Expert guidance for COVID-19 vaccine deployment in Switzerland: a Delphi process
DOI: https://doi.org/10.4414/SMW.2021.w30076
Kevin
Selby, Marc-Antoine
Bornet, Yann
Sancosme, Erik
von Elm, Valérie
d’Acremont, Serge
de Valliere, Jacques
Cornuz, Blaise
Genton
Centre for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland
Summary
BACKGROUND AND AIM: Vaccines providing protection against COVID-19 are a core tool for ending the pandemic. Though international organisations created guidance in 2020 for vaccine deployment, this had to be adapted for each country’s situation and values. We aimed to assist public health decision makers by identifying areas of consensus among Swiss experts for the deployment of one or more novel COVID-19 vaccines.
METHODS: An electronic, modified Delphi process between September and November 2020. We recruited a convenience sample of experts working in Switzerland from a variety of specialities, who completed two anonymous questionnaires. They voted on clarification questions and guidance statements from 0 (complete disagreement) to 10 (complete agreement). Responses for guidance statements with a median ≥8 and a lower inter-quartile range bound ≥7 were considered as reaching consensus.
RESULTS: Sixty-five experts accepted (66% response rate), with 47 completing the first questionnaire (72%), and 48 the second (74%). Statements reaching consensus included: in the first phase we should vaccinate front-line healthcare professionals and people ≥65 years with risk factors; widespread vaccination of children and adolescents should not be an early priority; and vaccines should be provided free of charge in the setting of national or cantonal vaccination campaigns. Statements not reaching consensus included: early vaccination of people living with someone with risk factors who are not themselves at risk; vaccination of people with previous confirmed or suspected COVID-19; and whether vaccination should be mandatory for individuals with certain activities, such as front-line healthcare professionals.
CONCLUSIONS: Experts reached consensus on several statements that were available for decision-makers when making key decisions for COVID-19 vaccine deployment in Switzerland. Statements without consensus highlighted areas requiring expert and public dialogue. The modified Delphi process allowed us to rapidly synthesise views from a broad panel of experts on sensitive topics, and could be considered for a broad range of issues during public health crises.
Introduction
Vaccines providing protection against COVID-19 are a core tool for ending the pandemic [1]. In mid to late 2020, significant uncertainty remained regarding early vaccine deployment. Several bodies developed expert guidance documents for decision makers outlining potential deployment strategies, including priority groups and means of improving vaccine uptake [2–7]. However, these documents had limitations. The World Health Organization’s global approach had to be refined and adapted to each country’s context [8]. A guidance document from France clearly identified priority populations, depending on levels of COVID-19 propagation when the vaccine became available, but did not give recommendations for specific populations such as children and pregnant women [6]. A German policy brief addressed difficult ethical issues such as mandatory vaccination and the need for a transparent vaccination strategy, but failed to address practical issues such as who should perform vaccination and whether to vaccinate those who have had a positive test for SARS-CoV-2 [7]. Importantly, none of the guidance documents provided information about controversial areas lacking consensus, thus simplifying reporting but potentially undermining trust and public dialogue. As data emerged that one or more vaccines would be safe and effective against COVID-19, Swiss authorities still needed to make critical decisions tailored to the country’s context and values. Indeed, countries differ in the value they place on the need for certain safety data, their access to early vaccines and the need to prioritise specific, marginalised populations for early vaccination (e.g., indigenous populations).
The Delphi method provides a transparent method for identifying areas of expert consensus in an environment of rapidly evolving evidence or on topics lacking evidence [9]. The Delphi method could be preferable to convening a task-force, for instance, because a large number of diverse experts can be convened asynchronously, with a written trace of all opinions and anonymous voting, allowing experts to express themselves more freely. We aimed to assist public health decision makers by identifying areas of consensus among Swiss experts for the deployment of one or more novel COVID-19 vaccines.
Methods
Setting and design
We conducted an electronic, modified Delphi process between September and November 2020. Our protocol (see appendix) planned for three Delphi rounds, but only two were needed as we had reached sufficient consensus to inform decision-makers. We recruited a convenience sample of Swiss experts from a variety of specialities by email. Experts were university-based professionals recognised for their knowledge areas of relevance, such as vaccinology, public health and medical ethics. We aimed to achieve a balance of language areas, specialities and gender. We specifically did not include key stakeholders such as government officials or members of the Federal Vaccination Commission. We provided experts with a summary of key literature, including guidance documents from other countries and a description of phase III trials in progress. During the study period COVID-19 cases were increasing rapidly in Switzerland and phase III vaccine results were not yet available. Ethics approval was not required as all information was collected anonymously. We informed key decision makers in Switzerland about our project (the Federal Office of Public Health (FOPH) and the Federal Commission for Vaccination) and worked in collaboration with the Swiss National COVID-19 Science Task Force.
Delphi questionnaires
Questionnaires were developed in English by the co-authors and submitted for feedback to an ad-hoc scientific committee with 10 members from institutions other than our own (8 of whom also participated in the Delphi process). Questionnaires were checked for clarity of language by five local physicians.
We sent participants open links to the survey questionnaires available on REDCap®, with up to two reminders. No remuneration was provided. Experts voted on clarification questions and guidance statements (all questions and responses are available in the appendix). Clarification questions were used, for example, to define minimum thresholds of vaccine efficacy and rank priority groups. Experts also provided free-text comments. Guidance statements were scored from 0 (complete disagreement) to 10 (complete agreement). Though an answer was required for all questions, experts could choose to opt out of individual questions, for example when lacking expertise. All questionnaires were complete.
Analyses
Because of the non-normal distribution of most responses, agreement scores were presented using medians and inter-quartile ranges (IQRs). Responses with a median ≥8 and a lower IQR bound ≥7 were considered as reaching consensus. All analyses were performed with STATA Version 16.0.
Results
Of 98 experts asked to participate, 65 (66%) accepted (table 1). Forty-seven experts completed the first questionnaire (72%) and 48 the second (74%).
Table 1Characteristics of study participants (n = 65).
|
|
Number (%)
|
|
Gender
|
|
| Female |
29 (45%) |
| Male |
36 (55%) |
|
Language region of professional activity
|
|
| German |
30 (46%) |
| French |
31 (48%) |
| Italian |
4 (6%) |
|
Speciality
|
|
| Infectious disease / vaccinology |
17 (26%) |
| Family medicine / integrative medicine |
12 (18%) |
| Public health |
8 (12%) |
| Paediatrician / Gynaecologist |
7 (11%) |
| Medical ethics / sociology / anthropology |
6 (9%) |
| Hospital internal medicine / intensive care |
5 (8%) |
| Geriatrics / nursing facilities |
5 (8%) |
| Pharmacist |
5 (8%) |
Key statements that did and did not reach consensus are listed in figures 1 and 2. The median of statements reaching consensus ranged between 8 and 10, while those not reaching consensus ranged between 5 and 9. Two statements included among those reaching consensus do not have a score; they were included based on a high number of votes to vaccinate people ≥65 years without risk factors, and those aged 18 to 65 with ≥1 risk factor in the second and third phases.
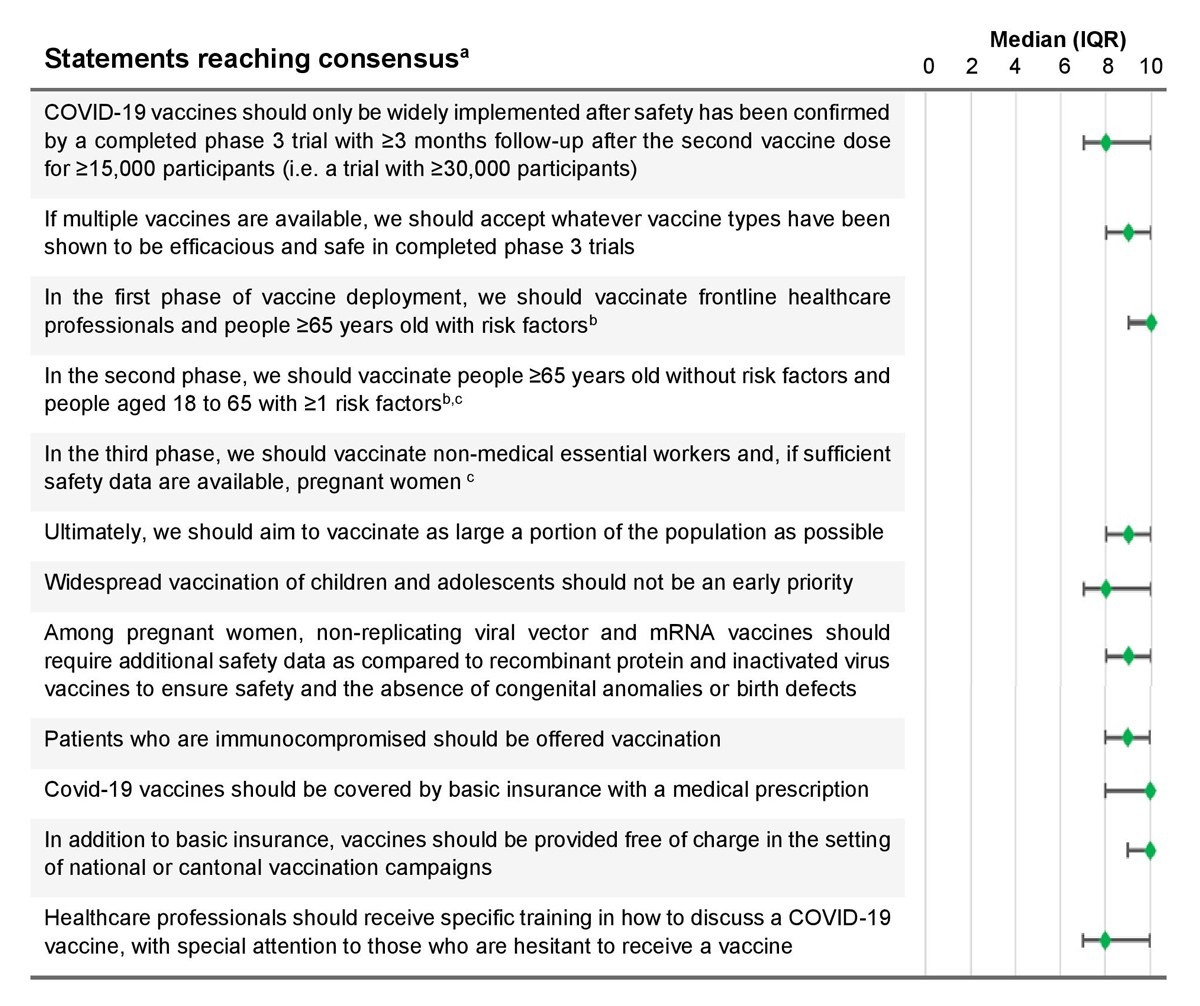
Figure 1 Key recommendation statements that reached consensus.a Agreement scores were on a scale from 0 (complete disagreement) to 10 (complete agreement). The green diamond is the median score, with horizontal bars representing the interquartile range (IQR).
a Consensus was defined as a median agreement score of ≥8 out of 10, with a lower IQR bound ≥7
b Risk factors as defined by the Swiss Federal Office of Public Health at https://www.bag.admin.ch/bag/en/home.html
c Experts voted on which groups to include in the second and third phases: (1) people aged ≥65 years old without FOPH risk factors (78%); (2) people aged 18 to 65 with ≥1 FOPH risk factor (62%); (3) non-medical essential workers (30%); and (4) Pregnant women (27%).
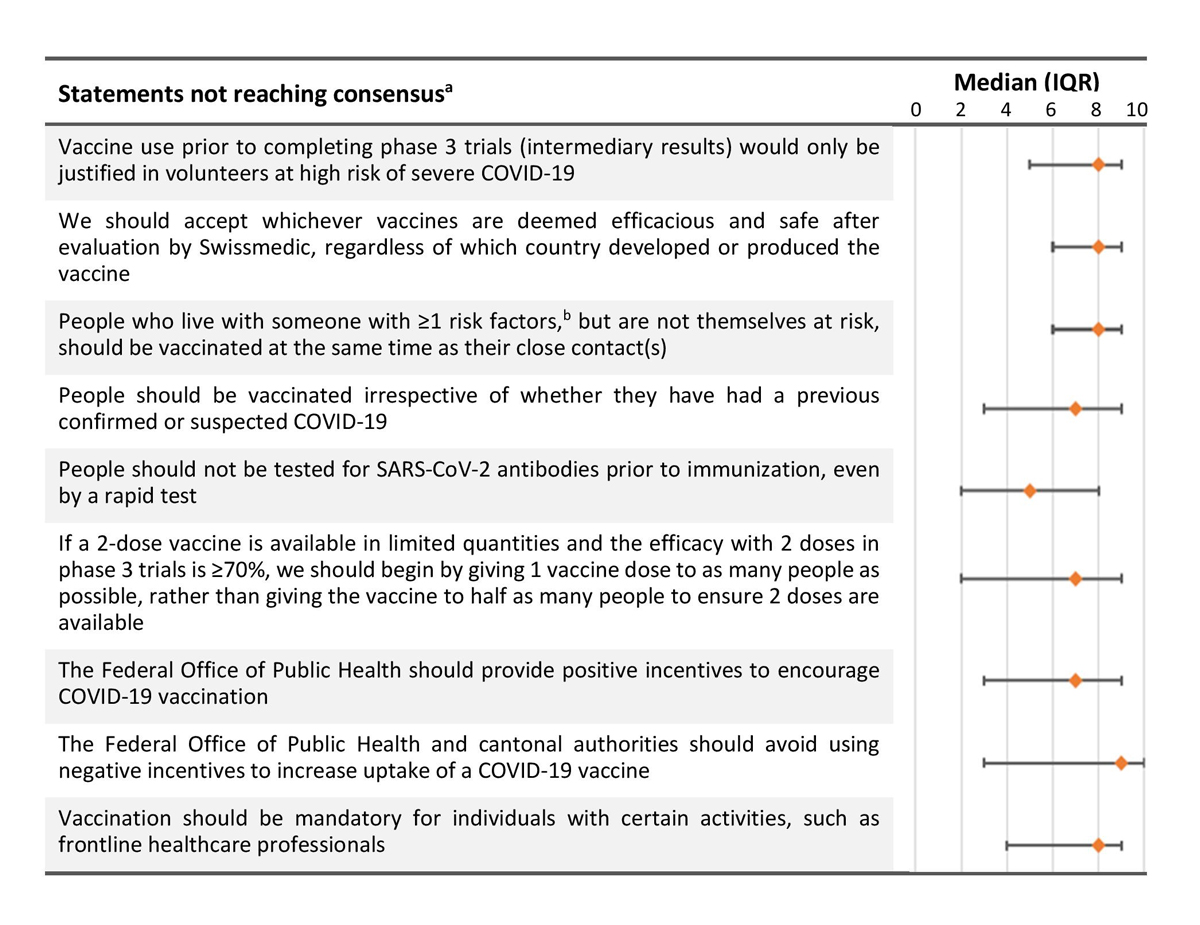
Figure 2 Key recommendations not reaching consensus. Agreement scores were on a scale from 0 (complete disagreement) to 10 (complete agreement). The orange diamond is the median score, with horizontal bars representing the interquartile range (IQR).
a Consensus was defined as a median agreement score of ≥8 out of 10, with a lower IQR bound ≥7.
b Risk factors as defined by the Swiss Federal Office of Public Health at https://www.bag.admin.ch/bag/en/home.html
Various distributions of level of agreement were seen for questions not reaching consensus (fig. 3). Whereas some statements approached consensus (fig. 3A), others had a wide distribution of answers fig. (3D), or even strong opposing views (figs 3B and C). Full results of both Delphi rounds are available in the appendix.
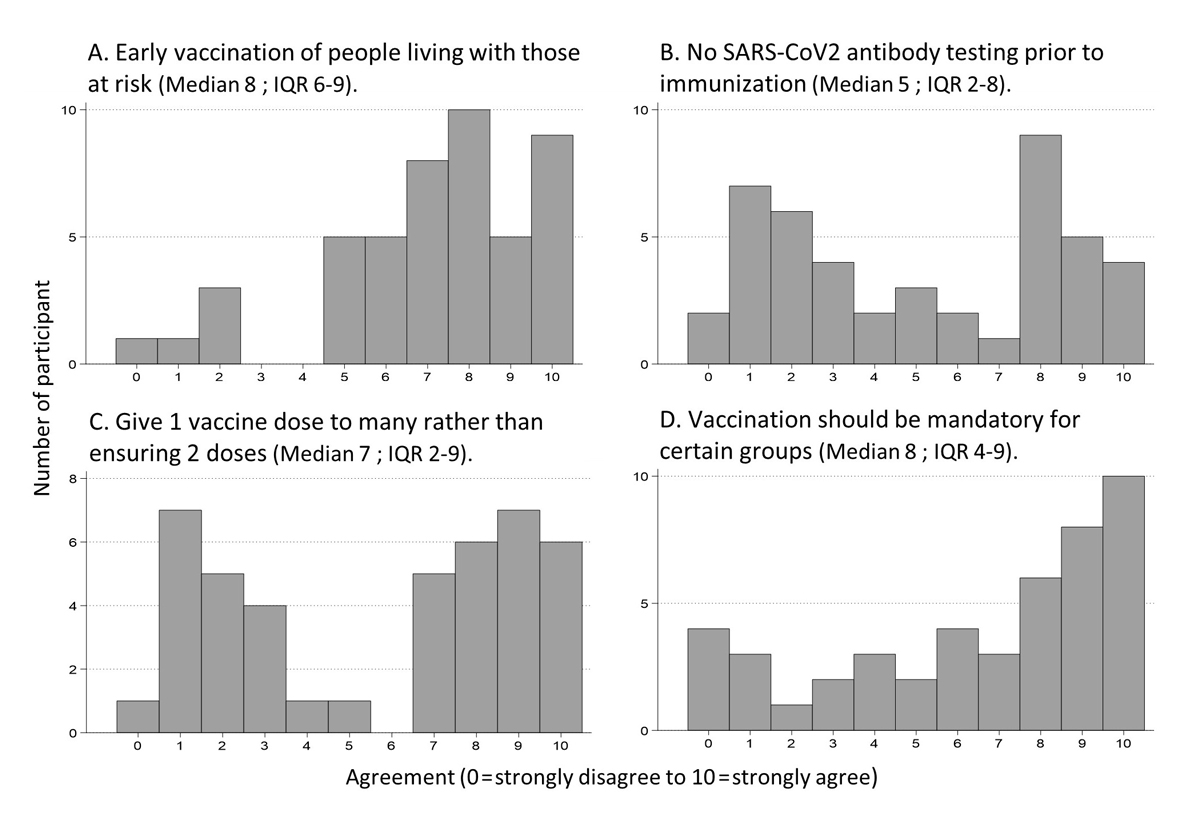
Figure 3 Distribution of responses for agreement with four selected statements not reaching consensus.
Discussion
Using a Delphi process, we reached consensus on several key aspects of COVID-19 vaccine deployment in a short time-frame, including desired vaccine characteristics, priority groups for early vaccination, approaches to subgroups such as children and pregnant women, and means of improving vaccine acceptability. Equally importantly, we identified relevant areas without consensus. Some approached consensus, such as prioritising early vaccination of individuals living with someone at high risk. Others revealed strong opposing views, such as whether vaccination should be mandatory for some groups such as health professionals. The Delphi process is an important means of advancing discourse on controversial, evolving topics.
Our primary results were published online on 18 November 2020 after two Delphi rounds [10]. We had met the predefined consensus criteria on several important points and an additional round would have delayed results beyond the time when they could still aid decision makers. The vaccination strategy of the FOPH and the Federal Commission for Vaccination was published on 16 December 2020 [11]. Their strategy overlapped with our results in most key areas. However, it prioritised vulnerable populations (≥65 years of age with risk factors and ≥75 years) above front-line healthcare workers, instead of considering them in parallel. It supported not pursuing early vaccination of children, adolescents and pregnant women. Important decisions were also made in areas where we did not find consensus. For instance, people who live with someone at high risk were prioritised above essential workers or adults <65 years living in community settings. Further, the chosen strategy did not include testing for SARS-CoV-2 antibodies; the decision was made not to vaccinate as many people as quickly as possible, but to conserve a sufficient number of vaccine doses to ensure that anyone receiving a first dose can get a second; and that vaccination should in no case be obligatory.
More recently, as of August 2021, just under half the Swiss population had been fully vaccinated and the rate of vaccine administration was slowing despite adequate vaccine supply [12]. There is now less focus on the fair allocation of vaccines and increasing pressure to use incentives to increase vaccination rates, especially with the threat of COVID-19 variants.
Several of our statements reaching consensus overlapped with international guidelines available at the time, including early vaccination of those ≥65 years with risk factors for severe COVID-19 and health professionals [6,7,13]. We further offered guidance for some specific subgroups, for example, to vaccinate immunosuppressed persons or pregnant women if sufficient safety data become available, but to delay vaccination of children and adolescents. Similar decisions were subsequently taken by the American Centers for Disease Control and several European countries [14]. Some areas where there was consensus in our study but not in other countries may reflect distinctly ‘Swiss’ values, underlining the importance of country-specific studies. For instance, there was consensus that non-replicating viral vector and mRNA vaccines should require additional, specific safety information before being given to pregnant women. Other countries, such as the United States, offered mRNA vaccines to pregnant women very early. Even 8 months later, in August 2021, written consent by the woman and her gynaecologist is required in Switzerland.
Lack of consensus in some areas in our study was sometimes reflected internationally by countries taking differing positions. For example, the United Kingdom chose to prioritise giving as many people as possible one vaccine dose [15], while the Food and Drug Administration in the USA clearly stipulated that two doses should be given at recommended intervals [16]. Sixteen European Union countries extended timing between doses [17]. One advantage of identifying statements not reaching consensus was to demonstrate areas where experts disagree, which could help the public to understand why government decisions change over time. Swiss decision makers made pragmatic decisions based on the currently available evidence, but evolving guidance can create confusion and frustration for the public.
Very large IQRs of certain statements, such as for the questions whether to use incentives or make vaccination mandatory (fig. 2), might be explained in different ways: some participants might not have been familiar with evidence in these areas; the scores might reflect differences in individual values, such as the tension between personal liberty and collective security; or there could be cultural differences between groups, such as by age or regions of Switzerland. All responses were fully anonymous, so we could not analyse potential differences between groups of experts.
Despite some limitations, the Delphi method proved useful in the context of planning COVID-19 vaccine deployment as a means of rapid, but transparent, synthesis of expert opinion. The Delphi method is limited by the time necessary to design, distribute and follow-up structured questionnaires, followed by analysis of responses and preparation of the next round [18]. However, these steps leave a trace, allowing interested parties to understand the diversity of opinions. We took steps to ensure full anonymity, allowing participants to express themselves fully. However, that prevented us from performing analyses linking responses to experts from certain specialities or regions of Switzerland. When using the Delphi methods for surveys in crisis contexts in the future, we suggest that researchers consider: (1) simultaneously involving non-experts, such as a citizen committee, to contrast their views with those of experts; and (2) using very short, frequent questionnaires giving automated real-time results, rather than longer, more formal questionnaires, to allow greater reactivity.
Strengths of our study included anonymous voting (without influence or judgement by others), rigorous criteria for consensus, and a diverse panel of experts. Weaknesses included convenience sampling, possibly having experts vote on questions outside their fields of expertise and limited generalisability to other countries and future decisions. A majority of our experts worked in healthcare and were of high socioeconomic status; they may have been more likely to vote for early vaccination of medical personnel than other groups. Some issues that occupied an important portion of our questionnaires, such as the minimum vaccine efficacy that would justify deployment, became quasi obsolete within a few weeks after obtaining our results. Further, we were unable to address issues that subsequently emerged, such as whether to mix different vaccines based on availability, or the use of vaccination certificates [17].
In conclusion, we rapidly generated a list of expert guidance statements, some with high levels of consensus allowing straightforward decisions, and others requiring ongoing expert and public dialogue. The Delphi method was a useful means of collecting information from a diverse panel of experts with a transparent trace of all opinions; other researchers may wish to use this technique when there is intense public scrutiny but evidence is lacking.
Kevin Selby, MD
Department of Ambulatory Care
Centre for Primary Care and Public Health (Unisanté)
Rue de Bugnon 44
CH-1011 Lausanne
kevin.selby[at]unisante.ch
References
1.
Anderson RM
,
Vegvari C
,
Truscott J
,
Collyer BS
. Challenges in creating herd immunity to SARS-CoV-2 infection by mass vaccination. Lancet. 2020 Nov;396(10263):1614–6. https://doi.org/10.1016/S0140-6736(20)32318-7
2.
Lee GM
,
Bell BP
,
Romero JR
. The Advisory Committee on Immunization Practices and Its Role in the Pandemic Vaccine Response. JAMA. 2020 Aug;324(6):546–7. https://doi.org/10.1001/jama.2020.13167
3.
National Academies of Sciences Engineering and Medicine
. Framework for Equitable Allocation of COVID-19 Vaccine. Washington, DC: The National Academies Press; 2020.
4.
World Health Organization
. Framework for decision-making: implementation of mass vaccination campaigns in the context of COVID-19. Geneva: World Health Organization;2020.
5
Food and Drug Administration
. Development and Licensure of Vaccines to Prevent COVID-19. Washington, D.C.: U.S. Department of Health and Human Services;June 2020.
6.
Haute Autorité de Santé
. Stratégie de vaccination contre le COVID-19. 2020; https://www.has-sante.fr/upload/docs/application/pdf/2020-07/rapport_strategie_vaccination_covid_19_vf.pdf. Accessed 2020 August 23.
7.
Kompetenznetz Public Health COVID-19
. Vaccination Policy. 2020; https://www.public-health-covid19.de/images/2020/Ergebnisse/PolicyBrief_vaccination_2020_final-1.pdf. Accessed 2020 September 3.
8.
World Health Organization
. WHO SAGE Roadmap for Prioritizing Uses of COVID-19 Vaccines in the Context of Limited Supply. 2020; https://www.who.int/publications/m/item/who-sage-roadmap-for-prioritizing-uses-of-covid-19-vaccines-in-the-context-of-limited-supply. Accessed 2020 November 26.
9.
Dalkey NC
. The Delphi Method: An Experimental Study of Group Opinion. RAND Corporation; 1969.
10. Covid-19 vaccine: experts submit their proposals for deployment in Switzerland [press release]. 2020; https://www.newsd.admin.ch/newsd/message/attachments/64534.pdf. Accessed 2020 December 12.
11. FOPH FOoPH. Vaccinations, Switzerland and Liechtenstein: Information on the current situation, as of 5 August 2021. COVID-19 2021; https://www.covid19.admin.ch/en/vaccination/doses. Accessed 2021 August 6.
12.
World Health Organization
. WHO SAGE values framework for the allocation and prioritization of COVID-19 vaccination. 2020; https://apps.who.int/iris/bitstream/handle/10665/334299/WHO-2019-nCoV-SAGE_Framework-Allocation_and_prioritization-2020.1-eng.pdf. Accessed 2020 21 September.
13.
European Center for Disease Prevention and Control
. https://www.ecdc.europa.eu/sites/default/files/documents/Overview-of-EU_EEA-UK-vaccination-deployment-plans.pdf. Stockholm: ECDC;2020.
14.
Iacobucci G
,
Mahase E
. Covid-19 vaccination: what’s the evidence for extending the dosing interval? BMJ. 2021 Jan;372(18):n18. https://doi.org/10.1136/bmj.n18
15.FDA Statement on Following the Authorized Dosing Schedules for COVID-19 Vaccines [press release]. FDA, 4 Jan 2021 2021.
16. Control; ECfDPa. Overview of the implementation of COVID-19 vaccination strategies and deployment plans in the EU/EEA – 6 May 2021. 2021; https://www.ecdc.europa.eu/en/publications-data/overview-implementation-covid-19-vaccination-strategies-and-vaccine-deployment#no-link. Accessed May 22, 2021.
17.
Goodman CM
. The Delphi technique: a critique. J Adv Nurs. 1987 Nov;12(6):729–34. https://doi.org/10.1111/j.1365-2648.1987.tb01376.x
Appendices
The appendices are available in the pdf version of the article.


