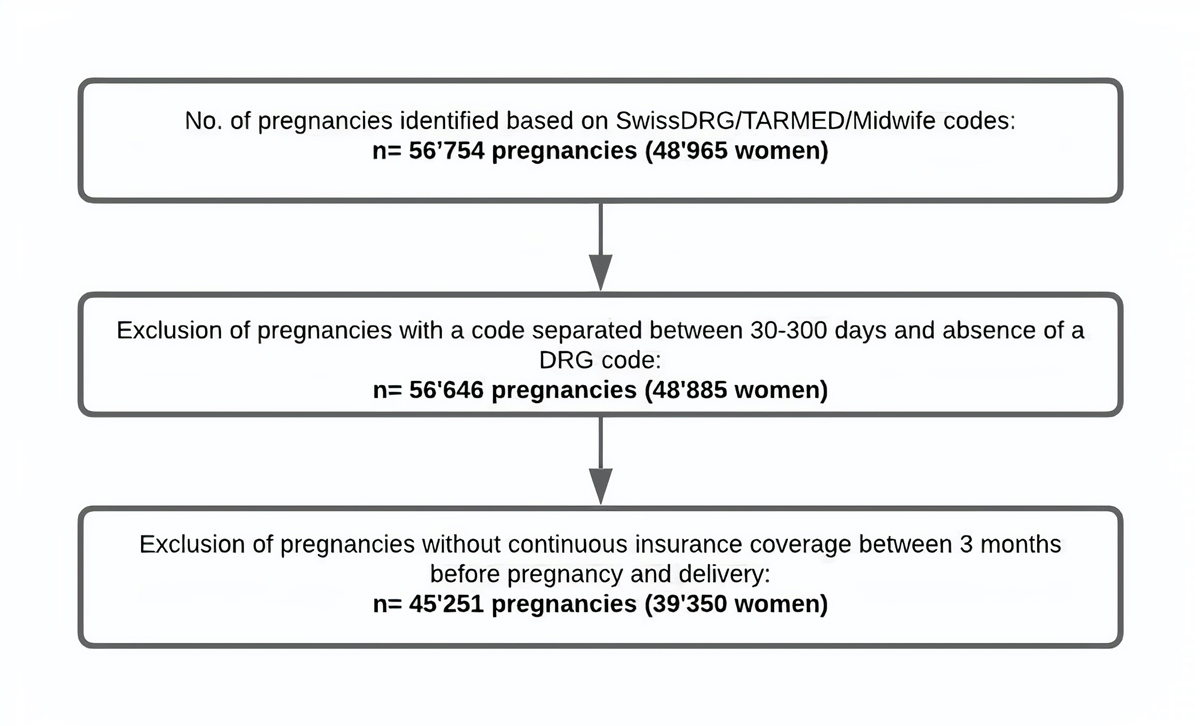
Figure 1 Flowchart of the study population (crude numbers).
DOI: https://doi.org/10.4414/SMW.2021.w30048
Pregnant women are typically excluded from interventional trials. Therefore, the safety of most drugs during pregnancy is not well characterised [1–4]. Nevertheless, many women require treatment of acute conditions, chronic illnesses, or obstetric complications during pregnancy. A study based on French national claims data reported that 90% of women filled at least one prescription for a drug during pregnancy between 2010 and 2013 [5], whereas 62% of pregnant women in Norway billed at least one drug to national health insurance between 2005 and 2015 [6]. In a multinational web-based survey [7], 81.2% of pregnant women reported using at least one drug, prescribed or over the counter (OTC), during pregnancy between 2011 and 2012. A total of 618 Swiss women took part in the survey, of whom 82.8% reportedly used at least one drug during pregnancy. However, these results may not be representative due to volunteer bias. The most frequently reported drugs were similar in all studies [5–7] and included analgesics (mainly paracetamol), antibiotics, drugs for gastro-oesophageal reflux disease and drugs for functional gastrointestinal disorders (mainly treatment of nausea). Iron, vitamins and folic acid were the most commonly used supplements.
We aimed to evaluate the utilisation of prescription drugs dispensed in outpatient care during pregnancy in Switzerland, using the data of the Helsana claims database. This study focuses on the utilisation of drugs (including potentially teratogenic/fetotoxic drugs) to treat acute conditions that frequently occur during pregnancy, such as pain of different origins, infections, gastro-oesophageal reflux, nausea and vomiting, and constipation. Utilisation of drugs to treat chronic illnesses will be evaluated in a separate study.
We conducted a descriptive study using data from the Swiss Helsana claims database between January 2014 and December 2018. The Helsana claims database includes data of approximately 1.1 million individuals from all 26 cantons in Switzerland (approximately 15% of the Swiss population) who are insured with Helsana mandatory insurance.
Recorded information includes outpatient medical encounters coded by the tariff system (TARMED), information on inpatient stays coded as Swiss Diagnosis Related Group (SwissDRG) codes as well as billing codes submitted by outpatient midwives. Furthermore, all reimbursed claims for drugs (recorded as Anatomical Therapeutic Chemical [ATC] codes) dispensed in outpatient care are captured [8].
This study population of pregnant women has been described previously [9]. To identify pregnancies, we captured all inpatient and outpatient deliveries between 2014 and 2018 using the SwissDRG, TARMED and midwife billing codes. Delivery codes that were recorded within 30 days following the first recorded delivery code were considered as pertaining to the same delivery and the date of the first record was defined as the delivery date. A delivery code recorded more than 300 days after an initial delivery code was considered as pertaining to a subsequent delivery. When two subsequent codes were separated by 30 to 300 days, the date of delivery was set at the SwissDRG code, whereas women (n = 80, crude number) were excluded if no SwissDRG code was recorded (fig. 1, flowchart of the unextrapolated study population). Thus, the same woman may have contributed several pregnancies to the cohort. Deliveries of twins were identified in the same way as deliveries of singletons and were counted as a single pregnancy.

Figure 1 Flowchart of the study population (crude numbers).
Because the beginning of pregnancy is not recorded in claims data, we used an algorithm to estimate the date of the last menstrual period (LMP), which was validated in US administrative claims data [10]. If a billing code indicating preterm delivery (<37 gestational weeks, see appendix for respective SwissDRG codes) was recorded, we defined LMP as 245 days before the delivery date. For all other pregnancies, LMP was defined as 270 days before the delivery date. Each pregnancy trimester was defined as a 90-day period and in case of prematurity, the third trimester was shortened (i.e., trimester 1: LMP until 89 days after LMP; trimester 2: 90 days after LMP until 179 days after LMP; trimester 3: 180 days after LMP until delivery). We also defined a pre-pregnancy period, which started 90 days before LMP and ended one day before LMP (LMP–90 until LMP–1). We excluded women (n = 9535, crude number) who were not continuously covered by mandatory insurance at Helsana between the date of their last menstrual period and delivery (fig. 1).
We extracted maternal age at delivery, the year of delivery and the mode of delivery (caesarean section vs vaginal delivery, see appendix for respective SwissDRG, TARMED and Midwife codes).
We defined drug groups to treat acute conditions frequently associated with pregnancy using the ATC classification. Included drug groups were analgesics (opioids N02A, other analgesics N02B, antimigraine preparations N02C, and nonsteroidal anti-inflammatory drugs [NSAIDs] M01A), systemic antibiotics (J01), drugs for gastro-oesophageal reflux (antacids A02A, proton pump inhibitors [PPIs] A02BC, H2 inhibitors A02BA, and others A02BX), nausea drugs (anti-emetics A04A and propulsives A03FA, antihistamines are not reimbursed), and laxatives (A06).
Within each drug group, we identified active substances dispensed to >1% of pregnancies. We further identified dispensations of active substances, which are potentially teratogenic or fetotoxic, or which have been associated with adverse events in the newborn. These substances were identified using the online teratogen information platforms ‘Le CRAT’ (Centre de Référence sur les Agents Tératogènes; French) [11] and ‘Embryotox’ (German) [12]. Additionally, we screened all warnings issued by Swissmedic (Swiss authorisation and supervisory authority for drugs and medical products [13]) between 2008 and 2020.
Finally, we evaluated the most prescribed supplements during pregnancy: folic acid (including multivitamins), intravenous iron and oral iron (not including multivitamins), vitamin D, and magnesium (not including multivitamins).
We quantified the prevalence of exposure to different drug substances and supplements overall, during each pregnancy trimester, and during pre-pregnancy. Exposure to potentially teratogenic or fetotoxic substances was quantified during specific risk periods. Prevalence of exposure was defined as the proportion of pregnancies during which at least one prescription was filled for the respective active substance, divided by the total number of enrolled pregnancies during the respective time period.
Prevalence of exposure is presented as absolute numbers per 100 pregnancies and is presented separately for all drug groups and for all active substances dispensed in >1% of pregnancies (1% cut off does not apply to potentially teratogenic and fetotoxic substances).
To present results that are representative of the overall Swiss population, all results were extrapolated/weighted relative to the demographic distribution of the overall female population of Switzerland, taking into account calendar year, canton, age, and the sex distribution within cantons.
The weighted sums (extrapolated number of pregnancies), weighted mean and standard deviation of age were calculated using the survey package in R [14].
All data are anonymous, and all analyses were conducted by the Helsana Department of Health Sciences using the statistical programming language R (version 3.6.1, [15]).
Ethics committee approval was not required because data used for the study were anonymous.
We identified an extrapolated population of 369,371 pregnancies from 323,632 women, with a weighted mean maternal age at delivery of 32.0 years (standard deviation 5.1 years). In total, 33.7% of all pregnancies resulted in caesarean section (table 1, unextrapolated pregnancy cohort is displayed in table S1 in the appendix).
Table 1Description of the extrapolated/weighted study population.
| Year | No. of extrapolated deliveries in our study population | Extrapolated mean age at delivery in the cohort (weighted SD) | Mean maternal age at delivery ins Switzerland (BfS) | Extrapolated percentage of caesarean sections in the cohort (%, 95% CI) | Percentage of caesarean section ins Switzerland (BfS) |
| 2014 | 71,933 | 31.96 (5.04) | 31.7 | 34.4 (33.4–35.5) | 33.7 |
| 2015 | 71,844 | 31.97 (5.15) | 31.8 | 34.3 (33.3–35.4) | 33.3 |
| 2016 | 74,149 | 31.93 (5.11) | 31.8 | 33.4 (32.4–34.5) | 33.2 |
| 2017 | 79,610 | 32.06 (5.14) | 31.9 | 33.4 (32.4–34.6) | 32.3 |
| 2018 | 71,836 | 32.15 (5.00) | 32.0 | 33.1 (32.0–34.2) | 32.1 |
CI: confidence interval; BfS: Bundesamt für Statistik, Swiss Federal Statistical Office; SD: standard deviation
Analgesics were the most frequently recorded drug group, with dispensing of prescribed analgesics during 34.5% (95% CI 33.9–35.0%) of pregnancies. The most frequently dispensed active substance was paracetamol (30.3%, 95% CI 29.8–30.8% of pregnancies) (fig. 2).
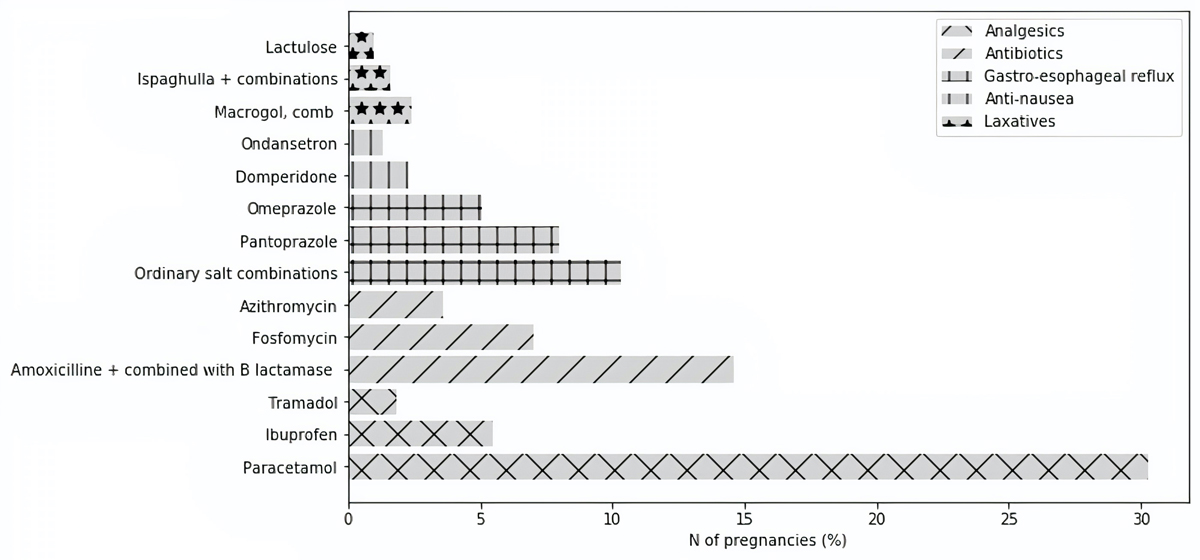
Figure 2 Exposure prevalence to the three most prescribed outpatient active substances per drug group during pregnancy (T1–T3) (extrapolated numbers).
NSAIDs were dispensed in 8.6% (8.3–8.8%) of pregnancies, and 1.3% (1.2–1.4%) of pregnancies had a claim for an NSAID after week 24 (table 2, unextrapolated numbers are displayed in table S2). Ibuprofen was the most commonly dispensed NSAID (5.5%, 95% CI 5.3–5.7%). Opioids were recorded in 2.6% (2.4–2.8%) of pregnancies overall, with tramadol being the most frequently dispensed opioid (1.8%, 95% CI 1.6–1.9%). In total, 1.3% (1.2–1.4%) of pregnancies had a recorded claim for an opioid in trimester 3 (table 2).
Table 2Exposure to potentially teratogenic or fetotoxic drugs during risk period and associated potential risks (extrapolated numbers).
| Potentially teratogenic or fetotoxic drugs (ATC code) | Warnings regarding use during critical period | Risk period | Pregnancies exposed during risk period during study period (n) | Pregnancies exposed during risk period during study period (%, 95% CI) |
| NSAIDs (M01A) | Premature closure of ductus arteriosus and renal toxicity [19] | After week 24 | 4657 | 1.3 (1.2–1.4) |
| Opioids (N02A) | Neonatal abstinence syndrome and respiratory distress [20–23] | Trimester 3 | 4747 | 1.3 (1.2–1.4) |
| Trimethoprim/sulphonamide (J01E) | Risk of neural tube defects [25, 26] | Trimester 1 | 1257 | 0.3 (0.3–0.4) |
| Quinolone (J01M) | Cartilage and bone damage in animal studies but not found in human studies [29, 30] | Trimester 1 | 2093 | 0.6 (0.5–0.6) |
| Tetracycline (J01A) | Tooth staining [27, 28] | Trimester 2; trimester 3 | 185; 93 | 0.1 (0.0–0.1); 0.0 (0.0–0.0) |
| Ondansetron (A04AA01) | Potentially increased risk of orofacial clefts [37] | Trimester 1 | 3726 | 1.0 (0.9–1.1) |
| Contact laxatives (A06AB); especially senna (anthraquinone derivative, A06AB06) | Theoretical risk of intestinal and uterine contractions [42] | Trimester 3 | 943; 0 | 0.3 (0.2–0.3); 0.0 (0.0–0.0) |
ATC: anatomic therapeutic chemical; CI: confidence interval: NSAID: nonsteroidal anti-inflammatory drug
The prevalence of exposure to antibiotics in outpatient care during pregnancy was 26.3% (25.8–26.8%) with amoxicillin being the most frequently dispensed antibiotic (14.6%, 95% CI 14.2–14.9%) (table 3, unextrapolated numbers are displayed in table S3).
Table 3Exposure prevalence to different drug groups and active substances during pregnancy overall and within trimester of pregnancy and pre-pregnancy separately (extrapolated numbers).
| ATC code | Drug substance | Pre-pregnancy | Trimester 1 | Trimester 2 | Trimester 3 | Trimesters 1 to 3 | |||||
| n (%) | 95% CI | n (%) | 95% CI | n (%) | 95% CI | n(%) | 95% CI | n (%) | 95% CI | ||
| N02+M01A | Analgesics | 67,143 (18.2) | 17.8–18.6 | 63,289 (17.1) | 16.7–17.5 | 61,167 (16.6) | 16.2–17.0 | 48,105 (13.0) | 12.7–13.4 | 127,353 (34.5) | 33.9–35.0 |
| N02B | Other analgesics | 39,288 (10.6) | 10.3–11.0 | 52,233 (14.1) | 13.8–14.5 | 54,460 (14.7) | 14.4–15.1 | 43,312 (11.7) | 11.4–12.1 | 113,435 (30.7) | 30.2–31.2 |
| N02BE01 | Paracetamol | 34,415 (9.3) | 9.0–9.6 | 50,470 (13.7) | 13.3–14.0 | 54,071 (14.6) | 14.3–15.0 | 43,045 (11.7) | 11.3–12.0 | 111,869 (30.3) | 29.8–30.8 |
| N02BB02 | Metamizole | 9447 (2.6) | 2.4–2.7 | 3008 (0.8) | 0.7–0.9 | 627 (0.2) | 0.1–0.2 | 627 (0.2) | 0.1–0.1 | 3896 (1.1) | 1.0–1.2 |
| M01A | NSAIDs | 45,169 (12.2) | 11.9–12.6 | 19,216 (5.2) | 5.0–5.4 | 11,205 (3.0) | 2.9–3.2 | 4134 (1.1) | 1.0–1.2 | 31,586 (8.6) | 8.3–8.8 |
| M01AE01 | Ibuprofen | 26,230 (7.1) | 6.8–7.4 | 12,057 (3.3) | 3.1–3.4 | 7589 (2.1) | 1.9–2.1 | 2132 (0.6) | 0.5–0.7 | 20,255 (5.5) | 5.3–5.7 |
| M01AG01 | Mefenamic acid | 8’848 (2.4) | 2.2–2.5 | 3286 (0.9) | 0.8–1.0 | 2066 (0.6) | 0.5–0.6 | 1093 (0.3) | 0.2–0.3 | 6088 (1.6) | 1.5–1.8 |
| M01AB05 | Diclofenac | 8918 (2.4) | 2.3–2.6 | 2907 (0.8) | 0.7–0.9 | 1232 (0.3) | 0.3–0.4 | 570 (0.2) | 0.1–0.2 | 4510 (1.2) | 1.1–1.3 |
| N02A | Opioids | 5633 (1.5) | 1.4–1.6 | 2962 (0.8) | 0.7–0.9 | 2762 (0.7) | 0.7–0.8 | 4747 (1.3) | 1.2–1.4 | 9593 (2.6) | 2.4–2.8 |
| N02AX02 | Tramadol | 2521 (0.7) | 0.6–0.8 | 1456(0.4) | 0.3–0.5 | 1763(0.5) | 0.4–0.5 | 3644 (1.0) | 0.9–1.1 | 6471 (1.8) | 1.6–1.9 |
| J01 | Antibiotics | 34,237 (9.3) | 9.0–9.6 | 39,265 (10.6) | 10.3–10.9 | 43,859 (11.9) | 11.5–12.2 | 39,001 (10.6) | 10.2–10.9 | 97167 (26.3) | 25.8–26.8 |
| J01CR02+ J01CA04 | Amoxicillin + combined with beta-lactamase inhibitor | 11,494 (3.1) | 2.9–3.3 | 18,137 (4.9) | 4.7–5.1 | 23,114 (6.3) | 6.0–6.5 | 20,326 (5.5) | 5.3–5.7 | 53,871 (14.6)) | 14.2–14.9 |
| J01XX01 | Fosfomycin | 5142 (1.4) | 1.3–1.5 | 9188 (2.5) | 2.3–2.6 | 11,101 (3.0) | 2.8–3.2 | 9321 (2.5) | 2.42.7 | 25,922 (7.0) | 6.87.3 |
| J01FA10 | Azithromycin | 4596 (1.2) | 1.1–1.4 | 5012 (1.4) | 1.2–1.5 | 5011 (1.4) | 1.21.5 | 4457 (1.2) | 1.1–1.3 | 13,116 (3.6) | 3.4–3.7 |
| J01DC02 | Cefuroxime | 2129 (0.6) | 0.5–0.7 | 2222 (0.6) | 0.5–0.7 | 2804 (0.8) | 0.7–0.8 | 2618 (0.7) | 0.6–0.8 | 6977 (1.9) | 1.7–2.0 |
| J01XE01 | Nitrofurantoin | 1301 (0.4) | 0.3–0.4 | 2492 (0.7) | 0.6–0.8 | 2953 (0.8) | 0.7–0.9 | 2811 (0.8) | 0.7–0.9 | 7342 (2.0) | 1.8–2.1 |
| J01FF01 | Clindamycin | 437 (0.1) | 0.1–0.2 | 853 (0.2) | 0.2–0.3 | 1710 (0.3) | 0.4–0.5 | 2054 (0.6) | 0.5–0.6 | 4369 (1.2) | 1.1–1.3 |
| A02 | Gastro-oesophageal reflux | 17,406 (4.7) | 4.5–4,9 | 26,297 (7.1) | 6.9–7.4 | 36,178 (9.8) | 9.5–10.1 | 56,099 (15.2) | 14.8–15.6 | 91,079 (24.7) | 24.2–25.1 |
| A02BC | Proton pump inhibitors | 16,878 (4.6) | 4.4–4.8 | 19,200 (5.2) | 5.0–5.4 | 22,181 (6.0) | 5.8–6.3 | 34,021 (9.2) | 8.9–9.5 | 58,957 (16.0) | 15.6–16.3 |
| A02BC02 | Pantoprazole | 11,827 (3.2) | 3.0–3.4 | 10,845 (2.9) | 2.8–3.1 | 10,108 (2.7) | 2.6–2.9 | 14,949 (4.0) | 3.8–4.3 | 29,664 (8.0) | 7.7–8.3 |
| A02BC01 | Omeprazole | 1871 (0.5) | 0.4–0.6 | 4568 (1.2) | 1.1–1.3 | 7419 (2.0) | 1.9–2.2 | 11,125 (3.0) | 2.8–3.2 | 18,705 (5.1) | 4.8–5.3 |
| A02BC05 | Esomeprazole | 3428 (0.9) | 0.8–1.0 | 4445 (1.2) | 1.1–1.3 | 4700 (1.3) | 1.2–1.4 | 7699 (2.1) | 1.9–2.2 | 13,858 (3.8) | 3.6–3.9 |
| A02A | Antacids | 682 (0.2) | 0.1–0.2 | 8359 (2.3) | 2.1–2.4 | 14,824 (4.0) | 3.8–4.2 | 21,897 (5.9) | 5.7–6.2 | 39,332 (10.6) | 10.3–11.0 |
| A02AD01 | Ordinary salt combinations | 522 (0.1) | 0.1–0.2 | 7941 (2.1) | 2.0–2.3 | 14,346 (3.9) | 3.7–4.1 | 21,247 (5.8) | 5.5–6.0 | 37,984 (10.3) | 10.0–10.6 |
| A02BA | H2 receptor antagonists (ranitidine only) | 267 (0.1) | 0.0–0.1 | 1158 (0.3) | 0.3–0.4 | 2143 (0.6) | 0.5–0.7 | 4789 (1.3) | 1.2–1.4 | 6830 (1.8) | 1.7–2.0 |
| A03 | Anti-nausea drugs | 10,303 (2.8) | 2.6–3.0 | 48,140 (13.0) | 12.7–13.4 | 14,085 (3.8) | 3.6–4.0 | 7380 (2.0) | 1.9–2.1 | 60,404 (16.4) | 16.0–16.7 |
| A03FA01 | Metoclopramide | 4074 (1.1) | 1.0–1.2 | 42,076 (11.4) | 11.1–11.7 | 11,520 (3.1) | 2.9–3.3 | 6242 (1.7) | 1.6–1.8 | 53,021 (14.4) | 14.0–14.7 |
| A03FA03 | Domperidone | 4980 (1.3) | 1.2–1.5 | 432 (1.7) | 1.6–1.9 | 1596 (0.4) | 0.4–0.5 | 765 (0.2) | 0.2–0.3 | 8278 (2.2) | 2.1–2.4 |
| A04AA01 | Ondansetron | 1784 (0.5) | 0.4–0.6 | 3726 (1.0) | 0.9–1.1 | 1562 (0.4) | 0.4–0.5 | 644 (0.2) | 0.1–0.2 | 4659 (1.3) | 1.2–1.4 |
| A11HA02 | Pyridoxine* | 116 (0.0) | 0.0–0.0 | 446 (0.1) | 0.1–0.2 | 72 (0.0) | 0.0–0.0 | 45 (0.0) | 0.0–0.0 | 522 (0.1) | 0.1–0.2 |
| A06 | Laxatives | 6030 (1.6) | 1.5–1.8 | 10,139 (2.7) | 2.6–2.9 | 9586 (2.6) | 2.4–2.8 | 8374 (2.3) | 2.1–2.4 | 23',821 (6.4) | 6.2–6.7 |
| A06AD | Osmotically active | 3675 (1.0) | 0.9–1.1 | 5713 (1.5) | 1.4–1.7 | 4941 (1.3) | 1.2–1.5 | 4480 (1.2) | 1.1–1.3 | 13,167 (3.6) | 3.4–3.8 |
| A06AD15+ A06AD65 | Macrogol, comb + macrogol | 3026 (0.8) | 0.7–0.9 | 3969 (1.1) | 1.0–1.2 | 3166 (0.9) | 0.8–0.9 | 2835 (0.8) | 0.7–0.9 | 8756 (2.4) | 2.2–2.5 |
| A06AD11+ A06AD61 | Lactulose | 543 (0.1) | 0.1–0.2 | 1499 (0.4) | 0.3–0.5 | 1507 (0.4) | 0.3–0.5 | 1217 (0.3) | 0.3–0.4 | 3834 (1.0) | 0.9–1.1 |
| A06AC | Bulk forming laxatives | 1352 (0.4) | 0.3–0.4 | 3479 (0.9) | 0.8–1.0 | 3528 (1.0) | 0.9–1.1 | 2659 (0.7) | 0.6–0.8 | 8593 (2.3) | 2.2–2.5 |
| A06AC01+ A06AC51 | Ispaghulla + combinations | 1057 (0.3) | 0.2–0.3 | 2507 (0.7) | 0.6–0.8 | 2534 (0.7) | 0.6–0.8 | 1821 (0.5) | 0.4–0.6 | 6033 (1.6) | 1.5–1.8 |
ATC: anatomic therapeutic chemical; CI: confidence interval: NSAID: nonsteroidal anti-inflammatory drug
* Pyridoxine is shown even though <1% of pregnancies were exposed to it because it is a first line therapy to treat nausea and vomiting in pregancy
Regarding potentially teratogenic antibiotics, tetracycline antibiotics were dispensed in 0.1% (95% CI 0.0–0.1%) of pregnancies in trimester 2 and in 93 pregnancies (0.0%, 95% CI 0.0–0.0%) in trimester 3. Sulfonamide/trimethoprim was dispensed in 0.3% (95% CI 0.3–0.4%) of pregnancies in trimester 1 and quinolones were recorded in 0.6% (95% CI 0.5–0.6%) in trimester 1 (table 2).
Drugs for gastro-oesophageal reflux were dispensed during 24.7% of pregnancies (24.2–25.1%), most frequently in trimester 3 (15.2%, 95% CI 14.8–15.6%). Proton pump inhibitors were the most frequently dispensed drug class, with claims during 16.0% (15.6–16.3%) of pregnancies (table 3).
Anti-nausea drugs were claimed in 16.4% (95% CI 16.0–16.7%) of pregnancies, most frequently in trimester 1 (13.0%, 95% CI 12.7–13.4%), with metoclopramide (14.4%, 95% CI 14.0–14.7%) being the most frequently claimed drug. Ondansetron was claimed during 1.0% (95% CI 0.9–1.1%) of pregnancies in trimester 1 (table 3).
Laxatives were reimbursed by health insurance in 6.4% (95% CI 6.2–6.7%) of pregnancies, most frequently in trimester 2 (2.6%, 95% CI 2.4–2.8%). The most frequently dispensed laxative was macrogol (2.4%, 95% CI 2.2–2.5%) (table 3).
Claims for contact laxatives were recorded in 0.3% (95% CI 0.2–0.3%) of pregnancies in trimester 3, mostly for sodium picosulphate, which was dispensed in 36 pregnancies (0.0%, 95% CI 0.0–0.0%). No claims were recorded for senna (anthraquinone derivative) (table 2).
Folic acid preparations were claimed to health insurance in 9.8% (9.5–10.1%) of pregnancies during pre-pregnancy and in 18.4% (18.0–18.8%) in trimester 1. In total, 18.5% (18.0–18.9%) and 45.9% (45.3–46.5%) of pregnancies had a recorded claim for intravenous and oral iron during pregnancy, most frequently in trimester 2 (6.1% and 26.3%) and 3 (13.1% and 27.2%) (table 4, unextrapolated numbers are displayed in table S4).
Table 4Exposure prevalence to supplements (extrapolated numbers).
| ATC code | Drug substance | Pre pregnancy N (%) | 95% CI | T1 N (%) | 95% CI | T2 N (%) | 95% CI | T3 N (%) | 95% CI | T1-T3 N (%) | 95% CI |
| B03AC | IV iron | 6'891 (1.9) | 1.7, 2.0 | 5'299 (1.4) | 1.3, 1.5 | 22'635 (6.1) | 5.9, 6.4 | 48'265 (13.1) | 12.7, 13.4 | 68'182 (18.5) | 18.0, 18.9 |
| B03AA+ B03AB+ B03AD + B03AE | Oral iron | 11'524 (3.1) | 3.0, 3.3 | 44'498 (12.0) | 11.7, 12.4 | 97'322 (26.3) | 25.9, 26.8 | 100'501 (27.2) | 26.7, 27.7 | 169'563 (45.9) | 45.3, 46.5 |
| Unspecified* | Unspecified | 284 (0.1) | 0.0, 0.1 | 338 (0.1) | 0.1, 0.1 | 2'185 (0.6) | 0.5, 0.7 | 4'562 (1.2) | 1.1, 1.3 | 6'895 (1.9) | 1.7, 2.0 |
| B03BB | Folic acid | 36’350 (9.8) | 9.5, 10.1 | 67’974 (18.4) | 18.0, 18.8 | 8’277 (2.2) | 2.1, 2.4 | 3'735 (1.0) | 0.9, 1.1 | 71'913 (19.5) | 19.1, 19.9 |
| A12CC | Magnesium | 8’804 (2.4) | 2.2, 2.5 | 93’188 (25.2) | 24.8, 25.7 | 160’337(43.4) | 42.8, 44.0 | 154'625 (41.9) | 41.3, 42.5 | 246'891 (66.8) | 66.2, 67.5 |
| A11CC | Vitamin D | 6’772 (1.8) | 1.7, 2.0 | 14’965 (4.1) | 3.9, 4.2 | 11’627 (3.1) | 3.0, 3.3 | 7'532 (2.0) | 1.9, 2.2 | 24'869 (6.7) | 6.5, 7.0 |
ATC: anatomic therapeutic chemical; CI: confidence interval: IV: intravenous; NSAID: nonsteroidal anti-inflammatory drug
* The form of iron dispensed (oral or intravenous) was not obtained from the ATC codes but from the information directly captured in the Helsana data. Therefore, for some prescriptions, this information was not available and is marked as “unspecified”.
This drug utilisation study used Swiss health care claims data to evaluate the use of prescription drugs dispensed in outpatient care to treat acute conditions frequently associated with pregnancy in Switzerland between 2014 and 2018. Our results allow a representative evaluation of which drugs are commonly prescribed to pregnant women in outpatient care in Switzerland.
We identified an extrapolated study population of 369,371 deliveries per year between 2014 and 2018. Mean maternal age at delivery (32.0 years) as well as the proportion of caesarean sections (33.7%) were consistent with results reported by the Swiss Federal Statistical Office for the overall population in Switzerland for this time period [16]. Thus, our extrapolated study population can be assumed to be representative of all pregnancies in Switzerland during this time period.
In our cohort of pregnant women, paracetamol, which is recommended as the first-line drug to treat pain during pregnancy [17], was the most frequently reimbursed analgesic during pregnancy (30.3%, 95% CI 29.8–30.8%), followed by NSAIDs (8.6%, 95% CI 8.3–8.8%). Lupattelli et al. reported that among pregnant women in Western Europe who responded to a web-based survey, 51.7% indicated having used OTC paracetamol and 2.2% used OTC NSAIDs at least once during pregnancy [7]. Even though such surveys may be affected by volunteer bias and may thus not be entirely representative, our study only captures dispensing of prescribed drugs and therefore, likely underestimates the actual use of analgesics during pregnancy in Switzerland, because paracetamol and most NSAIDs are available OTC [18], which is not captured in claims databases.
Of all pregnancies, 1.3% (95% CI 1.2–1.4%) had a recorded claim for an NSAID after week 24. Use of NSAIDs after week 24 has been associated with premature closure of the ductus arteriosus and renal toxicity and is therefore not recommended. [19].
In our cohort, 1.3% (95% CI 1.2–1.4%) of pregnancies had a prescription for an opioid in trimester 3. Opioids have been associated with neonatal abstinence syndrome and neonatal respiratory distress, especially when administered near delivery [2023]. In clinical situations in which the use of opioids in trimester 3 is required, neonatal surveillance and special care during delivery and the early post-partum period should be provided.
Infections are a frequent complication of pregnancy, which require treatment in order to prevent complications. The most frequently reported indications for antibiotic use during pregnancy are respiratory infections, pelvic inflammatory disease and urinary tract infections [24].
Exposure to antibiotics during pregnancy was 26.3% in our cohort, which is comparable to a Norwegian national claims-based study (27.9%) [6], but lower than in a French claims based study (40.6%) [5]. As opposed to analgesics, antibiotics cannot be purchased OTC and therefore we expect our results to accurately reflect exposure to antibiotics in outpatient care in Switzerland.
Overall, potentially teratogenic or fetotoxic antibiotics were rarely dispensed during risk periods; sulfonamide/trimethoprim, which is associated with a theoretical increased risk of neural tube defects if administered in trimester 1 [25, 26], was dispensed during 0.3% of pregnancies in trimester 1. Tetracyclines, which may cause tooth staining if administered after week 14 of pregnancy and especially in trimester 3 [27, 28], were dispensed during 0.1% of pregnancies in trimester 2 and in 93 pregnancies in trimester 3. Quinolones have been associated with cartilage and bone damage in animal models. Even though similar effects have not been found in human fetuses, quinolone use, especially in trimester 1, should be avoided unless better alternatives are lacking [29, 30]. In our cohort, quinolone exposure was recorded in 0.6% of pregnancies in trimester 1.
Gastro-oesophageal reflux is a common complication during pregnancy, which affects between 30% and 50% of pregnant women [31], especially towards the end of pregnancy. According to Le Crat [32], PPIs and antacids, which were the most frequently claimed drugs for gastro-oesophageal reflux in our cohort during pregnancy (16% and 10.6%) can be used safely throughout pregnancy. In Switzerland, antacids as well as some PPIs may be purchased OTC and many aluminum-free antacids are not reimbursed by health insurance (e.g., Riopan gel® (Magaldrat), Rennie® (salts of magnesium/calcium). Thus, the actual use of drugs for gastro-oesophageal reflux, and especially use of antacids, is likely underestimated in our cohort. Lupattelli et al. reported a high proportion of OTC antacids (14.7%) during pregnancy in Western Europe in their web-based survey [7]. Self-reported use of OTC PPIs was lower (1.2%) in that study [7].
Nausea and vomiting during pregnancy affect up to 85% of pregnant women during trimester 1, and usually subside after week 14 of pregnancy [33]. Metoclopramide was the most frequently reimbursed nausea drug during pregnancy (14.4%, 95% CI 14.0–14,7%) in our cohort. Specific treatment guidelines for Switzerland are lacking. Both, the British Royal College of Obstetricians and Gynaecologists (RCOG) [34] and the American College of Obstetricians and Gynecologists (ACOG) [35] recommend metoclopramide as second- or third-line anti-nausea drug, after pyridoxine, as mono-preparation or in combination in an antihistamine (ACOG), or an antihistamine (RCOG). We observed claims for pyridoxine monopreparations in 0.1% (95% CI 0.1–0.2%) of pregnancies. Furthermore, in the web-based survey, pyridoxine was not among the four most frequently self-reported treatments against nausea in Western Europe, suggesting it is only rarely used [36]. In the same survey [36], 19.0% of Swiss women self-reported use of antihistamines during pregnancy, which was reportedly the most frequently used anti-nausea drug. In Switzerland, a combination of the antihistamine meclozine and pyridoxine is frequently prescribed to treat nausea and vomiting during pregnancy, but is not reimbursed by health insurance. Thus, our results underestimate the overall use of anti-nausea drugs during pregnancy and presumably only reflect exposure to second- and third-line anti-nausea drugs.
Ondansetron was claimed during 1.3% (95% CI 1.2–1.4%) of pregnancies in our cohort (1.0%, 95% CI 0.9-1.1% in trimester 1). In 2020, Swissmedic followed the EMA by issuing a warning regarding a potentially increased risk of orofacial clefts in association with ondansetron exposure in trimester 1 [37]. The warning was based on an observational study in US claims data, which observed a moderately increased risk of 3 additional cases of oral clefts per 10,000 children exposed to ondansetron during trimester 1 compared with unexposed children [38]. Debate on whether this reported association is causal or not is ongoing [39]. The US Food and Drug Administration (FDA) has not issued a comparable warning, although ondansetron has become the most frequently used anti-nausea drug during pregnancy in the US (22.2% of pregnancies in a US claims-based study in 2014) [40]. The RCOG classifies ondansetron as safe and effective to treat nausea and vomiting during pregnancy, but states that it should be reserved as a second-line therapy given the limited data [34], whereas the ACOG states that it should be reserved as a third-line therapy [35], for cases of persistent nausea and vomiting of pregnancy.
Constipation is one of the most common gastrointestinal complaints during pregnancy, affecting almost half of pregnant women, most commonly during the first and second trimester [31].
Bulk and osmotic laxatives are the first-line treatment of constipation during pregnancy [41]. Lubricants should be limited to short-term use since they may diminish absorption of lipophilic vitamins [41]. Exposure to prescribed laxatives during pregnancy was 6.4% (95% CI 6.2-6.7%) in our cohort, which likely underestimates overall use of laxatives during pregnancy in Switzerland. Lupattelli et al. observed a self-reported use of OTC laxatives (most laxatives are available OTC in Switzerland) of 7.5% during pregnancy in Western Europe in their web-based survey [7].
Claims for contact laxatives were recorded in 0.3% (95% CI 0.2–0.3%) of pregnancies in trimester 3. Contact laxatives are recommended for short-term use only, if osmotic and bulk laxatives were ineffective, especially senna preparations, which may cause intestinal and uterine contractions if taken in trimester 3 (0 exposed pregnancies in trimester 3) [42].
Claims for folic acid were recorded in 9.8% (95% CI 9.5–10.1%) of pregnancies in pre-pregnancy and in 18.4% (95% CI 18.018.8%) in trimester 1. Folic acid supplementation is recommended for every woman who intends to become pregnant between 2–3 months before conception and until week 12 of pregnancy [43]. The observed exposure to folic acid in our cohort underestimates overall use of folic acid in pregnant women in Switzerland, as folic acid may be purchased OTC and most prenatal vitamins also include folic acid in an appropriate dose but are not reimbursed by basic health insurance in Switzerland. Thus, only one out of ten women in Switzerland is prescribed reimbursable folic acid prior to pregnancy and one out of five during trimester 1. Given the important role of folic acid in the prevention of neural tube defects, more comprehensive prescribing may be desirable to ensure sufficient folic acid supplementation around the time of conception.
According to the Swiss Society of Gynaecology and Obstetrics [44], iron deficiency without anaemia and iron deficiency anaemia should be screened for and supplemented during pregnancy. It has been reported that one in three pregnant women in Switzerland presents with iron deficiency and one in ten with anaemia due to iron deficiency [44]. In our cohort, 18.5% of women had a claim for intravenous iron and 45.9% for oral iron.
To our knowledge, this study is the first to evaluate outpatient drug utilisation during pregnancy on a population-based level in Switzerland. Our findings originate from a representative claims database including longitudinal data on 15% of pregnancies in Switzerland. Data are recorded as a by-product of routine clinical care, independently of the research question, and our results are thus not vulnerable to volunteer or recall bias. However, some limitations need to be considered. First, our extrapolated study population is representative of the overall female population of Switzerland in terms of demographic factors. However, given the lack of socioeconomic information in claims data, we were not able to account for potential socioeconomic differences in the extrapolation process. Nevertheless, given that the average maternal age at delivery, which is a well-known proxy for socioeconomic status [45], was consistent between our extrapolated cohort and overall maternal age reported by the Swiss Federal Statistical Office, major channeling by socioeconomic status is unlikely. Second, we only included pregnancies that ended in live births or stillbirths because early abortions and terminations are not reliably captured in healthcare claims data. This may have led to an underestimation of the prevalence of exposure to drugs that can cause spontaneous abortions due to early death of the embryo or fetus, or which are associated with an increased rate of medical or surgical abortions. Third, inpatient drug use could not be evaluated because of the bundled DRG-based reimbursement system for inpatient stays in Switzerland. In a survey among Swiss obstetric clinics, Schenkel et al. reported drugs that were routinely used to treat various pregnancy and post-partum indications [46]. Forth, gestational age at delivery is not recorded in Swiss claims data, and LMP and trimester dates had to be estimated based on relatively crude information on gestational age provided by DRG billing codes. The applied algorithm to estimate LMP has been validated in US claims data [10]. However, validation of the algorithm in Swiss claims data would require linkage of different external data sources providing exact information on gestational age at delivery. Unlike other countries, such linkage of different data sources is not routinely feasible in Switzerland yet, for legal and political reasons. Thus, some misclassification of the dispensing timing by trimester is possible. Fifth, healthcare claims data only provide information on when a prescribed drug was dispensed but not on actual drug use or drug adherence. Finally, the TARMED coding system for outpatient care does not capture medical diagnoses, and thus we cannot determine whether use of potentially teratogenic/fetotoxic drugs was clinically necessary.
The observed pattern of claimed drugs during pregnancy is in line with existing treatment guidelines. Exposure to potentially teratogenic or fetotoxic drugs was small, but given the lack of recorded diagnoses, we cannot determine if their use was clinically indicated. Our study demonstrates that Swiss healthcare claims databases are a valuable tool to evaluate drug utilization during pregnancy in Switzerland.
Pregnant women are a vulnerable and yet under-investigated patient population, and appropriate research methods and tools to further understand their medical needs are required.
Financial support for this study was provided by the LOA IV fund, managed by curafutura, pharmaSuisse and santésuisse, Switzerland.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.
1. Lyerly AD , Little MO , Faden RR ; Anne Drapkin Lyerly; Margaret Olivia Little; Ruth R. Faden . Pregnancy and clinical research. Hastings Cent Rep. 2008 Nov-Dec;38(6):3. https://doi.org/10.1353/hcr.0.0089
2. Frost Widnes SK , Schjøtt J . Advice on drug safety in pregnancy: are there differences between commonly used sources of information? Drug Saf. 2008;31(9):799–806. https://doi.org/10.2165/00002018-200831090-00008
3. Noh Y , Yoon D , Song I , Jeong HE , Bae JH , Shin JY . Discrepancies in the Evidence and Recommendation Levels of Pregnancy Information in Prescription Drug Labeling in the United States, United Kingdom, Japan, and Korea. J Womens Health (Larchmt). 2018 Sep;27(9):1086–92. https://doi.org/10.1089/jwh.2017.6792
4. Dashraath P , Nielsen-Saines K , Madhi SA , Baud D . COVID-19 vaccines and neglected pregnancy. Lancet. 2020 Sep;396(10252):e22. https://doi.org/10.1016/S0140-6736(20)31822-5
5. Bérard A , Abbas-Chorfa F , Kassai B , Vial T , Nguyen KA , Sheehy O , et al. The French Pregnancy Cohort: medication use during pregnancy in the French population. PLoS One. 2019 Jul;14(7):e0219095. https://doi.org/10.1371/journal.pone.0219095
6. Engeland A , Bjørge T , Klungsøyr K , Hjellvik V , Skurtveit S , Furu K . Trends in prescription drug use during pregnancy and postpartum in Norway, 2005 to 2015. Pharmacoepidemiol Drug Saf. 2018 Sep;27(9):995–1004. https://doi.org/10.1002/pds.4577
7. Lupattelli A , Spigset O , Twigg MJ , Zagorodnikova K , Mårdby AC , Moretti ME , et al. Medication use in pregnancy: a cross-sectional, multinational web-based study. BMJ Open. 2014 Feb;4(2):e004365. https://doi.org/10.1136/bmjopen-2013-004365
8. WHOCC - ATC/DDD Index . Accessed January 21, 2021. https://www.whocc.no/atc_ddd_index/
9. Spoendlin J , Blozik E , Graber S , Rauch M , Marxer C , Rüegg S , et al. Use of valproate in pregnancy and in women of childbearing age between 2014 and 2018 in Switzerland: a retrospective analysis of Swiss healthcare claims data. Swiss Med Wkly. 2021 Jan;151:w20386. https://doi.org/10.4414/smw.2021.20386
10. Margulis AV , Setoguchi S , Mittleman MA , Glynn RJ , Dormuth CR , Hernández-Díaz S . Algorithms to estimate the beginning of pregnancy in administrative databases. Pharmacoepidemiol Drug Saf. 2013 Jan;22(1):16–24. https://doi.org/10.1002/pds.3284
11. CRAT - Centre de référence sur les agents tératogènes chez la femme enceinte. Accessed November 26, 2020. http://www.lecrat.fr/
12. Embryotox - Sécurité des médicaments pendant la grossesse et l’allaitement: Accueil. Accessed November 26, 2020. https://www.embryotox.de/
13. Swissmedic 2019 © Copyright. (Direct) Healthcare Professional Communications. Accessed March 26, 2021. https://www.swissmedic.ch/swissmedic/fr/home/humanarzneimittel/marktueberwachung/healthcare-professional-communications.html
14. Lumley T . Analysis of Complex Survey Samples. J Stat Softw. 2004;9(1):1–19. https://doi.org/10.18637/jss.v009.i08
15. R: The R Project for Statistical Computing. Accessed March 26, 2021. https://www.r-project.org/
16. Santé des nouveau-nés | Office fédéral de la statistique. Accessed November 26, 2020. https://www.bfs.admin.ch/bfs/fr/home/statistiques/sante/etat-sante/sante-nouveau-nes.html
17. Antalgiques - Grossesse . Lecrat.fr. https://lecrat.fr/spip.php?page=article&id_article=18. Accessed March 26, 2021.
18. compendium.ch . Accessed January 21, 2021. https://compendium.ch/fr
19. Ibuprofène - Grossesse et allaitement. Lecrat.fr. https://lecrat.fr/spip.php?page=article&id_article=402. Accessed March 26, 2021.
20. Morphine - Grossesse et allaitement. Lecrat.fr. https://lecrat.fr/spip.php?page=article&id_article=40. Accessed March 26, 2021.
21. Embryotox - Sécurité des médicaments pendant la grossesse et l’allaitement: la morphine. Accessed December 22, 2020. https://www.embryotox.de/arzneimittel/details/morphin/
22. Tramadol - Grossesse et allaitement. Lecrat.fr. https://lecrat.fr/spip.php?page=article&id_article=42. Accessed March 26, 2021.
23. Embryotox - Sécurité des médicaments pendant la grossesse et l’allaitement: Tramadol. Accessed December 22, 2020. https://www.embryotox.de/arzneimittel/details/tramadol/
24. Santos F , Oraichi D , Bérard A . Prevalence and predictors of anti-infective use during pregnancy. Pharmacoepidemiol Drug Saf. 2010 Apr;19(4):418–27. https://doi.org/10.1002/pds.1915
25. Triméthoprime - Grossesse et allaitement. Lecrat.fr. https://lecrat.fr/spip.php?page=article&id_article=1012. Accessed March 26, 2021.
26. Embryotox - sécurité des médicaments pendant la grossesse et l’allaitement: cotrimoxazole. Accessed December 22, 2020. https://www.embryotox.de/arzneimittel/details/co-trimoxazol/
27. Cyclines - Grossesse et allaitement. Lecrat.fr. https://lecrat.fr/spip.php?page=article&id_article=1021. Accessed March 26, 2021.
28. Embryotox - Sécurité des médicaments pendant la grossesse et l’allaitement: la minocycline. Accessed December 22, 2020. https://www.embryotox.de/arzneimittel/details/minocyclin/
29. Fluoroquinolones - Grossesse et allaitement. Lecrat.fr. https://lecrat.fr/spip.php?page=article&id_article=491. Accessed March 26, 2021.
30. Embryotox - Sécurité des médicaments pendant la grossesse et l’allaitement: Ciprofloxacine. Accessed December 22, 2020. https://www.embryotox.de/arzneimittel/details/ciprofloxacin/
31. Zielinski R , Searing K , Deibel M . Gastrointestinal distress in pregnancy: prevalence, assessment, and treatment of 5 common minor discomforts. J Perinat Neonatal Nurs. 2015 Jan-Mar;29(1):23–31. https://doi.org/10.1097/JPN.0000000000000078
32. Reflux gastro-oesophagien - Grossesse et allaitement. Lecrat.fr. https://lecrat.fr/spip.php?page=article&id_article=1049. Accessed March 26, 2021.
33. Netgen. Nausées et vomissements chez la femme enceinte. Revue Médicale Suisse. Accessed February 5, 2021. https://www.revmed.ch/RMS/2018/RMS-N-614/Nausees-et-vomissements-chez-la-femme-enceinte
34. The Management of Nausea and Vomiting of Pregnancy and Hyperemesis Gravidarum (Green-top Guideline No. 69). Royal College of Obstetricians & Gynaecologists. Accessed March 26, 2021. https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg69/
35. Committee on Practice Bulletins-Obstetrics . ACOG Practice Bulletin No. 189: Nausea And Vomiting Of Pregnancy. Obstet Gynecol. 2018 Jan;131(1):e15–30. https://doi.org/10.1097/AOG.0000000000002456
36. Heitmann K , Holst L , Lupattelli A , Maltepe C , Nordeng H . Treatment of nausea in pregnancy: a cross-sectional multinational web-based study of pregnant women and new mothers. BMC Pregnancy Childbirth. 2015 Dec;15(1):321. https://doi.org/10.1186/s12884-015-0746-2
37. Swissmedic 2019 © Copyright. DHPC – Études épidémiologiques publiées récemment visant à évaluer le risque de malformations congénitales. Accessed December 18, 2020. https://www.swissmedic.ch/swissmedic/fr/home/humanarzneimittel/marktueberwachung/healthcare-professional-communications/dhpc-veroeffentlichte-epidemiologische-studien.html
38. Parker SE , Van Bennekom C , Anderka M , Mitchell AA ; National Birth Defects Prevention Study . Ondansetron for Treatment of Nausea and Vomiting of Pregnancy and the Risk of Specific Birth Defects. Obstet Gynecol. 2018 Aug;132(2):385–94. https://doi.org/10.1097/AOG.0000000000002679
39. Huybrechts KF , Hernández-Díaz S , Straub L , Gray KJ , Zhu Y , Patorno E , et al. Association of Maternal First-Trimester Ondansetron Use With Cardiac Malformations and Oral Clefts in Offspring. JAMA. 2018 Dec;320(23):2429–37. https://doi.org/10.1001/jama.2018.18307
40. Taylor LG , Bird ST , Sahin L , Tassinari MS , Greene P , Reichman ME , et al. Antiemetic use among pregnant women in the United States: the escalating use of ondansetron. Pharmacoepidemiol Drug Saf. 2017 May;26(5):592–6. https://doi.org/10.1002/pds.4185
41. Laxatifs - Grossesse et allaitement. Lecrat.fr. https://lecrat.fr/spip.php?page=article&id_article=10. Accessed March 26, 2021.
42. Embryotox - sécurité des médicaments pendant la grossesse et l’allaitement: feuilles de séné. Accessed November 26, 2020. https://www.embryotox.de/arzneimittel/details/sennesblaetter/
43. OSAV O fédéral de la sécurité alimentaire et des affaires vétérinaires. Acide folique (2002). Accessed March 26, 2021. https://www.blv.admin.ch/blv/fr/home/das-blv/organisation/kommissionen/eek/folsaeure.html
44. Breymann C , Honegger C , Hösli I , Surbek D . Diagnostic et traitement de l’anémie ferriprive durant la grossesse et le postpartum (mise à jour remplaçant la version du 24.12.2009). :5.
45. De Wit ML , Rajulton F . Education and timing of parenthood among Canadian women: a cohort analysis. Soc Biol. 1992;39(1-2):109–22. https://doi.org/10.1080/19485565.1992.9988808
46. Schenkel L , Simões-Wüst AP , Hösli I , von Mandach U . Drugs in Pregnancy and Lactation - Medications Used in Swiss Obstetrics. Z Geburtshilfe Neonatol. 2018 Feb;222(4):152–65. https://doi.org/10.1055/s-0043-124975
47. http://www.mysign.ch WM 2021 S. Risikoausgleich. Gemeinsame Einrichtung KVG. Accessed June 23, 2021. https://www.kvg.org/de/risikoausgleich-_content---1--1047.html
Table S1Description of the study population (crude numbers).
| Year | No. of deliveries in the cohort | Mean of maternal age at delivery in the cohort (SD) | Mean maternal age at delivery in Switzerland (BfS) | Percentage of caesarean sections in the cohort | Percentage of caesarean sections in Switzerland (BfS) |
| 2014 | 9560 | 32.09 (5.06) | 31.7 | 34.6 | 33.7 |
| 2015 | 9409 | 32.10 (5.20) | 31.8 | 34.8 | 33.3 |
| 2016 | 9163 | 32.03 (5.25) | 31.8 | 34.1 | 33.2 |
| 2017 | 8811 | 32.12 (5.33) | 31.9 | 33.8 | 32.3 |
| 2018 | 8300 | 32.09 (5.23) | 32.0 | 33.4 | 32.1 |
CI: confidence interval; BfS: Bundesamt für Statistik, Swiss Federal Statistical Office; SD: standard deviation
Table S2Exposure to potentially teratogenic or fetotoxic drugs during risk period and associated potential risks (crude numbers).
| Potentially teratogenic or fetotoxic drugs (ATC code) | Warnings regarding use during critical period | Risk period | Pregnancies exposed during risk period during study period (n, %) |
| NSAIDs (M01A) | Premature closure of ductus arteriosus and renal toxicity [19] | After week 24 | 710 (1.6) |
| Opioids (N02A) | Neonatal abstinence syndrome and respiratory distress [20–23] | Trimester 3 | 739 (1.6) |
| Trimethoprim/sulphonamide (J01E) | Risk of neural tube defects [25, 26] | Trimester 1 | 152 (0.3) |
| Quinolone (J01M) | Cartilage and bone damage in animal studies but not found in human studies [29, 30] | Trimester 1 | 252 (0.6) |
| Tetracycline (J01A) | Tooth staining [27, 28] | Trimester 2 and 3 | 25; 13 (0.1; 0.0) |
| Ondansetron (A04AA01) | Potentially increased risk of orofacial clefts [37] | Trimester 1 | 469 (1.0) |
| Contact laxatives (A06AB), especially senna (anthraquinone derivative, A06AB06) | Theoretical risk of intestinal and uterine contractions [42] | Trimester 3 | 27; 0 (0.1; 0.0) |
ATC: anatomic therapeutic chemical; NSAID: nonsteroidal anti-inflammatory drug
Table S3Exposure prevalence to different drug groups and active substances during pregnancy overall and within trimester of pregnancy and pre-pregnancy separately (crude numbers).
| ATC code | Drug substance | Pre-pregnancy n (%) | T1 n (%) | T2 n (%) | T3 n (%) | T1–T3 n (%) |
| N02+M01A | Analgesics | 8226 (18.2) | 7713 (17.0) | 7372 (16.3) | 6318 (14.0) | 15757 (34.8) |
| N02B | Other analgesics | 4778 (10.6) | 6324 (14.0) | 6545 (14.5) | 5616 (12.4) | 13989 (30.9) |
| N02BE01 | Paracetamol | 4183 (9.2) | 6109 (13.5) | 6499 (14.4) | 5582 (12.3) | 13801 (30.5) |
| N02BB02 | Metamizole | 1140 (2.5) | 366 (0.8) | 74 (0.2) | 45 (0.1) | 470 (1.0) |
| M01A | NSAIDs | 5550 (12.3) | 2361 (5.2) | 1354 (3.0) | 568 (1.3) | 3908 (8.6) |
| M01AE01 | Ibuprofen | 3204 (7.1) | 1474 (3.3) | 915 (2.0) | 291 (0.6) | 2486 (5.5) |
| M01AG01 | Mefenamic acid | 1082 (2.4) | 400 (0.9) | 260 (0.6) | 153 (0.3) | 766 (1.7) |
| M01AB05 | Diclofenac | 1125 (2.5) | 364 (0.8) | 148 (0.3) | 84 (0.2) | 570 (1.3) |
| N02A | Opioids | 678 (1.5) | 347 (0.8) | 328 (0.7) | 739 (1.6) | 1308 (2.9) |
| N02AX02 | Tramadol | 298 (0.7) | 159 (0.4) | 209 (0.5) | 593 (1.3) | 911 (2.0) |
| J01 | Antibiotics | 4256 (9.4) | 4853 (10.7) | 5265 (11.6) | 5010 (11.1) | 12016 (26.6) |
| J01CR02+J01CA04 | Amoxicillin + combined with beta lactamase inhibitor | 1173 (2.6) | 1912 (4.2) | 2404 (5.3) | 2236 (4.9) | 5799 (12.8) |
| J01XX01 | Fosfomycin | 642 (1.4) | 1101 (2.4) | 1270 (2.8) | 1161 (2.6) | 3088 (6.8) |
| J01FA10 | Azithromycin | 567 (1.3) | 644 (1.4) | 620 (1.4) | 544 (1.2) | 1615 (3.6) |
| J01DC02 | Cefuroxime | 257 (0.6) | 267 (0.6) | 332 (0.7) | 335 (0.7) | 848 (1.9) |
| J01XE01 | Nitrofurantoin | 153 (0.3) | 272 (0.6) | 310 (0.7) | 329 (0.7) | 803 (1.8) |
| J01FF01 | Clindamycin | 57 (0.1) | 105 (0.2) | 217 (0.5) | 286 (0.6) | 579 (1.3) |
| A02 | Gastro-oesophageal reflux | 2601 (5.7) | 3242 (7.2) | 4374 (9.7) | 7506 (16.6) | 11651 (25.7) |
| A02BC | Proton pump inhibitors | 2082 (4.6) | 2285 (5.0) | 2607 (5.8) | 4212 (9.3) | 7134 (15.8) |
| A02BC02 | Pantoprazole | 1434 (3.2) | 1260 (2.8) | 1157 (2.6) | 1800 (4.0) | 3491 (7.7) |
| A02BC01 | Omeprazole | 241 (0.5) | 539 (1.2) | 871 (1.9) | 1344 (3.0) | 2237 (4.9) |
| A02BC05 | Esomeprazole | 436 (1.0) | 559 (1.2) | 578 (1.3) | 1059 (2.3) | 1825 (4.0) |
| A02A | Antacids | 80 (0.2) | 1083 (2.4) | 1848 (4.1) | 2835 (6.3) | 5008 (11.1) |
| A02AD01 | Ordinary salt combinations | 63 (0.1) | 1030 (2.3) | 1787 (3.9) | 2754 (6.1) | 4838 (10.7) |
| A02BA | H2 receptor antagonists (ranitidine only) | 31 (0.1) | 150 (0.3) | 276 (0.6) | 1176 (2.6) | 1432 (3.2) |
| A03 | Anti-nausea drugs | 1235 (2.7) | 5707 (12.6) | 1702 (3.8) | 971 (2.1) | 7271 (16.1) |
| A03FA01 | Metoclopramide | 481 (1.1) | 4962 (11.0) | 1385 (3.1) | 821 (1.8) | 6350 (14.0) |
| A03FA03 | Domperidone | 603 (1.3) | 778 (1.7) | 203 (0.4) | 107 (0.2) | 1025 (2.3) |
| A04AA01 | Ondansetron | 211 (0.5) | 469 (1.0) | 196 (0.4) | 83 (0.2) | 592 (1.3) |
| A11HA02 | Pyridoxine* | 14 (0.0) | 54 (0.1) | 10 (0.0) | 6 (0.0) | 64 (0.1) |
| A06 | Laxatives | 746 (1.6) | 1252 (2.8) | 1170 (2.6) | 1106 (2.4) | 3000 (6.6) |
| A06AD | Osmotically active | 446 (1.0) | 689 (1.5) | 585 (1.3) | 563 (1.2) | 1596 (3.5) |
| A06AD15+A06AD65 | Macrogol, comb + macrogol | 372 (0.8) | 468 (1.0) | 382 (0.8) | 354 (0.8) | 1061 (2.3) |
| A06AD11+ A06AD61 | Lactulose | 63 (0.1) | 185 (0.4) | 166 (0.4) | 149 (0.3) | 451 (1.0) |
| A06AC | Bulk forming laxatives | 167 (0.4) | 430 (1.0) | 431 (1.0) | 338 (0.7) | 1069 (2.4) |
| A06AC01+ A06AC51 | Ispaghulla + combinations | 133 (0.3) | 321 (0.7) | 316 (0.7) | 241 (0.5) | 775 (1.7) |
ATC: anatomic therapeutic chemical; NSAID: nonsteroidal anti-inflammatory drug
* Pyridoxine is shown even though <1% of pregnancies were exposed to it because it is a first-line therapy to treat nausea and vomiting in pregancy
Table S4Exposure prevalence to supplements (crude numbers).
| ATC code | Drug substance | Pre-pregnancy n (%) | T1 n (%) | T2 n (%) | T3 n (%) | T1–T3 n (%) |
| B03AC | IV iron | 837 (1.8) | 667 (1.5) | 2768 (6.1) | 5789 (12.8) | 8236 (18.2) |
| B03AA+ B03AB+ B03AD + B03AE | Oral iron | 1399 (3.1) | 5551 (12.3) | 12075 (26.7) | 12365 (27.3) | 20960 (46.3) |
| Unspecified* | Unspecified | 29 (0.1) | 42 (0.1) | 263 (0.6) | 551 (1.2) | 834 (1.8) |
| B03BB | Folic acid | 4397 (9.7) | 8337 (18.4) | 1011 (2.2) | 456 (1.0) | 8826 (19.5) |
| A12CC | Magnesium | 1100 (2.4) | 11681 (25.8) | 19614 (43.3) | 19104 (42.2) | 30333 (67.0) |
| A11CC | Vitamin D | 836 (1.8) | 1849 (4.1) | 1416 (3.1) | 977 (2.2) | 3092 (6.8) |
ATC: anatomic therapeutic chemical; CI: confidence interval: IV: intravenous; NSAID: nonsteroidal anti-inflammatory drug
* The form of iron dispensed (oral or intra venous) was not obtained from the ATC codes but from the information directly captured in the Helsana data. Therefore, for some prescriptions, this information was not available and is marked as “unspecified".
In order to represent numbers of all Switzerland and due to potential small biases in the Helsana data set, all results were extrapolated/weighted relative to the demographic distribution of the overall Swiss population, taking into account the stratification by calendar year, canton, age, and sex. The extrapolations/weightings were based on individual weighting factors (wi), which were calculated as the inverse of the sampling probability (pi = NHelsana ,i / NSwitzerland, i) of a given stratum (i): wi = 1 / pi. The strata are defined by a woman’s demographic characteristics at the time of the delivery. The corresponding sample sizes (NHelsana ,i, NSwitzerland, i) for the different strata come from the risk equalization statistics [47].
The weighted sums (extrapolated number of pregnancies), weighted mean and standard deviation of age were calculated using the survey package in R [14]. The package uses a simple inverse-probability weighting as described above. Besides the weighted estimates, the survey package provides standard estimators that incorporate the effects of stratification. These are used to calculate normal-based 95% confidence intervals of the extrapolated number of pregnancies and corresponding prevalences.
Table S5Relevant codes to identify vaginal or caesarean delivery.
| Codes | Definition | Type of delivery |
| TarMed codes version (V01.08.00, 01.08.01, 01.09) | Grossesse et obstétrique | |
| 22.2110 | Surveillance de la naissance et conduite de l'accouchement, risque normal | Vaginal delivery |
| 22.2120 | + Césarienne secondaire | Cesarean delivery |
| 22.2130 | + Hystérectomie lors d’une césarienne | Cesarean delivery |
| 22.2200 | Surveillance de la naissance et conduite de l'accouchement, haut risque | Vaginal delivery |
| 22.2210 | Surveillance de la naissance et conduite de l'accouchement, très haut risque | Vaginal delivery |
| 22.2410 | Césarienne, planifiée ou primaire | Cesarean delivery |
| 22.2420 | Césarienne itérative | Cesarean delivery |
| SwissDRG Codes | MDC 14: Grossesse, naissance et suites de couches | |
| O01A (V3.0, V4.0, V 5.0, V6.0) | Césarienne avec plusieurs diagnostics de complication, durée de la grossesse jusqu'à 25 semaines complètes ou avec thérapie intra-utérine | Cesarean delivery |
| O01A (V7.0) | Césarienne et dialyse, ou thérapie intra-utérine complexe du fœtus | Cesarean delivery |
| O01B (V3.0, V4.0) | Césarienne avec plusieurs diagnostics de complication, durée de la grossesse de 26 à 33 semaines complètes, sans thérapie intra-utérine ou avec diagnostic de complication, jusqu'à 25 semaines complètes, ou thromboembolie pendant la période de gestation avec procédure opératoire | Cesarean delivery |
| O01B (V5.0) | Césarienne avec plusieurs diagnostics de complication, durée de la grossesse de 26 à 33 semaines complètes, jusqu'à 25 semaines complètes, ou thromboembolie pendant la période de gestation avec procédure opératoire ou procédure complexe | Cesarean delivery |
| O01B (V6.0, V7.0) | Césarienne avec plusieurs diagnostics de complication, durée de la grossesse de 26 à 33 semaines ou CC extrêmement sévères ou diagnostic complexe ou procédure de complication, jusqu'à 33 semaines de grossesse ou diagnostic complexe et CC extrêmement sévères, ou jusqu'à 25 semaines de grossesse et diagnostic de complication | Cesarean delivery |
| O01C (V3.0, V4.0, V5.0) | Césarienne avec plusieurs diagnostics de complication, durée de la grossesse > 33 semaines complètes, sans thérapie intra-utérine ou avec diagnostic de complication, de 26 à 33 semaines ou avec diagnostic complexe ou jusqu'à 33 semaines ou avec diagnostic complexe, avec CC extrêmement sévères | Cesarean delivery |
| O01C (V6.0, V7.0) | Césarienne secondaire avec plusieurs diagnostics de complication ou procédure complexe, ou jusqu'à 33 semaines de grossesse ou diagnostic complexe ou diagnostic de complication et grossesse de 26 à 33 semaines ou diagnostic complexe | Cesarean delivery |
| O01D (V3.0, V4.0, V5.0) | Césarienne avec plusieurs diagnostics de complication, durée de la grossesse > 33 semaines complètes, sans thérapie intra-utérine ou avec diagnostic de complication, de 26 à 33 semaines ou avec diagnostic complexe ou jusqu'à 33 semaines ou avec diagnostic complexe, sans CC extrêmement sévères | Cesarean delivery |
| O01D (V6.0, V7.0) | Césarienne secondaire avec diagnostic de complication, durée de la grossesse plus de 33 semaines complètes | Cesarean delivery |
| O01E (V3.0, V4.0, V5.0) | Césarienne avec diagnostic de complication, durée de la grossesse plus de 33 semaines complètes, sans diagnostic complexe | Cesarean delivery |
| O01E (V6.0, V7.0) | Césarienne avec plusieurs diagnostics de complication ou procédure complexe, ou jusqu'à 33 semaines de grossesse ou diagnostic complexe, ou diagnostic de complication et grossesse de 26 à 33 semaines ou diagnostic complexe, ou césarienne secondaire | Cesarean delivery |
| O01F (V3.0, V4.0, V5.0) | Césarienne sans diagnostic de complication, durée de la grossesse plus de 33 semaines complètes, sans diagnostic complexe | Cesarean delivery |
| O01F (V6.0 V7.0) | Césarienne avec diagnostic de complication, durée de la grossesse plus de 33 semaines complètes | Cesarean delivery |
| O01G (V6.0, V7.0) | Césarienne, durée de la grossesse > 33 semaines complètes | Cesarean delivery |
| O01H (V7.0) | Césarienne, durée de la grossesse plus de 33 semaines complètes | Cesarean delivery |
| O02A (V3.0, V4.0) | Accouchement par voie basse avec procédure opératoire de complication, durée de la grossesse jusqu'à 33 semaines complètes ou avec thérapie intra-utérine | Vaginal delivery |
| O02A (V5.0, V6.0, V7.0) | Accouchement par voie basse avec procédure opératoire de complication, avec thérapie intra-utérine ou traitement complexe de soins intensifs > 119 points ou procédure de complication ou procédure complexe | Vaginal delivery |
| O02B (V3.0, V4.0) | Accouchement par voie basse avec procédure opératoire de complication, durée de la grossesse plus de 33 semaines complètes, sans thérapie intra-utérine | Vaginal delivery |
| O02B (V5.0, V6.0, V7.0) | Accouchement par voie basse avec procédure opératoire de complication | Vaginal delivery |
| O60A (V3.0) | Accouchement par voie basse avec plusieurs diagnostics de complication, au moins une complication sévère, durée de la grossesse jusqu’à 33 semaines complètes ou avec procédure de complication | Vaginal delivery |
| 060A (V 4.0, V5.0, V6.0, V7.0) | Accouchement par voie basse avec plusieurs diagnostics de complication, au moins une complication sévère, durée de la grossesse jusqu’à 33 semaines complètes ou avec procédure de complication ou thromboembolie pendant la période de gestation | Vaginal delivery |
| 060B (V3.0) | Accouchement par voie basse avec plusieurs diagnostics de complication, au moins une complication sévère, durée de la grossesse plus de 33 semaines complètes, sans procédure de complication ou thromboembolie pendant la période de gestation sans procédure opératoire | Vaginal delivery |
| 060B (V4.0, V5.0) | Accouchement par voie basse avec plusieurs diagnostics de complication, au moins une complication sévère, durée de la grossesse plus de 33 semaines complètes, sans procédure de complication ou thromboembolie pendant la période de gestation | Vaginal delivery |
| O60B (V6.0, V7.0) | Accouchement par voie basse avec plusieurs diagnostics de complication, au moins une complication sévère, durée de la grossesse plus de 33 semaines complètes | Vaginal delivery |
| O60C (V3.0, V4.0, V5.0, V6.0, V7.0) | Accouchement par voie basse avec diagnostic de complication sévère ou moyennement sévère | Vaginal delivery |
| O60D (V3.0, V4.0) | Accouchement par voie basse sans diagnostic de complication | Vaginal delivery |
| 060D (V5.0, V6.0, V7.0 ) | Accouchement par voie basse | Vaginal delivery |
| Midwife codes | ||
| B1 | Leitung einer ambulanten Geburt | Vaginal delivery |
| B2 | Zweithebamme für ambulante Geburt oder Verlegung | Vaginal delivery |
| B3 | Verbrauchsmaterial für unvollendete ambulante Geburt | Vaginal delivery |
| B4 | Verbrauchsmaterial für ambulante Geburt | Vaginal delivery |