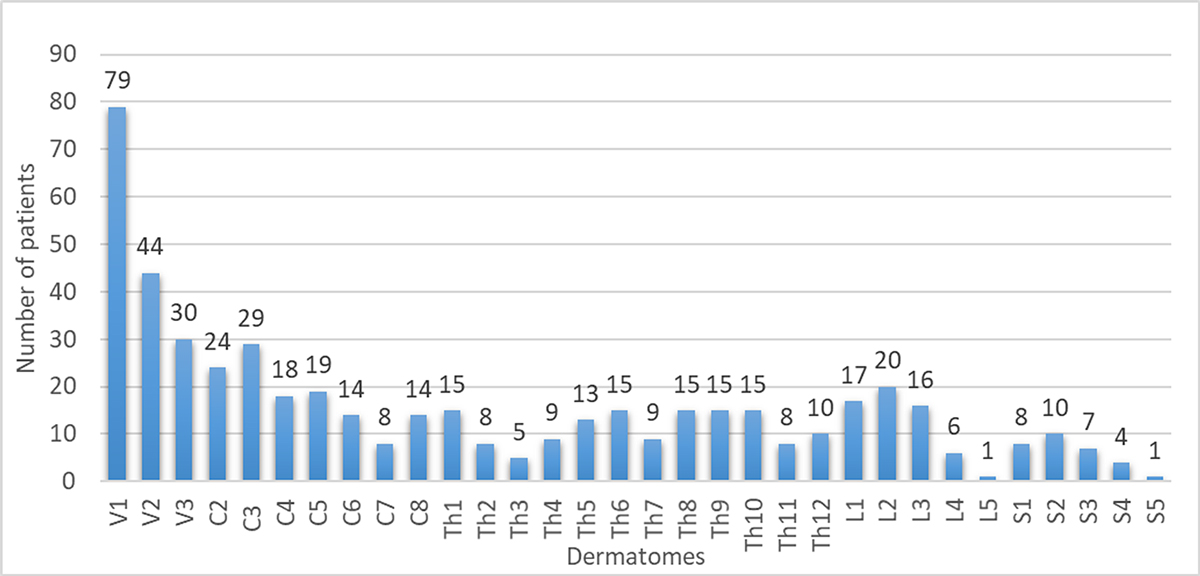
Figure 1 Affected dermatomes (506 in total, as 100 of the 355 patients had >1 dermatome affected).
DOI: https://doi.org/10.4414/SMW.2021.w30081
Herpes zoster, or shingles, is a painful cutaneous reactivation of the varicella zoster virus (VZV), which remains in latent form in trigeminal and dorsal root ganglia [1]. The lifetime risk of herpes zoster in the general population was reported to be 20–30%, resulting in substantial morbidity and high direct and indirect costs [2–4]. According to incidence estimates for Switzerland, approximately 21,400 individuals are affected by herpes zoster cases per year [5] of whom 10–20% suffer from post-herpetic neuralgia (PHN) as a common long-term complication. PHN adds to the patient’s and the economic burden of disease through its impact on the quality of life and the chronic use of painkillers their side effects [6, 7].
The exact pathophysiology of the VZV latency in trigeminal and dorsal root ganglia and the sequence of viral reactivation is not yet fully understood [8, 9]. Nevertheless, several risk factors for herpes zoster have been reported, such as cancer, immunosuppression (e.g., solid organ transplant recipients; human immunodeficiency virus [HIV]; immunosuppressive drugs) and diabetes mellitus [10–12]. However, many aspects of herpes zoster such as the types, rates and predisposing cofactors of complications remain ill-defined [13]. Furthermore, the impact of these complications on herpes zoster-related costs are lacking for central Europe including Switzerland.
Currently, two herpes zoster vaccines are approved by the US Food and Drug Administration (FDA) and European Medicines Administration (EMA): the herpes zoster live vaccine (LZV, Zostavax®) and the recombinant, adjuvanted subunit vaccine (RZV, Shingrix®), which prevent herpes zoster in about 50% (LZV) and >95% (RZV), respectively. In Switzerland, the RZV was approved in October 2021. The LZV is contraindicated in immunosuppressed individuals and has poor effectiveness in persons aged >80 years. RZV is effective in immunosuppressed and in the elderly. The recommendations for the RZV by the German Vaccination Advisory Board (Ständige Impfkommission, STIKO) include individuals aged >60 years, but if specific risk factors are coincident also in patients aged >50 years, these include: HIV infection, rheumatoid arthritis, systemic lupus erythematosus, diabetes, chronic obstructive pulmonary disease or asthma, immunosuppression, inflammatory bowel disease or chronic kidney disease.
As previously emphasised, there is still a need to match health-economic estimations in herpes zoster with clinical real-life data to refine vaccination strategies [13, 14]. The goals of our study were to identify complication rates of herpes zoster, determine risk groups and to evaluate the direct costs associated with herpes zoster in different clinical scenarios.
This single-centre, investigator-initiated retrospective study was approved by the Ethics Committee Northwest and Central Switzerland (#2018–02270).
We reviewed the medical records of patients with herpes zoster treated by the Department of Dermatology of the University Hospital Basel, Switzerland (a tertiary referral centre) between 2005 and 2019, and extracted the following herpes zoster-related data: age, sex, comorbidities, predisposing factors, seasonal occurrence, clinical manifestations, complications, and information on management and therapies.
Assessments of the clinical presentation included the number and location of affected dermatome(s), or dissemination (i.e., more than 20 vesicles outside the primary and immediately adjacent dermatomes [15]). If allocation to one single dermatome was not possible (e.g., recorded as “Th9/10”), the region (“thoracic”) was counted only once.
The following documented conditions were considered as herpes zoster complications: necrotising and/or haemorrhagic herpes zoster, bacterial superinfections, dissemination, PHN, VZV infection of the cerebrospinal fluid, Ramsay-Hunt syndrome type 2 (zoster oticus), peripheral herpes zoster-associated neuropathies, ocular complications (such as keratitis, glaucoma, visual impairment or blepharoconjunctivitis; each patient with V1 herpes zoster was referred to an ophthalmologist), chronification (defined as a persistent herpes zoster rash for over 30 days [16]) and herpes zoster-related death.
The selection of the risk factors for herpes zoster was based on previous publications [11, 17–20], expert opinion and the feasibility to retrieve the data in our retrospective study design. We assessed the following variables as potential risk factors in addition to age and sex: (i) cancers/malignancies (e.g. solid cancers, leukaemia, lymphoma, and melanoma; for simplicity summarised as “cancer”, [non-melanoma skin cancers and localised cutaneous lymphoma were not classified into this group]); (ii) diabetes mellitus (independent of type or therapy); (iii) immunosuppression including glucocorticosteroid doses >10 mg/d prednisone equivalent for any given reason, immunosuppression following solid organ transplantation (e.g., tacrolimus, mycophenolate mofetil), therapy of rheumatic or autoimmune disease with biologics or non-biologic disease modifying anti-rheumatic drugs (DMARDs) or HIV infection irrespective of the viral load or CD4+ T-cell count (since this information was not available). Only patients with disseminated herpes zoster were screened for HIV, because the pretest probability of HIV in common herpes zoster cases is very low in the University Hospital Basel.
Given the potentially additive herpes zoster risk of the three assessed risk factors, patients with more than one risk factor (e.g., kidney transplanted patients with diabetes) were classified into a fourth group termed “high-risk group”.
According to an exploratory analysis, the clinical presentation and complications were not consistently documented in non-dermatological specialties; we therefore chose to focus on herpes zoster patients seen by dermatologists for the reasons of quality, consistency and comparability of data.
We analysed herpes zoster-related treatment costs in both outpatient (including re-visits) and inpatient settings by checking invoices issued to the health insurance companies. For outpatients we added the estimated medication costs (calculated according to figure S1 in the appendix). Patients for whom herpes zoster was not the main reason for hospitalisation were excluded from cost analyses since it would have been impossible to determine the herpes zoster related costs in these cases. Indirect costs such as herpes zoster-related absence from work were not considered. We converted the costs from Euro to Swiss Francs with the exchange rate of EUR 1 = CHF 1.0638 and from Dollars to Swiss Francs with the exchange rate of USD 1 = CHF 0.9476 based on the exchange rate on 28 June 2020.
To compare patient characteristics, we used Fisher’s exact test for binary variables and t-tests or Mann-Whitney U tests for continuous variables where appropriate. To assess the impact of risk factors on complications we first used a logistic regression model to assess the univariate relationship between complications and patients` characteristics (age, cancer, diabetes, immunosuppression); we adjusted for age. Secondly, we analysed the data using a multivariate logistic regression model to better understand the relationships among the variables. Statistical analysis was performed using “R” software (Version 4.0.2).
The study population consisted of 355 herpes zoster patients (mean age 61.7 ± 18.7 years) with 201 females (56.6%) and a female predominance in the outpatient (55.9%) and inpatient (58.4%) settings. Out of 355 patients, 101 (28.5%) were hospitalised, 58 of whom (57.4%) were treated in the dermatology ward. The remaining 43 (42.6%) were treated in other wards, but were seen by our dermatologists. In 60% of the hospitalised patients (66/110) herpes zoster was the primary reason for the hospitalisation. The hospitalised patients were older than the outpatients (mean age 71.1 ± 18.7 years vs 58.0 ± 18.6 years). The 254 outpatients had on average 1.7 herpes zoster-related consultations (range 1–12 consultations).
There was no seasonality of herpes zoster cases with 29%, 25%, 25% and 21% of the cases occurring in winter, spring, summer and autumn, respectively.
Herpes zoster risk factors were present in 81/355 patients (22.8%) including 28 patients (7.9%) with solid cancers or haematological malignancies, 24 (6.7%) with immunosuppression (most frequently corticosteroids, mycophenolate mofetil, adalimumab) and 22 (6.2%) with diabetes. Seven patients (2.0%) had more than one risk factor.
In 255 patients (71.8%) herpes zoster was monosegmental, in 100 (28.2%) multisegmental. The prevalence of the affected dermatomes was as follows: trigeminal 30.2% (153/506 affected dermatomes, V1 being the most commonly affected dermatome of all [79/506, 16%]), cervical 24.9% (126/506), thoracic 27.1% (137/506), lumbar 11.9% (60/506) and sacral 5.9% (30/506) (fig. 1).

Figure 1 Affected dermatomes (506 in total, as 100 of the 355 patients had >1 dermatome affected).
A total of 141 complications were recorded in 107 patients (30.1%; 107/355). PHN (37/141, 26.2%), herpes zoster dissemination (29/141, 20.6%) and skin necrosis (21/141, 14.9%) were the most frequent, accounting for 61.7% of all complications (fig. 2). The herpes zoster-related mortality rate was 0.85% (n = 3).
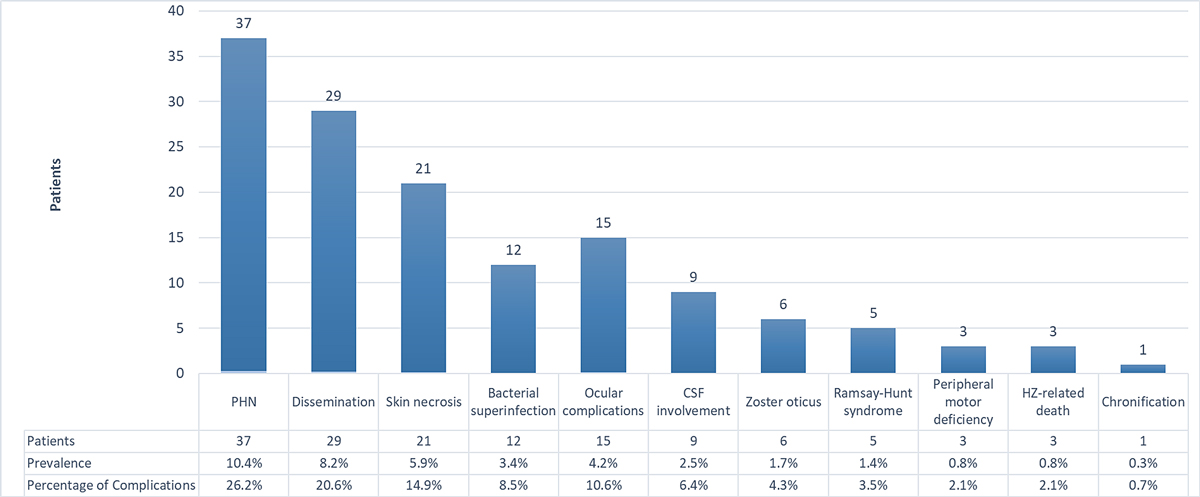
Figure 2 Types and prevalence of complications (percentage per 355 patients). CSF: cerebrospinal fluid; HZ: herpes zoster; PHN: post-herpetic neuralgia
According to the preliminary age-adjusted univariate logistic regression model (table 1), the complication rate was significantly increased in patients with cancer (odds ratio [OR] 2.38, 95% confidence interval [CI] 1.06–5.35), but not in patients with diabetes (OR 1.60, 95% CI 0.64–3.93), in immunosuppressed patients (OR 0.94, 95% CI 0.32–2.43) or patients with more than one risk factor (the "high-risk group") (OR 1.06, 95% CI 0.15–5.10).
The multivariate logistic regression model (table 2) yielded similar results for cancer (OR 2.52, 95% CI 1.12–5.72) diabetes (OR 1.79, 95% CI 0.71–4.42), immunosuppressed patients (OR 1.08, 95% CI 0.37–2.80) and patients with more than one risk factor (the "high-risk group") (OR 1.2, 95% CI 0.17–5.82). An exploratory analysis indicated that cancer patients aged <60 years showed a similarly high complication rate as older cancer patients (57.1%, 4/7). However, this subgroup was very small.
Age was a significant risk factor (OR 1.035, 95% CI 1.02–1.051; p = 0.0005). For each additional year of age, the odds for a complication increased by 3.5% and for 10 years by 41.4%. The complication rates per age group are shown in figure 3, which indicates a marked increase between 50 and 60 years of age. Women and men had comparable complication rates (females 32.3%, 65/201; males 33.1%, 51/154).
Table 1Univariate analysis.
| Risk group | OR for complications (95% CI) | p-value |
| Age | 1.037 (1.023–1.053) per 1 year | <0.01 |
| 1.441 per 10 years | ||
| Cancer1 | 2.38 (1.06—5.35) | 0.03 |
| Diabetes mellitus1 | 1.60 (0.64—3.93) | 0.30 |
| Immunosuppression1 | 0.94 (0.32—2.43) | 0.91 |
| Patients with >1 risk factor1 | 1.06 (0.15—5.10) | 0.95 |
CI: confidence interval; OR: odds ratio
1 Age adjusted univariate analysis
Table 2Multivariate analysis.
| Risk group | OR for complications (95% CI) | p-value |
| Age | 1.035 (1.020—1.051) per 1 year | <0.01 |
| 1.414 per 10 years | ||
| Cancer | 2.52 (1.12—5.72) | 0.02 |
| Diabetes mellitus | 1.79 (0.71—4.42) | 0.21 |
| Immunosuppression | 1.08 (0.39—2.80) | 0.88 |
| Patients with >1 risk factor | 1.20 (0.16—5.82) | 0.82 |
CI: confidence interval; OR: odds ratio
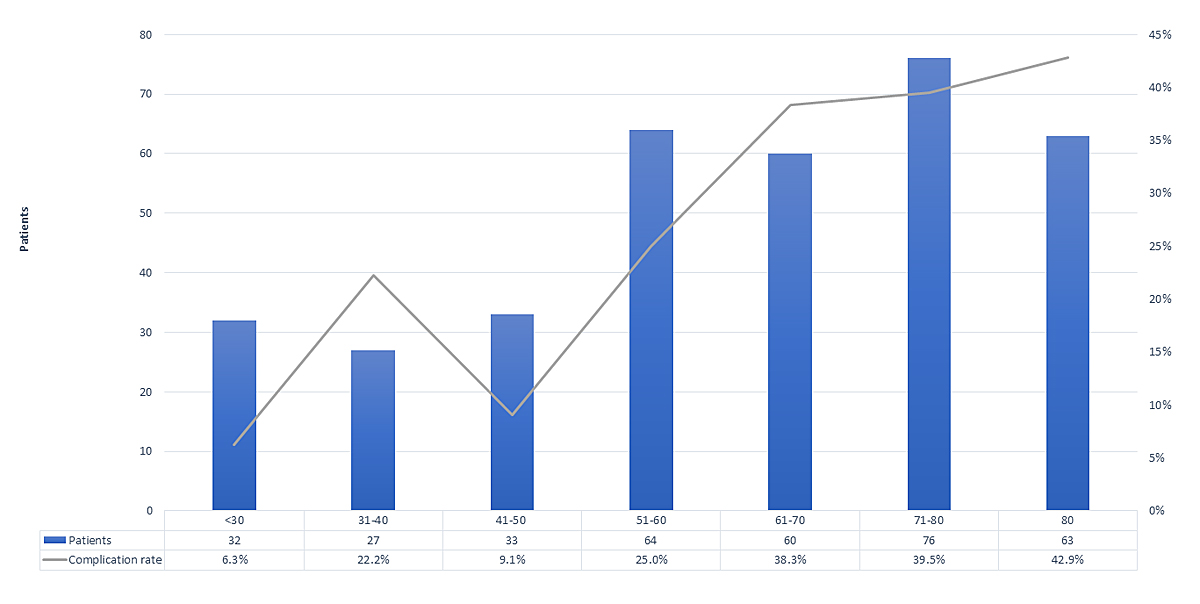
Figure 3 Complication rate per age group (n = 355).
Cost evaluation was available for 246/254 (96.9%) outpatients. The average estimated total cost (median) per outpatient (i.e., consultation plus medication) was CHF 335 (interquartile range [IQR] 283) (table 3). The costs were 4.3 times higher in outpatients with complications than in outpatients without complications (CHF 1331, IQR 1489) vs CHF 306 CHF, IQR 196).
In 62.2% (158/254) of outpatients a standard therapy with valaciclovir, analgesics and topical treatment was prescribed. In 16.5% of outpatients (42/254) no antiviral treatment was necessary. The remaining (21.3%) outpatients (54/254) needed higher doses, longer duration or additional use of analgesics (i.e., tramadol, metamizole, pregabalin or gabapentin) and/or antivirals (see appendix for details) resulting in higher costs per case.
Table 3Herpes zoster-related treatment costs.
| Group comparison | Median cost estimate per patient; IQR (CHF) | Mean (CHF) | Factor more expensive | |
| Hospitalized vs. outpatients | Hospitalised (n = 62) | 9029; 8178 | 11463 | 26.9 |
| Outpatient (n = 246) | 335; 283 | 554 | 1 | |
| Outpatients with vs. without complication | With complications (n = 52) | 1331; 1489 | 1300 | 4.3 |
| Without complications (n = 194) | 306; 196 | 355 | 1 | |
| All cases with vs. without complications | With complications (n = 91) | 2203; 7835 | 6557 | 6.6 |
| Without complications (n = 217) | 332; 268 | 1154 | 1 | |
Of 101 inpatients, 66.3% (67/101) were hospitalised because of herpes zoster and intravenous antiviral therapy was administered in 71.6% (48/67) of these patients. The remaining 34 patients (33.7%; 34/101) were hospitalised for other reasons but developed herpes zoster during hospitalisation, eight of whom were treated with intravenous antiviral therapy (23.5%). The cost evaluation focused on the patients hospitalised because of herpes zoster and was available for 62 of the 67 inpatients hospitalised because of herpes zoster.
The average cost (median) of patients hospitalised for Herpes zoster was CHF 9029 (IQR 8178). Overall, costs for hospitalised herpes zoster patients were 26.9 times higher in comparison with outpatients (CHF 9029 vs 335).
The overall costs of patients with complications were 6.6 times higher than the costs in herpes zoster-patients without complications (CHF 2203, IQR 7835 vs CHF 332, IQR 268).
In this single-centre, investigator-driven, retrospective study we analysed the clinical presentation, complication rates, risk factors and treatment-related costs of in- and outpatients treated by dermatologists in our tertiary referral centre between 2005 and 2019. The complication rate amongst the 355 patients analysed was 30.1% (n = 107), the herpes zoster-related hospitalisation rate was 19.2% (n = 68) and the herpes zoster-related mortality rate 0.85% (n = 3). Age and cancer were identified as factors that significantly increase the complication rate. The cost ratio of in- vs outpatient treatment was 26.9, the ratio of complicated vs non-complicated patients was 6.6 and the ratio in complicated vs non-complicated outpatients was 4.3.
The age and sex distribution in our population was comparable to previous studies [2, 21–23]. Therefore, we assume that our study reflects a population of herpes zoster patients treated in a tertiary hospital, allowing comparison with previous studies from other countries. To put our data into context, the Swiss Federal Statistical Office (FSO) reported approximately 542 herpes zoster-related hospitalisations per year between 2008 and 2013. In 2016, approximately 21,400 herpes zoster cases were reported, resulting in a hospitalisation rate of 2.53% [24, 25].
In our patients, the most frequently affected dermatomes were trigeminal (30.2%), with a higher prevalence than previously reported [26, 27]. We also observed a higher rate of multi-segmental herpes zoster (28.1%) compared with the 20% reported in the literature [28]. These differences were most likely due to a selection bias, as our clinic is a specialised tertiary referral centre.
The overall complication rate of 30.1% in our study population, including in- and outpatients, was comparable to other studies, ranging from 20% in a population-based study by Yawn et al. [29] up to 61.4% in a study with inpatients by Stein et al. [30]. The higher complication rate in our study compared with Yawn’s is attributable to higher non-PHN complications, possibly caused by different study designs and study populations.
Although herpes zoster complications are of paramount importance for patients and herpes zoster-related health-economic aspects, data on overall (non-PHN) complication rates are scarce, especially in an outpatient setting [13]. In addition, the term “complication” in herpes zoster studies is inconsistently used in these studies [30]. The definition of “PHN” has been a matter of discussion for decades (as reviewed by Tontodonati et al. [31]) with inconsistent definitions of the pain duration varying from 1–6 months. Therefore, rates of PHN range widely in the literature (depending on the definition and the setting [32]), from 4.5% [2] in a population-based study and up to 56% in a retrospective study [33]. It is probable that in our population PHN is underestimated as we did not systematically monitor subsequent PHN development.
Our study is the first to demonstrate the rate of specific herpes zoster complications in a sample of in- and outpatients in Switzerland. We found the same relationships of complications and risk factors in the age-adjusted univariate and multivariate analysis. The type and frequencies of complications in our group were comparable to the study by Yawn et al. except for disseminated herpes zoster, which was ten times higher in our population, possibly attributable to differing definitions of “dissemination” and the study settings [34]. The mortality rate of 0.85% was in the range of existing data from population-based studies [2, 35, 36], but lower than in an inpatient study of a tertiary referral centre in Connecticut (USA) between 1986 and 1995, in which a mortality rate of 5.3 was reported [37], possibly explained by varying study settings and improved treatment options (e.g., valaciclovir, brivudin).
Age was associated with higher incidence, and more complications and hospitalisations. In contrast to a Korean study, there was no decline in complication rate in patients >80 years [38]. Our data support the current recommendations that individuals over 60 years are at higher risk both for herpes zoster and its complications, suggesting that this group should be prioritised for vaccination.
However, approximately a quarter of the patients aged <60 years also suffered from complications, leading to significant morbidity and costs. Vaccination of healthy individuals aged <50 years may not be cost effective in general because of the high costs of the RZV, but potentially may be so in subgroups having additional risk factors.
The second factor associated with a significantly higher risk (p <0.05) for complications in our population was cancer (10 solid cancer patients and 14 lymphoma/leukaemia patients). Notably, the complication rate was also increased in young cancer patients, suggesting an age-independent association. In line with our data, Yenikomshian et al. reported both a significantly higher incidence of herpes zoster and complication rate in patients with a diagnosis of solid or haematological cancers compared with healthy controls [39]. Combined with these previous data, our results clearly suggest that cancer patients should be evaluated for vaccine strategies as a group at greater risk of complications – a fact not yet considered in guidelines [40].
Although the complication rate in diabetes patients was higher than the overall complication rate, the difference was not statistically significant. In consideration of several previous reports of significantly increased PHN in diabetes patients, we hypothesise that our sample size might have been too low to reach statistical significance [12, 41–43].
A second aim of our study was to characterise the additional direct costs if complications occur and the average case costs of in- and outpatient treatment for herpes zoster in Switzerland. Although there are data on this available from other central European countries, this is the first study to address this topic in Switzerland. The indirect costs were not assessed in this study, but are also relevant for estimating overall costs.
Generally, comparability of costs with other studies is limited for various reasons, including national differences in healthcare systems, insurance coverage, taxing of medical services, treatment policies and longitudinal changes of herpes zoster-related costs.
Complications increased treatment costs in our outpatients by a factor 4.3, and 6.6 when including the inpatients, highlighting complications as a major driver for costs in herpes zoster. Yawn et al. reported an even higher factor of 6.1 in patients with PHN and of 7.1 for non-PHN complications [44]. The lower costs of PHN in our study (CHF 1905) in comparison to Yawn et al. CHF 4158 (USD 4388) may be attributable to a shorter estimated average treatment time (6 months in our study) and the inclusion in their study of costs of visits to general practitioners. In the study by Ultsch et al. conducted in Germany, however, similar costs of CHF 1418–1595 (EUR 1333–1499) for PHN were reported [2].
In central Europe, the outpatient costs range approximately from EUR 80–500 (CHF 84–532) [2, 13, 45], which is lower than in our population. This may be partly explained by more severe and therefore more costly cases being referred to tertiary referral centres such as ours, whereas the studies mentioned above included general practitioners in their cost analyses.
Hospitalisation increased the treatment cost by a factor of 26.9. The average hospitalisation costs were CHF 9029. Very similarly, a study from Italy also showed a 20-fold increase in treatment costs in hospitalised patients [19]. Others reported lower costs in hospitalised patients (e.g., Germany EUR 2984 (CHF 3174; 2010) [2], Spain EUR 3719 (CHF 3956; 1998–2004) [46]). Healthcare costs are generally high in Switzerland [47] and the aforementioned studies included smaller hospitals, presumably with less severe cases.
To put our data into perspective, the only available study from Switzerland is the study by Szucs et al. In their modelling analysis, the direct costs per case in Switzerland were estimated to be between CHF 362–386 and the indirect costs betweenCHF 403–430. Costs of mild PHN were CHF 127–389, of moderate PHN CHF 600–1040, and of severe PHN CHF 1532–2493. Severe herpes zoster cases including hospitalizations – resulted in estimated costs of CHF 1227–1874. Notably, a study from the USA evaluated the costs of insurance claims related to herpes zoster complications: more than 20,000 patients with complicated herpes zoster were compared with matched patients with uncomplicated herpes zoster. The authors found that the mean annual incremental unadjusted costs for herpes zoster-related complications were USD 4753 (CHF 5016) [48]. As mentioned above, an Italian study reported that inpatient care for complicated herpes zoster was >20 times the outpatient costs per herpes zoster case [21]. Thus, in Switzerland and abroad, real-world costs for complicated herpes zoster are substantially higher than suggested by some modelling studies.
In most countries, herpes zoster vaccination is recommended for persons aged >60 years. Our results support the notion that people aged over 60 years are at particular risk for herpes zoster and its complications, as is stated in the German and the Swiss vaccine recommendations [40]. Since we have also observed a marked rise in herpes zoster cases and complications in patients aged over 50 years, we hypothesise that this “late middle aged” group could also benefit from herpes zoster vaccination. This aligns with the US Advisory Committee on Immunization Practices (ACIP), which recommends general herpes zoster vaccination in people aged over 50 years based on similar data (a two-fold increase of herpes zoster incidence >50 years) [49].
This study may have several limitations. Generalisability may be limited by national differences and by a potential selection bias of more severe cases at our tertiary referral centre, with consequently more complications and higher costs compared with primary or secondary care. We did not differentiate between the different forms of immunosuppression in the calculations, which could explain the insignificant association of immunosuppression and complication rates. A recent meta-analysis showed that specific immunosuppressive therapies (e.g., cyclophosphamide; Januskinase inhibitors) increase the risk for herpes zoster more than others (e.g., anti-tumour necrosis factor-alpha blockers, DMARDs) [50]. The selection strategy of the risk factors was not based on prior statistical analysis of our data. The calculations of the medication costs in outpatients were based on approximations depending on the most common treatment course, which may lead to some inaccuracies. Furthermore, the costs were not corrected for inflation over time (cases over a period of 14 years were included). Consequently, the costs may have been slightly underestimated for the earlier cases. Since not all patients were further followed-up at our clinic, we may have underestimated the incidence and costs of PHN. Side effects or adverse events (e.g., gastric ulcers or nephrotoxicity) of treatment for PHN is a further aspect that should be considered in future cost estimations. Finally, since this study was planned and conducted, the evidence for stroke and myocardial infarction being herpes zoster complications has increased [51]. We therefore suggest taking these two complications also into account in future cost assessments.
Our results support the notion that patients aged >60 years are the primary risk patients for complicated herpes zoster and thus should be the focus of vaccination strategies. However, our data identify complications and hospitalisations as the main drivers of herpes zoster-related health costs, which applied to patients aged <60 years as well. Thus, cost-effectiveness studies should also include younger patients and consider risk conditions for complicated herpes zoster. In our study, cancer was an age-independent risk factor for complications, suggesting the need to consider adult cancer patients for herpes zoster vaccination irrespective of age.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.
The authors are indebted to Pia Steinger and Annet Tiemessen, study nurses Department of Dermatology, University Basel, for their administrative support and to Laura Werlen PhD, Dr. Jonas Fischer and Phillip Dehio for their support in the statistical analyses of this study.

Figure S1 Cost estimates of the herpes zoster-related drugs in an outpatient setting. As many patients with post-herpetic neuralgia (PHN) were referred to us for the treatment of PHN only and it was unclear from our documents whether or not they had been treated with basic antiviral standard treatment, we did not include the basic antiviral standard treatment costs for this estimation in our patients. NSAIDs = Ibuprufen, diclofenac.
1. Hope-Simpson RE . The Nature of Herpes Zoster: A Long-Term Study and a New Hypothesis. Proc R Soc Med. 1965 Jan;58(1):9–20. https://doi.org/10.1177/003591576505800106
2. Ultsch B , Köster I , Reinhold T , Siedler A , Krause G , Icks A , et al. Epidemiology and cost of herpes zoster and postherpetic neuralgia in Germany. Eur J Health Econ. 2013 Dec;14(6):1015–26. https://doi.org/10.1007/s10198-012-0452-1
3. Johnson RW , Alvarez-Pasquin MJ , Bijl M , Franco E , Gaillat J , Clara JG , et al. Herpes zoster epidemiology, management, and disease and economic burden in Europe: a multidisciplinary perspective. Ther Adv Vaccines. 2015 Jul;3(4):109–20. https://doi.org/10.1177/2051013615599151
4. Yawn BP , Gilden D . The global epidemiology of herpes zoster. Neurology. 2013 Sep;81(10):928–30. https://doi.org/10.1212/WNL.0b013e3182a3516e
5. Szucs TD , Kressig RW , Papageorgiou M , Kempf W , Michel JP , Fendl A , et al. Economic evaluation of a vaccine for the prevention of herpes zoster and post-herpetic neuralgia in older adults in Switzerland. Hum Vaccin. 2011 Jul;7(7):749–56. https://doi.org/10.4161/hv.7.7.15573
6. Saguil A , Kane S , Mercado M , Lauters R . Herpes Zoster and Postherpetic Neuralgia: prevention and Management. Am Fam Physician. 2017 Nov;96(10):656–63.
7. Eckert N , Masserey Spicher V . [Chickenpox and shingles: one virus, two diseases and current vaccination recommendations in Switzerland]. Ther Umsch. 2016;73(5):247–52. https://doi.org/10.1024/0040-5930/a000787
8. Kennedy PG . Varicella-zoster virus latency in human ganglia. Rev Med Virol. 2002 Sep-Oct;12(5):327–34. https://doi.org/10.1002/rmv.362
9. Gershon AA , Gershon MD , Breuer J , Levin MJ , Oaklander AL , Griffiths PD . Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J Clin Virol. 2010 May;48 Suppl 1:S2–7. https://doi.org/10.1016/S1386-6532(10)70002-0
10. Kawai K , Yawn BP . Risk Factors for Herpes Zoster: A Systematic Review and Meta-analysis. Mayo Clin Proc. 2017 Dec;92(12):1806–21. https://doi.org/10.1016/j.mayocp.2017.10.009
11. Papagianni M , Metallidis S , Tziomalos K . Herpes Zoster and Diabetes Mellitus: A Review. Diabetes Ther. 2018 Apr;9(2):545–50. https://doi.org/10.1007/s13300-018-0394-4
12. Forbes HJ , Bhaskaran K , Thomas SL , Smeeth L , Clayton T , Langan SM . Quantification of risk factors for herpes zoster: population based case-control study. BMJ. 2014 May;348:g2911. https://doi.org/10.1136/bmj.g2911
13. Panatto D , Bragazzi NL , Rizzitelli E , Bonanni P , Boccalini S , Icardi G , et al. Evaluation of the economic burden of Herpes Zoster (HZ) infection. Hum Vaccin Immunother. 2015;11(1):245–62. https://doi.org/10.4161/hv.36160
14. Blank PR , Ademi Z , Lu X , Szucs TD , Schwenkglenks M . Herpes zoster vaccine: A health economic evaluation for Switzerland. Hum Vaccin Immunother. 2017 Jul;13(7):1495–504. https://doi.org/10.1080/21645515.2017.1308987
15. McCrary ML , Severson J , Tyring SK . Varicella zoster virus. J Am Acad Dermatol. 1999 Jul;41(1):1–14. https://doi.org/10.1016/S0190-9622(99)70398-1
16. Wauters O , Lebas E , Nikkels AF . Chronic mucocutaneous herpes simplex virus and varicella zoster virus infections. J Am Acad Dermatol. 2012 Jun;66(6):e217–27. https://doi.org/10.1016/j.jaad.2010.07.011
17. Hansson E , Forbes HJ , Langan SM , Smeeth L , Bhaskaran K . Herpes zoster risk after 21 specific cancers: population-based case-control study. Br J Cancer. 2017 Jun;116(12):1643–51. https://doi.org/10.1038/bjc.2017.124
18. Chen SY , Suaya JA , Li Q , Galindo CM , Misurski D , Burstin S , et al. Incidence of herpes zoster in patients with altered immune function. Infection. 2014 Apr;42(2):325–34. https://doi.org/10.1007/s15010-013-0550-8
19. Marra F , Lo E , Kalashnikov V , Richardson K . Risk of Herpes Zoster in Individuals on Biologics, Disease-Modifying Antirheumatic Drugs, and/or Corticosteroids for Autoimmune Diseases: A Systematic Review and Meta-Analysis. Open Forum Infect Dis. 2016 Sep;3(4):ofw205. https://doi.org/10.1093/ofid/ofw205
20. Marra F , Parhar K , Huang B , Vadlamudi N . Risk Factors for Herpes Zoster Infection: A Meta-Analysis. Open Forum Infect Dis. 2020 Jan;7(1):ofaa005. https://doi.org/10.1093/ofid/ofaa005
21. Gialloreti LE , Merito M , Pezzotti P , Naldi L , Gatti A , Beillat M , et al. Epidemiology and economic burden of herpes zoster and post-herpetic neuralgia in Italy: a retrospective, population-based study. BMC Infect Dis. 2010 Aug;10(1):230. https://doi.org/10.1186/1471-2334-10-230
22. Li Y , An Z , Yin D , Liu Y , Huang Z , Xu J , et al. Disease Burden Due to Herpes Zoster among Population Aged ≥50 Years Old in China: A Community Based Retrospective Survey. PLoS One. 2016 Apr;11(4):e0152660. https://doi.org/10.1371/journal.pone.0152660
23. Johnson RW , Wasner G , Saddier P , Baron R . Herpes zoster and postherpetic neuralgia: optimizing management in the elderly patient. Drugs Aging. 2008;25(12):991–1006. https://doi.org/10.2165/0002512-200825120-00002
24 Network TSSS . 2016.
25. Federal Statistical Office (FSO) . Neuchâtel, Switzerland. 2015.
26. Chen LK , Arai H , Chen LY , Chou MY , Djauzi S , Dong B , et al. Looking back to move forward: a twenty-year audit of herpes zoster in Asia-Pacific. BMC Infect Dis. 2017 Mar;17(1):213. https://doi.org/10.1186/s12879-017-2198-y
27. Meister W , Neiss A , Gross G , Doerr H , Höbel W , Malin J , et al. Demography, symptomatology, and course of disease in ambulatory zoster patients. A physician-based survey in Germany. Intervirology. 1998;41(6):272–7. https://doi.org/10.1159/000024949
28. Gnann JW Jr , Whitley RJ . Clinical practice. Herpes zoster. N Engl J Med. 2002 Aug;347(5):340–6. https://doi.org/10.1056/NEJMcp013211
29. Yawn BP , Saddier P , Wollan PC , St Sauver JL , Kurland MJ , Sy LS . A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007 Nov;82(11):1341–9. https://doi.org/10.4065/82.11.1341
30. Stein AN , Britt H , Harrison C , Conway EL , Cunningham A , Macintyre CR . Herpes zoster burden of illness and health care resource utilisation in the Australian population aged 50 years and older. Vaccine. 2009 Jan;27(4):520–9. https://doi.org/10.1016/j.vaccine.2008.11.012
31. Tontodonati M , Ursini T , Polilli E , Vadini F , Di Masi F , Volpone D , et al. Post-herpetic neuralgia. Int J Gen Med. 2012;5:861–71.
32. Kawai K , Gebremeskel BG , Acosta CJ . Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014 Jun;4(6):e004833. https://doi.org/10.1136/bmjopen-2014-004833
33. Kanbayashi Y , Onishi K , Fukazawa K , Okamoto K , Ueno H , Takagi T , et al. Predictive factors for postherpetic neuralgia using ordered logistic regression analysis. Clin J Pain. 2012 Oct;28(8):712–4. https://doi.org/10.1097/AJP.0b013e318243ee01
34. . Billroth — surgeon, teacher, musician. JAMA. 1964 May;188(8):749–51. https://doi.org/10.1001/jama.1964.03060340047014
35. Gonzalez Chiappe S , Sarazin M , Turbelin C , Lasserre A , Pelat C , Bonmarin I , et al. Herpes zoster: burden of disease in France. Vaccine. 2010 Nov;28(50):7933–8. https://doi.org/10.1016/j.vaccine.2010.09.074
36. Bricout H , Haugh M , Olatunde O , Prieto RG . Herpes zoster-associated mortality in Europe: a systematic review. BMC Public Health. 2015 May;15(1):466. https://doi.org/10.1186/s12889-015-1753-y
37. Lin F , Hadler JL . Epidemiology of primary varicella and herpes zoster hospitalizations: the pre-varicella vaccine era. J Infect Dis. 2000 Jun;181(6):1897–905. https://doi.org/10.1086/315492
38. Kim YJ , Lee CN , Lim CY , Jeon WS , Park YM . Population-based study of the epidemiology of herpes zoster in Korea. J Korean Med Sci. 2014 Dec;29(12):1706–10. https://doi.org/10.3346/jkms.2014.29.12.1706
39. Yenikomshian MA , Guignard AP , Haguinet F , LaCasce AS , Skarin AT , Trahey A , et al. The epidemiology of herpes zoster and its complications in Medicare cancer patients. BMC Infect Dis. 2015 Feb;15(1):106. https://doi.org/10.1186/s12879-015-0810-6
40. Siedler A , Koch J , Garbe E , Hengel H , von Kries R , Ledig T , et al. Background paper to the decision to recommend the vaccination with the inactivated herpes zoster subunit vaccine : Statement of the German Standing Committee on Vaccination (STIKO) at the Robert Koch Institute. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2019 Mar;62(3):352–76. https://doi.org/10.1007/s00103-019-02882-5
41. Jih JS , Chen YJ , Lin MW , Chen YC , Chen TJ , Huang YL , et al. Epidemiological features and costs of herpes zoster in Taiwan: a national study 2000 to 2006. Acta Derm Venereol. 2009 Nov;89(6):612–6. https://doi.org/10.2340/00015555-0729
42. Weitzman D , Shavit O , Stein M , Cohen R , Chodick G , Shalev V . A population based study of the epidemiology of Herpes Zoster and its complications. J Infect. 2013 Nov;67(5):463–9. https://doi.org/10.1016/j.jinf.2013.06.016
43. Forbes HJ , Thomas SL , Smeeth L , Clayton T , Farmer R , Bhaskaran K , et al. A systematic review and meta-analysis of risk factors for postherpetic neuralgia. Pain. 2016 Jan;157(1):30–54. https://doi.org/10.1097/j.pain.0000000000000307
44. Yawn BP , Itzler RF , Wollan PC , Pellissier JM , Sy LS , Saddier P . Health care utilization and cost burden of herpes zoster in a community population. Mayo Clin Proc. 2009 Sep;84(9):787–94. https://doi.org/10.4065/84.9.787
45. Bilcke J , Ogunjimi B , Marais C , de Smet F , Callens M , Callaert K , et al. The health and economic burden of chickenpox and herpes zoster in Belgium. Epidemiol Infect. 2012 Nov;140(11):2096–109. https://doi.org/10.1017/S0950268811002640
46. Gil A , Gil R , Alvaro A , San Martín M , González A . Burden of herpes zoster requiring hospitalization in Spain during a seven-year period (1998-2004). BMC Infect Dis. 2009 May;9(1):55. https://doi.org/10.1186/1471-2334-9-55
47. De Pietro C , Camenzind P , Sturny I , Crivelli L , Edwards-Garavoglia S , Spranger A , et al. Switzerland: Health System Review [xix.]. Health Syst Transit. 2015;17(4):1–288.
48. Meyers JL , Candrilli SD , Rausch DA , Yan S , Patterson BJ , Levin MJ . Costs of herpes zoster complications in older adults: A cohort study of US claims database. Vaccine. 2019 Feb;37(9):1235–44. https://doi.org/10.1016/j.vaccine.2018.11.079
49. Dooling KL , Guo A , Patel M , Lee GM , Moore K , Belongia EA , et al. Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines. MMWR Morb Mortal Wkly Rep. 2018 Jan;67(3):103–8. https://doi.org/10.15585/mmwr.mm6703a5
50. McKay SL , Guo A , Pergam SA , Dooling K . Herpes Zoster Risk in Immunocompromised Adults in the United States: A Systematic Review. Clin Infect Dis. 2020 Oct;71(7):e125–34. https://doi.org/10.1093/cid/ciz1090
51. Wu PH , Chuang YS , Lin YT . Does Herpes Zoster Increase the Risk of Stroke and Myocardial Infarction? A Comprehensive Review. J Clin Med. 2019 Apr;8(4):8. https://doi.org/10.3390/jcm8040547