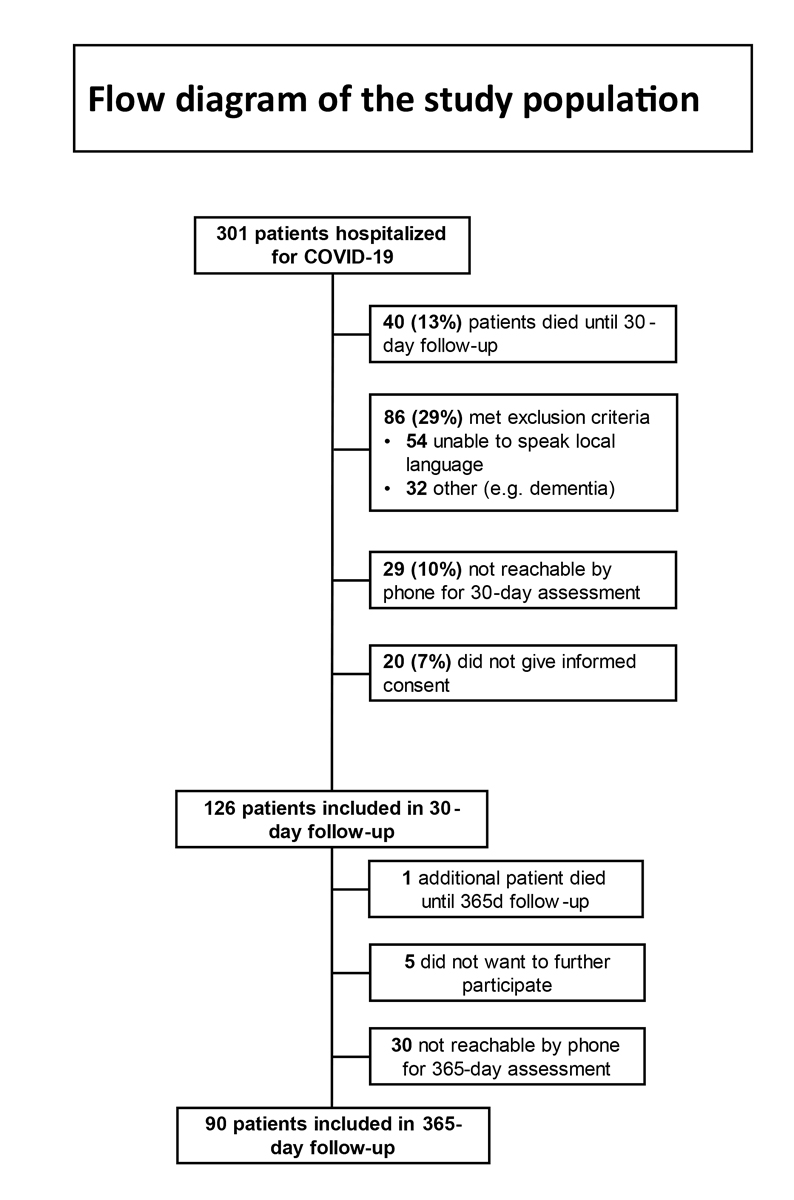
Figure 1 Flow diagram of the study population.
DOI: https://doi.org/10.4414/SMW.2021.w30091
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the coronavirus disease 2019 (COVID-19) pandemic, which has resulted in a global healthcare crisis affecting millions of patients worldwide. Whereas the initial focus was on the acute in-hospital treatment of patients, there are increasing reports of persistent and prolonged effects after acute COVID-19 – a syndrome called long COVID [1–5].
Long COVID has been defined as residual symptoms after acute disease, which persist for more than 4 weeks [6]. A recent systematic review found that almost three in four patients still suffer from sequelae several months after recovery from acute illness [3]. Long COVID may afflict a wide range of organ systems, with the most commonly reported symptoms being shortness of breath, chronic fatigue, cognitive impairment, cough and chronic pain syndromes [3, 4]. The pathophysiological mechanisms underlying long COVID are still incompletely understood and diagnostic consensus criteria have not yet been defined. The current literature considers direct viral toxicity, immune dysregulation with prolonged inflammation, endothelial and microvascular damage, or adverse effects of received treatments as potential triggers or mediators of long COVID [6–9]. Especially older patients, and patients with high symptom load and severe course of the disease appear to be at higher risk for developing long COVID [10–12].
Symptoms such as fatigue, pain, or mental sequelae were already seen in connection with other coronavirus diseases such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), and were prevalent in follow-ups of up to 15 years [13–17]. Further, several studies showed that patients with acute COVID-19 frequently experience psychological distress. According to a recent meta-analysis, about 17% (95% CI 10% to 23%) of patients suffer from anxiety, 38% (95% CI 29% to 46%) from depression and 42% (95% CI 9% to 74%) from symptoms of post-traumatic stress disorder (PTSD) [18]. Recent studies suggest that a substantial number of patients continue to suffer from mental health problems with around one fourth of patients reporting symptoms of anxiety, depression, and post-traumatic stress, several weeks to months later [3, 5, 19–21]. Yet for COVID-19, most studies have only followed patients for 3 to 6 months. Hence data regarding the persistence of long COVID symptoms and longer-term risks are scarce.
Our aim was to assess the prevalence of persisting symptoms suggestive of long COVID, as well as psychological distress and PTSD in patients 1 year after hospitalisation for COVID-19, and to understand risk factors associated with long COVID.
This prospective bicentric cohort study was conducted at two tertiary care hospitals in Switzerland, the University Hospital Basel, and the Kantonsspital Aarau, from March 2020 to July 2021. The local Ethics Committee (Ethics Committee Northwest and Central Switzerland, EKNZ) approved the study. All participating patients provided written informed consent. This manuscript adheres to the STROBE statement [22].
Patients with COVID-19 who were consecutively admitted for inpatient care during March to June 2020 were eligible for inclusion. Patients were hospitalised based on their clinical condition, clinical risk factors (e.g., age >65 years, respiratory rate >25/min, hypoxaemia or pulmonary infiltrates observed on chest imaging). Patients were excluded from our study if they had insufficient proficiency in one of the local languages, cognitive impairment such as dementia or serious psychiatric conditions (e.g., psychosis). Clinical patient characteristics and severity or duration of COVID-19 were no reasons for exclusion. We contacted patients about 1 month after hospital discharge, informed them about the aim of our study and asked for consent to participate. We sent a letter containing the study information and informed consent form, and included those patients who provided written informed consent.
Data were prospectively collected upon hospitalisation by specifically trained research staff. We reviewed patients’ medical records from their hospitalisation with COVID-19 to collect sociodemographic factors (e.g., age, gender, citizenship), vital signs and illness-related characteristics. Specifically, we calculated the National Early Warning Score (NEWS) [23], a commonly used tool to assess a patient’s severity of illness and detect patients prone to clinical deterioration. The NEWS is a scoring system that assesses the following physiological parameters: respiratory rate, oxygen saturation, temperature, systolic blood pressure, heart rate and level of consciousness. To each parameter a score between 0 and 3 is allocated, depending on how much the parameter deviates from its normal range. A sum score of 7 reflects a high risk for clinical deterioration.
In addition, we assessed patients’ severity of comorbidity using the Charlson Comorbidity Index (CCI) [24], intensive care unit (ICU) duration, intubation and duration of hospitalisation.
To assess the short-term health status of patients, specifically trained research staff conducted telephone interviews 30 and 90 days after hospital discharge. These structured interviews included internationally established, validated questionnaires as well as some items specifically designed for the purpose of this study (symptoms limiting life quality, predominant symptom, most concerning symptom). Psychological distress was assessed via the Hospital Anxiety and Depression Scale (HADS) as defined below [25]. We assessed patients’ self-perceived overall health status using the visual analogue scale (VAS) of the EuroQol index ranging from 0 (worst imaginable health) to 100 (best imaginable health) [26, 27]. We evaluated patients’ resilience through the 10-item version of the Connor-Davidson Resilience Scale (CD-RISC-10). The CD-RISC is commonly used in clinical research to assess resilience based on how well a person can cope with stress [28].
Finally, we determined patients’ perceived stress through the Perceived Stress Scale (PSS-10) [29]. The PSS is a widely used instrument to measure the perception of stress and whether people appraise life events as unpredictable or uncontrollable.
In a follow-up telephone interview 1 year after hospital discharge, specifically trained research staff assessed primary and secondary outcomes as outlined below.
Primary endpoint: The primary endpoint of our study was long COVID 1 year after hospitalisation, defined as the presence of at least one persisting symptom which newly occurred during or after acute infection with SARS-CoV-2. This definition of long COVID was based on previous studies [3, 4] and included different symptoms known to be associated with COVID-19, including shortness of breath, fatigue, cough, chest pain/tightness, dysosmia, dysgeusia, sleep disorders, headache, concentration difficulties, joint pain, muscle pain, palpitations, post-exertion malaise, loss of appetite or nausea, vomiting or diarrhoea, fever, paraesthesia, skin disorders (rash, pruritus) and other symptoms related to patients’ initial COVID-19. Patients also rated the intensity of symptoms on a VAS (ranging from 0 – not at all to 10 – very much).
Secondary endpoints: Secondary endpoints included psychological distress, defined as clinically relevant symptoms of anxiety and/or depression 1 year after discharge, measured with the HADS [25]. In line with previous research, a score of ≥8 on the depression and/or anxiety subscale (range: 0 to 21) of the HADS, indicating clinically relevant symptoms of depression and/or anxiety, was defined as clinically relevant psychological distress [25, 30].
Further, we assessed symptoms of PTSD through a German translation of the Impact of Event Scale-revised (IES-r), which measures symptoms of emotional distress caused by traumatic events [31]. The IES-r is a 22-item questionnaire containing three subscales covering the three symptom domains intrusion, avoidance and hyperarousal. A cut-off score of 1.5 was considered to indicate clinically relevant symptoms of PTSD as suggested in previous research [32].
During the interview, interviewers filled out a printed questionnaire form, entered the data into an electronic data file and conducted quality control for each variable.
Descriptive statistics, i.e., frequencies as well as means and standard deviations were used to present characteristics of the study population. To investigate the associations of potential risk and protective factors assessed at 30-day follow-up and outcomes at 1-year follow-up, we used logistic regression models. Multivariable regression models included age, gender and the factors significantly associated with the outcome in univariable analyses. We report odds ratios (ORs) and 95% confidence intervals (CIs) as a measure of association and the area under the receiver operating characteristic curve (AUC) as a measure of discrimination. A p-value of <0.05 (two-tailed) was considered statistically significant. All statistical analyses were conducted using Stata 15 (Stata Corp, College Station, Texas, USA).
Between March and June 2020, 301 patients with COVID-19 were hospitalised at the University Hospital of Basel (n = 198) and Kantonsspital Aarau (n = 103). Figure 1 shows the flowchart of patients regarding study inclusion. Forty-one patients had died before the end of follow-up, 86 met exclusion criteria such as insufficient proficiency in the local language, cognitive impairment or severe underlying psychiatric conditions, 59 were not reachable by phone and 25 did not provide informed consent or did not want to participate any further. The final sample therefore consisted of 90 patients.

Figure 1 Flow diagram of the study population.
The mean age of patients at hospitalisation was 60 years and 38% were female. The mean duration of hospitalisation was 9.4 days. Overall, there was a high burden of comorbidities with a CCI of 2.4, and 17% of patients required treatment in the intensive care unit (ICU). Patients who required ICU treatment were 58 years old and 27% were female. These patients stayed for 9 days in the ICU and for 18 days in the hospital. Additional sociodemographic and clinical characteristics of patients, as well as outcomes after 1 year are shown in table 1.
Table 1Characteristics of the study population.
| Factor | Value (n = 90) | |
| Sociodemographic factors | ||
| Age (years), mean (SD) | 60.09 (15.14) | |
| Gender (female), n (%) | 34 (38%) | |
| Citizenship, n (%) | Swiss | 66 (74%) |
| Non-Swiss | 23 (26%) | |
| Religious affiliation, n (%) | Christian | 50 (57%) |
| Non-Christian | 8 (9%) | |
| Not religious | 30 (34%) | |
| Civil status, n (%) | Married/partnership | 60 (67%) |
| Widowed/separated/single | 29 (33%) | |
| Having children (yes), n (%) | 61 (72%) | |
| Education, n (%) | High School | 6 (7%) |
| Apprenticeship | 58 (68%) | |
| College/university | 21 (25%) | |
| Acute illness-related factors | ||
| Duration of hospitalisation (days), mean (SD) | 9.44 (6.77) | |
| Severity of illness (NEWS score), mean (SD) | 6.60 (3.61) | |
| Comorbidity (CCI), mean (SD) | 2.36 (2.12) | |
| ICU stay, n (%) | 15 (17%) | |
| Requirement of oxygen, n (%) | None | 30 (34%) |
| Nasal canula/NIV | 49 (55%) | |
| Intubation | 10 (11%) | |
| Psychological factors at 30-day follow-up | mean (SD) | |
| Anxiety symptoms (HADS-A) | 4.01 (3.74) | |
| Depressive symptoms (HADS-D) | 2.53 (3.26) | |
| PTSD symptoms (IES-r) | -2.5 (3.26) | |
| Perceived stress (PSS-10) | 21.9 (7.54) | |
| Self-perceived overall health status (EuroQol VAS 0-100) | 73.82 (17.08) | |
| Resilience (CD-RISC) | 31.65 (6.04) | |
| Long COVID symptoms one year after hospitalisation | n (%) / mean (SD) | |
| overall | 63 (70%) | |
| Shortness of breath | 19 (21%) | |
| – if yes, intensity (VAS 0-10) | 4.16 (1.68) | |
| Fatigue | 41 (46%) | |
| – if yes, intensity (VAS 0-10) | 5.54 (2.34) | |
| Cough | 6 (7%) | |
| – if yes, intensity (VAS 0-10) | 4.17 (1.17) | |
| Chest pain/tightness | 11 (12%) | |
| – if yes, intensity (VAS 0-10) | 4.18 (2.52) | |
| Dysosmia | 14 (16%) | |
| – if yes, intensity (VAS 0-10) | 4.43 (2.47) | |
| Dysgeusia | 11 (12%) | |
| – if yes, intensity (VAS 0-10) | 4.27 (2.24) | |
| Sleep disorder | 15 (17%) | |
| – if yes, intensity (VAS 0-10) | 5.67 (2.41) | |
| Headache | 8 (9%) | |
| – if yes, intensity (VAS 0-10) | 3.25 (2.19) | |
| Concentration difficulties | 28 (31%) | |
| – if yes, intensity (VAS 0-10) | 5.11 (1.99) | |
| Joint pain | 16 (18%) | |
| – if yes, intensity (VAS 0-10) | 5.06 (2.29) | |
| Muscle pain | 13 (14%) | |
| – if yes, intensity (VAS 0-10) | 5.08 (2.60) | |
| Palpitations | 9 (10%) | |
| – if yes, intensity (VAS 0-10) | 4.33 (2.40) | |
| Post-exertion malaise | 18 (20%) | |
| – if yes, intensity (VAS 0-10) | 5.28 (2.30) | |
| Loss of appetite / Nausea | 6 (7%) | |
| – if yes, intensity (VAS 0-10) | 4.83 (1.72) | |
| Vomiting/Diarrhoea | 5 (6%) | |
| – if yes, intensity (VAS 0-10) | 7.00 (2.45) | |
| Fever | 2 (2%) | |
| Paraesthesia | 7 (8%) | |
| – if yes, intensity (VAS 0-10) | 5.00 (3.11) | |
| Skin disorders (Rash, pruritus) | 5 (6%) | |
| – if yes, intensity (VAS 0-10) | 4.80 (2.28) | |
| Other | 10 (11%) | |
| – if yes, intensity (VAS 0-10) | 4.70 (2.71) | |
| Symptoms limiting quality of life (yes), n (%) | 34 (38%) | |
| Which is the predominant symptom? | Fatigue | 18 (28%) |
| Dyspnoea | 9 (14.29%) | |
| Concentration difficulties | 9 (14.29%) | |
| Joint pain | 6 (9.5%) | |
| Post-exertion malaise | 3 (4.76%) | |
| Which is the most concerning symptom? | Fatigue | 8 (12.7%) |
| Concentration difficulties | 8 (12.7%) | |
| Dyspnoea | 6 (9.5%) | |
| Post-exertion malaise | 5 (7.94%) | |
| Joint pain | 4 (6.35%) | |
| Psychological outcomes one year after hospitalisation | ||
| Self-perceived overall health status (EuroQol VAS 0-100), mean (SD) | 77.11 (16.01) | |
| Anxiety symptoms (HADS-A), n (%) | 11 (13%) | |
| Depressive symptoms (HADS-D), n (%) | 9 (10%) | |
| Anxiety and/or depressive symptoms (HADS), n (%) | 16 (18%) | |
| PTSD symptoms (IES-r), n (%) | 3 (3.33%) | |
SD: standard deviation; HADS-A: anxiety subscale of the Hospital and Anxiety Depression Scale; HADS-D: depression subscale of the Hospital and Anxiety Depression Scale; PTSD: post-traumatic stress disorder; IES-r: Impact of Event Scale revised; PSS-10: Perceived Stress Scale; CD-RISC: Connor-Davidson Resilience Scale; VAS: visual analogue scale; NEWS: National Early Warning Score; CCI: Charlson Comorbidity Index; ICU: intensive care unit
One year after hospitalisation, 63 (70%) patients had symptoms of long COVID and thus met our definition of the primary endpoint (table 1). The most common symptoms experienced were fatigue (46%), concentration difficulties (31%), shortness of breath (21%) and post-exertion malaise (20%). Thirty-four (38%) patients indicated that their symptoms limited their quality of life. A total of 34% of patients reported one or two symptoms, and 36% reported ≥3 symptoms after 1 year. Figures 2 and 3 show the distribution of symptoms overall and the most important symptoms at 90 days and 1 year among patients in our cohort. The frequency of symptoms was relatively similar at 90 days and 1 year.
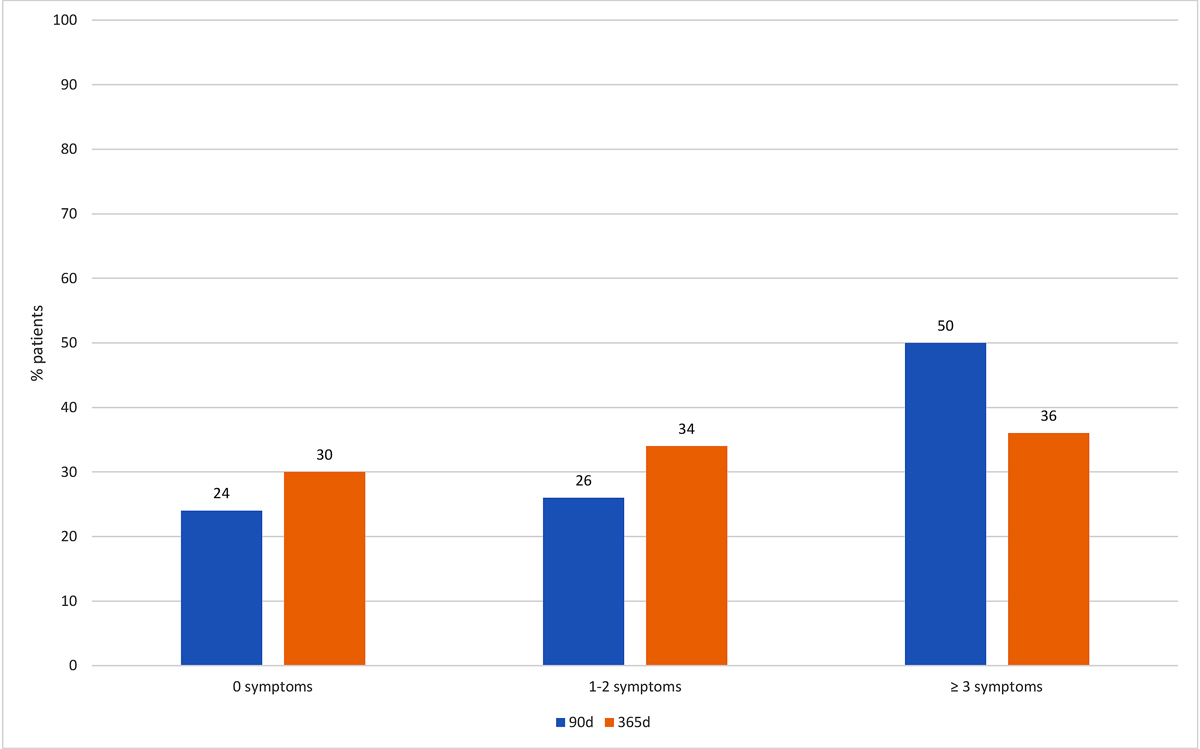
Figure 2 Number of persistent symptoms 90 and 365 days after hospitalisation.
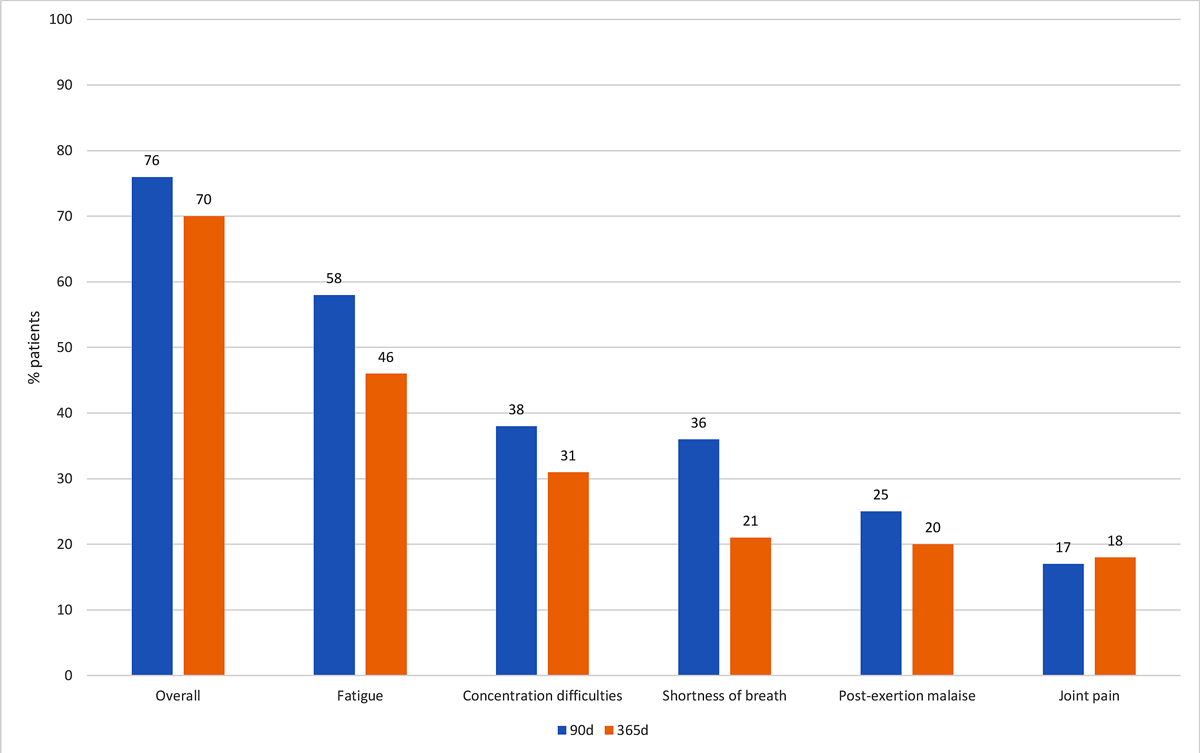
Figure 3 Prevalence of the most common persistent symptoms 90 and 365 days after hospitalisation.
We investigated associations between several predictor variables and long COVID (table 2). Three acute illness-related factors were associated with long COVID in univariable analyses, including duration of hospitalisation (OR 1.11, 95% CI 1.00–1.22; p = 0.041), severity of illness according to NEWS (OR 1.19, 95% CI 1.04–1.37; p = 0.013) and self-perceived overall health status (EuroQol) 30 days after hospitalisation (OR 0.97, 95% CI 0.94–1.00; p = 0.027). There were no significant associations of sociodemographic and psychological factors assessed at 30-day follow-up and the prevalence of long COVID at 1 year.
Table 2Prevalence and factors associated with long COVID.
| Factor | n | Long COVID (no) | Long COVID (yes) | Odds ratio, 95% CI | p-value | |
| n = 27 | n = 63 | |||||
| Age (years), mean (SD) | 90 | 61.70 (17.13) | 59.41 (14.30) | 0.99 (0.96–1.02) | 0.508 | |
| Gender (female), n (%) | 90 | 13 (48%) | 21 (33%) | 0.54 (0.21–1.35) | 0.187 | |
| Citizenship, n (%) | Swiss | 90 | 21 (78%) | 45 (73%) | 1 (Ref) | |
| Non-Swiss | 6 (22%) | 17 (27%) | 1.32 (0.46–3.84) | 0.607 | ||
| Education, n (%) | High school/apprenticeship | 85 | 18 (67%) | 46 (79%) | 1 (Ref) | |
| College/university | 9 (33%) | 12 (21%) | 0.52 (0.19–1.45) | 0.212 | ||
| Duration of hospitalisation (days), mean (SD) | 90 | 7.11 (5.02) | 10.45 (7.20) | 1.11 (1.00–1.22) | 0.041 | |
| Severity of illness (NEWS score), mean (SD) | 90 | 5.11 (3.75) | 7.24 (3.38) | 1.19 (1.04–1.37) | 0.013 | |
| Comorbidity (CCI), mean (SD) | 90 | 2.59 (2.10) | 2.26 (2.13) | 0.93 (0.75–1.15) | 0.492 | |
| ICU stay, n (%) | 90 | 2 (7%) | 13 (21%) | 3.32 (0.69–15.86) | 0.133 | |
| Anxiety symptoms 30 days after hospitalisation (HADS-A), mean (SD) | 89 | 3.19 (2.54) | 4.37 (4.12) | 1.1 (0.96–1.27) | 0.174 | |
| Depressive symptoms 30 days after hospitalisation (HADS-D), mean (SD) | 89 | 1.93 (2.11) | 2.79 (3.63) | 1.1 (0.93–1.31) | 0.258 | |
| PTSD symptoms 30 days after hospitalisation (IES-r), mean (SD) | 87 | –2.85 (1.49) | –2.34 (1.68) | 1.24 (0.90–1.7) | 0.183 | |
| Perceived stress during illness (PSS-10), mean (SD) | 67 | 21.86 (6.51) | 21.91 (8.04) | 1 (0.93–1.07) | 0.977 | |
| Self-perceived overall health status 30 days after hospitalisation (EuroQol VAS 0-100), mean (SD) | 88 | 80.00 (15.38) | 71.08 (17.20) | 0.97 (0.94–1.00) | 0.027 | |
| Resilience (CD-RISC) 30 days after hospitalisation, mean (SD) | 79 | 32.31 (5.16) | 31.32 (6.45) | 0.97 (0.89–1.06) | 0.494 | |
SD: standard deviation; OR: odds ratio; CI: confidence interval; HADS-A: anxiety subscale of the Hospital and Anxiety Depression Scale; HADS-D: depression subscale of the Hospital and Anxiety Depression Scale; IES-r: Impact of Event Scale revised; PSS-10: Perceived Stress Scale; CD-RISC: Connor-Davidson Resilience Scale; NEWS: National Early Warning Score; CCI: Charlson Comorbidity Index; ICU: intensive care unit
To further investigate the prognostic value of these predictors regarding long COVID, we calculated a multivariable model including age, gender and the three significant predictors. Results showed moderate discrimination of this model with an area under the receiver-operating characteristic curve (AUC) of 0.75 (supplementary table S1 in the appendix).
Sixteen patients (18%) experienced psychological distress, i.e., symptoms of depression and/or anxiety (table 3). Nine patients reported only anxiety, two only depression and five patients had both. Psychological factors at 30-day follow up were associated with psychological distress 1 year after hospitalisation: Anxiety symptoms (OR 1.25, 95% CI 1.07–1.45; p = 0.004), depressive symptoms (OR 1.45, 95% CI 1.15–1.82; p = 0.002), perceived stress (OR 1.11, 95% CI 1.02–1.22; p = 0.015) and resilience (OR 0.83, 95% CI 0.74–0.93; p = 0.002). There was no association between sociodemographic and acute illness-related factors and outcomes. Three patients (3.3%) had clinically relevant symptoms of PTSD at 1 year follow-up. There were no differences in the rates of psychological distress in patients with and without long COVID. Of the 61 patients with long COVID, 10 (16.4%) showed psychological distress and 51 (83.6%) did not (p = 0.513). Of 39 patients with fatigue, 9 (23.1%) experienced psychological distress whereas 30 (76.9%) did not (p = 0.288).
Table 3Prevalence and factors associated with psychological distress.
| Factor | n | Psychological distress (no) | Psychological distress (yes) | Odds ratio, 95% CI | p-value | |
| n = 72 | n = 16 | |||||
| Sociodemographic factors | ||||||
| Age (years), mean (SD) | 88 | 60.88 (13.76) | 57.06 (20.87) | 0.98 (0.95–1.02) | 0.365 | |
| Gender (female), n (%) | 88 | 26 (36%) | 6 (38%) | 1.06 (0.35–3.26) | 0.917 | |
| Citizenship, n (%) | Swiss | 88 | 52 (73%) | 12 (75%) | 1 (Ref) | |
| Non-Swiss | 19 (27%) | 4 (25%) | 0.91 (0.26–3.18) | 0.885 | ||
| Education, n (%) | High school/apprenticeship | 83 | 54 (79% | 9 (60%) | 1 (Ref) | |
| College/university | 14 (21%) | 6 (40%) | 2.57 (0.78–8.44) | 0.119 | ||
| Acute illness-related factors | ||||||
| Duration of hospitalisation (days), mean (SD) | 88 | 9.42 (7.06) | 9.63 (5.92) | 1 (0.93–1.09) | 0.914 | |
| Severity of illness (NEWS score), mean (SD) | 88 | 6.54 (3.58) | 6.63 (3.98) | 1.01 (0.87–1.16) | 0.928 | |
| Comorbidity (CCI), mean (SD) | 88 | 2.41 (2.09) | 2.19 (2.40) | 0.95 (0.73–1.24) | 0.708 | |
| ICU stay, n (%) | 88 | 12 (17%) | 2 (13%) | 0.7 (0.14–3.5) | 0.666 | |
| Psychological factors at 30-day follow-up | ||||||
| Anxiety symptoms (HADS-A), mean (SD) | 87 | 3.27 (3.16) | 6.50 (4.46) | 1.25 (1.07–1.45) | 0.004 | |
| Depressive symptoms (HADS-D), mean (SD) | 87 | 1.73 (1.95) | 5.44 (5.15) | 1.45 (1.15–1.82) | 0.002 | |
| Perceived stress (PSS-10), mean (SD) | 66 | 20.61 (6.28) | 27.00 (10.42) | 1.11 (1.02–1.22) | 0.015 | |
| Resilience (CD-RISC), mean (SD) | 77 | 32.98 (4.43) | 26.40 (8.58) | 0.83 (0.74–0.93) | 0.002 | |
SD: standard deviation; CI: confidence interval; HADS-A: anxiety subscale of the Hospital and Anxiety Depression Scale; HADS-D: depression subscale of the Hospital and Anxiety Depression Scale; PSS-10: Perceived Stress Scale; CD-RISC: Connor-Davidson Resilience Scale; NEWS: National Early Warning Score; CCI: Charlson Comorbidity Index; ICU: intensive care unit
The main finding of this two-centre, prospective cohort study is that 70% of patients report residual symptoms suggestive of long COVID 1 year after hospitalisation due to COVID-19. In more than a third of patients, symptoms were severe with a significant impact on quality of life, and 36% of patients reported ≥3 symptoms 1 year after infection. Additionally, 18% of patients suffered from psychological distress 1 year after hospitalisation for COVID-19. Several key findings need to be discussed.
First, our study shows that even 1 year after acute illness with COVID-19 a vast majority of patients still meet the criteria for long COVID and show at least one persistent symptom. The most common symptoms reported in our cohort (in order of decreasing frequency) were fatigue, concentration difficulties, shortness of breath, post-exertion malaise and joint pain. These results are largely in line with studies investigating the prevalence of long COVID in patients 3 to 6 months after COVID-19 [3–5, 33]. A recent meta-analysis reported residual symptoms in 72.5% of all patients between 2 and 7 months after acute infection [3]. Recent longer-term studies in outpatients indicated that between 39% and 53% of patients reported persisting symptoms after 7 to 12 months [34, 35]. The high proportion of patients with ongoing symptoms in our study may be explained by the fact that we only included hospitalised patients who presumably had a higher burden of disease and comorbidities. Correspondingly, we found illness-related factors to be associated with long COVID. In particular, patients with a longer duration of hospitalisation, more severe illness according to the NEWS during acute infection and lower self-perceived overall health status at baseline were at increased risk for developing long COVID. This finding is in line with previous studies with short-term follow-up, which demonstrated that high initial symptom load and disease severity are predictive for patients showing residual symptoms that go beyond the acute illness with COVID-19 [11, 36, 37].
Second, one in three patients reported at least three residual symptoms after COVID-19 and more than half of our patients with long COVID indicated a relevant impact of symptoms on daily life. Similarly, in a mixed cohort of outpatients and hospitalised patients, about one third of patients reported a persistent decline in health-related quality of life 9 months after COVID diagnosis [1]. Moreover, even a mild course of COVID-19 had a negative impact on psychosocial functioning [38, 39]. The long-term outcome in patients assessed beyond 9 months after acute illness with COVID-19 has not been well-described, so far. Our study adds to emerging evidence indicating that long COVID may well persist for more than 1 year after acute infection.
Third, we found that 18% of patients suffer from symptoms of anxiety and/or depression even 1 year after hospitalisation. The rates in our sample are higher than those in the general population in Switzerland in 2017 [40] and the European Union during 2013 to 2015 [41].
Although emotional distress during and shortly after the acute phase of COVID-19 may be self-limiting and thus does not necessarily require clinical care, several studies indicate that a substantial group of patients continues to suffer from clinically relevant symptoms of anxiety and depression up to 1 year later. A recent systematic review showed that about 15% of patients who had been diagnosed with COVID-19 suffered from persistent symptoms of depression and 22% from anxiety, which are both higher than in our sample [3]. However, the rates reported by the meta-analysis are based on 16 studies which mostly had follow-up durations of around 3 months and none followed patients for longer than 6 months. One of the included studies assessing Swiss patients 6 to 8 months after their COVID-19 diagnosis showed that 32% reported anxiety and 26% depressive symptoms [20]. As we assessed patients 1 year after hospitalisation, the longer follow-up duration might explain the lower rates in our sample compared with most other studies. Further, the higher rates of depression and anxiety in our sample at 1- and 3-month follow-up [42, 43], which more closely align with those of Nasserie et al. [3], suggest that the psychological symptoms might decrease over time. Additionally, differences in operationalisation of psychological distress might contribute to differences in the reported rates. Most studies analysed self-rated symptoms assessed with single items or questionnaires, and some evaluated psychiatric diagnoses. The latter emphasises the clinical relevance of the persisting psychological symptoms. Despite the high risk of symptoms of long COVID in our population, two thirds of patients did not have limitations to their quality of life, and we found no difference in psychological symptoms between long COVID and not long COVID patients after 1year. Although our study was limited by low power, resilience, social support and other protective factors might have prevented further adverse health outcomes in patients. Early preventive strategies regarding long-term mental health problems in patients with COVID-19 should be assessed by future studies.
So far, evidence on potential predictors of long-term psychological distress is scarce. One Swiss study evaluating a cohort of mainly non-hospitalised patients found a lower level of education as well as unemployment to be associated with symptoms of depression 6 to 8 months after COVID-19 [20]. In our cohort of hospitalised patients, sociodemographic factors as well as severity of illness were not associated with long-term psychological distress. However, patients with higher levels of perceived stress, anxiety and depression at 1-month follow-up were more likely to experience psychological distress after 1 year. However, our sample was small and there is thus a risk for type II error.
Finally, fatigue, sleep disorders and concentration difficulties are potential symptoms of depressive and anxiety disorders, but also commonly described as long COVID symptoms. We assessed depression and anxiety with the HADS, which was developed for patients with somatic diseases and does not include these symptoms. In our study, patients with long COVID did not have higher rates of anxiety or depression than patients without residual long COVID symptoms. However, it must be noted that in our sample only a relatively small number of patients had anxiety or depression, potentially concealing a possible difference between the two groups. Nonetheless, considering the increased long-term prevalence of anxiety and depressive disorders in patients with COVID-19, affective disorders should be considered as potential differential diagnoses, especially in patients who solely present with fatigue, sleep disorders or concentration difficulties.
This study has limitations including the small sample size and selection bias due to our exclusion criteria. Particularly, the exclusion of almost 20% of patients owing to a lack of language skills might be a selection bias. As previous research has shown that being in an ethnic minority is often associated with psychological symptoms [44, 45], the prevalence of psychological distress in our study might be underrepresented. Also, we excluded a large proportion of patients due to dementia who were significantly older than the remaining patients of our cohort. As age is an important predictor of long COVID [3, 36], again the prevalence could be underestimated from our study.
We evaluated several symptoms suggested as symptoms of long COVID by previous studies. The specificity of these different symptoms to long COVID needs validation in future studies. The observational design does not allow any conclusions regarding preventive effects and the study is thus rather hypothesis-generating.
Further, we did not assess fatigue and neurocognitive deficits objectively but through inquiring via interview only. Fatigue might play an important role in patients with long COVID similar to that in patients with multiple sclerosis, as fatigue prevents patients from functioning normally and affects quality of life. We assessed quality of life through the EuroQol. Further research should incorporate other questionnaires such as the INTERMED self-assessment questionnaire to better evaluate the influence of long COVID on activities of daily life or social activities.
Finally, we only quantitatively assessed symptoms of long COVID. Future research should also focus on qualitative data to better understand this new disease entity.
A high proportion of COVID-19 patients report symptoms of long COVID 1 year after hospitalisation, which negatively affect their quality of life. The main risk factors are associated with severity of illness and may not be modifiable. There is need for further research into prevention and treatment of long COVID, particularly in patients with a high initial severity of illness.
We will share deidentified data of patients included in this study after finalisation of secondary projects and upon reasonable request. Proposals should be directed to sabina.hunziker[at]usb.ch.
Sabina Hunziker and her research team received funding by the Swiss National Science Foundation (SNSF) (Ref 10001C_192850/1 and 10531C_182422) and the Gottfried and Julia Bangerter-Rhyner Foundation (8472/HEG-DSV).
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.
Table S1Multivariable analyses of factors associated with long COVID.
| Factor | n | Long COVID (no) | Long COVID (yes) | Multivariable OR, 95% CI | p-value | AUC |
| n = 27 | n = 63 | 0.75 | ||||
| Age (years), mean (SD) | 90 | 61.70 (17.13) | 59.41 (14.30) | 0.98 (0.95–1.02) | 0.298 | |
| Gender (female), n (%) | 90 | 13 (48%) | 21 (33%) | 0.51 (0.18–1.41) | 0.192 | |
| Duration of hospitalisation (days), mean (SD) | 90 | 7.11 (5.02) | 10.45 (7.20) | 1.04 (0.93–0) | 0.461 | |
| Severity of illness (NEWS score), mean (SD) | 90 | 5.11 (3.75) | 7.24 (3.38) | 1.15 (0.97–1.35) | 0.104 | |
| Self-perceived overall health status 30 days after hospitalisation (EuroQol VAS 0-100), mean (SD) | 88 | 80.00 (15.38) | 71.08 (17.20) | 0.97 (0.94–1) | 0.055 |
SD: standard deviation; OR: odds ratio; CI: confidence interval; AUC: area under the curve; NEWS: National Early Warning Score
1. Logue JK , Franko NM , McCulloch DJ , McDonald D , Magedson A , Wolf CR , et al. Sequelae in Adults at 6 Months After COVID-19 Infection. JAMA Netw Open. 2021;4(2):e210830. Epub 2021/02/20. doi: https://doi.org/10.1001/jamanetworkopen.2021.0830. PubMed PMID: 33606031; PubMed Central PMCID: PMCPMC7896197.
2. Carfì A , Bernabei R , Landi F ; Gemelli Against COVID-19 Post-Acute Care Study Group . Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020 Aug;324(6):603–5. https://doi.org/10.1001/jama.2020.12603
3. Nasserie T , Hittle M , Goodman SN . Assessment of the Frequency and Variety of Persistent Symptoms Among Patients With COVID-19: A Systematic Review. JAMA network open. 2021;4(5):e2111417. Epub 2021/05/27. doi: https://doi.org/10.1001/jamanetworkopen.2021.11417. PubMed PMID: 34037731; PubMed Central PMCID: PMCPMC8155823.
4. Nehme M , Braillard O , Alcoba G , Aebischer Perone S , Courvoisier D , Chappuis F , et al. COVID-19 Symptoms: Longitudinal Evolution and Persistence in Outpatient Settings. Ann Intern Med. 2021;174(5):723-5. doi: https://doi.org/10.7326/M20-5926. PubMed PMID: 33284676; PubMed Central PMCID: PMCPMC7741180.
5. Al-Aly Z , Xie Y , Bowe B . High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021 Jun;594(7862):259–64. https://doi.org/10.1038/s41586-021-03553-9
6. Nalbandian A , Sehgal K , Gupta A , Madhavan MV , McGroder C , Stevens JS , et al. Post-acute COVID-19 syndrome. Nat Med. 2021 Apr;27(4):601–15. https://doi.org/10.1038/s41591-021-01283-z
7. Maltezou HC , Pavli A , Tsakris A . Post-COVID Syndrome: An Insight on Its Pathogenesis. Vaccines (Basel). 2021;9(5). Epub 2021/06/03. doi: https://doi.org/10.3390/vaccines9050497. PubMed PMID: 34066007; PubMed Central PMCID: PMCPMC8151752.
8. Ortelli P , Ferrazzoli D , Sebastianelli L , Engl M , Romanello R , Nardone R , et al. Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: Insights into a challenging symptom. J Neurol Sci. 2021;420:117271. Epub 2020/12/29. doi: https://doi.org/10.1016/j.jns.2020.117271. PubMed PMID: 33359928; PubMed Central PMCID: PMCPMC7834526.
9. Stratton CW , Tang YW , Lu H . Pathogenesis-directed therapy of 2019 novel coronavirus disease. J Med Virol. 2021 Mar;93(3):1320–42. https://doi.org/10.1002/jmv.26610
10. Carvalho-Schneider C , Laurent E , Lemaignen A , Beaufils E , Bourbao-Tournois C , Laribi S , et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. 2021;27(2):258-63. Epub 2020/10/09. doi: https://doi.org/10.1016/j.cmi.2020.09.052. PubMed PMID: 33031948; PubMed Central PMCID: PMCPMC7534895.
11. Goertz YMJ , Van Herck M , Delbressine JM , Vaes AW , Meys R , Machado FVC , et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020;6(4). Epub 2020/12/02. doi: https://doi.org/10.1183/23120541.00542-2020. PubMed PMID: 33257910; PubMed Central PMCID: PMCPMC7491255
12. Stavem K , Ghanima W , Olsen MK , Gilboe HM , Einvik G . Prevalence and Determinants of Fatigue after COVID-19 in Non-Hospitalized Subjects: A Population-Based Study. Int J Environ Res Public Health. 2021;18(4). Epub 2021/03/07. doi: https://doi.org/10.3390/ijerph18042030. PubMed PMID: 33669714; PubMed Central PMCID: PMCPMC7921928.
13. Lee SH , Shin HS , Park HY , Kim JL , Lee JJ , Lee H , et al. Depression as a Mediator of Chronic Fatigue and Post-Traumatic Stress Symptoms in Middle East Respiratory Syndrome Survivors. Psychiatry Investig. 2019;16(1):59-64. Epub 2019/01/05. doi: https://doi.org/10.30773/pi.2018.10.22.3. PubMed PMID: 30605995; PubMed Central PMCID: PMCPMC6354037.
14. Moldofsky H , Patcai J . Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol. 2011;11:37. Epub 2011/03/26. doi: https://doi.org/10.1186/1471-2377-11-37. PubMed PMID: 21435231; PubMed Central PMCID: PMCPMC3071317.
15. Lam MH , Wing YK , Yu MW , Leung CM , Ma RC , Kong AP , et al. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med. 2009 Dec;169(22):2142–7. https://doi.org/10.1001/archinternmed.2009.384
16. Chau SWH , Wong OWH , Ramakrishnan R , Chan SSM , Wong EKY , Li PYT , et al. History for some or lesson for all? A systematic review and meta-analysis on the immediate and long-term mental health impact of the 2002-2003 Severe Acute Respiratory Syndrome (SARS) outbreak. BMC Public Health. 2021;21(1):670. Epub 2021/04/09. doi: https://doi.org/10.1186/s12889-021-10701-3. PubMed PMID: 33827499; PubMed Central PMCID: PMCPMC8025448.
17. Rogers JP , Chesney E , Oliver D , Pollak TA , McGuire P , Fusar-Poli P , et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020 Jul;7(7):611–27. https://doi.org/10.1016/s2215-0366(20)30203-0 https://doi.org/10.1016/S2215-0366(20)30203-0
18. Dong F , Liu HL , Dai N , Yang M , Liu JP . A living systematic review of the psychological problems in people suffering from COVID-19. J Affect Disord. 2021;292:172-88. Epub 2021/06/15. doi: https://doi.org/10.1016/j.jad.2021.05.060. PubMed PMID: 34126309; PubMed Central PMCID: PMCPMC8169237.
19. Taquet M , Luciano S , Geddes JR , Harrison PJ . Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021 Feb;8(2):130–40. https://doi.org/10.1016/s2215-0366(20)30462-4 https://doi.org/10.1016/S2215-0366(20)30462-4
20. Menges D , Ballouz T , Anagnostopoulos A , Aschmann HE , Domenghino A , Fehr JS , et al. Burden of post-COVID-19 syndrome and implications for healthcare service planning: A population-based cohort study. PLoS One. 2021;16(7):e0254523. Epub 2021/07/13. doi: https://doi.org/10.1371/journal.pone.0254523. PubMed PMID: 34252157; PubMed Central PMCID: PMCPMC8274847.
21. Frontera JA , Yang D , Lewis A , Patel P , Medicherla C , Arena V , et al. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J Neurol Sci. 2021;426:117486. Epub 2021/05/18. doi: https://doi.org/10.1016/j.jns.2021.117486. PubMed PMID: 34000678; PubMed Central PMCID: PMCPMC8113108.
22. von Elm E , Altman DG , Egger M , Pocock SJ , Gøtzsche PC , Vandenbroucke JP ; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008 Apr;61(4):344–9. https://doi.org/10.1016/j.jclinepi.2007.11.008
23. Smith GB , Prytherch DR , Meredith P , Schmidt PE , Featherstone PI . The ability of the National Early Warning Score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation. 2013 Apr;84(4):465–70. https://doi.org/10.1016/j.resuscitation.2012.12.016
24. Charlson ME , Pompei P , Ales KL , MacKenzie CR . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. https://doi.org/10.1016/0021-9681(87)90171-8
25. Zigmond AS , Snaith RP . The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983 Jun;67(6):361–70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x
26. Dolan P . Modeling valuations for EuroQol health states. Med Care. 1997 Nov;35(11):1095–108. https://doi.org/10.1097/00005650-199711000-00002
27. EuroQol Group . EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990 Dec;16(3):199–208. https://doi.org/10.1016/0168-8510(90)90421-9
28. Connor KM , Davidson JR . Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety. 2003;18(2):76–82. https://doi.org/10.1002/da.10113
29. Cohen S , Kamarck T , Mermelstein R . A global measure of perceived stress. J Health Soc Behav. 1983 Dec;24(4):385–96. https://doi.org/10.2307/2136404
30. Bjelland I , Dahl AA , Haug TT , Neckelmann D . The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002 Feb;52(2):69–77. https://doi.org/10.1016/s0022-3999(01)00296-3 https://doi.org/10.1016/S0022-3999(01)00296-3
31. Maercker A , Schützwohl M . Erfassung von psychischen Belastungsfolgen: Die Impact of Event Skala-revidierte Version (IES-R). Diagnostica. 1998;(44(3)):130–41.
32. Creamer M , Bell R , Failla S . Psychometric properties of the impact of event scale—revised. Behav Res Ther. 2003 Dec;41(12):1489–96. https://doi.org/10.1016/j.brat.2003.07.010
33. Huang C , Huang L , Wang Y , Li X , Ren L , Gu X , et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220-32. Epub 2021/01/12. doi: https://doi.org/10.1016/S0140-6736(20)32656-8. PubMed PMID: 33428867; PubMed Central PMCID: PMCPMC7833295.
34. Nehme M , Braillard O , Chappuis F , Courvoisier DS , Guessous I . Prevalence of Symptoms More Than Seven Months After Diagnosis of Symptomatic COVID-19 in an Outpatient Setting. Ann Intern Med. 2021. Epub 2021/07/06. doi: https://doi.org/10.7326/M21-0878. PubMed PMID: 34224254; PubMed Central PMCID: PMCPMC8280535.
35. Boscolo-Rizzo P , Guida F , Polesel J , Marcuzzo AV , Capriotti V , D’Alessandro A , et al. Sequelae in adults at 12 months after mild-to-moderate coronavirus disease 2019 (COVID-19). Int Forum Allergy Rhinol. 2021 Jun; :alr.22832. https://doi.org/10.1002/alr.22832
36. Sudre CH , Murray B , Varsavsky T , Graham MS , Penfold RS , Bowyer RC , et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626-31. doi: https://doi.org/10.1038/s41591-021-01292-y
37. Blomberg B , Mohn KG , Brokstad KA , Zhou F , Linchausen DW , Hansen BA , et al.; Bergen COVID-19 Research Group . Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021 Sep;27(9):1607–13. https://doi.org/10.1038/s41591-021-01433-3
38. Havervall S , Rosell A , Phillipson M , Mangsbo SM , Nilsson P , Hober S , et al. Symptoms and Functional Impairment Assessed 8 Months After Mild COVID-19 Among Health Care Workers. JAMA. 2021;325(19):2015-6. Epub 2021/04/08. doi: https://doi.org/10.1001/jama.2021.5612. PubMed PMID: 33825846; PubMed Central PMCID: PMCPMC8027932.
39. Frontera JA , Yang DO , Lewis A , Patel P , Medicherla C , Arena V , et al. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J Neurol Sci. 2021;426. doi: ARTN 117486.
40. Schuler D , Tuch A , Peter C . Psychische Gesundheit in der Schweiz. Monitoring 2020. (Obsan Bericht 15/2020). Neuchâtel: Schweizerisches Gesundheitsobservatorium, 2020.
41. Robert Koch-Institut . Depressive Symptomatik im europäischen Vergleich – Ergebnisse des European Health Interview Survey (EHIS) 2. Journal of Health Monitoring. 2019;4(4):62–70. https://doi.org/10.25646/6221
42. Vincent A , Beck K , Becker C , Zumbrunn S , Ramin-Wright M , Urben T , et al. Psychological burden in patients with COVID-19 and their relatives 90 days after hospitalization: A prospective observational cohort study. J Psychosom Res. 2021;147:110526. Epub 2021/05/30. doi: https://doi.org/10.1016/j.jpsychores.2021.110526. PubMed PMID: 34051515; PubMed Central PMCID: PMCPMC8132501.
43. Beck K , Vincent A , Becker C , Keller A , Cam H , Schaefert R , et al. Prevalence and factors associated with psychological burden in COVID-19 patients and their relatives: A prospective observational cohort study. PLoS One. 2021 May;16(5):e0250590. https://doi.org/10.1371/journal.pone.0250590
44. Lara-Cinisomo S , Akinbode TD , Wood J . A Systematic Review of Somatic Symptoms in Women with Depression or Depressive Symptoms: Do Race or Ethnicity Matter? Journal of women's health (2002). 2020;29(10):1273-82. doi: https://doi.org/10.1089/jwh.2019.7975. PubMed PMID: 32397866.
45. McKnight-Eily LR , Okoro CA , Strine TW , Verlenden J , Hollis ND , Njai R , et al. Racial and Ethnic Disparities in the Prevalence of Stress and Worry, Mental Health Conditions, and Increased Substance Use Among Adults During the COVID-19 Pandemic - United States, April and May 2020. MMWR Morb Mortal Wkly Rep. 2021;70(5):162-6. doi: https://doi.org/10.15585/mmwr.mm7005a3. PubMed PMID: 33539336; PubMed Central PMCID: PMCPMC7861483 Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.