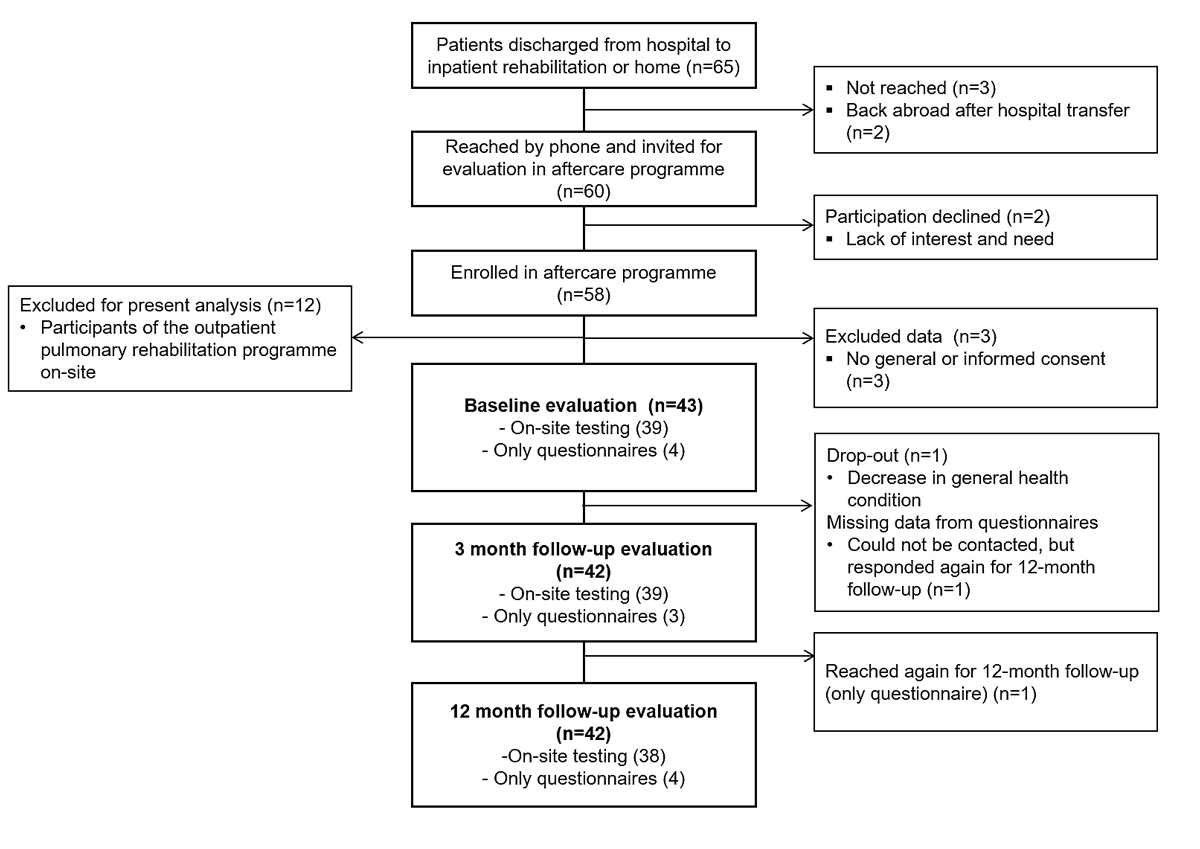
Figure 1 Flowchart of participants.
DOI: https://doi.org/10.4414/SMW.2021.w30072
From late 2019 onwards, there have been over 200 million confirmed cases of the coronavirus disease (COVID-19) worldwide, affecting 220 different countries and territories [1]. Unsurprisingly, there has been substantial clinical and research interest in both the physical and mental consequences of COVID-19. Research based on cohort and case-control studies showed immediate and longer-term consequences of COVID-19 in survivors three to eight months after their first diagnosis. For example, persistent damage to various organ systems was reported in studies from different countries, as summarized in a recent review [2]. Large studies reported that 51% of COVID-19 patients experienced at least one persisting infection-related symptom after four months, or 76% six months after diagnosis [3; 4]. Dyspnea, muscle weakness, fatigue and sleep difficulties were the most frequently reported symptoms in patients with different severity levels of the disease [3, 5-10]. Health-related quality of life (HRQoL) was reduced in 51%-62% of COVID-19 survivors after three to six months [3, 8, 9, 11]. Negative changes in mental health, such as increased anxiety, depression and post-traumatic stress disorder, were further frequently reported symptoms after several months [2, 3, 7-9]. Next to these mental and psychological long-term consequences, consistent findings suggest that physical performance, quantified with the six-minute walk test (6MWT) or the one-minute sit to stand test, is reduced after three to six months in 22%-50% of the cohorts analyzed [4, 10, 12-16]. To our knowledge, two studies reported physical performance one year after COVID-19 infection [17, 18]. Both concluded that COVID-19 survivors had a good recovery in physical performance after 12 months. However, in the large Wuhan Cohort (n = 1,276) health status was still lower than in the control population [18].
Increased care dependency and a loss of working productivity after several months have been found post-COVID-19 in further studies [9, 18, 19]. These findings point towards a longer recovery time than initially anticipated, with implications for on-going support and service provision. With a continuing pandemic in Switzerland, the interest in longer-term consequences in terms of health-related quality of life and physical performance is still of interest. Thus, this longitudinal cohort study aimed to describe the physical performance and HRQoL of Swiss cohort recovering from COVID-19 one year after hospitalization.
This prospective cohort study was registered at ClinicalTrials.gov (NCT04375709) and approved by the local ethics committee Zurich (2020-00899). The study is reported according to the STROBE statements [20]. A cohort of patients with COVID-19, hospitalized in the acute hospital Kantonsspital Winterthur in Switzerland, were followed over one year. Patients were contacted by telephone within two to five days of discharge and were invited for an on-site evaluation of physical performance and quality of life after discharge and at 3 and 12 months post-discharge. The phone-based invitations were conducted by in-house physiotherapists, who screened patients using the inclusion criteria described below. Furthermore, patients were informed about the content of the assessments and given the opportunity to ask questions about the disease and any remaining symptoms. Assessments were conducted by physiotherapists specialized in cardiopulmonary rehabilitation. Raw data from the phone-based screening and the assessments were gathered on paper and inputted into spreadsheets (Microsoft Excel) for practicality. The transfers from paper into spreadsheets were double-checked for correctness, coded and transferred into R-Studio for the statistical analysis. All cases were discussed with an in-house pulmonologist and patients continued to have access to their usual aftercare, which was not affected by participation in the study.
The study cohort was hospitalized between 3 March, 2020 and 26 June, 2020. Consecutive cases were not included, due to organizational and resource limitations during the second and third virus waves. Thus, the present cohort was hospitalized during the first wave of COVID-19 in Switzerland. During their stay, patients received respiratory physiotherapy and occupational, psychological or dysphagia therapy if necessary. Inclusion criteria for on-site evaluation were (a) a laboratory-confirmed positive test for Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) via nasopharyngeal swab, (b) hospitalized with COVID-19 for at least one day, (c) hospitalized at the Kantonsspital Winterthur with confirmed positive SARS-CoV-2 test more than 14 days ago and at least 4 days without COVID-19 related symptoms (i.e. axillary temperature >37.3 degrees Celsius, sore throat, cough [productive or non-productive, related to COVID-19] or common cold) [21, 22]) and (d) over 18 years of age. Patients provided written informed consent to subsequent use of their personal data. Participants were excluded if presenting a mental disability or impairments to reasoning or judgment, were immunocompromised due to medical treatment, or had a documented objection to subsequent use of their personal health data. Based on the outcomes of the evaluation after discharge, some participants were offered an outpatient pulmonary rehabilitation program at the study site. It was anticipated that participation in this program could impact patients’ physical performance and HRQoL, biasing the interpretation of the present cohort. Therefore, data from these participants were not used for the current analysis and are presented elsewhere [23].
The measurements described below were conducted at the following three points in time: after discharge and at 3 and 12 months post-discharge.
Primary outcome measures were physical performance and health-related quality of life (HRQoL). Physical performance was quantified with the 6MWT, conducted on a pre-marked corridor according to the statement by the American Thoracic Society [24]. HRQoL was measured using the 5-level EuroQol 5-dimension questionnaire (EQ-5D-5L) [25]. The questionnaire includes five dimensions (mobility, personal care, usual activities, pain/discomfort and anxiety/depression) and a visual analogue scale (VAS) ranging from 0-100%, with 100% representing “the best health you can imagine”. Additionally, the novel Post-COVID-19 Functional Status (PCFS) scale was used to quantify participants’ perceptions of COVID-19 specific functional restrictions in daily life [26]. This self-reported questionnaire is composed of five grades (0 = no functional limitations; 1 = negligible limitations; 2 = slight limitations; 3 = moderate limitations; 4 = severe limitations; 5 = death). The PCFS has been validated for patients with COVID-19 [27].
Secondary outcome measures were the following: the presence of hospital-related anxiety was screened for with the Hospital Anxiety and Depression Scale (HADS-D/-A) (Zigmond, 1983). The modified Medical Research Council (mMRC) dyspnea scale was used to evaluate the extent of breathlessness [28]. This five-point scale ranges from 0 (“not troubled by breathlessness”) to 4 (“I am too breathless to leave the house” or “I am breathless when dressing”). Pulmonary function was tested with a bedside spirometer, measuring the percentage of predicted forced expiratory volume in one second (FEV1), forced vital capacity (FVC) and the Tiffeneau-Pinelli index (FEV1:FVC ratio) (EasyOne Air portable; NDD Medical Technologies©). Body mass index, severity of pneumonia, pre-existing comorbidities and the presence of dysphagia were retrieved from the medical records on-site. Severity of pneumonia during hospitalization was categorized into mild (1), moderate (2), severe (3) and critical (4) according to the World Health Organization (WHO) interim guidance [29]. At 12-month follow-up, participants were asked about their current work status (% of former working status ranging from 0-100% or retirement).
The following aspects were considered to avoid potential biases in the data: (1) Therapists were already familiar with the assessments (except for the PCFS) and data monitoring due to their use in usual care for cardiopulmonary rehabilitation. (2) Participants were tested by the same assessor on all three test occasions to reduce any potential impact of inter-rater reliability. (3) Questionnaire data from participants who were not fluent in German and thus had difficulties understanding the questionnaires were excluded from the analysis, as described in Table 2. (4) Spirometry was assessed before the 6MWT to avoid pre-exhaustion of the participants and a negative influence on performance during the spirometry.
The normality of data was evaluated by visual inspection of histograms and Q-Q plots. Continuous variables were described using means and standard deviations (SD) and medians with interquartile ranges (IQR). Categorical variables were reported as frequencies (n) and percentages (%). Based on the data distribution, repeated measures ANOVA, Friedman’s Chi-square (X 2) tests or Fisher’s exact tests were applied to test the differences between the three evaluation time points. In case of significance in normally distributed data, Tukey’s post hoc contrast analysis was conducted to locate significance within the means obtained at the three time points. As a cut-off for normative values for distance covered in the six-minute walk test (6MWD), 82% of the percentage predicted (%-predicted) age- and gender-related norm was used, as suggested by Troosters and colleagues (1999) [30]. Perceived functional limitations were defined with a cut-off of >1 on the PCFS scale [27]. Perceived dyspnea was defined as ≥2 on the mMRC score ( =“I walk slower than people of the same age on the level because of breathlessness or have to stop for breath when walking at my own pace on the level”). Desaturation during the 6MWT was defined as a reduction of ≥4% of the resting saturation [31]. The level of significance was set at a p-value below 0.05. The entire analysis was conducted with R-studio version 4.1.0.
From March to June 2020, 65 individuals were discharged home or to nursing care (n = 2) after a median stay of 10 days (range 1-41). Sixty were reached by telephone and met the inclusion criteria. Two individuals declined to participate after the call due to a lack of interest. Among the remaining 58 participants, 55 provided written consent to participation in the study (figure 1). Data from 12 individuals were excluded from the present analysis due to their participation in an outpatient pulmonary rehabilitation program at the study site. Patients were followed up a median of 25 days post-discharge (10-53 days) for the initial evaluation, and 99 days (80-134) and 352 days (315-387) post-discharge for the 3- and 12-month follow-ups respectively.

Figure 1 Flowchart of participants.
Table 1Demographics.
| Total n = 43 | ||
| Gender, female (%) | 13 (30%) | |
| Age (years), mean (SD), range | 60 (14), 32-84 | |
| Length of stay (days), median (IQR) | 10 (9) | |
| Severity of pneumonia*, n (%) | ||
| Mild | 10 (23%) | |
| Moderate | 17 (40%) | |
| Severe | 10 (23%) | |
| Critical | 6 (14%) | |
| Comorbidities (median (IQR)) | 2 (2) | |
| Pre-existing comorbidities | ||
| Arterial hypertension, n (%) | 22 (51%) | |
| Internal disease, n (%) | 19 (45%) | |
| Cardiovascular disease, n (%) | 15 (35%) | |
| Diabetes mellitus, n (%) | 10 (23%) | |
| Cancerogenous disease, n (%) | 7 (16%) | |
| Obesity (BMI ≥25), n (%) | 4 (9%) | |
| Chronic obstructive pulmonary disease, n (%) | 3 (7%) | |
| Asthma, n (%) | 2 (5%) | |
| Mental health condition, n (%) | 3 (7%) | |
Values are presented for the entire group (total); * pneumonia is categorized according to the interim guidance of the WHO: (1) mild pneumonia (mild symptoms without radiographic appearance of pneumonia), (2) moderate pneumonia (having symptoms and the radiographic evidence of pneumonia, with no requirement for supplemental oxygen), (3) severe pneumonia (having pneumonia, including one of the following: respiratory rate >30 breaths/minute; severe respiratory distress; or SpO2 ≤93% on room air at rest), and (4) critical cases (e.g. respiratory failure requiring mechanical ventilation, septic shock, other organ failure occurrence or admission into the ICU) [29]; SD = standard deviation, IQR = interquartile range
At the first evaluation, 43 patients (13 female; aged 32-84 years) were successfully assessed, 39 attending an on-site assessment and 4 completing their questionnaires at home. Participants presented with mild (n = 10), moderate (n = 17), severe (n = 10) and critical (n = 6) manifestations of pneumonia during hospitalization (table 1). For 3-month and 12-month follow-up, 39 and 38 participants respectively returned for evaluation on-site.
After hospitalization, 24% (8/34) of the tested participants achieved less than their age- and gender-predicted norm for the 6MWT. As a group, changes in 6MWD or proportions under the norm (%-predicted) were not statistically significant over the three measurement time points (figure 2). As far as dyspnea is concerned, 9/38 perceived breathlessness (mMRC >1) during activity and 9/34 presented desaturation at the end of the 6MWT. At the three-month follow-up, 58% (19/35) of participants increased their 6MWD to a clinically relevant extent (>30 m) and the percentage of participants below the %-predicted value went from 24% (8/34) to 17% (6/35) when compared to the initial assessment. Breathlessness during activity was reported in 13% (5/39).
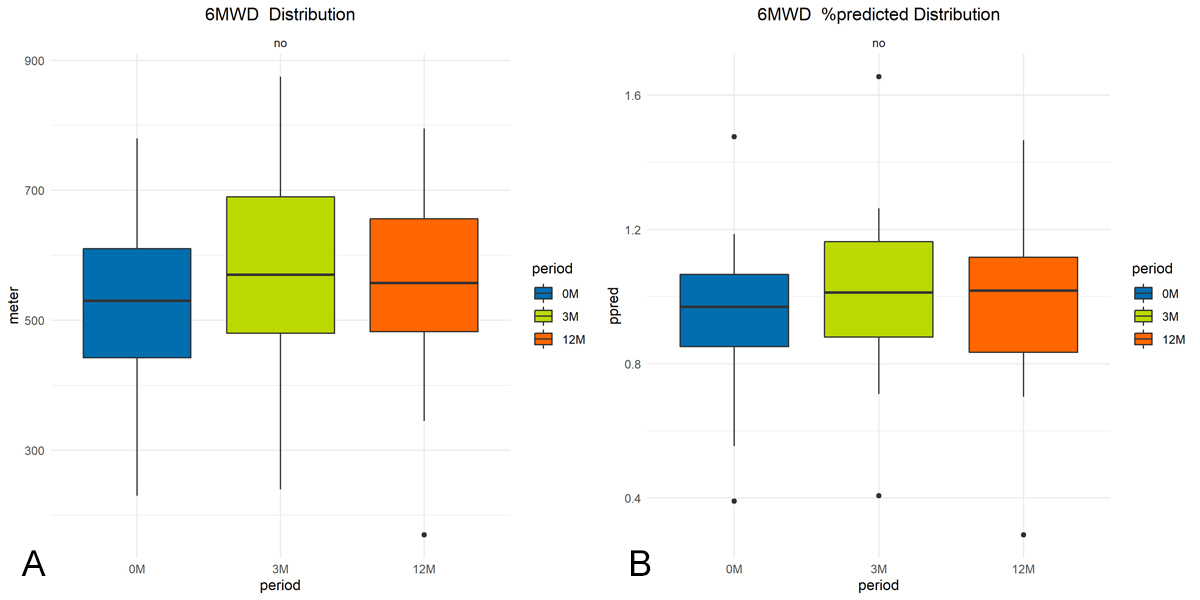
Figure 2 Illustration of changes in physical performance: group mean changes in (a) 6MWD (meters), (b) percentage predicted norm (%). No statistically significant changes in any of the three parameters over time (p >0.05).
At 12-month follow-up, 25% (9/36) of participants covered a distance below the %-predicted norm. Two out of nine patients were limited due to pre-existing musculoskeletal pain which worsened after COVID-19. One participant reported sleep disturbance and feeling depressed since hospitalization. From 3-month to 12-month follow-up, 47% (16/36) of participants’ walking distance decreased by >30 m (figure 3).
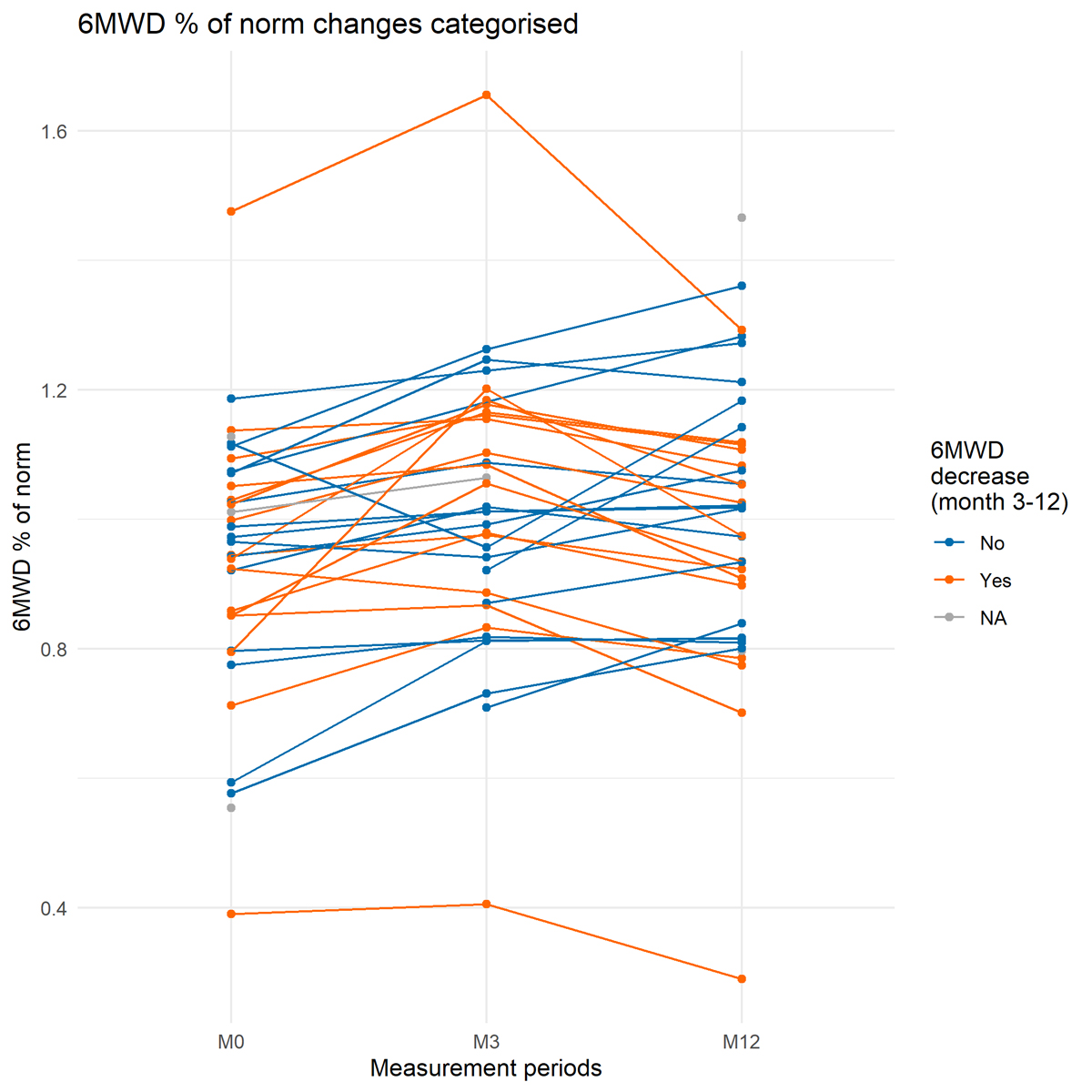
Figure 3 Individual changes in 6MWD percentage predicted norm (% of norm) for first evaluation (M0), 3-month follow-up (M3) and 12-month follow-up (M12). Individuals with decreases in 6MWD >30 m are colored in orange.
Perception of breathlessness on the mMRC scale was reported by 18% (7/40), with 12% (3/36) showing desaturation at the end of 6MWT. Among these three patients, two had no cardiorespiratory comorbidities but were severely to critically affected by the COVID-19 induced pneumonia.
Table 2 Clinical data from first evaluation to one-year evaluation.
| Performance | M0 (n = 34/40) | M3 (n = 35/39) | M12 (n = 36/38) | p-value |
| 6MWD (m),mean (SD) | 529 (118) | 578 (129) | 563 (124) | 0.23 |
| %-pred. 6MWD (%), mean (SD) | 94 (21) | 102 (21) | 100 (22) | 0.29 |
| 6MWD below 82% norm, n (%) | 8 (24%) | 6 (17%) | 9 (25%) | 0.70 |
| Desaturation yes, n (%) | 9 (26%) | 6 (17%) | 3 (8%) | 0.15 |
| mMRC dyspnea ≥2, n (%) | 9 (24%) | 5 (13%) | 7 (17%) | 0.46 |
| FEV1/FVC (%), mean (SD) (n)** | 80 (10)(n = 17) | 79 (10)(n = 25) | 77 (11)(n = 25) | 0.72 |
| HRQoL | ||||
| EQ-5D VAS (0-100%), mean (SD) | 75 (16) | 82 (16) | 75 (19) | 0.09 |
| HADS-A >7, n (%) | 5 (14%) | 2 (5%) | 6 (16%) | 0.29 |
| HADS-D >7, n (%) | 4 (11%) | 2 (5%) | 8 (22%) | 0.09 |
| PCFS ≥2, n (%) | 14 (44%) | 10 (26%) | 12 (29%) | 0.24 |
| PCFS (0), n (%) | 9 (28%) | 25 (64%) | 19 (46%) | |
| PCFS (1), n (%) | 9 (28%) | 4 (10%) | 10 (24%) | |
| PCFS (2), n (%) | 5 (15%) | 7 (18%) | 10 (24%) | |
| PCFS (3), n (%) | 9 (28%) | 3 (8%) | 2 (5%) | |
| PCFS (4), n (%) | 0 (0%) | 0 (0%) | 0 (0%) | |
Values are presented for the entire group (total) as means and standard deviations or as medians and interquartile ranges; m = meter; 6MWD = six-minute walking distance; %-pred. 6MWD = percentage predicted distance, age- and gender-specific norm value, in percent. Missing data for the 6MWT during all periods was due to musculoskeletal pain (pre-existing) or refusal to carry out the test (n = 1). HADS-A/-D >7 is considered as positive when screening for hospital-related anxiety and depression, with 8-10 indicating borderline signs and >10 indicating signs for requiring care. For questionnaires, missing data were due to language issues and therefore non-valid responses. Incomplete data for bedside spirometry was due to low quality of measurements at M0 and M3 caused by coughing or pain during the completion of the spirometry.
** Number of participants with measures of good quality (quality grade of A or B) at the spirometry; p-value is listed for time effect of all three measurement time points; no statistical significance for time-effect reported.
At initial assessment, 21% (9/42) of participants perceived moderate-to-severe limitations in at least one of the subcategories of the EQ-5D questionnaire (Figure 4). The strongest limitations were perceived for usual activity 22% (10/42) and pain 18% (8/42). Pain was frequently reported as musculoskeletal pain or pain during forced inspiration, for example during exercise or when coughing. The group mean VAS score was 75% (SD 17%) at initial testing. At three-month follow-up, moderate and extreme problems were still reported for usual activity in 12% (5/42) and severe-to-moderate problems for pain/discomfort in 12% (5/42), for mobility in 7% (3/42) and for anxiety/depression in 5% (2/42). After 12 months, problems with self-care and usual activity reduced and remained similar, respectively. However, the number of participants reporting moderate-to-severe problems with pain/discomfort increased again, to 30% (12/40), similar to the reports for anxiety/depression in 10% (4/40) and for mobility in 18% (7/40) (Figure 4). From a statistical point of view, changes in HRQoL were not significant. After discharge, 14% (5/37) reported signs of anxiety towards perceived breathlessness during physical exertion and 11% (4/37) reported signs of being at risk for depression (HADS-A/-D). Three participants reported pre-existing anxiety issues or signs of depression.
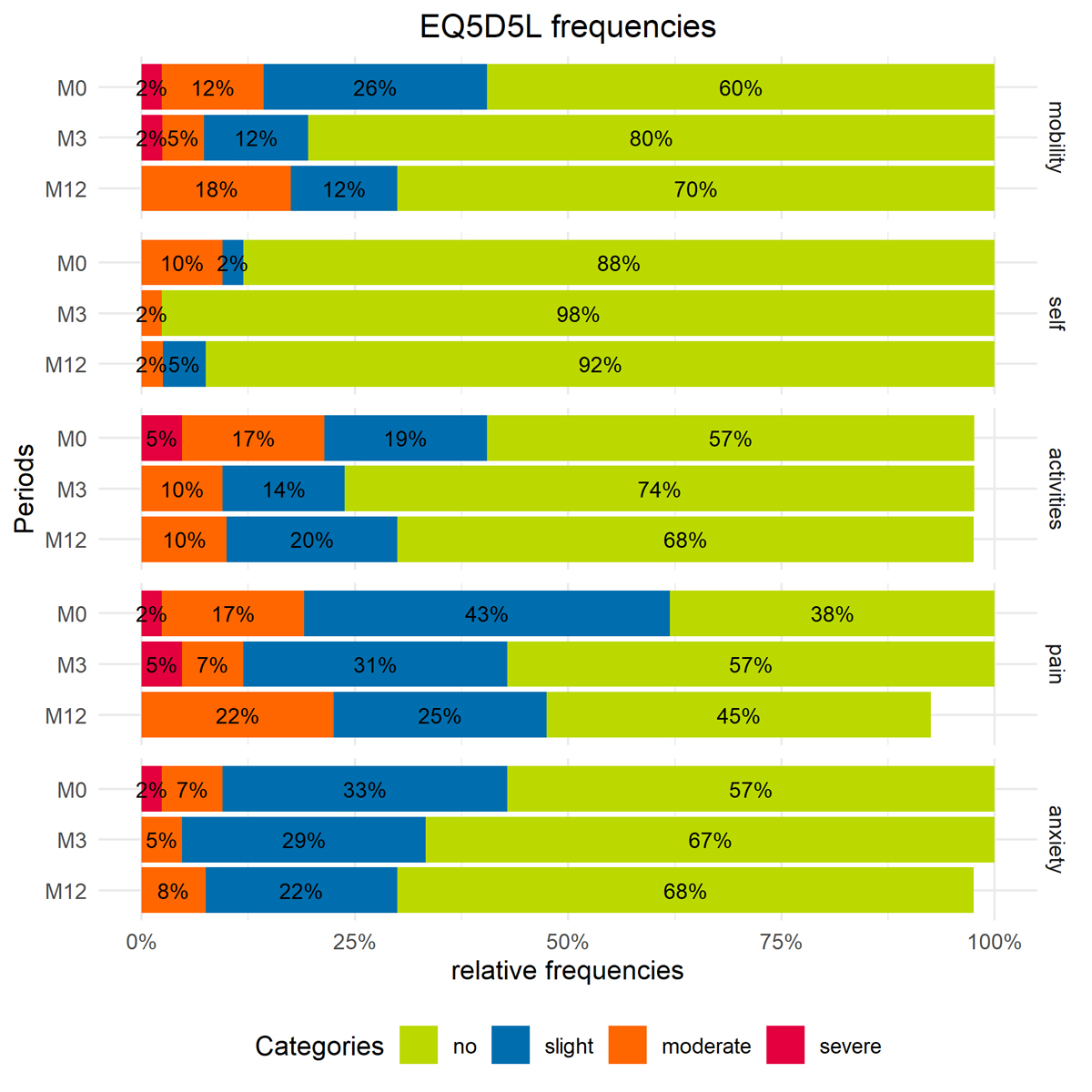
Figure 4 Distribution frequencies of problems on EQ-5D-5L dimensions for first evaluation (M0), 3-month follow-up (M3) and 12-month follow-up (M12). The categories are mobility ("mobility"), self-care ("self"), usual activities ("activities"), pain and discomfort ("pain") and anxiety and depression ("anxiety"). Values are presented as percentages of total responses for “no, slight, moderate and severe problems”.
In the weeks after discharge, 44% (14/32) of participants experienced COVID-19 specific limitations in daily life according to the PCFS scale (Figure 5). The percentage of participants with COVID-19 related limitations in daily life on the PCFS scale reduced to 26% after three months. At 12-month follow-up, 29% (12/41) still reported slight to moderate limitations on the PCFS scale. New impairments reported at 12-month follow-up were musculoskeletal pain (n = 5), difficulties in concentration and memory (n = 2) and myalgia (n = 1).
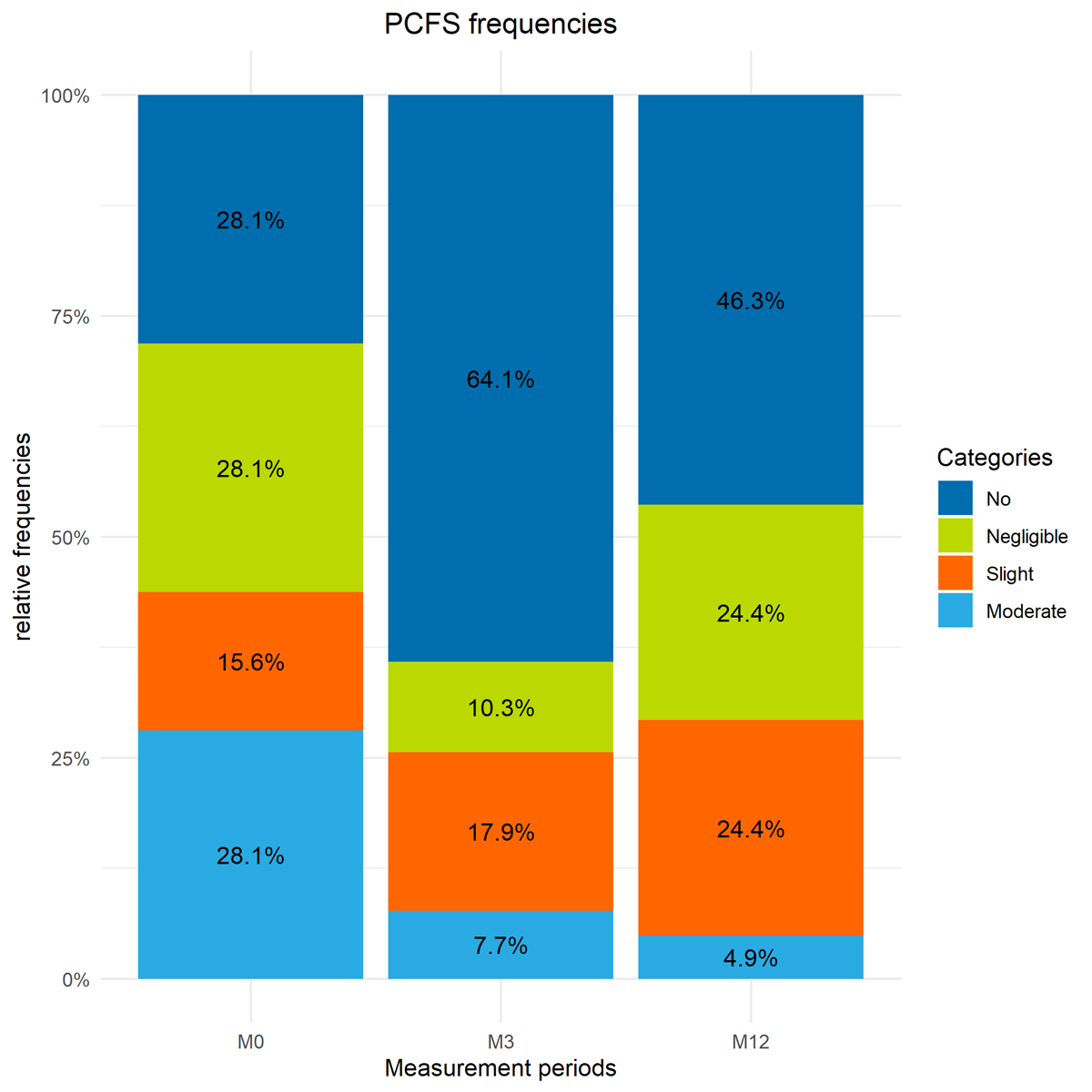
Figure 5 Distribution of categories for perceived limitations in functionality according to the PCFS scale. “No limitations” includes patients with no and negligible limitations on the PCFS scale (no = 0, negligible = 1). Individuals in the “limitations” category scored slight and moderatelimitations on the PCFS scale (2 = slight, 3 = moderate). The score “5 = dead” was not included.
Of the 21 participants with employment before hospitalization, 4 were not back in employment at 12-month follow-up due to the pandemic-related restrictions and COVID-19 induced limitations. In one participant this was due to COVID-19 induced physical limitations. Pandemic restrictions led to a shortage of certain job positions, which affected three of the participants.
To our knowledge, this is the first study to report recovery in physical performance and HRQoL after one year in patients hospitalized in Switzerland due to COVID-19 induced pneumonia. In a cohort of whom 62% (27/43) were affected by mild to moderate pneumonia during hospitalization, participants generally showed good physical performance. However, 18% (7/40) had slight to moderate dyspnea on the mMRC scale after one year and a third of participants perceived restrictions in function due to COVID-19 (PCFS ≥2). More precisely, HRQoL generally improved over one year, but 30% (12/40) of participants still perceived moderate-to-severe symptoms of pain and discomfort and 33% (13/40) slight-to-severe symptoms of anxiety and depression.
Group data for physical performance did not change significantly over one year. However, from the first evaluation after hospitalization to the 12-month follow-up 44% (15/34) of participants increased their walking distance in a clinically relevant manner (<30 meters) without particular training. In contrast, from the 3-month to the 12-month follow-up 47% (16/34) decreased their walking distance by more than 30 meters. Scrutiny of the individual data did not reveal any pattern or common factors among the parameters assessed or the patients’ demographics which could have explained this decrease. We found, to date, two studies using 6MWT outcomes one year after COVID-19 induced hospitalization [17, 18]. Similar to our findings, both studies report good recovery in physical performance, but they have limited comparability to our results. Wu and colleagues reported 6MWD at 3, 6, 9 and 12 months post-discharge from hospital after severe COVID-19 in participants without any cardiovascular or pulmonary comorbidities. In the present cohort, participants had between zero and six comorbidities. Therefore, some of the participants might have had limitations in physical performance before COVID-19, which could have influenced the course of their recovery. A second prospective cohort study analyzed a large Wuhan population with different initial severities of pneumonia and comorbidities [18], but outcomes were assessed only at 6 and 12 months.
As far as quality of life is concerned, the present cohort reported limitations mainly in the dimensions “pain or discomfort”, “anxiety/depression” and “usual activities” in the EQ-5D-5L questionnaire. These three EQ-5D-5L dimensions were also reported as the main restrictions in HRQoL after COVID-19 in previous literature in Brazilian and Belgian cohorts [11, 32]. The percentages of moderate-to-severe problems in these dimensions tended to be higher in both studies when compared to the data from the present cohort at three months post-discharge. At least moderate limitations in the usual activities and pain dimensions were found in 67% and 70% of the Belgian cohort, obtained from an online survey (n = 210 non-hospitalized respondents) [11]. The self-care dimension was virtually unaffected, as in our cohort. The EQ-5D VAS scores reported in the Belgian study were markedly lower, with a mean of 51% (SD 19) a mean of 79 days after symptom onset compared to the 82% (SD 16) at three-month follow-up in our cohort.
In the present analysis, information on HRQoL before the COVID-19 induced hospitalization was not collected. This information would have allowed more definite assertions on longer-term recovery of quality of life. However, from the entire group questioned, only two participants clarified that the restrictions reported in the EQ-5D-5L questionnaire were not related to COVID-19. Additionally, 29% reported slight to moderate limitations in functionality on the PCFS scale. This indicates that a third still perceived limitations in daily life related to COVID-19 after one year.
Previous literature suggests that limitations in HRQoL before hospitalization could still be present in healthy individuals. In large samples from a healthy German population (aged 20-90 years), the mean scores on the EQ-5D-5L VAS (0-100%) were 71.6% (SD 21.4) (n = 4,998) [33] and 91.5% (SD 13) (n = 2,469) [34]. Thus, the VAS scores from the present cohort of 82% (SD 16) at 3-month follow-up and 75% (SD 19) at 12-month follow-up fall within the range reported by the two previous studies from the healthy German population.
This study included several limiting aspects. First, the recruitment time between the day of discharge and the first evaluation ranged from 10-54 days. This range includes outliers, with two patients who were rehospitalized due to other medical issues and musculoskeletal disorders and were only able to participate 53 and 54 days after discharge. The period between the first evaluation and the three-month follow-up was, on average, two months.
Second, due to the observational nature of this study, new health issues were not systematically recorded or categorized (onset, duration), nor were any changes in existing symptoms (e.g. renal disease, gastrointestinal changes) measured during the evaluations. These health issues could have influenced physical performance and quality of life. Also, the present data were not compared to data from age- and gender-matched controls. Third, this cohort included 25% of participants with mild pneumonia during the acute state of infection and only 14% with critical pneumonia. This might not be representative for larger cohorts with more severe disease. Nevertheless, this was a representative cohort for the first wave experienced in Switzerland. The large proportion of participants with mild disease could also explain why patients were discharged into their former homes (including nursing care).
In the present analysis, 30 meters was considered a clinically important difference in the 6MWT. Since no reference values exist for COVID-19, the cut-off was based on findings from other pathologies, mainly respiratory diseases [35, 36]. A review of the minimal clinically important difference in the 6MWT in adults with different pathologies reported differences ranging from 14.0 to 30.5 meters [37]. Moreover, 20 to 30 meters was found to be the minimal important difference, derived from anchor- and distribution-based analysis in 641 patients after acute respiratory failure and acute respiratory distress syndrome [38]. For spirometry, no results are described due to a large number of missing values. Data are presented in Table 2.
In a small cohort of COVID-19 survivors who were predominantly mildly affected by pneumonia, limitations in physical performance during the follow-up analyses were small. Thus, negative consequences of COVID-19 tended to relate to HRQoL, also in individuals who had a milder course of COVID-19.
For those with moderate to severe COVID-19, related limitations in daily functionality and deficits in HRQoL were still reported one year after hospitalization. These results offer preliminary indications for ongoing support after hospitalization and point towards the need for specific, individualized follow-up to support patients’ recovery.
We direct special acknowledgements to the participants for agreeing to the use of their data and the therapists from the institute involved in the evaluations with the patients. We thank Giuseppe Mungo, Anna Göbel and Axel Boger for their organizational support and Michael Schläppi for his helpful contribution in the statistical analysis.
Author contributions: Conceptualization, S.R., I.U., D.G.; methodology and project administration M.B., S.R., I.U., N.O.; formal analysis, data curation, M.B., A.B.; writing—original draft preparation, M.B.; investigation, S.P., I.U., N.O., S.B.; resources, D.G.; writing—review and editing C.S., H.S., N.O., S.R., M.A.S. All authors have read and agreed to the published version of the manuscript.
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy regulations.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.
1 World Health Organisation , W. WHO Coronavirus (COVID-19) Dashboard. 2021.
2. Nalbandian A , Sehgal K , Gupta A , Madhavan MV , McGroder C , Stevens JS , et al. Post-acute COVID-19 syndrome. Nat Med. 2021 Apr;27(4):601–15. https://doi.org/10.1038/s41591-021-01283-z
3. Morin L , Savale L , Pham T , Colle R , Figueiredo S , Harrois A , et al.; Writing Committee for the COMEBAC Study Group . Four-Month Clinical Status of a Cohort of Patients After Hospitalization for COVID-19. JAMA. 2021 Apr;325(15):1525–34. https://doi.org/10.1001/jama.2021.3331
4. Huang C , Huang L , Wang Y , Li X , Ren L , Gu X , et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021 Jan;397(10270):220–32. https://doi.org/10.1016/S0140-6736(20)32656-8
5. Anastasio F , Barbuto S , Scarnecchia E , Cosma P , Fugagnoli A , Rossi G , et al. Medium-term impact of COVID-19 on pulmonary function, functional capacity and quality of life. Eur Respir J. 2021 Sep;58(3):2004015. https://doi.org/10.1183/13993003.04015-2020
6. Goërtz YM , Van Herck M , Delbressine JM , Vaes AW , Meys R , Machado FV , et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020 Oct;6(4):00542-2020. https://doi.org/10.1183/23120541.00542-2020
7. Huang C , Wang Y , Li X , Ren L , Zhao J , Hu Y , et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb;395(10223):497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
8. Wong AW , Shah AS , Johnston JC , Carlsten C , Ryerson CJ . Patient-reported outcome measures after COVID-19: a prospective cohort study. Eur Respir J. 2020 Nov;56(5):2003276. https://doi.org/10.1183/13993003.03276-2020
9 Vaes, A.W. ; Goertz, Y.M.J. ; Van Herck, M. ; Machado, F.V.C. ; Meys, R. ; Delbressine, J.M. ; Houben-Wilke, S. ; Gaffron, S. ; Maier, D. ; Burtin, C. ; Posthuma, R. ; Van Loon, N. ; Franssen, F.M. ; Hajian, B. ; Simons, S.O. ; M., v.B.J.F.; Klok, F.A.; Spaetgens, B.; Pinxt, C.M.H.; Liu, L.Y.L.; Wesseling, G.; Spies, Y.; Vijlbrief, H.; van't Hul, A.J.; Janssen, D.J.; Spruit, M.A. Recovery from COVID-19: a sprint or marathon? 6 months follow-up data of online long COVID-19 support group members. ERJ Open Res 2021. 2021.
10. van Gassel RJ , Bels J , Remij L , van Bussel BC , Posthuma R , Gietema HA , et al. Functional Outcomes and Their Association With Physical Performance in Mechanically Ventilated Coronavirus Disease 2019 Survivors at 3 Months Following Hospital Discharge: A Cohort Study. Crit Care Med. 2021 Oct;49(10):1726–38. https://doi.org/10.1097/CCM.0000000000005089
11. Meys R , Delbressine JM , Goërtz YM , Vaes AW , Machado FV , Van Herck M , et al. Generic and Respiratory-Specific Quality of Life in Non-Hospitalized Patients with COVID-19. J Clin Med. 2020 Dec;9(12):E3993. https://doi.org/10.3390/jcm9123993
12. Baricich A , Borg MB , Cuneo D , Cadario E , Azzolina D , Balbo PE , et al.; No-more Covid Group . Midterm functional sequelae and implications in rehabilitation after COVID-19: a cross-sectional study. Eur J Phys Rehabil Med. 2021 Apr;57(2):199–207. https://doi.org/10.23736/S1973-9087.21.06699-5
13. Aranda J , Oriol I , Martín M , Feria L , Vázquez N , Rhyman N , et al. Long-term impact of COVID-19 associated acute respiratory distress syndrome. J Infect. 2021 Aug;S0163-4453(21)00396-0.
14. Raman B , Cassar MP , Tunnicliffe EM , Filippini N , Griffanti L , Alfaro-Almagro F , et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021 Jan;31:100683. https://doi.org/10.1016/j.eclinm.2020.100683
15. Belli S , Balbi B , Prince I , Cattaneo D , Masocco F , Zaccaria S , et al. Low physical functioning and impaired performance of activities of daily life in COVID-19 patients who survived hospitalisation. Eur Respir J. 2020 Oct;56(4):2002096. https://doi.org/10.1183/13993003.02096-2020
16. van den Borst B , Peters JB , Brink M , Schoon Y , Bleeker-Rovers CP , Schers H , et al. Comprehensive health assessment three months after recovery from acute COVID-19. Clin Infect Dis. 2020 Nov.
17. Wu X , Liu X , Zhou Y , Yu H , Li R , Zhan Q , et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021 Jul;9(7):747–54. https://doi.org/10.1016/S2213-2600(21)00174-0
18. Huang L , Yao Q , Gu X , Wang Q , Ren L , Wang Y , et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021 Aug;398(10302):747–58. https://doi.org/10.1016/S0140-6736(21)01755-4
19. Vaes AW , Machado FV , Meys R , Delbressine JM , Goertz YM , Van Herck M , et al. Care Dependency in Non-Hospitalized Patients with COVID-19. J Clin Med. 2020 Sep;9(9):E2946. https://doi.org/10.3390/jcm9092946
20. Vandenbroucke JP , von Elm E , Altman DG , Gøtzsche PC , Mulrow CD , Pocock SJ , et al.; STROBE Initiative . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology. 2007 Nov;18(6):805–35. https://doi.org/10.1097/EDE.0b013e3181577511
21. Wolfel R , Corman VM , Guggemos W , Seilmaier M , Zange S , Muller MA , et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020 Apr;581(7809):465–9. https://doi.org/10.1038/s41586-020-2196-x
22. Li Q , Guan X , Wu P , Wang X , Zhou L , Tong Y , et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020 Mar;382(13):1199–207. https://doi.org/10.1056/NEJMoa2001316
23. Betschart M , Rezek S , Unger I , Beyer S , Gisi D , Shannon H , et al. Feasibility of an Outpatient Training Program after COVID-19. Int J Environ Res Public Health. 2021 Apr;18(8):3978. https://doi.org/10.3390/ijerph18083978
24. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002 Jul;166(1):111–7. https://doi.org/10.1164/ajrccm.166.1.at1102
25. EuroQol.org . EuroQol Research Foundation [Internet]; [cited 2020 March 21]. Available from: https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/
26. Klok FA , Boon GJ , Barco S , Endres M , Geelhoed JJ , Knauss S , et al. The Post-COVID-19 Functional Status scale: a tool to measure functional status over time after COVID-19. Eur Respir J. 2020 Jul;56(1):2001494. https://doi.org/10.1183/13993003.01494-2020
27. Machado FV , Meys R , Delbressine JM , Vaes AW , Goërtz YM , van Herck M , et al. Construct validity of the Post-COVID-19 Functional Status Scale in adult subjects with COVID-19. Health Qual Life Outcomes. 2021 Feb;19(1):40. https://doi.org/10.1186/s12955-021-01691-2
28. Mahler DA , Wells CK . Evaluation of clinical methods for rating dyspnea. Chest. 1988 Mar;93(3):580–6. https://doi.org/10.1378/chest.93.3.580
29. World Health Organisation . W. Clinical management of COVID-19: intermim guidance. [Internet] WHO; [modified 27 May 2020; cited 2021 26 January ]. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1
30. Troosters T , Gosselink R , Decramer M . Six minute walking distance in healthy elderly subjects. Eur Respir J. 1999 Aug;14(2):270–4. https://doi.org/10.1034/j.1399-3003.1999.14b06.x
31. Poulain M , Durand F , Palomba B , Ceugniet F , Desplan J , Varray A , et al. 6-minute walk testing is more sensitive than maximal incremental cycle testing for detecting oxygen desaturation in patients with COPD. Chest. 2003 May;123(5):1401–7. https://doi.org/10.1378/chest.123.5.1401
32. Todt BC , Szlejf C , Duim E , Linhares AO , Kogiso D , Varela G , et al. Clinical outcomes and quality of life of COVID-19 survivors: A follow-up of 3 months post hospital discharge. Respir Med. 2021 Aug;184:106453. https://doi.org/10.1016/j.rmed.2021.106453
33. Grochtdreis T , Dams J , König HH , Konnopka A . Health-related quality of life measured with the EQ-5D-5L: estimation of normative index values based on a representative German population sample and value set. Eur J Health Econ. 2019 Aug;20(6):933–44. https://doi.org/10.1007/s10198-019-01054-1
34. Hinz A , Kohlmann T , Stöbel-Richter Y , Zenger M , Brähler E . The quality of life questionnaire EQ-5D-5L: psychometric properties and normative values for the general German population. Qual Life Res. 2014 Mar;23(2):443–7. https://doi.org/10.1007/s11136-013-0498-2
35. Polkey MI , Spruit MA , Edwards LD , Watkins ML , Pinto-Plata V , Vestbo J , et al.; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Study Investigators . Six-minute-walk test in chronic obstructive pulmonary disease: minimal clinically important difference for death or hospitalization. Am J Respir Crit Care Med. 2013 Feb;187(4):382–6. https://doi.org/10.1164/rccm.201209-1596OC
36. Singh SJ , Puhan MA , Andrianopoulos V , Hernandes NA , Mitchell KE , Hill CJ , et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J. 2014 Dec;44(6):1447–78. https://doi.org/10.1183/09031936.00150414
37. Bohannon RW , Crouch R . Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract. 2017 Apr;23(2):377–81. https://doi.org/10.1111/jep.12629
38. Chan KS , Pfoh ER , Denehy L , Elliott D , Holland AE , Dinglas VD , et al. Construct validity and minimal important difference of 6-minute walk distance in survivors of acute respiratory failure. Chest. 2015 May;147(5):1316–26. https://doi.org/10.1378/chest.14-1808