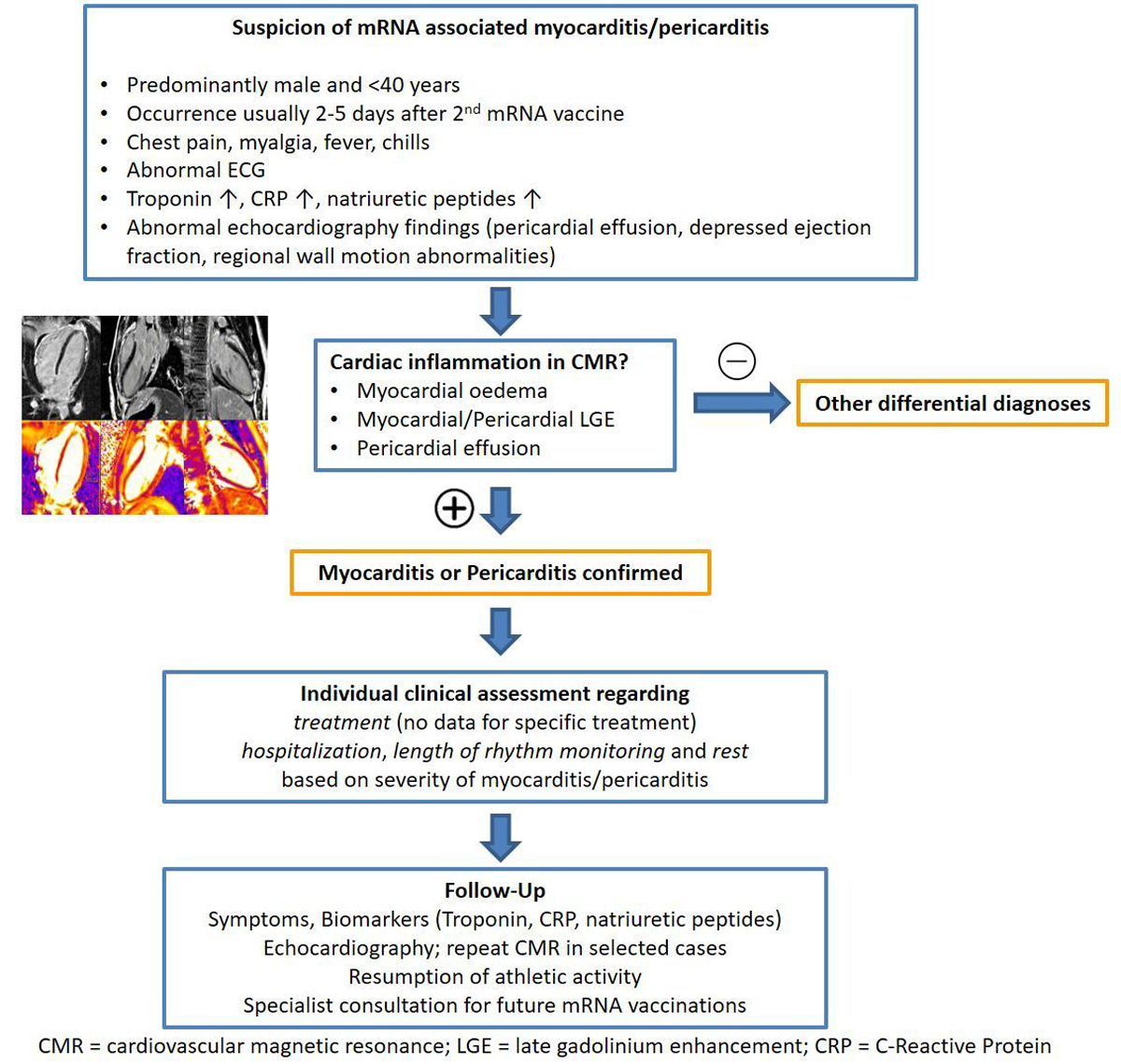
Figure 1 Summary of pretest probability, diagnostic work-up, management/treatment and follow-up in patients with mRNA vaccine-associated myocarditis/pericarditis.
DOI: https://doi.org/10.4414/SMW.2021.w30087
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing coronavirus disease 2019 (COVID-19) reached pandemic levels in March 2020 and has caused repeated waves of outbreaks across the globe. COVID-19 has imposed an enormous burden on global healthcare systems, with more than 221 million documented infections and more than 4.5 million deaths associated with SARS-CoV-2 as of 8 September 2021. It also has led to long-standing social restrictions and to a negative impact on global economies. Long-term socio-medical sequelae of COVID-19 including long COVID syndrome are not yet foreseeable in their full scope. The successful timely development and global deployment of COVID-19 vaccines and, most importantly, their administration play a key role in limiting disease severity and overcoming the pandemic. In Switzerland, two novel types of messenger ribonucleic acid (mRNA) vaccines, namely Comirnaty® (Pfizer/BioNTech) and Spikevax® (Moderna) were approved by Swissmedic in late 2020 after their safety and effectiveness had been demonstrated in large clinical trials [1, 2].
Robust data from these double-blind randomised controlled trials, as well as real-life studies, clearly established that mRNA vaccines are highly effective in the protection against COVID-19 and post-COVID complications [1,2]. Accordingly, the Eidgenössische Impfkommission recommends vaccination against COVID-19 for all persons above the age of 12 years [3].
After more than a billion doses of mRNA COVID-19 vaccinations were applied all over the world, several cases of myocarditis and pericarditis were reported as adverse events related to vaccination [4]. Given the enormous global impact of COVID-19 and vaccination currently have on our societies, there has been intensive media coverage. Most regulatory agencies now have added a warning about the risk of myocarditis and pericarditis for COVID-19 mRNA vaccines as new adverse effects [5].
Despite the broad availability of COVID-19 vaccine doses and the easy access to vaccination centres in Switzerland, Switzerland currently has one of the lowest vaccination rates in western Europe, with only 53% of the population being twice vaccinated as of 10 September 2021. At the same time, Switzerland has, tied with France, the highest rate of patients hospitalised in intensive care units (both numbers above according to the Federal Office of Public Health, Bundesamt für Gesundheit [BAG] and www.ourworldindata.org, 10 September 2021). The current, fourth wave of COVID-19 is predominantly caused by the more virulent delta variant and is a "wave of the unvaccinated" with approximately 9 out of 10 intensive care unit patients being unvaccinated. Additional efforts to increase vaccination rates are urgently needed.
Yet the uncertainty caused by reports of new adverse effects associated with COVID-19 vaccines is currently jeopardising the success of COVID-19 vaccines. The widespread general vaccine scepticism is in striking contrast to the huge success of former mass vaccination programmes in eradicating other diseases such as smallpox or diphtheria.
Based on the current, rapidly changing knowledge and experience, this article aims to give a short summary of what is currently known about mRNA COVID-19 vaccination-associated inflammation of the heart and to give some practical clinical recommendations on how to detect and manage it. This position paper has been approved by the Swiss Society of Cardiology (SSC), Working Group of Echocardiography and Cardiac Imaging of the SSC and the Swiss Society of Paediatric Cardiology.
COVID-19 is known to cause vascular and myocardial inflammation, with worse outcomes in particular in patients with pre-existing cardiovascular disease, hypertension and related conditions [6]. In contrast to COVID-19-associated myocarditis, cases of mRNA vaccine-associated myocarditis have been almost exclusively (96%) reported in younger, healthy males (12–29 years) [7]. They usually occur 3 to 5 days after administration of the second dose of vaccine, suggesting an immune-mediated mechanism (autoimmune/hypersensitivity myocarditis) [8–10]. Most patients present with chest pain (95100%), myalgia, fatigue or fever (63%) and elevated troponin levels (100%), as well as elevated markers of inflammation (C-reactive protein). Similarly, a large retrospective case series studied the occurrence of myocarditis following mRNA COVID-19 vaccination among members of the US military. After more than 2.8 million doses of mRNA COVID-19 vaccines had been administered, a total of 23 male patients with a median age of 25 years had the final diagnosis of vaccine-associated myocarditis without identifiable infectious, ischaemic or other autoimmune aetiology apart from the vaccine. Of note, only 8 of these 23 patients had cardiovascular magnetic resonance imaging (CMR) for diagnosis and none of them histopathological evidence (probable myocarditis) [11].
The initial work-up should include detailed history, a 12-lead electrocardiogram (ECG) and serological biomarkers, particularly high-sensitivity cardiac troponin T/I (hs-cTnT/I) in accordance with the assessments recommended in current clinical practice guidelines for patients presenting with acute chest pain [12]. The diagnosis of myocarditis is often challenging. Clinically, myocarditis may closely resemble acute myocardial infarction and also takotsubo syndrome regarding presentation, ECG abnormalities and biomarkers. Therefore, careful individual evaluation is necessary to select those patients in whom exclusion of acute coronary syndrome by invasive angiography or computed tomography angiography is deemed necessary. This includes assessment of the respective pretest probabilities and consideration of local and timely availability of diagnostic examinations. Of note, normal troponin levels and a normal ECG at presentation do not exclude isolated pericarditis. Assessment of serological evidence for prior SARS-CoV2 infection (determination of SARS-CoV2-Nucleocapsid-IgG) should be considered, especially in subjects with myocarditis after the first dose.
The majority of patients present with ECG abnormalities suggestive of perimyocarditis (87%) (fig. 1) [7]. Like patients with presumed myocarditis without previous COVID vaccination, further assessment of cardiac function and morphology should be undertaken, primarily with echocardiography and CMR for assessment of peri-/myocardial inflammation and tissue damage according to current CMR recommendations [13]. Of note, active myocarditis may engender regional or global contractile dysfunction. However, often there can be extensive tissue injury (subepicardial late gadolinium enhancement by CMR) with comparably minimal impact on cardiac contractility, as endocardial myocytes, the prime movers in normal ventricular function, are usually spared [14].

Figure 1 Summary of pretest probability, diagnostic work-up, management/treatment and follow-up in patients with mRNA vaccine-associated myocarditis/pericarditis.
Given the lack of well-designed contemporary clinical studies in the field of acute myocarditis and chronic inflammatory cardiomyopathies, there is no specific treatment. General heart failure treatment (primarily angiotensin converting enzyme [ACE] inhibitors and beta blockers) should be evaluated in all patients [15]. Its indication should be re-evaluated during follow-up consultation. There is no contraindication for nonsteroidal anti-inflammatory medication (NSAIDs). The use of immunomodulatory therapy such as colchicine (in cases with presumed pericardial inflammation) and intravenous globulins or corticosteroids should be individually assessed in severe cases [4, 10, 16]. Chest pain can be treated with paracetamol, novaminsulfone, NSAIDs, or morphine as needed [4, 10, 16].
Clinical outcome of mRNA vaccine-associated myocarditis has been mostly very favourable without relevant arrhythmias and with rapid complete spontaneous recovery [7, 10]. Only a few cases in older adults have been reported with outcomes varying depending on other pre-existing conditions [7], in addition to two cases with a fulminant course [17].
So far, Israel and the United States provide most information about vaccine-associated inflammation of the heart. There seems to be a slightly higher than expected occurrence of myocardial inflammation in male adolescents and younger adults. Based on the data available to date, myocarditis occurring after mRNA vaccination is still very rare [4]. The US Military Health System administered more than 2.8 million doses of mRNA-based vaccines in healthy individuals and detected only 23 myocarditis cases [9, 11]. Up to 21 September 2021 and after more than 10.2 million doses of mRNA COVID-19 vaccines had been administered in Switzerland, 151 potential cases of vaccine-associated myocarditis have been reported to Swissmedic [18].
All medical interventions need to be evaluated balancing benefit versus harm. The benefit of COVID-19 mRNA vaccination in terms of prevented hospitalisations compared with its risk of vaccine-associated myocarditis seems to be very clearly in favour of vaccination (fig. 2), even more with increasing age.
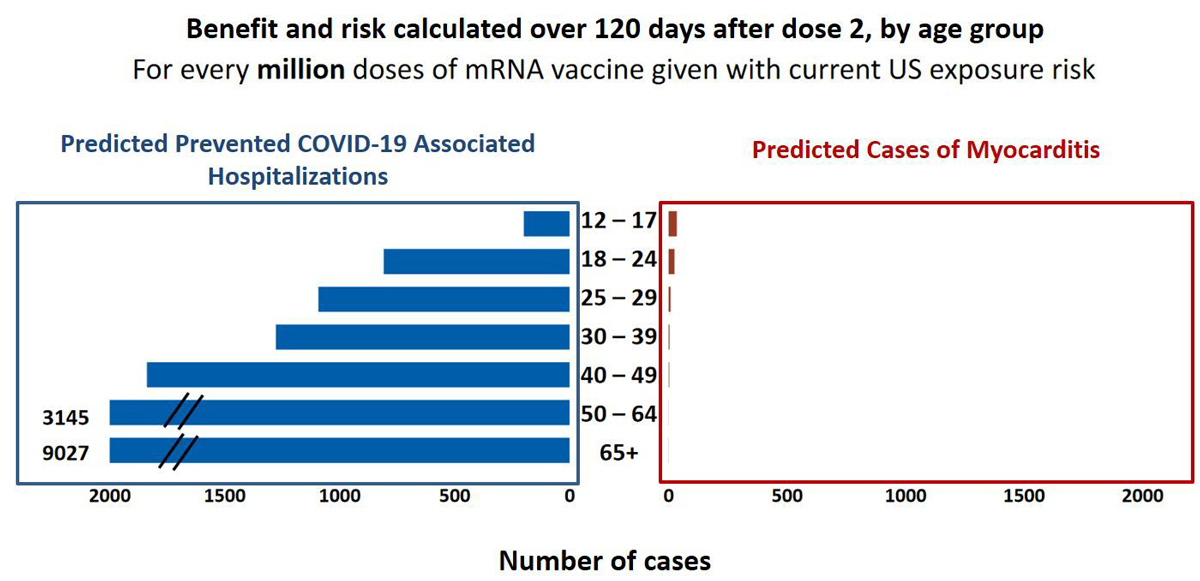
Figure 2 Potential risk of myocarditis with COVID-19 mRNA vaccination in the 120 days after vaccination and predicted prevention of COVID-19 hospitalisations. Adapted from Bozkurt B. et al. Circulation 2021 [7].
Given that SARS-CoV-2 is constantly mutating, it seems likely that globally most individuals will be contact with this increasingly virulent virus – both the vaccinated and unvaccinated. To vaccinate or not to vaccinate both incur certain risks: a recently published Israeli study tried to put risks for adverse events by the mRNA vaccine in the context of the risks of the same adverse events after documented infection with SARS-CoV-2 [1]. The risk ratio of myocarditis was estimated to increase by factor 3.2 after mRNA vaccination, with 1 to 5 events per 100,000 persons [1]. However, after SARS-COV-2 infection, the risk of myocarditis was increased by a factor of 18.3. Apart from myocarditis, the risk of multiple other serious adverse events was substantially higher after infection than after vaccination [1].
Currently, it is unknown whether cardiac inflammation is specific to the mRNA platform. It should also be noted, that a causal link has not yet been established between COVID-19 vaccination and perimoycarditis.
In general, there is a recommendation for COVID-19 vaccination for all individuals ≥12 years of age. Given the relatively short-term knowledge regarding adverse effects of mRNA vaccination, certain patients need individual evaluation of the indication for vaccination (table 1) [7].
Table 1Individual evaluation of risk and benefits of vaccination. Adapted from [4, 10, 16].
| Recommendation for vaccination | ≥12 years of age, including in patients with |
| – Coronary artery disease | |
| – Chronic heart failure | |
| – Cardiomyopathies | |
| – Arrhythmias, pacemaker, implantable cardioverter defibrillator | |
| – Any congenital heart disease | |
| Vaccination deferral | Acute decompensated heart failure |
| Acute inflammatory cardiac diseases (myo-, peri-, endocarditis) including acute mRNA vaccine-associated perimyocarditis1 | |
| Acute rheumatic fever | |
| Individual evaluation | 12–29 years and dilated cardiomyopathy |
| Cardiac transplant recipients | |
| Consultation with a vaccination expert regarding further mRNA vaccination in all patients with mRNA vaccine-associated perimyocarditis |
1 Currently, until additional safety data are available, experts recommend deferring of the second dose after mRNA vaccine-associated perimyocarditis at least until symptoms of peri-/myocarditis have completely resolved and until there are no more signs of ongoing myocardial inflammation. Administration of the second dose of an mRNA COVID-19 vaccine can be considered after individual assessment, including the risk of a severe COVID-19 infection course (e.g., age, underlying conditions) [4].
Based on all available current clinical and scientific evidence, the benefits of COVID-19 vaccines by far outweigh the potential risk of vaccine associated myocarditis and pericarditis. It is unlikely that there will ever be a completely risk-free vaccine. All medical interventions – including vaccines – incur potential risks that need to be balanced with their benefit. COVID-19 vaccines continue to be recommended for all eligible individuals. Population-wide vaccination is key to fighting the global pandemic, preventing COVID-19 infection, transmission, hospitalisation, long COVID syndrome, multi-system inflammatory syndrome in children and death, and is crucial to the further relaxation of all social restrictions. Nevertheless, active monitoring and research are needed to better understand and prevent vaccine-associated cardiac damage.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.
1. Barda N , Dagan N , Ben-Shlomo Y , Kepten E , Waxman J , Ohana R , et al. Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N Engl J Med. 2021 Sep;385(12):1078–90. https://doi.org/10.1056/NEJMoa2110475
2. Baden LR , El Sahly HM , Essink B , Kotloff K , Frey S , Novak R , et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 4. February 2021;384(5):403–16.
3. Eidgenössische Kommission für Impffragen EKIF . Covid-19-Impfstrategie (Status 22 June2021) (https://www.bag.admin.ch/dam/bag/de/dokumente/mt/k-und-i/aktuelle-ausbrueche-pandemien/2019-nCoV/impfstrategie-bag-ekif.pdf.download.pdf/Covid-19-Impfstrategie%20BAG%20EKIF.pdf)
4. Myocarditis and pericarditis following mRNA COVID-19 vaccination. Centers for Disease Control and Prevention, June 2021 (https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html)
5. Direct Healthcare Professional Communications – mRNA-Impfstoffe gegen COVID-19 (COVID-19 Vaccine Moderna und Comirnaty) (https://www.swissmedic.ch/swissmedic/en/home/humanarzneimittel/market-surveillance/health-professional-communication--hpc-/dhpc-mrna-impfstoffe-gegen-covid-19.html)
6. Liu PP , Blet A , Smyth D , Li H . The Science Underlying COVID-19: Implications for the Cardiovascular System. Circulation. 7. Juli 2020;142(1):68–78.
7. Bozkurt B , Kamat I , Hotez PJ . Myocarditis With COVID-19 mRNA Vaccines. Circulation. 10. August 2021;144(6):471–84.
8. Stone CA Jr , Rukasin CR , Beachkofsky TM , Phillips EJ . Immune-mediated adverse reactions to vaccines. Br J Clin Pharmacol. 2019 Dec;85(12):2694–706. https://doi.org/10.1111/bcp.14112
9. Shay DK , Shimabukuro TT , DeStefano F . Myocarditis Occurring After Immunization With mRNA-Based COVID-19 Vaccines. JAMA Cardiol. 2021 Oct;6(10):1115–7. https://doi.org/10.1001/jamacardio.2021.2821
10. Covid-19 vaccine-associated myocarditis/pericarditis. A report of the chief science advisor of Canada. 16 July 2021. (https://science.gc.ca/eic/site/063.nsf/vwapj/report-myocarditis-pericarditis-en.pdf/$file/report-myocarditis-pericarditis-en.pdf)
11. Montgomery J , Ryan M , Engler R , Hoffman D , McClenathan B , Collins L , et al. Myocarditis Following Immunization With mRNA COVID-19 Vaccines in Members of the US Military. JAMA Cardiol. 2021 Oct;6(10):1202–6. https://doi.org/10.1001/jamacardio.2021.2833
12. Collet J-P , Thiele H , Barbato E , Barthélémy O , Bauersachs J , Bhatt DL , et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 7. April 2021;42(14):1289–367.
13. Ferreira VM , Schulz-Menger J , Holmvang G , Kramer CM , Carbone I , Sechtem U , et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J Am Coll Cardiol. 18. December 2018;72(24):3158–76.
14. Ismail TF , Hua A , Giorgetti A , Haaf P . Imaging of Inflammation and Infection in Cardiovascular Diseases. Chapter 7: Myocarditis. Springer 1st edition 2021.
15. Ammirati E , Frigerio M , Adler ED , Basso C , Birnie DH , Brambatti M , et al. Management of Acute Myocarditis and Chronic Inflammatory Cardiomyopathy: An Expert Consensus Document. Circ Heart Fail. 2020 Nov;13(11):e007405. https://doi.org/10.1161/CIRCHEARTFAILURE.120.007405
16. Guidance on Myocarditis and Pericarditis after mRNA COVID-19 Vaccines by the Australian Technical Advisory Group on Immunisation (ATAGI) and the Cardiac Society of Australia and New Zealand (CSANZ). Version 1.1 - 6 August 2021 (https://www.health.gov.au/sites/default/files/documents/2021/08/covid-19-vaccination-guidance-on-myocarditis-and-pericarditis-after-mrna-covid-19-vaccines_1.pdf)
17. Verma AK , Lavine KJ , Lin CY . Myocarditis after Covid-19 mRNA Vaccination. N Engl J Med. 2021 Sep;385(14):1332–4. https://doi.org/10.1056/NEJMc2109975