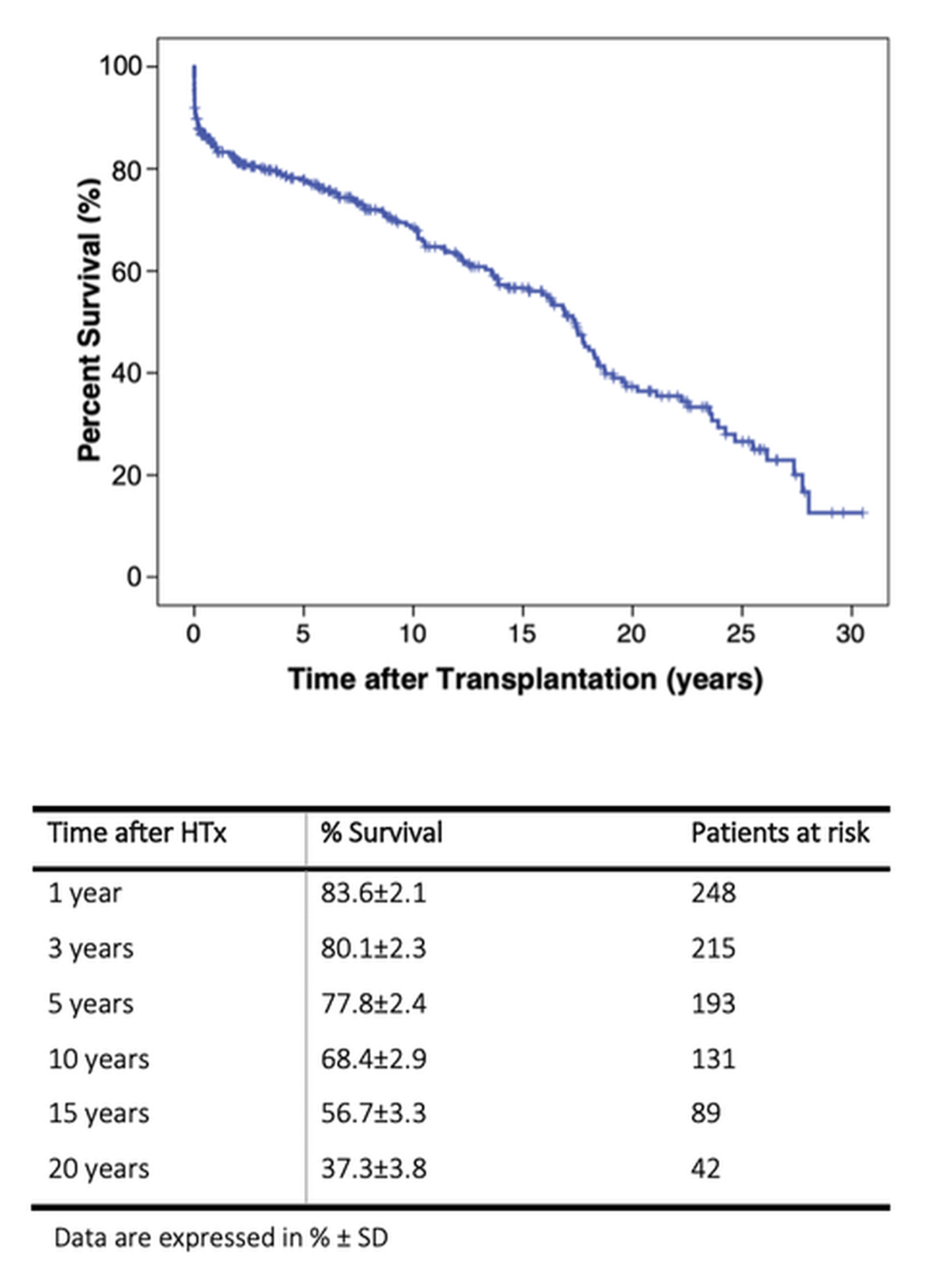
Figure 1 Kaplan-Meier estimate of overall survival after heart transplantation in the years 1987–2018.
DOI: https://doi.org/10.4414/SMW.2022.w30108
As of 1967, heart transplantation has remained the treatment of choice in end-stage heart failure [1]. However, this therapeutic option turned into a success story only after the early 1980s when ciclosporin became available for anti-rejection treatment. At the Lausanne University Hospital, heart transplantation started in 1987 and this uninterrupted activity resulted in a total of 328 heart transplantations up to 31 December 2018. The continuation of this activity for more than 31 years indicates that heart transplantation remains an indispensable option for heart failure treatment despite of the significant progress during the last decades, in particularly in the treatment of heart failure with reduced left ventricular ejection fraction (HFrEF).
Since 1987, angiotensin converting enzyme inhibitors (ACE-Is) have been shown to decrease mortality in HFrEF patients [2, 3]. In 1999, antagonists of the beta-adrenergic receptor and the mineralocorticoid receptor (MRA) were established as effective pharmacological therapies in severe heart failure when added to ACE-I [4, 5]. In 2001, angiotensin II type 1 receptor blockers (ARB) were shown to reduce the incidence of heart failure-related hospitalisations [6] and, in 2003, to decrease cardiovascular mortality [7]. Finally, since 2005, internal defibrillators and resynchronisation devices have become established HFrEF treatment because of a significant decrease in mortality when added to optimal pharmacological treatment [8, 9]. These achievements have reduced case fatality with heart failure in the real world [9, 10]. However, heart failure treatment only slows progression of the disease, which is why ventricular assist device (VAD) treatment has become a welcome therapeutic option for those patients progressing towards terminal heart failure, in particular while waiting for heart transplantation [10, 11].
Despite the dynamic development of therapeutic options for heart failure treatment, key listing criteria for heart transplantation have remained largely unchanged [12–14]. We hypothesised that improvement in heart failure therapy with a reduction of morbidity should result in selection of heart transplantation recipients of older age, since heart failure patients are less symptomatic for a longer period of time. Comorbidity load increases with age and the time living with heart failure. Therefore, we supposed that heart transplant recipients may present with a higher comorbidity load, which is known to affect post-transplant survival [14].
This cohort includes consecutive heart transplantation recipients at the Lausanne University Hospital from the first transplant operation on 5 June 1987 until study end on 31 December 2018. All patients were censored on the 31 December 2019. The protocol was approved by the local research ethics committee (CER VD 2019-704) and the study was conducted in accordance with the Declaration of Helsinki [15].
The primary aim of this retrospective monocentric cohort study was to investigate whether pretransplant characteristics of patients undergoing heart transplantation at the University Hospital of Lausanne differed in the periods 1987–1998 (period 1) versus 1999–2010 (period 2) versus 2011–2018 (period 3). The secondary aim was to investigate whether post-transplant mortality differed between these periods based on the hypothesis that differences in pretransplant patientcharacteristics may affect post-transplant survival.
Recipient-related demographic, anthropometric, biological and clinical data, as well as medical history and basic information on the transplant operation were collected from the individual patients’ electronic health report of the Lausanne University Hospital (AZ). For some old files, data were extracted from paper medical records. Biological and clinical data always refer to the day of transplant operation. Donor-related demographic, clinical and biological data were extracted either from the respective recipients’ electronic/paper medical file (AZ) or from the Swiss Organ Allocation System data bank (KL). Left ventricular ejection fraction of the donor heart was always assessed with echocardiography as of 1988. Data availability always varied between 92 and 100% but forserum iron levels which were available for 83% of all cohort patients. Data accuracy was confirmed by revisiting 20% patients’ data, revealing 97% accuracy (TA). Comprehensive pretransplant transthoracic echocardiography, right heart catheterisation and cardiopulmonary exercise testing were performed by board-certified cardiologists and represent the last examination before the transplant operation. All-cause mortality data derived from the Swiss national mortality registry and local documentation in the electronic/paper file of the individual patient.
For each patient, the acute cellular rejection score of the first postoperative year was calculated as the sum of histopathological results from all endomyocardial biopsies obtained divided by the number of endomyocardial biopsies [16]. Endomyocardial biopsies were graded according to the revised criteria of the International Society of Heart and Lung Transplantation, ISHLT–2004 [17]. For the purpose of this study, endomyocardial biopsy results graded by ISHLT-1990 recommendations (endomyocardial biopsies procured 1990–2004) or by the Texas Heart Institute classification (endomyocardial biopsies before 1990) [17, 18] were all converted into the ISHLT-2004 grading system [17].
Statistical analysis was performed using SPSS BASE 17.0 statistical software (SPSS Inc. Chicago, IL, USA). Categorical variables were expressed as percentages and compared using the Pearson’s chi-square or Fisher’s exact test (when n ≤5). Continuous variables were expressed as medians and interquartile ranges (IQRs). The groups were compared using the non-parametric Kruskal-Wallis test, in order to waive the assumption of normal distribution of variables. Post-hoc intergroup differences were assessed using the non-parametric Mann-Whitney test with Bonferroni correction. Survival data were analysed with standard Kaplan-Meier actuarial techniques for estimation of survival probabilities and compared using the log-rank test. A two-tailed p-value <0.05 was taken to indicate statistical significance.
A total of 323 patients underwent heart transplantation at the Lausanne University Hospital between 5 June 1987 and 31 December 2018 and were included in this study. Five of them had a retransplant, expanding the number of transplant operations to 328. Separation of the cohort by the time of operation resulted in 115 heart transplantation recipients for the first period, 106 for the second period, and 107 for the third period. The annual number of heart transplant operations varied between 3 (1987) and 22 (2018), yielding a mean of 10.6 heart transplantations/year (supplementary fig. S1 in the appendix). Three-month and 1-year all-cause mortality were not different when years with <12 heart transplantations/year and other years were compared (14 vs 10%, p = 0.27; 18 vs 15%, p = 0.42, respectively).
At the time of the transplant operation, 57 (17%) heart transplant recipients had urgent status on the waiting list, with 50 patients on positive inotropic treatment and 2 patients on veno-arterial extracorporeal membrane oxygenation support. In addition, three cases waited in urgent status because of multiple pre-existing anti-HLA antibodies and two patients suffered from persisting severe infection of the implanted left ventricular assist device (LVAD).
Table 1 shows patients’ characteristics. Median age at heart transplantation was 53.4 years; age was not different between groups. Twelve patients were <18 years old, representing altogether 4% of the total cohort; more patients <18 years had heart transplantation in the third period (period 1 vs 2 vs 3: 0 vs 3 vs 9, p = 0.003). Male gender was preponderant (79%) and not different between groups (83 vs 76 vs 77%, p = 0.34). Body mass index (BMI) and associated anthropometric measures were not different between groups (p = 0.29). The prevalence of a history of arterial hypertension was higher in the first and third period (38.3 vs 26 vs 40.2%, p = 0.04). The proportion of recipients with a history of tobacco use tended to be higher in the first and third era (57 vs 45.3 vs 54%, p = 0.1). In absolute numbers, more patients had diabetes in the third era (15.7 vs 14 vs 23%, p = 0.2). The prevalence of dyslipidaemia, chronic obstructive pulmonary disease (COPD) and renal replacement therapy was not different between groups.
Table 1Demographic and clinical parameters.
| Variable | All patients (n = 328) | 1987–1998 (n = 115) | 1999–2010 (n = 106) | 2011–2018 (n = 107) | p-value | |
| Demographics | Caucasians | 97% | 98% | 99% | 94% | |
| Age (years), median (IQR) | 53.4 (14.3) | 52.4 (13.0) | 53.7 (15.0) | 53.6 (18.9) | 0.71 | |
| Recipients <18 years(%) | 12 (4%) | 0 (0%) | 3 (3%) | 9 (8%) | 0.003 | |
| Male gender | 79% | 83% | 76% | 77% | 0.34 | |
| Clinical data | Height (m), median (IQR) | 1.71 (0.13) | 1.72 (0.11) | 1.72 (0.14) | 1.70 (0.14) | 0.68 |
| BMI (kg/m2), median (IQR) | 24.5 (6.8) | 24.0 (6.4) | 23.8 (7.0) | 24.9 (6.6) | 0.29 | |
| BSA (m2), median (IQR) | 1.86 (0.30) | 1.85 (0.25) | 1.85 (0.32) | 1.88 (0.32) | 0.51 | |
| Comorbidity | Smoking | 171 (52%) | 65 (57%) | 48 (45.3%) | 58 (54%) | 0.10 |
| Arterial hypertension | 115 (35%) | 44 (38.3%) | 28 (26%) | 43 (40.2%) | 0.04 | |
| Diabetes | 58 (18%) | 18 (15.7%) | 15 (14%) | 25 (23%) | 0.20 | |
| Dyslipidaemia | 147 (45%) | 49 (42.6%) | 48 (45.3%) | 50 (47%) | 0.99 | |
| COPD | 37 (11%) | 14 (12.2%) | 12 (11%) | 11 (10%) | 0.85 | |
| Haemodialysis | 10 (3%) | 3 (3%) | 3 (3%) | 4 (4%) | 0.90 | |
| Cardiovascular parameters | LVEF (%), median (IQR) | 20.0 (14.0) | 20.0 (8.0) | 20 (10.0) | 22.5 (20.0) | 0.23 |
| PVR, median (IQR) | 2.19 (1.49) | 1.76 (1.5) | 2.44 (2.1) | 2.22 (1.3) | 0.51 | |
| VO2max, (median IQR) | 13.0 (4.7) | 12.5 (4.9) | 12.2 (4.1) | 13.3 (4.5) | 0.36 | |
| HR (bpm), median (IQR) | 80.0 (18.0) | 88.0 (21.0) | 84.0 (27.0) | 76.5 (14.0) | 0.008 | |
| HR ≥80 bpm | 184 (56.1%) | 74 (64.3%) | 65 (61.3%) | 45 (42.1%) | 0.001 | |
| Medical therapy | Beta-blockers | 132 (40%) | 3 (3%) | 51 (39%) | 78 (73%) | 0.0001 |
| RAS antagonist | 247 (75%) | 95 (83%) | 78 (74%) | 74 (69%) | 0.06 | |
| Diuretics | 261 (80%) | 90 (78%) | 91 (86%) | 80 (75%) | 0.122 | |
| MRA | 172 (52%) | 27 (24%) | 64 (60%) | 81 (76%) | 0.0001 | |
| CRT | 60 (18.3%) | 0 (0%) | 23 (21.7%) | 37 (35%) | 0.0001 | |
| Pre-heart transplantation VAD | 54 (16.5%) | 0 (0%) | 16 (15%) | 38 (35.5%) | <0.0001 | |
| – VAD-type PF (n) | 14 | 10 | 4 | <0.0001 | ||
| – VAD-type CF (n) | 40 | 6 | 34 | <0.0001 | ||
Data are expressed in absolute numbers and percentages, if not otherwise specified, or as median (IQR).
ACE-I: angiotensin-converting-enzyme inhibitor; ARB: angiotensin II receptor blocker; BMI: body mass index; BSA: body surface area; COPD: chronic obstructive pulmonary disease; CRT: cardiac resynchronisation therapy; HR: heart rate; IQR: interquartile range; LVEF: left ventricular ejection fraction; MRA: mineralocorticoid receptor antagonist; PVR: pulmonary vascular resistance in Wood Units; VO2max: maximal oxygen consumption in ml/kg/min; RAS: renin-angiotensin system; VAD-type PF: ventricular assist device with pulsatile flow; VAD-type CF: ventricular assist device with continuous flow
Table 2 shows heart failure aetiology classified according to Maron et al. [19]. Non-ischaemic and ischaemic cardiomyopathy were the overall most prevalent aetiologies in the study cohort (36.8 and 34.7%, respectively). Less prevalent were congenital heart disease (4.9%) and secondary cardiomyopathies (4.8%), with half of them due to cardiotoxicity (2.4%). The distribution of the aetiologies of heart failure differed between the three periods with a higher prevalence of ischaemic cardiomyopathy in periods 1 and 3, whereas non-ischaemic cardiomyopathy was less frequent in period 3.
Table 2Aetiologies of heart failure.
| Aetiologies of heart failure | All patients (n = 328) | 1987–1998 (n = 115) | 1999–2010 (n = 106) | 2011–2018 (n = 107) |
| Primary cardiomyopathy | ||||
| – Mixed | 121 (36.8%) | 50 (44%) | 46 (43%) | 25 (23%) |
| – Genetic | 26 (7.9%) | 2 (1.7%) | 8 (7.5%) | 16 (15%) |
| – Acquired | 12 (3.7%) | 3 (2.6%) | 4 (3.8%) | 5 (4.7%) |
| Ischaemic heart disease | 114 (34.7%) | 40 (34.8%) | 32 (30.2%) | 42 (39.3%) |
| Valvular heart disease | 8 (2.4%) | 6 (5.2%) | 0 (0%) | 2 (1.9%) |
| Congenital heart disease | 16 (4.9%) | 3 (2.6%) | 6 (5.7%) | 7 (6.5%) |
| Cardiac allograft vasculopathy | 5 (1.5%) | 0 (0%) | 2 (1.9%) | 3 (2.8%) |
| COPD related | 1 (0.3%) | 1 (0.9%) | 0 (0%) | 0 (0%) |
| HTA, coarctation | 5 (1.5%) | 4 (3.5%) | 1 (0.9%) | 0 (0%) |
| Secondary cardiomyopathy | 16 (4.8%) | 2 (1.7%) | 7 (6.6%) | 7 (6.5) |
| Unknown | 4 (1.2%) | 4 (3.5%) | 0 (0%) | 0 (0%) |
Data are expressed in absolute numbers and percentages.
COPD: chronic obstructive pulmonary disease; HTA: systemic arterial hypertension; always p = 0.0001 between periods
As shown in table 1, pretransplant beta-blocker or MRA treatment increased from period 1 to 3 (3 vs 39 vs 73%, p = 0.0001; 24 vs 60 vs 76%, p = 0.0001, respectively) whereas renin-angiotensin system antagonist treatment and use of diuretics were not significantly different between periods (83 vs 74 vs 69%, p = 0.06; 78 vs 86 vs 75%, p = 0.122, respectively). The percentage of recipients with cardiac resynchronisation therapy (CRT) significantly increased between the second and third period (0 vs 21.7 vs 35%, p = 0.0001). Furthermore, the number of patients waiting for heart transplantation while on VAD treatment increased from period 2 to 3 (15 vs 35.5%, p <0.0001). The number of continuous-flow (CF) LVADs increased from 6 to 34 (p <0.0001) while the number of LVADs with a pulsatile flow (PF) decreased between periods 2 and 3 from 10 to 4 and was limited in period 3 to paediatric cases (p <0.0001).
Table 1 shows that the overall median left ventricular ejection fraction (LVEF) was 20% with LVEF being numerically higher in the third decade (20 vs 20 vs 22.5%, p = 0.23). Median pulmonary vascular resistance (PVR) was 2.19 Wood Units (WU) and median VO2max was 13 m/kg/min; PVR and peak VO2 did not significantly differ between groups. Median heart rate decreased over time (period 1 vs 2 vs 3: 88 vs 84 vs 76.5 bpm, p = 0.008) with a corresponding decrease of the proportion of patients with a heart rate ≥80 bpm (64.3 vs 61.3 vs 45%, p = 0.001) consistent with the increasing use of beta-blockade after 1999.
Supplementary table S2 (in the appendix) demonstrates that the haemoglobin levels were lower in period 2 and 3 (147 vs 130 vs 130 g/l, p = 0.0001), whereas leucocyte and platelet counts were not different between groups. Bilirubin and creatinine levels were lower in the third period (18.0 vs 15.0 vs 10.0 µmol/l, p = 0.0001; 110 vs 114 vs 98 µmol/l, p = 0.08). Aspartate and alanine aminotransferase (ASAT and ALAT) levels were always within normal range but varied between groups (30.0 vs 27.0 vs 31 U/l, p = 0.56; 36.0 vs 21.0 vs 31.0 U/l, p = 0.017; respectively) and the proportion of patients with ASAT or ALAT >3 times above the upper limit of normal was lowest in period 3 (p = 0.006, p = 0.0001, respectively). On the basis of the biological measures, heart transplantation recipients in period 3 seemed healthier and this may relate to lower serum levels of creatinine and ASAT in patients on LVAD treatment at the time of the transplant operation. In fact, patients with a VAD had significantly lower (always p <0.0001) median preoperative values of creatinine (85.0, IQR 53.5 vs 110.0, IQR 42.0 µmol/l), blood urea nitrogen (6.3, IQR5.3 vs 8.8, IQR 5.9 mmol/l) and total bilirubin (9.0, IQR 11.8 vs 16.0, IQR 14.5 µmol/l). Serum iron was significantly lower in the second period (13.1 vs 10.1 vs 14.2 µmol/l, p = 0.001) and the proportion of recipients with a serum iron <10 µmol/l was highest during period 2 (30 vs 46.7 vs 22%, p = 0.001).
Table 3 shows that median waiting time for heart transplantation was shortest during period 1 and longest for period 3 patients (1 vs 2 vs 3: 90 vs 129 vs 185 days, p = 0.006). Cold ischaemia time was 154 minutes for the total cohort and was lower in the first period (123.6 vs 180.0 vs 169.8 min, p = 0.0001). Significantly more heart transplant recipients had pretransplant cardiac surgery in the third era (32 vs 39 vs 58%, p = 0.001). The overall proportion of recipient/donor sex mismatches was 37% and was not significantly different between the three periods. Donor age was lower in period 1 and increased in periods 2 and 3 (32.0 vs 41.0 vs 49.0 years; p = 0.0001).
Table 3Risk factors for heart transplantation surgery.
| Variable | All patients (n = 328) | 1987–1998 (n = 115) | 1999–2010 (n = 106) | 2011–2018 (n = 107) | p-value |
| Time on waiting list (days) median (IQR) | 118.0 (289) | 90 (225) | 129 (229) | 184 (347) | 0.006; B 0.002 |
| Urgent status on waiting list | 57 (17%) | 6 (5%) | 32 (30%) | 19 (18%) | 0.0001 |
| Cold ischaemic time (min) median (IQR) | 154.0 (57.3) | 123.6 (82.7) | 180.0 (69.8) | 169.8 (62.3) | 0.0001; B, P1 vs (P2+P3) |
| Previous cardiac surgery | 136 (41.5%) | 35 (32%) | 39 (29%) | 62 (58%) | 0.001 |
| Donor age (years) median (IQR) | 41.5 (25.0) | 32.0 (22.0) | 41.0 (25.0) | 49.0 (22.0) | 0.0001; B, P1 vs (P2+P3) |
| Recipient/donor sex mismatch | 92 (37%) | 29 (32%) | 39 (38%) | 24 (26%) | 0.40 |
Data are presented as absolute numbers and percentages or median (IQR).
P1: period 1 (1987–1998); P2: period 2 (19992010); P3: period 3 (2011-2018)
From 1987 to 2018, one patient was lost to follow-up, five patients are known to be dead but the date and cause of death are unknown and five patients underwent a second heart transplantation. As shown in table 4, the median follow-up time was 85.3 months. Total follow-up was 2989.1 patient-years.
Table 4Follow-up and outcome.
| Variable | All patients (n = 328) | 1987–1998 (n = 115) | 1999–2010 (n = 106) | 2011–2018 (n = 107) | p-value |
| Mean follow-up time (months) median (IQR) | 85.3 (174.6) | 209.0 (194.2) | 121.4 (181.2) | 33.1 (57.0) | <0.0001; B, P3 vs (P2+P1) and P1 vs P2 |
| Early mortality <3 months | 39 (12%) | 8 (7%) | 27 (26%) | 4 (4%) | <0.0001 |
| Mean rejection score, median (IQR) | 0.54 (0.82) | 1.0 (0.10) | 0.49 (0.55) | 0.16 (0.23 ) | <0.0001; B, P1 vs (P2+P3) and P1 vs P2 |
Data are presented as median (IQR) or absolute numbers and percentages. Median rejection score was calculated as the sum of the results of endomyocardial biopises divided by the number of biopsies.
P1: period 1 (1987–1998); P2: period 2 (1999–2010); P3: period 3 (2011-–2018)
A: period 2 versus period 1; B: period 3 versus period 1; C: period 3 versus period 2
Figure 1 and the attached table show the Kaplan-Meier estimate of overall survival as censored by survival status on 31 December 2019. The 1-year overall survival was 83.6 ± 2.1%; the 10-year survival was estimated to be 68.4 ± 2.9%. A total of 12% of all heart transplant recipients died within the first 3 months; the deaths were related to graft failure (5%) and haemorrhagic shock (4%), whereas infection (2%), acute rejection (1%) and neurological causes (0.6%) were less frequent (supplementary table S3 in the appendix).

Figure 1 Kaplan-Meier estimate of overall survival after heart transplantation in the years 1987–2018.
Survival analyses of each period is presented in figure 2 and the attached table. Early mortality was significantly different between periods (period 1 vs 2 vs 3: 7 vs 26 vs 4%, p <0.0001). The difference in early mortality contributed to the significant difference in 1-year survival, which was 87.2% in period 1, 70.9% in period 2 and 93.0% in period 3 (p <0.022). The difference remained significant for 3- and 5-year survival. However, all-cause mortality in patients surviving at 1 year was not different between groups (figure 3) suggesting that the risk of mortality was largely associated with fatalities occurring within the first post-transplant year.
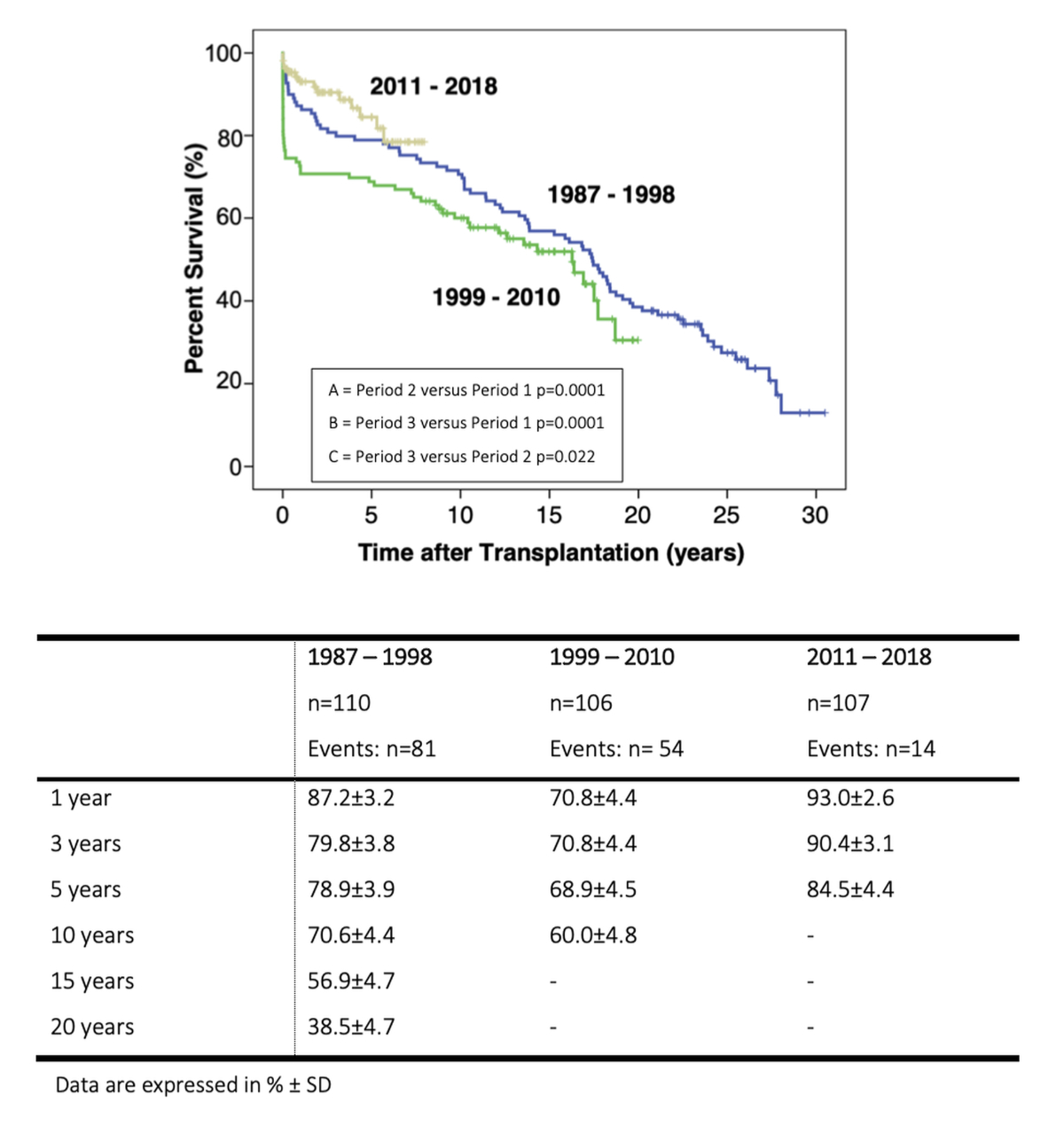
Figure 2 Survival after heart transplantation according to period (1987–1998, 1999–2010 and 2011–2018).
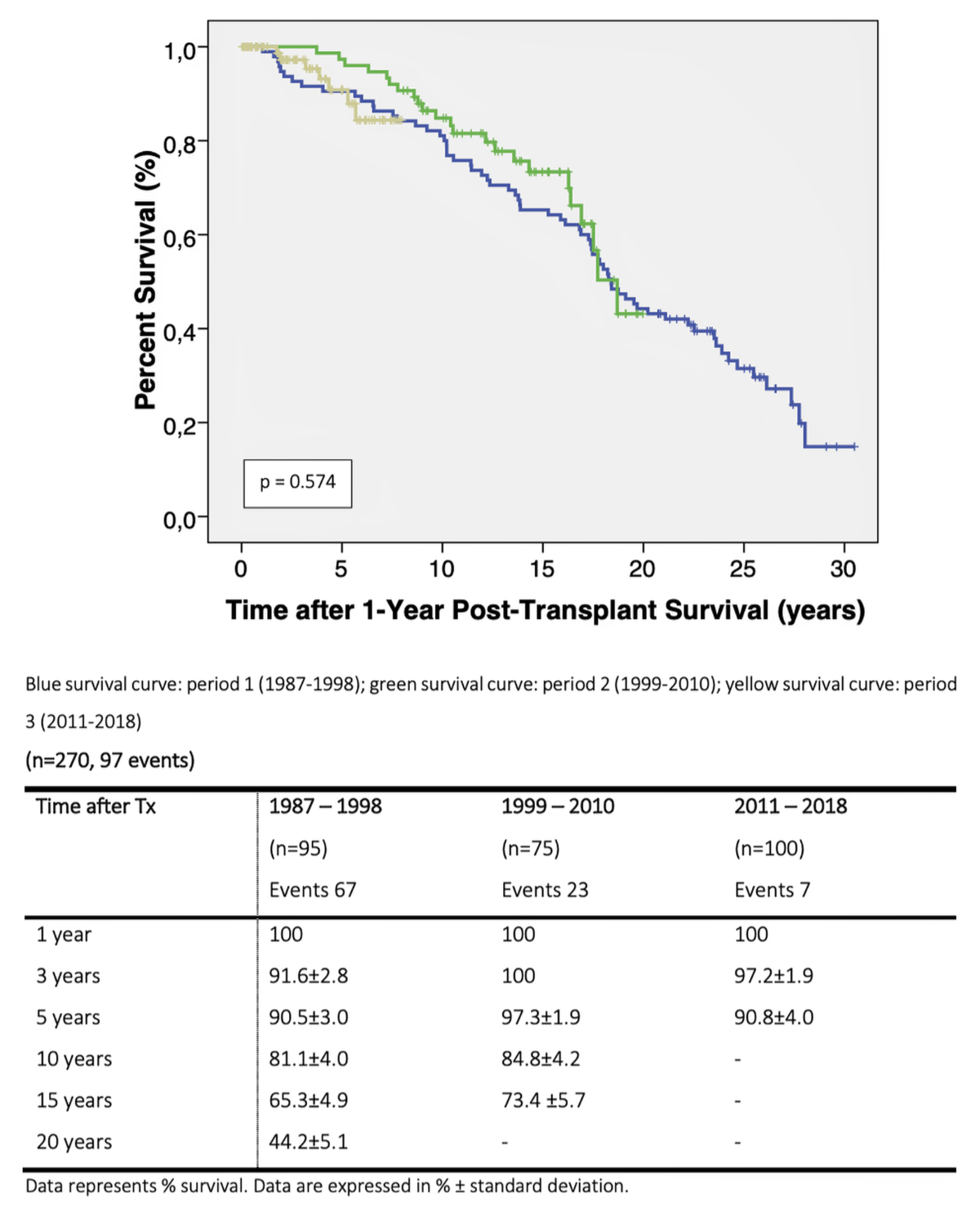
Figure 3 Kaplan-Meier estimate of all-cause mortality of patients surviving at 1 year post-transplant for the years 1987–1998, 1999–2010 and 2011–2018.
Since the first cardiac allograft implantation in 1967, heart transplantation has remained the treatment option of choice for selected patients with advanced-stage heart failure refractory to standard treatment. This retrospective monocentric study tested whether characteristics of heart transplantation candidates have changed since heart failure therapy has substantially improved quality of life and survival of patients while central elements of the listing criteria for heart transplantation remained largely unchanged. Neither demographic, clinical or biological patient characteristics, nor survival of patients surviving the first year posttransplant differed greatly between periods, suggesting that the largely unchanged listing criteria still select similar patients. However, early mortality was significantly higher in period 2 and therefore its relation to pretransplant patient characteristics was investigated further.
During the last decades the key elements indicating eligibility for heart transplantation listing, in particular peak VO2 and pulmonary vascular resistance, have been maintained without change of the respective cut-offs [12–14]. This could explain why these two parameters were not significantly different between the three periods. However, we were surprised that median patient age was also not different between the periods since drug treatment of heart failure not only prolongs survival but also retards progression of clinical symptoms and signs. Likewise, distribution between female and male gender remained unchanged, despite of the fact that more females survive acute myocardial infarction [20]. Furthermore, during the last two 2 decades the guidelines for listing of heart transplant recipients had less stringent comorbidity-related contraindications to heart transplantation . While this change should render patients with higher BMI, more severe renal dysfunction, or more severe diabetes eligible for heart transplantation [13, 14], neither BMI nor renal function parameters were significantly different between the three periods, although the prevalence of diabetes was numerically higher in period 3.
Altogether, these results suggest that patient selection remained conservative across the three periods and candidates with the best chances for long-term favourable outcome post-transplant were listed. In accordance with this conclusion, heart transplantation recipients across the three periods presented with a relatively benign comorbidity profile when compared with other patients with advanced heart failure [9]. In fact, pretransplant comorbidity was a determinant of postoperative long-term survival [21] and the superposition of the Kaplan-Meier estimates on the condition of 1-year survival across the three periods is in accordance with the not significantly different and overall benign comorbidity profile.
However, ischaemic cardiomyopathy was more prevalent in periods 1 and 3, which is of interest since this pathology is associated with an increased post-transplant mortality, as reported in a retrospective analysis by the International Society of Heart and Lung Transplantation (ISHLT) [22]. In fact, survival after heart transplantation was best in periods 1 and 3, with a 1-year survival of 87.2 / 93% and a 5-year survival of 78.9/84.5%, indicating that period 3 survival was even superior to the survival reported from the registry of the ISHLT for the years 2010–2017 [10]. In contrast, the Kaplan-Meier estimates of survival indicated a higher mortality in period 2 due to a significantly higher mortality within the first year post-transplant.
In order to better understand the increased early mortality in period 2, we compared across the three periods acknowledged pretransplant risk factors for early mortality post-transplant such as serum bilirubin, serum creatinine, haemoglobin, PVR and donor age [21, 22]. As monitoring and care in the operating room and in the intensive care unit, and patients’ management in the immediate and early postoperative period improved significantly between 1987 and 2018, we chose a descriptive approach for statistical analysis.
Serum bilirubin was significantly higher in the first period whereas mortality was lower in period 1 than period 2, suggesting that this parameter of hepatic function does not explain the high early mortality in period 2. Likewise, serum creatinine was numerically higher in the second period, which could theoretically explain the increased early mortality in this period, but serum creatinine levels were not significantly different when survivors were compared with non-survivors in period 2 (as shown in the supplementary table S4). In contrast, pretransplant VAD therapy decreased bilirubin and creatinine serum levels significantly, suggesting that this treatment might have contributed to the improved outcomes in period 3.
Furthermore, the haemoglobin level was lower in the second period, which is interesting since low pretransplant haemoglobin levels have been associated with increased 1-year mortality after transplantation [23]. Furthermore, the serum iron level was low in period 2 and the combination of low haemoglobin level and low serum iron suggests the presence of iron deficiency as common underlying pathology. Iron deficiency is highly prevalent in severe heart failure and is also associated with increased mortality [24]. Therefore, it is conceivable that heart transplant recipients from period 2 suffered from iron deficiency, although we cannot confirm this hypothesis since ferritin and transferrin saturation were at that time not regularly measured before transplantation. However, peak VO2 levels were lower in 1-year non-survivors of the second period when compared with survivors (10.4 ± 3.5 vs 13.7 ± 3.7 ml/min/kg, p = 0.03), in accordance with the negative impact of iron deficiency on maximum exercise capacity observed in heart failure patients [25]. Nonetheless, it remains to be shown whether pretransplant iron deficiency is associated with increased post-transplant mortality.
Last but not least, PVR and donor age have been associated with increased early mortality. However PVR was not different between groups and not related in univariate analysis. Donor age was highest in period 3 whereas all-cause mortality was lowest, suggesting that donor age alone cannot explain the increased early mortality in period 2.
Perioperative or post-transplant factors such as cold ischaemia time may explain the higher early mortality observed in period 2, since cold ischaemia time is an important determinant of early postoperative mortality [26, 27]. Cold ischaemia time was significantly longer in period 2 as compared with other periods and long cold ischaemia time increases the risk of early mortality, especially when combined with older donor age (>34 years) [26, 27]. However, early mortality predicted by the cold ischaemia time was similar for periods 2 and 3 on the basis of analyses in the the United Network of Organ Sharing (UNOS) and the ISHLT registry. This suggests that cold ischaemia time alone cannot explain the increased mortality in period 2. The longer cold ischaemia time in periods 2 and 3 as compared with period 1 are surprising, but may reflect changes in the organ allocation system, which was regional until 2007 and national thereafter [28].
More recently, a propensity-matching analysis of the UNOS showed that both 1-year and 5-year mortality is higher in heart transplantation recipients with LVAD implantation preceding transplant surgery [29]. In contrast, survival was best in period 3 when 38 of the 62 heart transplantation recipients had pretransplant VAD implantation. This is in accordance with other reports that CF-LVAD treatment results in more favourable clinical and biological presentation on the day of heart transplantation [30, 31] and this was more true for those patients on HeartMate 3 support [32].
Post-transplant factors may likewise impact on early postoperative mortality and, in particular, acute cellular rejection can play an important role. However, the mean rejection score significantly decreased from period 1 to period 3, although most of the immunosuppressive drugs were already available in period 1. A similar decrease of the mean rejection score was also documented in a more recent analysis of the ISHLT registry and this decrease was associated with improved survival [33]. Therefore, the decrease in the rejection score may have contributed to the favourable results in period 3, but its progressive decrease from period 1 to period 3 does not suggest that acute cellular rejection explains the high early mortality in period 2.
Last but not least, changes in immediate post-transplant care may also explain the favourable results in period 3. In 2010, a multidisciplinary team was established at the Lausanne University Hospital for the care of the heart transplantation patients. This team is composed of local transplant cardiologists and cardiac surgeons, trained cardiac anaesthesiologists, together with dedicated intensive care and infectious disease specialists, as well as transplant immunology specialists. This multidisciplinary team approach was shown to improve morbidity and mortality after heart transplantation in our cohort, whether heart transplantation recipients had been on pretransplant LVAD support or not [30, 34]. This multidisciplinary approach is not limited to heart transplantation candidates but extends to the follow-up of patients with advanced heart failure and can likewise explain why substantially more heart failure patients were on beta-blocker, MRA, and CRT treatment in period 3. This integrated approach to advanced heart failure care corresponds to the quality of care centre strategy set out by te European Society of Cardiology [35]. Follow-up by such structures was shown to improve heart failure symptoms and cardiac function [36, 37] and may explain why LVEF was numerically higher in period 3.
This retrospective cohort study spans 31 years of uninterrupted heart transplantation at the Lausanne University Hospital. The study stratified heart transplantation recipients as a function of the heart failure therapies available at the time of transplant operation, but we cannot exclude that heart transplantation outcome was affected by the great improvement in monitoring and care in the operating room and in the intensive care unit, and in patients’ management thereafter. In acknowledgement that this presents a confounder for any association with post-transplant mortality, we limited the analysis to descriptive statistics. Furthermore, we cannot exclude that changes in the availability of immunosuppressive medicines may have impacted on post-transplant survival too. In fact, ciclosporin arrived on the market in 1982, tacrolimus in 1989, mycophenolate mofetil in 1992, and everolimus after 2004. However, the guideline-based local immunosuppressive regimen initiated at our institution has remained largely unchanged since 1995 [38], suggesting that change in immunosuppression may not explain the increased early mortality in period 2.
The results of the present heart transplantation cohort study indicate that the overall profile of the local heart transplantation recipients has not changed between 1987 and 2018. This observation is in accordance with the current guidelines for the selection of heart transplantation candidates, which perpetuated central criteria of earlier guidelines on the one hand. On the other hand, other criteria for heart transplantation listing softened, in particular with respect to severity of comorbidity, but we did not observe an effect of this change. This suggests that the medical strategy for heart transplantation listing has remained conservative, most likely related to the intention to select the heart transplantation candidates with the best option for long-term survival post-transplant. In the absence of any significant association of survival with demographic, clinical and biological parameters in the descriptive statistical analysis, we hypothesise that the superior survival in the years 2011–2018 is based on the implementation of a multidisciplinary team approach achieving optimal arrival of the heart transplantation candidates at the time of their transplant operation. However, we cannot exclude a contribution of the favourable pretransplant characteristics in heart transplantation recipients with pretransplant LVAD treatment.
We acknowledge the support of the local team of transplant coordinators (Breton, MC, Chappuis N, Church C, Peuble C, Pilon N, Rogati G) and the nurses implicated in the follow-up of patients on CF-LVAD support (Collins J, Froideveaux O, Revelly ML).
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.
1. Barnard CN . The operation. A human cardiac transplant: an interim report of a successful operation performed at Groote Schuur Hospital, Cape Town. S Afr Med J. 1967 Dec;41(48):1271–4.
2. CONSENSUS Trial Study Group . Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987 Jun;316(23):1429–35. https://doi.org/10.1056/NEJM198706043162301
3. Packer M , Poole-Wilson PA , Armstrong PW , Cleland JG , Horowitz JD , Massie BM , et al.; ATLAS Study Group . Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. Circulation. 1999 Dec;100(23):2312–8. https://doi.org/10.1161/01.cir.100.23.2312 https://doi.org/10.1161/01.CIR.100.23.2312
4. CIBIS II investigators and committees . The cardiac insufficiency bisoprolol study II (CIBIS II): a randomised trial. Lancet. 1999 Jan;353(9146):9–13. https://doi.org/10.1016/S0140-6736(98)11181-9
5. Pitt B , Zannad F , Remme WJ , Cody R , Castaigne A , Perez A , et al.; Randomized Aldactone Evaluation Study Investigators . The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999 Sep;341(10):709–17. https://doi.org/10.1056/NEJM199909023411001
6. Cohn JN , Tognoni G ; Valsartan Heart Failure Trial Investigators . A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001 Dec;345(23):1667–75. https://doi.org/10.1056/NEJMoa010713
7. Granger CB , McMurray JJ , Yusuf S , Held P , Michelson EL , Olofsson B , et al.; CHARM Investigators and Committees . Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003 Sep;362(9386):772–6. https://doi.org/10.1016/S0140-6736(03)14284-5
8. Moss AJ , Zareba W , Hall WJ , Klein H , Wilber DJ , Cannom DS , et al.; Multicenter Automatic Defibrillator Implantation Trial II Investigators . Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002 Mar;346(12):877–83. https://doi.org/10.1056/NEJMoa013474
9. Cleland JG , Daubert JC , Erdmann E , Freemantle N , Gras D , Kappenberger L , et al.; Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators . The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005 Apr;352(15):1539–49. https://doi.org/10.1056/NEJMoa050496
10. Khush KK , Cherikh WS , Chambers DC , Harhay MO , Hayes D Jr , Hsich E , et al.; International Society for Heart and Lung Transplantation . The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult heart transplantation report - 2019; focus theme: Donor and recipient size match. J Heart Lung Transplant. 2019 Oct;38(10):1056–66. https://doi.org/10.1016/j.healun.2019.08.004
11. Mehra MR , Uriel N , Naka Y , Cleveland JC Jr , Yuzefpolskaya M , Salerno CT , et al.; MOMENTUM 3 Investigators . A Fully Magnetically Levitated Left Ventricular Assist Device - Final Report. N Engl J Med. 2019 Apr;380(17):1618–27. https://doi.org/10.1056/NEJMoa1900486
12. Mancini DM , Eisen H , Kussmaul W , Mull R , Edmunds LH Jr , Wilson JR . Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991 Mar;83(3):778–86. https://doi.org/10.1161/01.cir.83.3.778 https://doi.org/10.1161/01.CIR.83.3.778
13. Mehra MR , Kobashigawa J , Starling R , Russell S , Uber PA , Parameshwar J , et al. Listing criteria for heart transplantation: international Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates—2006. J Heart Lung Transplant. 2006 Sep;25(9):1024–42. https://doi.org/10.1016/ j.healun.2006.06.008 https://doi.org/10.1016/j.healun.2006.06.008
14. Mehra MR , Canter CE , Hannan MM , Semigran MJ , Uber PA , Baran DA , et al.; International Society for Heart Lung Transplantation (ISHLT) Infectious Diseases, Pediatric and Heart Failure and Transplantation Councils . The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J Heart Lung Transplant. 2016 Jan;35(1):1–23. https://doi.org/10.1016/j.healun.2015.10.023
15. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013 Nov;310(20):2191–4. https://doi.org/10.1001/jama.2013.281053
16. Dec GW , Narula J , Ballester M , Carrio I . Cardiac allograft rejection. New York: Springer Science&Business Media, LCC; 2001. 434 p.
17. Stewart S , Winters GL , Fishbein MC , Tazelaar HD , Kobashigawa J , Abrams J , et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005 Nov;24(11):1710–20. https://doi.org/10.1016/j.healun.2005.03.019
18. McAllister HA Jr , Schnee MJ , Radovancević B , Frazier OH . A system for grading cardiac allograft rejection. Tex Heart Inst J. 1986 Mar;13(1):1–3.
19. Maron BJ , Towbin JA , Thiene G , Antzelevitch C , Corrado D , Arnett D , et al.; American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention . Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006 Apr;113(14):1807–16. https://doi.org/10.1161/CIRCULATIONAHA.106.174287
20. Nanna MG , Hajduk AM , Krumholz HM , Murphy TE , Dreyer RP , Alexander KP , et al. Sex-based differences in presentation, treatment, and complications among older adults hospitalized for acute myocardial infarction: the SILVER-AMI study. Circ Cardiovasc Qual Outcomes. 2019 Oct;12(10):e005691. https://doi.org/10.1161/CIRCOUTCOMES.119.005691
21. Stehlik J , Edwards LB , Kucheryavaya AY , Aurora P , Christie JD , Kirk R , et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult heart transplant report—2010. J Heart Lung Transplant. 2010 Oct;29(10):1089–103. https://doi.org/10.1016/j.healun.2010.08.007
22. Lund LH , Edwards LB , Dipchand AI , Goldfarb S , Kucheryavaya AY , Levvey BJ , et al.; International Society for Heart and Lung Transplantation . The registry of the International Society of Heart and Lung Transplantation : Thirty-third adult heart transplantation report - 2016; Focus theme: primary diagnostics for transplant. J Heart Lung Transplant. 2016 Oct;35(10):1158–69. https://doi.org/10.1016/j.healun.2016.08.017
23. Taegtmeyer AB , Rogers P , Breen JB , Barton PJ , Banner NR , Yacoub MH . The effects of pre- and post-transplant anemia on 1-year survival after cardiac transplantation. J Heart Lung Transplant. 2008 Apr;27(4):394–9. https://doi.org/10.1016/j.healun.2008.01.014
24. Klip IT , Comin-Colet J , Voors AA , Ponikowski P , Enjuanes C , Banasiak W , et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J. 2013 Apr;165(4):575–582.e3. https://doi.org/10.1016/j.ahj.2013.01.017
25. Jankowska EA , Rozentryt P , Witkowska A , Nowak J , Hartmann O , Ponikowska B , et al. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail. 2011 Nov;17(11):899–906. https://doi.org/10.1016/j.cardfail.2011.08.003
26. Russo MJ , Chen JM , Sorabella RA , Martens TP , Garrido M , Davies RR , et al. The effect of ischemic time on survival after heart transplantation varies by donor age: an analysis of the United Network for Organ Sharing database. J Thorac Cardiovasc Surg. 2007 Feb;133(2):554–9. https://doi.org/10.1016/j.jtcvs.2006.09.019
27. Lund LH , Khush KK , Cherikh WS , Goldfarb S , Kucheryavaya AY , Levvey BJ , et al.; International Society for Heart and Lung Transplantation . The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Heart Transplantation Report-2017; Focus Theme: Allograft ischemic time. J Heart Lung Transplant. 2017 Oct;36(10):1037–46. https://doi.org/10.1016/j.healun.2017.07.019
28. Weiss J , Beyeler F , Immer FF , Swisstransplant H ; Swisstransplant Heart Working Group Stah . Heart allocation and transplantation in Switzerland since the introduction of the Swiss Organ Allocation System (SOAS). Swiss Med Wkly. 2014 Nov;144:w14057. https://doi.org/10.4414/smw.2014.14057
29. Truby LK , Farr MA , Garan AR , Givens R , Restaino SW , Latif F , et al. Impact of Bridge to Transplantation With Continuous-Flow Left Ventricular Assist Devices on Posttransplantation Mortality. Circulation. 2019 Aug;140(6):459–69. https://doi.org/10.1161/CIRCULATIONAHA.118.036932
30. Nowacka A , Hullin R , Tozzi P , Barras N , Regamey J , Yerly P , et al. Short-term single-centre experience with the HeartMate 3 left ventricular assist device for advanced heart failure. Eur J Cardiothorac Surg. 2020 Sep;58(3):511–8. https://doi.org/10.1093/ejcts/ezaa075
31. Tozzi P , Banfi C , Ahmadov K , Hullin R , Meyer P , Giraud R , et al. HeartMate 3 in Lowest INTERMACS Profile Cohort: the Swiss Experience. ASAIO J. 2017 Nov/Dec;63(6):752–8. https://doi.org/10.1097/MAT.0000000000000589
32. Tozzi P , Nowacka A , Hullin R , Yerly P , Kirsch M . The role of Heart Failure Team in managing Mechanical Circulatory Support in a Swiss low-volume institution. Heart Surg Forum. 2018 Jun;21(4):E257–62. https://doi.org/10.1532/hsf.1979
33. Söderlund C , Öhman J , Nilsson J , Higgins T , Kornhall B , Johansson L , et al. Acute cellular rejection the first year after heart transplantation and its impact on survival: a single-centre retrospective study at Skåne University Hospital in Lund 1988-2010. Transpl Int. 2014 May;27(5):482–92. https://doi.org/10.1111/tri.12284
34. Schmidhauser M , Regamey J , Pilon N , Pascual M , Rotman S , Banfi C , et al. The impact of multidisciplinary care on early morbidity and mortality after heart transplantation. Interact Cardiovasc Thorac Surg. 2017 Sep;25(3):384–90. https://doi.org/10.1093/icvts/ivx151
35. Seferović PM , Piepoli MF , Lopatin Y , Jankowska E , Polovina M , Anguita-Sanchez M , et al.; Heart Failure Association Board of the European Society of Cardiology . Heart Failure Association of the European Society of Cardiology Quality of Care Centres Programme: design and accreditation document. Eur J Heart Fail. 2020 May;22(5):763–74. https://doi.org/10.1002/ejhf.1784
36. Januzzi JL Jr , Prescott MF , Butler J , Felker GM , Maisel AS , McCague K , et al.; PROVE-HF Investigators. Association of Change in N-Terminal Pro-B-Type Natriuretic Peptide Following Initiation of Sacubitril-Valsartan Treatment With Cardiac Structure and Function in Patients With Heart Failure With Reduced Ejection Fraction. JAMA. 2019;322(11):1–11. https://doi.org/10.1001/jama.2019.12821
37. Costanzo MR , Dipchand A , Starling R , Anderson A , Chan M , Desai S , et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients J Heart Lung Transplant 2010; 29 8):914-956. doi:https://doi.org/10.1016/j.healun.2010.05.034
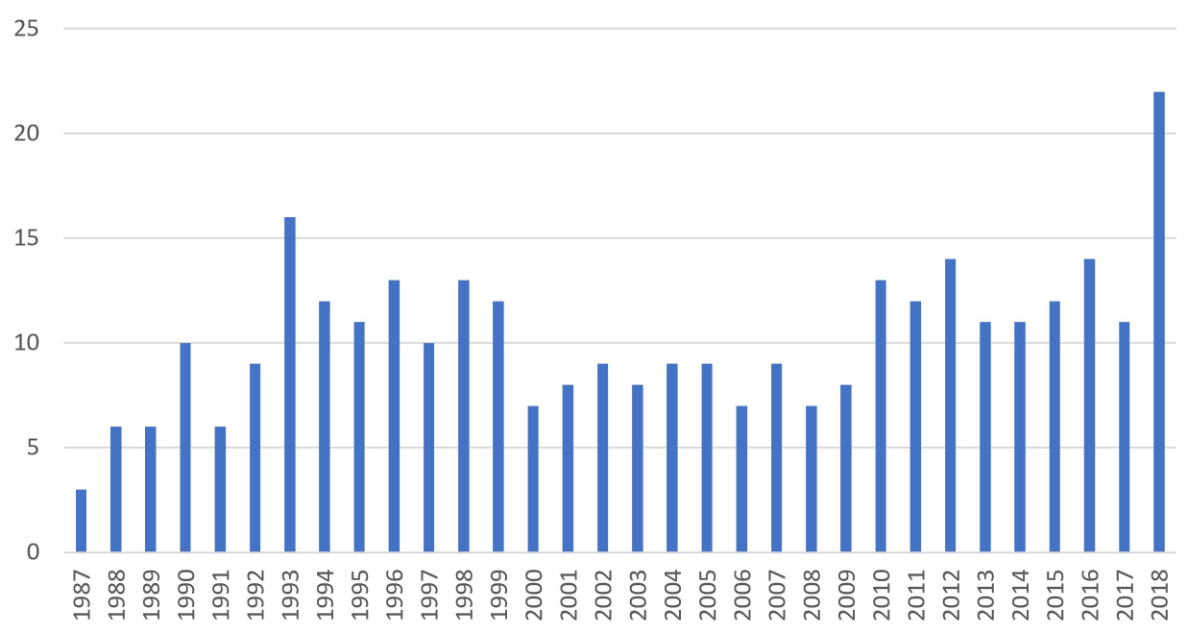
Figure S1 Number of annual heart transplantation operations from 1987 to 2018. Bars represent absolute numbers.
Table S1 Kaplan-Meier estimate of 1-year mortality of patients surviving at 1 year post-transplant (n = 270, 97 events).
| Time after transplantation | % Survival | Patients at risk |
| 1 year | 100% | 270 |
| 3 years | 95.8 ± 1.3 | 215 |
| 5 years | 93.0 ± 1.7 | 193 |
| 10 years | 81.9 ± 2.8 | 131 |
| 15 years | 67.8 ± 3.6 | 89 |
| 20 years | 44.6 ± 4.4 | 42 |
Data are expressed as % ± standard deviation
Table S2Pretransplantation laboratory values.
| Variable | All patients(n = 328) | 1987–1998(n = 115) | 1999–2010(n = 106) | 2011–2018(n = 107) | p-value |
| Renal function | |||||
| Creatinine (µmol/l), median (IQR) | 103.5 (48.3) | 110.0 (33.0) | 114.0 (57.0) | 98.0 (45.8) | 0.08 |
| Creatinine >150 µmol/l | 42 (12.8%) | 12 (10.4%) | 17 (16%) | 13 (12.1%) | 0.66 |
| BUN (mmol/l), median (IQR) | 8.2 (5.4) | 8.4 (5.7) | 9.9 (11.6) | 7.7 (4.4) | 0.06 |
| Blood count | |||||
| Hb (g/l), median (IQR) | 131.5 (26.8) | 147.0 (32.0) | 130.0 (31.0) | 130.0 (244.0) | 0.0001 B, P1 vs (P2+P3) |
| Leucocytes (G/l), median (IQR) | 7.7 (3.1) | 7.9 (5(IQR).0) | 7.6 (3.1) | 7.6 (2.8) | 0.16 |
| Platelets (G/l), median (IQR) | 209.5 (92.3) | 208.0 (237.0) | 217.0 (93.0) | 204.5 (96.8) | 0.96 |
| Hepatic function | |||||
| Total bilirubin (µmol/l), median (IQR) | 13.0 (10.0) | 18.0 (23.0) | 15.0 (12.0) | 10.0 (10.8) | 0.0001 B |
| Total bilirubin >3N (63 U/l) | 1 (0.6%) | 1 (2.4%) | 0 (0%) | 0 (0%) | 0.24 |
| ASAT (U/l), median (IQR) | 29.0 (17.0) | 30.0 (18.0) | 27.0 (10.0) | 31.0 (17.8) | 0.56 B 0.04 |
| ASAT >3N (150 U/l) | 15 (5.3%) | 9 (11.7) | 5 (5.0%) | 1 (0.9%) | 0.006 |
| ALAT (U/l), median (IQR) | 27.5 (25.3) | 36.0 (29.0) | 21.0 (10.0) | 31.0 (25.8) | 0.017 A,B |
| ALAT >3N (180 U/l) | 11 (3.9%) | 9 (11.5%) | 1 (1.0%) | 1 (0.9%) | 0.0001 |
| Iron level | |||||
| Iron (µmol/l), median (IQR) | 13.3 (9.4) | 13.1 (5.9) | 10.1 (10.9) | 14.2 (8.7) | 0.0001 B,C |
| Iron <10 µmol/l | 76 (33%) | 12 (30%) | 42 (46.7%) | 22 (22%) | 0.001 |
Data are presented as absolute number and percentage or median (IQR).
ALAT: alanine aminotransferase; ASAT: aspartate aminotransferase; BUN: blood urea nitrogen; Hb: haemoglobin
A: period 2 versus period 1; B: period 3 versus period 1; C: period 3 versus period 2
Table S3Causes of death <3 months in the total cohort (n = 39).
| Cardiac | 16 (5%) |
| Bleeding | 12 (4%) |
| Infection | 6 (2%) |
| Rejection | 3 (1%) |
| Neurological | 2 (0.6%) |
Table S4Comparison of survivors and non-survivors in period 2.
| 1 year survivors (n = 75) | 1 year non-survivors (n = 31) | p-value | |
| Sex (M/F) | 58/17 | 23/8 | 0.8 |
| Age (years) | 50 ± 13.9 | 50 ± 15.4 | 0.9 |
| Smoking | 36 (49%) | 12 (39%) | 0.4 |
| Arterial hypertension | 19 (25%) | 9 (29%) | 0.7 |
| Diabetes | 9 (12%) | 6 (19%) | 0.3 |
| Dyslipidaemia | 32 (44%) | 16 (53%) | 0.4 |
| COPD | 8 (11%) | 4 (13%) | 0.7 |
| Dialysis | 2 (3%) | 1 (3%) | 0.9 |
| Time on waiting list (days) | 210.3 ± 239 | 247.4 ± 208 | 0.5 |
| LVEF (%) | 22.9 ± 11.8 | 26.6 ± 13.7 | 0.2 |
| PVR | 2.7 ± 1.6 | 2.6 ± 0.8 | 0.5 |
| VO2max | 13.7 ± 3.7 | 10.4 ± 3.5 | 0.03 |
| HR >80 bpm | 13 (19%) | 8 (26%) | 0.4 |
| CRT | 18 (24%) | 5 (16%) | 0.4 |
| Preoperative LVAD | 12 (16%) | 4 (13%) | 0.7 |
| Diuretic | 65 (87%) | 26 (84%) | 0.8 |
| MRA | 45 (60%) | 19 (61%) | 1.0 |
| ACE-I or ARB | 59 (79%) | 19 (61%) | 0.07 |
| Beta-blocker | 37 (49%) | 14 (45%) | 0.7 |
| Creatinine (µmol/l) | 131.0 ± 85.8 | 121.3 ± 68.1 | 0.6 |
| Creatinine >150 µmol/l | 14 (19%) | 3 /10%) | 0.23 |
| BUN (mmol/l) | 12.0±8.8 | 10.7±6.1 | 0.5 |
| Total bilirubin (µmol/l) | 17.8±7.5 | 23.6±9.7 | 0.03 |
| Total bilirubin >3N | 0 (0%) | 0 (0%) | - |
| ASAT (U/l) | 46.0 ± 69.4 | 59.7 ± 127.6 | 0.5 |
| ASAT >3N | 4 (6%) | 1 (3%) | 0.6 |
| ALAT (U/l) | 43.8 ± 70.9 | 28.4 ± 17.5 | 0.3 |
| ALAT >3N | 1 (1%) | 0 (0%) | 1.0 |
| Iron <10 µmol/l | 36 (55%) | 12 (48%) | 0.5 |
| Hb (g/l) | 124.9 ± 19.3 | 127.2 ± 24.7 | 0.6 |
| WBC (G/l) | 8.8 ± 3.5 (n = 73) | 7.2 ± 2.0 (n = 31) | 0.02 |
| Platelets (G/l) | 225.1 ± 78.7 | 206.9 ± 68.8 | 0.3 |
| Time on waiting list (days) | 210.3 ± 239 | 247.4 ± 208 | 0.5 |
| Cold ischaemia time (min) | 180.5 ± 47.2 | 185.3 ± 58.4 | 0.7 |
| Redo surgery | 27 (36%) | 12 (39%) | 0.8 |
| Gender mismatch | 28 (38%) | 11 (36%) | 0.8 |
ALAT: alanine aminotransferase; AST: aspartate aminotransferase; ACE-I: angiotensin converting enzyme inhibitor; ARB: angiotensin II receptor blocker; BUN: blood urea nitrogen; COPD: chronic obstructive pulmonary disease; CRT: cardiac resynchronisation therapy; LVEF: left ventricular ejection fraction; Hb: haemoglobin; HR: heart rate; LVAD: left ventricular assist device; MRA: mineralocorticoid receptor antagonist; PVR: pulmonary vascular resistance in Wood Units; VO2max: maximal oxygen consumption in ml/kg/min; WBC: white blood cell count.