Long-term follow-up of medically treated patients with spontaneous coronary artery dissection: a prospective, Swiss single-centre cohort study
DOI: https://doi.org/10.4414/SMW.2021.w30067
Sebastian
Seidl, Hans
Rickli, Sebastian
Rogowski, Daniel
Weilenmann, Peter
Ammann, Philipp K.
Haager, Lucas
Joerg, Franziska
Rohner, Joannis
Chronis, Johannes
Rigger, Micha T.
Maeder
Cardiology Division, Kantonsspital St. Gallen, Switzerland
Summary
AIMS OF THE STUDY: Spontaneous coronary artery dissection (SCAD) is an increasingly diagnosed cause of acute myocardial infarction. However, there is still a limited number of larger cohorts with long-term follow-up. We report on the largest Swiss single-centre cohort to date, with follow-up of up to 22 years.
METHODS: We prospectively collected SCAD cases from June 1998 until December 2020. A strategy of systematic follow-up angiography was applied. Information on long-term follow-up was collected up to the end of 2020. Major cardiovascular events (MACE) were defined as all-cause death, non-fatal MI, and non-fatal cardiac arrest.
RESULTS: We identified 105 SCAD patients (mean age 53 ± 11 years, 98 female, 5 peripartum). Presentation was myocardial infarction in all patients. In 102 patients, there was one contiguous dissection. Three patients had two (n = 2) or three (n = 1) non-contiguous dissections. In the majority of patients (n = 97), the primary treatment approach was conservative (dual antiplatelet therapy for 12 months in 90% of patients, statins in 91%). Seven patients were treated with percutaneous coronary intervention (PCI) and one patient underwent bypass surgery. Elective follow-up angiograms were performed in 73 asymptomatic patients after a median follow-up of 6.0 months (interquartile range [IQR] 5.5–6.5). These showed healing of the dissection (n = 65) or a good result after PCI (n = 5) in 70 patients. Three patients had a persistent dissection but conservative treatment was continued. After a median follow-up of 7.5 years (IQR 3.6–12.5) (longest follow-up: 22.5 years) there were 15 MACE. Five MACE occurred within 30 days of the index event: death following catastrophic peripartum left main SCAD (n = 1), out-of-hospital cardiac arrest with successful resuscitation 16 days after SCAD (n = 1), ST-segment elevation myocardial infarction due to occlusion of the dissected artery 10 hours after the index angiogram with subsequent PCI (n = 1), SCAD of a second vessel 8 days after the index SCAD (n = 1), and non-ST-segment elevation myocardial infarction with persistent, multisite SCAD 10 days after the index event (n = 1). There were 10 late MACE, including myocardial infarction and recurrent SCAD (different vessel/lesion) a median of 7.6 years (IQR 3.9–9.6) after the index event in eight patients and death with unclear cause in two patients.
CONCLUSION: This SCAD series highlights its highly variable clinical course during the acute phase and in the long term. Although most SCAD patients can be treated conservatively with subsequent healing of the dissection and good clinical outcome, there are also patients with dramatic acute presentation or MACE several years after the initial presentation.
Introduction
Spontaneous coronary artery dissection (SCAD) is an important cause of acute myocardial infarction (MI), particularly in women with otherwise normal coronary arteries [1, 2]. The use of high-sensitivity cardiac troponin assays for the diagnosis of acute myocardial infarction, an earlier, more aggressive and more invasive strategy in patients with non-ST-segment elevation myocardial infarction (NSTEMI), and a rising awareness of SCAD have led to a marked increase in the number of SCAD cases being diagnosed over the last decade. Thus, SCAD can no longer be considered a rare disease, and it is even likely that the condition is still underdiagnosed [3]. Position papers from North America and Europe regarding the evaluation and management of SCAD have been published recently [4, 5]. However, the optimal management of patients with SCAD is still poorly defined, given the observational nature of all available studies. Importantly, the natural history of the condition is still not known exactly, since the follow-up times of all previous studies are limited when considering the typically very long post-SCAD life expectancy in these young patients. The median follow-up periods of the largest series in the literature [6–11] range from 30 days [6] to 4.7 years [10]. Thus, additional outcome data in SCAD patients are crucial to understanding this disease and its long-term trajectories and to forming a basis for the development of appropriate therapies. In the present paper, we report on the largest Swiss cohort to date, representing one of the largest European studies, with a median follow-up of 7.5 years and a maximum follow-up of more than 22 years.
Materials and methods
Patients
This was a prospective, single-centre cohort study. Consecutive patients diagnosed with SCAD at the Kantonsspital St. Gallen from 1998 to December 2020 were included in a registry, which was led by the same group of interventional cardiologists during the entire study period. We have had a strong interest in SCAD for more than 20 years and established this registry at a time when there were no larger SCAD case series. The Cardiology Department of the Kantonsspital St. Gallen is the largest cardiology referral centre in Eastern Switzerland, with one of the largest interventional centres in Switzerland (2019: 2,931 coronary angiographies [12]). Management of SCAD was at the discretion of the treating interventional cardiologist but was fairly uniform within the interventional team and over the entire study period. Decisions on the mode of therapy were typically based on clinical presentation, coronary anatomy, and other clinical factors, and are thereby still in line with current recommendations [4, 5]. In general, only patients with a large area of myocardium at risk, flow-limiting dissection, and evidence of ongoing ischaemia underwent revascularisation by percutaneous coronary intervention (PCI) or coronary artery bypass grafting. Patients without evidence of ongoing ischaemia and/or without flow-limiting dissection of a major vessel were treated conservatively. Medical treatment typically included dual antiplatelet therapy (DAPT; in the early years of the registry aspirin 100 mg/d + clopidogrel 75 mg/d, later on aspirin 100 mg/d + ticagrelor 2x90 mg or aspirin 100 mg/d + prasugrel 10 mg/d) for 12 months, followed by aspirin 100 mg/d alone. Statins were also routinely prescribed, and beta blockers and angiotensin converting-enzyme inhibitors or angiotensin receptor blockers were given depending on left ventricular ejection fraction (LVEF). Treatment with aspirin and statins was suggested for an unlimited period of time.
Angiographic SCAD diagnosis
Diagnostic angiograms were performed by femoral or radial approach using 4F or 5F catheters. All angiograms were reviewed by at least two experienced interventional cardiologists. The diagnosis of SCAD was established based on the following findings: (i) pathognomonic contrast dye staining of arterial wall with multiple radiolucent lumina, with or without the presence of dye hang-up or slow contrast clearing from the lumen or (ii) diffuse and smooth narrowing that could vary in severity plus no response to intracoronary nitroglycerin and no atherosclerotic lesions in other coronary arteries and/or a repeat coronary angiogram showing angiographic resolution of the dissected segment [13]. Angiographic stenosis by visual estimation, lesion length, and thrombolysis in myocardial infarction (TIMI) flow grade were recorded. The angiographic SCAD classification defined by Jaqueline Saw was applied [14]: in brief, type 1 SCAD refers to the typical angiographic feature of a dissection with a visible double lumen, type 2 is a long, diffuse, and smooth stenosis (2A: normalisation of the vessel calibre distally, 2B: extension of the vessel abnormality into the periphery), and type 3 is a lesion that cannot be distinguished angiographically from an atherosclerotic stenosis. We did not perform intravascular ultrasound or optical coherence tomography in SCAD patients. Therefore, we did not diagnose any patients with type 3 SCAD. As mentioned below, follow-up angiograms were performed routinely, and these were also used to confirm a suspected diagnosis of type 2 SCAD in ambiguous cases.
Percutaneous coronary interventions
In selected patients, ad hoc PCI was performed using 6F or 7F guiding catheters and standard techniques. Unfractionated heparin was used for periprocedural anticoagulation. More detailed information on PCI is provided in the results section.
Follow-up
For all patients treated conservatively and for those who underwent PCI, a follow-up angiogram was suggested, which typically was scheduled 6 months after the index event. Repeat angiograms were performed by the femoral or radial approach using 4F or 5F catheters during a short hospital stay or on an ambulatory basis. Patients also had outpatient appointments at the Kantonsspital St. Gallen, the referring hospital, or with cardiologists in private practice, typically 1 to 3 months and 12 months after the index event, and thereafter depending on the clinical context. Data on the clinical follow-up and all available follow-up examinations were collected by three physician scientists. Information was collected up to the end of 2020 by extensive manual and electronic chart review, by systematic telephone follow-up directly with patients, and from treating general practitioners and cardiologists. Patients provided written informed consent for the study, i.e. for the scientific use of the registry. The local ethics committee approved the study [BASEC 2016-01514 (EKSG 14/100)], and a waiver of consent was granted for patients who had died or could not be contacted during follow-up. We have previously reported on the first 64 consecutive patients of this registry, with a median follow-up of 4.5 years [13, 15, 16]. The present analysis includes a substantially higher number of patients with a considerably longer follow-up. Major cardiovascular events (MACE) were defined as all-cause death, non-fatal myocardial infarction, and non-fatal cardiac arrest. Information on other important events such as major bleeding and extracardiac vascular events was also collected.
Statistical analysis
Categorical data are given as numbers and percentages, continuous data with a normal distribution are given as mean ± standard deviation, and continuous data with a skewed distribution are always reported as median (interquartile range [IQR]). Patients with and without MACE were compared using chi-square tests, unpaired t-tests, and Mann-Whitney U tests for the respective types of data. The follow-up was censored at the time of the first MACE or at the most recent follow-up. Survival free of MACE is presented as Kaplan-Meier curves, and comparisons of outcomes between subgroups were performed using log-rank tests. Cox regression was performed to identify independent predictors of MACE.
Results
Patient characteristics
We included 105 SCAD patients [mean age 53 ± 11 years, 98 (93%) female]. All patients presented with acute myocardial infarction: 55 (52%) patients had ST-segment elevation myocardial infarction (STEMI), 49 (47%) patients had NSTEMI, and one (1%) patient had acute myocardial infarction in the presence of a pre-existing left bundle branch block. The baseline characteristics of the study population are shown in table 1.
Table 1Baseline characteristics of the study population and patient with versus without a major cardiac event (MACE).
|
All patients (n = 105)
|
MACE (n = 15)
|
No MACE (n = 90)
|
p-value
|
| Age (years) |
53.4 ± 10.9 |
48.3 ± 11.0 |
54.2 ± 10.7 |
0.049 |
| Female gender |
98 (93%) |
14 (93%) |
84 (93%) |
>0.99 |
| Height (cm) |
165±8 |
165±5 |
165±8 |
0.74 |
| Weight (kg) |
69±13 |
70±13 |
69±13 |
0.78 |
| Body mass index (kg/m2) |
25.5 ± 4.7 |
25.6 ± 4.6 |
25.5 ± 4.8 |
0.91 |
| Race |
|
|
|
0.68 |
| – Caucasian |
104 (99%) |
15 (100%) |
89 (99%) |
|
| – South Asian |
1 (1%) |
0 |
1 (1%) |
|
| Haemoglobin (g/l) |
137 ± 11 |
131 ± 16 |
137 ± 10 |
0.08 |
| Serum creatinine (μmol/l) |
70±17 |
68±14 |
71±18 |
0.63 |
| eGFR (ml/min/1.73 m2) |
89 ± 23 |
95 ± 22 |
88 ± 23 |
0.31 |
|
Clinical presentation
|
0.47 |
| ST segment elevation MI |
55 (52%) |
10 (67%) |
45 (50%) |
|
| Non-ST segment elevation MI |
49 (47%) |
5 (33%) |
44 (49%) |
|
| Left bundle branch block MI |
1 (1%) |
0 |
1 (1%) |
|
|
Peripartum status and previous pregnancies
|
| Peripartum |
5 (5%) |
3 (20%) |
2 (2%) |
0.003 |
| Number of previous pregnancies |
|
|
|
0.92 |
| – None |
21 (20%) |
3 (20%) |
18 (20%) |
|
| – One |
17 (16%) |
3 (20%) |
14 (16%) |
|
| – Two |
25 (24%) |
4 (27%) |
21 (23%) |
|
| – ≥3 |
20 (19%) |
2 (13%) |
18 (20%) |
|
| – Unknown |
22 (21%) |
3 (20%) |
19 (21%) |
|
|
Tradition
al cardiovascular risk factors
|
| Hypertension |
46 (44%) |
5 (33%) |
41 (46%) |
0.38 |
| Family history of coronary disease |
20 (19%) |
1 (7%) |
19 (21%) |
0.18 |
| Smoking |
32 (30%) |
3 (20%) |
29 (32%) |
0.34 |
| Diabetes |
0 |
0 |
0 |
– |
| Dyslipidaemia |
63 (60%) |
6 (40%) |
57 (63%) |
0.09 |
| – Total cholesterol (mmol/l) n = 85 |
5.1 ± 1.3 |
4.7 ± 1.1 |
5.2 ± 1.3 |
0.27 |
| – LDL cholesterol (mmol/l) n = 70 |
3.3 ± 0.9 |
3.1 ± 1.1 |
3.3 ± 0.9 |
0.61 |
| – HDL cholesterol (mmol/l) n = 75 |
1.6 ± 0.4 |
1.5 ± 0.03 |
1.6 ± 0.4 |
0.76 |
| – Triglcerides (mmol/l) n = 83 |
1.1 ± 0.7 |
1.3 ± 0.9 |
1.1 ± 0.6 |
0.29 |
|
Cardiac history
|
| Atrial fibrillation (paroxysmal/permanent) |
7 (7%) |
1 (7%) |
6 (7%) |
>0.99 |
| Mitral valve replacement |
2 (2%) |
0 |
2 (2%) |
0.56 |
| Moderate mitral regurgitation |
1 (1%) |
0 |
1 (1%) |
0.68 |
| Moderate aortic stenosis |
2 (2%) |
0 |
2 (2%) |
0.46 |
|
Non-cardiac history
|
| Panic disorder |
1 (1%) |
0 |
1 (1%) |
0.68 |
| Parkinson’s disease |
1 (1%) |
1 (7%) |
0 |
0.01 |
| Depression |
4 (4%) |
0 |
4 (4%) |
0.41 |
| Stroke |
2 (2%) |
0 |
2 (2%) |
0.56 |
| Bronchial asthma |
8 (8%) |
0 |
8 (9%) |
0.23 |
| Obstructive sleep apnoea |
1 (1%) |
0 |
1 (1%) |
0.68 |
| Hypothyroidism |
3 (3%) |
0 |
3 (3%) |
0.47 |
| Hyperthyroidism |
1 (1%) |
0 |
1 (1%) |
0.68 |
| Psoriasis |
2 (2%) |
0 |
2 (2%) |
0.56 |
| Ulcerative colitis |
1 (1%) |
0 |
1 (1%) |
0.68 |
| Irritable bowel syndrome |
1 (1%) |
0 |
1 (1%) |
0.68 |
| Coeliac disease |
1 (1%) |
1 (7%) |
0 |
0.01 |
| Gastric ulcer |
1 (1%) |
0 |
1 (1%) |
0.68 |
| Lactose intolerance |
1 (1%) |
0 |
1 (1%) |
0.68 |
| Fibromyalgia |
2 (2%) |
0 |
2 (2%) |
0.56 |
| Osteoporosis |
2 (2%) |
0 |
2 (2%) |
0.56 |
| Osteoarthritis |
1 (1%) |
0 |
1 (1%) |
0.68 |
| Endometriosis |
1 (1%) |
0 |
1 (1%) |
0.68 |
| Cancer |
3 (3%) |
0 |
3 (3%) |
0.47 |
Coronary anatomy and SCAD lesion characteristics
There were 102 patients with one contiguous dissection (including dissections extending from a main branch to a side branch, e.g., left main and left anterior descending artery (LAD) or LAD and diagonal branch, two patients had two non-contiguous dissections, in two different vessels, and one patient had dissections in three different vessels. Detailed information about coronary anatomy and SCAD lesions is given in table 2. All patients had otherwise normal coronary arteries or angiographically only minimal atherosclerosis.
Table 2Coronary anatomy of all patients with spontaneous coronary artery dissection (SCAD) and those with versus without major cardiac events (MACE).
|
All patients (n = 105)
|
MACE (n = 15)
|
No MACE (n = 90)
|
p-value
|
|
Artery affected by SCAD
|
0.15 |
|
Single-site SCAD (n = 102)
|
| Right coronary artery |
|
|
|
|
| – Proximal |
4 (4%) |
1 (7%) |
3 (3%) |
|
| – Mid |
1 (1%) |
0 |
1 (1%) |
|
| – Distal |
0 |
0 |
0 |
|
| – Posterior descending artery (PDA) |
9 (9%) |
1 (7%) |
8 (9%) |
|
| – Posterolateral branch |
2 (2%) |
0 |
2 (2%) |
|
| Left main (LM) |
|
|
|
|
| – LM + left anterior descending artery (LAD) |
1 (1%) |
1 (7%) |
0 |
|
| – LM + left circumflex artery (LCX) |
1 (1%) |
0 |
1 (1%) |
|
| – LM + LAD + LCX |
1 (1%) |
1 (7%) |
0 |
|
| LAD |
|
|
|
|
| – Ostial/proximal |
3 (3%) |
0 |
3 (3%) |
|
| – Mid |
16 (15%) |
1 (7%) |
15 (17%) |
|
| – Distal |
18 (17%) |
2 (13%) |
16 (18%) |
|
| – First diagonal branch |
5 (5%) |
0 |
5 (6%) |
|
| – Second diagonal branch |
2 (2%) |
0 |
2 (2%) |
|
| LCX |
|
|
|
|
| – Proximal |
1 (1%) |
1 (7%) |
0 |
|
| – Mid |
4 (4%) |
0 |
4 (4%) |
|
| – Distal |
5 (5%) |
1 (7%) |
4 (4%) |
|
| – First obtuse marginal branch |
12 (11%) |
3 (20%) |
9 (10%) |
|
| – Second obtuse marginal branch |
13 (12%) |
2 (13%) |
11 (12%) |
|
| – Fourth obtuse marginal branch |
1 (1%) |
0 |
1 (1%) |
|
| – Intermediate branch |
3 (3%) |
0 |
3 (3%) |
|
|
Multisite SCAD (n = 3)
|
| Distal LCX + PDA of ACD |
1 (1%) |
0 |
1 (1%) |
|
| LAD + intermediate branch |
1 (1%) |
0 |
1 (1%) |
|
| Mid LCX + intermediate branch + distal ACD |
1 (1%) |
1 (7%) |
0 |
|
|
Lesion characteristics
|
| Reference diameter (mm) |
2.5 (2.0–2.5) |
2.5 (2.0–3.0) |
2.5 (2.0–2.5) |
0.28 |
| Percent stenosis (%) |
95 (80–99) |
90 (70–99) |
97 (80–99) |
0.30 |
| Lesion length (mm) |
30 (20–50) |
30 (20–50) |
30 (20–50) |
0.68 |
| SCAD type |
|
|
|
0.77 |
| – Type 1 |
70 (67%) |
10 (67%) |
60 (67%) |
|
| – Type 2A |
23 (22%) |
4 (26%) |
19 (21%) |
|
| – Type 2B |
22 (21%) |
1 (7%) |
11 (12%) |
|
| TIMI flow |
|
|
|
0.49 |
| – 0 |
21 (20%) |
3 (20%) |
18 (20%) |
|
| – 1 |
21 (20%) |
3 (20%) |
18 (20%) |
|
| – 2 |
29 (28%) |
2 (13%) |
27 (30%) |
|
| – 3 |
34 (32%) |
7 (47%) |
27 (30%) |
|
Cardiovascular risk factors and predisposing factors
As shown in table 1, many patients had classical cardiovascular risk factors, though none had diabetes. Five cases occurred during the peripartum period. One woman was still pregnant at the time of the event, whereas the other four cases occurred during the puerperium.
Treatment strategy and in-hospital clinical course
Treatment is summarised in table 3, and the patient flow is shown in figure 1. One patient with SCAD of the LAD and a large first diagonal branch underwent urgent coronary artery bypass grafting. The perioperative course was uneventful. One patient with electrocardiogram (ECG) evidence of severe global ischaemia and impaired LVEF during late pregnancy had an emergency caesarean section where she suffered in-hospital cardiac arrest due to ventricular fibrillation. She was transferred to the catheterisation laboratory under ongoing cardiopulmonary resuscitation, where a diagnosis of extensive left main, LAD and left circumflex artery (LCX) SCAD was established. Percutaneous coronary intervention (PCI) was performed and extracorporeal membrane oxygenation was installed, but the patient died from refractory cardiogenic and haemorrhagic shock in the catheterisation laboratory, while the baby survived. Six additional patients underwent PCI with good angiographic and clinical results. Figure 2 shows an example of a challenging PCI for SCAD of a large right coronary artery (RCA) in a patient with inferior STEMI. The other 97 patients (age 54 ± 10 years, 90 female, 4 peripartum) were treated conservatively.
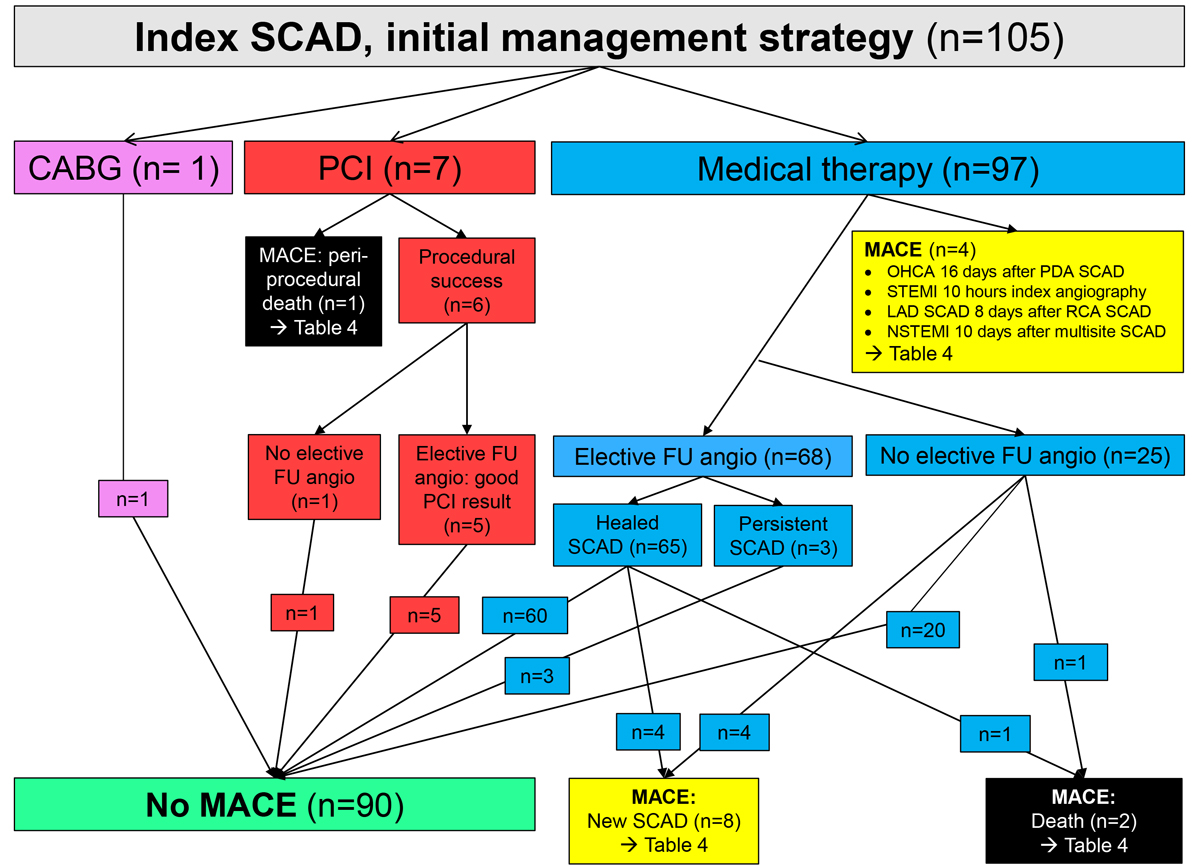
Figure 1 Patient flow for the entire cohort of patients with spontaneous coronary artery dissection (SCAD). For details see text and table 4.
CABG: coronary artery bypass grafting; FU: follow-up; LAD: left anterior descending artery; MACE: major cardiovascular events; OHCA: out-of-hospital cardiac arrest; PCI: percutaneous coronary intervention; PDA: posterior descending artery; RCA: right coronary artery; STEMI: ST-segment elevation myocardial infarction
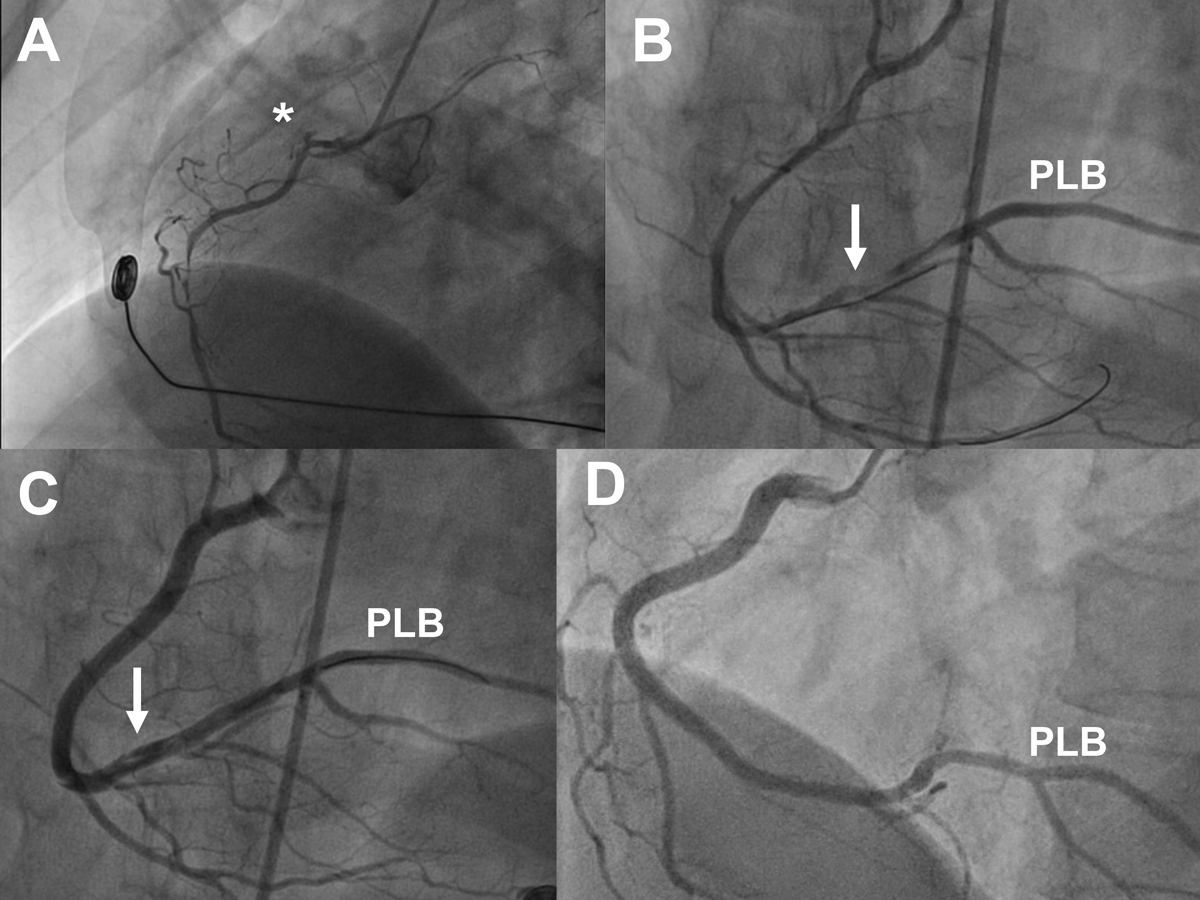
Figure 2 Angiograms of a 40-year-old female with inferolateral ST-segment elevation myocardial infarction, ongoing chest pain, and massive ECG ST-segment elevation at the time of angiography, treated by percutaneous coronary intervention. Panel A: left anterior oblique (LAO) 60° view showing extensive dissection of the right coronary artery (RCA) with minimal flow. B: cranial view showing extensive dissection of the RCA and the large posterolateral branch (PLB) after wiring of a large right ventricular branch and a side branch of the PLB. C: cranial view showing situation after correct wiring of the PLB and stenting of the mid to ostial RCA. The distal RCA and PLB are still dissected but were not stented. D: follow-up angiogram after 6 months. LAO 50°/cranial 15° view showing patent stents and angiographic healing of the dissection in the distal RCA and PLB.
Table 3Acute management and medication (n = 105).
|
All patients (n = 105)
|
MACE (n = 15)
|
No MACE (n = 90)
|
p-value
|
|
Initial treatment strategy
|
0.92 |
| Medical therapy |
97 (92%) |
14 (93%) |
83 (92%) |
|
| Percutaneous coronary intervention |
7 (7%) |
1 (7%) |
6 (7%) |
|
| Bypass surgery |
1 (1%) |
0 |
1 (1%) |
|
|
Discharge medication (n = 104)
|
|
n = 14 |
n = 90 |
|
| Aspirin |
101 (97%) |
14 (100%) |
87 (97%) |
0.49 |
| Any dual antiplatelet therapy |
95 (90%) |
12 (86%) |
83 (92%) |
0.42 |
| Type of dual antiplatelet therapy (n = 95) |
|
|
|
0.56 |
| – Aspirin + clopidogrel |
48 (51%) |
7 (58%) |
41 (49%) |
|
| – Aspirin + prasugrel |
9 (9%) |
0 |
9 (11%) |
|
| – Aspirin + ticagrelor |
38 (40%) |
5 (42%) |
33 (40%) |
|
| Oral anticoagulation |
8 (8%) |
1 (7%) |
7 (8%) |
0.93 |
| Statin |
95 (91%) |
13 (93%) |
82 (91%) |
0.83 |
| Beta-blocker |
83 (80%) |
10 (71%) |
73 (81%) |
0.40 |
| ACE inhibitor or ARB |
44 (42%) |
3 (21%) |
41 (46%) |
0.09 |
| Calcium channel blocker |
25 (24%) |
2 (14%) |
23 (26%) |
0.36 |
The in-hospital clinical course was uneventful in all but three patients: First, the above-mentioned patient with fatal peripartum left main SCAD. Second, a 41-year-old woman with postpartum SCAD of the proximal to the distal RCA experienced extensive LAD SCAD eight days after the index angiogram, which was also treated medically (fig. 1, fig. 3, supplementary table S1 in the appendix). Third, a 56-year-old woman with NSTEMI due to LAD SCAD, in whom a decision for a conservative approach had been made, suffered STEMI 10 hours later and underwent successful PCI for the acutely occluded LAD (fig. 1, fig. 4, table S1).
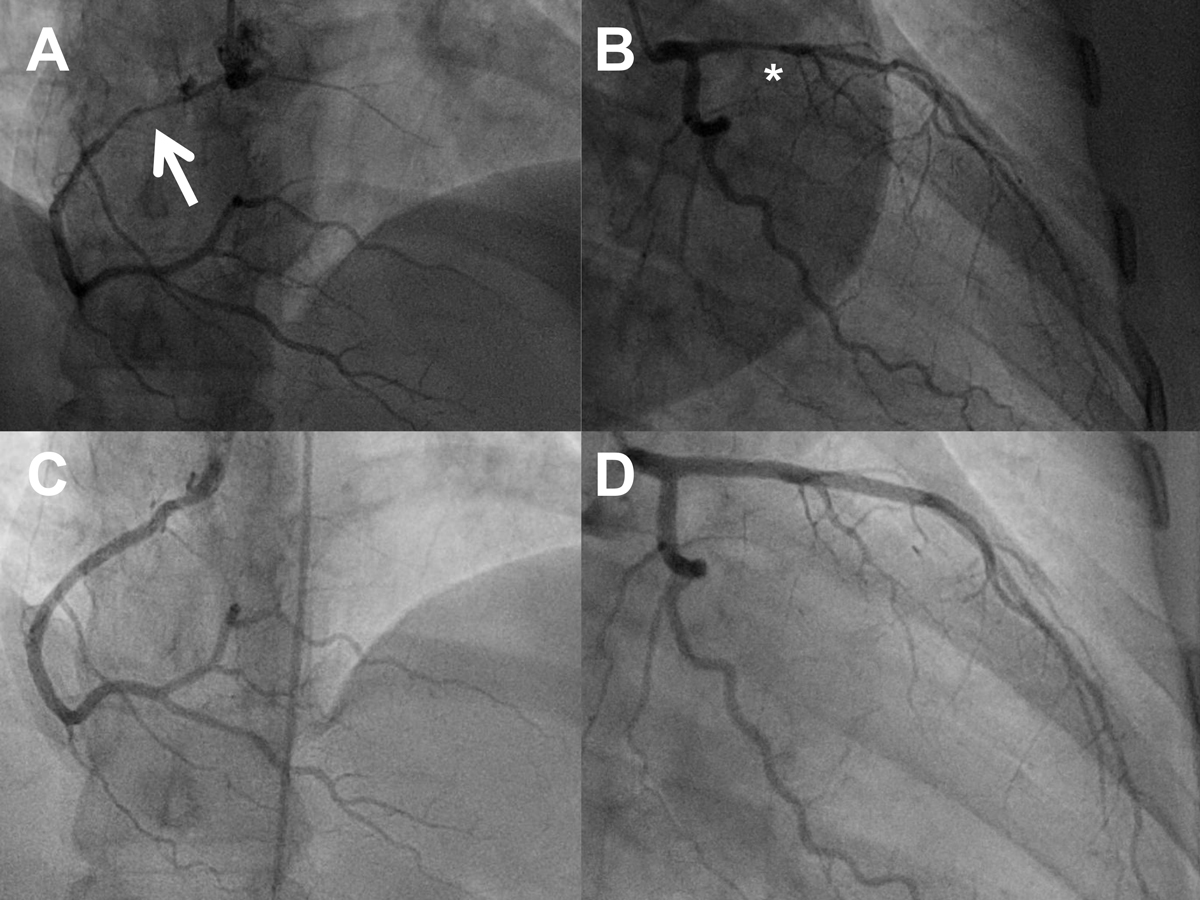
Figure 3 Serial angiograms of a 41-year-old woman (postpartum) with spontaneous coronary artery dissection (SCAD) of the right coronary artery (RCA; panel A, cranial view, arrow pointing to the dissection) and SCAD of the left anterior descending artery (LAD) eight days later (panel B, right anterior oblique 30°/caudal 20° view, asterisk highlighting the dissection). Panels C and D: repeat angiogram after 6 months showing healing of the dissections (same projections; see also Table 4)
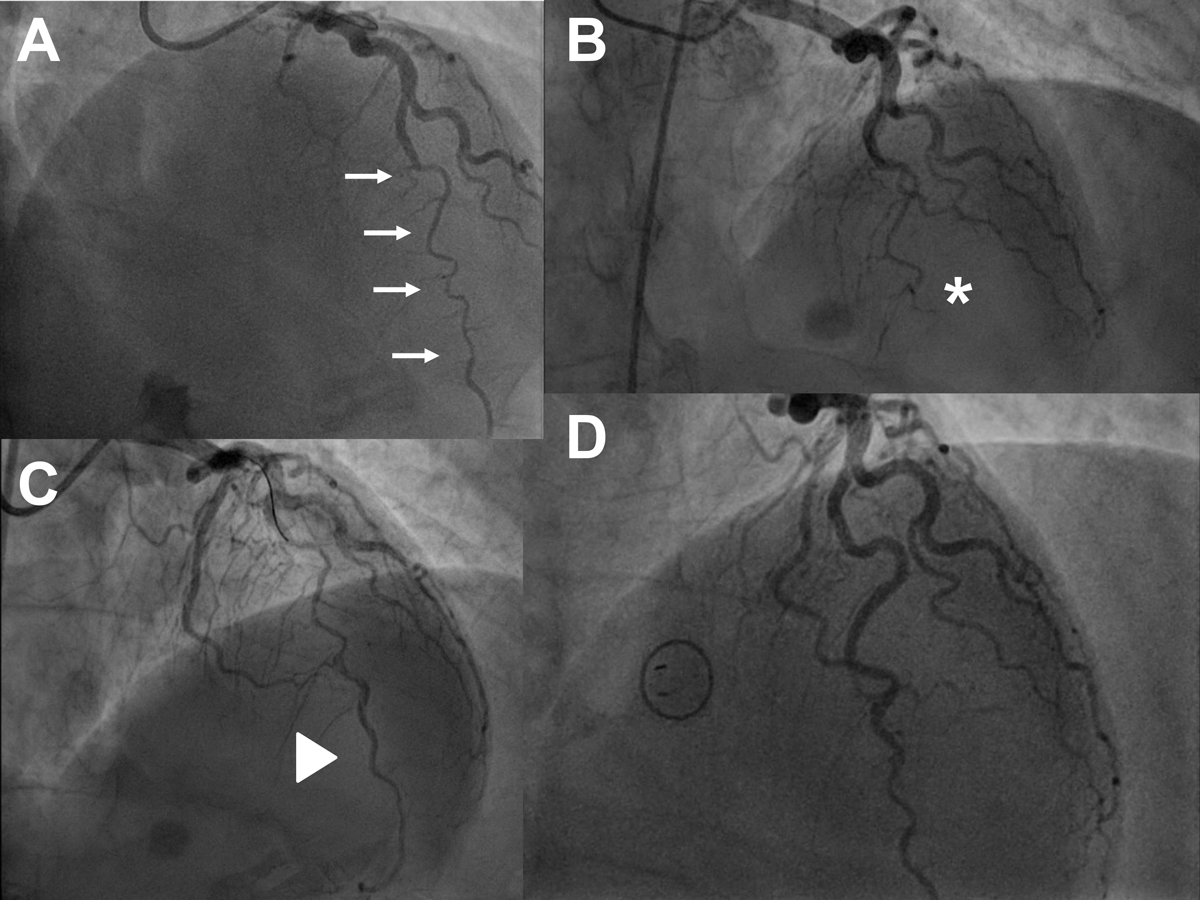
Figure 4 Serial angiograms of a 56-year-old woman with extensive spontaneous coronary artery dissection (SCAD) of the mid to distal left anterior descending artery (LAD; panel A, cranial view, as also in B-D, arrows highlighting the extent of the dissection). A decision for conservative management was made at that time. The guiding catheter and the guidewire were used to optimise angiography but no percutaneous coronary intervention (PCI) was attempted. Panel B: emergency repeat angiogram 10 hours later because of ST-segment elevation myocardial infarction showing complete occlusion of the distal LAD (asterisk). The LAD was treated by PCI using balloon dilation and application of a drug-coated balloon with the aim of preventing secondary re-stenosis after injury to the vessel wall caused by balloon inflation. Panel C: result after PCI, triangle highlighting the re-opened LAD. Panel D: repeat angiography after 6 months showing an open LAD with minimal irregularities of the vessel wall (see also table 4).
The mean LVEF in the entire population was 61 ± 7% (n = 99). Discharge medication is shown in table 3. The vast majority of patients (95/104; 90%) were treated with DAPT. Eight patients were discharged on oral anticoagulation for pre-existing indications with or without aspirin, and one patient was treated with aspirin monotherapy. Statins, beta-blockers, angiotensin converting-enzyme inhibitors or angiotensin receptor blockers, and calcium channel blockers were prescribed in 91%, 80%, 42% and 24% of patients respectively.
Non-invasive follow-up
An exercise stress was performed in 63 patients a median of 3.1 months (IQR 0.5–12.3) after the index event. Exercise capacity, expressed as a percentage of predicted work rate, was 95 ± 26%. All patients were free of chest pain at that time. The LVEF during follow-up was 58 ± 12%.
Angiographic follow-up
Overall, 73/105 (70%) asymptomatic patients had a planned repeat angiogram after an uncomplicated course following PCI or a decision for medical therapy, which was performed 6.0 months (IQR 5.5–6.5) after the index event (fig. 1). There were no major complications during any of these repeat angiograms. In five patients treated with PCI and surviving the acute phase, the follow-up angiogram revealed a good result after PCI. In 65/68 (96%) of the conservatively treated patients the follow-up angiogram revealed healing of the dissection, whereas in 3/68 (4%) there was a persistent dissection. These three patients all had a dissection in a small vessel with normal flow and were asymptomatic. Medical treatment was continued in these three patients. In addition, the patient with LAD occlusion and PCI early after the index angiography (fig. 4) and the postpartum patient with LAD SCAD early after RCA SCAD (fig. 3) underwent another angiogram 6 months later, showing complete healing of all dissections.
Long-term clinical follow-up and MACE
The median follow-up in the entire population was 7.5 years (IQR 3.6–12.5; maximum 22.5). There were 12 additional MACE (not including the above-mentioned three MACE occurring during the index hospitalisation), which are summarised in supplementary table S1 (appendix). The overall MACE rate was 1.7 events/100 patient years. A 39-year-old woman with SCAD of the posterior descending artery (PDA) of the RCA suffered an out-of-hospital cardiac arrest only a few days after discharge (16 days after the initial angiography) and was found to have a persistent PDA dissection but no occlusion and normal flow at the second angiogram. She was still treated medically. She had a complicated clinical course, including multiorgan failure, but finally survived with only minimal neurological sequelae (fig. 1, table S1). The LVEF was severely depressed after resuscitation, but it returned to nearly normal after 1 year, and exercise capacity was normal at that time. Eight conservatively treated patients (age at the initial presentation 48 ± 11 years, all female) experienced acute myocardial infarction and recurrent SCAD a median of 7.6 years (IQR 3.9–9.6) after the index event. All these dissections were at different sites to the index SCAD (table S1, fig. 5). All patients were treated conservatively again, including DAPT, and remained free of symptoms during follow-up. In four of these eight patients another angiogram was performed 6 months later, showing healing of the dissection in all cases. One patient with multisite SCAD, i.e., dissections of the mid LCX, the intermediate branch and the distal RCA, suffered another NSTEMI a few days after hospital discharge. A repeat angiogram showed persistent, extensive dissections of the mid to distal LCX and the intermediate branch, and medical therapy was intensified. Symptoms could be controlled sufficiently, and a repeat angiogram 6 months later showed healing of all three dissection sites (table S1).
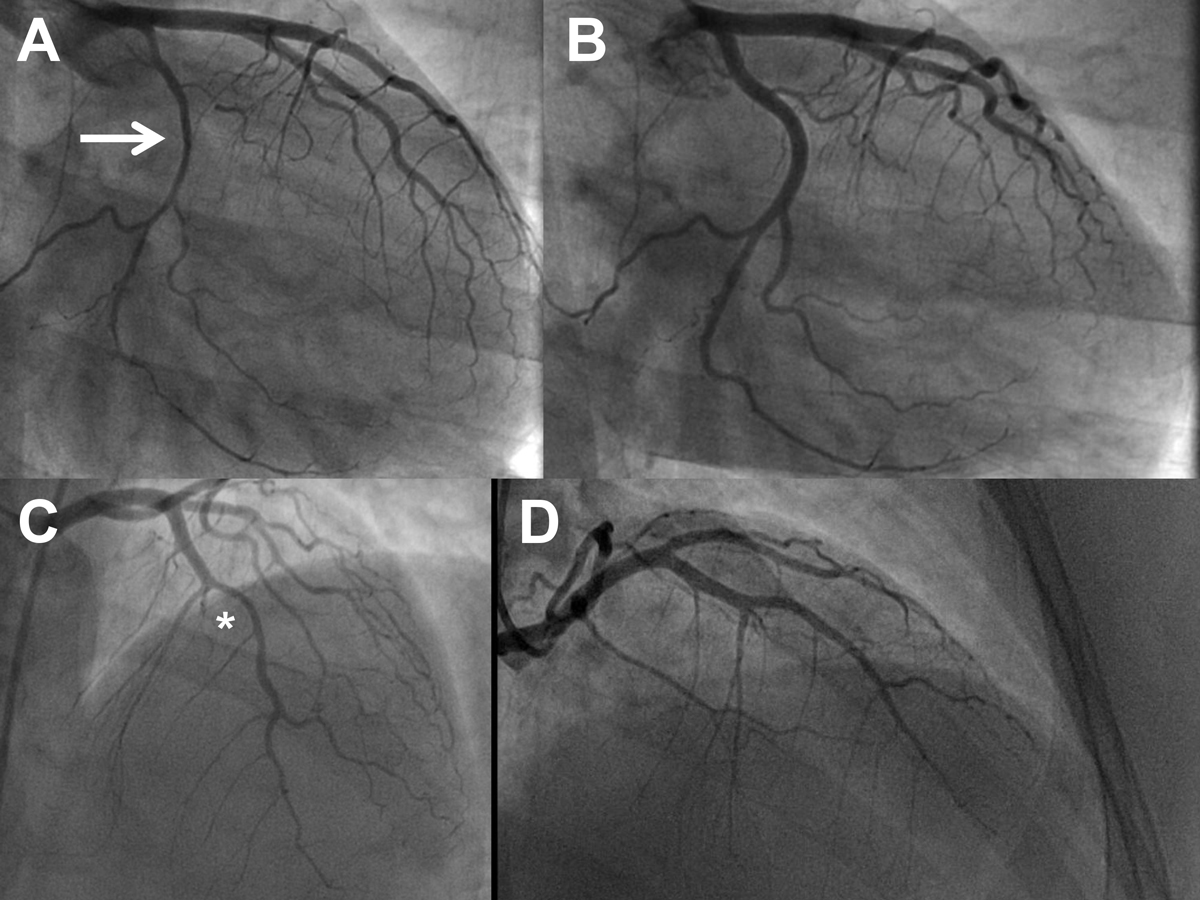
Figure 5 Serial angiogram of a 39-year-old woman with spontaneous coronary artery dissection (SCAD) on the entire left circumflex artery (LCX; panel A, right anterior oblique 35° view, arrow) and complete healing of the dissection at the repeat angiogram after 6 months (panel B, same view). Panel C: SCAD of the left anterior descending artery (LAD; asterisk) and the first diagonal branch 7.9 years later. Panel D: repeat angiogram 6 months later after conservative therapy showing healing of the dissections (cranial/right anterior oblique view) (see also table 4).
A 53-year-old man with SCAD of the first obtuse marginal branch of the LCX and otherwise normal coronary arteries, with angiographically documented healing of the dissection by repeat angiography, and with an LVEF of 60% at the time of the index event and at the 12-month follow-up was found dead at home 15 months after the index event. Resuscitation attempts were not successful. An autopsy was not performed. Another patient experiencing SCAD of the first obtuse marginal branch of the LCX at the age of 69 years was found dead at home 12.7 years later. An autopsy was not performed, but the patient was 80 years old at that time and had an advanced form of Parkinson’s disease (table S1).
Comparison of patients with MACE versus no MACE
As shown in tables 1–3, patients suffering MACE were younger and more likely to have peripartum SCAD compared to those without MACE. Otherwise, there were no significant differences between patients with and without MACE. MACE-free survival in the entire cohort (panel A) and in patients with peripartum SCAD versus non-peripartum cases (panel B) is shown in figure 6. Cox regression identified peripartum status as the only significant predictor of the time-dependent occurrence of MACE (hazard ratio 5.55, 95% confidence interval 1.56–19.75; p = 0.008). The association between age and MACE was not statistically significant in the Cox regression (hazard ratio 0.97, 95% confidence interval 0.92–1.01 per year; p = 0.11).
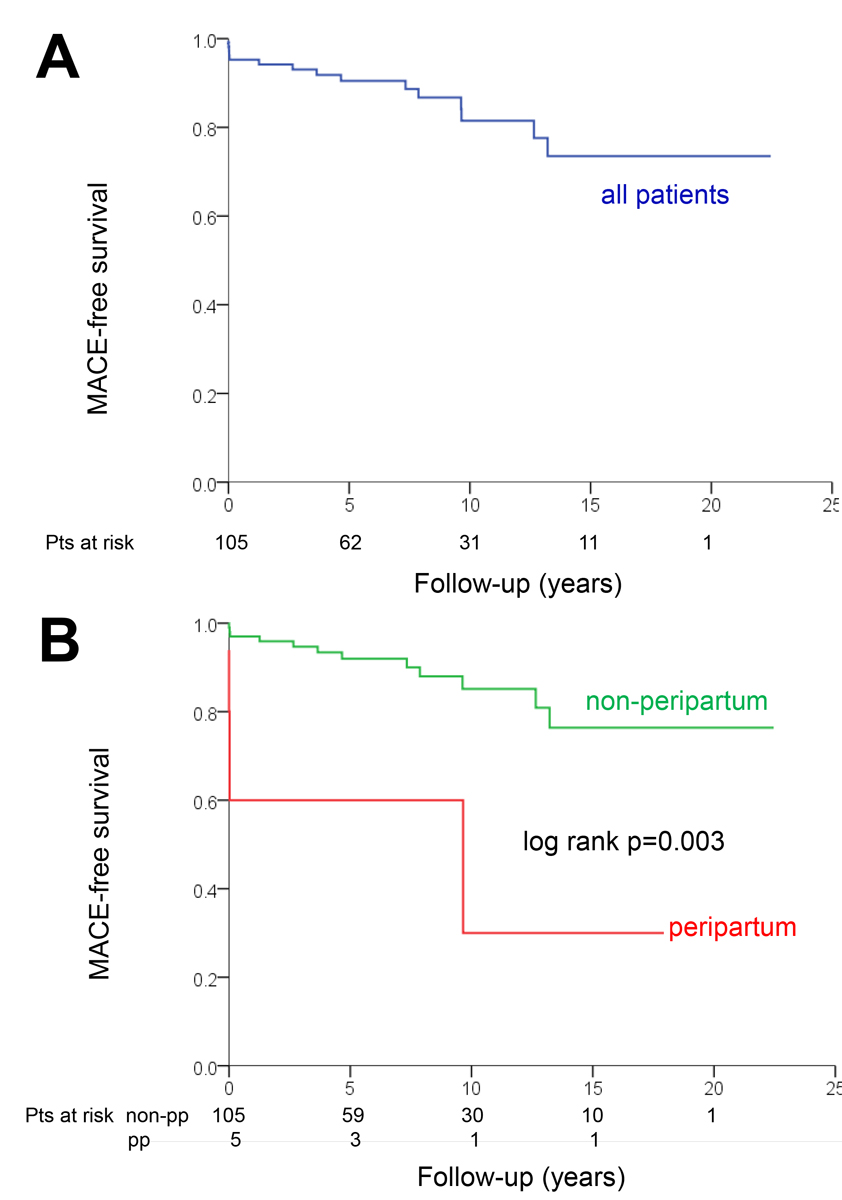
Figure 6 Kaplan Meier plots showing survival free of major cardiovascular events (MACE) in the entire cohort (panel A) and in peripartum versus non-peripartum cases (panel B).
Discussion
In the present study, we report the following key findings. First, the typical SCAD patient is an approximately 50-year-old woman with some cardiovascular risk factors presenting with myocardial infarction. Second, the majority of SCADs can be treated medically, which we documented by healing of SCAD in the follow-up angiogram and by a very long follow-up period. Third, we found an early MACE rate of 4.8%, including catastrophic and life-threatening events. Fourth, we observed recurrent SCAD in 7.6% of patients after a median follow-up of 7.6 years, although healing of the index SCAD had been documented by follow-up angiography in many of these patients.
The demographic characteristics of our population are in line with previous studies, in particular with the Canada/US multicentre cohort (n = 750), by far the largest cohort in the field [6]. The mean age is typically approximately 50 years and the vast majority are women. The presence of some cardiovascular risk factors (except diabetes) is common but coronary arteries are typically normal apart from the dissection or have only mild atherosclerosis [2, 4]. A variety of factors potentially predisposing to SCAD have been suggested, but evidence is weak and mainly based on case reports [4]. Patients in the present population had some co-morbidities, but there was no clustering of certain conditions. The most frequently discussed condition potentially related to SCAD is fibromuscular dysplasia [2, 4]. A prevalence of fibromuscular dysplasia of up to 86% has been described in other series [17], which has led to the recommendation for screening for extracardiac arteriopathies [2]. In the early phase of the present registry, angiograms were performed by the femoral approach, and femoral and renal angiograms were performed on a regular basis. There was possible evidence of fibromuscular dysplasia in only 5/40 screened patients [13]. More recently, radial angiography was systematically implemented, although this can be challenging in the SCAD population (small arteries, spasm, small aorta with consequently challenging intubation of the coronary arteries) and even hazardous due to catheter-induced dissections [18]. Thus, fibromuscular dysplasia screening was not performed any more given the low yield in both the previous period and also in other series, including an analysis from a small Swiss population [19]. More recently, screening for extracardiac vascular abnormalities by computed tomography or magnetic resonance angiography has been recommended for SCAD patients [1]. This was not performed in our patients, which is a limitation. However, we are not aware of any patient in our cohort experiencing an event potentially related to an extracardiac arteriopathy. We must acknowledge, however, that certain important vascular events may have been missed. For example, we cannot exclude the possibility that the unexplained death in the 53-year-old male patient was due to intracranial bleeding from an aneurysm or another vascular event. Notably, with an LVEF of 60% the patient was not at high risk for ventricular tachycardia / ventricular fibrillation (VT/VF). An LVEF <50% and VT/VF at the index presentation have been identified as independent predictors of VT/VF during follow-up in SCAD patients [20].
Although many patients presented with STEMI, sustained complete occlusion of a large artery by the dissection was rare, and the vast majority of our patients were treated medically. This is in line with the current expert recommendation [2, 4, 5], which is based on several considerations. First, there is usually no atherosclerosis, and most dissections will heal over time [21]. Second, PCI is often very challenging given the risks of false lumen wiring, haematoma propagation by stenting, and extensive and potentially undersized stenting of healthy vessels [2, 4, 5]. Acute and long-term results of PCI for SCAD have been reported to be unsatisfactory, even in expert centres [2, 4]. In the present cohort, we performed PCI in approximately 7% of patients. In these cases, there was ongoing ischaemia with a large area of myocardium at risk. All these procedures were challenging but finally led to a good result, except in the case of the patient with catastrophic left main SCAD. Third, bypass surgery is a bail-out option but unlikely to provide a good long-term result in terms of graft patency as competitive flow following healing of the dissection will prevent the optimal maturation of the arterial grafts [4].
The conservative approach was successful in the vast majority of our patients, as shown by an uncomplicated acute and long-term clinical course. A strength of our cohort is that we applied a strategy of systematic angiographic follow-up. Among 68 asymptomatic patients who underwent a planned follow-up angiogram after a conservative treatment approach, the dissection had healed in 96%. Our study thereby represents one of the largest SCAD series with repeat angiograms. In the largest available series, from the Vancouver group, 157/182 SCAD (86%) lesions in 156 patients were found to have healed after a median follow-up of 154 days [21]. In a smaller, earlier series (n = 88), the same group of investigators were able to demonstrate that in those undergoing repeat angiography very early (<20 days) after the index event, healing of the SCAD had not yet occurred, whereas in all those undergoing repeat angiography later (≥26 days) there was complete angiographic healing [22]. These three series help our understanding of the natural history of SCAD and support the concept of a conservative approach as the primary option. Availability of angiograms demonstrating complete healing of the index SCAD is also very important for understanding the process of recurrent SCAD as new events as opposed to persistent dissection with secondary extension. Given that this evidence on the natural history of SCAD is now available and has been consistent across studies, experts now argue against the routine use of follow-up angiograms and even suggest avoiding repeat angiograms in patients with recurrent symptoms potentially reflecting myocardial ischaemia [2]. This reluctance regarding repeat angiography is based on the increased risk of catheter-induced dissections in these patients with fragile coronary arteries [5, 18].
Although experts agree that a conservative therapy is preferred, the exact medical regimen for SCAD patients is still poorly defined [2, 4]. Many physicians prescribe DAPT in SCAD patients, given the beneficial impact of this therapy in patients with a non-SCAD myocardial infarction. However, there are concerns regarding progression of the intramural haematoma and extension of the dissection with aggressive antiplatelet therapy. These are primarily based on a fatal SCAD case where there was clinical deterioration after the administration of thrombolysis [23]. Therefore, prolonged heparin administration is discouraged after a diagnosis of SCAD has been established, and there is wide variation regarding the use of DAPT in clinical practice [2]. In a very recent registry study [24], use of DAPT was associated with a significantly higher MACE (all-cause, non-fatal myocardial infarction, unplanned PCI) rate after 12 months compared to single antiplatelet therapy (18.9% versus 6.0%), which was driven by an excess MACE rate during the index hospitalisation in the DAPT group (11.4% versus 1.5%). In contrast, there was no difference in terms of major bleedings. We treated the vast majority of patients with DAPT for 12 months, followed by aspirin monotherapy. There were no major bleeding events in our cohort either, but we cannot exclude the possibility that some of the MACE occurring during the first 12 months (i.e., cardiac arrest and persistence of SCAD early after the index event, vessel occlusion early after the index angiography, recurrent NSTEMI and persistence of SCAD) were related to enhanced intramural bleeding and propagation of the dissection and/or delayed resorption of the intramural haematoma with DAPT. However, all late recurrent SCAD events occurred while patients were on aspirin monotherapy or not on antiplatelet therapy at all. Notably, the event rate in our population was relatively low compared to large series from North America, e.g., 1.7 MACE/100 patient years in the present study versus 5.8 MACE/100 patient years in the study by Saw et al. [9], although the proportion of patients on DAPT was substantially higher in our cohort (90% versus 62%). There is also uncertainty regarding the utility of other drugs typically prescribed to patients with MI, including statins, angiotensin converting-enzyme inhibitors, and beta-blockers. One study found a reduced risk of recurrent SCAD in patients treated with beta-blockers [9]. Beta-blockers are currently suggested for all patients with SCAD and hypertension to reduce the risk of recurrent SCAD [2]. There are conflicting studies regarding statin therapy, one of them suggesting possible harm (recurrent SCAD) [25]. We did not observe any differences in medical therapy between patients with and without MACE. Statins were prescribed routinely in our cohort based on the idea of an at least transient endothelial injury and dysfunction. Experts now recommend statin therapy only for patients with dyslipidaemia [2]. The relatively low event rate in our cohort compared to large cohorts from North America is difficult to explain given the lack of major differences in baseline characteristics. Obviously, the high proportion of patients treated with a statin (91% versus 54% in the study by Saw et al. [9]) did not negatively impact outcomes.
Peripartum cases represented a small minority in our cohort, but these patients had a high risk of complications, which is in line with other reports [6, 26]. The only fatal index myocardial infarction occurred in a patient with catastrophic peripartum left main SCAD, one peripartum patient had a dissection in a second vessel a few days after the index event, and one presented with two non-contiguous dissections. Peripartum status was the only covariate significantly associated with MACE in the Kaplan-Meier analysis, although this analysis is limited by the small number of peripartum cases.
Limitations
There are further limitations in addition to those mentioned previously. First, the size of the population of the present study is limited. However, it should be noted that our study represents one of the largest single-centre series from Europe, and that there was a very long follow-up period. Still, efforts to diagnose SCAD even more systematically and to collect detailed information on these patients through prospective, multicentre registries are needed. This is the aim of the recently launched Swiss SCAD registry (https://clinicaltrials.gov/ct2/show/NCT04457544). Second, as discussed above, the purely angiographic diagnosis of SCAD can be considered a limitation. However, we feel that the systematic use of follow-up angiograms was very helpful in retrospectively confirming the diagnosis. Third, despite major efforts to collect complete follow-up data it remains possible that some cardiac or extracardiac vascular events or bleeding remained unrecognised.
Conclusions
This SCAD series highlights its highly variable clinical course during the acute phase and in the long term. Although most SCAD patients can be treated conservatively with subsequent healing of the dissection and good clinical outcome, there are also some patients with dramatic acute presentation or MACE several years after the initial presentation. Thus, optimal medical therapy needs further investigation and the optimal follow-up and surveillance strategy needs to be defined.
Data sharing statement
All relevant data have been reported in the manuscript and the supplementary table.
Acknowledgment
The excellent support of the nursing staff of the cardiac catheterisation laboratory of the Kantonsspital St. Gallen and the excellent administrative assistance of Mrs Irene Schneider are greatly appreciated.
Micha T. Maeder, MD, PhD
Cardiology Division
Kantonsspital St. Gallen
Rorschacherstrasse 95
CH-9007 St. Gallen
micha.maeder[at]kssg.ch
References
1.
Kim ES
. Spontaneous Coronary-Artery Dissection. N Engl J Med. 2020 Dec;383(24):2358–70. https://doi.org/10.1056/NEJMra2001524
2.
Hayes SN
,
Tweet MS
,
Adlam D
,
Kim ES
,
Gulati R
,
Price JE
, et al.
Spontaneous Coronary Artery Dissection: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020 Aug;76(8):961–84. https://doi.org/10.1016/j.jacc.2020.05.084
3.
Adlam D
,
García-Guimaraes M
,
Maas AH
. Spontaneous coronary artery dissection: no longer a rare disease. Eur Heart J. 2019 Apr;40(15):1198–201. https://doi.org/10.1093/eurheartj/ehz048
4.
Adlam D
,
Alfonso F
,
Maas A
,
Vrints C
,
Writing C
; Writing Committee
. European Society of Cardiology, acute cardiovascular care association, SCAD study group: a position paper on spontaneous coronary artery dissection. Eur Heart J. 2018 Sep;39(36):3353–68. https://doi.org/10.1093/eurheartj/ehy080
5.
Hayes SN
,
Kim ES
,
Saw J
,
Adlam D
,
Arslanian-Engoren C
,
Economy KE
, et al.; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council
. Spontaneous Coronary Artery Dissection: Current State of the Science: A Scientific Statement From the American Heart Association. Circulation. 2018 May;137(19):e523–57. https://doi.org/10.1161/CIR.0000000000000564
6.
Saw J
,
Starovoytov A
,
Humphries K
,
Sheth T
,
So D
,
Minhas K
, et al.
Canadian spontaneous coronary artery dissection cohort study: in-hospital and 30-day outcomes. Eur Heart J. 2019 Apr;40(15):1188–97. https://doi.org/10.1093/eurheartj/ehz007
7.
Kok SN
,
Hayes SN
,
Cutrer FM
,
Raphael CE
,
Gulati R
,
Best PJ
, et al.
Prevalence and Clinical Factors of Migraine in Patients With Spontaneous Coronary Artery Dissection. J Am Heart Assoc. 2018 Dec;7(24):e010140. https://doi.org/10.1161/JAHA.118.010140
8.
Tweet MS
,
Eleid MF
,
Best PJ
,
Lennon RJ
,
Lerman A
,
Rihal CS
, et al.
Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv. 2014 Dec;7(6):777–86. https://doi.org/10.1161/CIRCINTERVENTIONS.114.001659
9.
Saw J
,
Humphries K
,
Aymong E
,
Sedlak T
,
Prakash R
,
Starovoytov A
, et al.
Spontaneous Coronary Artery Dissection: Clinical Outcomes and Risk of Recurrence. J Am Coll Cardiol. 2017 Aug;70(9):1148–58. https://doi.org/10.1016/j.jacc.2017.06.053
10.
Clare R
,
Duan L
,
Phan D
,
Moore N
,
Jorgensen M
,
Ichiuji A
, et al.
Characteristics and Clinical Outcomes of Patients With Spontaneous Coronary Artery Dissection. J Am Heart Assoc. 2019 May;8(10):e012570. https://doi.org/10.1161/JAHA.119.012570
11.
Lettieri C
,
Zavalloni D
,
Rossini R
,
Morici N
,
Ettori F
,
Leonzi O
, et al.
Management and Long-Term Prognosis of Spontaneous Coronary Artery Dissection. Am J Cardiol. 2015 Jul;116(1):66–73. https://doi.org/10.1016/j.amjcard.2015.03.039
12.
Wagener M
,
Twerenbold R
,
Cook S
,
Gämperli O
,
Meier P
,
Muller O
, et al.; On Behalf Of The Swiss Working Group Interventional Cardiology Of The Swiss Society Of Cardiology
. Coronary and structural heart interventions in Switzerland 2019. Swiss Med Wkly. 2021 May;151:w20495.
13.
Rogowski S
,
Maeder MT
,
Weilenmann D
,
Haager PK
,
Ammann P
,
Rohner F
, et al.
Spontaneous Coronary Artery Dissection: Angiographic Follow-Up and Long-Term Clinical Outcome in a Predominantly Medically Treated Population. Catheter Cardiovasc Interv. 2017 Jan;89(1):59–68. https://doi.org/10.1002/ccd.26383
14.
Saw J
. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter Cardiovasc Interv. 2014 Dec;84(7):1115–22. https://doi.org/10.1002/ccd.25293
15.
Maeder M
,
Ammann P
,
Drack G
,
Rickli H
. Pregnancy-associated spontaneous coronary artery dissection: impact of medical treatment. Case report and systematic review. Z Kardiol. 2005 Dec;94(12):829–35. https://doi.org/10.1007/s00392-005-0302-6
16.
Maeder M
,
Ammann P
,
Angehrn W
,
Rickli H
. Idiopathic spontaneous coronary artery dissection: incidence, diagnosis and treatment. Int J Cardiol. 2005 Jun;101(3):363–9. https://doi.org/10.1016/j.ijcard.2004.03.045
17.
Saw J
,
Ricci D
,
Starovoytov A
,
Fox R
,
Buller CE
. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv. 2013 Jan;6(1):44–52. https://doi.org/10.1016/j.jcin.2012.08.017
18.
Prakash R
,
Starovoytov A
,
Heydari M
,
Mancini GB
,
Saw J
. Catheter-Induced Iatrogenic Coronary Artery Dissection in Patients With Spontaneous Coronary Artery Dissection. JACC Cardiovasc Interv. 2016 Sep;9(17):1851–3. https://doi.org/10.1016/j.jcin.2016.06.026
19.
Toggweiler S
,
Puck M
,
Thalhammer C
,
Manka R
,
Wyss M
,
Bilecen D
, et al.
Associated vascular lesions in patients with spontaneous coronary artery dissection. Swiss Med Wkly. 2012 Mar;142:w13538. https://doi.org/10.4414/smw.2012.13538
20.
Cheung CC
,
Starovoytov A
,
Parsa A
,
Andrade JG
,
Krahn AD
,
Bennett M
, et al.
In-hospital and long-term outcomes among patients with spontaneous coronary artery dissection presenting with ventricular tachycardia/fibrillation. Heart Rhythm. 2020 Nov;17(11):1864–9. https://doi.org/10.1016/j.hrthm.2020.06.019
21.
Hassan S
,
Prakash R
,
Starovoytov A
,
Saw J
. Natural History of Spontaneous Coronary Artery Dissection With Spontaneous Angiographic Healing. JACC Cardiovasc Interv. 2019 Mar;12(6):518–27. https://doi.org/10.1016/j.jcin.2018.12.011
22.
Saw J
,
Aymong E
,
Sedlak T
,
Buller CE
,
Starovoytov A
,
Ricci D
, et al.
Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv. 2014 Oct;7(5):645–55. https://doi.org/10.1161/CIRCINTERVENTIONS.114.001760
23.
Zupan I
,
Noc M
,
Trinkaus D
,
Popovic M
. Double vessel extension of spontaneous left main coronary artery dissection in young women treated with thrombolytics. Catheter Cardiovasc Interv. 2001 Feb;52(2):226–30. https://doi.org/10.1002/1522-726X(200102)52:2<226::AID-CCD1054>3.0.CO;2-R
24.
Cerrato E
,
Giacobbe F
,
Quadri G
,
Macaya F
,
Bianco M
,
Mori R
, et al.
Antiplatelet therapy in patients with conservatively managed spontaneous coronary artery dissection from the multicentre DISCO registry. Eur Heart J. 2021;42(33):3161–71. https://doi.org/10.1093/eurheartj/ehab372
25.
Tweet MS
,
Hayes SN
,
Pitta SR
,
Simari RD
,
Lerman A
,
Lennon RJ
, et al.
Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012 Jul;126(5):579–88. https://doi.org/10.1161/CIRCULATIONAHA.112.105718
26.
Saw J
. Pregnancy-Associated Spontaneous Coronary Artery Dissection Represents an Exceptionally High-Risk Spontaneous Coronary Artery Dissection Cohort. Circ Cardiovasc Interv. 2017 Mar;10(3):e005119. https://doi.org/10.1161/CIRCINTERVENTIONS.117.005119
Appendix: Supplementary table
Table S1Major cardiac events (MACE; please see also text)
|
Patient characteristics
|
Index event and management
|
MACE and management
|
|
MACE during the index hospitalisation
|
| 1 |
32 years, female, peripartum, left main SCAD, cardiogenic shock, in-hospital cardiac arrest |
Emergency caesarean section, cardiopulmonary resuscitation, PCI, percutaneous extracorporal membrane oxygenation |
Death due to cardiogenic and haemorrhagic shock |
| 2 |
41 years, female, postpartum SCAD of proximal to distal RCA, STEMI (see fig. 3) |
Medical therapy (aspirin, ticagrelor, pravastatin, metoprolol, amlodipine, nitrate) |
SCAD of LAD 8 days after index event, NSTEMI, medical therapy, clinical follow-up thereafter uneventful |
| 3 |
56 years, female, SCAD of mid to distal LAD, NSTEMI (see fig. 4) |
Medical therapy (aspirin, ticagrelor, atorvastatin, bisoprolol) |
STEMI 10 hours after initial angiogram, re-angiography: LAD occluded, successful dilation with drug-coated balloon, good result in follow-up angiogram 6 months later |
|
MACE after discharge from the initial hospitalisation
|
| 4 |
39 years, female, SCAD of PDA of the RCA, STEMI |
Medical therapy (aspirin, clopidogrel, pravastatin, bisoprolol) |
Out-of-hospital cardiac arrest 16 days after index event, successful resuscitation, repeat angiography showing persisting PDA dissection, continued medical treatment, complicated clinical course but finally survival with minimal neurological sequelae, normal exercise capacity and LVEF 55% at follow-up |
| 5 |
62 years, female, SCAD of apical LAD, STEMI |
Medical therapy (aspirin, clopidogrel, bisoprolol, amlodipine) |
SCAD of proximal LCX 4.7 years after index event, LAD healed, NSTEMI, medical therapy, documentation of healing of the LCX dissection by angiography 6 months later, clinical follow-up thereafter uneventful |
| 6 |
53 years, female, SCAD of second obtuse marginal branch of LCX, NSTEMI |
Medical therapy (aspirin, clopidogrel, pravastatin, bisoprolol) |
SCAD of first marginal branch of LCX and PDA of RCA 3.7 years after index event, healed dissection of the second obtuse marginal branch, NSTEMI, medical therapy, clinical follow-up thereafter uneventful |
| 7 |
39 years, female, SCAD of proximal LCX, NSTEMI (see fig. 5) |
Medical therapy (aspirin, clopidogrel, atorvastatin, bisoprolol), healing of dissection documented in follow-up angiography after6 months |
SCAD of LAD and first diagonal branch 7.9 years after index event, NSTEMI, medical therapy, healing of the dissection documented by follow-up angiography after 6 months, clinical follow-up thereafter uneventful |
| 8 |
50 years, female, SCAD of apical LAD, STEMI |
Medical therapy (aspirin, clopidogrel, rosuvastatin, lisinopril); healing of dissection documented in follow-up angiography after 6 months |
SCAD of PDA of RCA 2.7 years after index event, NSTEMI, medical therapy, clinical follow-up thereafter uneventful |
| 9 |
44 years, female, SCAD of distal LCX, STEMI |
Medical therapy (aspirin, clopidogrel, atorvastatin, bisoprolol) |
Re-SCAD of LCX (more proximally) 9.6 years after index event, NSTEMI, medical therapy, clinical follow-up thereafter uneventful, healing of dissection documented by follow-up angio 6 months later |
| 10 |
29 years, female, SCAD of ostial LAD and distal left main 7 days postpartum (second child), STEMI (transient ST elevation) |
Medical therapy (aspirin, clopidogrel, bisoprolol, lisinopril); healing of the dissection at 6 months (follow-up angiography) |
SCAD of mid to distal LAD 9.7 years after index event, NSTEMI, medical therapy; initial dissection site angiographically normal |
| 11 |
50 years, female, SCAD of PDA, NSTEMI |
Medical therapy (aspirin, clopidogrel, atorvastatin, bisoprolol); healing of the dissection at 2.5 months (follow-up angiography) |
SCAD of distal LCX 7.3 years after index event, NSTEMI, medical therapy |
| 12 |
57 years, female, SCAD of the first obtuse marginal branch of the LCX, STEMI |
Medical therapy (aspirin, rivaroxaban, bisoprolol, rosuvastatin, amlodipine) |
SCAD of second obtuse marginal branch of the LCX 13.2 years after index event, NSTEMI, medical therapy, initial dissection site angiographically normal |
| 13 |
45 years, female, multisite SCAD: mid LCX, intermediate branch, and distal RCA, NSTEMI |
Medical therapy (aspirin, ticagrelor, atorvastatin, bisoprolol) |
Recurrent chest pain and new troponin rise 9 days after the index angiography; repeat angiogram showing persistent dissections with TIMI 1 flow in LCX and intermediate branch; stabilisation with medical therapy; third angiogram 6 months later showing healing of all three dissections |
| 14 |
53 years, male, SCAD of the first obtuse marginal branch of the LCX, STEMI |
Medical therapy (aspirin, ticagrelor, statin, bisoprolol, lisinopril, calcium channel blocker); healing of the dissection at 6 months (folllow-up angiography) |
Death 15 months after index event |
| 15 |
69 years, female, SCAD of the first obtuse marginal branch of the LCX, NSTEMI |
Medical therapy (aspirin, clopidogrel, atorvastatin) |
Death 12.7 years after index event |





