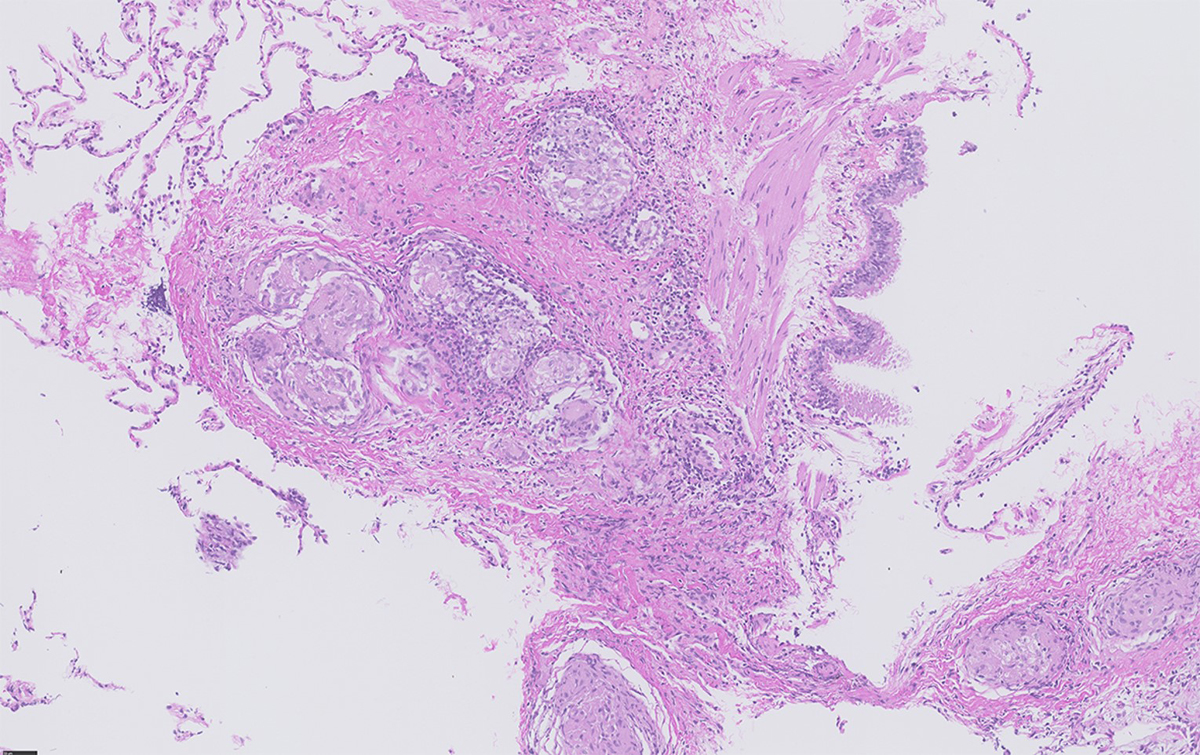
Figure 1 Transbronchial biopsy showing multiple non-necrotising granulomas situated in close proximity to the bronchial mucosa (bronchovascular distribution) (courtesy Dr Bart Vrugt, Cantonal Hospital Muensterlingen).
DOI: https://doi.org/10.4414/SMW.2022.w30049
Sarcoidosis is a systemic inflammatory disease, characterised by granuloma formation upon an unknown trigger. Innate and adaptive immune response in genetically predisposed individuals has been associated with its pathogenesis [1] .
Pulmonary involvement is the most common manifestation in sarcoidosis, although all organs can be affected, with several distinguishable clinical phenotypes. The disease course can vary significantly (table 1), ranging from spontaneous recovery, through chronic active inflammation to a post-inflammatory state, eventually resulting in an irreversible impairment of organ function such as pulmonary fibrosis, congestive heart failure or renal failure. In addition, quality of life is often substantially compromised owing to direct organ damage, but also because of sarcoidosis-associated comorbidities, of which fatigue has been increasingly recognised and investigated in recent past years [2].
Table 1Organ involvements and symptoms in sarcoidosis.
| Organ | Possible related signs and symptoms | Prevalence |
| Lung | Cough, wheezing, stridor, dyspnoea, chest pain | 80–90% |
| Lymph nodes | Lymphadenopathy, pain | 90% hilar/paratracheal/mediastinal, 40% in the periphery |
| Eyes | Red eye, pain, vision loss | 25–50% |
| Skin | Mild to moderate tender, itchy, papules, nodules, plaques, subcutaneous nodules, infiltrated scars and tattoos, lupus pernio, erythema nodosum | Up to 25% |
| Liver | Abdominal pain and elevated liver enzymes | Up to 20% |
| Nervous system | Cranial mononeuropathy (facial palsy, visual loss,trigeminal neuralgia, hearing loss, vertigo), gait abnormality, headache, seizure, weakness, numbness, paraesthesia, paresis, fatigue | 510% (facial palsy up to 50%) |
| Arthropathy | Pain, tenderness, stiffness, swelling, dysfunction, inflammation, warm erythematous skin, weakness | Up to 15% |
| Muscle | Usually asymptomatic; could also involve diaphragm or extraocular muscles | Up to 10% |
| Heart | Pain, arrhythmias, conductance disturbances, dyspnoea, syncope, fatigue | 27%, in post-mortem studies up to 83% |
| Spleen | Abdominal pain, swelling | Up to 6% |
| Kidney | Decreased renal function (reduced eGFR) due to interstitial nephritis, hypercalcaemia or nephrocalcinosis; flank/abdominal pain due to nephrolithiasis; interstitial nephritis (urinalysis normal or displaying sterile pyuria or mild tubular proteinuria, rarely haematuria or glycosuria); sarcoidosis patients may present with glomerulonephritis although causal relationship to sarcoidosis has not been proven | Nephrocalcinosis about 5%, interstitial nephritis about 20% |
| Exocrine glands (parotid and salivary) | Sicca syndrome, xerostomia | About 5% |
| Bone and bone marrow | Pain, tenderness, and sometimes swelling; cytopenia (anaemia, leukopenia, lymphopenia) | About 5% |
| Gastrointestinal tract | (Apart from liver) most commonly oral cavity and stomach, rarely oesophagus, small intestine, appendix, colon, rectum, pancreas, peritoneum | About 1% |
| Upper respiratory tract (laryngeal and sinonasal) | Dysphagia, dyspnoea, cough, stridor and hoarseness. Nasal obstruction / crusting / polyps, anosmia, epistaxis | About 1% |
| Parasarcoidosis symptoms | Fatigue, sleep disturbances, cognitive deficits. hypercalcaemia, hypercalciuria | Up to 70%. Up to 20% and 40%, respectively |
eGFR: estimated glomerular filtration rate
The incidence of sarcoidosis is variable, depending on age, gender and ethnicity, with seasonal and geographic variations, indicating the possible influence of environmental factors such as microorganisms or inorganic materials in genetically predisposed individuals, carrying certain human leucocyte antigen (HLA) variants.
The formation of non-necrotising epithelioid cell granulomas, which are surrounded by activated T helper 1 (Th1) and Th17 cells suggests that specific antigens trigger an inflammatory process. Interestingly, in multiple individuals with sarcoidosis, identical CD4+ T cell receptor (TCR) repertoires, so called public TCRs, have been found. These T cells recognise the specific sarcoidosis-associated epitopes in the context of HLA-DR3 [3, 4]. Thus, these public TCRs could explain how similar (public) T cell responses are triggered in multiples individuals by certain antigens and lead to a sarcoidosis phenotype.
Histological confirmation should be sought whenever possible to strengthen diagnosis and exclude differential diagnoses such as infection or cancer. Assessments include laboratory, imaging and organ-specific investigations (table 2). Recent diagnostic approaches, such as 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET), cardiac magnetic resonance imaging (cMRI), detection of perfusion deficits in the myocardium with 13N-ammonia or 82Rb PET, allow early diagnosis and risk stratification. Although data are still limited, certain HLA types are associated with a self-limiting, rather than a chronic, course and could add information towards risk stratification. Finally, based on more diagnostic possibilities, a more personalised therapeutic approach can be pursued, leading towards a tailored selection of immunosuppressive treatment in selected cases, including glucocorticoids (GCs) and steroid-sparing agents such as azathioprine, methotrexate, leflunomide, hydroxychloroquine, mycophenolate mofetil and biologicals such as tumour necrosis factor-alpha (TNFα) inhibitors (TNFis).
Table 2Diagnostic approach for minimum organ screening assessment.
| Diagnostic approach | Measures |
| Medical history | Environmental and occupational factors (dust, beryllium, etc.), comorbidities, medication (current and former), family history of sarcoidosis |
| Symptom evaluation | All organ systems: particularly lungs, heart, central nervous system, eyes and skin |
| Physical examination | |
| Laboratory (minimal screening) | Differential blood count, liver (AP, γGT) and kidney (creatinine, eGFR) values, serum calcium, 25- and 1,25-OH vitamin D (calcidiol, calcitriol), total IgG (incl. subclasses), IgA, IgM, CRP |
| Sarcoidosis-associated biomarkers: ACE, sIL-2Rα, neopterin | |
| Urine: urine sediment, urine calcium/24 h, calcium/creatinine, urine protein/creatinine and albumin/creatinine ratios | |
| Microbiology: IFNγ release assay, e.g., QuantiFERON-TB Gold® | |
| Possible laboratory assessments in certain clinical settings (not mandatory) | CK, SAA, ESR, protein electrophoresis and further cytokines like TNFα or IL-17 |
| HLA typing | |
| Specific organ assessment | |
| Heart | Screening: ECG, 24h-ECG, echocardiography |
| In symptomatic patients or suspicion of cardiac involvement: troponin T, pro-BNP. cardiac MRI and cardiac PET-CT | |
| Eyes | Ophthalmological work-up |
| Lungs | High-resolution chest CT, spirometry, plethysmography, DLCO. |
| Bronchoscopy with BAL and histological confirmation in case of positive CT findings (or at the place lowest invasive burden, see text) | |
| In symptomatic individuals: cardio-pulmonary exercise testing / 6-minute walking test | |
| Abdomen / lymph nodes | Sonography |
| In the case of symptoms or suspicious organ involvement | |
| Skin | Biopsy |
| Kidney | Ultrasound or abdominal CT (highly sensitive for nephrocalcinosis or nephrolithiasis), renal biopsy |
| CNS | In the case of symptoms, cranial and spinal MRI, lumbar puncture (cerebrospinal fluid analyses: cell count, protein, immunoglobulins, oligoclonal band, ACE, sIL-2R, CD4/CD8 ratio) |
| Joints | Rheumatological referral, possibly ultrasond, MRI, puncture |
| Exocrine glands | Sonography, sicca assessment (e.g., sialography), Schirmer's test |
| Hypothalamic-hypophyseal system | Endocrinologist referral |
ACE: angiotensin converting-enzyme; AP: alkaline phosphatase; BAL: bronchoalveolar lavage; CK: creatine kinase; CRP: C-reactive protein; CT: computed tomography; DLCO: diffusing capacity of lung for carbon monoxide; eGFR: estimated glomerular filtration rate; ESR: erythrocyte sedimentation rate; γGT: gamma-glutamyltransferase; HLA: human leucocyte antigen; Ig: immunoglobulin; IFNγ: interferon gamma; IL: interleukin; MRI: magnetic resonance imaging: PET: positron emission tomography; pro-BNP: pro-B-type natriuretic peptide; SAA: serum amyloid A; sIL-2Rα: soluble interleukin-2 receptor alpha; TNFα: tumour necrosis factor-alpha
The awareness that sarcoidosis is recognised increasingly as an inflammatory, systemic granulomatous disease, requires also the inclusion of a multidisciplinary team if multi-organ involvement is suspected.
The incidence and prevalence of sarcoidosis vary across geographical regions, age, sex and ethnicities [5–7]. The highest incidence is found in Scandinavian countries (11–24 cases per 100,000 individuals per year) [8–10] and African Americans (18–71 cases per 100,000 individuals per year) [11-13], and the lowest incidence is found in Asian countries (1 case per 100,000 individuals per year) [14–16]. The incidence in Switzerland was reported to be 7 per 100,000 individuals per year with a higher regional occurrence in areas with metal industry and intense agriculture, especially production of potatoes, artificial meadows and bread grains [17].
The average age of onset is 40–55 years, with a peak in younger men between the ages of 30 and 50 years and middle-aged women between 50 and 60 years of age, without gender predilection [15, 18, 19]. African American ethnicity and low income are associated with more severe disease at diagnosis [20–22].
Genetic susceptibility has been shown in genome wide association studies with several HLA risk alleles predisposing for prognostic outcome [23, 24]. For example, DRB1*01 and DQB1*0501 seem to be protective, whereas DRB1*12 and *14 are associated with lung involvement [25]. Also, non-HLA different risk variants, for example in BTNL2, have been associated with sarcoidosis [26], and shown in Japanese [27] as well as British or Dutch [28] patients. However, sarcoidosis will occur in these susceptible individuals only if an external trigger is present, which activates the immune system. Since lung involvement is the most prevalent manifestation of sarcoidosis, inhalational exposure is a possible driving cause. Several studies have investigated associations between occupational exposures and sarcoidosis [17, 29]; for example an association in New York City Fire Department rescue workers after 09/11 has been suggested [30]. On the other hand, cigarette smoking and oestrogens, possibly due to immunomodulatory effects, are associated with lower risk of developing sarcoidosis [1].
Granuloma formation occurs to isolate potentially non-degradable antigens, such as pathogenic organisms such as certain Mycobacterium spp. or inorganic particles. In addition, granulomas may form as a result of drug reactions (interferon, immune checkpoint inhibitors, BRAF inhibitors, TNFis, antiretroviral therapy or immune reconstitution,), or due to potential self-antigens such as vimentin [31–34]. It is important to note that these peptide remnants, particularly from bacteria or viruses (e.g., mycobacterial catalase–peroxidase [KatG], superoxide dismutase, early-secreted antigenic target of 6 kDa [ESAT6] or heat-shock proteins), are seen as an initial and temporary trigger for the immune reaction rather than being continuously present [35–42].
Epithelioid non-necrotising granulomas (fig. 1) represent the pathological hallmark of sarcoidosis with an innate immune response characterised by activated macrophages (transforming into epithelioid cells fusing into giant cells) and dendritic cells. This fosters an adaptive immune response with a polarisation towards Th1 and Th17 cells and increased production of interferon-γ (IFN) and interleukin (IL)-17, as well as TNFα, IL-12, IL-18, IL-6, transforming growth factor β and IL-10. The activation of the Janus kinase (JAK)–STAT signaling pathway by INFγ for STAT1 has been shown in peripheral blood, lung tissue and lymph nodes of sarcoidosis patients [43–46] and IL-17 for STAT3 [47]. Additionally, activation of mechanistic target of rapamycin complex 1 (mTORC1) in progressive disease [48] and Toll-like or NOD-like receptors (TLRs, NLRs) have been associated with innate immune activation [49]. Maintenance and progression of granuloma formation can lead to chronic inflammation and tissue fibrosis.

Figure 1 Transbronchial biopsy showing multiple non-necrotising granulomas situated in close proximity to the bronchial mucosa (bronchovascular distribution) (courtesy Dr Bart Vrugt, Cantonal Hospital Muensterlingen).
Abundance of TCR-restricted, for example TRAV2.3+, T cells in bronchoalveolar lavage (BAL) fluid of HLA-DR3+ patients is associated with a good prognosis in Löfgren’s syndrome [50, 51]. Vimentin-specific TRAV2.3+ TRBV22+ T cells and production of anti-vimentin antibodies have been found in BAL of sarcoidosis patients [52, 53]. Increased production of serum amyloid A by macrophages and its accumulation in granulomas could provide an additional mechanism for Th1 activation and disease progression in the absence of a microbiological trigger [36].
The immune activation is accompanied by impaired regulatory T cell-mediated immune controls [1] and their survival [54]. TNF receptor-2 positive (TNFR2+ ) regulatory T (Treg) cells and soluble TNFR2 were also shown to be elevated in sarcoidosis patients with higher levels correlating with treatment response [55]. Additionally, in Löfgren’s syndrome, higher levels of Tregs were found, which could explain its self-limiting course [56].
Sarcoidosis is a multisystem disease, which may present with a broad range of symptoms, over various time-points, and organ manifestations (see table 1). It can be defined by onset (acute or gradual), disease course (self-limiting, chronic-stable or chronic-progressive) and major organ involvement. The most severe and potentially life-threatening organ manifestations include pulmonary, cardiac and neurological sarcoidosis. The disease resolves within approximately two years in 50% of patients. Conversely, full remission is less probable after a disease course of five years [57].
Pulmonary sarcoidosis includes involvement of the lungs and/or mediastinal/hilar lymph nodes and is the most common manifestation, affecting 80–90% of sarcoidosis patients. Clinical presentation includes cough, dyspnoea, and chest pain, which can be accompanied by fatigue, weight loss, fever and malaise [58]. The classification by John ‘Guy’ Scadding based on chest radiography is still in use and associated with a prognostic value [59]. However, there are several limitations of this system, such as lack of information on disease severity due to extrapulmonary involvement or on the risk of progression [1]. Lung function test findings are highly nonspecific, since pulmonary sarcoidosis can present with obstructive, restrictive, mixed or normal patterns, but are important tools for disease severity assessment, treatment indication and response. Interstitial lung disease (ILD) is typically present in Scadding stages 2, 3 and 4, ranging from subclinical manifestations to end-stage pulmonary fibrosis (stage 4). The latter is irreversible organ damage, whereas mild to moderate ILD due to sarcoidosis is a potentially treatable and reversible condition (fig. 2).
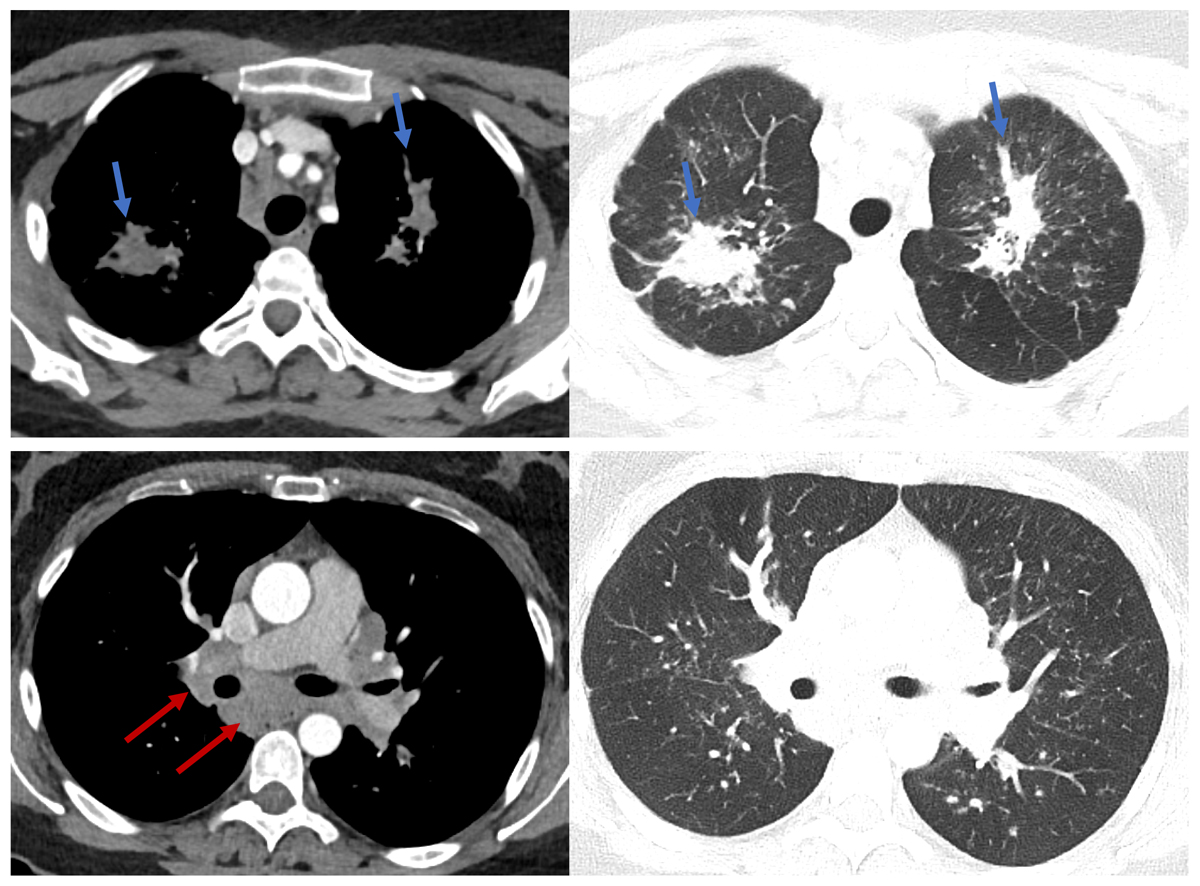
Figure 2 Axial chest computed tomography (CT) images of a 41-year-old female with histologically proven sarcoidosis. Upper panel: extensive, bilateral pulmonary consolidation along peri-bronchovascular bundle (blue arrow) and multiple micronodular changes in upper lobes. Lower panel: extensive mediastinal and bi-hilar lymphadenopathy (red arrow). Clearly less nodular changes in lower lobes (courtesy Dr D. Franzen, USZ).
Extrapulmonary manifestations occur in up to 30% of patients, and basically every organ can be affected including skin (about 25%), eye (about 25–50%), liver (about 20%), secondary lymphoid organs (lymph nodes 40% in the periphery, spleen about 6%), heart (2–7%), nervous system (5–10%), kidney (interstitial nephritis 20%, nephrocalcinosis 5%), arthropathy (up to 15%), muscle (up to 10%), exocrine glands (parotid and salivary, about 5%), bone marrow, gastrointestinal tract (about 1%) and upper airways (about 1%) [20, 60–62]. Manifestations vary based on gender, age and ethnicity, for example skin and eye involvements are more common in African Americans [63].
Ocular sarcoidosis is found in 25–50% of cases [64]. The most frequent ocular manifestation is uveitis. Although anterior uveitis occurs more frequently, the presence of intermediate or posterior uveitis often decreases visual prognosis [64]. It can be the first sign in 20% of sarcoidosis cases and may precede pulmonary manifestations by several years. Almost all parts of the eye, adnexa and orbit may be affected by granulomatous involvement, which is usually bilateral [65].
Cutaneous involvement is seen in up to 25% of patients, often presenting with papules and plaques, followed by subcutaneous nodules, scar or lupus pernio and plenty of rare manifestations resembling other diseases. These manifestations show non-caseating granulomas in histology. Erythema nodosum is the most common nonspecific cutaneous lesion, characterised as a reactive process without granuloma formation, and associated with a good prognosis. Treatment indication depends on symptoms and cosmetic disfigurement, usually beginning with topical treatments (mainly glucocorticoids), which can be extended to systemic treatments in refractory cases [66, 67].
Cardiac sarcoidosis is a rare but potentially life-threatening manifestation, which is observed in 2–7% of sarcoidosis patients and in up to 83% of cases in autopsy series [68–72]. Thus, it is assumed that a significant proportion of at least subclinical cardiac involvement is unrecognised. This applies also to other organs that are not routinely biopsied. According to unpublished data from several authors of this article (DF, JN, AK, JDS), a high prevalence of cardiac involvement (approximately 50%) can be observed in highly specialised sarcoidosis clinics, where modern imaging technologies may lead to detection of early cases and increased diagnostic yield in general. Cardiac involvement can result in ventricular arrhythmias, high-degree heart blocks or progressive heart failure due to myocardial granulomatous infiltration and/or fibrosis at a later stage of the disease (fig. 3). Symptoms include chest pain, palpitations, dizziness and syncope. As sudden cardiac death can occur in up to 25% of patients with cardiac sarcoidosis, an early diagnosis and appropriate treatment is crucial [73].
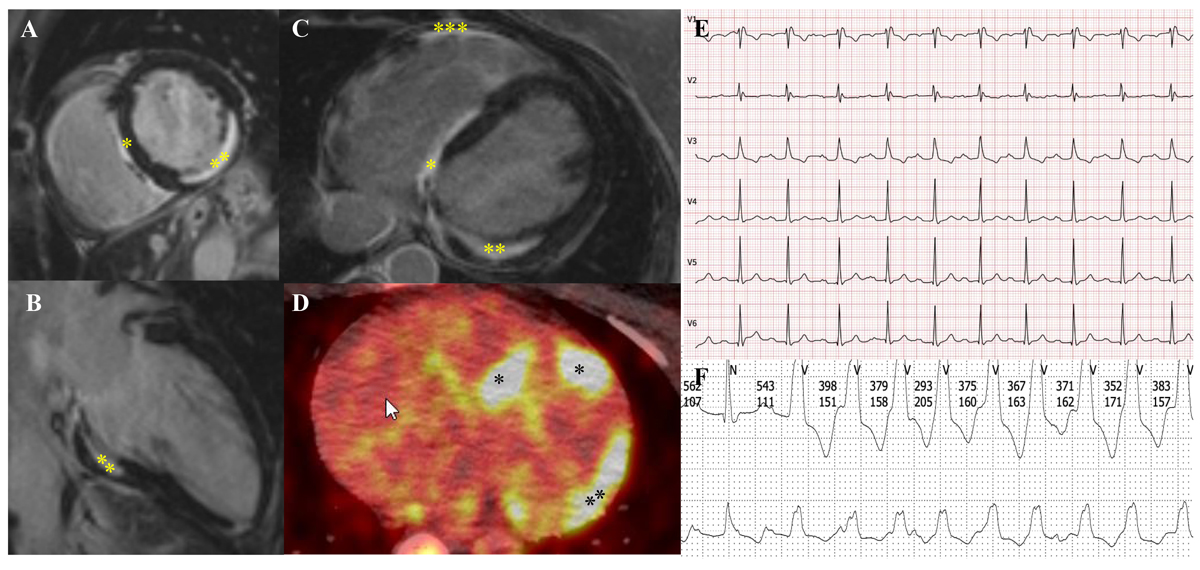
Figure 3 51-year-old woman with sarcoidosis and cardiac involvement. A–C demonstrates myocardial fibrosis in the interventricular septum (*), inferolateral wall (**) and the right ventricle (***) on cardiac magnetic resonance imaging. D shows the corresponding cardiac FDG-PET/CT with mild inflammation of the septum (*) and the lateral wall (**). E: Her ECG shows a compromised conduction system with first degree atrioventricular block (PQ time 264 ms) and complete right bundle branch block. F: She had repeated nonsustained ventricular tachycardia on Holter monitor. With consideration of all findings together, she was recommended to undergo insertion of a two-chamber intracardiac cardioverter defibrillator for primary prophylaxis of sudden cardiac death (courtesy Dr C. Gruner, USZ).
Neurosarcoidosis occurs in 5–10% of patients. Any level of the neuroaxis can be affected, which is reflected by the wide spectrum of symptoms (fig. 4) and varying disease severity. Whereas 10% of patients remain asymptomatic, one third to half of the patients with neurosarcoidosis exhibit more than one neurological manifestation [74]. Cranial nerve palsies occur frequently (50–70%), with (bilateral) facial nerve palsy being predominantly observed [75, 76], followed by involvement of the optic or vestibulocochlear nerves. If multiple nerves are affected a chronic course can be assumed, whereas isolated cranial neuropathies often resolve spontaneously. Aseptic meningitis (mostly affecting the basal meninges) ranks second to the most common manifestations, showing a considerable variety from asymptomatic to acute or chronic forms. Chronic granulomatous inflammation can impair cerebrospinal fluid (CSF) circulation leading to a hydrocephalus. The third most common manifestation is cerebral parenchymal infiltration, mainly involving the hypothalamus and the pituitary gland. As a result, neuroendocrine dysfunction can occur, including disturbances in appetite, thirst, temperature, sleep and/or libido [77]. Polyuria is usually caused by diabetes insipidus, which develops either centrally as a result of hypothalamic involvement or peripherally due to hypercalcaemia-related renal dysfunction [78]. Cortical involvement can cause cognitive or behavioural impairments, focal neurological deficits and seizures. Cognitive or behavioural disturbances were reported in up to 20% of neurosarcoidosis patients [79]. Encephalopathy can be caused by both vasculopathy or diffuse parenchymal inflammation. Granulomatous invasion of cerebral vessels is prevalent, though vascular complications such as ischaemic stroke solely occur [80, 81]. Cerebral mass lesions are also a rare finding. Compared with older reports, the emergence of MRI revealed a considerably higher involvement of the spinal cord [82], with lesions spreading over several segments of mostly the cervical and thoracic spinal cord [83]. In addition, radiculitis and cauda equina syndromes can occur. In later stages of the disease extra-cerebral manifestation can involve the peripheral nervous system (4–14% of the cases; including mononeuropathy, mononeuritis multiplex, and generalised motor, sensorimotor and sensory polyneuropathies) [84] and the muscles (sarcoidosis-related myopathy in 7–12% of the cases) [85].
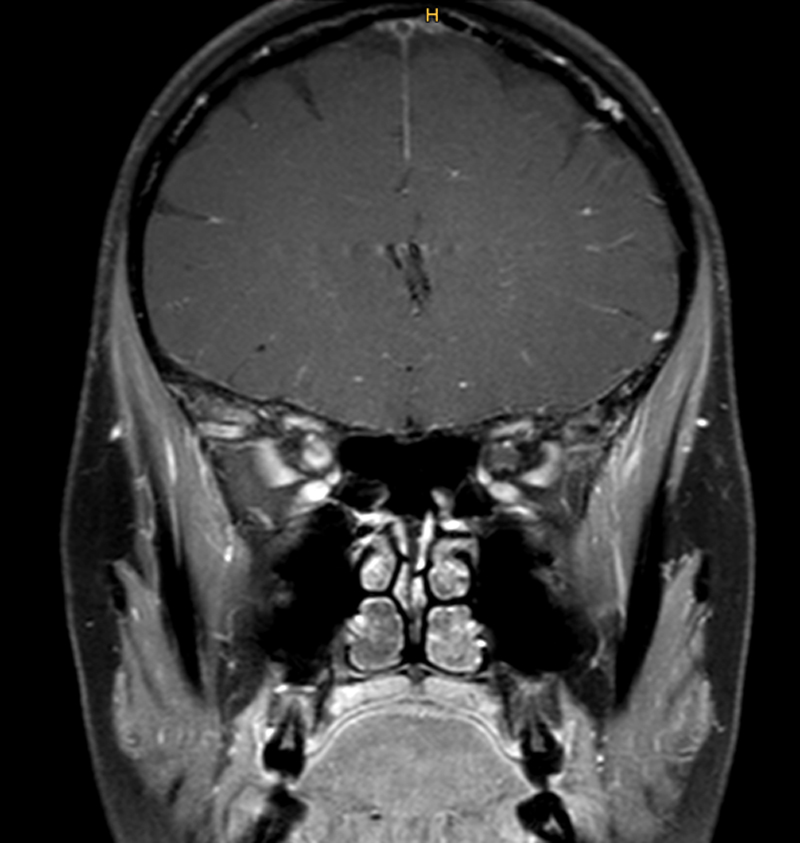
Figure 4 Coronal T1-weighted magnetic resonance imaging scan of the brain showing contrast-media enhancement of the right optic nerve (arrow) in a 43-year-old woman who presented with progressive vision loss on the right. Meningioma was expected; however a biopsy showed non-necrotising granulomas leading to the diagnosis of neurosarcoidosis (courtesy Dr H. Hayward-Könnecke, USZ).
Renal sarcoidosis occurs in up to one third of patients and can manifest in several ways [60, 86]. The most common is granulomatous interstitial nephritis leading to acute or chronic renal failure. Since this manifestation is clinically asymptomatic until severe renal failure occurs, it must be actively sought. Obstructive uropathy leading to abdominal/flank pain and/or deteriorating renal function is typically caused by nephrolithiasis, but may also be a consequence of retroperitoneal fibrosis or obstructing retroperitoneal lymph nodes. Chronic renal failure may also be a result of diffuse nephrocalcinosis caused by persistent hypercalciuria. Glomerular involvement is rare and, if present, may manifest with various degrees of albuminuria and glomerular haematuria.
Para-sarcoidosis syndromes are not caused by organ-specific granuloma manifestations but result from release of mediators and can even persist after adequate treatment and disease remission. Post-sarcoidosis fatigue syndrome is one of the most challenging manifestations, as no specific treatment options exist and quality of life is severely affected. This is also the case for depression and cognitive impairment [87]. Clinically relevant in patients with systemic sarcoidosis is the development of a small-fibre neuropathy (in up to 70% of the cases). Apart from a painful hyper- or hypoaesthesia, life-threatening autonomic dysfunction, including cardiac arrythmia, can occur [88]. Hypercalcaemia (up to 20% of patients) and hypercalcuria (up to 40% of patients) are caused by extensive synthesis of calcitriol by activated macrophages increasing gastrointestinal calcium absorption and osteoclast-mediated bone resorption [89].
Löfgren’s syndrome is an acute form of sarcoidosis presenting with fever, bilateral ankle arthritis, and/or erythema nodosum and bilateral hilar lymphadenopathy [90]. It has a good prognosis with a remission in 70–80% of these patients [91].
Heerfordt's Syndrome is an extremely rare variant defined by uveitis, enlargement of the parotid and submaxillary salivary glands and paresis of the cranial nerves, particularly the facial nerve.
Sarcoidosis is characterised by compact non-necrotising granulomas with a lymphangitic distribution along the bronchovascular bundle, interlobular septa and pleura. In open lung biopsies sarcoidosis is frequently associated with a granulomatous vasculitis without destruction of the vessel walls. During progression of the disease hyalinised fibrosis with remnants of granuloma dominate the picture. The main differential diagnoses are berylliosis and infliximab-induced granulomatous disease, especially because these entities are histologically indistinguishable from sarcoidosis. Other differentials include conditions associated with sarcoid-like disorders such as malignancies (lymphoma, carcinoma), collagen vascular diseases (systemic lupus erythematosus, Sjögren’s syndrome, primary biliary cirrhosis, familial granulomatous arthritis), infections (human immunodeficiency virus, tuberculosis), vasculitis (granulomatosis with polyangiitis, Takayasu arteritis, giant cell arteritis), hypersensitivity pneumonitis, hard metal pneumoconiosis, IgG4-related disease and common variable immunodeficiency [33, 58, 92]. However, morphology and distribution of the granulomas in these diseases differ from sarcoidosis.
Infectious diseases such as tuberculosis, Mycobacterium avium, histoplasmosis, coccidiomycosis and Whipple disease demonstrate a peribronchial or random distribution of the granulomas and are frequently associated with necrosis. The application of special stains (Ziehl-Neelsen, auramin and silver stains] or polymerase chain reaction (PCR) tests for Mycobacterium tuberculosis complex and atypical mycobacteria help to identify the microorganisms. In contrast to sarcoidosis, hypersensitivity pneumonitis is characterised by loose aggregates of histiocytes in close proximity of the bronchiole. Granulomatosis with polyangiitis (formerly Wegener's granulomatosis) is characterised by basophilic, geographic necrosis surrounded by a cellular infiltrate containing giant cells. Nodular sarcoid granulomatosis, currently regarded as a variant of sarcoidosis, also shows extensive necrosis, which, in contrast to granulomatosis with polyangiitis, is eosinophilic, demarcated from numerous compact granulomas and accompanied by granulomatous vasculitis without destruction of the vessel walls. Differential diagnosis in the case of a neurological manifestation should be based on MRI findings (predominant periventricular, focal lesions vs parenchymal mass lesions or meningeal lesions). New MRI techniques increased sensitivity; however, due to the lack of specificity the spectrum of diseases to be considered is extensive. They range from autoimmune, inflammatory or idiopathic (e.g., multiple sclerosis, neuromyelitis optica spectrum disease, systemic lupus erythematosus, Sjögren`s syndrome, Behçet disease, primary central nervous system vasculitis) to infectious (e.g., tuberculosis, Lyme disease, neurosyphilis, toxoplasmosis) entities and neoplasms (primary central nervous system neoplasms, lymphomas and others).
Sarcoidosis patients bear an increased risk of comorbidities, such as infections (hazard ratio [HR] 2.13, depending on immunosuppressive therapy) [93, 94], autoimmune diseases (Sjögren`syndrome: HR 11.6; ankylosing spondylitis: HR 3.8; systemic lupus erythematosus: HR 3.0; and autoimmune thyroiditis: HR 1.3) [93–95], cerebrovascular diseases (HR 3.3) [96], venous thromboembolism (HR 2–4) [97, 98], congestive heart failure (HR 1.7–2.7) [96, 99], and also cancer (skin: relative risk [RR] 2.00; haematological: RR 1.92; upper digestive: RR 1.73; colorectal: RR 1.33; liver: RR 1.79,;and kidney: RR 1.55) [100]. A Swiss analysis compared hospitalisations for sarcoidosis to hospitalisations for other causes and demonstrated an increased re-hospitalisation rate and significantly more comorbidities in sarcoidosis patients [101].
The mortality of sarcoidosis is higher if patients have more severe disease manifestations at time of diagnosis. Overall, it ranges from 9–14 cases per 1000 person-years. The 5-year overall survival is estimated to be 93–95%. The mortality risk varies depending on gender and race, with a 2.4-fold increase in African American women [102–107]. A Swiss evaluation showed a significantly higher in-hospital mortality of sarcoidosis patients compared with age matched controls (2.6% vs 1.8%) with age being a risk factor [101].
As sarcoidosis is a multisystem disease and shows varying courses, we recommend an interdisciplinary approach for a minimum organ screening assessment (see table 2). Organ involvement can change over time leading to new symptoms. Hence, follow-up examinations are highly recommended and depend on disease severity and activity, symptoms and treatment (see below).
Histological confirmation should be sought in almost all cases, whenever possible [108], to foster diagnosis and exclude other causes of organ dysfunction, particularly those in which immunosuppressive treatment could cause an adverse outcome. However, a clinical diagnosis alone is sufficient in Löfgren’s syndrome presenting with its pathognomonic triad of ankle arthritis, erythema nodosum and bi-hilar lymphadenopathy, or in Heerfordt’s syndrome (see above) [109]. In principle, histological specimens should be sampled at the site with the lowest invasive burden and the highest chances for diagnosis – this is typically done via flexible bronchoscopy. Extrapulmonary biosampling, such as from the skin, parotid or lacrimal glands, palpable lymph nodes or conjunctival lesions, is also possible, but somewhat less specific. In any case, histology needs to be associated with compatible clinical and radiographic manifestations and exclusion of other diseases. If there is a histological confirmation at extrapulmonary sites and concomitant lung involvement with suspicion of infection, such as cavitary pulmonary disease, bronchoscopy may still be required to exclude infectious causes such as mycobacteria and fungi.
So far, no specific test for sarcoidosis exists. We recommend that minimum screening laboratory tests include differential blood count, liver (alkaline phosphatase [AP], gamma-glutamyltransrease [γGT] and kidney (creatinine, glomerular filtration rate [GFR]) tests, serum calcium, 25- and 1,25-OH vitamin D (calcidiol and calcitriol), total IgG including IgG subclasses, IgM, IgA, C-reactive protein (CRP) and sarcoidosis-associated biomarkers such angiotensin converting-enzyme (ACE), soluble IL-2 receptor alpha (sIL-2Rα) and neopterin. Urine assessment includes urine sediment, and calcium/creatinine, albumin/creatinine and protein/creatinine ratios.
Serum amyloid A A, erythrocyte sedimentation rate and certain cytokines such TNFα were found to be elevated and can be useful but are not widely available or performed. Certain centres perform HLA typing to assess risk alleles for potential major organ involvement. For example, DRB1 ∗0803 is associated with increased risk for developing cardiac or neurosarcoidosis [25]. Furthermore, interferon-γ release assay (e.g., QuantiFERON-TB GOLD®) to exclude a (latent) M. tuberculosis infection and serological testing of Histoplasma and Coccidioides infection can be potentially useful and should be considered during initial diagnostic work-up [110].
Since the lung is affected in most cases, bronchoscopy serves as a safe and minimally invasive procedure (table 2). There are several diagnostic procedures during a bronchoscopy with different diagnostic yields, indications, and risks, including endobronchial (mucosa) biopsy, transbronchial lung biopsy (TBLB), transbronchial needle aspiration (TBNA) of hilar/mediastinal lymph nodes and bronchoalveolar lavage (BAL). The latter is the least invasive procedure, but the exclusive finding suggestive of sarcoidosis, with lymphocytosis and an increased CD4+/CD8+ T cell ratio (>3.5), has a low sensitivity of 54% [111] but a high specificity of 94–96% [112]. Therefore, BAL fluid analysis is only a supportive finding in addition to other bronchoscopy biopsy techniques. Endobronchial ultrasonography-guided transbronchial needle aspiration (EBUS-TBNA) is an elegant way to provide samples from hilar/mediastinal lymph nodes with a diagnostic yield of up to 79% (sensitivity 84%, specificity 100%), depending on the skill level of the operator [111, 113]. In the case of radiologically evident involvement of lung parenchyma (ILD), escalation to TBLB (including cryobiopsies) can be added. Using a combination of EBUS-TBNA, mucosal biopsies with BAL and/or TBLB with cryobiopsies, the diagnostic yield can be increased to 93–100% [114, 115].
Pulmonary function testing (spirometry, body plethysmography and CO-diffusion) and high-resolution CT (HRCT) are the preferred methods for the assessment of pulmonary involvement and its severity, as well as treatment response (table 2) [116]. HRCT is superior to conventional chest X-ray as it better defines the extent of parenchymatous involvement and fibrosis [117]. However, in some cases, such as Löfgren’s syndrome, a chest X-ray may still be sufficient.
Additionally, we recommend ECG, 24-hour ECG and echocardiography, as well as abdominal and lymph node sonography for initial minimum organ screening assessment (table 2).
In the case of clinical signs suggestive of other organ involvement, specific further assessments should be evaluated (table 2). In patients with suspected cardiac sarcoidosis the combination of cardiac MRI and 18F-FDG-PET/CT are considered as the diagnostic tools of choice [118].
If neurosarcoidosis is suspected, a brain MRI and a lumbar puncture should be performed. However, pathognomonic cerebrospinal fluid findings do not exist. In the majority of patients, lymphocytic pleocytosis, elevated protein and IgG can be detected. In up to 50% oligoclonal bands are positive [119, 120], also elevated beta-2-microglobulin values can be found [121]. Elevated ACE CSF levels were shown in only 28% of the individuals in a large retrospective analysis [122], thus ACE levels are not necessarily of help in diagnosing neurosarcoidosis. A biopsy should be obtained, especially in the case of isolated CNS involvement, if accessibility and safety permit it (both are often restrictions).
Monitoring depends on the severity and organ involvement. Patients with active and severe disease during immunosuppression should be seen at least every 12 weeks. Follow-up in patients with a self-limiting course during remission should be seen twice yearly for 2 years, then at yearly intervals for 3 years, followed by on-demand consultations.
Generally, the disease course is highly variable ranging from mild courses with spontaneous resolution within several weeks, to irreversible organ damage requiring transplantation in the case of progressive pulmonary or cardiac sarcoidosis. There are currently no uniformly accepted predictors for disease progression or relapses, although high FDG uptake, assessed as standardised uptake value (SUVmax) >6.0, was independently associated with disease recurrence [159].
Eventually, it is difficult to decide when and how a treatment should be initiated. In mild forms, topical treatment and a watch-and-wait approach may be justified, depending on the patients symptoms, but needs a close follow-up to intervene early enough in the case of disease progression. For example, a sudden increase of sarcoidosis-associated biomarkers (e.g., ACE, sIL-2Rα, neopterin), BAL lymphocyte cell count or FDG-uptake in PET/CT may indicate ongoing or progressive inflammation.
Treatment decisions aiming at symptom relief or remission should be personalised during the disease course, with consideration of therapeutic advantage and adverse effects, and depending on organ manifestations [1]. This includes immunosuppression, as well as supportive treatment for comorbidities or complications such as oxygen therapy or an implantable cardioverter–defibrillator (ICD). Absolute indications for a systemic treatment in the case of persisting disease activity include clinically apparent impairment of lung function, significant radiographic manifestations or progression towards pulmonary fibrosis, pulmonary hypertension, cardiac involvement (myocardial inflammation, high-degree heart block, ventricular arrhythmias, congestive heart failure), nervous system manifestations, pronounced reduction of hepatic function, hypercalcaemia and renal involvement, lupus pernio, obstructive lymph nodes, eye manifestation, splenomegaly causing thrombocytopenia and diabetes insipidus. As no immunosuppressive drugs are approved for sarcoidosis, selection of an appropriate treatment is based on consensus decisions [1, 123].
It is unknown if, due to better diagnostic procedures, an early treatment intervention in the case of relevant inflammation without concurrent functional impairment yet, will result in a more favourable disease course or outcome, less organ damage and reduced morbidity. Currently, longitudinal data are lacking and need verification over the upcoming years.
Apart from lung, eye and skin/mucosa manifestations (which can be treated topically), oral glucocorticoids are the first-line therapy of choice, although randomised trials are lacking [124–126]. A recent European Respiratory Society (ERS) guideline summarises extensively the current treatment options for sarcoidosis [127]. A typical dose regimen consists of an initial daily dose of 0.5–0.75 mg prednisone per kg body weight for four weeks depending on disease response [1]. Thereafter, glucocorticoid dose can be slowly tapered over 6–12 months (up to 24 months in selected and severe cases). In refractory or chronic active cases, glucocorticoids should be continued at a low dose in combination with one (or even more) steroid-sparing agents. For second-line treatment (in severe organ involvement, in glucocorticoid-refractory, chronic active or relapsing cases) or as steroid-sparing regimen (in the case of intolerable side effects and contraindications to glucocorticoids), there are several alternative immunosuppressants to be considered. Even though drugs, such as azathioprine, methotrexate, mycophenolate mofetil or leflunomide have been investigated only in small randomised trials or case series, beneficial effects have been reported [128–132]. TNFis, particularly infliximab or adalimumab, may serve as further off-label agents, also with some beneficial effects on inflammation, predominantly in extrapulmonary sarcoidosis [133–136]. TNFis were shown to be effective in randomised trials of pulmonary, extrapulmonary (lymph nodes, skin, bone/joint, liver, eyes, muscle, heart, peripheral and central nervous system, nose, spleen, kidney, bone marrow, throat, parotid/salivary glands, ear and gastrointestinal tract) and cutaneous sarcoidosis [137–139]. Chloroquine and hydroxychloroquine, with the latter showing less ocular toxicity, are immunomodulatory agents originally known as antimalarial drugs. Hydroxychloroquine is used as first-line treatment for cutaneous involvement, and shows beneficial effects in hypercalcaemia, in some patients with polymyalgia/arthralgia and fatigue [130]. A treatment algorithm for systemic treatment options is summarised in figure 5.
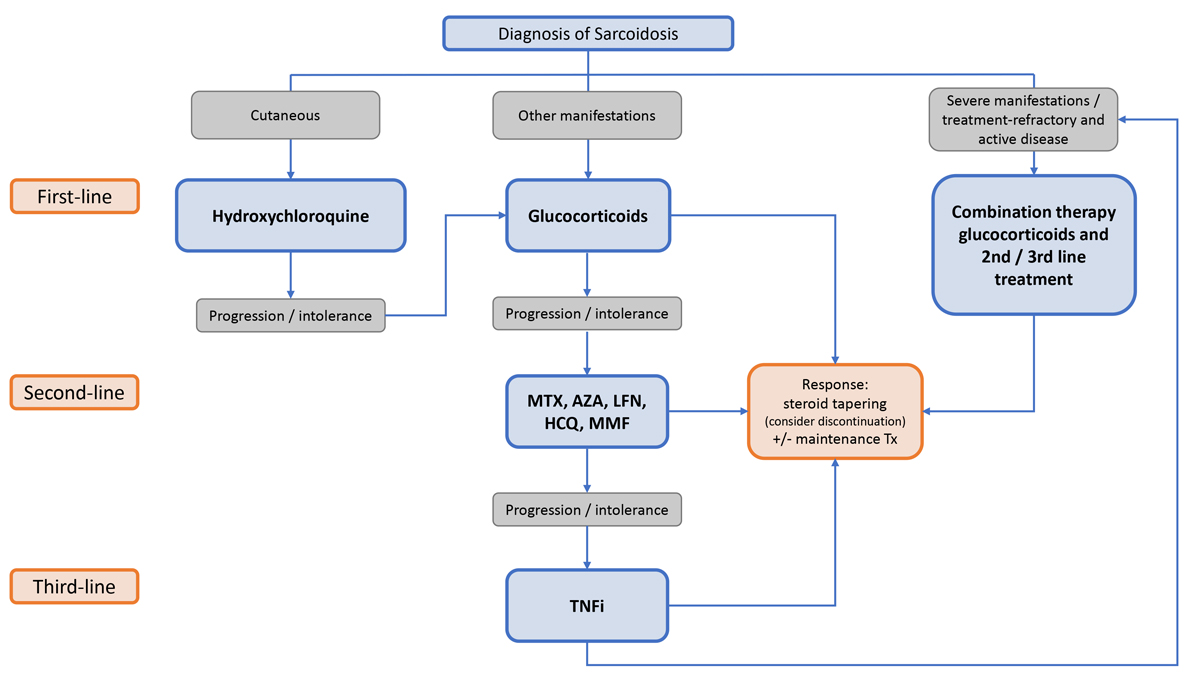
Figure 5 Algorithm of systemic treatments in sarcoidosis. MTX: methotrexate; AZA: azathioprine; LFN: leflunomide; HCQ: hydroxychloroquine; MMF: mycophenolate mofetil; TNFi: TNFα inhibitor.
Since TNFα seems to play a key role in the pathogenesis of sarcoidosis, several anti-inflammatory drugs interacting with TNF metabolism, such as thalidomide, lenalidomide, and pentoxifylline, have also shown therapeutic effects in small case series or reports [140–142]. On the other hand, TNFis may also induce sarcoid-like diseases [143, 144]. Eventually, pathogenesis of sarcoidosis seems more complex with derangements in several pathways of acquired and innate immunity, including IL-23/Th17, IL-1 and IL-6 pathways. Thus, alternative approaches have been published in case series and reports with the IL-17 inhibitor secukinumab [143], the IL-6 receptor antagonist tocilizumab [145], or the JAK-1/3 inhibitor tofacitinib [146, 147], and the monoclonal chimeric anti-CD20 antibody rituximab [148]. Studies are currently under way with the phosphodiesterase inhibitor roflumilast and the fusion protein abatacept. In severe, therapy-refractory cases or in neurosarcoidosis, cyclophosphamide can also be considered [149–151], although the evidence level is low.
In general, in the case of persisting disease activity and severe organ involvement, combination therapies should be evaluated early in the treatment process to avoid complications due to long-term glucocorticoid treatment and provide sufficient disease control, with consideration of comorbidities and potential side effects, which could lead to higher hospitalisation rates and higher mortality, especially in older patients [101]. Furthermore, caution is required to avoid overtreatment in cases of burned-out sarcoidosis. This could lead to unnecessary treatment toxicity. If kidney function is decreased, therapy with methotrexate should be executed with great caution and only if no other options are available. When kidney function is severely impaired, methotrexate is contraindicated.
In addition to anti-inflammatory treatments, ICD/pacemaker implantation should be considered in cardiac sarcoidosis with severe ventricular arrhythmias, high degree atrioventricular block or impaired left ventricular function, as well as extensive myocardial fibrosis. Nitedanib, a tyrosine kinase inhibitor, which was approved for idiopathic pulmonary fibrosis, recently received extended approval by the US Food and Drug administration (FDA) for chronic fibrosing interstitial lung diseases with a progressive phenotype, which among others also includes sarcoidosis (12 of 663 participants, about 1.8%) [152, 153].
In end-stage disease, allotransplantation of the lungs, heart, kidney or liver might be the last opportunity. In the case of long-term immunosuppression by glucocorticoids >20 mg/day for longer than one month with an additional immunocompromising factor (immunosuppressive or predisposing disease), prophylaxis against Pneumocystis jiroveci with trimethoprim-sulfamethoxazole 160/800 mg three times weekly is recommended [154].
Comprehensive care of sarcoidosis patients also includes non-pharmacological measures such as oxygen therapy, physiotherapy and occupational therapy, physical training, pulmonary rehabilitation, cognitive behavioural therapy, psychosocial counselling, multimodal speech pathology therapy, mindfulness-based therapy and more [155]. For regular assessment of quality of life, there are several tools available, such as the 36-item Short Form Health Survey and EuroQol Group 5-dimension questionnaire or disease-specific questionnaires such as the King’s Sarcoidosis Questionnaire [156], Sarcoidosis Health Questionnaire [157] or the Sarcoidosis Assessment Tool [158].
As a multi-systemic granulomatous disease with various clinical symptoms, sarcoidosis management requires an interdisciplinary approach to investigate all possibly affected organs and to seek the best treatment approach. Besides organ involvement and pharmacological treatments, comprehensive care also includes non-pharmacological measures and should address the psychosocial burden and para-sarcoidosis symptoms such as fatigue.
The increasing awareness of sarcoidosis as a systemic disease also requires the involvement of a multidisciplinary team. Registries and real-world data of rare diseases, by combining clinical and research efforts, have proven advantageous in multiple medical fields for patients and specialists. Therefore, the authors are currently attempting to generate an inter- and intra-disciplinary national network for sarcoidosis (SARNET).
DPF received speaker and consultancy honoraries from Boehringer Ingelheim. OD has/had consultancy relationship and/or has received research funding in the area of fibrosis and fibrotic diseases from (last three years): Abbvie, Acceleron Pharma, Amgen, AnaMar, Bayer, Boehringer Ingelheim, Catenion, Drug Development International Ltd, CSL Behring, ChemomAb, GSK, Horizon (Curzion) Pharmaceuticals, Inventiva, Italfarmaco, iQvia, Lilly, Medac, Medscape, Mitsubishi Tanabe Pharma, MSD, Novartis, Pfizer, Roche, Sanofi, Serodapharm, Target Bio Science and UCB. AGAK has served as an investigator, speaker, and/or advisor for AbbVie, Abbott, AstraZeneca, Janssen, Eli Lilly, MSD, Pfizer, Celgene, Novartis, Actelion, Leo, Amgen, Alk-Abello, and does not hold any shares or other financial interest in any related pharmaceutical company. The other authors do not have any conflict of interests related to the manuscript.
1. Grunewald J , Grutters JC , Arkema EV , Saketkoo LA , Moller DR , Müller-Quernheim J . Sarcoidosis. Nat Rev Dis Primers. 2019 Jul;5(1):45. https://doi.org/10.1038/s41572-019-0096-x
2. Marcellis RG , Lenssen AF , de Vries J , Drent M . Reduced muscle strength, exercise intolerance and disabling symptoms in sarcoidosis. Curr Opin Pulm Med. 2013 Sep;19(5):524–30. https://doi.org/10.1097/MCP.0b013e328363f563
3. Grunewald J , Hultman T , Bucht A , Eklund A , Wigzell H . Restricted usage of T cell receptor V alpha/J alpha gene segments with different nucleotide but identical amino acid sequences in HLA-DR3+ sarcoidosis patients. Mol Med. 1995 Mar;1(3):287–96. https://doi.org/10.1007/BF03401553
4. Mitchell AM , Kaiser Y , Falta MT , Munson DJ , Landry LG , Eklund A , et al. Shared alphabeta TCR Usage in Lungs of Sarcoidosis Patients with Lofgren's Syndrome. Journal of immunology (Baltimore, Md : 1950). 2017;199(7):2279-90.
5. Cozier YC . Assessing the worldwide epidemiology of sarcoidosis: challenges and future directions. Eur Respir J. 2016 Dec;48(6):1545–8. https://doi.org/10.1183/13993003.01819-2016
6. Jamilloux Y , Maucort-Boulch D , Kerever S , Gerfaud-Valentin M , Broussolle C , Eb M , et al. Sarcoidosis-related mortality in France: a multiple-cause-of-death analysis. Eur Respir J. 2016 Dec;48(6):1700–9. https://doi.org/10.1183/13993003.00457-2016
7. Judson MA , Boan AD , Lackland DT . The clinical course of sarcoidosis: presentation, diagnosis, and treatment in a large white and black cohort in the United States. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG. 2012;29(2):119-27.
8. Arkema EV , Grunewald J , Kullberg S , Eklund A , Askling J . Sarcoidosis incidence and prevalence: a nationwide register-based assessment in Sweden. Eur Respir J. 2016 Dec;48(6):1690–9. https://doi.org/10.1183/13993003.00477-2016
9. Byg KE , Milman N , Hansen S . Sarcoidosis in Denmark 1980-1994. A registry-based incidence study comprising 5536 patients. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG. 2003;20(1):46-52.
10. Milman N , Selroos O . Pulmonary sarcoidosis in the Nordic countries 1950-1982. Epidemiology and clinical picture. Sarcoidosis. 1990 Mar;7(1):50–7.
11. Baughman RP , Field S , Costabel U , Crystal RG , Culver DA , Drent M , et al. Sarcoidosis in America. Analysis Based on Health Care Use. Ann Am Thorac Soc. 2016 Aug;13(8):1244–52. https://doi.org/10.1513/AnnalsATS.201511-760OC
12. Cozier YC , Berman JS , Palmer JR , Boggs DA , Serlin DM , Rosenberg L . Sarcoidosis in black women in the United States: data from the Black Women’s Health Study. Chest. 2011 Jan;139(1):144–50. https://doi.org/10.1378/chest.10-0413
13. Dumas O , Abramovitz L , Wiley AS , Cozier YC , Camargo CA Jr . Epidemiology of Sarcoidosis in a Prospective Cohort Study of U.S. Women. Ann Am Thorac Soc. 2016 Jan;13(1):67–71. https://doi.org/10.1513/AnnalsATS.201508-568BC
14. Morimoto T , Azuma A , Abe S , Usuki J , Kudoh S , Sugisaki K , et al. Epidemiology of sarcoidosis in Japan. Eur Respir J. 2008 Feb;31(2):372–9. https://doi.org/10.1183/09031936.00075307
15. Rybicki BA , Iannuzzi MC . Epidemiology of sarcoidosis: recent advances and future prospects. Semin Respir Crit Care Med. 2007 Feb;28(1):22–35. https://doi.org/10.1055/s-2007-970331
16. Yoon HY , Kim HM , Kim YJ , Song JW . Prevalence and incidence of sarcoidosis in Korea: a nationwide population-based study. Respir Res. 2018 Aug;19(1):158. https://doi.org/10.1186/s12931-018-0871-3
17. Deubelbeiss U , Gemperli A , Schindler C , Baty F , Brutsche MH . Prevalence of sarcoidosis in Switzerland is associated with environmental factors. Eur Respir J. 2010 May;35(5):1088–97. https://doi.org/10.1183/09031936.00197808
18. Arkema EV , Cozier YC . Epidemiology of sarcoidosis: current findings and future directions. Ther Adv Chronic Dis. 2018 Aug;9(11):227–40. https://doi.org/10.1177/2040622318790197
19. Ungprasert P , Crowson CS , Matteson EL . Influence of Gender on Epidemiology and Clinical Manifestations of Sarcoidosis: A Population-Based Retrospective Cohort Study 1976-2013. Lung. 2017 Feb;195(1):87–91. https://doi.org/10.1007/s00408-016-9952-6
20. Baughman RP , Teirstein AS , Judson MA , Rossman MD , Yeager H Jr , Bresnitz EA , et al.; Case Control Etiologic Study of Sarcoidosis (ACCESS) research group . Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001 Nov;164(10 Pt 1):1885–9. https://doi.org/10.1164/ajrccm.164.10.2104046
21. Rabin DL , Richardson MS , Stein SR , Yeager H Jr . Sarcoidosis severity and socioeconomic status. Eur Respir J. 2001 Sep;18(3):499–506. https://doi.org/10.1183/09031936.01.00056201
22. Rabin DL , Thompson B , Brown KM , Judson MA , Huang X , Lackland DT , et al. Sarcoidosis: social predictors of severity at presentation. Eur Respir J. 2004 Oct;24(4):601–8. https://doi.org/10.1183/09031936.04.00070503
23. Rivera NV , Ronninger M , Shchetynsky K , Franke A , Nöthen MM , Müller-Quernheim J , et al. High-Density Genetic Mapping Identifies New Susceptibility Variants in Sarcoidosis Phenotypes and Shows Genomic-driven Phenotypic Differences. Am J Respir Crit Care Med. 2016 May;193(9):1008–22. https://doi.org/10.1164/rccm.201507-1372OC
24. Schürmann M , Reichel P , Müller-Myhsok B , Schlaak M , Müller-Quernheim J , Schwinger E . Results from a genome-wide search for predisposing genes in sarcoidosis. Am J Respir Crit Care Med. 2001 Sep;164(5):840–6. https://doi.org/10.1164/ajrccm.164.5.2007056
25. Sato H , Woodhead FA , Ahmad T , Grutters JC , Spagnolo P , van den Bosch JM , et al. Sarcoidosis HLA class II genotyping distinguishes differences of clinical phenotype across ethnic groups. Hum Mol Genet. 2010 Oct;19(20):4100–11. https://doi.org/10.1093/hmg/ddq325
26. Valentonyte R , Hampe J , Huse K , Rosenstiel P , Albrecht M , Stenzel A , et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat Genet. 2005 Apr;37(4):357–64. https://doi.org/10.1038/ng1519
27. Suzuki H , Ota M , Meguro A , Katsuyama Y , Kawagoe T , Ishihara M , et al. Genetic characterization and susceptibility for sarcoidosis in Japanese patients: risk factors of BTNL2 gene polymorphisms and HLA class II alleles. Invest Ophthalmol Vis Sci. 2012 Oct;53(11):7109–15. https://doi.org/10.1167/iovs.12-10491
28. Spagnolo P , Sato H , Grutters JC , Renzoni EA , Marshall SE , Ruven HJ , et al. Analysis of BTNL2 genetic polymorphisms in British and Dutch patients with sarcoidosis. Tissue Antigens. 2007 Sep;70(3):219–27. https://doi.org/10.1111/j.1399-0039.2007.00879.x
29. Liu H , Patel D , Welch AM , Wilson C , Mroz MM , Li L , et al. Association Between Occupational Exposures and Sarcoidosis: An Analysis From Death Certificates in the United States, 1988-1999. Chest. 2016 Aug;150(2):289–98. https://doi.org/10.1016/j.chest.2016.01.020
30. Izbicki G , Chavko R , Banauch GI , Weiden MD , Berger KI , Aldrich TK , et al. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest. 2007 May;131(5):1414–23. https://doi.org/10.1378/chest.06-2114
31. Heckmann JG , Stefan H , Heuss D , Hopp P , Neundörfer B . Isolated muscular sarcoidosis. Eur J Neurol. 2001 Jul;8(4):365–6. https://doi.org/10.1046/j.1468-1331.2001.00229.x
32. Heyder T , Kohler M , Tarasova NK , Haag S , Rutishauser D , Rivera NV , et al. Approach for Identifying Human Leukocyte Antigen (HLA)-DR Bound Peptides from Scarce Clinical Samples. Mol Cell Proteomics. 2016 Sep;15(9):3017–29. https://doi.org/10.1074/mcp.M116.060764
33. Valeyre D , Bernaudin JF , Uzunhan Y , Kambouchner M , Brillet PY , Soussan M , et al. Clinical presentation of sarcoidosis and diagnostic work-up. Semin Respir Crit Care Med. 2014 Jun;35(3):336–51. https://doi.org/10.1055/s-0034-1381229
34. Wahlström J , Dengjel J , Persson B , Duyar H , Rammensee HG , Stevanović S , et al. Identification of HLA-DR-bound peptides presented by human bronchoalveolar lavage cells in sarcoidosis. J Clin Invest. 2007 Nov;117(11):3576–82. https://doi.org/10.1172/JCI32401
35. Chen ES , Moller DR . Sarcoidosis—scientific progress and clinical challenges. Nat Rev Rheumatol. 2011 Jul;7(8):457–67. https://doi.org/10.1038/nrrheum.2011.93
36. Chen ES , Song Z , Willett MH , Heine S , Yung RC , Liu MC , et al. Serum amyloid A regulates granulomatous inflammation in sarcoidosis through Toll-like receptor-2. Am J Respir Crit Care Med. 2010 Feb;181(4):360–73. https://doi.org/10.1164/rccm.200905-0696OC
37. Chen ES , Wahlström J , Song Z , Willett MH , Wikén M , Yung RC , et al. T cell responses to mycobacterial catalase-peroxidase profile a pathogenic antigen in systemic sarcoidosis. Journal of immunology (Baltimore, Md : 1950). 2008;181(12):8784-96.
38. Drake WP , Dhason MS , Nadaf M , Shepherd BE , Vadivelu S , Hajizadeh R , et al. Cellular recognition of Mycobacterium tuberculosis ESAT-6 and KatG peptides in systemic sarcoidosis. Infect Immun. 2007 Jan;75(1):527–30. https://doi.org/10.1128/IAI.00732-06
39. Dubaniewicz A . Mycobacterium tuberculosis heat shock proteins and autoimmunity in sarcoidosis. Autoimmun Rev. 2010 Apr;9(6):419–24. https://doi.org/10.1016/j.autrev.2009.11.015
40. Dubaniewicz A . Microbial and human heat shock proteins as ‘danger signals’ in sarcoidosis. Hum Immunol. 2013 Dec;74(12):1550–8. https://doi.org/10.1016/j.humimm.2013.08.275
41. Oswald-Richter K , Sato H , Hajizadeh R , Shepherd BE , Sidney J , Sette A , et al. Mycobacterial ESAT-6 and katG are recognized by sarcoidosis CD4+ T cells when presented by the American sarcoidosis susceptibility allele, DRB1*1101. J Clin Immunol. 2010 Jan;30(1):157–66. https://doi.org/10.1007/s10875-009-9311-y
42. Song Z , Marzilli L , Greenlee BM , Chen ES , Silver RF , Askin FB , et al. Mycobacterial catalase-peroxidase is a tissue antigen and target of the adaptive immune response in systemic sarcoidosis. J Exp Med. 2005 Mar;201(5):755–67. https://doi.org/10.1084/jem.20040429
43. Li H , Zhao X , Wang J , Zong M , Yang H . Bioinformatics analysis of gene expression profile data to screen key genes involved in pulmonary sarcoidosis. Gene. 2017 Jan;596:98–104. https://doi.org/10.1016/j.gene.2016.09.037
44. Rosenbaum JT , Pasadhika S , Crouser ED , Choi D , Harrington CA , Lewis JA , et al. Hypothesis: sarcoidosis is a STAT1-mediated disease. Clin Immunol. 2009 Aug;132(2):174–83. https://doi.org/10.1016/j.clim.2009.04.010
45. Zhou T , Casanova N , Pouladi N , Wang T , Lussier Y , Knox KS , et al. Identification of Jak-STAT signaling involvement in sarcoidosis severity via a novel microRNA-regulated peripheral blood mononuclear cell gene signature. Sci Rep. 2017 Jun;7(1):4237. https://doi.org/10.1038/s41598-017-04109-6
46. Zhou T , Zhang W , Sweiss NJ , Chen ES , Moller DR , Knox KS , et al. Peripheral blood gene expression as a novel genomic biomarker in complicated sarcoidosis. PLoS One. 2012;7(9):e44818. https://doi.org/10.1371/journal.pone.0044818
47. Celada LJ , Kropski JA , Herazo-Maya JD , Luo W , Creecy A , Abad AT , et al. PD-1 up-regulation on CD4+ T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-β1 production. Sci Transl Med. 2018 Sep;10(460):eaar8356. https://doi.org/10.1126/scitranslmed.aar8356
48. Linke M , Pham HT , Katholnig K , Schnöller T , Miller A , Demel F , et al. Chronic signaling via the metabolic checkpoint kinase mTORC1 induces macrophage granuloma formation and marks sarcoidosis progression. Nat Immunol. 2017 Mar;18(3):293–302. https://doi.org/10.1038/ni.3655
49. Wikén M , Grunewald J , Eklund A , Wahlström J . Higher monocyte expression of TLR2 and TLR4, and enhanced pro-inflammatory synergy of TLR2 with NOD2 stimulation in sarcoidosis. J Clin Immunol. 2009 Jan;29(1):78–89. https://doi.org/10.1007/s10875-008-9225-0
50. Grunewald J , Janson CH , Eklund A , Ohrn M , Olerup O , Persson U , et al. Restricted V alpha 2.3 gene usage by CD4+ T lymphocytes in bronchoalveolar lavage fluid from sarcoidosis patients correlates with HLA-DR3. Eur J Immunol. 1992 Jan;22(1):129–35. https://doi.org/10.1002/eji.1830220120
51. Moller DR , Konishi K , Kirby M , Balbi B , Crystal RG . Bias toward use of a specific T cell receptor beta-chain variable region in a subgroup of individuals with sarcoidosis. J Clin Invest. 1988 Oct;82(4):1183–91. https://doi.org/10.1172/JCI113715
52. Grunewald J , Kaiser Y , Ostadkarampour M , Rivera NV , Vezzi F , Lötstedt B , et al. T-cell receptor-HLA-DRB1 associations suggest specific antigens in pulmonary sarcoidosis. Eur Respir J. 2016 Mar;47(3):898–909. https://doi.org/10.1183/13993003.01209-2015
53. Kinloch AJ , Kaiser Y , Wolfgeher D , Ai J , Eklund A , Clark MR , et al. In Situ Humoral Immunity to Vimentin in HLA-DRB1*03+ Patients With Pulmonary Sarcoidosis. Front Immunol. 2018 Jul;9:1516. https://doi.org/10.3389/fimmu.2018.01516
54. Broos CE , van Nimwegen M , Kleinjan A , ten Berge B , Muskens F , in ’t Veen JC , et al. Impaired survival of regulatory T cells in pulmonary sarcoidosis. Respir Res. 2015 Sep;16(1):108. https://doi.org/10.1186/s12931-015-0265-8
55. Verwoerd A , Hijdra D , Vorselaars AD , Crommelin HA , van Moorsel CH , Grutters JC , et al. Infliximab therapy balances regulatory T cells, tumour necrosis factor receptor 2 (TNFR2) expression and soluble TNFR2 in sarcoidosis. Clin Exp Immunol. 2016 Aug;185(2):263–70. https://doi.org/10.1111/cei.12808
56. Idali F , Wikén M , Wahlström J , Mellstedt H , Eklund A , Rabbani H , et al. Reduced Th1 response in the lungs of HLA-DRB1*0301 patients with pulmonary sarcoidosis. Eur Respir J. 2006 Mar;27(3):451–9. https://doi.org/10.1183/09031936.06.00067105
57. Strookappe B , De Vries J , Elfferich M , Kuijpers P , Knevel T , Drent M . Predictors of fatigue in sarcoidosis: the value of exercise testing. Respir Med. 2016 Jul;116:49–54. https://doi.org/10.1016/j.rmed.2016.05.010
58. Spagnolo P , Rossi G , Trisolini R , Sverzellati N , Baughman RP , Wells AU . Pulmonary sarcoidosis. Lancet Respir Med. 2018 May;6(5):389–402. https://doi.org/10.1016/S2213-2600(18)30064-X
59. Scadding JG . Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. BMJ. 1961 Nov;2(5261):1165–72. https://doi.org/10.1136/bmj.2.5261.1165
60. Berliner AR , Haas M , Choi MJ . Sarcoidosis: the nephrologist’s perspective. Am J Kidney Dis. 2006 Nov;48(5):856–70. https://doi.org/10.1053/j.ajkd.2006.07.022
61. Löffler C , Löffler U , Tuleweit A , Waldherr R , Uppenkamp M , Bergner R. Renal sarcoidosis: epidemiological and follow-up data in a cohort of 27 patients. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG. 2015;31(4):306-15.
62. Müller NL , Kullnig P , Miller RR . The CT findings of pulmonary sarcoidosis: analysis of 25 patients. AJR Am J Roentgenol. 1989 Jun;152(6):1179–82. https://doi.org/10.2214/ajr.152.6.1179
63. James WE , Koutroumpakis E , Saha B , Nathani A , Saavedra L , Yucel RM , et al. Clinical Features of Extrapulmonary Sarcoidosis Without Lung Involvement. Chest. 2018 Aug;154(2):349–56. https://doi.org/10.1016/j.chest.2018.02.003
64. Salah S , Abad S , Monnet D , Brézin AP . Sarcoidosis. J Fr Ophtalmol. 2018 Dec;41(10):e451–67. https://doi.org/10.1016/j.jfo.2018.10.002
65. Bradley D , Baughman RP , Raymond L , Kaufman AH . Ocular manifestations of sarcoidosis. Semin Respir Crit Care Med. 2002 Dec;23(6):543–8. https://doi.org/10.1055/s-2002-36518
66. Haimovic A , Sanchez M , Judson MA , Prystowsky S . Sarcoidosis: a comprehensive review and update for the dermatologist: part I. Cutaneous disease. J Am Acad Dermatol. 2012;66(5):699 e1-18; quiz 717-8.
67. Noe MH , Rosenbach M . Cutaneous sarcoidosis. Curr Opin Pulm Med. 2017 Sep;23(5):482–6. https://doi.org/10.1097/MCP.0000000000000402
68. Birnie DH , Nery PB , Ha AC , Beanlands RS . Cardiac Sarcoidosis. J Am Coll Cardiol. 2016 Jul;68(4):411–21. https://doi.org/10.1016/j.jacc.2016.03.605
69. Chau EM , Fan KY , Chow WH . Cardiac sarcoidosis: a potentially fatal but treatable form of infiltrative heart disease. Hong Kong medical journal = Xianggang yi xue za zhi. 2006;12(1):65-7.
70. Sayah DM , Bradfield JS , Moriarty JM , Belperio JA , Lynch JP 3rd . Cardiac Involvement in Sarcoidosis: Evolving Concepts in Diagnosis and Treatment. Semin Respir Crit Care Med. 2017 Aug;38(4):477–98. https://doi.org/10.1055/s-0037-1602381
71. Schupp JC , Freitag-Wolf S , Bargagli E , Mihailović-Vučinić V , Rottoli P , Grubanovic A , et al. Phenotypes of organ involvement in sarcoidosis. Eur Respir J. 2018 Jan;51(1):1700991. https://doi.org/10.1183/13993003.00991-2017
72. Silverman KJ , Hutchins GM , Bulkley BH . Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978 Dec;58(6):1204–11. https://doi.org/10.1161/01.CIR.58.6.1204
73. Hamzeh N , Steckman DA , Sauer WH , Judson MA . Pathophysiology and clinical management of cardiac sarcoidosis. Nat Rev Cardiol. 2015 May;12(5):278–88. https://doi.org/10.1038/nrcardio.2015.22
74. Nowak DA , Widenka DC . Neurosarcoidosis: a review of its intracranial manifestation. J Neurol. 2001 May;248(5):363–72. https://doi.org/10.1007/s004150170175
75. Stern BJ , Krumholz A , Johns C , Scott P , Nissim J . Sarcoidosis and its neurological manifestations. Arch Neurol. 1985 Sep;42(9):909–17. https://doi.org/10.1001/archneur.1985.04060080095022
76. Gascón-Bayarri J , Mañá J , Martínez-Yélamos S , Murillo O , Reñé R , Rubio F . Neurosarcoidosis: report of 30 cases and a literature survey. Eur J Intern Med. 2011 Dec;22(6):e125–32. https://doi.org/10.1016/j.ejim.2011.08.019
77. Culver DA , Ribeiro Neto ML , Moss BP , Willis MA . Neurosarcoidosis. Semin Respir Crit Care Med. 2017 Aug;38(4):499–513. https://doi.org/10.1055/s-0037-1604165
78. Stuart CA , Neelon FA , Lebovitz HE . Disordered control of thirst in hypothalamic-pituitary sarcoidosis. N Engl J Med. 1980 Nov;303(19):1078–82. https://doi.org/10.1056/NEJM198011063031902
79. Joseph FG , Scolding NJ . Neurosarcoidosis: a study of 30 new cases. J Neurol Neurosurg Psychiatry. 2009 Mar;80(3):297–304. https://doi.org/10.1136/jnnp.2008.151977
80. O’Dwyer JP , Al-Moyeed BA , Farrell MA , Pidgeon CN , Collins DR , Fahy A , et al. Neurosarcoidosis-related intracranial haemorrhage: three new cases and a systematic review of the literature. Eur J Neurol. 2013 Jan;20(1):71–8. https://doi.org/10.1111/j.1468-1331.2012.03783.x
81. González-Aramburu I , Ruiz-Pérez E , Gómez-Román J , Quirce R , Larrosa D , Pascual J . Sarcoidosis presenting as transient ischemic attack status. J Stroke Cerebrovasc Dis. 2012 Aug;21(6):515–7. https://doi.org/10.1016/j.jstrokecerebrovasdis.2010.12.003
82. Ginat DT , Dhillon G , Almast J . Magnetic resonance imaging of neurosarcoidosis. J Clin Imaging Sci. 2011;1:15.
83. Shah R , Roberson GH , Curé JK . Correlation of MR imaging findings and clinical manifestations in neurosarcoidosis. AJNR Am J Neuroradiol. 2009 May;30(5):953–61. https://doi.org/10.3174/ajnr.A1470
84. Ibitoye RT , Wilkins A , Scolding NJ . Neurosarcoidosis: a clinical approach to diagnosis and management. J Neurol. 2017 May;264(5):1023–8. https://doi.org/10.1007/s00415-016-8336-4
85. Stern BJ . Neurological complications of sarcoidosis. Curr Opin Neurol. 2004 Jun;17(3):311–6. https://doi.org/10.1097/00019052-200406000-00013
86. Rajakariar R , Sharples EJ , Raftery MJ , Sheaff M , Yaqoob MM . Sarcoid tubulo-interstitial nephritis: long-term outcome and response to corticosteroid therapy. Kidney Int. 2006 Jul;70(1):165–9. https://doi.org/10.1038/sj.ki.5001512
87. Korenromp IH , Grutters JC , van den Bosch JM , Heijnen CJ . Post-inflammatory fatigue in sarcoidosis: personality profiles, psychological symptoms and stress hormones. J Psychosom Res. 2012 Feb;72(2):97–102. https://doi.org/10.1016/j.jpsychores.2011.10.001
88. Hoitsma E , Marziniak M , Faber CG , Reulen JP , Sommer C , De Baets M , et al. Small fibre neuropathy in sarcoidosis. Lancet. 2002 Jun;359(9323):2085–6. https://doi.org/10.1016/S0140-6736(02)08912-2
89. Tebben PJ , Singh RJ , Kumar R . Vitamin D-Mediated Hypercalcemia: Mechanisms, Diagnosis, and Treatment. Endocr Rev. 2016 Oct;37(5):521–47. https://doi.org/10.1210/er.2016-1070
90. Grunewald J , Eklund A . Sex-specific manifestations of Löfgren’s syndrome. Am J Respir Crit Care Med. 2007 Jan;175(1):40–4. https://doi.org/10.1164/rccm.200608-1197OC
91. Grunewald J , Eklund A . Löfgren’s syndrome: human leukocyte antigen strongly influences the disease course. Am J Respir Crit Care Med. 2009 Feb;179(4):307–12. https://doi.org/10.1164/rccm.200807-1082OC
92. Hunninghake GW , Costabel U , Ando M , Baughman R , Cordier JF , du Bois R , et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG. 1999;16(2):149-73.
93. Ungprasert P , Crowson CS , Matteson EL . Sarcoidosis Increases Risk of Hospitalized Infection. A Population-based Study, 1976-2013. Ann Am Thorac Soc. 2017 May;14(5):676–81. https://doi.org/10.1513/AnnalsATS.201610-750OC
94. Wu CH , Chung PI , Wu CY , Chen YT , Chiu YW , Chang YT , et al. Comorbid autoimmune diseases in patients with sarcoidosis: A nationwide case-control study in Taiwan. J Dermatol. 2017 Apr;44(4):423–30. https://doi.org/10.1111/1346-8138.13654
95. Fallahi P , Ferrari SM , Ruffilli I , Elia G , Biricotti M , Vita R , et al. The association of other autoimmune diseases in patients with autoimmune thyroiditis: review of the literature and report of a large series of patients. Autoimmun Rev. 2016 Dec;15(12):1125–8. https://doi.org/10.1016/j.autrev.2016.09.009
96. Ungprasert P , Crowson CS , Matteson EL . Risk of cardiovascular disease among patients with sarcoidosis: a population-based retrospective cohort study, 1976-2013. Eur Respir J. 2017 Feb;49(2):1601290. https://doi.org/10.1183/13993003.01290-2016
97. Ungprasert P , Crowson CS , Matteson EL . Association of Sarcoidosis With Increased Risk of VTE: A Population-Based Study, 1976 to 2013. Chest. 2017 Feb;151(2):425–30. https://doi.org/10.1016/j.chest.2016.09.009
98. Yaqoob ZJ , Al-Kindi SG , Zein JG . Sarcoidosis and Risk of VTE: Validation With Big Data. Chest. 2017 Jun;151(6):1398–9. https://doi.org/10.1016/j.chest.2017.03.022
99. Crawshaw AP , Wotton CJ , Yeates DG , Goldacre MJ , Ho LP . Evidence for association between sarcoidosis and pulmonary embolism from 35-year record linkage study. Thorax. 2011 May;66(5):447–8. https://doi.org/10.1136/thx.2010.134429
100. Bonifazi M , Bravi F , Gasparini S , La Vecchia C , Gabrielli A , Wells AU , et al. Sarcoidosis and cancer risk: systematic review and meta-analysis of observational studies. Chest. 2015 Mar;147(3):778–91. https://doi.org/10.1378/chest.14-1475
101. Pohle S , Baty F , Brutsche M . In-Hospital Disease Burden of Sarcoidosis in Switzerland from 2002 to 2012. PLoS One. 2016 Mar;11(3):e0151940. https://doi.org/10.1371/journal.pone.0151940
102. Gribbin J , Hubbard RB , Le Jeune I , Smith CJ , West J , Tata LJ . Incidence and mortality of idiopathic pulmonary fibrosis and sarcoidosis in the UK. Thorax. 2006 Nov;61(11):980–5. https://doi.org/10.1136/thx.2006.062836
103. Nardi A , Brillet PY , Letoumelin P , Girard F , Brauner M , Uzunhan Y , et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011 Dec;38(6):1368–73. https://doi.org/10.1183/09031936.00187410
104. Park JE , Kim YS , Kang MJ , Kim CJ , Han CH , Lee SM , et al. Prevalence, incidence, and mortality of sarcoidosis in Korea, 2003-2015: A nationwide population-based study. Respir Med. 2018 Nov;144S:S28–34. https://doi.org/10.1016/j.rmed.2018.03.028
105. Rossides M , Kullberg S , Askling J , Eklund A , Grunewald J , Arkema EV . Sarcoidosis mortality in Sweden: a population-based cohort study. Eur Respir J. 2018 Feb;51(2):1701815. https://doi.org/10.1183/13993003.01815-2017
106. Tukey MH , Berman JS , Boggs DA , White LF , Rosenberg L , Cozier YC . Mortality among African American women with sarcoidosis: data from the Black Women's Health Study. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG. 2013;30(2):128-33.
107. Ungprasert P , Carmona EM , Utz JP , Ryu JH , Crowson CS , Matteson EL . Epidemiology of Sarcoidosis 1946-2013: A Population-Based Study. Mayo Clin Proc. 2016 Feb;91(2):183–8. https://doi.org/10.1016/j.mayocp.2015.10.024
108. Crouser ED , Maier LA , Wilson KC , Bonham CA , Morgenthau AS , Patterson KC , et al. Diagnosis and Detection of Sarcoidosis. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020 Apr;201(8):e26–51. https://doi.org/10.1164/rccm.202002-0251ST
109. Wessendorf TE , Bonella F , Costabel U . Diagnosis of Sarcoidosis. Clin Rev Allergy Immunol. 2015 Aug;49(1):54–62. https://doi.org/10.1007/s12016-015-8475-x
110. Kuberski T , Yourison I , Coccidioidomycosis A . Cause of Sarcoidosis. Open Forum Infect Dis. 2017 Jul;4(3):ofw117. https://doi.org/10.1093/ofid/ofw117
111. von Bartheld MB , Dekkers OM , Szlubowski A , Eberhardt R , Herth FJ , in ’t Veen JC , et al. Endosonography vs conventional bronchoscopy for the diagnosis of sarcoidosis: the GRANULOMA randomized clinical trial. JAMA. 2013 Jun;309(23):2457–64. https://doi.org/10.1001/jama.2013.5823
112. Costabel U . Sarcoidosis: clinical update. Eur Respir J Suppl. 2001 Sep;32:56s–68s.
113. Trisolini R , Lazzari Agli L , Tinelli C , De Silvestri A , Scotti V , Patelli M . Endobronchial ultrasound-guided transbronchial needle aspiration for diagnosis of sarcoidosis in clinically unselected study populations. Respirology. 2015 Feb;20(2):226–34. https://doi.org/10.1111/resp.12449
114. Aragaki-Nakahodo AA , Baughman RP , Shipley RT , Benzaquen S . The complimentary role of transbronchial lung cryobiopsy and endobronchial ultrasound fine needle aspiration in the diagnosis of sarcoidosis. Respir Med. 2017 Oct;131:65–9. https://doi.org/10.1016/j.rmed.2017.08.003
115. Navani N , Booth HL , Kocjan G , Falzon M , Capitanio A , Brown JM , et al. Combination of endobronchial ultrasound-guided transbronchial needle aspiration with standard bronchoscopic techniques for the diagnosis of stage I and stage II pulmonary sarcoidosis. Respirology. 2011 Apr;16(3):467–72. https://doi.org/10.1111/j.1440-1843.2011.01933.x
116. Walsh SL , Wells AU , Sverzellati N , Keir GJ , Calandriello L , Antoniou KM , et al. An integrated clinicoradiological staging system for pulmonary sarcoidosis: a case-cohort study. Lancet Respir Med. 2014 Feb;2(2):123–30. https://doi.org/10.1016/S2213-2600(13)70276-5
117. Huitema MP , Spee M , Vorselaars VM , Boerman S , Snijder RJ , van Es HW , et al. Pulmonary artery diameter to predict pulmonary hypertension in pulmonary sarcoidosis. Eur Respir J. 2016 Feb;47(2):673–6. https://doi.org/10.1183/13993003.01319-2015
118. Yatsynovich Y , Valencia D , Petrov M , Linares JD , Rahman MM , Dittoe N . Updates on the Role of Imaging in Cardiac Sarcoidosis. Curr Treat Options Cardiovasc Med. 2018 Aug;20(9):74. https://doi.org/10.1007/s11936-018-0670-7
119. Borucki SJ , Nguyen BV , Ladoulis CT , McKendall RR . Cerebrospinal fluid immunoglobulin abnormalities in neurosarcoidosis. Arch Neurol. 1989 Mar;46(3):270–3. https://doi.org/10.1001/archneur.1989.00520390036012
120. McLean BN , Miller D , Thompson EJ . Oligoclonal banding of IgG in CSF, blood-brain barrier function, and MRI findings in patients with sarcoidosis, systemic lupus erythematosus, and Behçet’s disease involving the nervous system. J Neurol Neurosurg Psychiatry. 1995 May;58(5):548–54. https://doi.org/10.1136/jnnp.58.5.548
121. Stern BJ , Griffin DE , Luke RA , Krumholz A , Johns CJ . Neurosarcoidosis: cerebrospinal fluid lymphocyte subpopulations. Neurology. 1987 May;37(5):878–81. https://doi.org/10.1212/WNL.37.5.878
122. Dale JC , O’Brien JF . Determination of angiotensin-converting enzyme levels in cerebrospinal fluid is not a useful test for the diagnosis of neurosarcoidosis. Mayo Clin Proc. 1999 May;74(5):535. https://doi.org/10.1016/S0025-6196(11)65143-4
123. Schutt AC , Bullington WM , Judson MA . Pharmacotherapy for pulmonary sarcoidosis: a Delphi consensus study. Respir Med. 2010 May;104(5):717–23. https://doi.org/10.1016/j.rmed.2009.12.009
124. Hunninghake GW , Gilbert S , Pueringer R , Dayton C , Floerchinger C , Helmers R , et al. Outcome of the treatment for sarcoidosis. Am J Respir Crit Care Med. 1994 Apr;149(4 Pt 1):893–8. https://doi.org/10.1164/ajrccm.149.4.8143052
125. James WE , Baughman R . Treatment of sarcoidosis: grading the evidence. Expert Rev Clin Pharmacol. 2018 Jul;11(7):677–87. https://doi.org/10.1080/17512433.2018.1486706
126. Selroos O , Löfroos AB , Pietinalho A , Niemistö M , Riska H . Inhaled budesonide for maintenance treatment of pulmonary sarcoidosis. Sarcoidosis. 1994 Sep;11(2):126–31.
127. Baughman RP , Valeyre D , Korsten P , Mathioudakis AG , Wuyts WA , Wells A , et al. ERS clinical practice guidelines on treatment of sarcoidosis. Eur Respir J. 2021 Jun;2004079. https://doi.org/10.1183/13993003.04079-2020
128. Baughman RP , Winget DB , Lower EE . Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG. 2000;17(1):60-6.
129. Müller-Quernheim J , Kienast K , Held M , Pfeifer S , Costabel U . Treatment of chronic sarcoidosis with an azathioprine/prednisolone regimen. Eur Respir J. 1999 Nov;14(5):1117–22. https://doi.org/10.1183/09031936.99.14511179
130. Nunes H , Jeny F , Bouvry D , Uzunhan Y , Valeyre D . Indications for treatment of sarcoidosis. Curr Opin Pulm Med. 2019 Sep;25(5):505–18. https://doi.org/10.1097/MCP.0000000000000604
131. Rahaghi FF , Baughman RP , Saketkoo LA , Sweiss NJ , Barney JB , Birring SS , et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur Respir Rev. 2020 Mar;29(155):190146. https://doi.org/10.1183/16000617.0146-2019
132. Hamzeh N , Voelker A , Forssén A , Gottschall EB , Rose C , Mroz P , et al. Efficacy of mycophenolate mofetil in sarcoidosis. Respir Med. 2014 Nov;108(11):1663–9. https://doi.org/10.1016/j.rmed.2014.09.013
133. Baughman RP , Drent M , Kavuru M , Judson MA , Costabel U , du Bois R , et al.; Sarcoidosis Investigators . Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med. 2006 Oct;174(7):795–802. https://doi.org/10.1164/rccm.200603-402OC
134. Crommelin HA , van der Burg LM , Vorselaars AD , Drent M , van Moorsel CH , Rijkers GT , et al. Efficacy of adalimumab in sarcoidosis patients who developed intolerance to infliximab. Respir Med. 2016 Jun;115:72–7. https://doi.org/10.1016/j.rmed.2016.04.011
135. Milman N , Graudal N , Loft A , Mortensen J , Larsen J , Baslund B . Effect of the TNF-α inhibitor adalimumab in patients with recalcitrant sarcoidosis: a prospective observational study using FDG-PET. Clin Respir J. 2012 Oct;6(4):238–47. https://doi.org/10.1111/j.1752-699X.2011.00276.x
136. Sweiss NJ , Noth I , Mirsaeidi M , Zhang W , Naureckas ET , Hogarth DK , et al. Efficacy Results of a 52-week Trial of Adalimumab in the Treatment of Refractory Sarcoidosis. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG. 2014;31(1):46-54.
137. Judson MA , Baughman RP , Costabel U , Flavin S , Lo KH , Kavuru MS , et al.; Centocor T48 Sarcoidosis Investigators . Efficacy of infliximab in extrapulmonary sarcoidosis: results from a randomised trial. Eur Respir J. 2008 Jun;31(6):1189–96. https://doi.org/10.1183/09031936.00051907
138. Pariser RJ , Paul J , Hirano S , Torosky C , Smith M . A double-blind, randomized, placebo-controlled trial of adalimumab in the treatment of cutaneous sarcoidosis. J Am Acad Dermatol. 2013 May;68(5):765–73. https://doi.org/10.1016/j.jaad.2012.10.056
139. Rossman MD , Newman LS , Baughman RP , Teirstein A , Weinberger SE , Miller W, Jr ., et al. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG. 2006;23(3):201-8.
140. Baughman RP , Judson MA , Teirstein AS , Moller DR , Lower EE . Thalidomide for chronic sarcoidosis. Chest. 2002 Jul;122(1):227–32. https://doi.org/10.1378/chest.122.1.227
141. Giv MJ , Yoosuff A , Bazargan A . Use of Lenalidomide in 5q-Myelodysplastic Syndrome Provides Novel Treatment Prospects in Management of Pulmonary Sarcoidosis. Chest. 2015 Aug;148(2):e35–7. https://doi.org/10.1378/chest.14-2529
142. Park MK , Fontana, Jr., Babaali H, Gilbert-McClain LI, Stylianou M, Joo J, et al. Steroid-sparing effects of pentoxifylline in pulmonary sarcoidosis. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG. 2009;26(2):121-31.
143. Eichhoff G . Management with secukinumab of tumour necrosis factor inhibitor-induced pulmonary sarcoidosis-like reaction in a patient with psoriasis. Clin Exp Dermatol. 2020 Jun;45(4):455–6. https://doi.org/10.1111/ced.14101
144. Perez-Alvarez R , Pérez-de-Lis M , Ramos-Casals M ; BIOGEAS study group . Biologics-induced autoimmune diseases. Curr Opin Rheumatol. 2013 Jan;25(1):56–64. https://doi.org/10.1097/BOR.0b013e32835b1366
145. Sharp M , Donnelly SC , Moller DR . Tocilizumab in sarcoidosis patients failing steroid sparing therapies and anti-TNF agents. Respir Med X. 2019;1:1. https://doi.org/10.1016/j.yrmex.2019.100004
146. Damsky W , Thakral D , Emeagwali N , Galan A , King B . Tofacitinib Treatment and Molecular Analysis of Cutaneous Sarcoidosis. N Engl J Med. 2018 Dec;379(26):2540–6. https://doi.org/10.1056/NEJMoa1805958
147. Kerkemeyer KL , Meah N , Sinclair RD . Tofacitinib for cutaneous and pulmonary sarcoidosis: a case series. J Am Acad Dermatol. 2020(In Press Journal Pre-Proof).
148. Sweiss NJ , Lower EE , Mirsaeidi M , Dudek S , Garcia JG , Perkins D , et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J. 2014 May;43(5):1525–8. https://doi.org/10.1183/09031936.00224513
149. Doty JD , Mazur JE , Judson MA . Treatment of corticosteroid-resistant neurosarcoidosis with a short-course cyclophosphamide regimen. Chest. 2003 Nov;124(5):2023–6. https://doi.org/10.1378/chest.124.5.2023
150. Sodhi M , Pearson K , White ES , Culver DA . Infliximab therapy rescues cyclophosphamide failure in severe central nervous system sarcoidosis. Respir Med. 2009 Feb;103(2):268–73. https://doi.org/10.1016/j.rmed.2008.08.016
151. Zuber M , Defer G , Cesaro P , Degos JD . Efficacy of cyclophosphamide in sarcoid radiculomyelitis. J Neurol Neurosurg Psychiatry. 1992 Feb;55(2):166–7. https://doi.org/10.1136/jnnp.55.2.166-a
152. Wells AU , Flaherty KR , Brown KK , Inoue Y , Devaraj A , Richeldi L , et al.; INBUILD trial investigators . Nintedanib in patients with progressive fibrosing interstitial lung diseases-subgroup analyses by interstitial lung disease diagnosis in the INBUILD trial: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Respir Med. 2020 May;8(5):453–60. https://doi.org/10.1016/S2213-2600(20)30036-9
153. Flaherty KR , Wells AU , Cottin V , Devaraj A , Walsh SL , Inoue Y , et al.; INBUILD Trial Investigators . Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N Engl J Med. 2019 Oct;381(18):1718–27. https://doi.org/10.1056/NEJMoa1908681
154. Stern A , Green H , Paul M , Vidal L , Leibovici L . Prophylaxis for Pneumocystis pneumonia (PCP) in non-HIV immunocompromised patients. Cochrane Database Syst Rev. 2014 Oct;(10):CD005590. https://doi.org/10.1002/14651858.CD005590.pub3
155. Moor CC , Kahlmann V , Culver DA , Wijsenbeek MS . Comprehensive Care for Patients with Sarcoidosis. J Clin Med. 2020 Feb;9(2):E390. https://doi.org/10.3390/jcm9020390
156. Patel AS , Siegert RJ , Creamer D , Larkin G , Maher TM , Renzoni EA , et al. The development and validation of the King’s Sarcoidosis Questionnaire for the assessment of health status. Thorax. 2013 Jan;68(1):57–65. https://doi.org/10.1136/thoraxjnl-2012-201962
157. Cox CE , Donohue JF , Brown CD , Kataria YP , Judson MA . The Sarcoidosis Health Questionnaire: a new measure of health-related quality of life. Am J Respir Crit Care Med. 2003 Aug;168(3):323–9. https://doi.org/10.1164/rccm.200211-1343OC
158. Judson MA , Mack M , Beaumont JL , Watt R , Barnathan ES , Victorson DE . Validation and important differences for the Sarcoidosis Assessment Tool. A new patient-reported outcome measure. Am J Respir Crit Care Med. 2015 Apr;191(7):786–95. https://doi.org/10.1164/rccm.201410-1785OC
159. Vorselaars AD , Verwoerd A , van Moorsel CH , Keijsers RG , Rijkers GT , Grutters JC . Prediction of relapse after discontinuation of infliximab therapy in severe sarcoidosis. Eur Respir J. 2014 Feb;43(2):602–9. https://doi.org/10.1183/09031936.00055213