maps obtained from PET/CT data. Such features were then classified using support vector machines as “cardiac sarcoidosis” or “non-cardiac sarcoidosis”, yielding sensitivity and specificity of 83.9% and 87.0%, respectively [10].
Besides application in clinical practice, the use of AI in [18F]-FDG PET/CT is also expected to reduce technical issues such as uncertainties in image reconstruction and administered radioactivity.
Widely adopted reconstruction algorithms, such as ordered subset expectation maximization [18] may lead to inaccurate results in the case of poor counts statistics. Therefore, a method able to compensate for the loss of image quality may be of great importance, and may also reduce the radioactivity dose. A stacked sparse auto-encoder-based reconstruction framework for dynamic PET imaging was proposed, wherein the dynamic reconstruction problem was formulated in a DL representation. The encoding layers extracted the prototype features, such as edges, so that the reconstructed results were obtained through a combination of those features [19]. With these advantages, a significant dose reduction of 1–10% can be achieved for [18F]-FDG PET/CT in the setting of cardiac viability testing, without significant loss of diagnostic accuracy, when using a common deep-learning network model for noise reduction [11].
[18F-]sodium fluoride ([18F]-NaF) PET coupled with CT-coronary angiography (CCTA)-based quantitative plaque analysis represents a valid tool to assess risk in patients with CAD [20].
Augmented-PET noninvasive techniques might provide the opportunity to identify vulnerable plaques and patients in broad populations. However, today few articles with specific AI applications on PET images are available. The study of Kolossváry et al. [12], despite a low number of patients (n = 25), aimed to assess whether radiomics analysis outperforms conventional assessment of CCTA images to identify invasive and [18F]-Na-F PET radionuclide imaging markers of plaque vulnerability. CCTA radiomics identified invasive and radionuclide imaging markers of plaque vulnerability with good to excellent diagnostic accuracy, significantly outperforming conventional quantitative and qualitative high-risk plaque features.
Kwiecinski et al. [13] developed a machine-learning model for prediction of the future risk of myocardial infarction in patients with stable CAD undergoing [18F]-NaF PET/CCTA. The machine learning included clinical data, CT quantitative plaque analysis measures and [18F]-NaF PET findings of 293 subjects. In their model, quantitative plaque analysis-based outperformed clinical data, but even more interestingly, if all available data were included in the model, a substantial diagnostic improvement was achieved (c-statistic 0.85, 95% confidence interval 0.79–0.91). As such, the combination of various parameters is likely to yield the best clinical value in predicting myocardial infarction, similarly to what had been already reported in the literature in the usual analyses not featuring AI.
In a sample of 47 subjects (13 with transthyretin-related amyloidosis – ATTR – cardiac amyloidosis, 15 patients with immunoglobulin light-chain – AL – amyloidosis, and 19 control patients), Santarelli et al. [14] investigated the potential of deep-learning tools for identifying cardiac amyloidosis from PET images acquired as early as 15 minutes after [18F]-florbetaben tracer injection. They designed a deep convolutional neural network (CAclassNet) consisting of five 2D convolutional layers, three fully connected layers and a final classifier returning scores for each of the subgroups. Applyication of this neural network resulted in good diagnostic performance, with an accuracy of 93.6% for the diagnosis of ATTR amyloidosis and 97.2% for the identification of AL amyloidosis. While their results showed promise, the low number of subjects involved requires further investigation to assess the real clinical potential.
Artificial intelligence in medical imaging has gained increasing interest in the recent years and new applications in cardiovascular imaging, including PET, have been tested. However, although many optimistic announcements were made, many of the predicted advantages are still far from coming to fruition in clinical practice, partly due to the large amount of unpredictability, raising doubts on the practicability even in the mid-term [21].
The aim of AI algorithms would be to perform tasks that are hardly feasible for humans given the high amount of data involved. Alternatively, AI may simply facilitate physicians’ tasks in order to save time, thus streamlining patients' care. As a matter of fact, in recent years, user-friendly AI software packages have been made available and have been introduced in the medical research environment [22]. In the field of PET imaging, there is also great interest in new applications of AI, although the number of possible applications is to date very limited.
From the analysis of the literature, AI in cardiovascular PET imaging has been mainly tested in overcoming technical issues such as reconstruction and dose reduction, and for the diagnostic and prognostic assessment of patients with CAD and cardiac sarcoidosis. Therefore, on one hand there is enough room for further applications, but on the other, there are growing uncertainties as to whether this approach can really provide advantages over the current methods [4]. In fact, some major drawbacks should be mentioned and need to be overcome to secure the role of AI in cardiac PET imaging. (1) The appropriate balance between fully autonomous AI and physician supervision should be clarified; what can a machine do without the need for human supervision? Or in other words, how much can a doctor be confident in the results provided by a fully automated method? (2) Ethical aspects should be considered, as recently reported [23]. (3) Legal aspects should also be a matter of concern, especially with regard to privacy and the lack of firm and universal agreement on the legal basis for the use of data. Not less important, the risk of potential threats from hacker attacks both during training and use of AI algorithms should be considered.
Despite these major concerns, the use of AI may improve patients' care in many different scenarios, starting from the semi-automatisation of a part of medical work from the technical aspect of image preparation, through image interpretation, calculation of additional factors based on data obtained during scanning, to prognosis prediction and risk-group selection [1]. In our opinion, the most important advantage empowered by AI in cardiac PET imaging may be the invaluable chance to expand on the prognostic value of myocardial perfusion imaging, which has already secured a pivotal role of these techniques in the management of patients with CAD. This may lead to better clinical decisions, thus optimising the therapeutic approach in selected patients.
In this sense, deep learning as a building block of AI-based support systems may have clinical value, not limited to PET imaging but translatable to other modalities, on the basis of the encouraging primary results in quantitative myocardial perfusion PET and in the identification of patients who developed MACE [4].
What should we expect then? The most conceivable scenario seems to be a gradual process, through the creation of big datasets, in which AI tools are progressively integrated into clinical practice. At the end of the path, medical doctors will probably play a role as supervisors of automatically generated data, in view of their capability to integrate data based on their clinical experience. Once this technology becomes standard in clinical routine, guidelines will need to be developed in order to standardise broad applications of AI in medicine. Consistent with this hypothesis, AI would be a helpful tool to systematise the interpretation process, which will be, however, supervised by medical imaging doctors by integrating information coming from automatic and visual assessment.
The use of AI in cardiac PET imaging is to date limited, although first, important results have been shown. To date, the small number of studies in the literature and thoughtful drawbacks limit the wide implementation of such methods in clinical practice. Consideration of these limitations is a prerequisite to providing an efficient approach to improve AI research, facilitating the interpretation of medical imaging.
Data are available for bona fide researchers who request it from the authors.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed..
None.
1. Laudicella R , Comelli A , Stefano A , Szostek M , Crocè L , Vento A , et al. Artificial Neural Networks in Cardiovascular Diseases and its Potential for Clinical Application in Molecular Imaging. Curr Radiopharm. 2021;14(3):209–19. https://doi.org/10.2174/1874471013666200621191259
2. Dey D , Slomka PJ , Leeson P , Comaniciu D , Shrestha S , Sengupta PP , et al. Artificial Intelligence in Cardiovascular Imaging: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019 Mar;73(11):1317–35. https://doi.org/10.1016/j.jacc.2018.12.054
3. Slart RH , Williams MC , Juarez-Orozco LE , Rischpler C , Dweck MR , Glaudemans AW , et al. Position paper of the EACVI and EANM on artificial intelligence applications in multimodality cardiovascular imaging using SPECT/CT, PET/CT, and cardiac CT. Eur J Nucl Med Mol Imaging. 2021 May;48(5):1399–413. https://doi.org/10.1007/s00259-021-05341-z
4. Juarez-Orozco LE , Martinez-Manzanera O , van der Zant FM , Knol RJ , Knuuti J . Deep Learning in Quantitative PET Myocardial Perfusion Imaging: A Study on Cardiovascular Event Prediction. JACC Cardiovasc Imaging. 2020 Jan;13(1 Pt 1):180–2. https://doi.org/10.1016/j.jcmg.2019.08.009
5. Juarez-Orozco LE , Knol RJ , Sanchez-Catasus CA , Martinez-Manzanera O , van der Zant FM , Knuuti J . Machine learning in the integration of simple variables for identifying patients with myocardial ischemia. J Nucl Cardiol. 2020 Feb;27(1):147–55. https://doi.org/10.1007/s12350-018-1304-x
6. Dey D , Diaz Zamudio M , Schuhbaeck A , Juarez Orozco LE , Otaki Y , Gransar H , et al. Relationship Between Quantitative Adverse Plaque Features From Coronary Computed Tomography Angiography and Downstream Impaired Myocardial Flow Reserve by 13N-Ammonia Positron Emission Tomography: A Pilot Study. Circ Cardiovasc Imaging. 2015 Oct;8(10):e003255. https://doi.org/10.1161/CIRCIMAGING.115.003255
7. Wang F , Xu W , Lv W , Du D , Feng H , Zhang X , et al. Evaluation of the diagnostic value of joint PET myocardial perfusion and metabolic imaging for vascular stenosis in patients with obstructive coronary artery disease. J Nucl Cardiol. 2020 May;https://doi.org/10.1007/s12350-020-02160-x
8. Wang X , Yang B , Moody JB , Tang J . Improved myocardial perfusion PET imaging using artificial neural networks. Phys Med Biol. 2020 Jul;65(14):145010. https://doi.org/10.1088/1361-6560/ab8687
9. Shi L , Lu Y , Dvornek N , Weyman CA , Miller EJ , Sinusas AJ et al. Automatic Inter-frame Patient Motion Correction for Dynamic Cardiac PET Using Deep Learning. IEEE Trans Med Imaging. 2021;doi:https://doi.org/10.1109/TMI.2021.3082578.
10. Togo R , Hirata K , Manabe O , Ohira H , Tsujino I , Magota K , et al. Cardiac sarcoidosis classification with deep convolutional neural network-based features using polar maps. Comput Biol Med. 2019 Jan;104:81–6. https://doi.org/10.1016/j.compbiomed.2018.11.008
11. Ladefoged CN , Hasbak P , Hornnes C , Højgaard L , Andersen FL . Low-dose PET image noise reduction using deep learning: application to cardiac viability FDG imaging in patients with ischemic heart disease. Phys Med Biol. 2021 Feb;66(5):054003. https://doi.org/10.1088/1361-6560/abe225
12. Kolossváry M , Park J , Bang JI , Zhang J , Lee JM , Paeng JC , et al. Identification of invasive and radionuclide imaging markers of coronary plaque vulnerability using radiomic analysis of coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging. 2019 Nov;20(11):1250–8. https://doi.org/10.1093/ehjci/jez033
13. Kwiecinski J , Tzolos E , Meah M , Cadet S , Adamson PD , Grodecki K et al. Machine-learning with 18F-sodium fluoride PET and quantitative plaque analysis on CT angiography for the future risk of myocardial infarction. J Nucl Med. 2021jnumed.121.262283. doi:https://doi.org/10.2967/jnumed.121.262283.
14. Santarelli MF , Genovesi D , Positano V , Scipioni M , Vergaro G , Favilli B , et al. Deep-learning-based cardiac amyloidosis classification from early acquired pet images. Int J Cardiovasc Imaging. 2021 Jul;37(7):2327–35. https://doi.org/10.1007/s10554-021-02190-7
15. Išgum I , de Vos BD , Wolterink JM , Dey D , Berman DS , Rubeaux M , et al. Automatic determination of cardiovascular risk by CT attenuation correction maps in Rb-82 PET/CT. J Nucl Cardiol. 2018 Dec;25(6):2133–42. https://doi.org/10.1007/s12350-017-0866-3
16. Dekker M , Waissi F , Bank IE , Lessmann N , Išgum I , Velthuis BK , et al. Automated calcium scores collected during myocardial perfusion imaging improve identification of obstructive coronary artery disease. Int J Cardiol Heart Vasc. 2019 Nov;26:100434. https://doi.org/10.1016/j.ijcha.2019.100434
17. Genovesi D , Bauckneht M , Altini C , Popescu CE , Ferro P , Monaco L , et al. The role of positron emission tomography in the assessment of cardiac sarcoidosis. Br J Radiol. 2019 Aug;92(1100):20190247. https://doi.org/10.1259/bjr.20190247
18. Laudicella R , Baratto L , Minutoli F , Baldari S , Iagaru A . Malignant Cutaneous Melanoma: updates in PET Imaging. Curr Radiopharm. 2020;13(1):14–23. https://doi.org/10.2174/1874471012666191015095550
19. Cui J , Liu X , Wang Y , Liu H . Deep reconstruction model for dynamic PET images. PLoS One. 2017 Sep;12(9):e0184667. https://doi.org/10.1371/journal.pone.0184667
20. Joshi NV , Vesey AT , Williams MC , Shah AS , Calvert PA , Craighead FH , et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet. 2014 Feb;383(9918):705–13. https://doi.org/10.1016/S0140-6736(13)61754-7
21. Caobelli F . Artificial intelligence in medical imaging: game over for radiologists? Eur J Radiol. 2020 May;126:108940. https://doi.org/10.1016/j.ejrad.2020.108940
22. Slomka PJ , Dey D , Sitek A , Motwani M , Berman DS , Germano G . Cardiac imaging: working towards fully-automated machine analysis & interpretation. Expert Rev Med Devices. 2017 Mar;14(3):197–212. https://doi.org/10.1080/17434440.2017.1300057
23. Geis JR , Brady AP , Wu CC , Spencer J , Ranschaert E , Jaremko JL , et al. Ethics of Artificial Intelligence in Radiology: Summary of the Joint European and North American Multisociety Statement. Radiology. 2019 Nov;293(2):436–40. https://doi.org/10.1148/radiol.2019191586