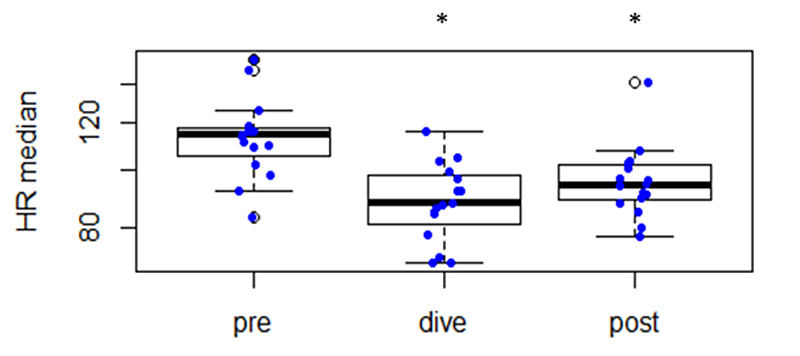
Figure 1 Changes in heart rate before submersion (“pre”), during diving (“dive”) and after resurfacing (“post”).
* Statistically significant different (p <0.05) from “pre”.
DOI: https://doi.org/10.4414/SMW.2021.w30039
autonomic nervous system
body mass index
power in high frequency range (high frequency spectral component)
heart rate variability
power in low frequency range (low frequency spectral component)
proportion derived by dividing NN50 (number of interval differences of successive normal-to-normal (NN) intervals greater than 50 ms) by the total number of NN intervals
square root of the mean squared differences of successive normal-to-normal (NN) intervals
self-contained underwater breathing apparatus
standard deviation of the normal-to-normal (NN) interval
total power (variance of all normal-to-normal (NN) intervals)
power in ultralow frequency range (ultralow frequency spectral component)
power in very low frequency range (very low frequency spectral component)
Diving bradycardia is a primordial oxygen-conserving reflex by which the heart rate of air-breathing vertebrates slows down in response to apnoea and is accentuated by immersion of the face or whole body in cold water [1].
More than 200 years ago, Edmund Goodwyn described this reflex in his doctoral thesis, which was originally published in Latin in 1786 [2]. Later followed the observations of Paul Bert, published in 1870 as part of a series of experiments that examined physiological adaptations to asphyxia in ducks and other animals [3]. The reflex nature of the bradycardia was investigated almost two decades later by Charles Richet, who reported that average survival time after complete tracheal ligation was tripled by immersion and that bradycardia could be induced by the mere contact of water with a duck’s nostrils and beak [4]. Transection of the vagus nerve abrogated both the bradycardia and the survival advantage of the immersed duck, suggesting that bradycardia was an oxygen-conserving reflex by which stimulation of the trigeminal nerve with water resulted in a deceleration of the heart rate via the vagus nerve [5]. Beside the bradycardia, two other reflex responses to water immersion, namely a redirection of blood flow from peripheral to central organs by vasoconstriction of selected vascular beds and a suppression of metabolism, have been described in the early twentieth century [6, 7]. The sum of these reactions (reflex bradycardia, vasoconstriction associated with a blood flow redistribution, metabolic changes) is known as “dive response” and represents a multidimensional oxygen-conserving response to immersion, protecting oxygen-sensitive organs such as the heart and brain [8, 9].
To date, this response has been found in all air-breathing vertebrate species including humans [10]. The diving response appears to be more pronounced in mammals than in birds. Heart rates during dives of big marine mammals such as the blue whale are typically at 4 to 8 bpm and as low as 2 bpm, whereas after-dive surface heart rates go up to 25 to 37 bpm, near the estimated maximum possible heart rate [11]. In humans, the bradycardic response to diving varies greatly from person to person; the reduction in heart rate generally ranges from 15% to 40%, but a small proportion of healthy individuals can develop bradycardia below 20 bpm [9].
The aim of the present study was to describe heart rate profiles and autonomic nervous system activation (measured by heart rate variability [HRV]) in scuba divers of different ages under real-world conditions.
From April to December 2018 we recruited adult divers of the Zollikon Coast Guard (Seerettungsdienst Zollikon) and commercial/private dive clubs (Tauchsport Uster) performing scheduled dives in the Lake Zürich. The Lake Zürich is a continental freshwater lake, extending south-east of the city of Zürich with an average depth of 49 metres (maximum depth 136 metres) and a surface level at 406 metres above see level. All divers willing to participate in the study were eligible; however divers with critical medical conditions (without attested fitness to dive) were excluded from participation. Divers participating in this study gave written informed consent. The study was reviewed and approved by the ethics committee of the Canton of Zurich, Switzerland (KEK-ZH-NR: 2012-0524).
Primary data relevant to the subsequent statistical analysis, such as information concerning the diver’s health, the setting and technical data were acquired by means of a questionnaire that was filled in prior to each dive. In the questionnaire, health data for each of the divers was recorded (age, gender, biometrics). Furthermore, the divers stated their form of the day, their diving education certificate, the number of years of diving experience, the number of dives performed overall and the amount of days passed since the diver’s last dive. The diving equipment used by the divers was also recorded. The diver’s fitness level was assessed by a healthcare professional based on subjective categorical variables (athletic, normal, sedentary/inactive)..
After each dive, the level of physiological stress the divers experienced during the dive was assessed using the Borg CR10 (category ratio 10) scale. The Borg scale is a psychophysical scaling system used to assess the degree of perceived exertion. Numbers from 1 to 10 are anchored to verbal expressions according to their quantitative meaning, thus creating a category scale with ratio properties [12]. In addition to the level of physiological stress, the level psychological stress was assessed for each diver during each dive. For this, we also used a category ratio scale similar to the Borg scale, indicating the subjectively experienced level of psychological stress the divers felt they were exposed to. After the dive, the divers were asked whether they experienced decompression symptoms or nitrogen narcosis, and if so, for how long they lasted. Dive time and maximum depth were reconstructed after the dive using the divers dive computer. In addition, data concerning the setting of the dive, such as weather and water conditions were recorded.
Underwater ECGs were recorded with a custom-built setup. Due to the challenges with the performance of clinical gel electrodes when submerged [13], silver-loaded polydimethylsiloxane dry electrodes [14] (Dryode, IDUN Technologies AG, Switzerland) were used for this study (two conductive circular electrodes, placed on the lower edge of the left rib cage, and under the left clavicle, forming a two-channel measurement arrangement). All recordings were taken with custom recording hardware based on an open-source research biopotential recorder design (Cyton, OpenBCI, Brooklyn, NY) at a sampling rate of 250 Hz for each of the two channels and stored locally on the device. The electronics were carried by the divers in a purpose-built waterproof enclosure inside their scuba suits. Dive characteristics were recorded with the divers' personal dive computers, recording at least the duration and depth of the dive.
Heart rate variability (HRV) describes the cyclic variations in heart rate and offers non-invasive opportunities for investigating alterations of autonomic nervous system (ANS) activity and its modulatory effects on intrinsic heart rate. Variations in heart rate may be evaluated by a number of methods. According to standards of measurements [15], we analysed in the present study several time domain parameters: pNN50 (proportion derived by dividing NN50 (number of interval differences of successive normal-to-normal [NN] intervals greater than 50 ms) by the total number of NN intervals), SDNN (standard deviation of the NN interval), RMSSD (square root of the mean squared differences of successive NN intervals). We also analysed a number of frequency domain parameters: HF (power in high frequency range), LF (power in low frequency range), ULF (power in ultra low frequency range), VLF (power in very low frequency range), LF/HF ratio, TTLPWR (total power [variance of all NN) intervals]). Automated R-peak detection and further analysis based thereon was conducted with PhysioNet’s Cardiovascular Signal Toolbox [16, 17]. All metrics were calculated for both recording channels on time windows of 300 s duration with an overlap of 30 s between adjacent windows. For every window, the channel with higher signal quality was selected as data for statistical evaluation (based on correlation of R-marks of three different peak detection algorithms). Finally, experienced ECG readers assessed the recordings visually and excluded tracings with insufficient R-peak detection from analysis.
Continuous data are expressed as medians and interquartile ranges (IQRs) or as mean ± standard deviation (SD) as appropriate, and categorical data as number and percentage (%). The effect of dive phases on heart rrate and heart rate variability was analysed using a Friedman rank sum test. Post hoc Nemenyi test was used for pairwise comparison of mean ranks of the dive phases. A p-value of <0.05 was considered statistically significant. Statistical analyses were performed on R Studio (V 1.2.5033) using the PMCMR (V 4.3) and LME4 (V 1.1-17) packages.
Diver characteristics (biometrics, dive experience) and diving features (equipment, meteorological circumstances, subjective assessments, dive profiles) are shown in table 1.
Table 1Diver and diving characteristics.
| Median | IQR 25–75 | Min | Max | |
| Diver characteristics | ||||
| Age (years) | 51.5 | 46–55 | 24 | 61 |
| Height (cm) | 181 | 170.5–181.75 | 163 | 196 |
| Weight (kg) | 90 | 80–98 | 63 | 101 |
| BMI (kg/m2) | 27.297 | 24.419–30.829 | 20.825 | 37.638 |
| Diving experience (years) | 9 | 8.25–17.25 | 2 | 22 |
| Total number of dives | 385 | 317–587.5 | 59 | 1500 |
| Time since last dive (days) | 5 | 3–6.75 | 1 | 49 |
| Dive settings | ||||
| Dive time (minutes) | 35 | 31–39 | 16 | 44 |
| ECG recording time (minutes) | 53.5 | 47–55.75 | 37 | 66 |
| Maximum depth | 34.5 | 16.5–38.675 | 5.7 | 42.5 |
| Gas consumption (bar) | 113.5 | 81.25–130 | 36 | 178 |
| Physiological stress (1–10) | 2 | 1.25–3 | 1 | 7 |
| Psychological stress (1–10) | 2 | 1–3 | 1 | 4 |
| Weather and water conditions | ||||
| Outside temperature (°C) | 21.4 | 19–27 | 15 | 30 |
| Surface water temperature (°C) | 22.95 | 20–25.025 | 18.6 | 25.7 |
| Minimum water temperature (°C) | 6 | 5.25–11.25 | 5 | 20 |
A total of 13 divers were included in the study, 5 (38.5%) of whom performed multiple dives. At 92%, most of the participants were male, with only 1 female diver participating in the study. Median age was 51.5 years, ranging from 24 to 61 (IQR 46–55). Median height was 181 cm, with an interquartile range from 170.5 to 181.75 cm. The average weight amounted to 88.12 kg, with a median weight of 90 kg (IQR 80–98). The average BMI calculated based on the diver’s height and weight distribution therefore amounted to 27.96 kg/m2 (median 27.3, IQR 24.42–30.83). 61.12% of the divers had a normal body shape. 22.23% an athletic figure and 16.67% were inactive. In terms of diving education, 16.67% of the divers had a CMAS 2-Star certificate or a dive master certificate.One of the divers (5.56%) was an assistant instructor, while 61.12% were instructors. The divers had a median of 9 years of diving experience (IQR 8.25–17.25), with a median of 385 dives performed overall (IQR 317–587.5).
Overall, data of 18 dives were recorded over the course of 3 months. 94.45% of the divers used compressed air, with only one diver (5.56%) using a 32% nitrox mixture. Median gas consumption was 113.5 bar with an interquartile range from 81.25 to 130. The majority (77.78%) wore a neoprene wet suit during the dive. Only 16.67% used a semi-dry alternative, leaving only one diver who used a dry suit (5.56%).
The weather conditions were mostly good, with a median outside temperature of 21.4°C (IQR 19–27). The median surface water temperature was 22.95°C (IQR 20–25.025), while the median minimum water temperature was 6°C (IQR 5.25-–1.25), ranging from 5 to 20°C depending on immersion depth. In 61.1% of the dives the weather was sunny or clear (44.44% and 16.67%, respectively) and in only 38.89% was the sky.
In the questionnaire, 50% of the divers stated they were in a normal daily form, whereas 44.45% felt good on the day of the dive. One (5.56%) of the divers felt he was in a particularly good form on the day of the dive. Furthermore, a median of 5 days (IQR 3–6.75) since the last dive was reported. Subjective stress levels during the dives were in general low. The divers reported an average physiological (Borg-scale) and psychological stress level of 2.3 and 2.1, respectively, with a median stress level 2 for both categories (IQR 1.25–3 and 1–3, respectively).
The median dive time was 35 minutes, with an interquartile range from 31 to 39 minutes. Cardiac activity was recorded over a median period of 53.5 minutes (IQR 47–55.75). The divers submerged from 5.7 up to 40.1 metres water depth, with a median diving depth of 34.5 metres (IQR 16.5–38.68).
One of the divers experienced slight nitrogen narcosis during the dive, which lasted for 3 minutes. Relevant symptoms of decompression sickness were not observed in any of the divers.
Signal quality – assessed visually by experienced ECG readers – was sufficient (>80% evaluable) in 72.22% and good (>90% evaluable) in 61.11 % of the samples. Only three (16.67%) of the samples showed poor signal quality (<50% evaluable) and only one (5.56%) sample showed very poor quality (< 30% evaluable). Overall, median evaluability was 92.13%.
No relevant brady- or tachyarrhythmias occurred on land or underwater. Extrasystoles (either ventricular or supraventricular) were recorded in 90% of divers older than 50 years and 50% of the younger divers. Nevertheless, the amount of extrasystoles – expressed as extrasystoles per minute – was generally low (mean 0.18 extrasystoles/min, range 0–1.45). The occurrence of extrasystoles was individually very variable, and no specific pattern could be documented: some divers showed more pronounced occurrence of extrasystoles during and after the dive, whereas in others extrasystoles were rather suppressed during diving.
Heart rates in the divers just before descent were remarkably high (median 114 bpm, IQR 105–117). After immersion, heart rates rapidly decreased to a median of 90 bpm (IQR 85–97) representing a statistically significant reduction (p = 0.008). The percentage heart rate reduction by immersion was individually very variable (median 21%, range 5–39%). After surfacing, median heart rates were 95 bpm (IQR 90–103), which was statistically significantly lower than before immersion (p = 0.013) but overall quite comparable to heart rates during diving (p = 0.06).

Figure 1 Changes in heart rate before submersion (“pre”), during diving (“dive”) and after resurfacing (“post”).
* Statistically significant different (p <0.05) from “pre”.
Heart rate changes are listed in table 2.
Table 2Changes in heart rate and different heart rate variability parameters before submersion, during diving and after resurfacing.
| Pre-dive | Dive | Post-dive | |
| Median (IQR 25–75) | Median (IQR 25–75) | Median (IQR 25–75) | |
| Heart rate (bpm) | 114 (105–117) | 90 (85–97) | 95 (90–103) |
| Heart Rate Variability Parameters | |||
| pNN50 | 0.04 (0.02-0.16) | 0.30 (0.15–0.42) | 0.29 (0.15–0.39) |
| SDNN (ms) | 52 (36–59) | 76 (42–104) | 90 */** (67–120) |
| RMSSD (ms) | 34 (16–45) | 59 (42–107) | 60 (41–130) |
| HF (ms2 ) | 555 (104–855) | 1802 * (535–4650) | 980 (651–1829) |
| LF (ms2 ) | 723 (250–963) | 2133 * (616–4448) | 1293 (693–5044) |
| ULF (ms2 ) | 997 (782–1346) | 556 (382–1074) | 2876 */** (2321–4183) |
| VLF (ms2 ) | 2053 (1277–2458) | 1278 (780–2961) | 4156 */** (3026–6178) |
| LF/HF ratio | 1.33 (1.03–2.69) | 1.22 (0.75–2.11) | 1.03 (0.88–1.54) |
| TTLPWR (ms2 ) | 3949 (2389–5650) | 7101 (1944–11117) | 12332 */** (7157–17293) |
* Statistically significant different (p <0.05) compared with “pre”
** Statistically significant different (p <0.05) compared with “dive”
Changes of the different HRV parameters before submersion (“pre”), during diving (“dive”) and after resurfacing (“post”) are listed in figure 2 and table 2.
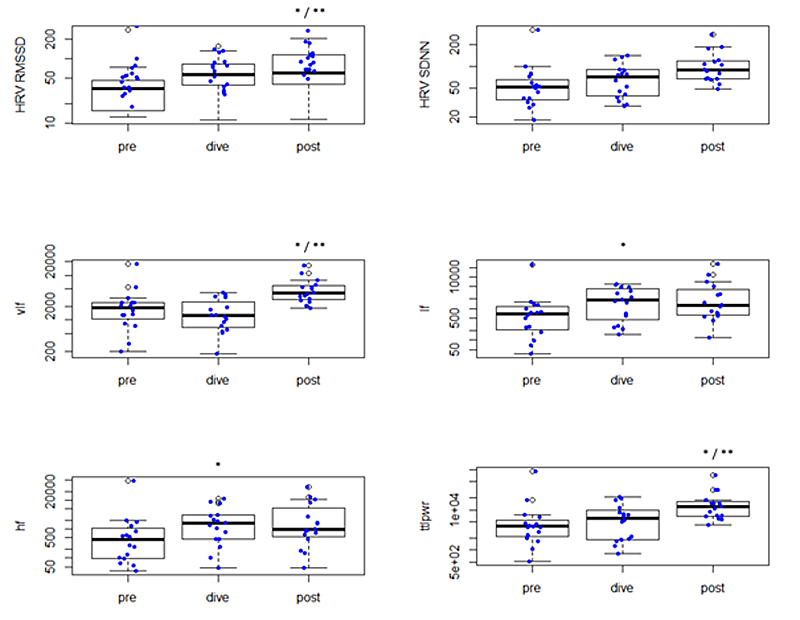
Figure 2 Changes in different HRV parameters before submersion (“pre”), during diving (“dive”) and after resurfacing (“post”).
* Statistically significant different (p <0.05) compared with “pre”
** statistically significant different (p <0.05) compared with “dive”
Scuba diving represents an unusual (or even unnatural) challenge to the physiological adaption of the human organism to hyperbaric conditions combined with submersion, apparatus-assisted breathing of gas mixtures, temperature changes, underwater physical activity, altered haemodynamic conditions and much more. ANS modulations and neurohumoral changes produce a unique and complex multidimensional reaction known as “dive response”.
The present study describes the heart rate changes during recreational scuba diving under real-world conditions. Heart rates of the divers just before descent were remarkably high (median 114 bpm, IQR 83–154) with a statistically significant rapid decrease after immersion (median 90 bpm, IQR 70–116; p = 0.008). The percentage heart rate reduction by immersion – as described in previous studies [9] – was individually very variable (median 21%, range 5–39%). Despite exceptionally low self-reported subjective physical stress in the present study (median Borg-scale 2, IQR 1.25–3) and the general perception of scuba diving being a relaxed leisure activity, the tachycardic heart rates just before immersion could represent a clinically relevant mechanism in diving incidents. Scuba -diving is officially classified as a vigorous physical activity (7 METS) [18] and heavy physical exertion one hour before acute coronary syndromes are common [19]. Physical activities are reported to cause sympathetic activation, catecholamine secretion, systemic vasoconstriction, and increase heart rate and blood pressure, thereby modifying myocardial oxygen demand, which may precipitate the rupture of an already vulnerable atherosclerotic plaque. Since the highest heart rates (and therefore cardiovascular stress) are recorded just before immersion, an eventual subsequent coronary event has a high probability to occur underwater during the dive. Indeed, a recent forensic study identified myocardial ischaemia as one of the most frequent disabling injuries in diving-related fatalities [20). Furthermore, we would like to emphasise that the even a small potential non-fatal myocardial infarction under diving conditions can cause panic, rapid ascent (with subsequent lung overexpansion, arterial gas embolism, organ barotrauma, etc.) or drowning.
The diving response is a complex physiological process mediated by ANS activity. Beside ANS stimulation due to increased vagal activity when a face is immersed in water [21], additional ANS responses are complex and sometimes conflicting in scuba diving [22]: During immersion in thermoneutral water, the hydrostatic pressure of the surrounding water shifts blood from the lower part of body to the central circulation, thereby contributing to bradycardia, increased stroke volume and cardiac output, reduced muscle sympathetic nerve activity and systemic vascular resistance, and unaltered blood pressure [23, 24]. Furthermore, the increased partial pressure of oxygen in the scuba breathing gas increases cardiac parasympathetic tone and decreases the sympathetic activity to the heart and peripheral circulation [25]. On the other hand, being immersed in cold water may enhance both sympathetic and parasympathetic activity [26, 27], and mental stress and proficiency of diving skills are also known to influence autonomic nervous function [28]. Several studies used HRV to determinate parasympathetic and sympathetic-adrenergic activity during scuba diving [22, 29-31]. All studies noted a general increase of ANS activity by scuba diving. In healthy young divers, there wasa dominance in cardiac parasympathetic activity (increased RMSSD and LF, decreased HF and LF/HF ratio). The present study could not reproduce these findings: we noted a general HRV increase without predominance of parasympathetic parameters (parallel increase of HF and LF without change in LF/HF ratio), suggesting a concomitant sympathetic-adrenergic activation. This finding could be explained on one hand by the demanding diving conditions (cold water diving, limited visibility, heavy equipment, boat-based diving) and on the other hand by different and heterogenous diver characteristics compared with previous studies with standardised settings. Our study population included divers of different and rather advanced age (median age 51.5 years, ranging from 24 to 61 years), rather non-athletic body shape (median BMI 27 kg/m2, IQR 24–31) and varying diving experience (2–22 years of diving experience, 59–1500 total number of dives). The parallel increase of parasympathetic and sympathetic-adrenergic ANS activity (“autonomic conflict”) has been postulated to account for arrhythmias and sudden death in drowning accidents(32]. Beside immediate increase of ANS activity after submersion (previously described), we found in the present study a sustained elevation of HRV parameters after the dive. This finding reflects to our opinion the complexity and sustainability of the dive response under real-world circumstances during scuba diving, with several influencing factors far more than just vagal activation by immersion and hydrostatic-pressure mediated haemodynamic responses.
Diver characteristics and dive settings were unusual in this study and probably not comparable to most of the populations studied in the quoted publications. First, the divers were relatively old and experienced. Furthermore, more than half of the divers were overweight (median BMI 27 kg/m2). This brings a certain elevation of cardiovascular risk for the majority of the study population, as well as some probability of a reduced HRV. Women were underrepresented (only one female diver in the entire study population).
The dives were quite deep and beyond the scope of average recreational diving, since a substantial part of the divers were members of the Zollikon Coast Guard (performing underwater tasks such as search missions, buoy mooring checks, etc.). In terms of safe diving, we would like to emphasise that these dives are considered as dives with elevated risk and that it is not advisable for less experienced divers to dive in this way . Furthermore, the use of compressed air at this depth range might represent a confounding factor through the high nitrogen burden [33).
Heart rates of the divers just before descent were remarkably high (median 114 bpm, IQR 83–154) with a statistically significant rapid decrease after immersion (median 90 bpm, IQR 70116; p = 0.008). The percentage heart rate reduction by immersion was individually very variable (median 21%, range 539%). Interestingly, the bradycardic dive response was independent of the diver’s age. Since the highest heart rates (and therefore cardiovascular stress) are recorded just before immersion, an eventual subsequent coronary event has a high probability to occur underwater during the dive, underlining the importance of cardiovascular risk stratification in diving eligibility assessment (especially in older divers).
Scuba diving under real-world conditions by non-selected divers induces an immediate and sustained parallel increase of parasympathetic and sympathetic-adrenergic ANS activity. This “autonomic conflict” is a potential trigger for arrhythmias, (with subsequent fatal consequences such as sudden death or drowning) and could attenuate the general cardiovascular protective effect of the dive response.
We would like to thank Katja Locher (HerzKlinik Zürich) for technical counceling in ECG recording/fixation technique, the divers of the Zollikon Coast Guard (Seerettungsdienst Zollikon) for participation and Sandro Scagliola (Tauchsport Uster) for assistance.
Author contributions: CS, AF, SB and CW contributed to the conception of the work. CS, AF, SB and YN contributed to the acquisition of data. CS, LO, CW and RM contributed to the analysis and interpretation of data for the work. CW, CS, RM and AF drafted the manuscript. PB critically revised the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Simon Bachmann has co-founded IDUN Technologies AG (IDUN), which is aiming for commercialisation of soft dry electrodes for non-invasive biopotential recordings in humans. Andrea Fümm is employed by IDUN. Both report holding shares in IDUN. The other authors have no conflict of interest to declare.
1 Vega JL . Edmund Goodwyn and the first description of diving bradycardia. J Appl Physiol (1985). 2017;123(2):275-7.
2. Goodwyn E . Dissertatio medica inauguralis, de morbo morteque submersorum investigandis [Diss - Edinburgh]. Edinburgi,1786.
3. Bert P . Leçons sur la physiologie comparée de la respiration professées au Muséum d'histoire naturelle. Paris: Baillière; 1870. xxxv, 588 p. p.
4. Richet C . La resistance des canards a l’asphyxie. C R Soc Biol Paris. 1894;1:244–5.
5. Richet C . De la resistance des canards a l’asphyxie. J Physiol Pathol Gen. 1899;1:641–50.
6. Irving L , Scholander P , Grinnell S . The regulation of arterial blood pressure in the seal during diving. Am J Physiol. 1942;135(3):557–66. https://doi.org/10.1152/ajplegacy.1942.135.3.557
7. Scholander PF . Hvalradets Skrifter. Experimental Investigations on the Respiratory Function in Diving Mammals and Birds. Oslo: Det Norske Videnskaps Akademi. 1940(22).
8. Panneton WM . The mammalian diving response: an enigmatic reflex to preserve life? Physiology (Bethesda). 2013 Sep;28(5):284–97. https://doi.org/10.1152/physiol.00020.2013
9. Alboni P , Alboni M , Gianfranchi L . Diving bradycardia: a mechanism of defence against hypoxic damage. J Cardiovasc Med (Hagerstown). 2011 Jun;12(6):422–7. https://doi.org/10.2459/JCM.0b013e328344bcdc
10. Song SH , Lee WK , Chung YA , Hong SK . Mechanism of apneic bradycardia in man. J Appl Physiol. 1969 Sep;27(3):323–7. https://doi.org/10.1152/jappl.1969.27.3.323
11. Goldbogen JA , Cade DE , Calambokidis J , Czapanskiy MF , Fahlbusch J , Friedlaender AS , et al. Extreme bradycardia and tachycardia in the world’s largest animal. Proc Natl Acad Sci USA. 2019 Dec;116(50):25329–32. https://doi.org/10.1073/pnas.1914273116
12. Borg G . Psychophysical scaling with applications in physical work and the perception of exertion. Scand J Work Environ Health. 1990;16 Suppl 1:55–8. https://doi.org/10.5271/sjweh.1815
13. Reyes BA , Posada-Quintero HF , Bales JR , Clement AL , Pins GD , Swiston A , et al. Novel electrodes for underwater ECG monitoring. IEEE Trans Biomed Eng. 2014 Jun;61(6):1863–76. https://doi.org/10.1109/TBME.2014.2309293
14. Stauffer F , Thielen M , Sauter C , Chardonnens S , Bachmann S , Tybrandt K , et al. Skin Conformal Polymer Electrodes for Clinical ECG and EEG Recordings. Adv Healthc Mater. 2018 Apr;7(7):e1700994. https://doi.org/10.1002/adhm.201700994
15. Malik M , Bigger JT , Camm AJ , Kleiger RE , Malliani A , Moss AJ , et al.; Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology . Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996 Mar;17(3):354–81. https://doi.org/10.1093/oxfordjournals.eurheartj.a014868
16. Goldberger AL , Amaral LA , Glass L , Hausdorff JM , Ivanov PC , Mark RG , et al. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000 Jun;101(23):E215–20. https://doi.org/10.1161/01.CIR.101.23.e215
17. Vest AN , Da Poian G , Li Q , Liu C , Nemati S , Shah AJ , et al. An open source benchmarked toolbox for cardiovascular waveform and interval analysis. Physiol Meas. 2018 Oct;39(10):105004. https://doi.org/10.1088/1361-6579/aae021
18. Ainsworth BE , Haskell WL , Herrmann SD , Meckes N , Bassett DR Jr , Tudor-Locke C , et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011 Aug;43(8):1575–81. https://doi.org/10.1249/MSS.0b013e31821ece12
19. Smyth A , O’Donnell M , Lamelas P , Teo K , Rangarajan S , Yusuf S ; INTERHEART Investigators . Physical Activity and Anger or Emotional Upset as Triggers of Acute Myocardial Infarction: the INTERHEART Study. Circulation. 2016 Oct;134(15):1059–67. https://doi.org/10.1161/CIRCULATIONAHA.116.023142
20. Casadesús JM , Aguirre F , Carrera A , Boadas-Vaello P , Serrando MT , Reina F . Diving-related fatalities: multidisciplinary, experience-based investigation. Forensic Sci Med Pathol. 2019 Jun;15(2):224–32. https://doi.org/10.1007/s12024-019-00109-2
21. Gooden BA . Mechanism of the human diving response. Integr Physiol Behav Sci. 1994 Jan-Mar;29(1):6–16. https://doi.org/10.1007/BF02691277
22. Noh Y , Posada-Quintero HF , Bai Y , White J , Florian JP , Brink PR , et al. Effect of Shallow and Deep SCUBA Dives on Heart Rate Variability. Front Physiol. 2018 Feb;9:110. https://doi.org/10.3389/fphys.2018.00110
23. Mano T , Iwase S , Yamazaki Y , Saito M . Sympathetic nervous adjustments in man to simulated weightlessness induced by water immersion. J UOEH. 1985 Mar;7 Suppl:215–27.
24. Epstein M . Renal effects of head-out water immersion in humans: a 15-year update. Physiol Rev. 1992 Jul;72(3):563–621. https://doi.org/10.1152/physrev.1992.72.3.563
25. Lund V , Kentala E , Scheinin H , Klossner J , Sariola-Heinonen K , Jalonen J . Hyperbaric oxygen increases parasympathetic activity in professional divers. Acta Physiol Scand. 2000 Sep;170(1):39–44. https://doi.org/10.1046/j.1365-201x.2000.00761.x
26. Mourot L , Bouhaddi M , Gandelin E , Cappelle S , Nguyen NU , Wolf JP , et al. Conditions of autonomic reciprocal interplay versus autonomic co-activation: effects on non-linear heart rate dynamics. Auton Neurosci. 2007 Dec;137(1-2):27–36. https://doi.org/10.1016/j.autneu.2007.06.284
27. Srámek P , Simecková M , Janský L , Savlíková J , Vybíral S . Human physiological responses to immersion into water of different temperatures. Eur J Appl Physiol. 2000 Mar;81(5):436–42. https://doi.org/10.1007/s004210050065
28. Flouris AD , Scott JM . Heart rate variability responses to a psychologically challenging scuba dive. J Sports Med Phys Fitness. 2009 Dec;49(4):382–6.
29. Schipke JD , Pelzer M . Effect of immersion, submersion, and scuba diving on heart rate variability. Br J Sports Med. 2001 Jun;35(3):174–80. https://doi.org/10.1136/bjsm.35.3.174
30. Chouchou F , Pichot V , Garet M , Barthélémy JC , Roche F . Dominance in cardiac parasympathetic activity during real recreational SCUBA diving. Eur J Appl Physiol. 2009 Jun;106(3):345–52. https://doi.org/10.1007/s00421-009-1010-0
31. Lundell RV , Räisänen-Sokolowski AK , Wuorimaa TK , Ojanen T , Parkkola KI . Diving in the Arctic: Cold Water Immersion’s Effects on Heart Rate Variability in Navy Divers. Front Physiol. 2020 Jan;10:1600. https://doi.org/10.3389/fphys.2019.01600
32. Shattock MJ , Tipton MJ . ‘Autonomic conflict’: a different way to die during cold water immersion? J Physiol. 2012 Jul;590(14):3219–30. https://doi.org/10.1113/jphysiol.2012.229864
33 Ostlund A , Linnarsson D . Slowing and attenuation of baroreflex heart rate control with nitrous oxide in exercising men. J Appl Physiol (1985). 1999;87(2):830-4.