
Figure 1 Flow chart describing patient inclusion into the study.
DOI: https://doi.org/10.4414/SMW.2021.w30050
The 30-day mortality and complication rate after open repair of an abdominal aortic aneurysm (AAA) by implanting a tube or bifurcated aorto-iliac graft have been well documented in many large patient series [1–4]. Similarly, the 30-day mortality and complication rate after aorto-femoral or aorto-bifemoral bypass used to treat aorto-iliac occlusive disease (AIOD) is well established [5–7]. The prevalence of severe AIOD in AAA patients or the presence of an AAA in patients with severe AIOD is reported to be around 20% [8]. However, very little is known about whether the patients who have concomitant AAA and severe AIOD and thus require a combination of open aneurysm replacement and aorto-femoral bypass have a worse outcome after surgery.
We performed a retrospective analysis of consecutive patients undergoing open AAA repair for isolated AAA, aorto-femoral bypass for isolated AIOD or a combination of open AAA repair and aorto-femoral bypass for a combination of AAA and AIOD. The primary endpoint was the occurrence of at least one surgical complication of grade III, IV or V according to the Clavien-Dindo classification. Secondary endpoints were 30-day and 1-year mortality and colon ischaemia.
This was a retrospective analysis of consecutive patients undergoing open AAA repair, aorto-femoral bypass or a combination of the two procedures at Basel University Hospital and the Kantonsspital Aarau between January 2003 and December 2013. Patients requiring emergency aneurysm repair because of ruptured AAA or aorto-femoral bypass because of acute lower limb ischaemia, patients with thoracoabdominal aneurysm and patients with aorto-femoral bypass with the proximal anastomosis to the thoracic aorta were excluded (fig. 1). All patients were preoperatively evaluated by CT-angiography for the possibility of endovascular aneurysm repair, which was rejected either because of anatomical criteria or because of the patient’s preference. The indication for the treatment of an aortic aneurysm was a diameter >5.0 cm, the indication for the treatment of an iliac artery aneurysm was a diameter >3.5 cm. Patients were identified by screening our electronic patient documentation programme (ISMed-eOPPS, ProtecData AG) for aortic procedures performed with bifurcated or tube grafts in the aortic position.

Figure 1 Flow chart describing patient inclusion into the study.
The notes of the operative procedure, charts and anaesthesia protocols were reviewed. The operation notes were analysed for the indication for surgery, site of the proximal clamp, configuration of the proximal graft anastomosis, need for bypass to the renal arteries, graft configuration (tube or bifurcated), reimplantation of the inferior mesenteric artery, selective revascularisation of the hypogastric artery and configuration and location of the distal graft anastomosis. Patient characteristics collected included patient age, gender, body mass index (BMI), preoperative American Society of Anesthesiologists (ASA) class, the presence of diabetes, coronary artery disease (defined as prior myocardial infarction, stable angina pectoris, acute coronary syndrome within the last 30 days, prior percutaneous transluminal coronary angioplasty [PTCA] or coronary artery bypass), creatinine clearance estimated with the modification of diet in renal disease (MDRD) formula and the presence of preoperative anticoagulant therapy with vitamin K antagonists.
For the characterisation of postoperative complications, we used the Clavien-Dindo classification [9]. Only complications of grade III (complication requiring an intervention), IVa (life-threatening complication with single organ dysfunction), IVb (life-threatening complication with multi-organ dysfunction), or V (death) were considered. Complications of grade I (deviation of normal course not requiring any treatment) or grade ll (complications requiring medical therapy, not causing organ dysfunction and not requiring intensive care therapy) were not included. This decision was taken because it was felt that grade I or II complications were not of sufficient clinical relevance and because the retrospective data collection did not permit us to reliably ascertain the presence or absence of grade I and II complications.
Surgical complications were classified as bleeding, cardiac (myocardial infarction, pulmonary oedema, new-onset atrial fibrillation), renal (temporary or permanent dialysis), pulmonary, neurological, deep vein thrombosis or pulmonary embolism, colon ischaemia, small bowel ischaemia, peripheral arterial embolism, graft occlusion, fascial dehiscence, bowel obstruction, laparotomy for other reason, abdominal compartment syndrome, anastomotic stenosis, surgical site infection at the laparotomy, surgical site infection in the groin, graft infection requiring graft replacement and lymphocele or lymph fistula. The diagnosis of colon ischaemia was made only if the patient developed an acute abdomen and full-thickness necrosis of the sigmoid colon requiring colon resection was confirmed at the time of laparotomy.
Follow-up information on long-term survival was obtained by contacting the patient or the family physician by phone or letter. Contact information for the patient or family physician was obtained from the patient chart. Patients were considered to be in the “combined” group if either (1) the primary indication for surgery was the AAA, and it was deemed necessary already before surgery to perform aorto-unilateral or aorto-bifemoral bypass because of severe AIOD or (2) if the primary indication for surgery was AIOD and the infrarenal aorta was aneurysmatic to an extent that warranted a proximal end-to-end anastomosis of the graft. AAA patients in whom a distal anastomosis to the aortic bifurcation or iliac arteries was attempted, but in whom intraoperative problems with the distal anastomosis warranted a femoral anastomosis were not included in the “combined” group. Similarly, AIOD patients with a small AAA that was considered not to preclude a proximal end-to-side anastomosis were not included in the “combined” group.
SR, AM and AZ were responsible for the data collection. Complications were classified by TW according to the definitions given above. Data were organised in Microsoft Excel (2016 MSO).
This study was designed and conducted according to the Declaration of Helsinki as well as the rules for Good Clinical Practice. All personal data of patients were anonymised prior to data analysis. The project was approved by the local ethics committee (Ethikkomission Nordwest – und Zentralschweiz; ID 2016 – 00185).
Continuous variables are presented as median and interquartile range (IQR) and the p-values were calculated using the Kruskal-Wallis rank-sum test. Categorical variables are shown as counts with percentages and the p-values were calculated using Pearson`s chi-square test or Fisher's exact test as appropriate. To study the associations between risk factors and in-hospital complications, we used logistic regression. The choice of the pre-specified variables used in the models was based on clinical judgment. Because of the low number of events for the primary outcome, we selected the variables that were deemed to be clinically important and statistically relevant (i.e., >10% change of the effect measure [odds ratio, OR] for the outcome) in the multivariable model(s) comparing odds of events between the AAA, AIOD and combined groups, adjusted for age (10-year increments) and proximal clamping (y/n). Because of the low number of events for mortality and also a complete follow-up of the patients at both 30 days and 1 year, the Poisson model was used to analyse mortality data. The occurrence of colon ischaemia was considered a count outcome and the Poisson model was used to investigate the associations. The variable selection for the multivariable model for mortality (30-day and 1-year) and also the occurrence of colon ischaemia followed the same approach as for the primary outcome. A Kaplan-Meier survival curve is presented for 1-year mortality, and the log-rank test was used to assess differences between the groups. The data set was complete, except for two patients with missing BMI. These were excluded from the data and we performed a complete-case data analysis. Data were collected in Excel (2016 Microsoft Office), and all statistical analyses and graphs were performed using Stata Version 15.1 for Windows (StataCorp, College Station, TX, USA).
During the study period 511 patients underwent open repair for isolated aortic aneurysm (group “AAA”), 104 patients underwent aorto-unifemoral or aorto-bifemoral bypass for isolated aorto-iliac occlusive disease (group “AIOD”) and 46 patients underwent open AAA repair and aorto-unifemoral or aorto-bifemoral bypass for concomitant AAA and AIOD (group “combined”). The patient baseline characteristics and details of the operations are presented in table 1.
Table 1Baseline and procedural characteristics.
| AAA (n = 511) | AIOD (n = 104) | Combined (n = 46) | p-value 1 | Total (n = 661) | |
| Baseline characteristics | |||||
| Age in years, median (IQR) | 71 (64–76) | 63 (57–69) | 71 (64–75) | 0.105 | 70 (63–75) |
| Gender male, n (%) | 462 (90%) | 70 (67%) | 35 (76%) | <0.001 | 567 (85%) |
| BMI in kg/m2, median (IQR) | 26 (24–30) | 24 (22–27) | 26 (22–27) | 0.801 | 26 (24–29) |
| ASA class, n (%) | 0.035 | ||||
| – I | 6 (1.2%) | 0 | 0 | - | 6 (0.9%) |
| – II | 137 (27%) | 14 (14%) | 10 (22%) | - | 161 (24%) |
| – III | 323 (63%) | 83 (8 %) | 34 (74%) | - | 440 (67%) |
| – IV | 45 (8.8%) | 7 (6.8%) | 2 (4.4%) | - | 54 (8.2%) |
| Diabetes | 62 (12%) | 24 (23%) | 6 (13%) | 0.013 | 92 (14%) |
| Coronary heart disease | 167 (33%) | 30 (29%) | 9 (20%) | 0.158 | 206 (31%) |
| Creatinine clearance in ml/min/1.73 m2, median (IQR) | 63 (43–82) | 78 (45–105) | 59 (42–85) | 0.098 | 65 (43–86) |
| Oral anticoagulation | 80 (16%) | 7 (7%) | 5 (11%) | 0.047 | 92 (14%) |
| Centre | 0.815 | ||||
| – Aarau | 222 (77.08%) | 44 (15.28%) | 22 (7.64%) | - | 288 (43.57%) |
| – Basel | 289 (77.48%) | 60 (16.09%) | 24 (6.43%) | - | 373 (56.43%) |
| Procedural characteristics | |||||
| Position of aortic clamp | <0.001 | ||||
| – Infrarenal | 410 (80%) | 78 (75%) | 29 (63%) | - | 517 (78%) |
| – Above left renal artery | 34 (6.7%) | 4 (3.8%) | 3 (6.5%) | - | 41 (6.2%) |
| – Above right renal artery | 11 (2.0%) | 0 (0.0%) | 3 (6.5%) | - | 14 (2.1%) |
| – Suprarenal | 52 (10%) | 13 (12%) | 11 (24%) | - | 76 (12%) |
| – Supracoeliac | 4 (0.8%) | 9 (8.7%) | 0 (0.0%) | - | 13 (2.0%) |
| Bypass to renal artery | 0.219 | ||||
| – None | 482 (94%) | 102 (98%) | 46 (100%) | - | 630 (95%) |
| – Bypass to one renal artery | 24 (4.7%) | 1 (1.0%) | 0 | - | 25 (3.8%) |
| – Bypass to both renal arteries | 5 (1%) | 1 (1.0%) | 0 | - | 6 (0.9%) |
| Configuration of proximal anastomosis | <0.001 | ||||
| – End-to-end | 511 (100%) | 31 (30%) | 46 (100%) | 588 (89%) | |
| – End-to-side | 0 | 73 (70%) | 0 | 73 (11%) | |
| Graft configuration | <0.001 | ||||
| – Tube graft | 221 (43%) | 1 (1.0%) | 1 (2.2%) | - | 223 (34%) |
| – Bifurcated graft to iliac arteries | 279 (55%) | 6 (6.0%) | 6 (13.0%) | - | 291 (44%) |
| – Bifurcated graft, unifemoral | 9 (1.8%) | 3 (2.9%) | 14 (30%) | - | 26 (3.9%) |
| – Bifurcated graft, bifemoral | 2 (0.4%) | 94 (90%) | 25 (54%) | - | 121 (18%) |
| Hypogastric artery revascularization | 85 (17%) | 5 (4.8%) | 16 (35%) | <0.001 | 106 (16%) |
| Replantation of inferior mesenteric artery | 31 (6.0%) | 4 (3.8%) | 8 (17%) | 0.006 | 43 (6.5%) |
| PAD stage according to Fontaine | n = 511 | n = 100 | n = 41 | <0.001 | n = 141 |
| – lIa | - | 4 (4%) | 10 (24.38%) | - | 14 (9.93%) |
| – IIb | - | 65 (65%) | 25 (60.98%) | - | 90 (63.83%) |
| – IIc | - | 1 (1%) | 0 | - | 1 (0.71%) |
| – III | - | 16 (16%) | 3 (7.32%) | - | 19 (13.48%) |
| – IV | - | 14 (14%) | 3 (7.32%) | - | 17 (12.05%) |
| AAA diameter in cm, median (IQR) | n = 511 | n = 57 | n = 45 | n = 613 | |
| 5.7 (5.1–6.5) | 0 (0- 0) | 5 (4–5.9) | <0.001 | 5.5 (5–6.2) | |
AAA: abdominal aortic aneurysm; AIOD: aorto-iliac occlusive disease; ASA: American Society of Anesthesiologists; BMI: body mass index; IQR: interquartile range; PAD: peripheral arterial disease
1 The p-values are calculated to show the difference between Indication groups for continuous variables. For categorical variables, the overall p-values are presented comparing the differences between different categories by considering the different categories for the indication variable.
Surgical complications grade lll, IV or V occurred in 17% of AAA, 23% of AIOD and 37% of combined patients (OR between combined and AAA patients 2.76, 95% CI 1.43–5.34; p = 0.003; tables 2, 3 and 4). The results of the univariable models were used to identify the risk factors included in the multivariable model. The results of the univariable models are added as supplemental tables.
Table 2Outcomes (n = 661).
| AAA n = 511 | AIOD n = 104 | Combined n = 46 | Total n = 661 | |
| Surgical complication | 87 (17%) | 24 (23%) | 17 (37%) | 128 (19%) |
| Colon ischaemia | 19 (3.7%) | 3 (2.9%) | 6 (13%) | 28 (4.2%) |
| 30-day mortality | 16 (3.1%) | 5 (4.8%) | 5 (11%) | 26 (3.9%) |
| 1-year mortality | 29 (5.7%) | 6 (5.8%) | 7 (15%) | 42 (6.4%) |
AAA: abdominal aortic aneurysm; AIOD: aorto-iliac occlusive disease
Table 3Details of surgical complications (n = 661).
| Details of surgical complication | AAA n = 511 | AIOD n = 104 | Combined n = 46 | Total n = 661 |
| Bleeding | 15 (2.9%) | 3 (2.9%) | 0 | 18 (2.7%) |
| Myocardial Infarction | 5 (1.0%) | 1 (1.0%) | 1 (2.1%) | 7 (1.1%) |
| Renal | 10 (2.0%) | 2 (1.9%) | 0 | 12 (1.8%) |
| Pulmonary | 14 (2.7%) | 5 (4.8%) | 2 (4.4%) | 21 (3.2%) |
| Neurological | 3 (0.6%) | 0 | 0 | 3 (0.5%) |
| Deep vein thrombosis | 2 (0.4%) | 0 | 0 | 2 (0.3%) |
| Colon ischaemia | 19 (3.7%) | 3 (2.9%) | 6 (13%) | 28 (4.2%) |
| Small bowel ischaemia | 3 (0.6%) | 0 | 2 (4.4%) | 5 (0.8%) |
| Peripheral arterial embolisation | 16 (3.1%) | 2 (1.9%) | 2 (4.4%) | 20 (3.0%) |
| Graft occlusion | 11 (2.2%) | 3 (2.9%) | 1 (2.2%) | 15 (2.3%) |
| Fascial dehiscence | 11 (2.2%) | 0 | 2 (4.4%) | 13 (2.0%) |
| Bowel obstruction | 3 (0.6%) | 1 (1.0%) | 1 (2.2%) | 5 (0.8%) |
| Laparotomy for other reason | 12 (2.4%) | 4 (3.9%) | 2 (4.4%) | 18 (2.7%) |
| Abdominal compartment syndrome | 2 (0.4%) | 1 (1.0%) | 0 | 3 (0.5%) |
| Anastomotic stenosis | 0 | 2 (1.9%) | 0 | 2 (0.3%) |
| Surgical site infection at the laparotomy | 9 (1.8%) | 2 (1.9%) | 2 (4.4%) | 13 (2.0%) |
| Surgical site infection in the groin | 1 (0.2%) | 2 (1.9%) | 1 (2.2%) | 4 (0.6%) |
| Graft infection | 1 (0.2%) | 0 | 0 | 1 (0.15%) |
| Lymphocele or lymph fistula | 0 | 3 (2.9%) | 4 (8.7%) | 7 (1.1%) |
AAA: abdominal aortic aneurysm; AIOD: aorto-iliac occlusive disease
Table 4Multivariable analysis of risk factors for surgical complications (Clavien-Dindo >II, n = 661)
| Indication | Odds ratio (95% CI) | p-value |
| – AIOD vs AAA | 1.82 (1.04–3.17) | 0.04 |
| – Combined vs AAA | 2.76 (1.43–5.34) | 0.003 |
| – Combined vs. AIOD | 1.52 (0.69–3.33) | 0.30 |
| Age (per 10-year increase) | 1.35 (1.05–1.74) | 0.02 |
| Suprarenal clamp | 1.82 (1.17–2.82) | 0.007 |
| Coronary heart disease | 1.24 (0.82–1.90) | 0.31 |
AAA: abdominal aortic aneurysm; AIOD: aorto-iliac occlusive disease; CI: confidence interval
Postoperative colon ischaemia requiring colon resection occurred in 3.7% of AAA, 2.9% of AIOD and 13% of combined patients (incidence rate ratio [IRR] between combined and AAA 3.27, 95% CI 1.37–7.81; p = 0.01; tables 2 and 6).
The 30-day mortality was 3.1% in AAA, 4.8% in AIOD and 11% in combined patients (IRR between combined and AAA 3.17, 95% CI 1.267.96; p = 0.01; tables 2 and 5). One-year mortality was 5.7% in AAA, 5.8% in AIOD and 15% in combined patients (IRR between combined and AAA 2.50, 95% CI 1.175.35; p = 0.02; tables 2 and 5). Kaplan Meier survival curves showed a significant difference in 1-year survival between AAA and combined patients (fig. 2).

Figure 2 One-year survival according to the indication for surgery.
The difference in 1-year survival between AIOD and combined patients was not statistically significant. Median follow-up time was 4.4 years. But survival more than 1 year after surgery did not differ between groups (supplementary fig. S1 in the appendix). Patient age and the need for suprarenal clamping strongly correlated with the occurrence of postoperative complications and 30-day mortality (tables 4 and 5). Preoperative creatinine clearance, BMI and concomitant coronary artery disease were not significantly associated with 30-day mortality (table 5).
Table 5Multivariable analysis of risk factors for 30-days and 1-year mortality (n = 661).
| Incidence rate ratio (95% CI) | p-value | |
| 30-day-mortality | ||
| – AIOD vs AAA | 2.57 (1.00–6.57) | 0.05 |
| – Combined vs AAA | 3.17 (1.26–7.96) | 0.01 |
| – Combined vs AIOD | 1.23 (0.37–4.10) | 0.73 |
| Age (per 10-year increase) | 1.73 (0.99–3.02) | 0.06 |
| Creatinine clearance (ml/min/1.73m2) | 1.00 (0.98–1.01) | 0.68 |
| Suprarenal clamp | 2.90 (1.33–6.31) | 0.007 |
| BMI (kg/m2) | 0.97 (0.85–1.09) | 0.58 |
| Coronary heart disease | 1.71 (0.81–3.60) | 0.16 |
| 1-year mortality | ||
| – AIOD vs AAA | 1.79 (0.77–4.19) | 0.18 |
| – Combined vs AAA | 2.50 (1.17–5.35) | 0.02 |
| – Combined vs AIOD | 1.39 (0.49–3.97) | 0.53 |
| Age (per 10-year increase) | 1.85 (1.25–2.72) | 0.002 |
| Creatinine clearance (ml/min/1.73m2) | 0.99 (0.98–1.01) | 0.27 |
| Suprarenal clamp | 2.88 (1.57–5.26) | <0.001 |
| BMI (kg/m2) | 0.99 (0.91–1.08) | 0.86 |
| Coronary heart disease | 1.52 (0.85–2.72) | 0.16 |
AAA: abdominal aortic aneurysm; AIOD: aorto-iliac occlusive disease; BMI: body mass index; CI: confidence interval
Table 6Multivariable analysis of risk factors for colon ischaemia (n = 661).
| Incidence rate ratio (95% CI) | p-value | |
| – AIOD vs AAA | 1.22 (0.38–3.88) | 0.74 |
| – Combined vs AAA | 3.27 (1.37–7.81) | 0.01 |
| – Combined vs AIOD | 2.69 (0.72–10.02) | 0.14 |
| Age (per 10-year increase) | 1.97 (1.19–3.27) | 0.01 |
| Suprarenal clamp | 2.38 (1.14–5.01) | 0.02 |
| Coronary heart disease | 1.55 (0.76–3.18) | 0.23 |
AAA: abdominal aortic aneurysm; AIOD: aorto-iliac occlusive disease; CI: confidence interval
We investigated whether patients requiring aortic surgery for concomitant AAA and severe AIOD have a worse outcome than patients who undergo open aneurym repair for isolated AAA or patients who undergo aorto-femoral bypass for isolated severe AIOD. Patients requiring open AAA repair combined with aorto-femoral bypass for concomitant AAA and AIOD had a significantly higher rate of surgical complications, 30-day mortality and 1-year mortality than patients with open AAA repair for isolated AAA. The most striking difference was the higher rate of colon ischaemia in the combined group than in the AAA group (13% vs 3.7%). In the majority of patients in the combined group who died within 30 days of the operation, colon ischaemia was the postoperative event that triggered other complications such as sepsis and multi-organ failure and finally led to a fatal outcome. This can be seen in an analysis of the impact of colon ischaemia on 30-day and 1-year mortality (see supplementary table S5). This observation is in agreement with other studies that document the severe impact of colon ischaemia after aortic surgery [10, 11].
This is the first study investigating whether concomitant aorto-femoral bypass for AIOD in patients requiring open AAA repair affects operative mortality and complication rates. Daniel et al. investigated the effect of concomitant AAA in patients requiring aorto-femoral bypass on perioperative mortality and complication rate using data from a nationwide inpatient sample in the United States [6]. Their comparison is analogous to the comparison of patients in the combined group with the AIOD group in our study. Daniel reported significantly higher in-hospital mortality in the combined group, particularly in the subgroup of patients who required aorto-femoral bypass because of gangrene rather than claudication. In our study, the difference in complication rate and 30-day mortality between the combined group and the AIOD group was not statistically significant, presumably because of the relatively small size of the AIOD group (104 patients). The patients in our AAA group had a 30-day mortality (3.1%) and complication rate (17%) similar to published data on open AAA repair, where 30-day mortality rates of 3.5–5.4% [1, 2, 12] and complication rates of 27–47% [1, 12] have been reported. Comparison of complication rates between different studies are, however, problematical, since definitions of complications vary greatly. Presumably our complication rate is lower than in other studies, because we included only complications that required a reintervention (complications grade III, IV and V according to the Clavien Dindo classification). When comparing the AIOD group with the AAA group, we observed a slightly higher 30-day mortality rate (4.8% vs 3.1%) and complication rate (23% vs 17%) in the AIOD group. Inconsistent results on this question have been reported in the literature: some studies report a slightly higher mortality and complication rate after aorto-femoral bypass compared with open AAA repair (7% vs 6.3%) [4] whereas other studies report a slightly lower mortality rate (30-day mortality 2% vs 3%, p = ns; 1-year mortality 4% vs 8%, p = 0.04) [7].
We hypothesise that the increased mortality and complication rate in the combined group is due to the fact that adding aorto-femoral bypass to open AAA repair leads to a more profound alteration of the perfusion of the hypogastric arteries. In isolated AAA repair, the distal anastomosis is commonly to the aortic bifurcation or common iliac arteries, thus the perfusion of the hypogastric arteries is not affected. Isolated aorto-femoral bypass is performed either with a proximal end-to-side anastomosis, which preserves the perfusion of the lumbar and hypogastric arteries, or, in cases of complete Leriche syndrome, where a proximal end-to-end anastomosis is frequently used, the antegrade perfusion of lumbar and hypogastric arteries is already absent before surgery. Thus, in both situations, the perfusion of the hypogastric and ileo-lumbar arteries is not affected. When aorto-femoral bypass is combined with AAA repair, the combination of a proximal end-to-end anastomosis and a distal anastomosis in the groin frequently leads to the loss of antegrade perfusion of the hypogastric or lumbar arteries. This may trigger colon ischaemia and the consequent complications. It is of note that the worse outcome in our combined group occurred despite a higher rate of selective revascularisation of the hypogastric artery (35% vs 17%) and a higher rate of replantation of the inferior mesenteric artery (17% vs 6.1%). An alternative explanation for the worse outcome in the combined group compared with the isolated AAA group might be an increased burden of atherosclerotic disease in the combined group, as suggested by Daniel [6].
Our findings that increased age and the need for suprarenal clamping were independent risk factors for increased 30-day mortality and complication rate are not surprising and agree with many published results [1, 3, 12–16]. The association between suprarenal clamping and the occurrence of colon ischaemia has been found in a large study on the occurrence of colonic ischaemia after aortic surgery [17]. No mechanism by which suprarenal versus infrarenal clamping should predispose to colon ischaemia has been proposed in the literature. It may just be due to the fact that the need for suprarenal clamping is often associated with advanced atherosclerosis and adds to haemodynamic instability during surgery. We were also surprised not to find a correlation between preoperative creatinine clearance and 30-day mortality and complication rate, as renal failure is considered to be one of the most important independent risk factors for 30-day mortality after open AAA repair [1–3, 16]. We have no explanation for this.
The strengths of our study are the facts that it is an analysis of consecutive patients with a nearly 100% follow-up, the detailed information on clinically relevant patient characteristics and details of surgery as potential risk factors and the rigorous statistical analysis using multivariable models. The retrospective study design, the relatively small size of the study population, potential systematic differences in baseline characteristics between groups and the unequal size of the three groups must be considered as limitations to our study. Furthermore, we did not consider the diameter of the aortic aneurysm as a potential risk factor in our analysis.
We believe that our study is of clinical relevance, as it identifies a subset of AAA patients – those with combined AAA and AIOD – as a population at high risk for 30-day mortality and occurrence of postoperative complications when undergoing open repair. This is relevant even in the era of endovascular aneurysm repair, because severe AIOD is often a contraindication for endovascular aneurysm repair and the AAA in these patients can only be treated by open repair combined with aorto-femoral bypass. Our data suggest that in these patients the indication for AAA repair should be evaluated particularly carefully, possibly even setting a larger aneurysm diameter than the commonly accepted 55 mm as the threshold for aneurysm repair. Our data also suggest that in patients with combined AAA and AIOD, particular attention should be given to the anatomy and patency of the inferior mesenteric and hypogastric arteries in the preoperative CT scan, because replantation of the inferior mesenteric artery and selective revascularisation of the hypogastric arteries may be particularly important in these patients. However, the benefit of these procedures has not been proven unequivocally [18].
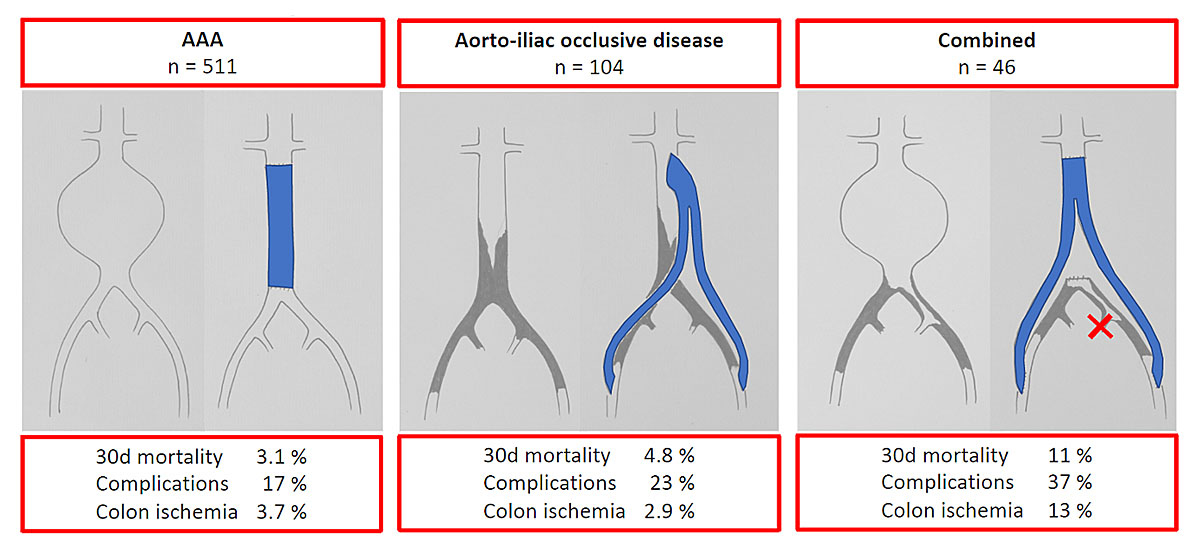
Figure 3 Graphical abstract summarising the results of our study.
In summary, our study shows that patients with AAA and AIOD requiring open AAA repair combined with aorto-femoral bypass have significantly higher 30-day mortality and postoperative complication rates than patients with isolated AAA undergoing open AAA repair. Of note was the much higher rate of colon ischaemia. We hypothesise that this is due to more profound changes in the perfusion of hypogastric and lumbar arteries in these patients. Our results suggest that patients with concomitant AAA and AIOD represent a high-risk population, which may have to be taken into account when deciding on the indication of AAA treatment.
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.
1. Biancari F , Leo E , Ylönen K , Vaarala MH , Rainio P , Juvonen T . Value of the Glasgow Aneurysm Score in predicting the immediate and long-term outcome after elective open repair of infrarenal abdominal aortic aneurysm. Br J Surg. 2003 Jul;90(7):838–44. https://doi.org/10.1002/bjs.4130
2. McPhee JT , Hill JS , Eslami MH . The impact of gender on presentation, therapy, and mortality of abdominal aortic aneurysm in the United States, 2001-2004. J Vasc Surg. 2007 May;45(5):891–9. https://doi.org/10.1016/j.jvs.2007.01.043
3. Brady AR , Fowkes FG , Greenhalgh RM , Powell JT , Ruckley CV , Thompson SG . Risk factors for postoperative death following elective surgical repair of abdominal aortic aneurysm: results from the UK Small Aneurysm Trial. On behalf of the UK Small Aneurysm Trial participants. Br J Surg. 2000 Jun;87(6):742–9. https://doi.org/10.1046/j.1365-2168.2000.01410.x
4. Bayly PJ , Matthews JN , Dobson PM , Price ML , Thomas DG . In-hospital mortality from abdominal aortic surgery in Great Britain and Ireland: Vascular Anaesthesia Society audit. Br J Surg. 2001 May;88(5):687–92. https://doi.org/10.1046/j.0007-1323.2001.01778.x
5. Bredahl K , Jensen LP , Schroeder TV , Sillesen H , Nielsen H , Eiberg JP . Mortality and complications after aortic bifurcated bypass procedures for chronic aortoiliac occlusive disease. J Vasc Surg. 2015 Jul;62(1):75–82. https://doi.org/10.1016/j.jvs.2015.02.025
6. Daniel VT , Gupta N , Raffetto JD , McPhee JT . Impact of coexisting aneurysms on open revascularization for aortoiliac occlusive disease. J Vasc Surg. 2016 Apr;63(4):944–8. https://doi.org/10.1016/j.jvs.2015.10.062
7. Bisgaard J , Gilsaa T , Rønholm E , Toft P . Aortic aneurysm disease vs. aortic occlusive disease: differences in outcome and intensive care resource utilisation after elective surgery: an observational study. Eur J Anaesthesiol. 2013 Feb;30(2):65–72. https://doi.org/10.1097/EJA.0b013e32835b9d7b
8. Costin JA , Watson DR , Duff SB , Edmonson-Holt A , Shaffer L , Blossom GB . Evaluation of the complexity of open abdominal aneurysm repair in the era of endovascular stent grafting. J Vasc Surg. 2006 May;43(5):915–20. https://doi.org/10.1016/j.jvs.2006.01.017
9. Dindo D , Demartines N , Clavien PA . Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004 Aug;240(2):205–13. https://doi.org/10.1097/01.sla.0000133083.54934.ae
10. Dovzhanskiy DI , Bischoff MS , Wilichowski CD , Rengier F , Klempka A , Böckler D . Outcome analysis and risk factors for postoperative colonic ischaemia after aortic surgery. Langenbecks Arch Surg. 2020 Nov;405(7):1031–8. https://doi.org/10.1007/s00423-020-01964-2
11. Williamson JS , Ambler GK , Twine CP , Williams IM , Williams GL . Elective Repair of Abdominal Aortic Aneurysm and the Risk of Colonic Ischaemia: Systematic Review and Meta-Analysis. Eur J Vasc Endovasc Surg. 2018 Jul;56(1):31–9. https://doi.org/10.1016/j.ejvs.2018.03.005
12. Landry GJ , Liem TK , Abraham CZ , Jung E , Moneta GL . Predictors of perioperative morbidity and mortality in open abdominal aortic aneurysm repair. Am J Surg. 2019 May;217(5):943–7. https://doi.org/10.1016/j.amjsurg.2018.12.054
13. Dillavou ED , Muluk SC , Makaroun MS . A decade of change in abdominal aortic aneurysm repair in the United States: have we improved outcomes equally between men and women? J Vasc Surg. 2006 Feb;43(2):230–8. https://doi.org/10.1016/j.jvs.2005.09.043
14. Beck AW , Goodney PP , Nolan BW , Likosky DS , Eldrup-Jorgensen J , Cronenwett JL ; Vascular Study Group of Northern New England . Predicting 1-year mortality after elective abdominal aortic aneurysm repair. J Vasc Surg. 2009 Apr;49(4):838–43. https://doi.org/10.1016/j.jvs.2008.10.067
15. Deery SE , Lancaster RT , Baril DT , Indes JE , Bertges DJ , Conrad MF , et al. Contemporary outcomes of open complex abdominal aortic aneurysm repair. J Vasc Surg. 2016 May;63(5):1195–200. https://doi.org/10.1016/j.jvs.2015.12.038
16. Dubois L , Durant C , Harrington DM , Forbes TL , Derose G , Harris JR . Technical factors are strongest predictors of postoperative renal dysfunction after open transperitoneal juxtarenal abdominal aortic aneurysm repair. J Vasc Surg. 2013 Mar;57(3):648–54. https://doi.org/10.1016/j.jvs.2012.09.043
17. Chong T , Nguyen L , Owens CD , Conte MS , Belkin M . Suprarenal aortic cross-clamp position: a reappraisal of its effects on outcomes for open abdominal aortic aneurysm repair. J Vasc Surg. 2009 Apr;49(4):873–80. https://doi.org/10.1016/j.jvs.2008.10.057
18. Senekowitsch C , Assadian A , Assadian O , Hartleb H , Ptakovsky H , Hagmüller GW . Replanting the inferior mesentery artery during infrarenal aortic aneurysm repair: influence on postoperative colon ischemia. J Vasc Surg. 2006 Apr;43(4):689–94. https://doi.org/10.1016/j.jvs.2005.12.016

Figure S1 10-year survival according to the indication for surgery.
Table S1Univariable analysis of risk factors for surgical complications (n = 661).
| Odds ratio (95% CI) | p-value | |
| Comparison of groups | ||
| – AIOD vs AAA | 1.46 (0.88–2.44) | 0.15 |
| – Combined vs. AAA | 2.86 (1.50–5.43) | 0.001 |
| – Combined vs AIOD | 1.95 (0.92–4.15) | 0.15 |
| Age (per 10-year increase) | 1.24 (0.99–1.57) | 0.07 |
| Creatinine clearance (ml/min/1.73m2) | 1.00 (0.99–1.01) | 0.82 |
| Suprarenal clamp | 1.97 (1.28–3.02) | 0.002 |
| BMI (kg/m2) | 0.98 (0.93–1.03) | 0.40 |
| Coronary heart disease | 1.15 (0.76–1.73) | 0.51 |
AAA: abdominal aortic aneurysm; AIOD: aorto-iliac occlusive disease; BMI: body mass index; CI: confidence interval
Table S2Univariable analysis of risk factors for 30-day mortality (n = 661).
| Incidence rate ratio (95% CI) | p-value | |
| Comparison of groups | ||
| – AIOD vs AAA | 1.54 (0.57–4.10) | 0.39 |
| – Combined vs AAA | 3.47 (1.33–9.05) | 0.01 |
| – Combined vs AIOD | 2.26 (0.69–7.44) | 0.18 |
| Age (per 10-year increase) | 1.74 (1.03–2.93) | 0.04 |
| Creatinine clearance (ml/min/1.73m2) | 0.99 (0.98–1.01) | 0.39 |
| Suprarenal clamp | 3.08 (1.45–6.51) | 0.003 |
| BMI (kg/m2) | 0.95 (0.85–1.07) | 0.41 |
| Coronary heart disease | 1.62 (0.76–3.47) | 0.21 |
AAA: abdominal aortic aneurysm; AIOD: aorto-iliac occlusive disease; BMI: body mass index; CI: confidence interval
Table S3Univariable analysis of risk factors for 1-year mortality (n = 661).
| Incidence rate ratio (95% CI) | p-value | |
| Comparison of groups | ||
| – AIOD vs AAA | 1.02 (0.43–2.39) | 0.97 |
| – Combined vs AAA | 2.68 (1.24–5.78) | 0.01 |
| – Combined vs AIOD | 2.64 (0.94–7.42) | 0.07 |
| Age (per 10-year increase) | 1.87 (1.30–2.70) | <0.001 |
| Creatinine clearance (ml/min/1.73m2) | 0.99 (0.98–1.00) | 0.09 |
| Suprarenal clamp | 2.97 (1.66–5.29) | <0.001 |
| BMI (kg/m2) | 0.98 (0.90–1.06) | 0.58 |
| Coronary heart disease | 1.50 (0.83–2.72) | 0.18 |
AAA: abdominal aortic aneurysm; AIOD: aorto-iliac occlusive disease; BMI: body mass index; CI: confidence interval
Table S4Univariable analysis of risk factors for colon ischaemia (n = 661).
| Incidence rate ratio (95% CI) | p-value | |
| Comparison of groups | ||
| – AIOD vs AAA | 0.78 (0.23–2.58) | 0.68 |
| – Combined vs AAA | 3.51 (1.47–8.35) | <0.001 |
| – Combined vs AIOD | 4.52 (1.18–17.32) | 0.03 |
| Age (per 10-year increase) | 1.80 (1.11–2.91) | 0.02 |
| Creatinine clearance (ml/min/1.73m2) | 0.99 (0.98–1.01) | 0.23 |
| Suprarenal clamp | 2.69 (1.30–5.56) | 0.01 |
| BMI (kg/m2) | 0.96 (0.86–1.07) | 0.42 |
| Coronary heart disease | 1.43 (0.68–3.00) | 0.34 |
AAA: abdominal aortic aneurysm; AIOD: aorto-iliac occlusive disease; BMI: body mass index; CI: confidence interval
Table S5Impact of colon ischaemia on mortality in the different groups.
| Mortality | AAA | AIOD | Combined | |||
| Colon ischaemia | Colon ischaemia | Colon ischaemia | ||||
| Yes | No | Yes | No | Yes | No | |
| 30-days | ||||||
| Yes | 7 (37%) | 9 (1.8%) | 1 (33%) | 3 (3.0%) | 4 (67%) | 1 (2.50%) |
| No | 12 (63%) | 483 (98%) | 2 (67%) | 98 (97%) | 2 (33%) | 39 (98%) |
| 1 year | ||||||
| Yes | 19 (3.9%) | 10 (53%) | 2 (67%) | 4 (4.0%) | 2 (5%) | 5 (83%) |
| No | 473 (96%) | 9 (47%) | 1 (33%) | 97 (96%) | 38 (95%) | 1 (17%) |
AAA: abdominal aortic aneurysm; AIOD: aorto-iliac occlusive disease