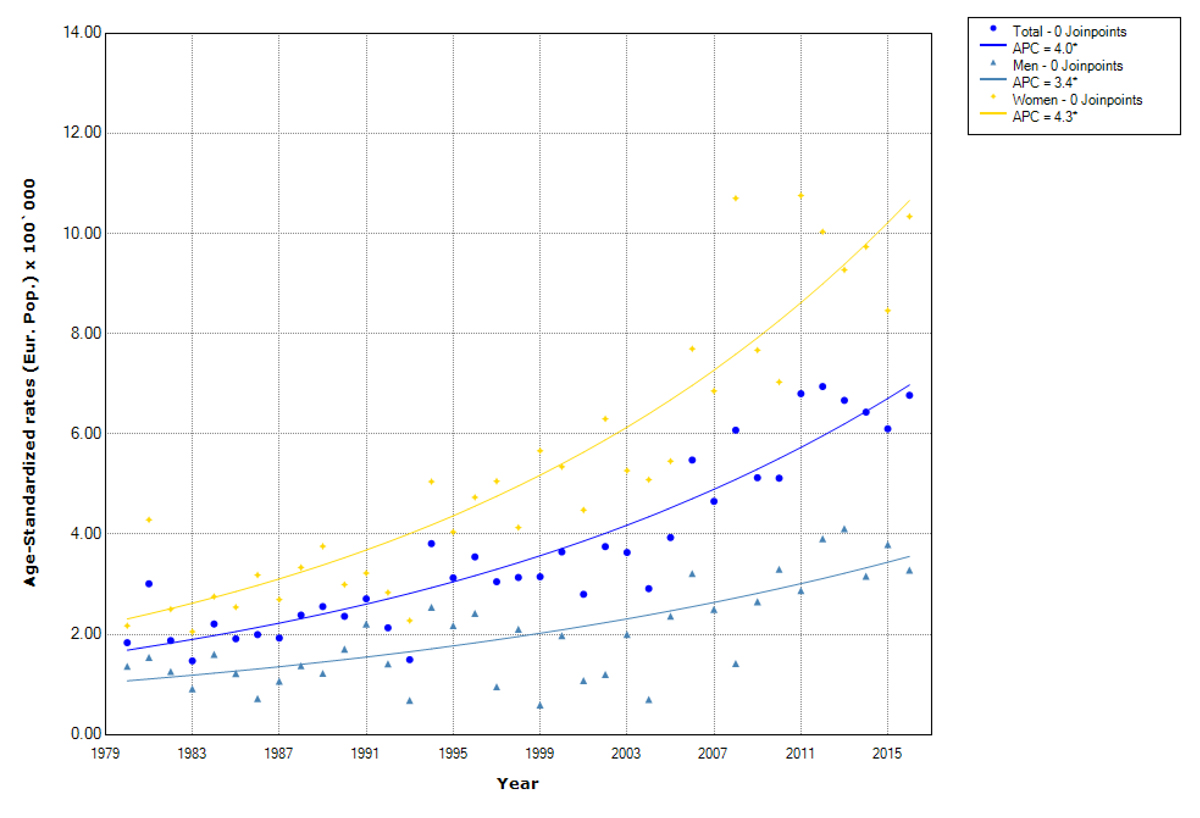
Figure 1 Trends in papillary thyroid cancer incidence overall and by sex. Canton of Zurich 1980–2016. APC (annual percentage change). Age-standardised rates (European population).
DOI: https://doi.org/10.4414/SMW.2021.w30029
Thyroid cancer is the most common endocrine cancer worldwide [1]. With nearly 570,000 cases being diagnosed globally in 2018, thyroid cancer accounts for 3.1% of all cancer cases (1.4% in men and 5.0% in women) [2]. The incidence rate adjusted to the world standard population for thyroid cancer in 2018 was 3.1 per 100,000 in men and three times higher in women at10.2 per 100,000. In the same year a total of 41,000 deaths (0.4% of all cancer deaths) were attributed to thyroid cancer, with age-standardised mortality rates of 0.4 per 100,000 in men and 0.5 per 100,000 in women [2, 3].
In Europe in 2018, the age-standardised incidence rate was 3.4 per 100,000 in men and 11.4 per 100,000 in women, which is above the world estimate [2]. The globally highest thyroid cancer incidence rates for both sexes were detected in French Polynesia, where women had incidence rates over five times higher than the European estimate. The lowest incidence rates were found in India, Pakistan and Sub-Saharan Africa [4]. High-income countries have an over two-fold higher incidence rate compared with low/middle-income countries. These findings were seen for both men and women [3]. Despite this large variability in incidence rates, a generally increasing trend was observed in most countries over time [5–7]. On the other hand, mortality rates decreased over time [3, 8, 9].
This global trend appears to be mainly driven by papillary thyroid cancer and could reflect the growing scrutiny of the thyroid gland with ultrasonography and other diagnostic techniques such as fine-needle aspiration biopsies [3, 5, 10] and has been interpreted as overdiagnosis in other studies. Overdiagnosis is the detection and histological verification of a disease that would not have been diagnosed in a person’s lifetime if testing had not been performed. Between 2008 and 2012, over 830,000 women and 220,000 men might have been overdiagnosed with thyroid cancer in 26 countries analysed; in South Korea the estimated proportion of thyroid cancer cases attributable to overdiagnosis was 83% in men and 93% in women [10].
Mortality rates are globally decreasing in men with an average annual percentage change (AAPC) of around –2% to –3% over the last decades, except in the United States where a significant increase in mortality rates was observed with an AAPC of 0.8% during the period 1993 to 2012 [3]. Mortality rates in women decreased globally as well, with an AAPC around –2% to –5% except in the United States, the United Kingdom and Australia, where the mortality rates slightly rose after the late 1990s. For the period 2008–2012, most countries reported mortality rates between 0.2 and 0.4 per 100,000 in men and between 0.2 and 0.6 per 100,000 in women (3, 11–14].
In thirteen Swiss cantons that were covered by cancer registration during 2012–2014, a total of 2317 thyroid cancer cases were diagnosed; 671 new cases in men (4.9 per 100,000) and 1646 new cases in women (12.0 per 100,000) [15]. Therefore, incidence rates in Switzerland are above the 2012 European estimate for men and women [16]. Furthermore, these numbers show that thyroid cancer is nearly three times more frequent in women than in men across Switzerland. A total of 184 deaths due to thyroid cancer were registered, 81 deaths in men and 103 deaths in women, accounting for less than 0.6% of all cancer deaths in both sexes in Switzerland [15].
As seen globally, the Swiss incidence rates also have increased since 1983 with a slight increase in men and a two-fold increase in women [17]. The increase is limited to people under the age of 70 years and was mainly driven by papillary thyroid cancer and early-stage tumours, while the mortality rates declined [15].
The aim of the study was to report 37-year trends in incidence of papillary vs non-papillary thyroid cancer and in mortality due to thyroid cancer in general in the canton of Zurich and compare them with other European rates.
A retrospective analysis was performed using data from the population-based Cancer Registry of the Cantons of Zurich, Zug, Schaffhausen and Schwyz.
Since 1980, the cancer registry has collected data of every patient diagnosed with cancer whose registered residence was in the canton of Zurich at the time of diagnosis. The canton of Zug joined the registry in 2011, and the cantons of Schaffhausen and Schwyz joined in 2020.
The cancer registry receives notifications from pathology and haematology laboratories, hospitals, and physicians, as well as death certificates from the Swiss Federal Statistical Office. Data include personal information and tumour characteristics, which are entered into the database by registry personnel. Tumours are coded by physicians and other appropriately trained personnel. Data completeness is annually monitored by calculating the percentage of cancers diagnosed by death certificate only (DCO) and the percentage of cancer that are morphologically verified [18].
The basis of the current analysis was the cases of thyroid cancer (C73 according to the International Classification of Diseases, ICD-10) diagnosed in the canton of Zurich from 1980–2016 (n = 3015). From these cases, we excluded patients aged below 20 years at diagnosis (n = 39) and patients with multiple primaries, for whom we included only the initial tumour (n = 4). Finally, 2972 cases of thyroid cancer were included.
We categorised thyroid tumors as follows: papillary thyroid cancer (International Classification of Diseases for Oncology, ICD-O-3 histological codes 8260, 8340–8344, 8350), follicular thyroid cancer (8290, 8310, 8330–8333, 8335, 8337), anaplastic thyroid cancer (8020, 8021), medullary thyroid cancer (8345, 8510, 8511) and others including unspecified or poorly specified, mixed subtypes, metastasis and unknown [19, 20]. These subtypes were further categorised into three groups: papillary, non-papillary (including follicular, anaplastic, and medullary), and others/unknown.
Incidence and mortality rates were estimated per 100,000 person-years and were age-standardised to the European standard population using the direct method [21]. For the age standardisation, we used data of the population of Zurich from 1981 to 2016 [22]. Because population data were not available for 1980, we assumed the same population distribution as for 1981. The annual thyroid cancer incidence and mortality rates were estimated overall and by sex, histological subtype and age group [23]. For incidence rates, to allow for comparability with other papers, we formed six age groups in 10-year steps. For mortality rates, because of the limited number of cases, we decided to create two age groups, 20–64 and 65+ years old. To identify changes in trends over time, we used the average annual percent change (AAPC) and 95% confidence intervals [CIs] of the age-standardised rates using Joinpoint regression (version 4.8.0) [24]. In the Joinpoint regression analyses, we used the Grid search method with a minimum of two observations from a changing point in trends to either end of the data and a minimum of two observations between two joinpoints. We applied the standard errors to model for heteroscedasticity. The program calculated the best-fitting log-linear regression model of our data to indicate the year where a statistically significant change in AAPCs happened using t-tests. Therefore, the smallest possible number of joinpoints was used and statistical significance was set at an α level of 0.05. All tests were two-sided.
Cancer cases in the Canton of Zurich are registered with presumed consent and registration based on a decision by the Zurich Government Council from 1980 and the general registry approval by the Federal Commission of Experts for professional secrecy in medical research from 1995. All data were used anonymously in this analysis, and no approval from the Ethics Committee of the Canton of Zurich was necessary.
A total of 2972 cases of thyroid cancer were assessed, 823 in men (27.7%) and 2149 in women (72.3%). Overall, women were younger than men when they received the diagnosis of thyroid cancer (53.5 years in women vs 56 years in men). The most frequent subtype of thyroid cancer was papillary (65.8%), followed by follicular (23.4%), anaplastic (5.2%), medullary (3.1%) and others (2.5%; including unspecified or mixed subtypes, and metastases). Summarising these subtypes into three groups resulted in papillary (65.8%), non-papillary (31.7%) and others (2.5%). Despite the sex differences in incidence rates, thyroid cancer histological distribution was similar in men and women (table 1).
Table 1Baseline characteristics of the study population.
| Men (n = 823) | Women (n = 2149) | Total (n = 2972) | |
| Age at incidence in years, mean ± SD | 56 ± 17.2 | 53.5 ± 17.6 | 54.3 ± 17.5 |
| Papillary, n (%) | 501 (60.9) | 1455 (67.7) | 1956 (65.8) |
| – Age at incidence in years, mean ± SD | 51.3 ± 15.9 | 49.4 ± 15.5 | 49.9 ± 15.6 |
| Non-papillary, n (%) | 295 (35.8) | 646 (30.1) | 941 (31.7) |
| – Age at incidence in years, mean ± SD | 62.1 ± 16.4 | 61.6 ± 18.7 | 61.7 ± 18.0 |
| – Follicular | 202 (24.5) | 492 (22.9) | 694 (23.4) |
| – Anapalstic | 58 (7.0) | 96 (4.5) | 154 (5.2) |
| – Medullary | 35 (4.3) | 58 (2.7) | 93 (3.1) |
| Other/unknown, n (%) | 27 (3.3) | 48 (2.2) | 75 (2.5) |
| – Age at incidence in years, mean ± SD | 76.2 ± 11.6 | 70.9 ± 15.5 | 72.8 ± 14.4 |
SD: standard deviation
Statistically significantly increasing trends in age-standardised incidence rates of thyroid cancer were observed during the whole study period, for men from 2.6 to 4.3 per 100,000 (AAPC 1.4%, 95 % CI 0.6% to 2.2%) and for women from 4.8 to 11.9 per 100,000 (AAPC 2.6%, 95% CI 2% to 3.1%). The increase is mainly driven by papillary thyroid cancer (fig. 1).

Figure 1 Trends in papillary thyroid cancer incidence overall and by sex. Canton of Zurich 1980–2016. APC (annual percentage change). Age-standardised rates (European population).
The rise in papillary thyroid cancer was significant for both sexes, but was higher for women (from 2.3 to 10.7 per 100,000, AAPC 4.3%) than for men (from 1.1 to 3.6 per 100,000, AAPC 3.4%) (fig. 1, table 2, supplementary table S1). For the non-papillary subtype, a statistically significant decrease was seen for both men (from 1.4 to 0.9 per 100,000, AAPC –1.1%) and women (from 2.6 to 1.8 per 100,000, AAPC –1.1%) (fig. 2, table 2, supplementary table S1).
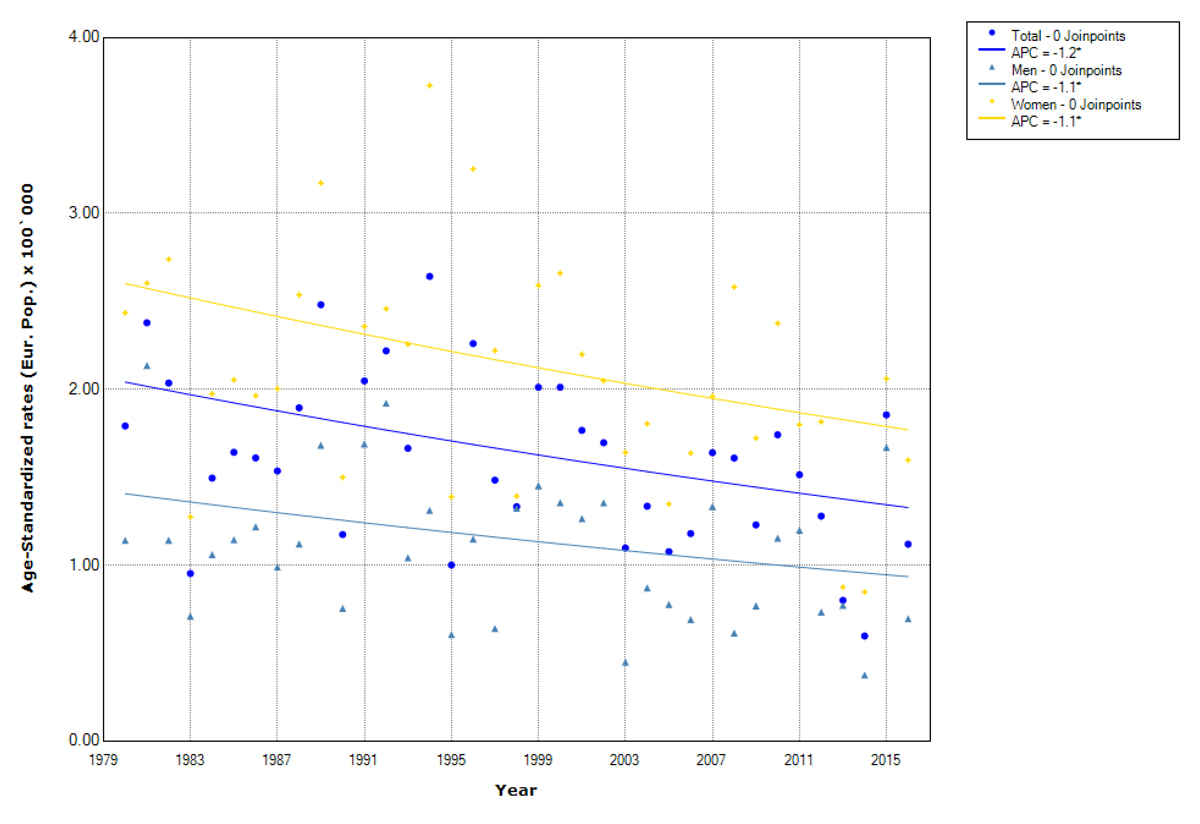
Figure 2 Trends in non-papillary thyroid cancer incidence by sex. Canton of Zurich 1980–2016. APC (annual percentage change). Age-standardised rates (European population)
Mean age at papillary thyroid cancer diagnosis was 49.9 (± 15.6) years overall, with 51.3 (± 15.9) years for men and 49.4 (± 15.5) years for women. We detected a significant increase in incidence in all age groups up to 74 years with the highest increase in the 45–54 years old individuals (AAPC 5%), followed by the 35–44 years old individuals (AAPC 4.3%). This was similar in men and women (table 2).
Mean age at diagnosis for the non-papillary subtype was 61.7 (± 18.0) years overall and similar in men (62.1 ± 16.4 years) and women (61.6 ± 18.7 years). We detected significant decreases in the incidence of non-papillary thyroid cancer in the age group 75+ years (AAPC –3.6%) and the age group 65–74 years (AAPC –2.5%). However, an increase limited to the age group 35–44 years (AAPC 1.7%) was observed. In men, significant decreases were restricted to the age groups above 65 years. The largest significant decline was seen for the age group 75+ years, during 1980–1992 (AAPC –11.7%) and during 1999–2016 (AAPC –6.4%). Together with the 65–74-year-old group (AAPC –1.9%), these age groups with a decreasing trend include 49.1% of all male cases. In women, we observed a significant decrease in women older than 45 years. The largest decrease was seen again for the age group 75+ years (AAPC –3.5%) followed by the 65-74 years old (AAPC –2.8%), 45-54 years old (AAPC –1.8%), and 55–64 years old (AAPC –1.5%). These age groups with a decreasing trend sum up to 75.6% of all female cases. In contrast to the decreasing incidence rates in older women, a significant increase was observed for the age group 35–44 years (AAPC 2.1%) (tables 2 and S1).
Table 2Trends in papillary thyroid cancer and non-papillary thyroid cancer incidence by sex and age groups in the canton of Zurich 1980–2016.
| Papillary thyroid cancer | Non-papillary thyroid cancer | ||||||
| Age group at diagnosis | Number of cases (%) | Average annual percentage change | 95% confidence Interval | Age group at diagnosis | Number of cases (%) | Average annual percentage change | 95% confidence Interval |
| Total | 1956 | Total | 941 | ||||
| 20–34 | 378 (19.3) | +3.8* | (+2.8, +4.8) | 20–34 | 91 (9.7) | –0.5 | (–2.0, +1.0) |
| 35–44 | 447 (22.9) | +4.3* | (+3.4, +5.2) | 35–44 | 124 (13.2) | +1.7* | (+0.0, +3.5) |
| 45–54 | 435 (22.2) | +5.0* | (+3.7, +6.2) | 45–54 | 105 (11.2) | –0.8 | (–2.0, +0.4) |
| 55–64 | 326 (16.7) | +3.6* | (+2.6, +4.6) | 55–64 | 151 (16.0) | –0.7 | (–2.0, +0.5) |
| 65–74 | 231 (11.8) | +2.2* | (+1.2, +3.3) | 65–74 | 196 (20.8) | –2.5* | (–4.0, –0.9) |
| 75+ | 139 (7.1) | –0.2 | (–1.5, +1.1) | 75+ | 274 (29.1) | –3.6* | (–4.6, –2.6) |
| All ages | 1956 (100) | +4.0* | (+3.4, +4.6) | All ages | 941 (100) | –1.2* | (–2.0, –0.3) |
| Men | 501 | Men | 295 | ||||
| 20–34 | 91 (18.2) | +1.1 | (–3.4, +5.8) | 20–34 | 21 (7.1) | –2.3 | (–4.9, +0.3) |
| 35–44 | 92 (18.4) | +3.2* | (+1.4, +5) | 35–44 | 36 (12.2) | –0.2 | (–2.3, +2.0) |
| 45–54 | 113 (22.6) | +4.0* | (+2.7, +5.4) | 45–54 | 32 (10.8) | +0.5 | (–1.8, +2.8) |
| 55–64 | 93 (18.6) | +1.0 | (–0.7, +2.7) | 55–64 | 61 (20.7) | –0.7 | (–2.8, +1.4) |
| 65–74 | 75 (15.0) | +2.1* | (+0.7, +3.5) | 65–74 | 72 (24.4) | –1.9* | (–3.5, –0.4) |
| 75+ | 37 (7.4) | +0.7 | (–1.0, +2.5) | 75+ | 73 (24.7) | –4.5b | (–9.4, +0.7) |
| All ages | 501 (100) | +3.4* | (+2.3, +4.4) | All ages | 295 (100) | –1.1* | (–2.2, –0.0) |
| Women | 1455 | Women | 646 | ||||
| 20–34 | 287 (19.7) | +3.7* | (+2.7, +4.8) | 20–34 | 70 (10.8) | +0.5 | (–1.1, +2.2) |
| 35–44 | 355 (24.4) | +4.4* | (+3.5, +5.3) | 35–44 | 88 (13.6) | +2.1* | (+0.4, +3.8) |
| 45–54 | 322 (22.1) | +4.9* | (+3.4, +6.3) | 45–54 | 73 (11.3) | –1.8* | (–3.0, –0.6) |
| 55–64 | 233 (16.0) | +4.2* | (+3.0, +5.4) | 55–64 | 90 (13.9) | –1.5* | (–2.7, –0.4) |
| 65–74 | 156 (10.7) | +2.1* | (+0.7, +3.5) | 65–74 | 124 (19.2) | –2.8* | (–4.6, –1.0) |
| 75+ | 102 (7.0) | –0.4 | (–1.7, +0.9) | 75+ | 201 (31.1) | –3.5* | (–4.8, –2.1) |
| All ages | 1455 (100) | +4.3* | (+3.7, +5) | All ages | 646 (100) | –1.1* | (–1.9, –0.2) |
* p <0.05
a 2 Joinpoints: 1980–2006: APC +0.6, 95% CI –1.8 to 3; 2006–2013: APC +17.5*, 95% CI 2.2 to 35.2]; 2013–2016: APC -25.6, 95% CI –51.3 to –1.4
b 2 Joinpoints: 1980–1992: APC –11.7*, 95% CI –17.6 to –5.4; 1992–1999: APC +14.7, 95% CI –9.7 to 45.6; 1999–2016: APC –6.4*, 95% CI –10.1 to –2.6]
During the study period, a total of 474 thyroid cancer deaths were registered in the canton of Zurich, 313 in women (65.8%) and 161 in men. Overall, men were younger than women when they died of thyroid cancer (73.6 ± 10.7 years in men vs 78.3 years ± 10.8 in women). The mortality data do not provide any information about subtypes; thus, we analysed the age-standardised mortality rates of thyroid cancer overall.
We observed a significant decrease in the age-standardised mortality rates during the whole study period for men (from 1.3 to 0.4 per 100,000, AAPC –3.6%) and for women (from 1.5 to 0.4 per 100,000, AAPC 3.7%) (table 3, and supplementary table S2).
A significant decrease in mortality rates was observed in both age groups, slightly larger in the 65+ years old (AAPC 3.8%) than in the 2064 years old individuals (AAPC 3.3) (fig. 3). In men, a similar significant decrease was observed for the age group 65+ years old (AAPC 3.5%) as well as for the 2064-year-old group (AAPC 3.4%). In women, a significant decrease in mortality was observed only in the older age group (AAPC 3.7%) (table 3).
Table 3Trends in thyroid cancer mortality by sex and age groups in the canton of Zurich 19802016.
| Thyroid cancer death, age group at death | Number of cases (%) | Average annual percentage change | 95% confidence Interval |
| Total | 474 | ||
| 20–64 | 67 (14.1) | –3.3* | (–5.2, –1.4) |
| 65+ | 407 (85.9) | –3.8* | (–4.8, –2.9) |
| All ages | 474 (100) | –3.9* | (–4.6, –3.1) |
| Men | 161 | ||
| 20–64 | 34 (21.1) | –3.4* | (–5.6, –1.0) |
| 65+ | 127 (78.9) | –3.5* | (–4.9, –2.2) |
| All ages | 161 (100) | –3.6* | (–4.7, –2.4) |
| Women | 313 | ||
| 20–64 | 33 (10.5) | –2.1 | (–4.2, –0) |
| 65+ | 280 (89.5) | –3.7* | (–4.9, –2.5) |
| All ages | 313 (100) | –3.7* | (–4.8, -–2.6) |
* p <0.05
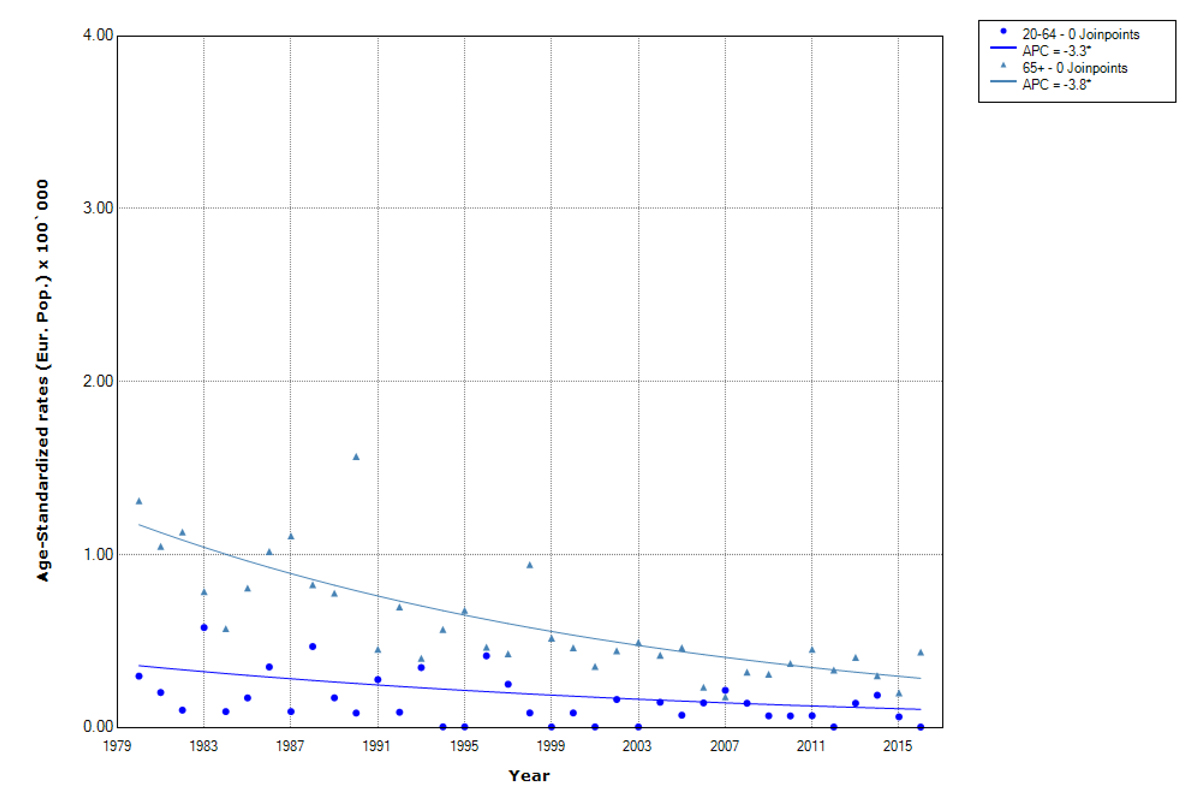
Figure 3 Trends in thyroid cancer mortality by age groups. Canton of Zurich 19802016. APC (annual percentage change). Age-standardised rates (European population).
In our study including data from a 37-year period, the incidence of thyroid cancer sharply increased in the canton of Zurich for men and women. This increase is consistent with the estimates observed in other countries [25-31]. papillary thyroid cancer, the most common subtype, mainly drives the overall increasing trends in thyroid cancer incidence. The increase in papillary thyroid cancer was significant for both men and women. In contrast, for non-papillary thyroid cancer, a statistically significant decrease was observed for men and women. Similarly, mortality rates significantly decreased for both sexes over the study period.
Our observations in the canton of Zurich are in line with those reported in other studies, showing a global and European trend of increasing incidence rates of papillary thyroid cancer while mortality rates remained stable or even decreased [2, 3, 8, 9]. This trend is common in high-income countries [3, 6, 32–38]. The extent of the observed increase in incidence over the last 37 years is similar to the increase in the United States, where the incidence tripled during 1974-2013 [6, 30]. However, the increase in incidence was smaller than in other countries where thyroid cancer screening with ultrasonography is used as a health checkup [6, 30, 39]. The incidence rates of the canton of Zurich for 2012 were above the European estimates of 2012 for men (3.1 per 100,000) and women (9.3 per 100,000) [16]. Compared with Switzerland in general, the incidence rates in the canton of Zurich for 2012 were below the Swiss overall estimate of 2012 for men (4.9 per 100,000) and for women (12.0 per 100,000) [15]. As in Europe and Switzerland overall, in the canton of Zurich the increasing incidence rates were mainly driven by the increase in papillary thyroid cancer, as in both sexes papillary is the major histological subtype that greatlyincreased whereas for the non-papillary subtypes a significant decrease was detected [3, 5, 15]. We detected a significant increase in incidence in all age groups up to 74 years. The highest rise in incidence rates was observed for the 45-54-year-old group (AAPC 5.0%) and 35-44-year-olds (AAPC 4.3%). The age group 35-54 years showed not only the highest increase but also the highest overall incidence rates; this group includes 45.1% of all cases. These findings are in line with previous Swiss incidence rates, which state an increase limited to people below 70 years of age [4]. Similarly, most affluent countries reported that increases in incidence peaked among individuals 45–54 years of age [10].
There are several factors that have been discussed as potential drivers of the increase in incidence rates of thyroid cancer, but only a few are well documented. High-dose ionising radiation exposure, especially during childhood, is a known risk factor for papillary thyroid cancer [40]. Overall radiation exposure in Switzerland has decreased due to wide-spread surveillance and protection measures [41]. It has to be taken into account that, after exposure, the minimum latency period until cancer development appears to be 5 to 10 years, which depends on the age at exposure and the type of cancer [40]. Furthermore, doses due to releases or direct radiation from nuclear power plants are irrelevant in Switzerland [42]. Additionally, the canton of Zurich has relatively low ground radiation compared with other regions [42]. Concerns about the Chernobyl nuclear power plant accident in 1986 may influence our results. The Federal Office of Public Health has estimated that the increase in cancers as a consequence of the Chernobyl nuclear power plant accident is less than 0.05%, due to extremely low additional long-term contamination in Switzerland [43]. Accordingly, radioactive exposure does not seem to explain the sharp increase in papillary thyroid cancer during past decades. Another reported risk factor is inadequate intake of iodine [44], which can also be neglected given that table salt has been iodised since 1922 in Switzerland and the median urinary iodine concentrations among the Swiss adult population is classified as at least marginal ( not insufficient) according to the WHO [45]. Obesity is a known risk factor as well [46, 47], which increased during the study period but to a far lesser extent than the incidence rates of papillary thyroid cancer [48]. Other mentioned risk factors such as female sex, nutrition and genetic factors might be important, but evidence is inconsistent or weak [38, 49-52].
We cannot exclude a partly true increase in the incidence of papillary thyroid cancer. However, the most likely reason for the increasing incidence rates of papillary thyroid cancer in Switzerland over past decades is the increased use of ultrasonography and fine-needle aspiration biopsies, which are performed much more systematically during the examination of thyroid problems [3, 6, 7, 10, 11, 17, 53]. In total, three different scenarios could explain the growing numbers of thyroid cancers diagnosed: opportunistic screening in asymptomatic patients, a diagnostic cascade to check unclear symptoms, and incidental findings not intended to examine the thyroid gland [39]. Even though they are not recommended, ultrasonography and fine-needle aspiration biopsies are commonly used for screening in Switzerland and in the canton of Zurich [54]. This leads to an increase in the incidental detection rate of subclinical very low-risk thyroid cancers, such as papillary microcarcinoma, which previously remained undetected [3, 6, 7, 11, 17, 53]. This may result in overdiagnosis and overtreatment [55] of indolent, very low-risk thyroid cancers which, if untreated, are very unlikely to cause morbidity or mortality. Overdiagnosis could expose some people to unneeded surgery and lifelong treatment, although treatment-related morbidity is not in any reasonable relation to the survival benefit [6, 9, 11, 39, 56-61]. Besides these physical and psychological consequences, overdiagnosis has large financial costs for the healthcare systems [10].
Overdiagnosis seems to be more common in women than in men in 26 countries analysed in a previous study, including Switzerland, potentially explaining the differences in incidence rates between sexes [10]. Other possible reasons for the sex-specific differences, such as reproductive factors and hormones, are discussed by different authors [50], but are poorly documented [38, 4952]. In Switzerland and many other countries, overdiagnosis and overtreatment are considered a major problem since rising incidence rates without a concomitant increase in mortality rates are suggestive of indirect evidence for overdiagnosis [9, 55, 62]. As mentioned previously, this is a major issue in South Korea, where the estimated proportion of thyroid cancer cases attributable to overdiagnosis is 93% in women and 83% in men. In 1999, South Korea started a government-funded national cancer screening programme to detect thyroid cancer in people without any symptoms [10]. In the United States, it was shown that the higher the level of health care access the greater the papillary thyroid cancer incidence rates, which also strongly suggests overdiagnosis [63].
We observed a significant decrease in mortality rates in the canton of Zurich over the whole study period, both for men and women. These results are consistent with previously published Swiss trends [4]. The mortality rates of the canton of Zurich for 2012 were below the 2012 European estimates for men (0.5 per 100,000) and women (0.7 per 100,000) [16]. Compared with Switzerland in general, the incidence rates in the canton of Zurich for 2012 were slightly below the Swiss overall estimate of 2012 for men (0.5 per 100,000) and for women (0.4 per 100,000) [15], which might be due to different health seeking behaviours in the different regions, for example with respect to screening [64]. The decreasing mortality rates can be mainly attributed to improved treatment and earlier diagnosis thanks to more commonly used and more sensitive ultrasonography and fine-needle aspiration biopsies [3, 65]. Therefore, diagnostics not only explain increasing incidences, but might also contribute to the decreasing mortality because of an earlier detection and initiation of treatment. An increasing incidence rate together with decreasing mortality serves as indirect evidence of thyroid cancer being overdiagnosed. It appears that many indolent thyroid cancers are increasingly diagnosed, especially papillary microcarcinomas, which, if undetected, would probably not have led to symptoms. It is debatable if the small decrease in mortality justifies the high increase in incidence, which leads to psychological consequences, overtreatment and life-long substitution therapy. Therefore, targeted diagnostic strategies are necessary to avoid overdiagnosis of thyroid cancer.
Our study had several strengths. We analysed population-based, high-quality data from the cancer registry of the canton of Zurich, including all thyroid cancer cases by subtype during a 37-year period from all individuals living in the canton of Zurich. We assume the chance of cases not being registered to be very low, as our registry has been shown to have good completeness [18, 66]. Nevertheless, our study had some limitations. Individual exposures to potential risk factors were not registered and we can only guess about the impact they might have. Although the canton of Zurich is the largest Swiss canton, we were not able to conduct meaningful analyses by non-papillary thyroid cancer subtypes or by size of the tumour. In addition, until recently, the cancer registry of the canton of Zurich did not routinely collect date and cause of death information or information on relapse. Finally, our study is a time-trend study, which provides only indirect evidence of overdiagnosis. The only direct way to reveal that thyroid cancer is overdiagnosed would be to follow untreated patients until they die of other causes [37, 62, 67, 68].
In conclusion, our results show significantly increasing incidence rates of thyroid cancer in the canton of Zurich in both sexes, with an almost two-fold increase in men and a nearly three-fold increase in women between 1980 and 2016. These increasing incidence trends are mainly driven by papillary thyroid cancer, the most frequent histological subtype, and the only subtype with a significant increase in both women and men. Simultaneously, the mortality rates significantly decreased in both sexes. These results are in accordance with existing Swiss, European and global trends in incidence and mortality of thyroid cancer, including subtypes and sex differences. To prevent the constant increase in thyroid cancer, it is highly recommended to understand the causes of these trends and to focus on mechanisms that are responsible for overdiagnosis.
Disclaimer: Where authors are identified as personnel of the International Agency for Research on Cancer / World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer / World Health Organization.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.
Table S1Thyroid cancer incidence rates by sex and histological subtypes in the canton of Zurich 1980–2016. Age-standardised rate (European Population) per 100,000 persons.
| Year | Men | Women | ||||
| Papillary | Non-papillary | All cases | Papillary | Non-papillary | All cases | |
| 1980 | 1.37 | 1.14 | 3.20 | 2.17 | 2.43 | 4.78 |
| 1981 | 1.55 | 2.14 | 4.4 | 4.29 | 2.60 | 7.70 |
| 1982 | 1.26 | 1.14 | 2.80 | 2.50 | 2.74 | 5.44 |
| 1983 | 0.92 | 0.71 | 2.05 | 2.05 | 1.27 | 3.95 |
| 1984 | 1.61 | 1.06 | 2.65 | 2.76 | 1.97 | 5.09 |
| 1985 | 1.23 | 1.15 | 2.50 | 2.55 | 2.05 | 4.72 |
| 1986 | 0.72 | 1.22 | 2.45 | 3.19 | 1.96 | 5.39 |
| 1987 | 1.07 | 0.99 | 2.49 | 2.70 | 2.00 | 4.94 |
| 1988 | 1.38 | 1.12 | 2.48 | 3.34 | 2.54 | 5.93 |
| 1989 | 1.23 | 1.68 | 3.42 | 3.76 | 3.17 | 7.37 |
| 1990 | 1.71 | 0.75 | 2.88 | 2.99 | 1.5 | 5.00 |
| 1991 | 2.21 | 1.69 | 4.07 | 3.23 | 2.36 | 5.88 |
| 1992 | 1.42 | 1.92 | 3.44 | 2.84 | 2.46 | 5.34 |
| 1993 | 0.69 | 1.04 | 1.88 | 2.28 | 2.26 | 4.68 |
| 1994 | 2.54 | 1.31 | 4.00 | 5.05 | 3.73 | 8.82 |
| 1995 | 2.18 | 0.61 | 2.76 | 4.05 | 1.39 | 5.48 |
| 1996 | 2.42 | 1.15 | 3.56 | 4.73 | 3.25 | 8.14 |
| 1997 | 0.96 | 0.64 | 1.89 | 5.06 | 2.22 | 7.54 |
| 1998 | 2.10 | 1.32 | 3.74 | 4.13 | 1.39 | 5.98 |
| 1999 | 0.60 | 1.45 | 2.17 | 5.66 | 2.59 | 8.44 |
| 2000 | 1.98 | 1.36 | 3.46 | 5.34 | 2.66 | 8.28 |
| 2001 | 1.08 | 1.26 | 2.33 | 4.48 | 2.20 | 6.97 |
| 2002 | 1.20 | 1.36 | 2.69 | 6.30 | 2.05 | 8.34 |
| 2003 | 2.00 | 0.45 | 2.59 | 5.27 | 1.64 | 7.03 |
| 2004 | 0.71 | 0.87 | 1.84 | 5.09 | 1.80 | 7.16 |
| 2005 | 2.37 | 0.78 | 3.25 | 5.46 | 1.35 | 7.21 |
| 2006 | 3.22 | 0.69 | 3.89 | 7.70 | 1.64 | 9.51 |
| 2007 | 2.50 | 1.33 | 4.06 | 6.86 | 1.96 | 8.81 |
| 2008 | 1.43 | 0.62 | 2.31 | 10.7 | 2.58 | 13.42 |
| 2009 | 2.65 | 0.77 | 3.54 | 7.67 | 1.72 | 9.60 |
| 2010 | 3.30 | 1.15 | 4.63 | 7.04 | 2.37 | 9.72 |
| 2011 | 2.88 | 1.20 | 4.21 | 10.76 | 1.80 | 12.82 |
| 2012 | 3.91 | 0.73 | 4.72 | 10.03 | 1.82 | 11.89 |
| 2013 | 4.11 | 0.77 | 4.87 | 9.27 | 0.88 | 10.14 |
| 2014 | 3.16 | 0.38 | 3.68 | 9.73 | 0.85 | 10.81 |
| 2015 | 3.79 | 1.67 | 5.78 | 8.46 | 2.06 | 10.66 |
| 2016 | 3.29 | 0.70 | 4.31 | 10.34 | 1.60 | 12.02 |
Table S2Thyroid cancer mortality rates by sex in the canton of Zurich 1980–2016. Age-standardised rate (European population) per 100,000 persons.
| Year | Men | Women |
| 1980 | 1.81 | 1.56 |
| 1981 | 1.24 | 1.30 |
| 1982 | 1.42 | 1.05 |
| 1983 | 1.23 | 1.32 |
| 1984 | 0.30 | 0.90 |
| 1985 | 1.29 | 0.71 |
| 1986 | 0.87 | 1.63 |
| 1987 | 0.91 | 1.28 |
| 1988 | 0.69 | 1.61 |
| 1989 | 1.21 | 0.76 |
| 1990 | 0.77 | 2.26 |
| 1991 | 0.82 | 0.68 |
| 1992 | 0.89 | 0.73 |
| 1993 | 0.73 | 0.71 |
| 1994 | 0.35 | 0.69 |
| 1995 | 0.68 | 0.61 |
| 1996 | 1.06 | 0.78 |
| 1997 | 0.67 | 0.61 |
| 1998 | 1.20 | 0.87 |
| 1999 | 0.14 | 0.72 |
| 2000 | 0.43 | 0.60 |
| 2001 | 0.26 | 0.45 |
| 2002 | 0.26 | 0.97 |
| 2003 | 0.34 | 0.65 |
| 2004 | 0.30 | 0.76 |
| 2005 | 0.53 | 0.54 |
| 2006 | 0.15 | 0.53 |
| 2007 | 0.54 | 0.28 |
| 2008 | 0.48 | 0.50 |
| 2009 | 0.15 | 0.54 |
| 2010 | 0.60 | 0.33 |
| 2011 | 0.23 | 0.67 |
| 2012 | 0.25 | 0.38 |
| 2013 | 0.72 | 0.39 |
| 2014 | 0.37 | 0.63 |
| 2015 | 0.40 | 0.15 |
| 2016 | 0.56 | 0.42 |
1. Bray F , Ferlay J , Soerjomataram I , Siegel RL , Torre LA , Jemal A . Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov;68(6):394–424. https://doi.org/10.3322/caac.21492
2. World Health Organization (WHO) . Cancer Today: World Health Organization; 2020 [Available from: https://gco.iarc.fr/today/online-analysis-table
3. La Vecchia C , Malvezzi M , Bosetti C , Garavello W , Bertuccio P , Levi F , et al. Thyroid cancer mortality and incidence: a global overview. Int J Cancer. 2015 May;136(9):2187–95. https://doi.org/10.1002/ijc.29251
4. Bouchardy C , Lutz JM , Kühni C . Krebs in der Schweiz Neuchâtel: NICER, FSO, SCCR; 2011 [Available from: https://www.nicer.org/assets/files/publications/others/krebs_in_der_schweiz_e_web.pdf].
5. Kilfoy BA , Zheng T , Holford TR , Han X , Ward MH , Sjodin A , et al. International patterns and trends in thyroid cancer incidence, 1973-2002. Cancer Causes Control. 2009 Jul;20(5):525–31. https://doi.org/10.1007/s10552-008-9260-4
6. Davies L , Welch HG . Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006 May;295(18):2164–7. https://doi.org/10.1001/jama.295.18.2164
7. Horn-Ross PL , Lichtensztajn DY , Clarke CA , Dosiou C , Oakley-Girvan I , Reynolds P , et al. Continued rapid increase in thyroid cancer incidence in california: trends by patient, tumor, and neighborhood characteristics. Cancer Epidemiol Biomarkers Prev. 2014 Jun;23(6):1067–79. https://doi.org/10.1158/1055-9965.EPI-13-1089
8. Raposo L , Morais S , Oliveira MJ , Marques AP , José Bento M , Lunet N . Trends in thyroid cancer incidence and mortality in Portugal. Eur J Cancer Prev. 2017 Mar;26(2):135–43. https://doi.org/10.1097/CEJ.0000000000000229
9. Vaccarella S , Franceschi S , Bray F , Wild CP , Plummer M , Dal Maso L . Worldwide Thyroid-Cancer Epidemic? The Increasing Impact of Overdiagnosis. N Engl J Med. 2016 Aug;375(7):614–7. https://doi.org/10.1056/NEJMp1604412
10. Li M , Dal Maso L , Vaccarella S . Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol. 2020 Jun;8(6):468–70. https://doi.org/10.1016/S2213-8587(20)30115-7
11. Kitahara CM , Sosa JA . The changing incidence of thyroid cancer. Nat Rev Endocrinol. 2016 Nov;12(11):646–53. https://doi.org/10.1038/nrendo.2016.110
12. Bosetti C , Bertuccio P , Malvezzi M , Levi F , Chatenoud L , Negri E , et al. Cancer mortality in Europe, 2005-2009, and an overview of trends since 1980. Ann Oncol. 2013 Oct;24(10):2657–71. https://doi.org/10.1093/annonc/mdt301
13. Kachuri L , De P , Ellison LF , Semenciw R ; Advisory Committee on Canadian Cancer Statistics . Cancer incidence, mortality and survival trends in Canada, 1970-2007. Chronic Dis Inj Can. 2013 Mar;33(2):69–80. https://doi.org/10.24095/hpcdp.33.2.03
14. Siegel R , Ma J , Zou Z , Jemal A . Cancer statistics, 2014. CA Cancer J Clin. 2014 Jan-Feb;64(1):9–29. https://doi.org/10.3322/caac.21208
15 Dyntar, D , Lorez M , Diebold J . Incidence-based mortality trends for thyroid cancer: Is there a "true" increase in incidence of thyroid cancer in Switzerland? : Schweizer Krebs-Bulletin = Bulletin Suisse du Cancer; 2018 [Available from: https://www.zora.uzh.ch/id/eprint/162152/].
16 European Network of Cancer Registries (ENCR) . Thyroid cancer Factsheet: ENCR Factsheets; 2017 [Available from: https://encr.eu/sites/default/files/factsheets/ENCR_Factsheet_Thyroid_2017-2.pdf].
17. Sant M , Allemani C , Santaquilani M , Knijn A , Marchesi F , Capocaccia R ; EUROCARE Working Group . EUROCARE-4. Survival of cancer patients diagnosed in 1995-1999. Results and commentary. Eur J Cancer. 2009 Apr;45(6):931–91. https://doi.org/10.1016/j.ejca.2008.11.018
18. Wanner M , Matthes KL , Korol D , Dehler S , Rohrmann S . Indicators of Data Quality at the Cancer Registry Zurich and Zug in Switzerland. BioMed Res Int. 2018 Jun;2018:7656197. https://doi.org/10.1155/2018/7656197
19. Fritz A , Percy C . A., Jack, K. Shanmugaratnam, L.H. Sobin, M.D. Parkin, C. WHO, Percy, V. Van, Holten,C. Muir: International Classification of Diseases for Oncology. 3rd ed., Geneva (2013).
20. WHO . World Health Organization. International statistical classification of diseases and related health problems. 10th revision. Geneva. (1992).
21. Boyle P , Parkin DM . Cancer registration: principles and methods. Statistical methods for registries. IARC Sci Publ. 1991;(95):126–58.
22. Federal Statistical Office . STAT-TAB – interactive tables (FSO). https://www.pxweb.bfs.admin.ch/pxweb/en/. 2020.
23. Surveillance E , Results E . (SEER) NCI. Rate Algorithms: National Cancer Institute (SEER); 2020 [Available from: https://seer.cancer.gov/
24. National Cancer Institute . Joinpoint Trend Analysis Software: National Cancer Institute; 2020 [Available from: https://surveillance.cancer.gov/joinpoint/
25. Pandeya N , McLeod DS , Balasubramaniam K , Baade PD , Youl PH , Bain CJ , et al. Increasing thyroid cancer incidence in Queensland, Australia 1982-2008 - true increase or overdiagnosis? Clin Endocrinol (Oxf). 2016 Feb;84(2):257–64. https://doi.org/10.1111/cen.12724
26. Harari A , Singh RK . Increased rates of advanced thyroid cancer in California. J Surg Res. 2016 Mar;201(1):244–52. https://doi.org/10.1016/j.jss.2015.10.037
27. Safavi A , Azizi F , Jafari R , Chaibakhsh S , Safavi AA . Thyroid Cancer Epidemiology in Iran: a Time Trend Study. Asian Pac J Cancer Prev. 2016;17(1):407–12. https://doi.org/10.7314/APJCP.2016.17.1.407
28. Keinan-Boker L , Silverman BG . Trends of Thyroid Cancer in Israel: 1980-2012. Rambam Maimonides Med J. 2016 Jan;7(1):e0001. https://doi.org/10.5041/RMMJ.10228
29. Colonna M , Uhry Z , Guizard AV , Delafosse P , Schvartz C , Belot A , et al.; FRANCIM network . Recent trends in incidence, geographical distribution, and survival of papillary thyroid cancer in France. Cancer Epidemiol. 2015 Aug;39(4):511–8. https://doi.org/10.1016/j.canep.2015.04.015
30. Lim H , Devesa SS , Sosa JA , Check D , Kitahara CM . Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA. 2017 Apr;317(13):1338–48. https://doi.org/10.1001/jama.2017.2719
31. Lukas J , Drabek J , Lukas D , Dusek L , Gatek J . The epidemiology of thyroid cancer in the Czech Republic in comparison with other countries. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2013 Sep;157(3):266–75. https://doi.org/10.5507/bp.2012.086
32. dos Santos Silva I , Swerdlow AJ . Thyroid cancer epidemiology in England and Wales: time trends and geographical distribution. Br J Cancer. 1993 Feb;67(2):330–40. https://doi.org/10.1038/bjc.1993.61
33. Pettersson B , Adami HO , Wilander E , Coleman MP . Trends in thyroid cancer incidence in Sweden, 1958-1981, by histopathologic type. Int J Cancer. 1991 Apr;48(1):28–33. https://doi.org/10.1002/ijc.2910480106
34. Akslen LA , Haldorsen T , Thoresen SO , Glattre E . Incidence of thyroid cancer in Norway 1970-1985. Population review on time trend, sex, age, histological type and tumour stage in 2625 cases. Acta Pathol Microbiol Scand Suppl. 1990 Jun;98(6):549–58. https://doi.org/10.1111/j.1699-0463.1990.tb01070.x
35. Montanaro F , Pury P , Bordoni A , Lutz JM , Network SC ; Swiss Cancer Registries Network . Unexpected additional increase in the incidence of thyroid cancer among a recent birth cohort in Switzerland. Eur J Cancer Prev. 2006 Apr;15(2):178–86. https://doi.org/10.1097/01.cej.0000197450.94980.36
36. Husson O , Haak HR , van Steenbergen LN , Nieuwlaat WA , van Dijk BA , Nieuwenhuijzen GA , et al. Rising incidence, no change in survival and decreasing mortality from thyroid cancer in The Netherlands since 1989. Endocr Relat Cancer. 2013 Mar;20(2):263–71. https://doi.org/10.1530/ERC-12-0336
37. Moynihan R , Doust J , Henry D . Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012 May;344(may28 4):e3502. https://doi.org/10.1136/bmj.e3502
38. Shin S , Sawada N , Saito E , Yamaji T , Iwasaki M , Shimazu T , et al.; JPHC Study Group . Menstrual and reproductive factors in the risk of thyroid cancer in Japanese women: the Japan Public Health Center-Based Prospective Study. Eur J Cancer Prev. 2018 Jul;27(4):361–9. https://doi.org/10.1097/CEJ.0000000000000338
39. Ahn HS , Kim HJ , Welch HG . Korea’s thyroid-cancer “epidemic”—screening and overdiagnosis. N Engl J Med. 2014 Nov;371(19):1765–7. https://doi.org/10.1056/NEJMp1409841
40. Iglesias ML , Schmidt A , Ghuzlan AA , Lacroix L , Vathaire F , Chevillard S , et al. Radiation exposure and thyroid cancer: a review. Arch Endocrinol Metab. 2017 Mar-Apr;61(2):180–7. https://doi.org/10.1590/2359-3997000000257
41 Bundesamt für Gesundheit (BAG). Jahresbericht Umweltradioaktivität und Strahlendosis in der Schweiz 2019 [Available from: https://www.bag.admin.ch/bag/de/home/das-bag/publikationen/taetigkeitsberichte/jahresberichte-umweltradioaktiviaet.html].
42. Rybach L , Bächler D , Bucher B , Schwarz G . Radiation doses of Swiss population from external sources. J Environ Radioact. 2002;62(3):277–86. https://doi.org/10.1016/S0265-931X(01)00169-2
43 Swissinfo. Swiss assess effects of Chernobyl disaster: Swissinfo; 2006 [Available from: https://www.swissinfo.ch/eng/swiss-assess-effects-of-chernobyl-disaster/5003874].
44. Cao LZ , Peng XD , Xie JP , Yang FH , Wen HL , Li S . The relationship between iodine intake and the risk of thyroid cancer: A meta-analysis. Medicine (Baltimore). 2017 May;96(20):e6734. https://doi.org/10.1097/MD.0000000000006734
45. Stalder E , Haldimann M , Blanc A , Dudler V , Ponte B , Pruijm M , et al. Use of day and night urinary iodine excretion to estimate the prevalence of inadequate iodine intakes via the estimated average requirement cut-point method. Swiss Med Wkly. 2019 Jun;149:w20090. https://doi.org/10.4414/smw.2019.20090
46. Kwon H , Han KD , Park CY . Weight change is significantly associated with risk of thyroid cancer: A nationwide population-based cohort study. Sci Rep. 2019 Feb;9(1):1546. https://doi.org/10.1038/s41598-018-38203-0
47. Xu L , Port M , Landi S , Gemignani F , Cipollini M , Elisei R , et al. Obesity and the risk of papillary thyroid cancer: a pooled analysis of three case-control studies. Thyroid. 2014 Jun;24(6):966–74. https://doi.org/10.1089/thy.2013.0566
48. Faeh D , Bopp M . Increase in the prevalence of obesity in Switzerland 1982-2007: birth cohort analysis puts recent slowdown into perspective. Obesity (Silver Spring). 2010 Mar;18(3):644–6. https://doi.org/10.1038/oby.2009.310
49. Peterson E , De P , Nuttall R . BMI, diet and female reproductive factors as risks for thyroid cancer: a systematic review. PLoS One. 2012;7(1):e29177. https://doi.org/10.1371/journal.pone.0029177
50. Zamora-Ros R , Rinaldi S , Biessy C , Tjønneland A , Halkjaer J , Fournier A , et al. Reproductive and menstrual factors and risk of differentiated thyroid carcinoma: the EPIC study. Int J Cancer. 2015 Mar;136(5):1218–27. https://doi.org/10.1002/ijc.29067
51. Zhu J , Zhu X , Tu C , Li YY , Qian KQ , Jiang C , et al. Parity and thyroid cancer risk: a meta-analysis of epidemiological studies. Cancer Med. 2016 Apr;5(4):739–52. https://doi.org/10.1002/cam4.604
52. Yi X , Zhu J , Zhu X , Liu GJ , Wu L . Breastfeeding and thyroid cancer risk in women: A dose-response meta-analysis of epidemiological studies. Clin Nutr. 2016 Oct;35(5):1039–46. https://doi.org/10.1016/j.clnu.2015.12.005
53. Baker SR , Bhatti WA . The thyroid cancer epidemic: is it the dark side of the CT revolution? Eur J Radiol. 2006 Oct;60(1):67–9. https://doi.org/10.1016/j.ejrad.2006.04.022
54. Brito JP , Morris JC , Montori VM . Thyroid cancer: zealous imaging has increased detection and treatment of low risk tumours. BMJ. 2013 Aug;347 aug27 4:f4706. https://doi.org/10.1136/bmj.f4706
55. Jegerlehner S , Bulliard JL , Aujesky D , Rodondi N , Germann S , Konzelmann I , et al.; NICER Working Group . Overdiagnosis and overtreatment of thyroid cancer: A population-based temporal trend study. PLoS One. 2017 Jun;12(6):e0179387. https://doi.org/10.1371/journal.pone.0179387
56. Brito JP , Davies L . Is there really an increased incidence of thyroid cancer? Curr Opin Endocrinol Diabetes Obes. 2014 Oct;21(5):405–8. https://doi.org/10.1097/MED.0000000000000094
57. Vaccarella S , Dal Maso L , Laversanne M , Bray F , Plummer M , Franceschi S . The Impact of Diagnostic Changes on the Rise in Thyroid Cancer Incidence: A Population-Based Study in Selected High-Resource Countries. Thyroid. 2015 Oct;25(10):1127–36. https://doi.org/10.1089/thy.2015.0116
58. Morris LG , Tuttle RM , Davies L . Changing Trends in the Incidence of Thyroid Cancer in the United States. JAMA Otolaryngol Head Neck Surg. 2016 Jul;142(7):709–11. https://doi.org/10.1001/jamaoto.2016.0230
59. Davies L . Overdiagnosis of thyroid cancer. BMJ. 2016 Nov;355:i6312. https://doi.org/10.1136/bmj.i6312
60. Brito JP , Davies L , Zeballos-Palacios C , Morris JC , Montori VM . Papillary lesions of indolent course: reducing the overdiagnosis of indolent papillary thyroid cancer and unnecessary treatment. Future Oncol. 2014 Jan;10(1):1–4. https://doi.org/10.2217/fon.13.240
61. Iñiguez-Ariza NM , Brito JP . Management of Low-Risk Papillary Thyroid Cancer. Endocrinol Metab (Seoul). 2018 Jun;33(2):185–94. https://doi.org/10.3803/EnM.2018.33.2.185
62. Welch HG , Black WC . Overdiagnosis in cancer. J Natl Cancer Inst. 2010 May;102(9):605–13. https://doi.org/10.1093/jnci/djq099
63. Morris LG , Sikora AG , Tosteson TD , Davies L . The increasing incidence of thyroid cancer: the influence of access to care. Thyroid. 2013 Jul;23(7):885–91. https://doi.org/10.1089/thy.2013.0045
64. Ulyte A , Wei W , Dressel H , Gruebner O , von Wyl V , Bähler C , et al. Variation of colorectal, breast and prostate cancer screening activity in Switzerland: influence of insurance, policy and guidelines. PLoS One. 2020 Apr;15(4):e0231409. https://doi.org/10.1371/journal.pone.0231409
65. Nguyen QT , Lee EJ , Huang MG , Park YI , Khullar A , Plodkowski RA . Diagnosis and treatment of patients with thyroid cancer. Am Health Drug Benefits. 2015 Feb;8(1):30–40.
66 Lorez M , Bordoni A , Bouchardy C , Bulliard JL , Camey B , Dehler S , et al. Evaluation of completeness of case ascertainment in Swiss cancer registration. Eur J Cancer Prev. 2017;26 Joining forces for better cancer registration in Europe:S139-S46.
67. Bulliard JL , Chiolero A . Screening and overdiagnosis: public health implications. Public Health Rev. 2015 Nov;36(1):8. https://doi.org/10.1186/s40985-015-0012-1
68. Chiolero A , Paccaud F , Aujesky D , Santschi V , Rodondi N . How to prevent overdiagnosis. Swiss Med Wkly. 2015 Jan;145:w14060.