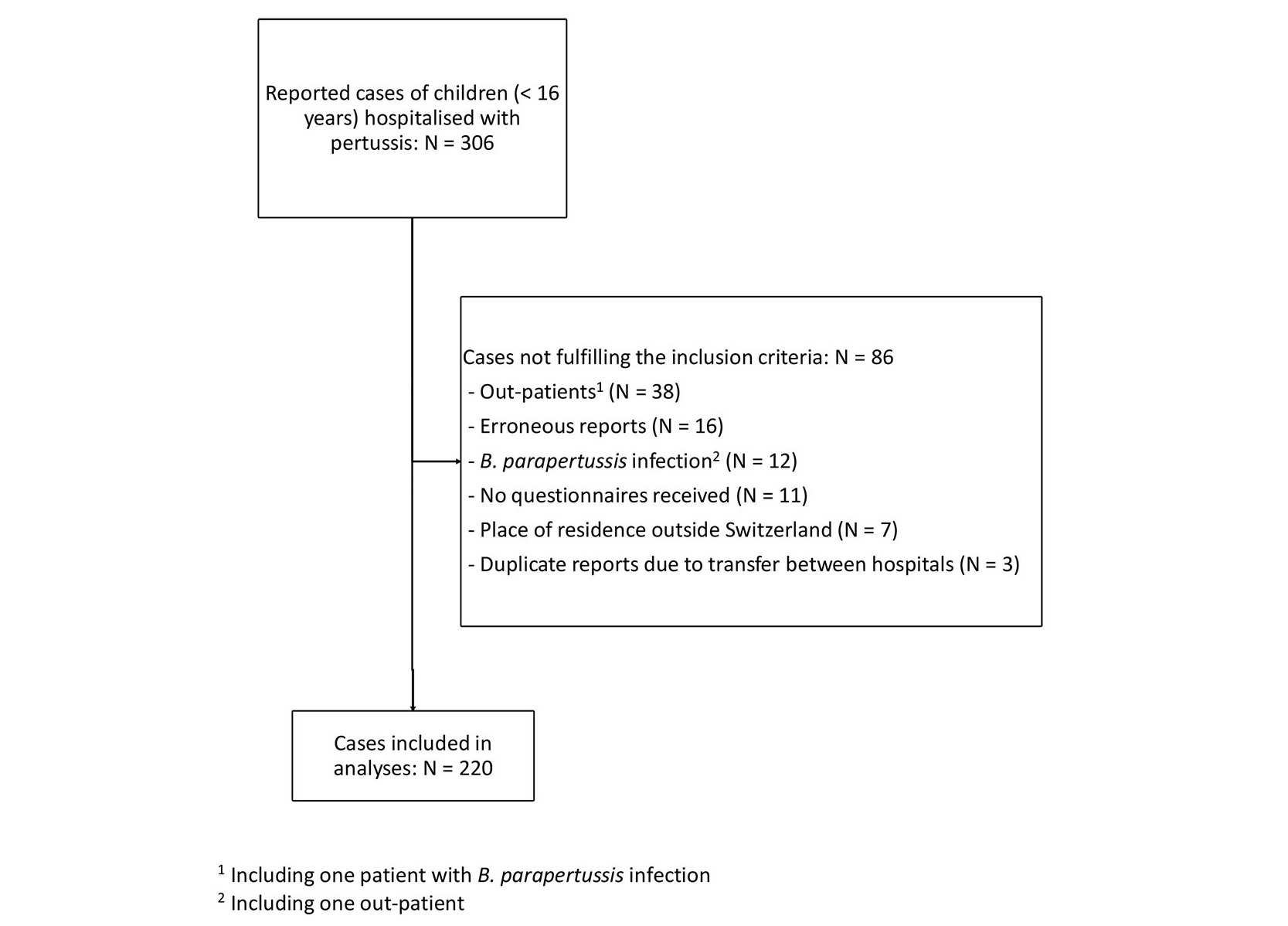
Figure 1 Flow chart. 1 Including one patient with B. parapertussis infection; 2 including one outpatient.
DOI: https://doi.org/10.4414/SMW.2021.w30064
The control of pertussis, a respiratory tract infection mainly caused by the bacterium Bordetella pertussis, remains a global challenge despite the availability of effective vaccines [1]. In this regard, longitudinal surveillance of pertussis at a national level is important [2, 3] to collect information on the epidemiology of the disease and to design an optimal immunisation strategy. The Swiss Sentinel Surveillance Network (SSSN) has been monitoring the incidence of pertussis in the general population in Switzerland since 1991. As pertussis is most severe in infants and children, we utilised the Swiss Paediatric Surveillance Unit (SPSU) to assess reported hospitalisations due to pertussis to survey the characteristics of the disease. After an initial surveillance period covering the years 2006–2010 [4], new recommendations regarding pertussis were implemented in the Swiss national immunisation schedule in 2013. Specifically, pertussis booster doses were introduced for anyone in close contact with infants <6 months of age and for pregnant women to protect the most vulnerable age group [5]. To assess the effects of these new recommendations, surveillance was resumed from 2013 to 2020. Having previously published some preliminary data [6], we now report the detailed and final results.
The SPSU is an active reporting system under the auspices of the Swiss Federal Office of Public Health (FOPH). It monitors nationwide hospitalisations of children due to defined childhood diseases in Switzerland. All paediatric hospitals or units in Switzerland participate on a voluntary basis. SPSU projects have been exempt from ethical approval throughout the duration of this study. Pertussis was reported to the SPSU from 2006 to 2010, and this resumed on 1 January 2013 until 31 December 2020.
Laboratory confirmation of a B. pertussis infection by polymerase chain reaction, culture or serology and/or a fulfilled clinical case definition in a hospitalised child under 16 years of age was mandatory for inclusion. The clinical case definition comprised (1) signs and symptoms compatible with pertussis (physician diagnosis) or (2) a cough lasting ≥ 14 days in combination with at least one of the following symptoms: paroxysmal cough, whooping or post-tussive vomiting or (3) apnoea in a patient under the age of 12 months not explained otherwise.
A standardised, anonymous questionnaire was sent to participating hospitals for every new case notification. All questionnaires were returned to the principal investigator (UH) and checked for completeness. In the case of missing data, the reporter was contacted for clarification. The data was converted into an Excel datasheet at the FOPH.
The statistical methods used have been reported before [6]. Briefly, we performed descriptive analyses for epidemiological data and used frequencies or proportions to report patient characteristics. Also, to compare the number of hospitalised cases of pertussis during the first and second halves of the total study surveillance period, we calculated age-specific proportionate changes. We used the GraphPad Prism software (version 8.4.3., San Diego, CA, USA) to calculate the 95% confidence intervals and the method of Wilson with modifications by Brown et al. for selected percentages, as appropriate [7]. Population statistics from the Swiss Federal Statistical Office were used to calculate age-dependent pertussis hospitalisation incidence values [8].
All paediatric units and hospitals in Switzerland participated in this SPSU project throughout the study period from January 2013 to December 2020. Compliance through the return of the monthly reporting forms was 100% over the whole study period.
In total, 306 cases of pertussis were reported to the SPSU. Of these, 86 were excluded for various reasons (fig. 1). The following analyses are based on the remaining 220 cases (95 females, 43%). Laboratory confirmation of B. pertussis infection was provided in 214 (97%) cases (209 by polymerase chain reaction and 5 by culture from nasopharyngeal specimens), of which 192 (87%) also met criteria for a clinical case of pertussis, as did a further six cases without laboratory confirmation (see supplementary table S1 in the appendix).

Figure 1 Flow chart. 1 Including one patient with B. parapertussis infection; 2 including one outpatient.
Figure 2 shows seasonal variations in incidence and the overall decline in reported cases across the whole surveillance period. The intermittent increase observed for the years 2016 and 2017 was also seen in outpatient pertussis cases reported via the SSSN (appendix, table S2). Notably, the last case of pertussis was reported to the SPSU in May 2020. The mean annual incidence of hospitalisation for pertussis was 27.9 per 100,000 in infants (<1 year of age) and 2.1 per 100,000 in all children under 16 years of age.
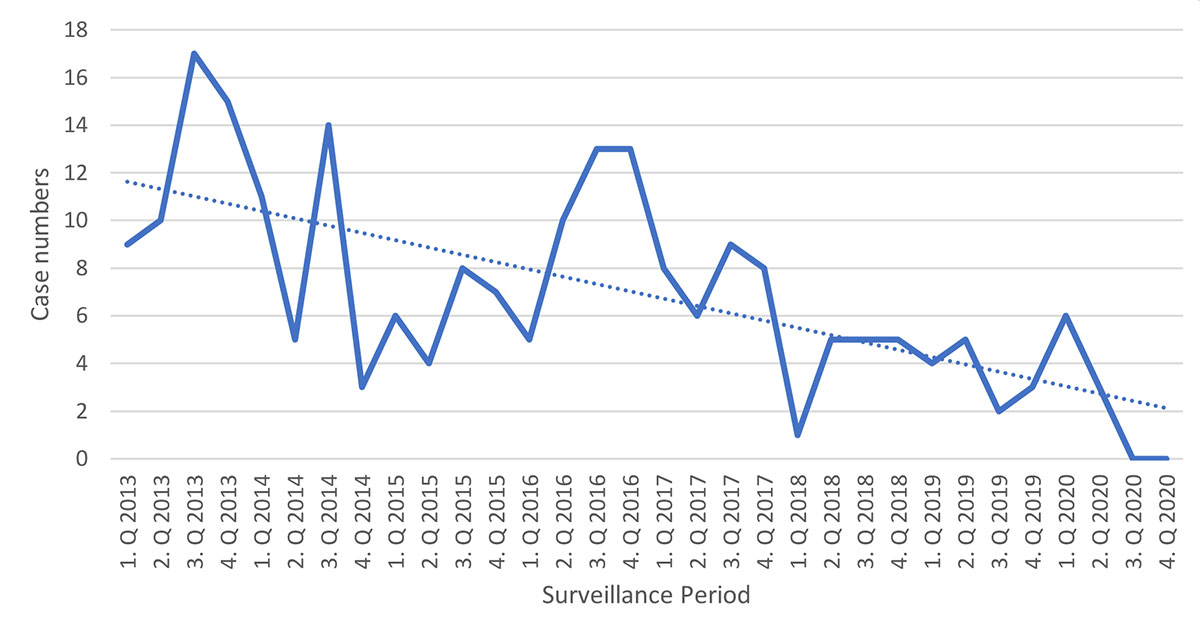
Figure 2 Seasonal variations in incidence by quarter (for 95% confidence intervals see table S8).
To evaluate the effects of the introduction of pertussis immunisation in pregnancy in 2013, we divided the surveillance period into two parts, 2013 to 2016 (early) and 2017 to 2020 (late). The decrease in case numbers from the early to the late surveillance period was most pronounced in infants (table 1).
Table 1Case counts by age category and surveillance period (modified from table 1 in [6]).
| 01.01.2013–31.12.2016 | 01.01.2017–31.12.2020 | Increase or decrease | Total (01.01.2013–31.12.2020) | |
| Age category | N (%)* | N (%)* | % (95% CI) | N (%) |
| <1 month | 16 (11) | 7 (10) | –56 (–33 to –77) | 23 (10) |
| 1 month | 43 (29) | 20 (29) | –54 (–39 to –68) | 63 (29) |
| <2 months (cumulative) | 59 (39) | 27 (39) | –54 (–42 to –66) | 86 (39) |
| 2 months | 28 (19) | 10 (14) | –64 (–46 to –79) | 38 (17) |
| 3–5 months | 34 (23) | 14 (20) | –59 (–42 to –74) | 48 (22) |
| <6 months (cumulative) | 121 (81) | 51 (73) | –58 (–49 to –66) | 172 (78) |
| 6–11 months | 13 (9) | 4 (6) | –69 (–42 to –87) | 17 (8) |
| 12–23 months | 5 (3) | 5 (7) | 0 (0 to –43) | 10 (5) |
| ≥2 years | 11 (7) | 10 (14) | –9 (–0.5 to –38) | 21 (10) |
| Total** | 150 (68) | 70 (32) | –53 (–45 to –61) | 220 (100) |
| Extrapolated number of cases according to Swiss Sentinel system*** | 34,845 (65) | 18,791 (35) | –46 (–46 to –47) | 53,636 (100) |
* Percentage of all reported cases in that period
** Percentage of total surveillance period 2013–2020
*** Personal Communication Damir Perisa and Tania Villeneuve (Federal Office of Public Health, Switzerland, March 16, 2021)
Age at day of hospitalisation ranged from 16 days to 15 years, with a median of 11.6 weeks (IQR: 6.4–20.6 weeks). The great majority of hospitalised children (172 out of 220, 78%) were infants <6 months of age, including 86 (39%) <2 months of age and 23 (10%) newborns, i.e. <1 month of age (table 1). The time interval between the onset of clinical signs or symptoms of pertussis and hospitalisation was known in 213 (97%) patients. The mean interval was 11.1 days (median: 10; range: 0–48) across all ages, with a mean of 5.4 days in neonates (median: 4.5; range: 1–16) and a mean of >10 days in children over two months of age (appendix, table S3). Mean and median duration of hospitalisation were highest in neonates and decreased with increasing age (table 2).
Table 2Duration of and reasons for hospitalisation.
| Patients* | Duration of first hospitalisation** | Cough/coughing spells | Cyanosis | Monitoring and observation | Feeding difficulties and/or vomiting | Apnea | Dyspnoea | Others | Reason Unknown | |
| Age category | N (%) | Mean/Median (Range) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) |
| <1 month | 23 (10) | 14.2/11 (3–46) | 15 (68) | 4 (18) | 1 (5) | 3 (14) | 4*** (18) | 2 (9) | 6 (27) | 1 (4) |
| 1 month | 63 (29) | 10.9/9.5 (2–34) | 36 (59) | 14 (23) | 16 (26) | 12 (20) | 7 (11) | 3 (5) | 8 (13) | 2 (3) |
| 2 months | 38 (17) | 6.6/4.5 (2–32) | 21 (60) | 8 (23) | 9 (26) | 3 (9) | 8 (23) | 0 (0) | 3 (9) | 3 (8) |
| 3–5 months | 48 (22) | 5.2/4 (2–21) | 33 (72) | 14 (30) | 11 (24) | 4 (9) | 8*** (17) | 2 (4) | 0 (0) | 2 (4) |
| 6–11 months | 17 (8) | 4.5/4 (2–11) | 13 (76) | 2 (12) | 4 (24) | 3 (18) | 1 (6) | 1 (6) | 2 (12) | 0 (0) |
| 12–23 months | 10 (5) | 5.1/4 (2–12) | 5 (50) | 1 (10) | 1 (10) | 0 (0) | 1 (10) | 2 (20) | 3 (30) | 0 (0) |
| ≥2 years | 21 (10) | 5.7/3 (2–47) | 14 (70) | 4 (20) | 1 (5) | 5 (25) | 1 (5) | 2 (10) | 7 (35) | 1 (5) |
| Total | 220 (100) | 8/5 (2–47) | 137 (65) | 47 (22) | 43 (20) | 30 (14) | 30 (14) | 12 (6) | 29 (14) | 9 (4) |
* In some patients more than one reason for hospitalisation was reported
** Duration in one patient unknown
*** Including one patient with an Apparent Life Threatening Event (ALTE)
Table updated from [4]
Paroxysmal cough was not only the main reason for hospitalisation, but also the most frequent symptom (97%), followed by rhinitis (65%) and post-tussive vomiting (43%), whereas fever was infrequent (17%), especially in infants <6 months of age (appendix, table S4). Only 15% of patients did not suffer from any complications due to pertussis. The most common complications were cyanosis and dyspnoea (both >50%). Severe complications, such as pneumonia, cerebral convulsions and encephalopathy, were rare (each ≤5%) (table 3). Over the whole surveillance period, three children died: two neonates (whose mothers were not vaccinated against pertussis during pregnancy) and an 18-month-old unvaccinated patient. All of them suffered from severe complications leading to death less than two weeks after admission to hospital. Therefore, the case fatality rate was 1.4% among all children hospitalised with pertussis, and 8.7% in neonates.
Table 3Complications by age category.
| Age category | Patients | Cyanosis | Dyspnoea | Sleep disturbed | Apnoea | Pneumonia | Cerebral convulsions | Encephalopathy | Others | Any |
| N (%) | N/N known (%) | N/N known (%) | N/N known (%) | N/N known (%) | N/N known (%) | N/N known (%) | N/N known (%) | N/N known (%) | N/N known (%) | |
| <1 month | 23 (10) | 18/22 (82) | 16/23 (70) | 10/22 (45) | 18/22 (82) | 6/23 (26) | 0/23 (0) | 2/23 (9) | 31,2/23 (13) | 21/23 (91) |
| 1 month | 63 (29) | 38/60 (63) | 38/63 (60) | 31/59 (53) | 35/63 (56) | 1/63 (2) | 1/63 (2) | 0/63 (0) | 11/63 (2) | 56/63 (89) |
| 2 months | 38 (17) | 16/37 (43) | 16/38 (42) | 13/38 (34) | 12/38 (32) | 2/38 (5) | 1/38 (3) | 0/38 (0) | 13/38 (3) | 26/38 (68) |
| 3–5 months | 48 (22) | 26/48 (54) | 20/48 (42) | 20/48 (42) | 17/48 (35) | 0/48 (0) | 0/48 (0) | 0/48 (0) | 0/48 (0) | 41/48 (85) |
| 6–11 months | 17 (8) | 9/16 (56) | 10/17 (59) | 10/14 (71) | 6/17 (35) | 0 /17 (0) | 0/17 (0) | 0/17 (0) | 0/17 (0) | 17/17 (100) |
| 12–23 months | 10 (5) | 5/10 (50) | 5/10 (50) | 2/9 (22) | 2/10 (20) | 0/10 (0) | 2/10 (20) | 1/10 (10) | 24,5/10 (20) | 9/10 (90) |
| ≥2 years | 21 (10) | 8/21 (38) | 9/21 (43) | 13/21 (62) | 1/21 (5) | 2/21 (10) | 0/21 (0) | 0/21 (0) | 25,6/21 (10) | 18/21 (86) |
| Total | 220 (100) | 120/214 (56) | 114/220 (52) | 99/211 (47) | 91/219 (42) | 11/220 (5) | 4/220 (2) | 3/220 (1) | 9/220 (4) | 188/220 (85) |
1 Hyperleucocytosis
2 Neonatal respiratory distress syndrome (NRDS), arterial hypertension
3 Weight loss
4 Hypoxia
5 Syncope during coughing spell
6 Fear of death
Two hundred and twelve patients (97%) received antibiotic treatment, most commonly a macrolide (appendix, table S5). Intensive care treatment was required in 52% of all neonates, with the proportions requiring this decreasing with increasing age (appendix, table S6).
A likely source of infection was identified in 162 patients (74%) and was most commonly a member of the same household (140 cases; 86%) (appendix, table S7).
Ninety-five patients (43%) were <2 months of age at onset of symptoms and therefore too young to be vaccinated. In 115 of the remaining 125 cases, all necessary information, such as number of doses, date of immunisations and exact age, was available (fig. 3). Of these, 50 patients (43%) were unimmunised. Three children (3%) had an incomplete pertussis immunisation status according to their age and a further 10 (9%) were still up to date but their next doses were due. Finally, 52 patients (45%) had an up-to-date pertussis immunisation status according to their age (fig. 3). Forty-four (85%) of these, however, were still too young to have completed their primary series. The remaining eight cases were vaccine failures (four after the third dose, three after the fourth dose and one after the sixth dose). Notably, no vaccine failures occurred after the switch from the 3+1 to the reduced 2+1 immunisation schedule in Switzerland in March 2019 [9].
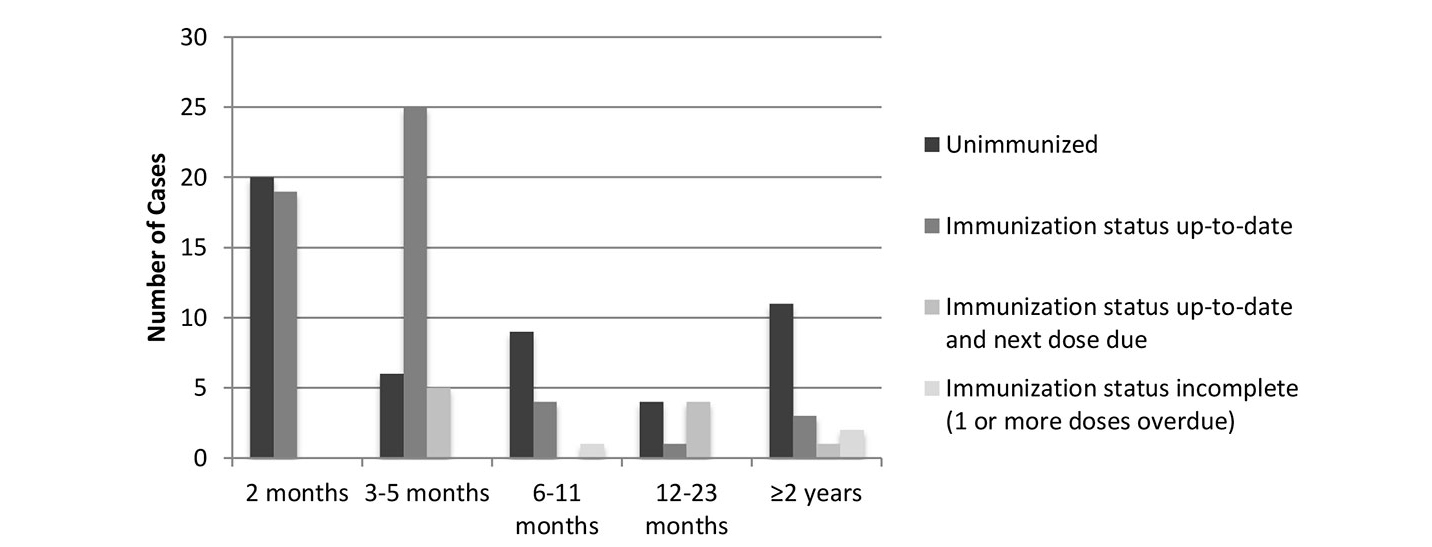
Figure 3 Immunisation status of children hospitalised due to pertussis.
Although information on the pertussis immunisation status was only known in 47 of the 220 (21%) mothers, we identified 5 out of 172 (3%) infants under the age of six months whose mothers had been vaccinated during the pregnancy. Three of these five mothers had received their vaccine dose at 17 to 31 weeks of gestation, whereas in the other two cases time points of the immunisation during pregnancy were not reported.
Here we report the results of a nationwide pertussis surveillance study in hospitalised children in Switzerland after the introduction of immunisation during pregnancy in 2013.
Figure 2 shows some seasonal variations but overall, a clear decrease in reported cases during the eight-year surveillance period was seen. We previously compared the mean hospitalisation rate of the current study period up to 2019 with the preceding period (2006–2010) and showed a decline in incidence, especially in infants, i.e. <1 year of age [6]. Here, with the case numbers from 2020 included, the decline is further extended, from mean annual hospitalisation rates of 38.8 per 100,000 children <1 year and 2.6 per 100,000 patients <16 years between 2006 and 2010 to 27.9 and 2.1 per 100,000 children <1 year and <16 years respectively between 2013 and 2020.
Different studies have shown that the implementation of public health measures to slow down the COVID-19 pandemic also had an impact on the transmission of other infectious diseases [10, 11], including pertussis [12]. With the introduction of strict measures (e.g. working from home, closing leisure facilities, limiting social gatherings etc.) in Switzerland in mid-March 2020, reported pertussis case numbers declined from six cases to three from the first to the second quarter of the year. After May 2020, no further cases were reported. This indicates a massive reduction in the transmission of B. pertussis in the Swiss paediatric population due to the public health measures established during 2020, as was also noted in the general population (table S2) and continues to be the case in 2021 [13].
Young infants are the age group at highest risk for complications and death due to pertussis and at the same time, they are too young to be completely immunised. In our surveillance study, 78% of all hospitalised children were under the age of six months and therefore not or incompletely immunised. Protecting this age group indirectly by immunising their close contacts, a strategy called “cocooning”, is challenging and – at least in our region – complicated by the lack of knowledge among parents about the importance of their own immunisation status [14, 15]. As in our previous surveillance period, covering the years 2006–2010 [4], the main suspected source of infection was members of the child’s household. However, even if cocooning was implemented with very high compliance, the limited efficacy of the currently used pertussis vaccines against disease – and especially against B. pertussis infection and transmission – would not prevent the exposure of infants in their own households sufficiently [16]. Moreover, the relatively high proportion of cases (26%) in which the source of infection was not known suggests that silent transmission (i.e. sporadic contact with unrecognised pertussis cases) of B. pertussis is a common phenomenon. This makes protecting young infants through cocooning even more difficult.
A more promising way to protect these infants from pertussis is via immunisation of their mothers against pertussis in every pregnancy. Studies have shown that this measure successfully reduces the risk of hospitalisation due to pertussis by approximately 80–90% [17, 18, 19]. Currently, pertussis immunisation in pregnancy can only be achieved by use of tetanus-diphtheria-acellular pertussis combination vaccines (Tdap) due to the lack of a stand-alone pertussis vaccine in most parts of the world, including Europe. For further pregnancies, repeated immunisation against pertussis alone would suffice, whereas pregnant women and their unborn children would not benefit from repeated tetanus and diphtheria toxoid doses. Although repeated doses of Tdap within a short period of time do not lead to increased reactogenicity when compared to longer intervals, i.e. up to 10 years, a stand-alone acellular pertussis vaccine would be highly welcome [20].
In Switzerland, immunisation during pregnancy was implemented in 2013 and acceptance of this recommendation during the early surveillance period was poor [15]. In the meantime, however, compliance has improved substantially. Although our study was not designed to formally assess the effectiveness of pertussis immunisation in pregnancy, the continuous decline in cases in infants under two months of age throughout the course of our study (table S2) is in accordance with this observation.
Similar to the findings from the previous surveillance period (2006–2010) [4], vaccine failure in children with complete primary immunisation series was infrequent.
We are not aware of any similar comprehensive, longitudinal, national pertussis surveillance data covering the whole paediatric age spectrum from other countries published in the recent past. However, a comparison between reports from the pre-acellular vaccine era, about three decades ago, in Germany and the USA [21, 22] and our current findings reveals very similar and unchanged clinical characteristics and epidemiological features of pertussis in children.
Our study has strengths and limitations. The duration of the study, over eight years, the high proportion of laboratory-confirmed cases and the nationwide reporting through SPSU make the results highly reliable. Although underreporting, underrecognition and underdiagnosis of pertussis may have led to falsely low numbers, there is no reason to believe that this is any different from previous years and other similar studies. One limitation is that white blood cell counts were not systemically demanded from the reporters. Extreme leucocytosis is a risk factor for severe pertussis and even death in young infants [23] and therefore, such information should be recorded in future pertussis surveillance projects.
In conclusion, respectable improvements have been achieved with the introduction of immunisation against pertussis in pregnancy. However, to better protect young infants from this life-threatening disease, further efforts to achieve better compliance through timely and complete immunisation in pregnant women, infants themselves and those in close contact with them are needed.
We thank the following representatives of the participating paediatric units in Switzerland for providing data: M. Albisetti; W. Bär; V. Bernet; M. Bianchetti; H. U. Bucher; M. Buettcher; L. Buetti; F. Cachat; A. Castiglione; V. Colombo; C. Däster; P. Diebold; Z. Dovhunovà; G. Duvoisin; S. Ferroni; S. Fluri; E. Galiart; M. Gebauer; M. Gehri; E. Giannoni; P. Goetschel; S. Gruppe; L. Hegi; K. Held-Egli; M. Horn; P. Imahorn; T. Karen; T. Keller; L. Kottanattu; B. Laubscher; R. Lauener; U. Lips; H. Madlon; V. Maghaouri-Slim; A. Malzacher; J. Mc Dougall; A. Merglen; J.-C. Minet; S. Minocchieri; M. Mönkhoff; A. Moser; V. Muehlethaler; A. Niederer; V. Pezzoli; N. Piol; K. Posfay Barbe; G. Ramos y Munoz; L. Reinhard; T. Riedel; H. Roten; C. Rudin; K. P. Rühs; M. Russo; P. Schillinger; V. Schlumbom; N. Schöbi; G. Simonetti; S. Stirnemann; F. Stollar; C. Stüssi; E. Süess; Z. Šufliarska; R. Tabin; M. Tomaske; R. Villiger; S. Wellmann; J. Wildhaber; K. Woll; M. Wopmann; B. Zanetti; G. Zeilinger; A. Zemmouri; U. Zimmermann; S.-A. Zoubir; and all the dedicated physicians for taking care of the patients and helping to complete the questionnaires.
We acknowledge administrative support by Daniela Beeli, data from SSSN by Tania Villeneuve, and co-designing of the study questionnaire with UH as well as database management by Damir Perisa (all Swiss Public Health Office, Berne). Thomas Erb and Nikola Stankovic, University of Basel Children’s Hospital, kindly calculated 95% confidence intervals for values in table 1.
Both authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.
1 Pertussis vaccines: WHO position paper - September 2015. Wkly Epidemiol Rec. 2015 Aug 28;90(35):433-58. English, French. PMID: 26320265.
2. Guiso N , Wirsing von König CH . Surveillance of pertussis: methods and implementation. Expert Rev Anti Infect Ther. 2016 Jul;14(7):657–67. https://doi.org/10.1080/14787210.2016.1190272
3. Forsyth KD , Tan T , von König CW , Heininger U , Chitkara AJ , Plotkin S . Recommendations to control pertussis prioritized relative to economies: A Global Pertussis Initiative update. Vaccine. 2018 Nov;36(48):7270–5. https://doi.org/10.1016/j.vaccine.2018.10.028
4. Heininger U , Weibel D , Richard JL . Prospective nationwide surveillance of hospitalizations due to pertussis in children, 2006-2010. Pediatr Infect Dis J. 2014 Feb;33(2):147–51. https://doi.org/10.1097/01.inf.0000435503.44620.74
5. Federal Office of Public Health Switzerland . Anpassung der Impfempfehlung gegen Pertussis: für Jugendliche, Säuglinge in Betreuungseinrichtungen und und schwangere Frauen. Bull BAG. 2013 Feb;9:118–23.
6. Zumstein J , Heininger U ; Swiss Paediatric Surveillance Unit . Clinical and Epidemiologic Characteristics of Pertussis in Hospitalized Children: A Prospective and Standardized Long-term Surveillance Study. Pediatr Infect Dis J. 2021 Jan;40(1):22–5. https://doi.org/10.1097/INF.0000000000002904
7. Brown LD , Cai TT , DasGupta A . Interval estimation for a binomial proportion. Stat Sci. 2001 May;16(2):101–33. https://doi.org/10.1214/ss/1009213286
8. Swiss Federal Statistical Office [Internet]. Basel: Demographic balance by age. [cited 2021 Apr 27]. Available from: https://www.pxweb.bfs.admin.ch/sq/7b29c6a9-6915-406c-9712-a5889194a53b
9. Federal Office of Public Health Switzerland . Das neue «2 + 1- Impfschema» zur Basisimpfung von Säuglingen gegen Diphtherie, Tetanus, Pertussis, Poliomyelitis, Haemophilus influenzae Typ b und Hepatitis B: eine Dosis weniger. Bull BAG. 2019 Mar;13:18–22.
10. Kuitunen I , Artama M , Mäkelä L , Backman K , Heiskanen-Kosma T , Renko M . Effect of Social Distancing Due to the COVID-19 Pandemic on the Incidence of Viral Respiratory Tract Infections in Children in Finland During Early 2020. Pediatr Infect Dis J. 2020 Dec;39(12):e423–7. https://doi.org/10.1097/INF.0000000000002845
11. Adlhoch C , Mook P , Lamb F , Ferland L , Melidou A , Amato-Gauci AJ , et al.; European Influenza Surveillance Network . Very little influenza in the WHO European Region during the 2020/21 season, weeks 40 2020 to 8 2021. Euro Surveill. 2021 Mar;26(11):2100221. https://doi.org/10.2807/1560-7917.ES.2021.26.11.2100221
12. Kitano T . The estimated burden of 15 vaccine-preventable diseases from 2008 to 2020 in Japan: A transition by the COVID-19 pandemic. J Infect Chemother. 2021 Oct;27(10):1482–8. https://doi.org/10.1016/j.jiac.2021.06.021
13. Federal Office of Public Health Switzerland [Internet]. Basel: Sentinella – Aktuelle Meldungen. [cited 2021 Jul 17]. Available from: https://www.bag.admin.ch/bag/de/home/zahlen-und-statistiken/zahlen-zu-infektionskrankheiten/sentinella---aktuelle-meldungen.html
14. Urwyler P , Heininger U . Protecting newborns from pertussis - the challenge of complete cocooning. BMC Infect Dis. 2014 Jul;14(1):397. https://doi.org/10.1186/1471-2334-14-397
15. Erb ML , Erlanger TE , Heininger U . Child-parent immunization survey: how well are national immunization recommendations accepted by the target groups? Vaccine X. 2019 Mar;1:100013. https://doi.org/10.1016/j.jvacx.2019.100013
16. Warfel JM , Zimmerman LI , Merkel TJ . Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci USA. 2014 Jan;111(2):787–92. https://doi.org/10.1073/pnas.1314688110
17. Amirthalingam G , Andrews N , Campbell H , Ribeiro S , Kara E , Donegan K , et al. Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet. 2014 Oct;384(9953):1521–8. https://doi.org/10.1016/S0140-6736(14)60686-3
18. Skoff TH , Blain AE , Watt J , Scherzinger K , McMahon M , Zansky SM , et al. Impact of the US Maternal Tetanus, Diphtheria, and Acellular Pertussis Vaccination Program on Preventing Pertussis in Infants <2 Months of Age: A Case-Control Evaluation. Clin Infect Dis. 2017 Nov;65(12):1977–83. https://doi.org/10.1093/cid/cix724
19. Romanin V , Acosta AM , Juarez MD , Briere E , Sanchez SM , Cordoba BL , et al. Maternal Vaccination in Argentina: Tetanus, Diphtheria, and Acellular Pertussis Vaccine Effectiveness During Pregnancy in Preventing Pertussis in Infants <2 Months of Age. Clin Infect Dis. 2020 Jan;70(3):380–7. https://doi.org/10.1093/cid/ciz217
20. Heininger U . Is There a Need for a Stand-alone Acellular Pertussis Vaccine? Pediatr Infect Dis J. 2018 Apr;37(4):359–60. https://doi.org/10.1097/INF.0000000000001767
21. Heininger U , Klich K , Stehr K , Cherry JD . Clinical findings in Bordetella pertussis infections: results of a prospective multicenter surveillance study. Pediatrics. 1997 Dec;100(6):E10. https://doi.org/10.1542/peds.100.6.e10
22. Farizo KM , Cochi SL , Zell ER , Brink EW , Wassilak SG , Patriarca PA . Epidemiological features of pertussis in the United States, 1980-1989. Clin Infect Dis. 1992 Mar;14(3):708–19. https://doi.org/10.1093/clinids/14.3.708
23. Winter K , Zipprich J , Harriman K , Murray EL , Gornbein J , Hammer SJ , et al. Risk Factors Associated With Infant Deaths From Pertussis: A Case-Control Study. Clin Infect Dis. 2015 Oct;61(7):1099–106. https://doi.org/10.1093/cid/civ472
Table S1Case definitions by age groups.
| Age category | Patients | Laboratory confirmed B. pertussis infection, clinical case definition fulfilled | Laboratory confirmed B. pertussis infection, clinical case definition not fulfilled | Clinical case definition fulfilled, no laboratory confirmation |
| N (%) | N (%) | N (%) | N (%) | |
| <1 month | 23 (10) | 22 (96) | 1 (4) | 0 (0) |
| 1 month | 63 (29) | 60 (95) | 3 (5) | 0 (0) |
| 2 months | 38 (17) | 30 (79) | 8 (21) | 0 (0) |
| 3–5 months | 48 (22) | 43 (90) | 3 (6) | 2* (4) |
| 6–11 months | 17 (8) | 15 (88) | 1 (6) | 1 (6) |
| 12–23 months | 10 (5) | 5 (50) | 4 (40) | 1 (10) |
| ≥2 years | 21 (10) | 17 (81) | 2 (10) | 2 (10) |
| Total | 220 (100) | 192 (87) | 22 (10) | 6 (3) |
* Including one patient with unknown duration of cough and physician diagnosed pertussis
Table S2Case counts by age categories, sex, and surveillance years.
| Age category | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | Total | Female | Incidence |
| N (%)* | N (%)* | N (%)* | N (%)* | N (%)* | N (%)* | N (%)* | N (%)* | N (%)** | N (%)*** | N+ | |
| <1 month | 6 (12) | 4 (12) | 3 (12) | 3 (7) | 0 (0) | 2 (13) | 5 (36) | 0 (0) | 23 (10) | 8 (35) | 40.8 |
| 1 month | 16 (31) | 9 (27) | 9 (36) | 9 (22) | 9 (29) | 5 (31) | 2 (14) | 4 (44) | 63 (29) | 26° (41) | 111.6 |
| <2 months (cumulative) | 22 (43) | 13 (39) | 12 (48) | 12 (29) | 9 (29) | 7 (44) | 7 (50) | 4 (44) | 86 (39) | 34 (40) | 76.2 |
| 2 months | 9 (18) | 4 (12) | 4 (16) | 11 (27) | 6 (19) | 1 (6) | 1 (7) | 2 (22) | 38 (17) | 16 (42) | 67.3 |
| 3–5 months | 13 (25) | 4 (12) | 4 (16) | 13 (32) | 8 (26) | 3 (19) | 3 (21) | 0 (0) | 48 (22) | 22 (46) | 28.3 |
| <6 months (cumulative) | 44 (86) | 21 (64) | 20 (80) | 36 (88) | 23 (74) | 11 (69) | 11 (79) | 6 (67) | 172 (78) | 72 (42) | 50.8 |
| 6–11 months | 1 (2) | 6 (18) | 3 (12) | 3 (7) | 1 (3) | 1 (6) | 2 (14) | 0 (0) | 17 (8) | 8 (47) | 5.0 |
| 12–23 months | 1 (2) | 1 (3) | 2 (8) | 1 (2) | 3 (10) | 1 (6) | 0 (0) | 1 (11) | 10 (5) | 5 (50) | 1.5 |
| ≥2 years | 5 (10) | 5 (15) | 0 (0) | 1 (2) | 4 (13) | 3 (19) | 1 (7) | 2 (22) | 21 (10) | 10 (48) | 0.2 |
| Total** | 51 (23) | 33 (15) | 25 (11) | 41 (19) | 31 (14) | 16 (7) | 14 (6) | 9 (4) | 220 (100) | 95 (43) | 2.1 |
| Extrapolated Cases by Swiss Sentinel System§,** | 10’140 (19) | 9’391 (18) | 7’001 (13) | 8’313 (15) | 8’873 (17) | 3’995 (7) | 4’374 (8) | 1’549 (3) | 53636 (100) | Not reported | – |
* Percentage of all reported cases in that year
** Percentage of total surveillance period 2013–2020
*** Percentage of all reported cases in that age group
° Information not available in one patient
+ Per 100’000 children in this age category
§ Personal Communication Damir Perisa and Tania Villeneuve (Federal Office of Public Health, Switzerland, March 16, 2021)
Table S3Duration from onset of signs and symptoms to hospitalisation (in days).
| Age category | Patients N (%) | Mean | Median | Range | Duration unknown |
| <1 month | 23 (10) | 5.4 | 4.5 | 1–16 | 1 (4) |
| 1 month | 63 (29) | 10.5 | 9 | 1–48 | 3 (5) |
| 2 months | 38 (17) | 10.7 | 9 | 1–36 | 0 (0) |
| 3–5 months | 48 (22) | 11.8 | 11.5 | 3–29 | 0 (0) |
| 6–11 months | 17 (8) | 14.8 | 11.5 | 4–43 | 1 (6) |
| 12–23 months | 10 (5) | 14.4 | 16 | 1–33 | 1 (10) |
| ≥2 years | 21 (10) | 14.2 | 14.5 | 0–31 | 1 (5) |
| Total | 220 (100) | 11.1 | 10 | 0–48 | 7 (3) |
Table S4Signs and symptoms by age categories.*
| Age category | Patients | Paroxysmal cough | Rhinitis | Post-tussive vomiting | Whooping | Fever (≥38 °C) | Others |
| N (%) | N/N known (%) | N/N known (%) | N/N known (%) | N/N known (%) | N/N known (%) | N/N known (%) | |
| <1 month | 23 (10) | 22/23 (96) | 18/23 (78) | 15/22 (68) | 9/22 (41) | 2/23 (9) | 11/23 (4) |
| 1 month | 63 (29) | 62/63 (98) | 41/63 (65) | 29/61 (48) | 15/59 (25) | 5/63 (8) | 21,2/63 (3) |
| 2 months | 38 (17) | 37/38 (97) | 25/37 (68) | 14/38 (37) | 9/37 (24) | 5/37 (14) | 12/38 (3) |
| 3–5 months | 48 (22) | 48/48 (100) | 28/46 (61) | 17/48 (35) | 10/47 (21) | 6/47 (13) | 42,3,4/48 (8) |
| 6–11 months | 17 (8) | 17/17 (100) | 11/17 (65) | 8/16 (50) | 4/14 (29) | 4/17 (24) | 0/17 (0) |
| 12–23 months | 10 (5) | 7/10 (70) | 8/10 (80) | 2/10 (20) | 1/10 (10) | 5/10 (50) | 13/10 (10) |
| ≥2 years | 21 (10) | 20/21 (95) | 10/20 (50) | 7/21 (33) | 4/19 (21) | 9/21 (43) | 31,5/21 (14) |
| Total | 220 (100) | 213/220 (97) | 141/216 (65) | 92/216 (43) | 52/208 (25) | 36/218 (17) | 12/220 (5) |
* Presence of signs and symptoms not in all patients known.
1 Feeding difficulties
2 Bradycardia
3 Exanthema
4 Reduced appetite, hypoxaemia
5 Aggressive behavior, nausea, pain
Table S5Antibiotic treatment.
| Age category | Patients | Clarithromycin | Azithromycin | Macrolides and/or other antibiotics 1 | Treated but drug unknown | No antibiotic treatment | Unknown if treated |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| <1 month | 23 (10) | 9 (39) | 10 (43) | 4 (17) | 0 (0) | 0 (0) | 0 (0) |
| 1 month | 63 (29) | 38 (60) | 23 (37) | 1 (2) | 1 (2) | 0 (0) | 0 (0) |
| 2 months | 38 (17) | 26 (70) | 8 (22) | 3 (8) | 0 (0) | 0 (0) | 1 (3) |
| 3–5 months | 48 (22) | 34 (72) | 11 (23) | 0 (0) | 0 (0) | 2 (4) | 1 (2) |
| 6–11 months | 17 (8) | 12 (71) | 4 (24) | 0 (0) | 0 (0) | 1 (6) | 0 (0) |
| 12–23 months | 10 (5) | 6 (60) | 2 (20) | 0 (0) | 1 (10) | 1 (10) | 0 (0) |
| ≥2 years | 21 (10) | 10 (48) | 6 (29) | 2 (10) | 1 (5) | 2 (10) | 0 (0) |
| Total | 220 (100) | 135 (62) | 64 (29) | 10 (5) | 3 (1) | 6 (3) | 2 (1) |
1 Usually other antibiotic was followed by a macrolide when a diagnosis of pertussis had been made
Table S6Intensive care and respiratory support.
| Age category | Patients | Intensive care | Duration of intensive care (in days) | Intubation | Duration of intubation (in days) | CPAP | Duration of CPAP (in days) | Oxygen substitution |
| N (%) | N (%) | Mean/median (range) | N (%) | Mean/median (range) | N (%) | Mean/median (range) | N (%) | |
| <1 month | 23 (10) | 12 (52) | 12.5/9 (1–39) | 41 (17) | 4/4 (2–6) | 3 (13) | 6/5 (1–12) | 7 (30) |
| 1 month | 63 (29) | 12 (19) | 7.7/7 (2–19) | 1 (2) | 2/2 (2) | 32 (5) | 4.7/5 (2–7) | 8 (13) |
| 2 months | 38 (17) | 3 (8) | 7.7/7 (2–14) | 2 (5) | 6.5/6.5 (4–9) | 0 (0) | 0/0 (0) | 4 (11) |
| 3–5 months | 48 (22) | 1 (2) | 7/7 (7) | 0 (0) | 0/0 (0) | 0 (0) | 0/0 (0) | 1 (2) |
| 6–11 months | 17 (8) | 0 (0) | 0/0 (0) | 0 (0) | 0/0 (0) | 0 (0) | 0/0 (0) | 2 (12) |
| 12–23 months | 10 (5) | 1 (10) | 8/8 (8) | 0 (0) | 0/0 (0) | 13 (10) | 8/8 (8) | 2 (20) |
| ≥ 2 years | 21 (10) | 0 (0) | 0/0 (0) | 0 (0) | 0/0 (0) | 0 (0) | 0/0 (0) | 1 (5) |
| Total | 220 (100) | 29 (13) | 9.7/7 (1–39) | 7 (3) | 4.4/4 (2–9) | 7 (3) | 5.7/5 (1–12) | 25 (11) |
1 Including one patient with Extracorporeal Membrane Oxygenation (ECMO)
2 Including one patient with Biphasic Positive Airway Pressure (BiPAP)
3 This patient had BiPAP
Table S7Source of infection*
| Age category | Patients | Parent(s) only | Sibling(s) only | Sibling(s) + parent(s) | Adults other than household members | Children other than household members | Source unknown |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| <1 month | 23 (10) | 9 (45) | 2 (10) | 7 (35) | 3 (15) | 1 (5) | 3 (13) |
| 1 month | 63 (29) | 20 (42) | 7 (15) | 15 (31) | 7 (15) | 8** (17) | 15 (24) |
| 2 months | 38 (17) | 11 (39) | 4 (14) | 8 (29) | 7 (25) | 3 (11) | 10 (26) |
| 3–5 months | 48 (22) | 10 (26) | 12 (32) | 10 (26) | 8 (21) | 1 (3) | 10 (21) |
| 6–11 months | 17 (8) | 5 (50) | 2 (20) | 3 (30) | 1 (10) | 1 (10) | 7 (41) |
| 12–23 months | 10 (5) | 2 (33) | 2 (33) | 2 (33) | 2 (33) | 0 (0) | 4 (40) |
| ≥2 years | 21 (10) | 3 (25) | 4 (33) | 2 (17) | 1 (8) | 4 (33) | 9 (43) |
| Total | 220 (100) | 60 (37) | 33 (20) | 47 (29) | 29 (18) | 18 (11) | 58 (26) |
* Some patients had more than one potential source of infection
** Including two patients with nosocomially acquired pertussis
Table S895% confidence intervals for figure 2.
| Lower 95%CI | Upper 95%CI | |
| 2013 Q1 | 0.2 | 1.1 |
| 2013 Q2 | 0.3 | 1.2 |
| 2013 Q3 | 0.7 | 1.9 |
| 2013 Q4 | 0.65 | 1.7 |
| 2014 Q1 | 0.3 | 1.3 |
| 2014 Q2 | 0.05 | 0.7 |
| 2014 Q3 | 0.5 | 1.6 |
| 2014 Q4 | 0 | 0.5 |
| 2015 Q1 | 0.1 | 0.8 |
| 2015 Q2 | 0 | 0.6 |
| 2015 Q3 | 0.2 | 1.0 |
| 2015 Q4 | 0.1 | 0.9 |
| 2016 Q1 | 0.05 | 0.7 |
| 2016 Q2 | 0.3 | 1.2 |
| 2016 Q3 | 0.5 | 1.5 |
| 2016 Q4 | 0.5 | 1.5 |
| 2017 Q1 | 0.2 | 1.0 |
| 2017 Q2 | 0.1 | 0.8 |
| 2017 Q3 | 0.2 | 1.1 |
| 2017 Q4 | 0.2 | 1.0 |
| 2018 Q1 | 0 | 0.2 |
| 2018 Q2 | 0.05 | 0.7 |
| 2018 Q3 | 0.05 | 0.7 |
| 2018 Q4 | 0.05 | 0.7 |
| 2019 Q1 | 0 | 0.6 |
| 2019 Q2 | 0.05 | 0.7 |
| 2019 Q3 | 0 | 0.4 |
| 2019 Q4 | 0 | 0.5 |
| 2020 Q1 | 0.1 | 0.8 |
| 2020 Q2 | 0 | 0.5 |
| 2020 Q3 | 0 | 0 |
| 2020 Q4 | 0 | 0 |