Resurrecting historical lessons from tuberculosis research on airborne transmission relevant to SARS-CoV-2
DOI: https://doi.org/10.4414/SMW.2021.w30096
Hans L.
Riedera, Marcel
Zwahlenb
aTuberculosis Consultant Services, Kirchlindach, Switzerland
bInstitute of Social and Preventive Medicine, University of Bern, Switzerland
Introduction
For those working in tuberculosis control, the reports and accompanying visuals from Bergamo, Italy, in early 2020 were not only horrifying, they were incredible. Why were those who work in tuberculosis control astonished by these images? They showed gowned, gloved healthcare workers wearing transparent face shields and insufficiently or ill-fitting surgical masks caring for their patients. These health professionals protected themselves against transmission via droplets and fomites, but were insufficiently protected with respirators against airborne SARS-CoV-2 transmission [1].
In Science, Wang and colleagues recently summarised existing evidence and “critical knowledge gaps” about the airborne transmission of respiratory viruses [2]; however, much has been known for quite some time in the case of tuberculosis. As evidence supporting the primordial quantitative role of aerosols in the transmission of SARS-CoV-2 continues to mount, fomites and their comparatively negligible role in transmitting the virus augments current understandings about SARS-CoV-2. Yet these findings resurrect long-held understandings about Mycobacterium tuberculosis transmission.
In this Viewpoint, we complement the comprehensive review of Wang et al. [2] by reviving three critical, historical contributions from the 1930s and 1960s about the airborne transmission of M. tuberculosis: characteristics of droplet nuclei; the production of droplets; and the deposition of inhaled particles in the respiratory tract. We suggest these are relevant to the ongoing discourse about the airborne transmission of SARS-CoV-2.
Characteristics of droplet nuclei
In 1882, Robert Koch suspected that M. tuberculosis was transmitted through an infectious patient’s sputum [3]. In 1899, Carl Flügge identified droplets expelled from the respiratory tract as the source of M. tuberculosis transmission [4, 5]. However, the experimental demonstration and the physics behind generating droplet nuclei from droplets expelled from the respiratory tract had to await research from William F. Wells in 1934 [6]. What is now often called the “Wells curve” (fig. 1) summarises the behaviour of droplets of varying sizes from >0 to 200 μm that are expelled from the respiratory tract in non-saturated air while falling.

Figure 1 Falling and evaporation times of droplets of varying diameters. On the left side the red lines indicate that the time for a droplet (nucleus) to cease to exist as a result of evaporation is identical to the falling time. This time is shorter than the falling time to the ground without evaporation. On the right side the blue lines indicate that the falling time is shorter for a larger droplet (nucleus), reaching the ground without evaporation and earlier than a smaller droplet (nucleus). 1 μm [micrometer] = 0.001 mm
Adapted reproduction from Wells [6] with permission of the publisher (Oxford University Press on behalf of Johns Hopkins University).
Depending on their size and the water saturation level of the air, droplets evaporate during falling. The smallest droplets never reach the ground. If a droplet contained a microorganism such as M. tuberculosis (a rod with an approximate length of 3–5 μm and diameter of 0.3–0.5 μm), the remaining droplet nucleus consists of just that organism or a clump of them. Notably, there is nothing in the report of Wells about the size defined as “droplet nucleus.” In this specific example (given the chosen water saturation and the height of fall), evaporation toward droplet nuclei ranges from >0 to 130 μm in size. (Wells also gives examples for varying air saturation.) The “life” (evaporation time) of droplets in unsaturated air is proportional to the square of their diameter, so rain drops can fall all the way from the sky, yet the smallest ones evaporate instantly [6]. Thus, Wells defined what today we call the generation of an aerosol, and he was not fixed on any specific constituent size of the individual droplet nuclei. Remarkably, Wang and collaborators suggest that sizes of up to 100 μm should be the formative quanta of an aerosol [2].
The production of droplets
In the 1960s, Robert G. Loudon studied respiratory manoeuvres, the force they produce, and the quantity and quality of droplets expelled by these manoeuvres [7, 8]. In ascending order of force, human respiratory manoeuvres are breathing, talking, singing, coughing and sneezing. A larger force produces (1) larger quantities of droplets, and (2) among those droplets, a larger proportion of a smaller size (fig. 2) [8].
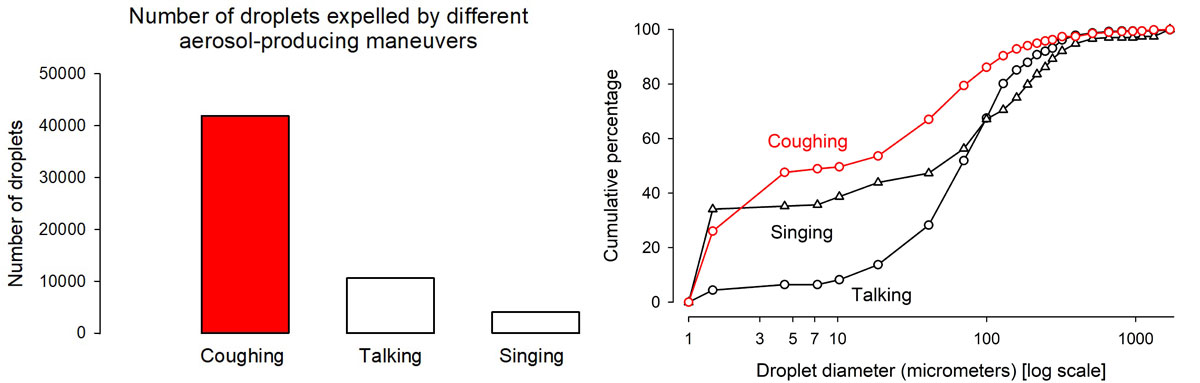
Figure 2 Comparative quantity of droplets produced by three respiratory manoeuvres (left) and cumulative proportional distribution of the sizes of expelled droplets by type of respiratory manoeuvre (right). Figure constructed from Loudon’s data [8], used with the permission of the publisher. The authors, editors, and The American Thoracic Society are not responsible for errors or omissions in adaptations. 1 μm [micrometer] = 0.001 mm
The deposition of inhaled particles in the respiratory tract
Often omitted from discussions about droplet nuclei size (and from where the often-mentioned size of 5 μm may originate) are elements that are historically rooted in M. tuberculosis and its port of entry. M. tuberculosis is ill-suited to implant itself in the upper respiratory tract (i.e., mouth, larynx and pharynx), nor in the trachea, or the bronchial tree. In the 1960s Theodore F. Hatch (fig. 3) summarised the then current knowledge showing that large particles can settle successfully in the upper respiratory tract, but only particles of sizes less than 10 μm have a reasonable probability to reach the deeper spaces of the lung [9].
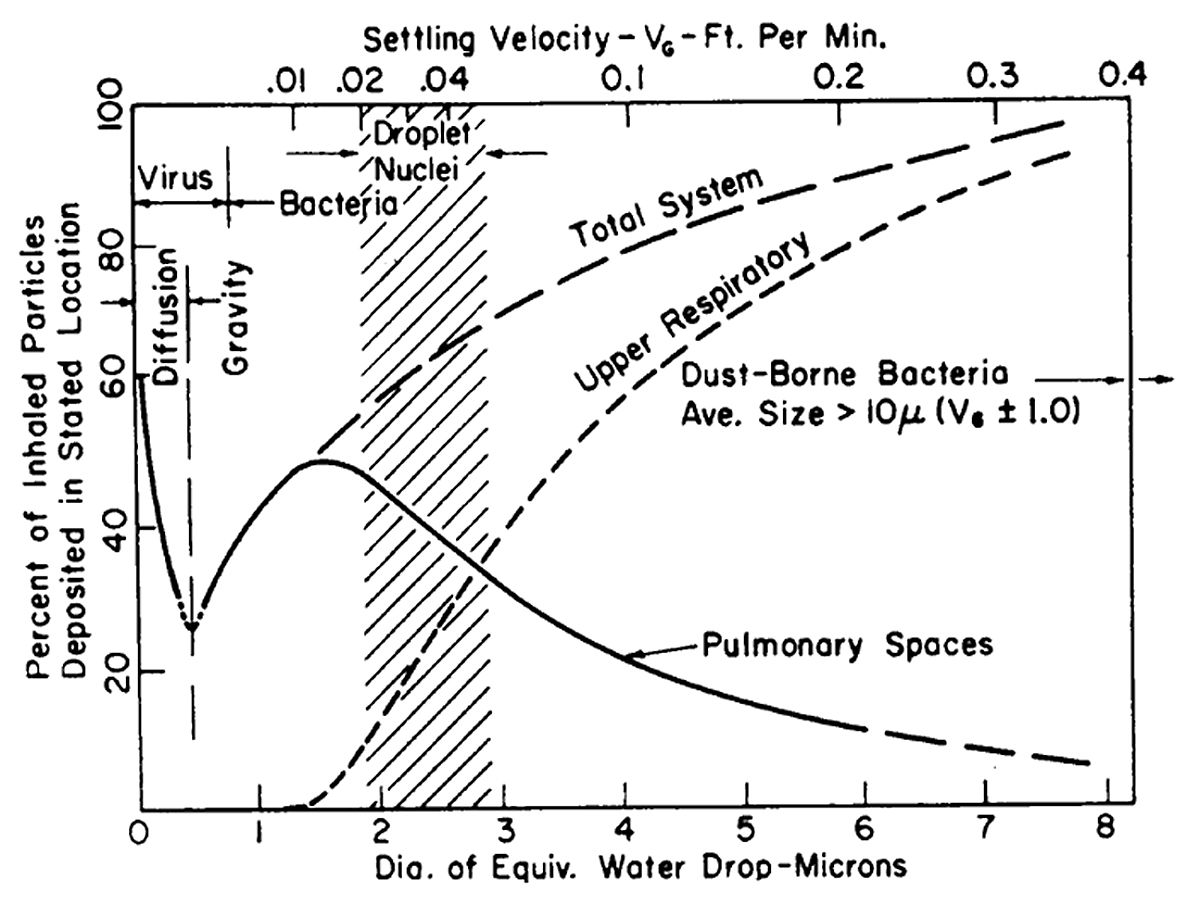
Figure 3 Total and regional deposition of inhaled particles, in relation to the aerodynamic particle size. Reproduced from Hatch [9] with permission from the American Society of Microbiology. 1 micron = 1 μm [micrometer] = 0.001 mm
Simply, M. tuberculosis must enter an alveolus, adhere to the cell wall and wait like a Trojan horse to be picked up by an alveolar macrophage. Then it must be transported into the lung parenchyma to initiate its intracellular replication. To be inhalable into an alveolus, there is an upper anatomical size restriction.
If M. tuberculosis droplet nuclei were not sized within 3–5 μm, they would not have been that successful as an obligate airborne pathogen that relies on access to the host’s alveolar cell wall. If another pathogen (either smaller or larger) can implant itself in the upper respiratory tract, there is no reason why transmission could not also be airborne to be successful, as so convincingly shown by Wang et al. for viruses [2].
SARS-CoV-2 can be isolated in abundance already in the pre-clinical stage [10]. Although viral presence is a prerequisite, it is not necessarily the sole sufficient condition for successful SARS-CoV-2 transmission, as the dependence on the viral load for successful transmission demonstrates [11]. The physics of M. tuberculosis droplet production tell us that coughing produces more droplets than breathing or talking (i.e., the most common forces that asymptomatic people mount). Thus, asymptomatically infected people likely produce fewer droplets laden with SARS-CoV-2 than symptomatic patients who cough.
As we increasingly learn to better understand transmission of SARS-CoV-2, we may very well take notice of the critical groundwork already laid by our forefathers in the field of tuberculosis. A comprehensive discussion of the history and current discourse about airborne transmission has been provided by Randall and colleagues [12].
Acknowledgement
We thank Kristin Bivens for editorial assistance.
Hans L. Rieder
Tuberculosis Consultant Services
Jetzikofenstrasse 12
CH-3038 Kirchlindach
tbrieder[at]tbrieder.org
References
1.
Fennelly KP
. Particle sizes of infectious aerosols: implications for infection control. Lancet Respir Med. 2020 Sep;8(9):914–24. https://doi.org/10.1016/S2213-2600(20)30323-4
2.
Wang CC
,
Prather KA
,
Sznitman J
,
Jimenez JL
,
Lakdawala SS
,
Tufekci Z
, et al.
Airborne transmission of respiratory viruses. Science. 2021 Aug;373(6558):eabd9149. https://doi.org/10.1126/science.abd9149
3.
Koch R
. The aetiology of tuberculosis. A translation by Berna Pinner and Max Pinner. With an introduction by Allen K. Krause. Am Rev Tuberc. 1932;25:284–323.
4.
Flügge C
. Die Verbreitung der Phthise durch staubförmiges Sputum und durch beim Husten verspritzte Tröpfchen. Med Microbiol Immunol (Berl). 1899;30(1):107–24. https://doi.org/10.1007/BF02198683
5.
Flügge C
. Ways of infection in the case of tuberculosis. Tuberculosis (Berlin). 1906;5:376–9.
6.
Wells WF
. On air-borne infection. Study II. Droplets and droplet nuclei. Am J Hyg. 1934;20:611–8.
7.
Loudon RG
,
Roberts RM
. Droplet expulsion from the respiratory tract. Am Rev Respir Dis. 1967 Mar;95(3):435–42. https://doi.org/10.1164/arrd.1967.95.3.435
8.
Loudon RG
,
Roberts RM
. Singing and the dissemination of tuberculosis. Am Rev Respir Dis. 1968 Aug;98(2):297–300. https://doi.org/10.1164/arrd.1968.98.2.297
9.
Hatch TF
. Distribution and deposition of inhaled particles in respiratory tract. Bacteriol Rev. 1961 Sep;25(3):237–40. https://doi.org/10.1128/br.25.3.237-240.1961
10.
Murata T
,
Sakurai A
,
Suzuki M
,
Komoto S
,
Ide T
,
Ishihara T
, et al.
Shedding of viable virus in asymptomatic SARS-CoV-2 carriers. MSphere. 2021 May;6(3):e00019–00021. https://doi.org/10.1128/mSphere.00019-21
11.
Marks M
,
Millat-Martinez P
,
Ouchi D
,
Roberts CH
,
Alemany A
,
Corbacho-Monné M
, et al.
Transmission of COVID-19 in 282 clusters in Catalonia, Spain: a cohort study. Lancet Infect Dis. 2021 May;21(5):629–36. https://doi.org/10.1016/S1473-3099(20)30985-3
12.
Randall K
,
Ewing ET
,
Marr LC
,
Jimenez JL
,
Bourouiba L
. How did we get here: what are droplets and aerosols and how far do they go? A historical perspective on the transmission of respiratory infectious diseases. SSRN, 2021. (Accessed 2021 October 24, at https://ssrn.com/abstract=3829873)


