Evolution of SARS-CoV-2 seroprevalence and clusters in school children from June 2020 to April 2021: prospective cohort study Ciao Corona
DOI: https://doi.org/10.4414/SMW.2021.w30092
Agne
Ulytea, Thomas
Radtkea, Irene A.
Abelabc, Sarah R.
Hailea, Priska
Ammanna, Christoph
Bergerd, Alexandra
Trkolab, Jan
Fehrac, Milo A.
Puhana, Susi
Kriemlera
aEpidemiology, Biostatistics and Prevention Institute (EBPI), University of Zurich, Switzerland
bInstitute of Medical Virology, University of Zurich, Switzerland
cDivision of Infectious Diseases & Hospital Epidemiology, University Hospital Zurich, Switzerland
dUniversity Children Hospital Zurich, Switzerland
*Shared first authorship
**Shared last authorship
Summary
BACKGROUND: Few studies have explored the spread of SARS-CoV-2 in schools in 2021, with the advent of variants of concern. We aimed to examine the evolution of the proportion of seropositive children at schools from June-July 2020 to March-April 2021. We also examined symptoms, under-detection of infections, potential preventive effect of face masks, and reasons for non-participation in the study.
METHODS: Children in lower (7–10 years), middle (8–13 years) and upper (12–17 years) school levels in randomly selected schools and classes in the canton of Zurich, Switzerland, were invited to participate in the prospective cohort study Ciao Corona. Three testing rounds were completed in June-July 2020, October-November 2020 and March-April 2021. From 5230 invited, 2974 children from 275 classes in in 55 schools participated in at least one testing round. We measured SARS-CoV-2 serology in venous blood, and parents filled in questionnaires on sociodemographic information and symptoms.
RESULTS: The proportion of children seropositive for SARS-CoV-2 increased from 1.5% (95% credible interval [CrI] 0.6–2.6%) by June-July 2020, to 6.6% (4.0–8.9%) by October-November, and to 16.4% (12.1–19.5%) by March-April 2021. By March-April 2021, children in upper school level (12.4%; 7.3–16.7%) were less likely to be seropositive than those in middle (19.5%; 14.2–24.4%) or lower school levels (16.0%; 11.0–20.4%). The ratio of PCR-diagnosed to all seropositive children changed from one to 21.7 (by June-July 2020) to one to 3.5 (by March-April 2021). Potential clusters of three or more newly seropositive children were detected in 24 of 119 (20%) classes, 17 from which could be expected by chance. Clustering was not higher than expected by chance in middle and upper school levels. Children in the upper school level, who were wearing face masks at school from November 2020, had a 5.1% (95% confidence interval 9.4% to 0.7%) lower than expected seroprevalence by March-April 2021 than those in middle school level, based on difference-in-differences analysis. Symptoms were reported by 37% of newly seropositive and 16% seronegative children. Fear of blood sampling (64%) was the most frequently reported reason for non-participation.
CONCLUSIONS: Although the proportion of seropositive children increased from 1.5% in June-July 2020 to 16.4% in March-April 2021, few infections were likely associated with potential spread within schools. In March-April 2021, significant clustering of seropositive children within classes was observed only in the lower school level.
Trial Registration: ClinicalTrials.gov NCT04448717
Introduction
In response to the high incidence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections and emerging variants of concern in the autumn and winter of 2020/2021 [1] attendance of schools has been disrupted in many countries. Half of the countries worldwide and in Europe interrupted physical attendance of schools for at least 30 weeks from March 2020 to June 2021 [2], but this was only for 7 weeks in Switzerland. Nevertheless, by November 2020, only minimal clustering of seropositive children was observed in the canton of Zurich, Switzerland, after 2–3 months of school attendance including 2–6 weeks of high community incidence [3]. Other studies showed that the spread of SARS-CoV-2 infection within schools was not larger than in the surrounding community in 2020, when variants of concern such as Alpha (B.1.1.7) and Delta (B.1.617.2) were not prevalent in most of the countries, and the rates of secondary attack and outbreaks low [4–7]. In contrast, the expected damage caused by school closures could result in worse mental health in children, reduced learning and subsequent income losses, and amplify gender and socioeconomic inequalities within and between countries [8].
However, only scarce information is available yet on SARS-CoV-2 infection in schools since December 2020, when alpha and subsequently delta variants of SARS-CoV-2 started dominating in Europe and other countries. In Switzerland, approximately 80% of SARS-CoV-2 infections were due to the alpha variant in March 2021 (see appendix 1) [9]. Children below the age of 12 will be the last group to be offered vaccination. Preventive measures in schools will likely need to be adjusted as new variants spread while more people, especially vulnerable populations, become vaccinated. Thus, monitoring the evolution of seroprevalence and clustering of infections within schools remains relevant.
The Ciao Corona study uniquely examines SARS-CoV-2 seroprevalence on the class, school, and district level. The objectives of this study were to assess longitudinally, with measurements in June-July and October-November 2020, and March-April 2021, the proportion of seropositive children and adolescents within school levels, cantonal districts and the region (canton of Zurich), the association of seropositivity with reported symptoms, and the frequency and evolution of clustering of seropositive children within classes in schools. In addition, we examined the potential effect of face masks on the evolution of seroprevalence in upper school level children and reasons for participation and non-participation in this cohort study, in order to address potential participation bias.
Materials and methods
The protocol [10] and previous results of this longitudinal study [3, 11] are reported elsewhere. This study is part of the nationally coordinated research network Corona Immunitas [12]. The study follows a cohort of randomly selected schools and classes in the canton of Zurich, Switzerland. The canton has a population of 1.5 million linguistically and ethnically diverse inhabitants in both rural and urban settings, and comprises 18% of the Swiss population [13].
During the COVID-19 pandemic in Switzerland, physical attendance of schools was interrupted only in March-May 2020. Preventive measures, such as distancing and reduced mixing of classes, were implemented with some variation between schools. All schools required ill children to stay home unless with very mild symptoms. Adults at school were required to wear masks from October 2020, secondary school children from November 2020, and primary school children in the middle school level grades from late January 2021.
School-specific contact tracing was implemented in school year 2020/2021. Testing and quarantine recommendations depended on the specific situation. As a general rule, the whole class was quarantined when two or more infected children were detected in the class simultaneously. If children were wearing masks, only close contacts were quarantined. Daily incidence of diagnosed SARS-CoV-2 cases between October 2020 and April 2021 in the canton of Zurich and Switzerland and the proportion of variants of concern is shown in appendix 1.
Ethics approval
The study was approved by the Ethics Committee of the Canton of Zurich, Switzerland (2020-01336). All participants provided written informed consent before being enrolled in the study.
Population
As described previously [3, 10, 11], in May-June 2020 we randomly selected primary schools in the canton of Zurich and matched the geographically closest secondary school. The number of schools invited in the 12 districts of the canton was proportional to population size.
We randomly selected classes within participating schools, stratified by school level: grades 1 to 2 in lower level (attended by 6-to 9-year-old-children), grades 4 to 5 in middle level (9- to 13-year-old children) and grades 7 to 8 in upper school level (12- to 16-year-old-children); grades were selected from the eligible grades in the school randomly. We aimed to invite at least three classes or at least 40 children in each invited school level of a school (i.e., ensuring that at least 40 children were invited if fewer than three classes were eligible within smaller schools, and a sufficient number of classes so that a total of at least 40 children are invited in schools with small classes). The random invitation of schools and classes ensured that the invited population is approximately representative for the school-aged children within the districts of the canton of Zurich.
Eligible children and adolescents (hereafter referred to as children) of the selected classes could participate in any of the testing rounds. Major exclusion criterion was suspected or confirmed SARS-CoV-2 infection during the testing (precluding child’s attendance of the testing at school).
Serological testing and questionnaire information
Venous blood samples were collected at schools from 16 June to 9 July 2020 (T1), 26 October to 19 November 2020 (T2); and 15 March to 16 April 2021 (T3). Blood samples were analysed with the ABCORA binding assay of the Institute of Medical Virology (IMV) of the University of Zurich, which is based on Luminex technology [14]. Binary results of the ABCORA 2.3 algorithm showed 98.2% sensitivity and 99.4% specificity [14]. Figure 1 shows the flowchart of participants, and appendix 2 includes counts of children with serological results at each testing round, and further details of the test.
We defined a composite (cumulative) serological outcome as the proportion of children who tested seropositive in at least one of the testing rounds (ever-seropositive children). The composite outcome allowed the proportion of all children who had SARS-CoV-2 antibodies at any time by a specific testing to be estimated, and was measured for T1, T2 and T3 separately (see detailed definitions of outcomes T1, T12, and T123 in appendix 2). To examine the clustering of new cases within tested classes, we defined the outcome of newly seropositive children at T3 (seropositive at T3 but not in the previous testing rounds), in a way similar to the approach in the previous publication [3].
Parents of participants filled in online questionnaires on sociodemographic and health information on the child and household. In March-April 2021, parents of eligible non-participating children were invited to complete an anonymous questionnaire on the reasons for non-participation (details in appendix 3). After T3 testing, we interviewed the principals of schools in which at least two classes with new clusters of seropositive children were detected (details in appendix 4).
We obtained official statistics of SARS-CoV-2 infections in the canton of Zurich [15] in order to calculate the cumulative incidence of diagnosed SARS-CoV-2 cases by T1, T2 and T3 testing, in children aged 7–17 years, and compare with the proportion of children who were seropositive by 30 June 2020 (median time point of T1), 6 November 2020 (T2), and 29 March 2021 (T3).
Statistical analysis
We report key characteristics of participants (age, school level, sex) as well as their reported symptoms summarised as median (range) or count (%).
To estimate the proportion of ever-seropositive children by T1, T2 and T3 testing, we employed Bayesian logistic regression [16], which was adjusted for participants’ grade at school, sex and geographic district of the school, and contained random effects for school levels (lower, middle and upper). The Bayesian approach permitted adjustment for the accuracy of serological test and the hierarchical structure of the cohort (individual, school and district levels). To compute estimates representative for the canton of Zurich even in the case of differential participation rates of children within districts or grades, we post-stratified our results according to the total population size at the school level and geographic district. We compared the estimates across school levels and districts.
Masks were introduced for upper school level children at the end of October 2020. Masks were also obligatory for middle school level children from late January 2021; however, the majority of SARS-CoV-2 infections between T2 and T3 happened earlier, in November 2020 to January 2021. We employed difference-in-differences analysis (DD) [17] to examine whether upper school level had a different from expected seroprevalence at T3, in regard to the previous trend at T1-T2 and in comparison with middle and lower school levels. Briefly, assuming that the outcome (seroprevalence) would develop in parallel over time in the compared groups (different school levels), DD allows potential deviation from the parallel trend in the intervention group to be identified . We modelled DD and examined the parallel trends assumption with a linear probability model [18]. The model included children who were tested three times (n = 1965). The binary outcome was ever-testing-positive by T1, T2 and T3. The models controlled for the measurement time point (T1, T2 or T3) and class of the child (thus, implicitly also for district and school grade). Robust cluster-corrected errors at the child level were used, to adjust for the heteroscedasticity of residuals and autocorrelation of outcomes at the three times points in the same child [19]. We applied the model to compare upper school level with lower, middle, and combined lower and middle school levels.
We compared the cumulative incidence of SARS-CoV-2 cases confirmed with reverse transcriptase polymerase chain-reaction testing (RT-PCR) with the proportion of ever-seropositive children, in order to estimate the ratio of undiagnosed to all SARS-CoV-2 cases.
Data analysis was performed with R version 4.0.3 [20]. Bayesian hierarchical modelling was performed using the R package rstan [21].
Analysis of potential clusters
We examined potential clusters (at least three newly T3 seropositive children) in classes in which at least five children were eligible and at least 50% of the children were tested. We compared the observed distribution of clusters with the distribution expected if SARS-CoV-2 infections were distributed among children across classes independently (randomly), as described previously [3]. In a simulation, we created 2500 hypothetical populations of children, corresponding to the study population in terms of the observed overall seroprevalence, number of classes and tested children within them. We further ran the simulation separately for classes within different school levels separately. By comparing the expected and the observed distributions, we could estimate whether the observed number of classes with clusters is compatible with the hypothesis of no association of SARS-CoV-2 infections within classes. We further compared the results of the simulation with the information from school principal interviews.
Results
In total, 2487 children from 275 classes in 55 schools in the canton of Zurich were tested between 15 March and 16 April 2021 (T3). Of these, 2237 (90%) participated in October-November 2020, and 2176 (87%) in June-July 2020. Retention rate from T1 to T2 was 84% (2176/2601) and 88% (2237/2552) from T2 to T3. The flowchart of participants is shown in figure 1.
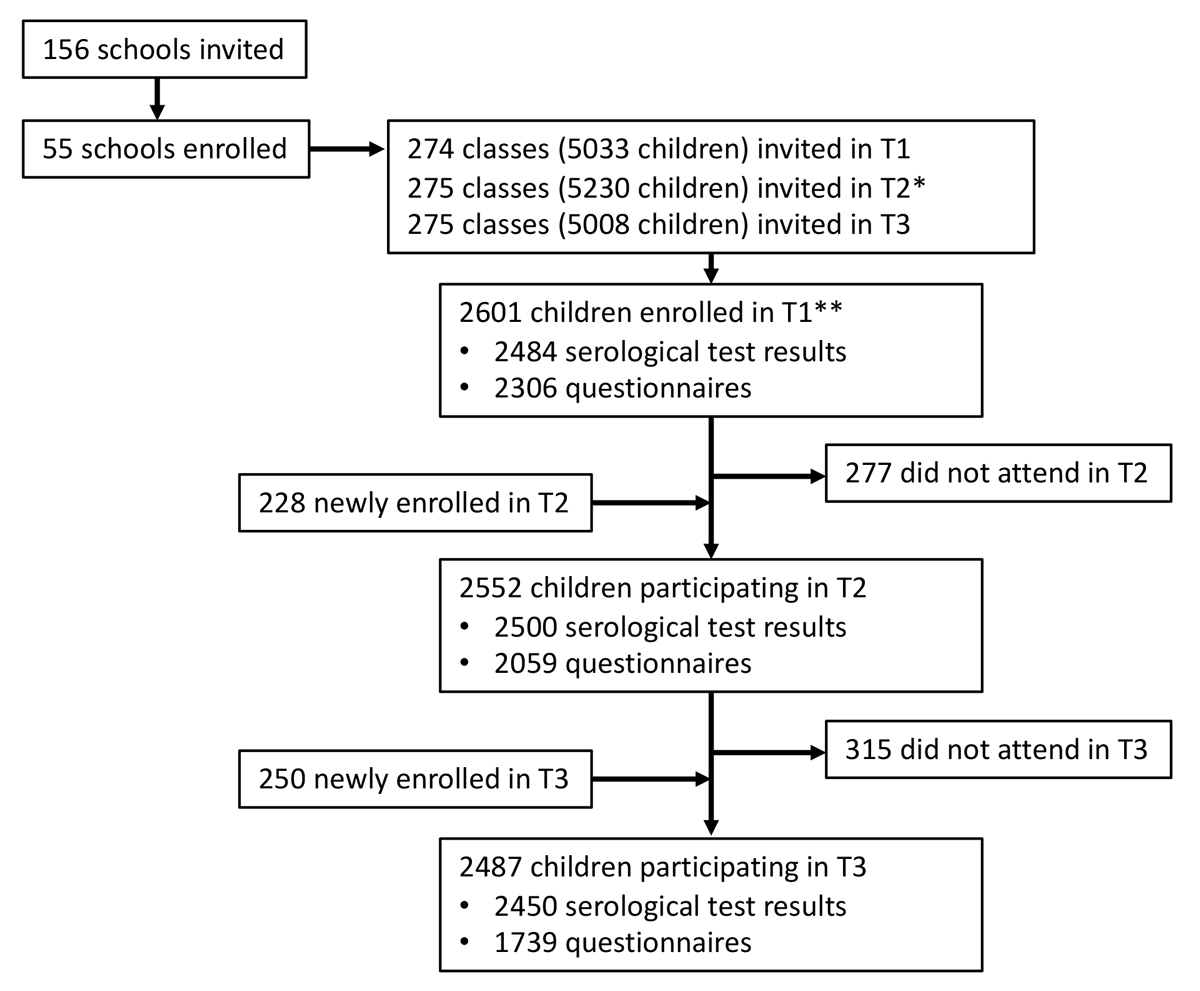
Figure 1 Flowchart of study participants.
T1 – testing in June-July 2020, T2 – testing in October-November 2020, T3 – testing in March-April 2021. Newly enrolled children were not tested in the previous round; did not attend means they did not attend the subsequent round.
* Some classes were split or rearranged after the summer break.
** 16 of these children were enrolled from late August to early September 2020 (10 serological results, 16 questionnaires available).
Serological results were available at T3 for 768 children in the lower school level (median age 8, age range 7–10 years), 845 children in the middle school level (median age 12, age range 8–13 years) and 837 children in the upper school level (median age 14, age range 12–17 years). Of these, 1279 children reported female, 1165 male and 6 other gender. The median participation rate within classes was 50% (interquartile range 35–63%; median 45% in lower, 54% in middle, and 50% in upper school levels).
Serological results
Table 1 presents the distribution of serological results at T1, T2 and T3 of children who were tested in all three testing rounds. Among children with serological results in the respective two rounds of testing, 33/52 (63%) of those seropositive at T1 were seropositive after 4 months at T2, 32/46 (70%) after 9 months at T3, and 101/115 (88%) of those seropositive at T2 were seropositive after 5 months at T3 (appendix 2).
Table 1Longitudinal serological results of children tested at all three testing rounds (n = 1965).
|
T1 result
|
T2 result
|
T3 result
|
| Positive |
44 |
Positive |
29 |
Positive |
26 |
| Negative |
3 |
| Negative |
15 |
Positive |
5 |
| Negative |
10 |
| Negative |
1921 |
Positive |
73 |
Positive |
65 |
| Negative |
8 |
| Negative |
1848 |
Positive |
209 |
| Negative |
1639 |
The proportion of ever seropositive children was 1.5% (95% credible interval [CrI] 0.6–2.6%) at T1, 6.6% (95% CrI 4.0–8.9%) at T2 and 16.4% (95% CrI 12.1–19.5%) at T3 (fig. 2). The proportion of ever seropositive children at T3 was 16.0% (11.0–20.4%) in lower, 19.5% (14.2–24.4%) in middle, and 12.4% (7.3–16.7%) in upper school levels. Seroprevalence was not statistically significantly different between lower and middle (p = 0.26) or lower and upper levels (p = 0.18), but different between middle and upper school levels (p = 0.02). The proportion of ever-seropositive children ranged from 9.2% to 25.7% in the districts of Zurich at T3. The proportion did not differ between boys and girls, although the estimates were slightly higher for boys (T1: 1.8% vs 1.1%; T2: 7.1% vs 6.1%; T3: 17.2% vs 15.6%).
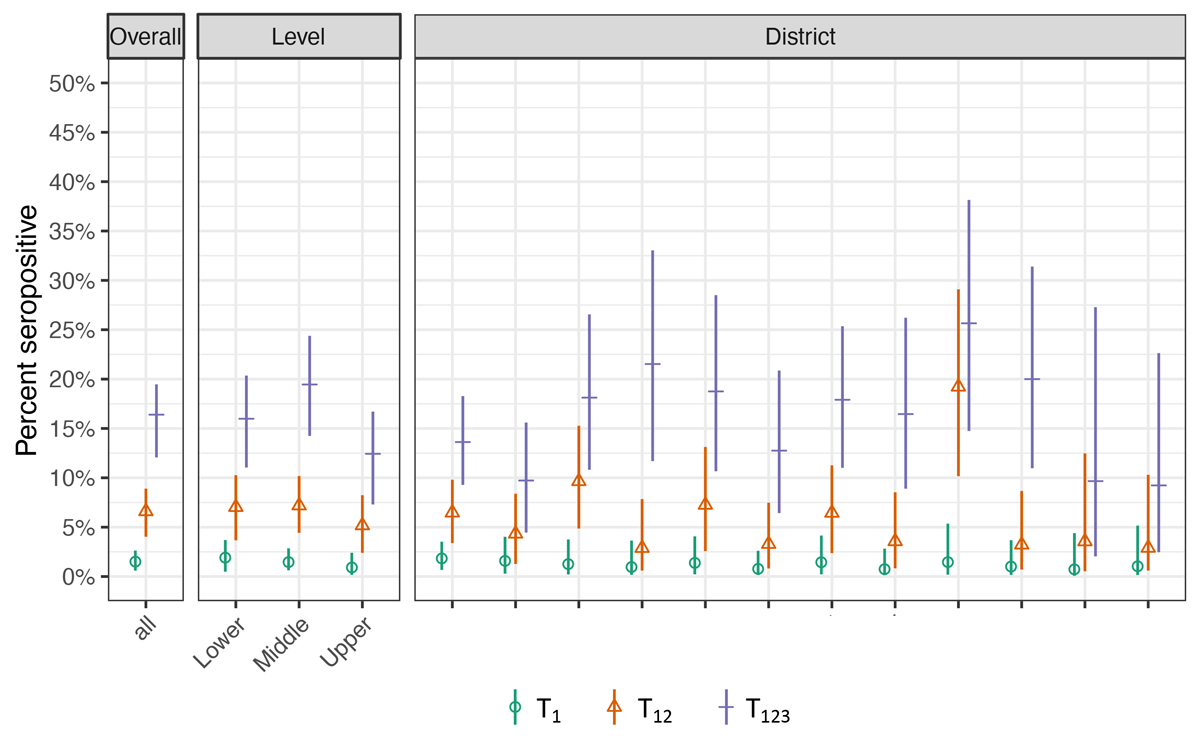
Figure 2 Proportion of ever-seropositive children in June-July 2020 (T1), October-November 2020 (T12) and March-April 2021 (T123).
T1 – proportion of children testing seropositive by June-July 2020, T12 – by October-November 2020, T123 – by March-April 2021.
Overall and school level specific estimates (lower school level: grades 1–2, children aged 6–10 years,; middle school level: grades 4–5, children aged 9–13 years; upper school level: grades 7–8, children aged 12–16 years; grades and age range are reported for time point of T1), and district specific estimates for the canton of Zurich, Switzerland. Districts are ranked by their population size, from largest to smallest.
In the difference-in-differences analysis, the parallel trends assumption between T1 and T2 was valid based on visual inspection (fig. 3A) and formal examination with the linear probability models (fig. 3B). The proportion of ever-seropositive children in the upper school level was lower than expected at T3 in comparison with lower and middle school levels (absolute difference of 3.2%, 95% confidence interval [CI] –7.0% to 0.6%; p = 0.098, relative difference of 19%). The proportion was lower by 5.1% (95% CI –9.4% to –0.7%, p = 0.022, relative difference of 27%) when compared only to the middle school level, and by 0.8% (95% CI –5.4% to 3.8%, p = 0.718, relative difference of 6%) when compared only to the lower school level.
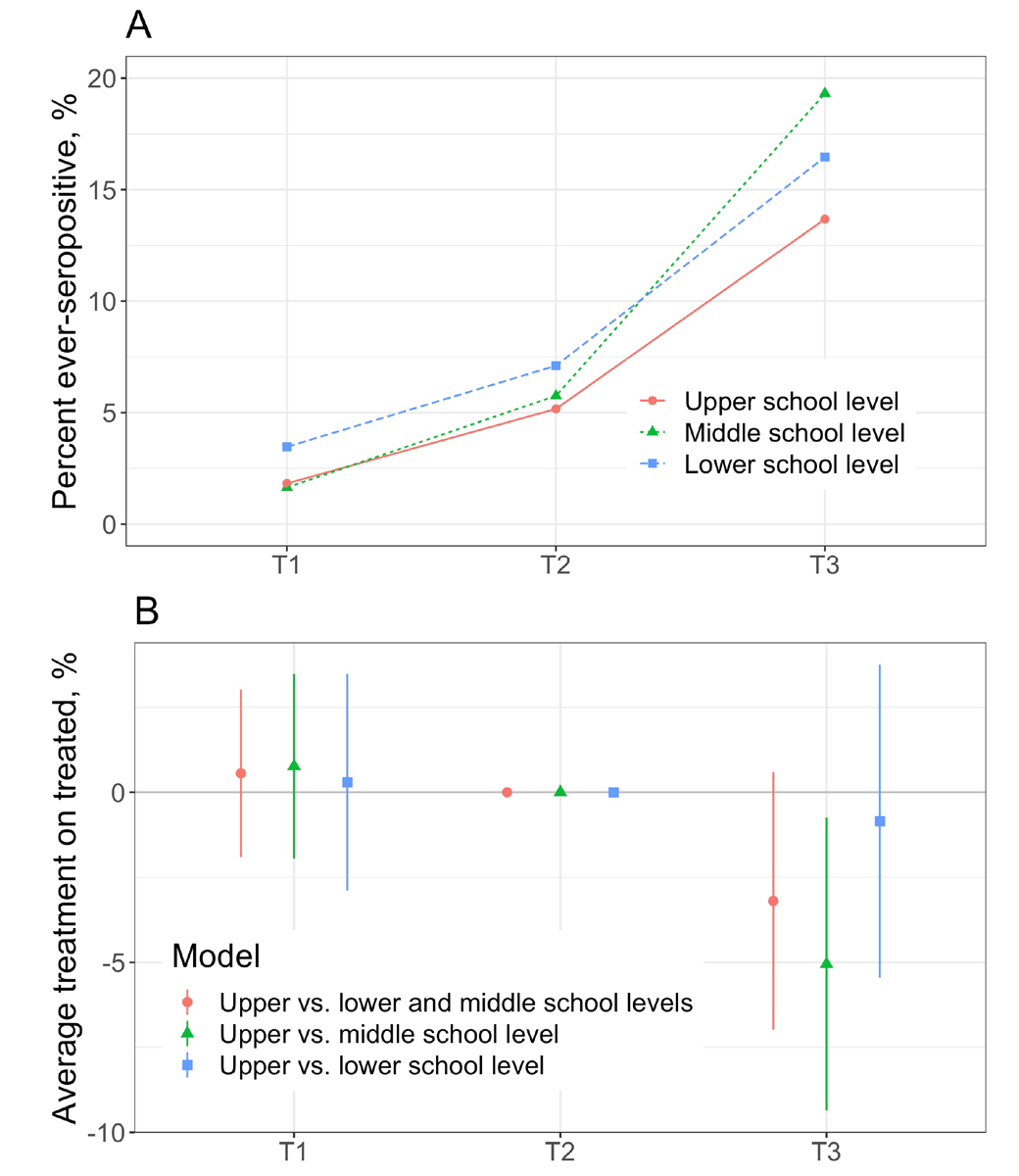
Figure 3 Difference-in-differences model of the change in ever-seroprevalence between T2 and T3 in upper school level.
A – Raw proportion of children, included in the models, ever testing seropositive by T1, T2 and T3 time points
B – Difference-in-differences estimates of linear probability models, with T2 as the reference. The parallel trends assumption is valid, as the estimates at T1 are not different from the T2 reference. Average treatment (effect) on the treated denotes the change in seroprevalence, potentially attributable to the effect of mask wearing by upper school level children.
The ratio of diagnosed to seropositive children was 1 to 21.7 by June-July 2020, 1 to 5.8 by October-November 2020, and 1 to 3.5 by March-April 2021.
Symptoms
Symptoms between T2 and T3 were reported in 67/182 (37%) of newly seropositive and 235/1443 (16%) of seronegative children (fig. 4). Symptoms most commonly reported by newly seropositive children were fatigue (30/182, 17%), sore throat (30/182, 17%), headache (27/182, 15%), fever (26/182, 14% for fever ≥38°C and 25/182, 14% for subjective fever) and runny or congested nose (22/182, 12%). Fatigue, sore throat, headache, fever, stomach ache, muscle or joint pain, and loss of smell or taste were reported more frequently by seropositive than seronegative children. Similar proportions of seropositive children reported symptoms in lower (35%, 22/63), middle (40%, 32/81) and upper (37%, 14/38) school levels. None of the participating children reported hospitalisation between T2 and T3 testing.
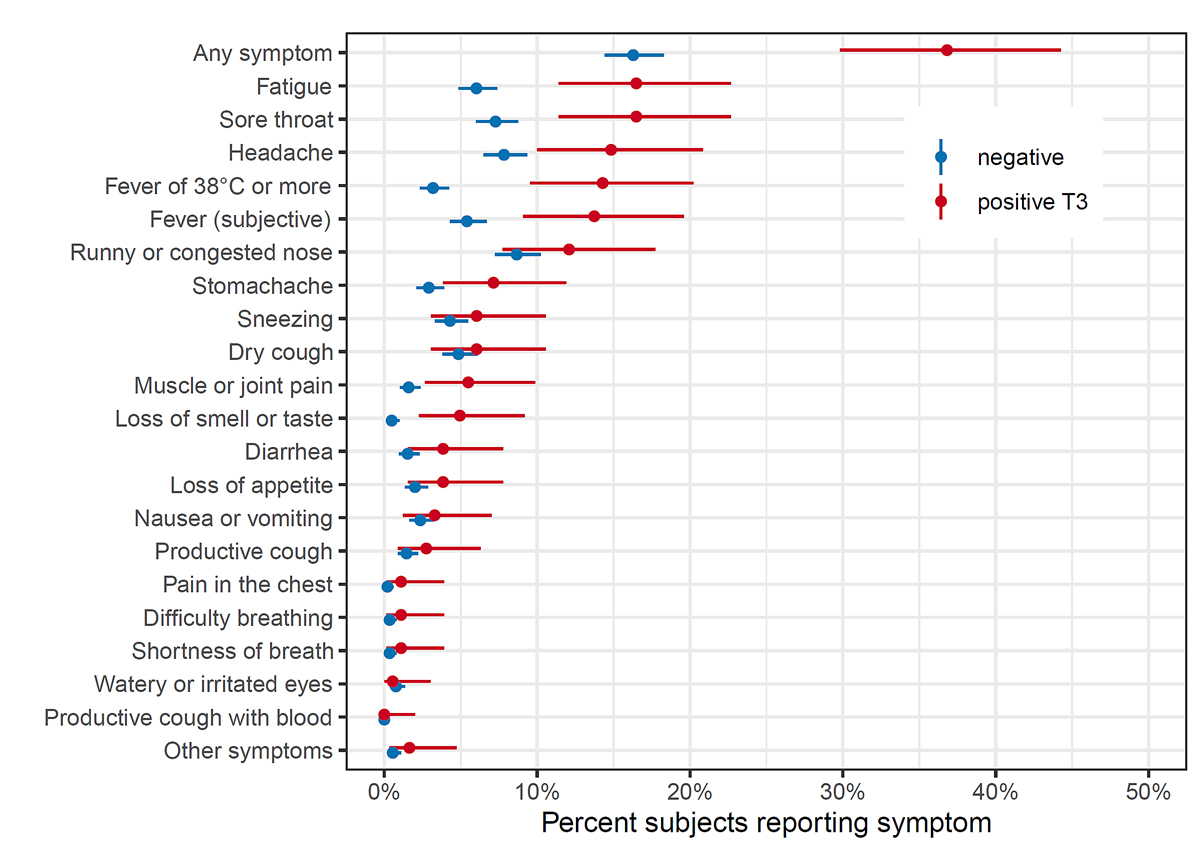
Figure 4 Symptoms reported between October-November 2020 and March-April 2021 in children who were seronegative and newly seropositive at T3 (March-April 2021).
Cluster analysis
At least one child newly seropositive at T3 was detected in 53 of 55 schools and 151 of 275 (55%) classes (76 of 119 [64%] classes with high participation rate). At least one child who was ever-seropositive at T3 was detected in all 55 schools and 184 of 275 (67%) classes (or in 95 of 119 [80%] classes with high participation rate). Figure 5 shows the distribution of seropositive children within classes with high participation rate.
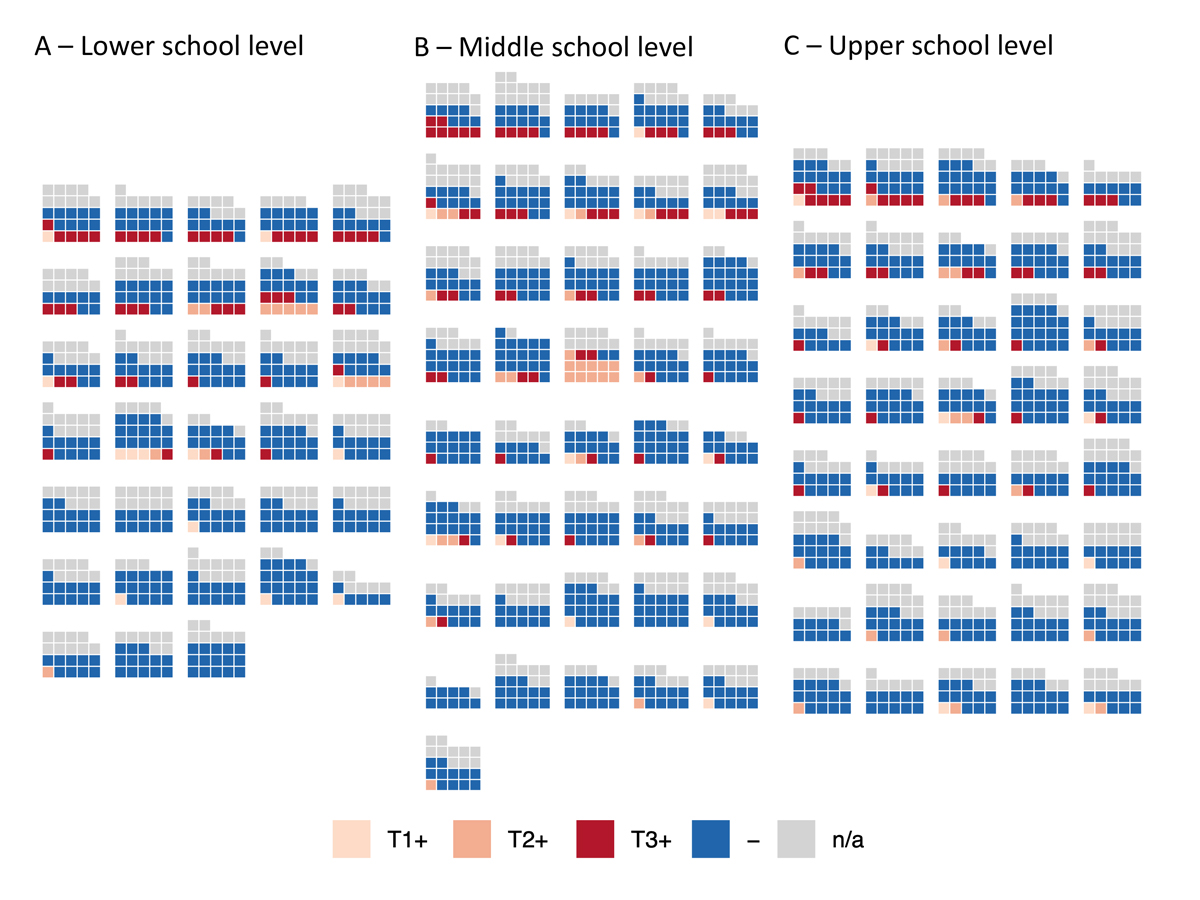
Figure 5 Distribution of children who were seropositive at T1, T2 and T3 in classes with high participation rate at T3.
Each block of squares represents a class. High participation rate means that at least 5 children were eligible and at least 50% children were tested in the class.
T1+ (light pink) – children who tested seropositive at T1 (June-July 2020), T2+ (middle orange) – children who tested seropositive at T2 (October-November 2020) but not T1, T3+ (dark red) – children who tested seropositive at T3 (March-April 2021) but not at T1 or T2, - (dark blue) – children who tested seronegative at T3 and were not test seropositive previously, n/a (grey) – children without a serological result at T3.
At T3, 39 of 275 (14%) classes had potential clusters of newly seropositive children. Twenty-four of 119 (20%) classes with high participation had clusters: 9 of 33 (27%) in the lower school level, 10 of 41 (24%) in the middle, and 5 of 45 (11%) in upper school level. Based on the interviews with school principals, intra-class transmission could have happened in 12 classes with potential clusters (63%), was improbable in 7 (37%), and could not be assessed because of insufficient information in 6 classes. The majority of RT-PCR-diagnosed infections and exposure resulting in quarantine of children originated from children’s households. Detailed results of the interviews are presented in appendix 4.
Assuming a uniform 10.9% rate of newly seropositive children among those not previously tested positive (158/1422 within classes with high participation), we would expect to observe a median of 17 (95% CrI 12–22) clusters within 119 classes with high participation. In comparison, we observed 24/119 (20%) such classes, making the hypothesis of completely independent distribution of seropositive children unlikely (p = 0.0052). The simulated distributions are shown in appendix 5. The expected and observed distributions of clusters were different in the lower school level (median of 5/33 expected vs 9/33 observed; p = 0.001) but not in middle ( 9/41 expected vs 10/41 observed; p = 0.27) and upper school levels (4/45 expected vs 5/45 observed; p = 0.29).
Reasons for participation and non-participation
On the day of testing at T3, 10 of 5517 (0.2%) children in the invited classes were not at school because of diagnosed or suspected SARS-CoV-2 infection, 47 (0.9%) because of quarantine, and 164 (3.0%) for other reasons. Parents of 712 of 2659 (27%) children provided reasons for non-participation; child’s fear of the blood sampling was reported the most often (358/564, 64% of children; 189/276, 69% of girls and 158/259, 61% of boys), especially in lower school levels (191/264, 72%). Among the participants, 28% (374/1331) reported participating for personal and 90% (1192/1331) for societal reasons. Detailed results are provided in appendix 3.
Discussion
In this cohort study of more than 2500 children in 55 schools, the proportion of children seropositive for SARS-CoV-2 increased from 1.5% in June-July 2020 to 6.6% in October-November and to 16.4% in March-April 2021. In March-April 2021, seroprevalence was lower in the upper (12.4%) than in middle (19.5%) or lower (16.0%) school levels. Although potential clusters of seropositive children were detected in approximately 20% of classes, the majority could be explained by infections not associated within a class, particularly in the middle and upper school levels. Seropositive children reported a history of acute symptoms more frequently than seronegative children, although 63% did not report any symptoms. Eighty-eight percent of seropositive children retained their antibodies for at least 5 months.
The canton of Zurich experienced an early second wave of the SARS-CoV-2 pandemic in 2020. Daily incidence of RT-PCR cases peaked at 88 in 100,000 inhabitants in late October 2020 [15], comparable to the peaks of 75 and 90 new cases in 100,000 inhabitants in the US and UK in early January 2021 [1]. Our study suggests an increasingly higher proportion of children diagnosed with RT-PCR, reflecting the revised indications for testing in children. Initially restrictive, the indications for testing children over 12 years matched those for adults in September 2020, and extended to children over 6 years from March 2021 [22]. However, the persisting high number of undiagnosed infections and thus the total spread of SARS-CoV-2 in children should be considered while planning preventive measures, such as masking in indoor spaces.
The increase of seropositive children by March-April 2021 was the smallest (and potential clusters least frequent) in upper school level. In contrast to our findings, SARS-CoV-2 has been observed to spread more among older children and adolescents [23, 24], partly explained by different patterns of contacts [25, 26] and susceptibility [27]. SARS-CoV-2 spread and secondary attack rates were associated with children’s age in a school contact tracing study in late 2020 [28]. In Switzerland, adolescents from grade 7 (approximately age 14) have been required to wear masks at school since November 2020. Potentially, consistent masking during the second wave of SARS-CoV-2 infections in November 2020 to January 2021, as well as different distancing behaviour of teenagers towards their parents compared with young children, could have contributed to fewer infections. The preventive effect of masking is supported by the results of the difference-in-differences analysis. In comparison with the middle school level, where masks were introduced 3 months later, the proportion of children ever testing seropositive in the upper school levels was lower by 5.1% (relative reduction of 27%) than could be expected if seroprevalence developed in the same trend as in the middle school level between T2 and T3. However, no significant difference was found in the development of seroprevalence between T2 and T3 between upper and lower school levels, potentially, as the age (and thus, susceptibility and behavioural patterns) are more different. Indeed, school contact tracing studies showing the positive association of SARS-CoV-2 infections with age were often conducted in countries with all school-aged children wearing masks (e.g., Italy [29], Catalonia [28, 30]), or no consistent mandate of masking for children at school (e.g., UK [31]). Although there are randomised studies on the effectiveness of masking to prevent SARS-CoV-2 spread for adult populations [32] and correlation studies of masking and SARS-CoV-2 infections at schools [33, 34], our study was unique in studying the effect of masking on SARS-CoV-2 infections in school-aged children.
Our results suggest that only a small part of potential clusters of seropositive children within classes were likely associated with intraclass transmission in 2020–2021. Clustering in 2021 was the most prevalent in lower school level, where masks for children were not mandated at any time point. Based on interviews with school principals, at least 33% of the clusters were unlikely to be due to intraclass transmission. These findings are supported by prospective studies of school contact tracing in late 2020, showing that the majority of SARS-CoV-2 infections identified in a school setting were not associated with further secondary cases within the school [29]. Furthermore, the secondary attack rates within families with a child index case were lower during school time as compared with the summer holiday [30].
In contrast to previous measurements in summer and autumn of 2020, three symptoms were reported more frequently in seropositive than seronegative children. The retrospective recall of symptoms could have been influenced by the knowledge of and increased attention to the symptoms, population incidence, and personal diagnosis of SARS-CoV-2. The influence of variants of concern on the symptoms of SARS-CoV-2 infection in children cannot be ruled out. However, the majority of symptom episodes were reported in December 2020 to February 2021 and variants of concern became predominant in Switzerland from March 2021 (see appendix 1). Although previously, 20–30% of child cases were estimated to be asymptomatic [35], a recent meta-analysis found almost half of SARS-CoV-2 infections in children, significantly more than in adults, to be asymptomatic [37].
The predominant reason for non-participation in the study was fear of blood sampling (not likely to be associated with particularly biased selection into the study), followed by lack of interest in participating in a research study. The non-participation rate was higher than the attrition rate (50% vs 16%), meaning that once enrolled, few children dropped out.
Most of the studies of SARS-CoV-2 spread in schools rely on contact tracing data [5, 6]. At the start of the 2020/2021 school year, such studies, a large online survey in the US [34], and the few available seroprevalence studies of school children [3, 38] pointed to the spread being low in schools with implemented protective measures. Prevalence of acute SARS-CoV-2 infections in school children and the general population tend to correlate [7, 11, 39]. Less evidence exists about how SARS-CoV-2 spread within schools might change owing to vaccination of adults and variants of concern with higher infectiousness [36], or in the case of implemented PCR-based screening of school children, which could substantially increase the proportion of diagnosed SARS-CoV-2 infections.
Strengths and limitations
The Ciao Corona cohort study is a population-based cohort of children within randomly selected schools and classes, with repeated measurements of SARS-CoV-2 serology and a high retention rate. Ascertainment of SARS-CoV-2 infections via serological testing means that the asymptomatic, previously not diagnosed children are also detected. It captures the whole spectrum of SARS-CoV-2 infections in children, which is important as under-detection remains significant.
The study has limitations. First, the exact timing of infection could not be ascertained. We could examine associations of infections within a class only indirectly (e.g., simulation study of distribution of clusters) or retrospectively (e.g., interviews with school principals). Second, although we used a highly accurate serological test and adjusted for accuracy in the Bayesian models, false positive and negative results cannot be avoided on an individual level. Low prevalence at T1 and T2 resulted in relatively lower positive predictive value of the serological test (approximately 0.75 at T1 and 0.90 at T2); however, it reached 0.97 at T3. Retention was slightly lower between T1 and T2 than between T2 and T3, potentially as more false positives were expected among T1 participants. Third, although 50% participation rate in our study is rather high for a study with venous blood sampling in children [40], it could still cause selection bias.
Conclusion
In Switzerland, an increase of SARS-CoV-2 seroprevalence in school children from 1.5% in June-July 2020 to 16.4% in March-April 2020 was accompanied by an increase of potential clusters of seropositive children in classes. Despite schools remaining open since May 2020, the majority of clusters in classes could be explained by unrelated, independent infections, likely stemming from household or community transmission. The increase in seroprevalence and clustering was lower in the upper school level, where masks were introduced in November 2020. Preventive measures in schools and improved detection of infections possibly contributed to mitigate the spread of SARS-CoV-2 within schools.
Data sharing statement
Data is still being collected for the longitudinal cohort study Ciao Corona. Upon study completion in 2022, de-identified and potentially aggregated participant data, together with required data dictionaries, will be available on reasonable request by email to the corresponding author. The purpose and methods of data analysis will be evaluated by the study team first to ensure that it complies with the ethics approval.
Acknowledgements
We would like to acknowledge Miquel Serra-Burriel (Epidemiology, Biostatistics and Prevention Institute, University of Zurich) for his valuable inputs on the implementation of the difference-in-differences analysis.
Contributors: SK and MAP initiated the project and preliminary design, with support of JF. SK, MAP, CB, TR and AU developed the design and methodology, with contribution of SRH. SK, AU, TR, PA recruited study participants, collected and managed the data. SRH performed statistical analysis. AT and IA developed the serology analysis plan, supervised, conducted and evaluated the serology tests. AU wrote the first draft of the manuscript. All authors contributed to the design of the study and interpretation of its results, and revised and approved the manuscript for intellectual content. SK, AU, TR and SRH had access to and verified all underlying data. The corresponding author SK attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Supplementary material
The appendices are available in the PDF version of the manuscript.
Appendix 1 Weekly incidence of SARS-CoV-2 detected cases and the proportion of the variants of concern (VOC) among them in September 2020 – April 2021
Appendix 2 Combinations of longitudinal serological results, at three testing time points, and serological outcomes
Appendix 3 Detailed results of survey for participants and non-participants on reasons for participation and non-participation
Appendix 4 Detailed information of classes with potential clusters (3 or more newly seropositive children at T3) in schools with at 2 or more classes with clusters
Appendix 5 Distribution of expected number of classes with clusters (3 or more newly seropositive children), standardised to 100 classes with high participation rate
Prof. Susi Kriemler
Epidemiology, Biostatistics and Prevention Institute (EBPI)
University of Zurich
Hirschengraben 84
CH-8001 Zürich
susi.kriemlerwiget[at]uzh.ch
References
1.
Roser M
,
Ritchie H
,
Ortiz-Ospina E
,
Hasell J
. Coronavirus Pandemic (COVID-19) [Internet]. Published online at OurWorldInData.org. 2020 [cited 2021 Jul 1]. Available from: https://ourworldindata.org/coronavirus
2. Education: From disruption to recovery [Internet]. [cited 2021 Jun 16]. Available from: https://en.unesco.org/covid19/educationresponse#durationschoolclosures
3.
Ulyte A
,
Radtke T
,
Abela IA
,
Haile SR
,
Berger C
,
Huber M
, et al.
Clustering and longitudinal change in SARS-CoV-2 seroprevalence in school children in the canton of Zurich, Switzerland: prospective cohort study of 55 schools [Internet]. BMJ. 2021 Mar;372:n616. [cited 2021 Apr 21] https://doi.org/10.1136/bmj.n616
4.
Zimmerman KO
,
Akinboyo IC
,
Brookhart MA
,
Boutzoukas AE
,
McGann KA
,
Smith MJ
, et al.; ABC SCIENCE COLLABORATIVE
. Incidence and Secondary Transmission of SARS-CoV-2 Infections in Schools [Internet]. Pediatrics. 2021 Apr;147(4):e2020048090. [cited 2021 Jan 29] Available from: www.aappublications.org/news https://doi.org/10.1542/peds.2020-048090
5.
Macartney K
,
Quinn HE
,
Pillsbury AJ
,
Koirala A
,
Deng L
,
Winkler N
, et al.
Transmission of SARS-CoV-2 in Australian educational settings: a prospective cohort study. Lancet Child Adolesc Heal [Internet]. 2020 Aug [cited 2020 Aug 30];4(11):807–16. Available from: www.thelancet.com/child-adolescent
6.
Ismail SA
,
Saliba V
,
Lopez Bernal J
,
Ramsay ME
,
Ladhani SN
. SARS-CoV-2 infection and transmission in educational settings: a prospective, cross-sectional analysis of infection clusters and outbreaks in England [Internet]. Lancet Infect Dis. 2021 Mar;21(3):344–53. [cited 2020 Dec 19] Available from: https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30882-3/fulltext https://doi.org/10.1016/S1473-3099(20)30882-3
7.
Willeit P
,
Krause R
,
Lamprecht B
,
Berghold A
,
Hanson B
,
Stelzl E
, et al.
Prevalence of RT-qPCR-detected SARS-CoV-2 infection at schools: First results from the Austrian School-SARS-CoV-2 prospective cohort study. Lancet Reg Heal - Eur. 2021 Jun 1;5:100086.
8.
Buonsenso D
,
Roland D
,
De Rose C
,
Vásquez-Hoyos P
,
Ramly B
,
Chakakala-Chaziya JN
, et al.
Schools Closures During the COVID-19 Pandemic: A Catastrophic Global Situation [Internet]. Pediatr Infect Dis J. 2021 Apr;40(4):e146–50. [cited 2021 Oct 11] Available from: https://journals.lww.com/10.1097/INF.0000000000003052 https://doi.org/10.1097/INF.0000000000003052
9. Covid-19 Schweiz | Coronavirus | Dashboard: Virus variants [Internet]. [cited 2021 Jun 29]. Available from: https://www.covid19.admin.ch/de/epidemiologic/virus-variants
10.
Ulyte A
,
Radtke T
,
Abela IA
,
Haile SR
,
Braun J
,
Jung R
, et al.
Seroprevalence and immunity of SARS-CoV-2 infection in children and adolescents in schools in Switzerland: design for a longitudinal, school-based prospective cohort study [Internet]. Int J Public Health. 2020 Dec;65(9):1549–57. [cited 2021 Jan 17] Available from: https://pubmed.ncbi.nlm.nih.gov/33063141/ https://doi.org/10.1007/s00038-020-01495-z
CrossMark reports this article was originally posted as a Preprint at https://doi.org/10.1101/2020.08.30.20184671. (Ref. 10 "Ulyte, Radtke, Abela, Haile, Braun, Jung, et al., 2020")
11.
Ulyte A
,
Radtke T
,
Abela IA
,
Haile SR
,
Blankenberger J
,
Jung R
, et al.
Variation in SARS-CoV-2 seroprevalence across districts, schools and classes: baseline measurements from a cohort of primary and secondary school children in Switzerland [Internet]. BMJ Open. 2021 Jul;11(7):e047483. [cited 2021 Jul 29] Available from: https://bmjopen.bmj.com/lookup/doi/10.1136/bmjopen-2020-047483 https://doi.org/10.1136/bmjopen-2020-047483
12.
West EA
,
Anker D
,
Amati R
,
Richard A
,
Wisniak A
,
Butty A
, et al.; Corona Immunitas Research Group
. Corona Immunitas: study protocol of a nationwide program of SARS-CoV-2 seroprevalence and seroepidemiologic studies in Switzerland. Int J Public Health. 2020 Dec;65(9):1529–48. https://doi.org/10.1007/s00038-020-01494-0
13. Portraits of the cantons - Up-to-date key figures on the 26 cantons | Federal Statistical Office [Internet]. [cited 2021 Jul 6]. Available from: https://www.bfs.admin.ch/bfs/en/home/statistics/regional-statistics/regional-portraits-key-figures/cantons.html
14.
Abela IA
,
Pasin C
,
Schwarzmüller M
,
Epp S
,
Sickmann ME
,
Schanz MM
, et al.
Multifactorial SARS-CoV-2 seroprofiling dissects interdependencies with human coronaviruses and predicts neutralization activity. medRxiv [Internet]. 2021 Apr 21 [cited 2021 Jun 8];2021.04.21.21255410. Available from: https://doi.org/https://doi.org/10.1101/2021.04.21.21255410
15.
Canton of Zurich
. Numbers and Facts on COVID-19 [Kanton Zürich. Zahlen & Fakten zu COVID-19] [Internet]. [cited 2021 Jul 14]. Available from: https://www.zh.ch/de/gesundheit/coronavirus/zahlen-fakten-covid-19.html?keyword=covid19#/home
16.
Stringhini S
,
Wisniak A
,
Piumatti G
,
Azman AS
,
Lauer SA
,
Baysson H
, et al.
Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study [Internet]. Lancet. 2020 Aug;396(10247):313–9. [cited 2020 Jun 22] Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673620313040 https://doi.org/10.1016/S0140-6736(20)31304-0
17.
Dimick JB
,
Ryan AM
. Methods for evaluating changes in health care policy: The difference-in-differences approach. JAMA - J Am Med Assoc [Internet]. 2014 Dec 10 [cited 2021 Jul 6];312(22):2401–2. Available from: https://jamanetwork.com/
18.
Gomila R
. Logistic or linear? Estimating causal effects of experimental treatments on binary outcomes using regression analysis [Internet]. J Exp Psychol Gen. 2021 Apr;150(4):700–9. [cited 2021 Oct 11] Available from: http://doi.apa.org/getdoi.cfm?doi=10.1037/xge0000920 https://doi.org/10.1037/xge0000920
19.
Bertrand M
,
Duflo E
,
Mullainathan S
. How Much Should We Trust Differences-In-Differences Estimates? [Internet]. Q J Econ. 2004 Feb;119(1):249–75. [cited 2021 Oct 11] Available from: https://academic.oup.com/qje/article-lookup/doi/10.1162/003355304772839588 https://doi.org/10.1162/003355304772839588
20.
R Core Team
. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Internet]. 2020 [cited 2020 Dec 17]. Available from: https://www.r-project.org/
21.
Stan Development Team
. RStan: the R interface to Stan. R package version 2.21.2. 2020.
22.
Pädiatrie Schweiz
. COVID-19: neue Test- und Schulausschlusskriterien - pädiatrie schweiz [COVID-19: new testing and absence from school criteria] [Internet]. [cited 2021 Jul 14]. Available from: https://www.paediatrieschweiz.ch/news/neue-test-und-schulausschlusskriterien/
23.
Viner RM
,
Russell SJ
,
Croker H
,
Packer J
,
Ward J
,
Stansfield C
, et al.
School closure and management practices during coronavirus outbreaks including COVID-19: a rapid systematic review [Internet]. Vol. 4, The Lancet Child and Adolescent Health. Elsevier B.V.; 2020 [cited 2020 Jul 19]. p. 397–404. Available from: www.thelancet.com/child-adolescentVol
24. Coronavirus (COVID-19) Infection Survey, UK - Office for National Statistics [Internet]. [cited 2021 Oct 12]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronaviruscovid19infectionsurveypilot/8october2021
25.
Mossong J
,
Hens N
,
Jit M
,
Beutels P
,
Auranen K
,
Mikolajczyk R
, et al.
Social Contacts and Mixing Patterns Relevant to the Spread of Infectious Diseases. Riley S, editor. PLoS Med [Internet]. 2008 Mar 25 [cited 2020 Jul 13];5(3):e74. Available from: https://dx.plos.org/10.1371/journal.pmed.0050074
26.
Zhang J
,
Litvinova M
,
Liang Y
,
Wang Y
,
Wang W
,
Zhao S
, et al.
Changes in contact patterns shape the dynamics of the COVID-19 outbreak in China. Science (80-) [Internet]. 2020 Jun 26 [cited 2021 Jul 6];368(6498):1481–6. Available from: http://science.sciencemag.org/
27.
Davies NG
,
Klepac P
,
Liu Y
,
Prem K
,
Jit M
,
Eggo RM
; CMMID COVID-19 working group
. Age-dependent effects in the transmission and control of COVID-19 epidemics [Internet]. Nat Med. 2020 Aug;26(8):1205–11. [cited 2021 Jul 6] https://doi.org/10.1038/s41591-020-0962-9
28.
Perramon A
,
Soriano-Arandes A
,
Pino D
,
Lazcano U
,
Andrés C
,
Català M
, et al.
Schools as a Framework for COVID-19 Epidemiological Surveillance of Children in Catalonia, Spain: A Population-Based Study [Internet]. Front Pediatr. 2021 Sep;9:754744. [cited 2021 Oct 11] Available from: https://www.frontiersin.org/articles/10.3389/fped.2021.754744/full https://doi.org/10.3389/fped.2021.754744
29.
Buonsenso D
,
De Rose C
,
Moroni R
,
Valentini P
. SARS-CoV-2 Infections in Italian Schools: Preliminary Findings After 1 Month of School Opening During the Second Wave of the Pandemic [Internet]. Front Pediatr. 2021 Jan;8:615894. [cited 2020 Dec 19] Available from: https://www.frontiersin.org/articles/10.3389/fped.2020.615894/full https://doi.org/10.3389/fped.2020.615894
30.
Soriano-Arandes A
,
Gatell A
,
Serrano P
,
Biosca M
,
Campillo F
,
Capdevila R
, et al.; COVID-19 Pediatric Disease in Catalonia Research Group
. Household Severe Acute Respiratory Syndrome Coronavirus 2 Transmission and Children: A Network Prospective Study [Internet]. Clin Infect Dis. 2021 Sep;73(6):e1261–9. [cited 2021 Oct 11] Available from: https://academic.oup.com/cid/article/73/6/e1261/6168547 https://doi.org/10.1093/cid/ciab228
31.
Aiano F
,
Mensah AA
,
McOwat K
,
Obi C
,
Vusirikala A
,
Powell AA
, et al.
COVID-19 outbreaks following full reopening of primary and secondary schools in England: Cross-sectional national surveillance, November 2020. Lancet Reg Heal - Eur [Internet]. 2021 Jul [cited 2021 Oct 12];6:100120. Available from: https://pubmed.ncbi.nlm.nih.gov/34278370/
32.
The Impact of Community Masking on COVID-19
. A Cluster-Randomized Trial in Bangladesh | Innovations for Poverty Action [Internet]. [cited 2021 Oct 12]. Available from: https://www.poverty-action.org/publication/impact-community-masking-covid-19-cluster-randomized-trial-bangladesh
33.
Oster E
,
Jack R
,
Halloran C
,
Schoof J
. Mcleod; Diana. COVID-19 Mitigation Practices and COVID-19 Rates in Schools: Report on Data from Florida, New York and Massachusetts. medRxiv [Internet]. 2021 May 21 [cited 2021 Oct 12];2021.05.19.21257467. Available from: https://doi.org/https://doi.org/10.1101/2021.05.19.21257467
34.
Lessler J
,
Grabowski MK
,
Grantz KH
,
Badillo-Goicoechea E
,
Metcalf JC
,
Lupton-Smith C
, et al.
Household COVID-19 risk and in-person schooling. Science (80-) [Internet]. 2021 Jun 4 [cited 2021 Jul 14];372(6546):1092–7. Available from: http://science.sciencemag.org/
35.
Buitrago-Garcia D
,
Egli-Gany D
,
Counotte MJ
,
Hossmann S
,
Imeri H
,
Ipekci AM
, et al.
Occurrence and transmission potential of asymptomatic and presymptomatic SARSCoV-2 infections: A living systematic review and meta-analysis [Internet]. Vol. 17, PLoS Medicine. Public Library of Science; 2020 [cited 2021 Oct 12]. p. e1003346. Available from: https://doi.org/https://doi.org/10.1371/journal.pmed.1003346
36.
Wiedenmann M
,
Goutaki M
,
Keiser O
,
Stringhini S
,
Tanner M
,
Low N
. The role of children and adolescents in the SARS-CoV-2 pandemic: a rapid review [Internet]. Swiss Med Wkly. 2021 Sep;151(37–38):w30058. [cited 2021 Sep 20] Available from: https://smw.ch/article/doi/SMW.2021.w30058
37.
Sah P
,
Fitzpatrick MC
,
Zimmer CF
,
Abdollahi E
,
Juden-Kelly L
,
Moghadas SM
, et al.
Asymptomatic SARS-CoV-2 infection: A systematic review and meta-analysis [Internet]. Vol. 118, Proceedings of the National Academy of Sciences of the United States of America. National Academy of Sciences; 2021 [cited 2021 Oct 12]. Available from: https://doi.org/https://doi.org/10.1073/pnas.2109229118
38.
Kirsten C
,
Unrath M
,
Lück C
,
Dalpke AH
,
Berner R
,
Armann J
. SARS-CoV-2 seroprevalence in students and teachers: a longitudinal study from May to October 2020 in German secondary schools [Internet]. BMJ Open. 2021 Jun;11(6):e049876. [cited 2021 Jul 1] Available from: http://bmjopen.bmj.com/ https://doi.org/10.1136/bmjopen-2021-049876
39. COVID-19 Schools Infection Survey Round 4, England - Office for National Statistics [Internet]. [cited 2021 Jul 2]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/covid19schoolsinfectionsurveyround4england/march2021
40.
Foster GD
,
Linder B
,
Baranowski T
,
Cooper DM
,
Goldberg L
,
Harrell JS
, et al.; HEALTHY Study Group
. A school-based intervention for diabetes risk reduction [Internet]. N Engl J Med. 2010 Jul;363(5):443–53. [cited 2021 Jul 1] Available from: https://pubmed.ncbi.nlm.nih.gov/20581420/ https://doi.org/10.1056/NEJMoa1001933




