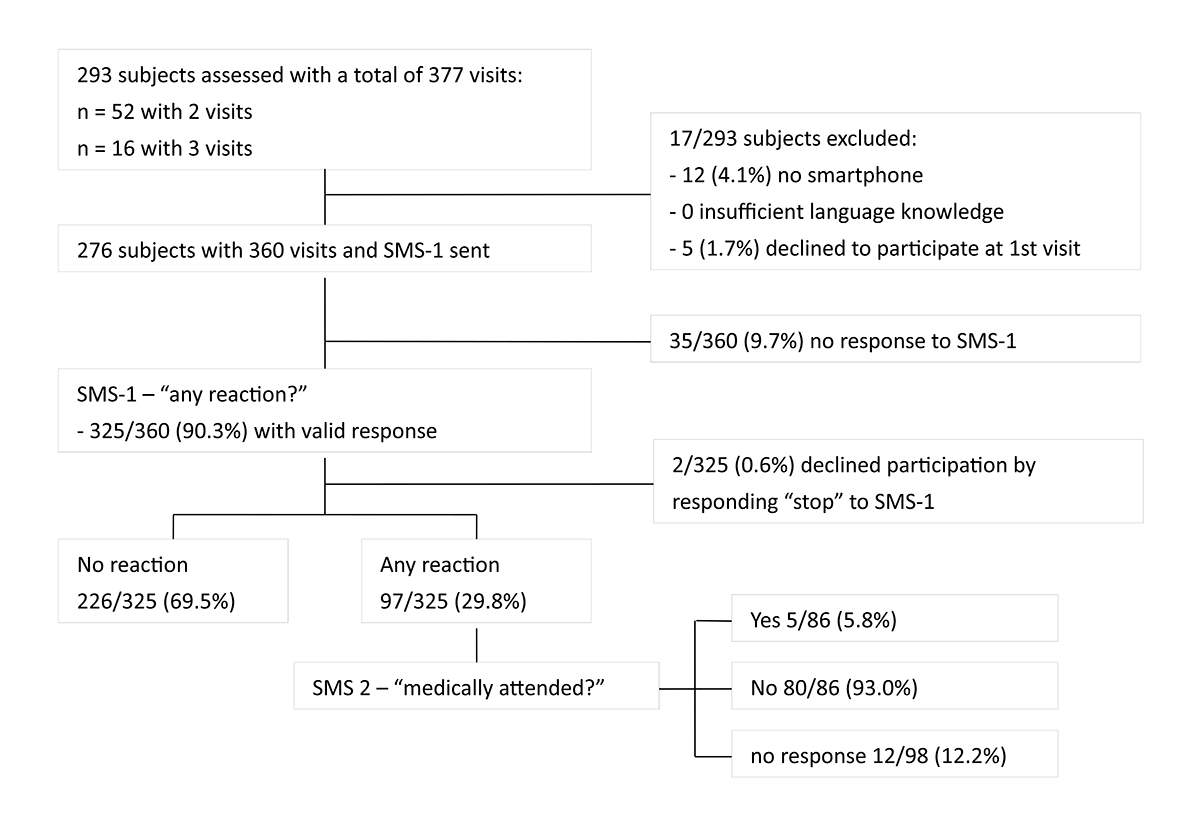
Figure 1 Study flow chart.
SMS: short message service; SMS-1: first SMS asking whether a reaction occurred following vaccination; SMS-2: second SMS asking whether the reaction resulted in a consultation with a physician.
DOI: https://doi.org/10.4414/SMW.2021.w30090
Vaccines are the most effective intervention to reduce the global burden of infectious diseases. Vaccines are preventive measures administered to healthy subjects. Therefore, the expectation regarding the safety profile is very high. The recently approved COVID vaccines are based on messenger RNA (mRNA) or viral vector technologies never applied on a broad scale in humans. Monitoring adverse events following immunisation (AEFI) is, therefore, an essential part of any vaccination programme. Non-significant or anticipated events are prevalent and include fever, local pain, rash, or swelling at the injection site [1, 2]. These are summarised as the vaccine's reactogenicity and are considered the physiological result of the vaccine-induced immune stimulation. Serious AEFI, most prominently seizures or anaphylactic reactions, occur at an estimated rate of about one in a million doses [3, 4]. Due to their rare incidence or delayed onset, serious AEFI may not be detected in phase III trials. Examples include Bell’s palsy cases after an E scherichia co li toxin adjuvanted inactivated virosomal-subunit influenza vaccine [5] or cases of narcolepsy after the 2009 H1N1 pandemic influenza vaccine [6]. Post-marketing surveillance of AEFI is essential to rapidly detect post-licensure safety concerns and limit the undermining of public confidence in vaccination programmes [2, 7–10].
Vaccine safety surveillance should have the ability to capture both vaccine event-based or adverse event-based events (AEFI), including those of special concern in relation to a given vaccine/s (adverse events of special interest – AESI); the latter is especially important for the COVID-19 vaccines. Passive AEFI monitoring systems exist, where medical professionals, pharmaceutical companies, or patients report AEFI online, by fax, or paper-based processes. Platforms such as the VAERS (Vaccine Adverse Event Reporting System) or the Swiss ElViS (Electronic Vigilance System) reporting to Swissmedic (Swiss Agency for Therapeutic Products) [10]. Passive monitoring has limitations, including low sensitivity, unknown denominators limiting calculation of incidence, the reporting of unconfirmed diagnoses, limited ability to assess causality, underreporting and reporting bias towards serious adverse events [10–13]. Furthermore, passive systems are slow, and a sudden increase in the incidence of a specific AEFI following a newly licensed vaccine may be detected only with significant delay [2, 11, 14–17]. Another problem with traditional passive, non-digital AEFI reporting systems is that people do not feel directly addressed. Subjects increasingly use social media to share their experiences with medications or vaccination, which could be leveraged by adding an active, digital AEFI monitoring system. Also, analysis of social media posts may contribute to improving public health decision-making by, for example, identifying under-vaccinated populations [18–21].
To address these limitations, several active surveillance systems for AEFI have been developed [10]. Active surveillance indicates that data on solicited AEFI are collected. Common approaches include diary cards, phone or clinic interviews [22–24], the use of email [25], mobile applications (e.g., [26, 27]), or SMS-based systems [28–31]. SmartVax is an automated system using smartphone and SMS technology in near real-time. Reports of AEFI are actively solicited via automated, opt-out SMS surveys to vaccine recipients 3 days following immunisation. With automated data extraction from any database, including practice software, to a locally installed platform, SmartVax complements and extends the existing passive surveillance systems. SmartVax, partnering with AusVaxSafety since 2016, has provided enhanced surveillance for over 3 million immunisation encounters and is an integral component of Australia’s post-marketing vaccine pharmacovigilance programme [28–32].
We report on findings from a single-centre pilot study defining the feasibility and acceptance of SMS-based active vaccine safety surveillance using SmartVax in an adult immunization clinic and employee health service at the University Hospital Basel, Switzerland.
This pilot study was performed at the adult immunisation clinic and the employee health service of the University Hospital Basel, Switzerland. The immunisation clinic runs once a week and has 400–800 immunisation visits per year. Most subjects receive one to two concomitant immunisations (range 0–6). Reasons for attending the immunisation consultation include travel consultations, general vaccination check-ups and immunisations of individuals with immune‐mediated inflammatory diseases (IMID). From February to September 2020, all clients of the immunisation clinic who received at least one immunisation were invited by the consulting physician (DSG or CTB) to participate in the pilot study. From June 2020 on, we expanded the recruitment to healthcare workers vaccinated at our hospital's employee health service (recruitment by FB). Extended recruitment was implemented in response to the COVID-19 pandemic, which slowed down study recruitment in the vaccination clinic. Subjects without access to an internet-enabled smartphone and those who could not reply to the English or German survey had to be excluded. We obtained written informed consent from each subject (in the case of minors from their legal guardians). The Swiss Ethics Committee approved the procedure (EKNZ-Req-2019-00499). All vaccines were administered by nurses of the immunisation clinic or from the employee health service according to the product information.
In collaboration with the developers of SmartVax in Australia (AL and IJP), we adapted the SmartVax software for use in Switzerland. Specifically, we translated the short message (SMS) text and user interface into German and set up a legal framework with the SMS provider. The SmartVax approach has been published previously [31]. Briefly, a short message (SMS-1) was sent to the participants 3 days after vaccination to ask whether they experienced any adverse event following the immunisation. The SMS-1 requests a “yes” or “no” reply by SMS. In the case of a “yes” reply to SMS-1, two additional SMS were sent: SMS-2 asking whether the reported adverse event was medically attended and SMS-3 providing a link to an online survey to be completed on the smartphone. The questions/data collected in the online survey followed the recommendations of the Brighton Collaboration on the analysis and presentation of vaccine safety data in pre- and post-licensure clinical studies [33, 34]. The online survey asked whether the subject experienced any of the following symptoms: fever/temperature, swelling or redness at the injection site, pain at the injection site, tiredness/fatigue, irritability, sleep pattern change, rash, headache, vomiting, diarrhoea, convulsions/seizures, rigours (shaking or shivering with high temperature), non-responsiveness/loss of consciousness or other, non-listed symptoms. In the case of "other", the symptoms could be typed in as free text. Participants could indicate the time to occurrence and duration for each reported symptom. Furthermore, they were asked (i) if they took any anti-inflammatory or pain medication to treat the reaction, (ii) if they contacted or visited a doctor, and (iii) if the symptoms had resolved ("yes", "no" or "unsure") (supplementary fig. S1 in the appendix). A study doctor (DSG) contacted by telephone all participants who indicated the symptoms had not resolved or were unsure about it.
Because this was a pilot study, Swiss SmartVax was not taking full advantage of the automated data extraction feature of SmartVax. The demographic data (age, gender) and telephone numbers were exported from the hospital database in an Excel spreadsheet and uploaded into the SmartVax software. All data were stored on a secured hospital server. Information on vaccine type and which arm the vaccine was administered was entered manually. Notably, upon full implementation of SmartVax, the programme features an automated import of these data for all subjects who received a vaccine. Three days after vaccination, SmartVax automatically sent the SMS via an SMS gateway provider (MessageMedia, UK). The gateway provider is ISO27001 and HIPAA (Health Insurance Portability and Accountability Act) compliant, ensuring the highest data safety standards. The "yes" or "no" replies to the SMS were directly transmitted to the SmartVax software. If an AEFI occurred, a survey link was sent to the participant. All the replies were stored anonymously in ISO 27018 compliant data centres, linked to a unique code, and transmitted back to the SmartVax software. Only within the software, i.e., on the hospital server, replies were assigned back to the respective person. Thus, data were only accessible by the investigator team in a non-patient-identifiable manner. For the data analysis, the SmartVax workspace features a user-friendly "one-click" export function to extract the data into a standard format such as Excel. For our pilot study, we manually added the information on the vaccination setting (adult immunisation clinic, employee health service), the inclusion/exclusion criteria (availability of an internet-enabled smartphone, German or English language knowledge, informed consent), the immunisation procedure (vaccine name, left or right arm) and diagnosed immune-mediated inflammatory diseases and their treatment.
Four weeks after the immunisation, an anonymous questionnaire was sent to the participants by post. The questionnaire used a three-grade scale to explore how the participant rated the SMS-based, active surveillance of AEFI. The questionnaire also asked if they had experienced any AEFI and whether they answered the SMS or not. If they had not answered the SMS, they were offered options for possible reasons, such as "no smartphone", "no SMS receive"’, "forgot to answer", "do not want to complete a questionnaire". Finally, the questionnaire asked whether additional adverse events occurred after the 3 days after immunisation covered by the SMS survey. In case such potential late reactions occurred, we gave the option to leave a phone number to be contacted by the study team for a follow-up interview (fig. S2 in the appendix). Participants contributing more than one visit received a questionnaire after each visit to avoid missing a delayed AEFI.
For the statistical analyses and data visualisation we used Prism (version 9.1.0) and Microsoft Excel 365. We compared demographic characteristics of those who responded to SMS-1 with those who did not. The response rate was defined as the proportion of patients who answered SMS-1 with a valid reply (i.e., "Y" or "yes", "N" or "no"). The primary outcome of our study was the feasibility and acceptance of SmartVax in an adult population of an immunisation clinic and the employee health service at a Swiss University Hospital. This outcome was measured by the response rate and secondarily descriptively analysed as "time to response" and based on the responses to the questionnaire sent 4 weeks after vaccination. The following secondary endpoints were assessed: differences in response rate and time to response in subgroups (time of day SMS-1 was sent, clinical setting, age, sex, IMID), differences in reported AEFI in different subgroups (clinical setting, sex, age, IMID and if yes if treated) and frequencies of reported AEFI for different vaccines (local vs systemic vs local and systemic). Anonymised data can be made available upon request by interested scientists or health authorities.
We calculated the response rate and time-to-respond to SMS-1 for the whole cohort and the following subgroups: (i) by the time of day SMS-1 was sent (6:00–9:00, 9:00–11:00, 11:00–14:00, 14:00–18:00, 18:00–22:00), (ii) the clinic setting (adult immunisation clinic vs employee health service), (iii) age (<60 y vs ≥60 y), (iv) gender and (v) those with vs. without an immune‐mediated inflammatory disease. For the time-to-respond analysis, we used non-parametric t-tests (Mann-Whitney test comparing two groups and Kruskal-Wallis test comparing more than two groups), whereas response rates were analysed using Fisher’s test for contingency tables.
We calculated the proportion of immunisation visits with at least one reported AEFI (isolated local reactions, isolated systemic reaction, combined local plus systemic reaction) in relation to all immunisation visits with a valid response. To do so, we used contingency tables and Fisher's exact test. The same calculation was performed for the following subgroups: setting (adult immunisation clinic vs employee health service), sex (female vs male), age (<60 y vs ≥60 y), and if an immune-mediated inflammatory disease was diagnosed or not. We further divided the subgroup of patients with immune-mediated inflammatory disease into patients with versus without immunosuppressive therapy. Patients were considered immunosuppressed if immunosuppressive therapy was taken in the last 28 days before the vaccination date. Ocrelizumab, rituximab, and leflunomide were considered exceptions, where we assumed immunosuppression until 12 months after the end of therapy. Oral steroid therapy was considered immunosuppressive if >20 mg of prednisone equivalent was taken for more than 2 weeks. After the dose dropped below 20 mg, the patient was still considered immunosuppressed for 28 days. Subjects receiving pulsed high-dose intravenous glucocorticoid therapy (Solumedrol [methylprednisolone sodium succinate] 500 mg to 1 g over 1–3 days) were also considered immunosuppressed for 28 days after the last dose.
For all vaccines administered more than five times in the study, the association with different AEFI was analysed. The proportion of visits after which participants reported a specific AEFI was calculated based on visits with valid survey replies. We compared proportions of AEFI for the most frequently used vaccines. Since up to three vaccinations are administered in the same arm per visit, we compared (i) how often each vaccine was applied to an arm "with" versus "without a reported local reaction", (ii) how often each vaccine was associated with a reported systemic reaction, and (iii) how often each vaccine was associated with "any" versus "no reaction". We compared each vaccine with all other vaccines combined, using Fisher’s test for contingency tables.
Because this was a pilot study, we did not specifically analyse potential confounders due to multiple visits by the same individual.
Between February and September 2020, we screened 293 subjects, with 95.9% (281/293) having an internet-enabled smartphone. The screening process is shown in figure 1. In total, we collected information on 360 immunisation visits. Fifty-two subjects contributed two vaccination visits, and 16 subjects contributed three visits. The age, gender, access to a smartphone, and immune-mediated inflammatory diseases are summarised in Table 1. The group of subjects with immune-mediated inflammatory diseases accounted for 20.3% (56/276) of the study population. The majority of the immunisation visits (268/360, 74.4%) took place at the adult immunisation clinic, the remaining 92/360 (25.6%) visits at the hospital's employee health service (supplementary fig. S3 in the appendix). During the 360 visits, a total of 625 vaccinations were administered (391 in the left and 234 in the right arm) with a median of 2 (interquartile range [IQR] 1–2) immunisations per visit and the most common being against tick-borne encephalitis (31.4%), hepatitis A and/or B (24.9%) and diphtheria/tetanus/ pertussis (19.8%) (table 1).

Figure 1 Study flow chart.
SMS: short message service; SMS-1: first SMS asking whether a reaction occurred following vaccination; SMS-2: second SMS asking whether the reaction resulted in a consultation with a physician.
Table 1Baseline characteristics.
| Included cohort (n = 276 persons) | Excluded cohort (n = 17 persons) | p-value | |
| Age, median (IQR) | 37.4 (28.4–49.7) | 67.6 (61.8–73.2) (n = 12 w/o smartphone) | <0.0001* |
| 35.4 (28.0–40.9) (n = 5 w/o IC) | ns | ||
| ≥60 y | 25/276 (9.1%) | 11/17 (64.7%) | <0.0001* |
| Female, n (%) | 169/276 (61.2) | 8/17 (47.1) | ns |
| Access to smartphone, n (%) | 276/276 (100) | 5/17 (29.4) | <0.0001* |
| Immune‐mediated inflammatory disease, n (%) | 56/276 (20.3) | na | |
| – Age, median (IQR) | 41.0 (34–53.2) | na | |
| – Female, n (%) | 34/56 (60.7) | na | |
| – Multiple cclerosis, n (%) | 34/56 (61) | na | ns |
| – Rheumatic disease, n (%) | 12/56 (21) | na | ns |
| – Others, n (%) | 10/56 (18) | na | |
| Included cohort (n = 360 visits) | |||
| Visits recruited at vaccination clinic, n (%) | 270/360 (75.0) (including 80/360 visits in IMID) | ||
| Visits recruited at employee health service, n (%) | 90/360 (25.0) | ||
| Total number of vaccinations | 625 | ||
| – Left arm, n (%) | 391 (62.6) | ||
| – Right arm, n (%) | 234 (37.4) | ||
| Vaccinations per visit, median (IQR) | 2 (1–2) | ||
| Immunisations by vaccine type, n (%) | 625 (100) | ||
| - TBE (FSME-Immun®) | 196 (31.4) | ||
| - dTpa (Boostrix®) | 87 (13,9) | ||
| - HBV (EngerixB20®) | 62 (9.9) | ||
| - HAV/HBV (Twinrix®) | 60 (9.6) | ||
| - MMR (Priorix®) | 48 (7.7) | ||
| - PCV13 (Prevenar13®) | 39 (6.2) | ||
| - dTpa-Polio (Boostrix Polio®) | 37 (5.9) | ||
| - HAV (Havrix1440®) | 34 (5.4) | ||
| - RZV (Shingrix®) | 27 (4.3) | ||
| - Rabies (Rabipur®) | 16 (2.6) | ||
| - Varicella (Varilix®) | 7 (1.1) | ||
| - HPV (Gardasil9®) | 5 (0.8) | ||
| - YF (Stamaril®) | 5 (0.8) | ||
| - JE (Menveo®) | 2 (0.3) | ||
* Significant p<0.05; ns: not significant with significance level alpha <0.05. TBE: tick-borne encephalitis vaccine; dTpa(-Polio): diphtheria-tetanus-pertussis(-poliomyelitis) vaccine; HBV: hepatitis B vaccine, HAV/HBV: combined hepatitis A and B vaccine; MMR: combined measles-mumps-rubella vaccine; PCV13: pneumococcal conjugate vaccine; HAV: hepatitis A vaccine; RZV: recombinant zoster vaccine; HPV: human papillomavirus vaccine; YF: yellow fever: JE: Japanese encephalitis
The overall response rate to SMS-1 was 90.3% (325/360), with a median time-to-response of 47 minutes (IQR 11–205). There was no significant difference in response rate or median time-to-response between age groups (≥60 y vs <60 y), gender, setting (adult immunisation clinic vs employee health service), or the time of day SMS-1 was sent (groups 1–5). Neither was there any difference between participants with and without diagnosed immune-mediated inflammatory disease (table 2). Of all those who responded to SMS-1, 64% responded within 2 h, 72% within 3 h, 78% within 4 h.
Table 2SMS response rates and time-to-response across different settings.
| Response rate % | p-value | Time-to-response(minutes), median (IQR) | p-value | |
| Overall | 90.3 (325/360) | 47 (11–205) | ||
| By clinical group | ||||
| Adult immunisation clinic | 90.0 (243/270) | ns | 45 (11–188) | ns |
| Healthcare workers | 88.9 (80/90) | 89 (11–399) | ||
| IMID patients | 90.0 (72/80) | 48 (14–197) | ||
| By time SMS-1 sent | ||||
| 6:00–9:00 | 87.7 (149/170) | ns | 79 (18–212) | ns |
| 9:00–11:00 | 91.1 (51/56) | 40 (13–186) | ||
| 11:00–14:00 | 94.2 (65/69) | 39 (6–246) | ||
| 14:00–18:00 | 93.0 (40/43) | 27 (4–153) | ||
| 18:00–22:00 | 90.0 (18/20) | 38 (4–543) | ||
| By age | ||||
| Age <60 years | 90.3 (298/330) | ns | 47.5 (10–202) | ns |
| Age ≥60 years | 89.3 (25/28) | 45.0 (15–239) | ||
| By sex | ||||
| Female | 89.6 (189/211) | ns | 41 (7–184) | ns |
| Male | 91.3 (136/149) | 72 (17–245) | ||
IMID: immune-mediated inflammatory disease; "By time SMS-1 sent" indicates comparison of response rates depending on time of day the SMS-1 was sent. ns: not significant with significance level alpha <0.05
Overall, 29.8% (97/325) of immunisation visits were associated with a reported AEFI (i.e. participants replied "yes" to SMS-1). The detailed response rates and drop-outs are indicated in figure 1. Online surveys were completed for 88.7% (86/97) of the visits with at least one AEFI reported. Of these, 36.1% (31/86) were isolated local reactions, 11.6% (10/86) isolated systemic reactions and 52.3% (45/86) a combination of local and systemic reactions. For descriptive statistics of AEFI subgroups we excluded 11 visits with reported AEFI but without subsequent completion of the online survey and two participants declining participation by replying "stop" to SMS-1. The most frequently reported local reactions were pain (70/312, 22.4%) and swelling/redness (32/312, 10.3%). Two subjects reported extensive limb swelling, which did not lead to medical consultation in either of the cases. Local reactions usually occurred within 24 h after vaccination. In more than half of the cases, these symptoms lasted 3 days or longer. Systemic reactions were most frequently fatigue (fig. 2A). The remaining symptoms were reported in 5 of the 312 visits. There was no reported case of convulsions/seizures or non-responsiveness / loss of consciousness. There were seven reported free-text AEFI: arthralgia/myalgia (n = 3), labial herpes (n = 2), axillary lymph node swelling, and rhinitis with swollen tonsils (n = 1 each). All were classified as systemic reactions. Systemic AEFI mainly occurred no later than the day after vaccination and usually lasted only 1–2 days (fig. 2A, tables S1 and S2).
Other than the elderly (≥60 y) reporting more isolated systemic reactions (p = 0.04, 12.0% vs 2.4% <60 y), we observed no difference in the frequency or pattern of reported AEFI amongst thesub-groups. Patients with immune-mediated inflammatory diseases (IMID), healthcare workers, or those vaccinated in routine vaccination check-up visits had comparable levels of AEFI (fig. 2B). There was also no difference in reported AEFI between patients with immune-mediated inflammatory diseases with versus without immunosuppressive treatment, table S1). Furthermore, the AEFI frequency did not differ between the group with a single administered vaccine (72.7%) versus the group with 2–6 vaccines administered in the same visit (72.2%). As part of the online survey, 15.5% (13/84) reported using an anti-inflammatory/-pyretic drug.
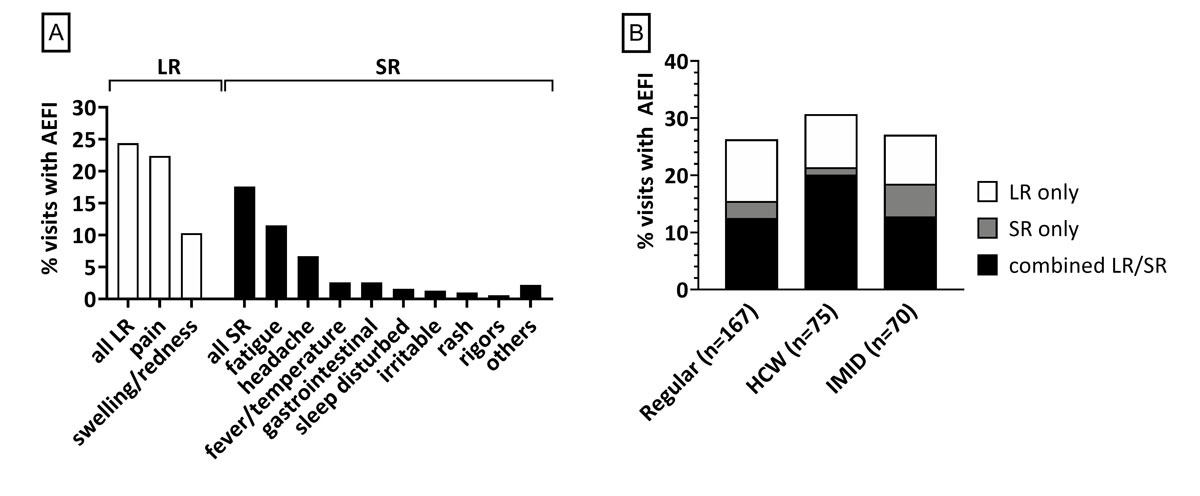
Figure 2 Frequency and type of local and systemic AEFI in different clinical settings.
(A) The overall frequency of reported AEFI is displayed by reaction type. LR: local reaction; SR: systemic reaction; AEFI: adverse events following immunisation. (B) The frequency of immunisation visits associated with AEFI in the three clinical groups is displayed. Regular: routine vaccination check-up visits; HCW: healthcare workers; IMID: subjects with diagnosed immune-mediated inflammatory disease; LR only: reporting a local reaction only; SR only: reporting a systemic reaction only: combined LR/SR: subjects reporting at least one local and systemic symptom following the immunisation visit. Data include all visits with a valid reply to SMS-1 and, in the case of a reported AEFI, with a completed online survey.
Of the 97 subjects who reported a reaction, 88.7% (86/97) responded to SMS-2, which asked whether the reaction was medically attended. Only 5.8% (5/86) reported that they had consulted a physician: two for strong local reactions, two for systemic reactions and one with combined local and systemic symptoms. Medically attended systemic reactions were gastrointestinal disturbance, rash, tiredness, irritability, fever, and headache. No specific vaccination was associated with medically attended visits.
The recombinant zoster vaccine (RZV) Shingrix® was recently approved to prevent herpes zoster and post-herpetic neuralgia. Shingrix® is highly reactogenic [35]. As proof of principle that SmartVax is a reliable tool to detect AEFI, we next compared the reported reactions by administered vaccines. We found that after 44% and 48% of the RZV immunisations, respetively, a local or systemic reaction was reported (fig. 3, table S3). Reactogenicity of RZV was significantly higher compared to the group of all other vaccines combined (local 44% vs 16%, p = 0.0012; systemic 48% vs 15%, p = 0.0002). However, AEFI following RZV were less frequently reported than in the phase III trials where, depending on age, 74.1–81.5% reported local reactions and 53–66.1% systemic reactions [35, 36]. After visits when a dTpa vaccine (Boostrix®) was administered, participants reported a local reaction more often than the group of all other vaccines combined (30.1% vs 17.2%, p = 0.015).
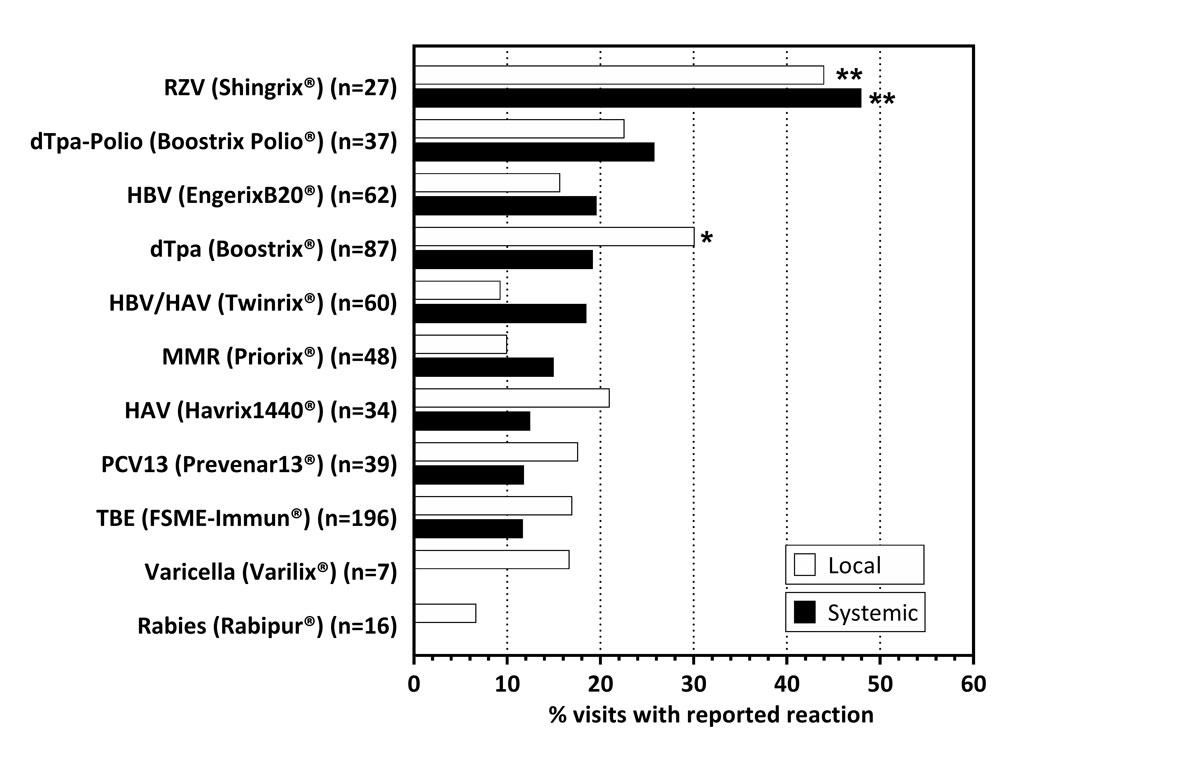
Figure 3 Frequency of local and systemic reactions by vaccine type.
Data expressed as % of visits, with the administration of the respective vaccine that was associated with a local (white) or systemic (black) reaction. Subjects receiving the respective vaccine were compared with the rest of the cohort using Fisher’s exact test. *p <0.05; **p <0.001. RZV: recombinant zoster vaccine (Shingrix®); dTpa(-Polio): diphtheria-tetanus-pertussis (with/without poliomyelitis) vaccine: HBV: hepatitis B vaccine; HAV/HBV: combined hepatitis A and B vaccine; MMR: combined measles-mumps-rubella vaccine; HAV: hepatitis A vaccine; PCV13: pneumococcal conjugate vaccine; TBE: tick-borne encephalitis vaccine.
The acceptance of an SMS-based active vaccine safety surveillance is essential for its broad implementation. We assessed acceptance by a short, anonymous paper questionnaire sent by the postal service 4 weeks after the immunisation visit. Fifty-six percent (200/357) of the questionnaires were returned completed, with 94% (188/200) considering the SMS-based surveillance system as being "useful" or "excellent", and 3.5% (7/200) thought it was not useful. In the questionnaire, 36.7% (73/199; 1 participant did not answer this question) affirmed having experienced at least one AEFI, compared with 27.6% in the SmartVax survey, suggesting that those with an AEFI were more likely to return the questionnaire. Notably, 20 participants (10%) reported in the paper questionnaire that they had not responded to SMS-1. The most common reasons for not having responded were "not having received the SMS" (12/20, 60%) or "forgot to answer" (2/20, 10%). Only two subjects (1%) reported AEFI that emerged after completing the SMS-based surveillance. One reported extensive limb swelling starting 3 days after Shingrix® immunisation, which was medically unattended and resolved entirely within 10 days. The second subject reported no details on the delayed AEFI and declined to be contacted by telephone.
The recently approved COVID-19 vaccines have been rolled out in unprecedented mass vaccination programmes. The rapid immunisation of millions of people highlights the need for ongoing post-licensure surveillance of AEFI. For example, the clustering of possible postvaccination myocarditis after mRNA COVID-19 vaccination was detected only as a result of close post-licensure monitoring, whereas the phase III trials detected no such safety signal [37–39]. In most countries, including Switzerland, such post-licensure vaccine safety monitoring is done by means of a passive surveillance system. The limitations of such passive systems include underreporting, biased reporting, the inability to predict incidences, and the risk that sudden increases in AEFI incidence are only detected with substantial delay [2, 11, 14–17]. Active safety surveillance mitigates some of these limitations. Here, we performed a pilot study to assess the feasibility and acceptance of SmartVax, a participant-centred, SMS-based, active vaccine safety monitoring software at a university clinic in Switzerland [31]. The study population included adults vaccinated at our immunisation clinic as regular immunisation check-ups, subjects with IMID and healthcare workers. We compared the response rates, time-to-response, frequency of solicited local or systemic AEFI, and the acceptance of SmartVax among these groups.
The SMS-based safety monitoring approach was highly accepted in the study population, with 98.3% (288/293) agreeing to participate in the study, a response rate of 90.3% to the first SMS and 94% indicating in a questionnaire 4 weeks post-immunisation that they found the system helpful or excellent. Notably, the response rate was comparable between subjects after a routine vaccination check-up, IMID patients, or healthcare workers. Thus, SmartVax is an appropriate tool to actively monitor AEFI in different clinical settings. Only 4.1% (12/293) of the screened subjects had no internet-enabled smartphone.
In Australia, previous studies using SmartVax in different populations (parents of vaccinated children, vaccinated adults) had 70-75 % response rates to the first SMS. The higher response rate (90.3%) across all our study groups (regular vaccination check-up, IMID, healthcare workers) may have been influenced by the personal contact between the study physician and participants to obtain informed consent. The median time-to-respond to the first SMS was 47 minutes (IQR 11-205) and not influenced by the time of day the SMS was sent. Time-to-response was longer compared to previous studies: 2 hours after the first SMS, only 64% had responded in our study compared with 80% in studies in Australia [28, 31]. This may have been related to different smartphone usage habits between the two countries or adults' working hours.
Age and gender have been suggested to impact the reporting of AEFI [40, 42], but this was not the case in our study. Our overall solicited AEFI rate of 27.6% was substantially higher than in previous Australian studies, in which 8.2% of parents in a children population and 11.3% of participants in a mixed population (children and adults) reported any kind of AEFI [28, 31]. Still, the rates of solicited AEFI were lower than those typically reported in phase III studies [35]. Interestingly, we found no significant difference in the incidence of AEFI between the IMID patients, healthcare workers and regular immunisation check-ups. Nor was there a difference in reported AEFI frequency between IMID with and without immunosuppressive therapy or depending on the number of vaccines administered simultaneously (1 vs >1). Whether a symptom is perceived and reported as an AEFI depends on various individual and external factors. The study information and informed consent procedure may have affected the perception of AEFI and may have led to higher reporting, since we typically explained the study using the example of local reactions. Indeed, most events were mild injection site reactions or mild general symptoms. In this pilot study, only solicited AEFIs were assessed. In general, the rate of solicited AEFI is significantly higher than that of unsolicited events. For example, following hepatitis A vaccination with Epaxal®, solicited (diary cards) versus unsolicited AEFI were reported in 29.6% versus 19.3% for local AEFI and 33.8% versus 18.2% systemic AEFI (n = 2675 healthy travelers) [43]. Similarly, the recombinant VZV vaccine Shingrix® caused more solicited than unsolicited AEFI in the phase III trials ZOE-50/70: 68.1% versus 22.98% for local pain, 32.9% versus 3.26% for myalgia, and 32.9 versus 3.56% for fatigue [44].
We also analysed the occurrence of AEFI depending on the vaccines used. We detected the highest frequency of AEFI following vaccination with the RZV (Shingrix®), which is known to be more reactogenic than most other licensed vaccines [35, 36]. Moreover, and as expected, we observed increased local reactions associated with the dTpa-combination vaccine Boostrix®. These examples support as a proof-of-concept that SmartVax is a suitable and sensitive tool for active monitoring of AEFI without relevant over- or underreporting.
The limitations of this study include the underrepresentation of people over the age of 60 (25/276, 9.1%) and their lower availability of an internet-enabled smartphone (72.2% vs 99.3% in the age group <60 y). However, the relatively high proportion of smartphone owners in the >60 age group combined with a response rate and time-to-response comparable to the younger group suggests that representative monitoring of AEFI using SmartVax is still feasible in the older population. Another limitation is the exclusive recruitment at a university hospital, with a possible selection bias towards patients with chronic disease and those on immunomodulatory therapies. Moreover, the population size was too small to study AEFI systematically. The analysis of potential AEFIs for individual vaccines was complicated because often multiple vaccines were administered simultaneously and, in some cases, in the same arm during one visit. More extensive studies focusing on real-life data (i.e., no study setting) in a larger group of individuals are needed to assess AEFI in various healthcare provider settings such as general practitioners’ offices, paediatric hospitals and paediatric practices. Moreover, the 3-day interval between the immunisation and the reporting of solicited adverse events misses later AEFI. Notably, most life-threatening reactions to immunisations occur within 24 hours of vaccine administration. In our study, only two subjects reported a delayed AEFI in the questionnaire sent 4 weeks after the immunisation. Nevertheless, extending the monitoring period by sending an additional SMS at 14 days after vaccination will be considered for future application of SmartVax in Switzerland. The post-marketing safety surveillance of the COVID-19 vaccines has taught us that this window may still detect critical AEFI, as vaccine-induced thrombosis with thrombocytopenia following the AstraZeneca vaccine [45] or vaccination-associated myocarditis following COVID-19 mRNA vaccines [38] occurred within a 2-week post-immunization period.
In conclusion, the SmartVax software as an active, SMS-based surveillance system of AEFI is highly accepted by clients of an adult immunisation clinic and vaccinated healthcare workers at a Swiss university hospital. It allows near real-time monitoring of AEFI and can therefore contribute to the early identification of adverse events not reported in phase III trials. As such, SmartVax is an ideal tool to monitor the post-licensure vaccine safety of newly approved vaccines, such as the COVID-19 vaccines. With additional surveillance SMS at later time points, potential long-term effects could be monitored at a population level. This could prevent health damage and improve public confidence in the COVID-19 vaccines.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper. AL is the founder of SmartVax. CTB is a member of the Federal Committee for Immunization (Eidgenössische Kommission für Impffragen; EKIF) in Switzerland.
The study was funded by research funds of the medical outpatient clinic (M.M.). C.T.B. was supported by a career development grant by the Margot aund Erich Goldschmidt and & Peter René Jacobson Foundation.
We are grateful to all participants and the staff of the immunisation clinic. We thank Denis Schiemann for local IT support and Edgar Ebel for the help with the data extraction.
1. Braun MM , Ellenberg SS . Descriptive epidemiology of adverse events after immunization: reports to the Vaccine Adverse Event Reporting System (VAERS), 1991-1994. J Pediatr. 1997 Oct;131(4):529–35. https://doi.org/10.1016/S0022-3476(97)70056-8
2. Zhou W , Pool V , Iskander JK , English-Bullard R , Ball R , Wise RP , et al. Surveillance for safety after immunization: Vaccine Adverse Event Reporting System (VAERS)—United States, 1991-2001. MMWR Surveill Summ. 2003 Jan;52(1):1–24.
3. Ahmadipour N , Watkins K , Fréchette M , Coulby C , Anyoti H , Johnson K . Vaccine safety surveillance in Canada: reports to CAEFISS, 2013-2016. Can Commun Dis Rep. 2018 Sep;44(9):206–14. https://doi.org/10.14745/ccdr.v44i09a04
4. McNeil MM , DeStefano F . Vaccine-associated hypersensitivity. J Allergy Clin Immunol. 2018 Feb;141(2):463–72. https://doi.org/10.1016/j.jaci.2017.12.971
5. Mutsch M , Zhou W , Rhodes P , Bopp M , Chen RT , Linder T , et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N Engl J Med. 2004 Feb;350(9):896–903. https://doi.org/10.1056/NEJMoa030595
6. Barker CI , Snape MD . Pandemic influenza A H1N1 vaccines and narcolepsy: vaccine safety surveillance in action. Lancet Infect Dis. 2014 Mar;14(3):227–38. https://doi.org/10.1016/S1473-3099(13)70238-X
7. Fescharek R , Nicolay U , Arras-Reiter C . Monitoring and safety assessment in Phase I to III clinical trials. Dev Biol Stand. 1998;95:203–9.
8. Bonhoeffer J , Kohl K , Chen R , Duclos P , Heijbel H , Heininger U , et al.; The Brighton Collaboration . The Brighton Collaboration: addressing the need for standardized case definitions of adverse events following immunization (AEFI). Vaccine. 2002 Dec;21(3-4):298–302. https://doi.org/10.1016/S0264-410X(02)00449-8
9. Di Pasquale A , Bonanni P , Garçon N , Stanberry LR , El-Hodhod M , Tavares Da Silva F . Vaccine safety evaluation: practical aspects in assessing benefits and risks. Vaccine. 2016 Dec;34(52):6672–80. https://doi.org/10.1016/j.vaccine.2016.10.039
10. Crawford NW , Clothier H , Hodgson K , Selvaraj G , Easton ML , Buttery JP . Active surveillance for adverse events following immunization. Expert Rev Vaccines. 2014 Feb;13(2):265–76. https://doi.org/10.1586/14760584.2014.866895
11. Hazell L , Shakir SA . Under-reporting of adverse drug reactions : a systematic review. Drug Saf. 2006;29(5):385–96. https://doi.org/10.2165/00002018-200629050-00003
12. Monteiro SA , Takano OA , Waldman EA . [Evaluation of the Brazilian surveillance system for adverse events following vaccination]. Rev Bras Epidemiol. 2011 Sep;14(3):361–71. https://doi.org/10.1590/S1415-790X2011000300002
13. Clothier HJ , Selvaraj G , Easton ML , Lewis G , Crawford NW , Buttery JP . Consumer reporting of adverse events following immunization. Hum Vaccin Immunother. 2014;10(12):3726–30. https://doi.org/10.4161/hv.34369
14. Centers of Disease Control and Prevention . Vaccine Adverse Event Reporting System (VAERS). 2017, January 27 2020, April 8]; Available from: https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vaers/index.html
15. Hasford J , Goettler M , Munter KH , Müller-Oerlinghausen B . Physicians’ knowledge and attitudes regarding the spontaneous reporting system for adverse drug reactions. J Clin Epidemiol. 2002 Sep;55(9):945–50. https://doi.org/10.1016/S0895-4356(02)00450-X
16. Isaacs D , Lawrence G , Boyd I , Ronaldson K , McEwen J . Reporting of adverse events following immunization in Australia. J Paediatr Child Health. 2005 Apr;41(4):163–6. https://doi.org/10.1111/j.1440-1754.2005.00580.x
17. Rosenthal S , Chen R . The reporting sensitivities of two passive surveillance systems for vaccine adverse events. Am J Public Health. 1995 Dec;85(12):1706–9. https://doi.org/10.2105/AJPH.85.12.1706
18. Salathé M , Khandelwal S . Assessing vaccination sentiments with online social media: implications for infectious disease dynamics and control. PLOS Comput Biol. 2011 Oct;7(10):e1002199. https://doi.org/10.1371/journal.pcbi.1002199
19. Salathé M , Vu DQ , Khandelwal S , Hunter DR . The dynamics of health behavior sentiments on a large online social network. EPJ Data Sci. 2013;2(1):4. https://doi.org/10.1140/epjds16
20. Salathé M . Digital Pharmacovigilance and Disease Surveillance: Combining Traditional and Big-Data Systems for Better Public Health. J Infect Dis. 2016 Dec;214 suppl_4:S399–403. https://doi.org/10.1093/infdis/jiw281
21. Freifeld CC , Brownstein JS , Menone CM , Bao W , Filice R , Kass-Hout T , et al. Digital drug safety surveillance: monitoring pharmaceutical products in twitter. Drug Saf. 2014 May;37(5):343–50. https://doi.org/10.1007/s40264-014-0155-x
22. Thoon KC , Soh SB , Liew WK , Gunachandran A , Tan NW , Chong CY , et al. Active surveillance of adverse events following childhood immunization in Singapore. Vaccine. 2014 Sep;32(39):5000–5. https://doi.org/10.1016/j.vaccine.2014.07.020
23. Sebastian J , Gurumurthy P , Ravi MD , Ramesh M . Active surveillance of adverse events following immunization (AEFI): a prospective 3-year vaccine safety study. Ther Adv Vaccines Immunother. 2019 Nov;7:2515135519889000. https://doi.org/10.1177/2515135519889000
24. Spila Alegiani S , Alfonsi V , Appelgren EC , Ferrara L , Gallo T , Alicino C , et al. Active surveillance for safety monitoring of seasonal influenza vaccines in Italy, 2015/2016 season. BMC Public Health. 2018 Dec;18(1):1401. https://doi.org/10.1186/s12889-018-6260-5
25. Pillsbury A , Quinn H , Cashman P , Leeb A , Macartney K ; AusVaxSafety consortium . Active SMS-based influenza vaccine safety surveillance in Australian children. Vaccine. 2017 Dec;35(51):7101–6. https://doi.org/10.1016/j.vaccine.2017.10.091
26. Wilson K , Atkinson KM , Westeinde J , Bell C , Marty K , Fergusson D , et al. An evaluation of the feasibility and usability of a proof of concept mobile app for adverse event reporting post influenza vaccination. Hum Vaccin Immunother. 2016 Jul;12(7):1738–48. https://doi.org/10.1080/21645515.2016.1152434
27. Nguyen MT , Ott JJ , Caputo M , Keller-Stanislawski B , Klett-Tammen CJ , Linnig S , et al. User preferences for a mobile application to report adverse events following vaccination. Pharmazie. 2020 Jan;75(1):27–31.
28. Westphal DW , Williams SA , Leeb A , Effler PV . Continuous active surveillance of adverse events following immunisation using SMS technology. Vaccine. 2016 Jun;34(29):3350–5. https://doi.org/10.1016/j.vaccine.2016.05.015
29. Regan AK , Blyth CC , Tracey L , Mak DB , Richmond PC , Effler PV . Comparison of text-messaging to voice telephone interviews for active surveillance of adverse events following immunisation. Vaccine. 2015 Jul;33(31):3689–94. https://doi.org/10.1016/j.vaccine.2015.06.022
30. Stockwell MS , Marchant CD , Wodi AP , Barnett ED , Broder KR , Jakob K , et al. A multi-site feasibility study to assess fever and wheezing in children after influenza vaccines using text messaging. Vaccine. 2017 Dec;35(50):6941–8. https://doi.org/10.1016/j.vaccine.2017.10.073
31. Leeb A , Regan AK , Peters IJ , Leeb C , Leeb G , Effler PV . Using automated text messages to monitor adverse events following immunisation in general practice. Med J Aust. 2014 Apr;200(7):416–8. https://doi.org/10.5694/mja13.11166
32. Pillsbury A , Cashman P , Leeb A , Regan A , Westphal D , Snelling T , et al.; AusVaxSafety, surveillance team . Real-time safety surveillance of seasonal influenza vaccines in children, Australia, 2015. Euro Surveill. 2015;20(43). https://doi.org/10.2807/1560-7917.ES.2015.20.43.30050
33. Bonhoeffer J , Bentsi-Enchill A , Chen RT , Fisher MC , Gold MS , Hartman K , et al.; Brighton Collaboration Methods Working Group . Guidelines for collection, analysis and presentation of vaccine safety data in pre- and post-licensure clinical studies. Vaccine. 2009 Apr;27(16):2282–8. https://doi.org/10.1016/j.vaccine.2008.11.036
34. Bonhoeffer J , Imoukhuede EB , Aldrovandi G , Bachtiar NS , Chan ES , Chang S , et al.; Brighton Collaboration Clinical Trial Protocol Working Group . Template protocol for clinical trials investigating vaccines—focus on safety elements. Vaccine. 2013 Nov;31(47):5602–20. https://doi.org/10.1016/j.vaccine.2013.02.041
35. Lal H , Cunningham AL , Godeaux O , Chlibek R , Diez-Domingo J , Hwang SJ , et al.; ZOE-50 Study Group . Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015 May;372(22):2087–96. https://doi.org/10.1056/NEJMoa1501184
36. Cunningham AL , Lal H , Kovac M , Chlibek R , Hwang SJ , Díez-Domingo J , et al.; ZOE-70 Study Group . Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. N Engl J Med. 2016 Sep;375(11):1019–32. https://doi.org/10.1056/NEJMoa1603800
37. Diaz GA , Parsons GT , Gering SK , Meier AR , Hutchinson IV , Robicsek A . Myocarditis and Pericarditis After Vaccination for COVID-19. JAMA. 2021 Sep;326(12):1210–2. https://doi.org/10.1001/jama.2021.13443
38. Bozkurt B , Kamat I , Hotez PJ . Myocarditis With COVID-19 mRNA Vaccines. Circulation. 2021 Aug;144(6):471–84. https://doi.org/10.1161/CIRCULATIONAHA.121.056135
39. Baden LR , El Sahly HM , Essink B , Kotloff K , Frey S , Novak R , et al.; COVE Study Group . Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021 Feb;384(5):403–16. https://doi.org/10.1056/NEJMoa2035389
40. Cook IF . Sex differences in injection site reactions with human vaccines. Hum Vaccin. 2009 Jul;5(7):441–9. https://doi.org/10.4161/hv.8476
41. Harris T , Nair J , Fediurek J , Deeks SL . Assessment of sex-specific differences in adverse events following immunization reporting in Ontario, 2012-15. Vaccine. 2017 May;35(19):2600–4. https://doi.org/10.1016/j.vaccine.2017.03.035
42. Mok CC , Ho LY , Fong LS , To CH . Immunogenicity and safety of a quadrivalent human papillomavirus vaccine in patients with systemic lupus erythematosus: a case-control study. Ann Rheum Dis. 2013 May;72(5):659–64. https://doi.org/10.1136/annrheumdis-2012-201393
43. Hatz C , Beck B , Steffen R , Genton B , d’Acremont V , Loutan L , et al. Real-life versus package insert: a post-marketing study on adverse-event rates of the virosomal hepatitis A vaccine Epaxal® in healthy travellers. Vaccine. 2011 Jul;29(31):5000–6. https://doi.org/10.1016/j.vaccine.2011.04.099
44. López-Fauqued M , Campora L , Delannois F , El Idrissi M , Oostvogels L , De Looze FJ , et al.; ZOE-50/70 Study Group . Safety profile of the adjuvanted recombinant zoster vaccine: pooled analysis of two large randomised phase 3 trials. Vaccine. 2019 Apr;37(18):2482–93. https://doi.org/10.1016/j.vaccine.2019.03.043
45. Oldenburg J , Klamroth R , Langer F , Albisetti M , von Auer C , Ay C , et al. Diagnosis and Management of Vaccine-Related Thrombosis following AstraZeneca COVID-19 Vaccination: guidance Statement from the GTH. Hamostaseologie. 2021 Jun;41(3):184–9. https://doi.org/10.1055/a-1469-7481
Figure S1 Screenshots of the online survey.

Figure S2 Questionnaire.
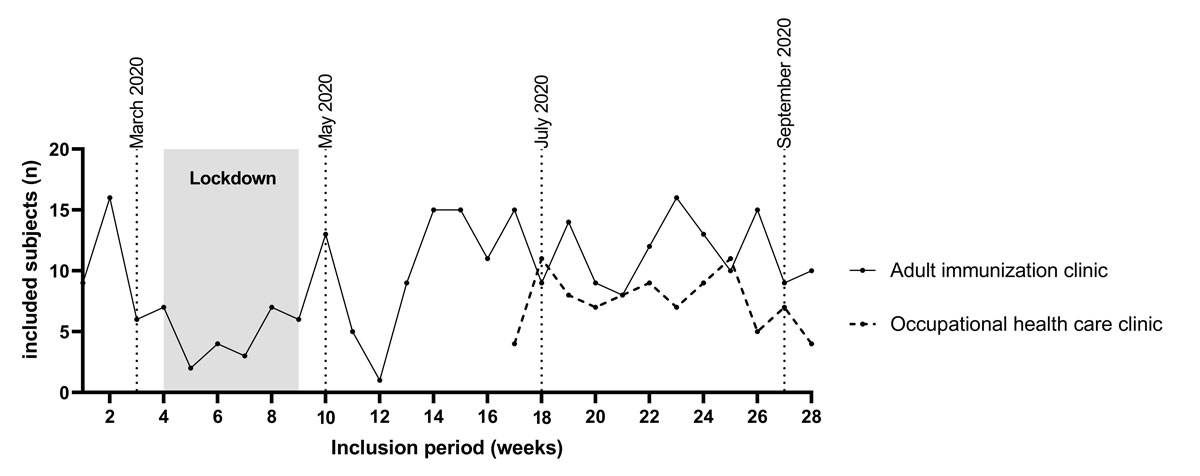
Figure S3 Number of subjects included per week in relation to the lockdown due to the COVID-19 pandemic. Lockdown: due to the COVID-19 pandemic from 16 March until 27 April 2020 all non-emergency medical services were deferred in Switzerland. The few visits at the adult immunisation clinic were subjects with immune-mediated inflammatory diseases needing immunisation before immunosuppressive therapy. Dotted lines indicate months for better orientation.
Table S1Comparison of reported AEFI in different groups.
| No data 1 | No reaction | Any reaction | p-value | Isolated local reaction | p-value | Isolated systemic reaction | p-value | Combined reaction | p-value | |
| Overall (n = 360) | 48/360 (13.3%) | 226/312 (72.4%) | 86/312 (27.6%) | 31/312 (9.9%) | 10/312 (3.2%) | 45/312 (14.4%) | ||||
| Setting: | ||||||||||
| Adult immunisation clinic (n = 270) | 33/270 (12.2%) | 174/237 (73.4%) | 63/237 (26.6%) | 0.55 | 24/237 (10.1%) | >0.99 | 9/237 (3.8%) | 0.46 | 30/237 (12.7%) | 0.13 |
| Employee health service (n = 90) | 15/90 (16.7%) | 52/75 (69.3%) | 23/75 (30.7%) | 7/75 (9.3%) | 1/75 (1.3%) | 15/75 (20.0%) | ||||
| Gender: | ||||||||||
| Female (n = 211) | 31/211 (14.7%) | 125/180 (69.4%) | 55/180 (30.6%) | 0.20 | 22/180 (12.2%) | 0.13 | 4/180 (2.2%) | 0.33 | 29/180 (16.1%) | 0.33 |
| Male (n = 149) | 17/149 (11.4%) | 101/132 (76.5%) | 31/132 (23.5%) | 9/132 (6.8%) | 6/132 (4.5%) | 16/132 (12.1%) | ||||
| Age: | ||||||||||
| <60 y (n = 330) | 43/330 (13.0%) | 209/287 (72.8%) | 78/287 (27.2%) | 0.64 | 31/287 (10.8%) | 0.15 | 7/287 (2.4%) | 0.04* | 40/287 (13.9%) | 0.38 |
| ≥60 y (n = 28) | 3/28 (10.7%) | 17/25 (68.0%) | 8/25 (32.0%) | 0/25 (0%) | 3/25 (12.0%) | 5/25 (20.0%) | ||||
| Immune‐mediated inflammatory diseases: | ns | ns | ns | ns | ||||||
| Overall (n = 80) | 10/80 (12.5%) | 51/70 (72.9%) | 19/70(27.1%) | 6/70(8.6%) | 4/70 (5.7%) | 9/70 (12.9%) | ||||
| MS (n = 48) | 10/48 (20.8%) | 29/38 (76.3%) | 9/38 (23.7%) | 4/38 (10.5%) | 1/38 (2.6%) | 4/38 (10.5%) | ||||
| Rheumatic (n = 21) | 0/21 (0%) | 13/21 (62.0%) | 8/21 (38.1%) | 1/21 (4.8%) | 3/21 (14.3%) | 4/21 (19.0%) | ||||
| Others (n = 11) | 0/11 (0%) | 9/11 (81.8%) | 4/11 (36.4%) | 1/11 (9.1%) | 0/11 (0%) | 3/11 (27.3%) | ||||
| Immunosuppressive therapy2: yes (n = 56) | 8/56(14.3%) | 33/48(68.8%) | 15/48(31.3%) | 3/48(6.3%) | 4/48(8.3%) | 8/48(16.7%) | ||||
| Immunosuppressive therapy2: no (n = 24) | 2/24(8.3%) | 18/22(81.8%) | 4/22(18.2%) | 3/22(13.6%) | 0/22(0%) | 1/22(4.5%) | ||||
| No immune‐mediated inflammatory diseases (n = 280) | 38/280 (13.6%) | 175/242 (62.9%) | 67/242 (24.1%) | 25/242 (9.0%) | 6/242 (2.2%) | 36/242 (12.9%) | ||||
| Regular (n = 190)3 | 23/190(12.1%) | 123/167(73.7%) | 44/167(26.3%) | ns4 | 18/167(10.8%) | ns | 5/167(3.0%) | ns 4 | 21/167(12.6%) | ns |
n: visits; MS: multiple sclerosis; ns; not significant with significance level alpha <0.05 * significant difference with significance level alpha <0.05
1 No response to SMS-1 or survey not completed
2 Immunosuppressive therapy: =immunosuppressive medication ≤28 d before the immunisation date; exceptions: ocrelizumab, rituximab, leflunomide immunosuppressive for 12 months after the last dose; oral steroids: immunosuppressive if >2 weeks >20 mg prednisone, until 28 d after dropping below 20 mg; intravenous Solumedrol 500 g to 1 g for 1–3d immunosuppressive for 28 days
3 subjects vaccinated in routine vaccination check-up visits
4 compared with employee health service and immune‐mediated inflammatory diseases
Table S2 Onset and duration of AEFI.
| Local pain, % (n = 70) | Swelling/redness, % (n = 32) | Fever, % (n = 8) | Tiredness/fatigue, % (n = 36) | Irritable % (n = 4) | Sleep pattern change, % (n = 5) | Rash, % (n = 3) | Headache, % (n = 21) | Gastrointestinal, % (n = 8) | Rigors, % (n = 2) | |
| Onset | ||||||||||
| Within hours | 80.0 (56) | 62.5 (20) | 75.0 (6) | 52.8 (19) | 50.0 (2) | 40.0 (2) | 0 (0) | 52.4 (11) | 12.5 (1) | 100.0 (2) |
| The day after vaccination | 12.9 (9) | 21.9 (7) | 25.0 (2) | 36.1 (13) | 0 (0) | 40.0 (2) | 33.3 (1) | 33.3 (7) | 62.5 (5) | 0 (0) |
| >1 day after vaccination | 2.9 (2) | 9.4 (3) | 0 (0) | 5.6 (2) | 25.0 (1) | 20.0 (1) | 66.7 (2) | 14.3 (3) | 25.0 (2) | 0 (0) |
| Unsure | 4.3 (3) | 6.3 (2) | 0 (0) | 5.6 (2) | 25.0 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Duration | ||||||||||
| 24 h or less | 21.4 (15) | 6.3 (2) | 75.0 (6) | 41.7 (15) | 25.0 (1) | 60.0 (3) | 33.3 (1) | 47.6 (10) | 75.0 (6) | 50.0 (1) |
| 2 days | 30.0 (21) | 21.9 (7) | 25.0 (2) | 41.7 (15) | 25.0 (1) | 0 (0) | 0 (0) | 28.6 (6) | 12.5 (1) | 50.0 (1) |
| 3 days or more | 41.4 (29) | 50.0 (16) | 0 (0) | 13.9 (5) | 0 (0) | 20.0 (1) | 66.7 (2) | 19.0 (4) | 12.5 (1) | 0 (0) |
| Unsure | 7.1 (5) | 21.9 (7) | 0 (0) | 2.8 (1) | 50.0 (2) | 20.0 (1) | 0 (0) | 4.8 (1) | 0 (0) | 0 (0) |
Supplementary Table 3AEFI profile by vaccine type.
Comparison of reported AEFI after each vaccine compared to the group of all other vaccines combined. The following differences were statistically significant:
a Boostrix® is associated with local AEFI more often than average (p = 0.015).
b Shingrix® is associated with local (p = 0.0012) and systemic (p = 0.0002) reactions more often than average.
Boostrix (Polio)®: dTpa(-Polio) = diphtheria-tetanus-pertussis(-poliomyelitis) vaccine; Varilix®: varicella vaccine; Shingrix®: RZV = recombinant zoster vaccine; Priorix®: MMR = combined measles-mumps-rubella vaccine; Gardasil9®: HPV = human papillomavirus vaccine; EngerixB20®: HBV = hepatitis B vaccine; Havrix1440®: HAV = hepatitis A vaccine; Twinrix®:HAV/HBV = combined hepatitis A and B vaccine; FSME-Immun®: TBE = tick-borne encephalitis vaccine; Prevenar13®: PCV13 = pneumococcal conjugate vaccine; Menveo®: JE = Japanese encephalitis vaccine; Rabipur®: rabies vaccine; Stamaril®: YF = yellow fever vaccine