Post-COVID-19 bifacial weakness and paraesthesia: a case report
DOI: https://doi.org/10.4414/SMW.2021.w30066
Johann
Stubya, René
Rotha, Nico
Streckerb, Jonas
Teubnerb, Alain
Rudigera
aInternal Medicine, Limmattal Hospital Zurich, Schlieren, Switzerland
bNeurology, Limmattal Hospital Zurich, Schlieren, Switzerland
Summary
OBJECTIVES: We present a patient with bifacial weakness and paraesthesia subtype of Guillain-Barré syndrome (GBS), which occurred 1 month after a SARS-CoV-2 infection. While GBS as complication of SARS-CoV-2 infection has been described many times, only a few cases of post-COVID-19 bifacial weakness and paraesthesia are known to date.
RESULTS: A 59-year-old man presented with thoracoradicular pain, paraesthesias of hands and feet, as well as progressive bilateral facial palsy. Neurological examination revealed a hyporeflexia of his lower limbs and hypoaesthesia of his hands and feet. Clinical and electrophysiological findings as well as CSF analysis were consistent with bifacial weakness and paraesthesia. The patient’s condition improved promptly after 5 days of intravenous immunoglobulin therapy.
DISCUSSION: We suspect bifacial weakness and paraesthesia to be a possible post-infectious complication of COVID-19. Hence, it is a differential diagnosis of facial nerve palsy in association with SARS-CoV-2 infection. Considering the rarity of GBS and bifacial weakness and paraesthesia, it appears unlikely that bigger trials elucidating the causal relation between them and SARS-CoV-2 infection will be available in the future.
Introduction
Coronavirus disease 2019 (COVID-19), mainly a respiratory infection, can lead to long-term health issues even after primary recovery. Next to cardiovascular events, neurological complications add substantially to the morbidity and mortality. While GBS is a known complication of SARS-CoV-2 infection, only few cases of post COVID-19 bifacial weakness and paraesthesia (BFP) have been reported [1, 2]. We present a case of the BFP subtype of Guillain-Barré syndrome (GBS) that developed 1 month after infection with SARS-CoV-2.
Case description
A 59-year-old man presented to the emergency room with a 1--week history of progressive chest and back pain resistant to non-opioid and opioid analgesics. Other than hypothyroidism and hypercholesterolaemia, for which he was taking levothyroxine and ezetimibe, his medical history was unremarkable. The reported pain radiated symmetrically from the thoracic spine to the ribs along T3 to T6. Furthermore, he reported burning sensations in his hands and feet. One month earlier, he had been hospitalised for severe, but non-critical COVID-19 (according to World Health Organization Therapeutics and COVID-19: living guideline [3]). His symptoms had been fever, frailty, cough and dyspnoea. Signs of neurological involvement had not been present. He received supplemental oxygen delivered by nasal cannula, as well as intravenously administered remdesivir, amoxicillin/clavulanic acid and clarithromycin.
At the current presentation, neurological examination revealed hyporeflexia of his lower limbs as well as hypoaesthesia of his hands and feet. Laboratory tests (high-sensitive troponin T, creatine kinase) and an electrocardiogram excluded myocardial infarction. Computed tomography of his chest did not show any signs of pulmonary embolism, aortic dissection or Boerhaave's syndrome. Magnetic resonance imaging found no myelopathy, spinal stenosis or disc herniation (data not shown). Following the clinical suspicion of polyradiculoneuropathy, an electroneurogram was performed, which demonstrated signs of demyelination in the lower limbs (presence of complex A-waves in left tibial nerve, fig. 1).
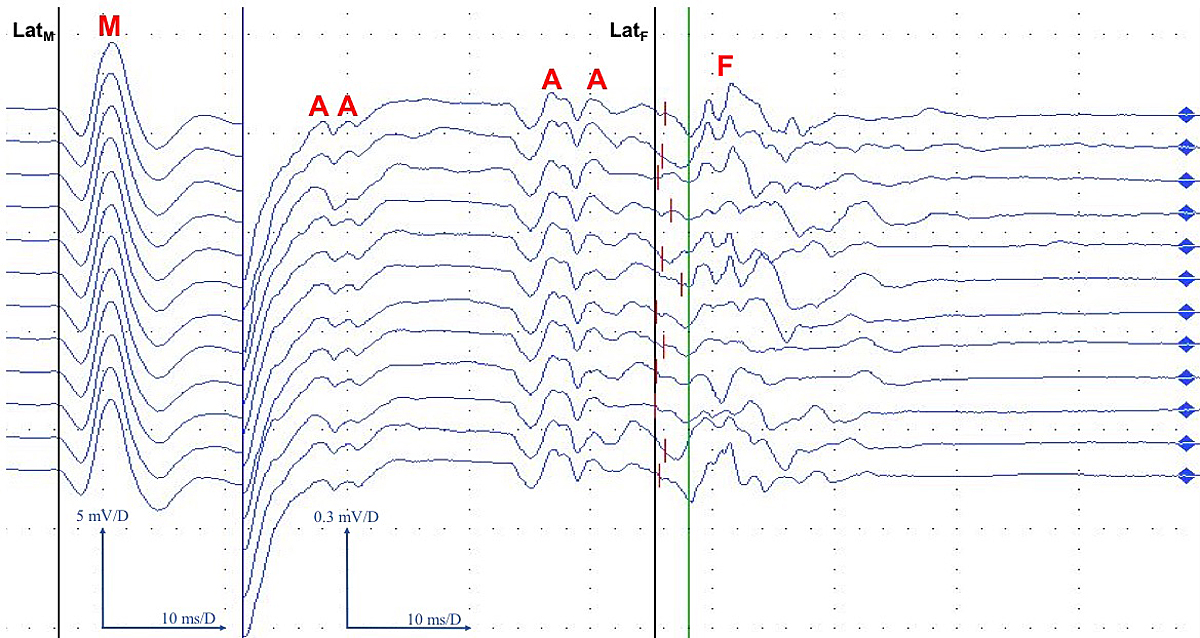
Figure 1 F-wave tracing of the left tibial nerve shows normal F-wave latencies but multiple A-waves indicating demyelination 10 days after symptom onset.
Black vertical line LatM: M response latency; M: (direct) motor response; A: A waves; black vertical line LatF: best F wave latency (55.3 ms); green vertical line: F wave latency cutoff (58 ms); F: F waves.
F waves are late responses on the electroneurogram evoked by an antidromic excitation of the alpha motor neuron (here: left tibial nerve, stimulated at the medial malleolus) towards the anterior horn and an orthodromic "backfiring" back down towards the target muscle (here: abductor hallucis brevis muscle). Characteristically, they are polymorphic and their latencies variable. F wave latency corresponds to the nerve conduction velocity over the whole peripheral motor neuron and thus yields additional information about the proximal nerve parts. Latencies can be calculated as absolute (time span between stimulus and best late response) or as difference between absolute latency and the latency of the direct motor response (F-M). Demyelinating processes can result in increased latencies or (partial) loss / reduced persistence of F wave responses (not the case here). Furthermore, demyelination can also cause pathological A waves to occur (as seen in figure 1), very monomorphic graphoelements localised between the M response and the F waves, which are thought to be caused (among other causes) by ephaptic activation from a neighbouring axon.
Serology for Borrelia burgdorferi (IgM, IgG), Treponema pallidum and human immunodeficiency virus (antibodies and p24 antigen) infection, as well as anti-ganglioside antibodies (anti-GM1 IgG, anti-GM2 IgG, anti-GD1a IgG, anti-GD1b, a-GQ1b IgG, anti-GM1 IgM, anti-GM2 IgM, anti-GD1a IgM, anti-GD1b IgM, anti-GQ1b IgM) were negative. Vitamin B12, folic acid, electrolytes, creatinine, liver function tests, glycated haemoglobin and thyroid stimulating hormone were within normal ranges (data not shown). Lumbar puncture for cerebrospinal fluid (CSF) analysis revealed albuminocytological dissociation with borderline pleocytosis of 5/μl (100% mononuclear) leucocytes (normal <5/ μl) and protein levels of 1901 mg/l (normal 150–450 mg/l). CSF polymerase chain reaction assays for herpes simplex virus type 1 and type 2 were negative (data not shown). On day four after admission, the patient complained of burning sensations of the tongue; later the same day he presented with a progressive bilateral facial palsy. Blink reflex demonstrated prolonged latencies of R1 of both eyes as well as prolonged latencies of ipsilateral R2 and contralateral R2 of the right eye and not measurable R2 latency of the left eye (fig. 2).
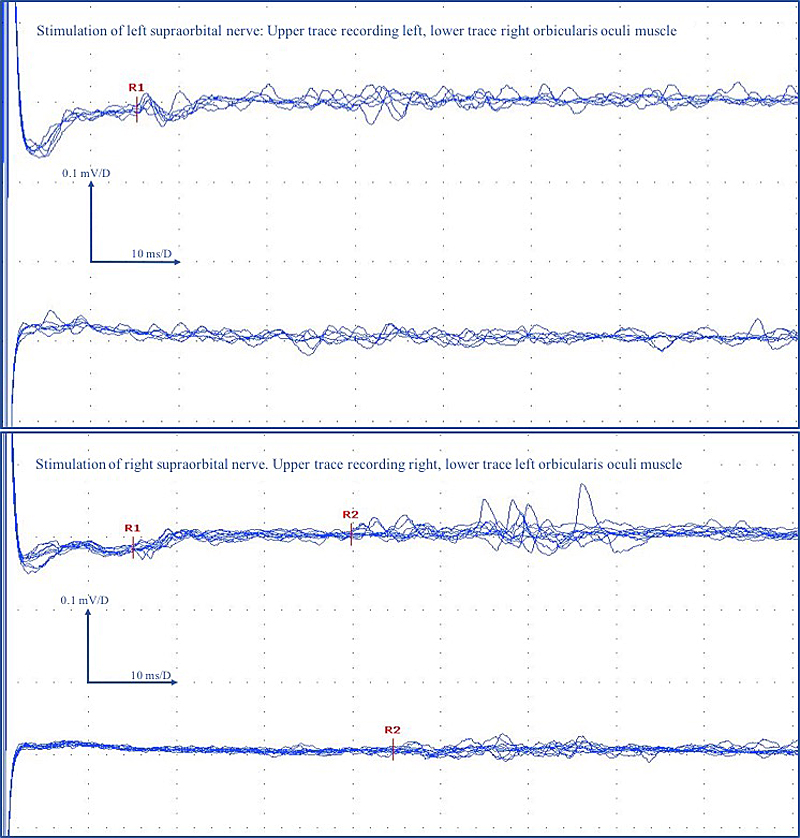
Figure 2 Asymmetric, bilateral pathological blink reflex suggestive of peripheral demyelination and partial interruption of the polysynaptic trigeminal-facial reflex.
Prolonged R1 latency on both sides (15.3 ms right, 15.1 ms left; normal <12.1 ms), prolonged R2 latencies on the right side (39.8 ms right, 44.6 ms left; normal <37.3 ms) and missing R2 response on the left side.
R1 is a response due to a disynaptic pathway between the ipsilateral principal sensory nucleus of trigeminal nerve and the ipsilateral facial motor nucleus. R2 is a response due to a multisynaptic pathway between the ipsilateral spinal trigeminal nucleus and interneurons to the ipsilateral and contralateral facial motor nucleus.
These findings were in line with a demyelinating process. Diagnosis of GBS subtype BFP was made. Paralysis of the extremities or respiratory muscles was not present at any time. An anti-inflammatory medication with intravenous immunoglobulins at a dose of 0.4g/kg for 5 days and analgesic treatment with pregabalin and amitriptyline was initiated. The patient’s condition improved promptly. At 1-month follow-up, only minor paraesthesia of hands and feet persisted, while bifacial weakness as well as chest and back pain had completely resolved. A follow-up ENG did not show significant differences. A visual representation of events can be found in figure 3.
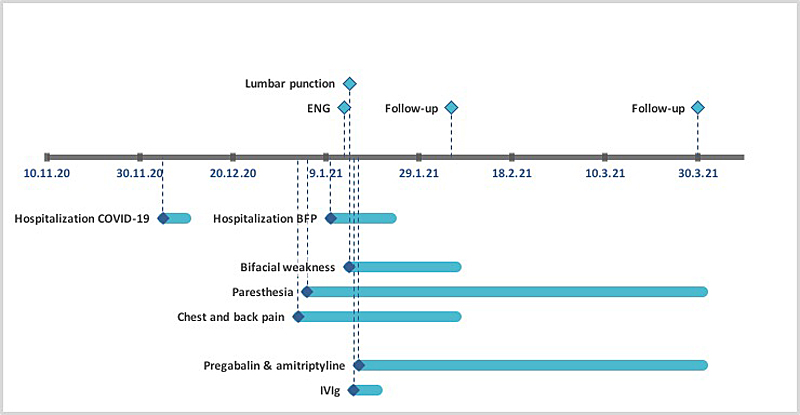
Figure 3 Case report timeline.
COVID-19: Coronavirus disease 2019; BFP: bifacial weakness and paresthesia; ENG: electroneurogram; IVIg: intravenous immunoglobulins
Discussion
We report a patient with thoracoradicular pain, paraesthesia of the hands and feet, as well as progressive bilateral facial palsy 1 month after COVID-19. According to the diagnostic criteria of BFP [4], clinical and electrophysiological findings together with CSF analysis were consistent with the bifacial weakness and paraesthesia subtype of Guillain-Barré syndrome.
Beside the classic sensorimotor form of GBS with ascending weakness, areflexia and sensory deficits, various subtypes such as Miller Fisher syndrome, Bickerstaff brainstem encephalitis, pharyngeal-cervical-brachial weakness and BFP are possible clinical manifestations. The incidence of bilateral facial palsy is about 1 per 5,000,000 population [5] and GBS is just one possibly aetiology, whereof BFP is a rare subtype. BFP is defined by rapidly progressive bilateral facial weakness, distal limb paraesthesia and hyporeflexia/areflexia, while other cranial neuropathies, ataxia, or limb weakness are absent [4]. In BFP, anti-ganglioside IgG antibodies are usually not present. Nevertheless, testing for anti-ganglioside antibodies can be helpful to exclude alternative diagnoses of facial weakness, such as Miller Fisher syndrome or pharyngeal-cervical-brachial weakness [4]. Given the lack of anti-ganglioside antibodies, ophthalmoparesis and ataxia in our patient, we suspected BFP and not the Miller Fisher subtype of GBS [6].
Viral (e.g., influenza A virus, cytomegalovirus, Epstein–Barr virus, MERS-CoV) and bacterial (e.g., Campylobacter jejuni, Mycoplasma pneumoniae, Haemophilus influenzae) infections are frequent antecedents of GBS [7, 8]. Due to molecular mimicry, post-infectious antibodies cross-react with neuronal antigens, causing demyelination and/or axonal damage [1]. Various case reports have been published hypothesising an induction of GBS by SARS-CoV-2 [9, 10]. Whether there is an increase of GBS incidence during COVID-19 pandemic is currently controversial [11–13]. Common neurological symptoms of COVID-19 such as hypogeusia and hyposmia seem to reflect a direct viral infiltration of the nervous system, whereas GBS is more likely to be triggered by autoimmunity as described for other pathogens above [8, 9]. In a systematic review of 73 cases, SARS-CoV-2 RNA in the CSF was absent in all tested patients [9]. This feature, together with the latency of neurological symptoms after COVID-19 (median 14 days) and clinical improvement after intravenous immunoglobulin therapy contribute to the theory of an immune-mediated mechanism of post COVID-19 GBS.
In the case by Hutchins et al. [1], a patient developed BFP in temporal relationship to antecedent SARS-CoV-2 infection (16 days after onset of COVID-19 symptomatology), but positive herpes simplex virus serology raised the question of causality. BFP has been described as a post infectious complication of COVID-19 in another case report [2], in which a pregnant woman with SARS-CoV-2 infection 6 weeks previously presented with rapidly progressive bilateral facial palsy, extremity paraesthesia, and right vestibulocochlear neuritis. In line with our findings, facial palsy rapidly resolved after intravenous immunoglobulin therapy, whereas paraesthesia was still present at 2-week follow-up.
Inherent to the clinical nature of a case report, we can only speculate about the causality of COVID-19 and BFP in our patient, and cannot provide evidence that SARS-CoV-2 was truly responsible. However, given that GBS is a known rare after-effect of viral infections, it seems highly plausible that SARS-CoV-2 can be a trigger. Other aetiologies such as drugs or subclinical diseases cannot be ruled out. Potential associations between an antibiotic therapy with fluoroquinolones or penicillin and the development of GBS have been described, but no definite cause-effect relationships have been established [14, 15].
Considering the rarity of GBS and especially the BFP subtype, it appears to be very unlikely that bigger trials elucidating the causal relation between BFP and COVID-19 will be available in the future. Hence advancing medical scientific knowledge through case reports seems the most appropriate way.
Conclusion
GBS and its subtype BFP are potential postinfectious immune-mediated complications of SARS-CoV-2 infection. Diagnosis is based on clinical features, electrophysiological assessment and CSF analysis. When it is treated with intravenous immunoglobulins, the outcome is favourable. Physicians must be aware of rare COVID-19-related immune-mediated disorders. BFP is an important differential diagnosis of facial nerve palsy in association with SARS-CoV-2 infection.
Availability of data and material: The data that support the findings of this study are available on request from the corresponding author JS.
Acknowledgements
Author contributions: JS, RR, NS and JT cared for the patient and managed the case. JS wrote the paper. All authors were involved in editing the manuscript.
Consent for publication: Written consent for publication was obtained from the patient.
Johann Stuby, MD
Internal Medicine
Limmattal Hospital
Urdorferstrasse 100
CH-8952 Schlieren
johannstuby[at]aol.com
References
1.
Hutchins KL
,
Jansen JH
,
Comer AD
,
Scheer RV
,
Zahn GS
,
Capps AE
, et al.
COVID-19–associated bifacial weakness with paresthesia subtype of Guillain-Barré syndrome. AJNR Am J Neuroradiol. 2020 Sep;41(9):1707–11. https://doi.org/10.3174/ajnr.A6654
2.
Aasfara J
,
Hajjij A
,
Bensouda H
,
Ouhabi H
,
Benariba F
. A unique association of bifacial weakness, paresthesia and vestibulocochlear neuritis as post-COVID-19 manifestation in pregnant women: a case report. Pan Afr Med J. 2021 Jan;38(30):30.
3.
World Health Organization
. Therapeutics and COVID-19: living guideline 6 July 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.2 (accessed on 2021 August 14) [
4.
Wakerley BR
,
Yuki N
. Isolated facial diplegia in Guillain-Barré syndrome: bifacial weakness with paresthesias. Muscle Nerve. 2015 Dec;52(6):927–32. https://doi.org/10.1002/mus.24887
5.
Kumar P
,
Charaniya R
,
Bahl A
,
Ghosh A
,
Dixit J
. Facial Diplegia with Paresthesia: An Uncommon Variant of Guillain-Barre Syndrome. J Clin Diagn Res. 2016 Jul;10(7):OD01–02. https://doi.org/10.7860/JCDR/2016/19951.8092
6.
Susuki K
,
Koga M
,
Hirata K
,
Isogai E
,
Yuki N
. A Guillain-Barré syndrome variant with prominent facial diplegia. J Neurol. 2009 Nov;256(11):1899–905. https://doi.org/10.1007/s00415-009-5254-8
7.
Jacobs BC
,
Rothbarth PH
,
van der Meché FG
,
Herbrink P
,
Schmitz PI
,
de Klerk MA
, et al.
The spectrum of antecedent infections in Guillain-Barré syndrome: a case-control study. Neurology. 1998 Oct;51(4):1110–5. https://doi.org/10.1212/WNL.51.4.1110
8.
Costello F
,
Dalakas MC
. Cranial neuropathies and COVID-19: neurotropism and autoimmunity. AAN Enterprises; 2020.
9.
Abu-Rumeileh S
,
Abdelhak A
,
Foschi M
,
Tumani H
,
Otto M
. Guillain–Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol. 2021;<span class="docsum-journal-citation full-journal-citation">268(4)</span>:1–38. https://doi.org/10.1007/s00415-020-10124-x
10.
Sriwastava S
,
Kataria S
,
Tandon M
,
Patel J
,
Patel R
,
Jowkar A
, et al.
Guillain Barré Syndrome and its variants as a manifestation of COVID-19: A systematic review of case reports and case series. J Neurol Sci. 2021 Jan;420:117263. https://doi.org/10.1016/j.jns.2020.117263
11.
Palaiodimou L
,
Stefanou MI
,
Katsanos AH
,
Fragkou PC
,
Papadopoulou M
,
Moschovos C
, et al.
Prevalence, clinical characteristics and outcomes of Guillain-Barré syndrome spectrum associated with COVID-19: A systematic review and meta-analysis. Eur J Neurol. 2021 Oct;28(10):3517–29. https://doi.org/10.1111/ene.14860
12.
Fragiel M
,
Miró Ò
,
Llorens P
,
Jiménez S
,
Piñera P
,
Burillo G
, et al.; SIESTA (Spanish Investigators in Emergency Situations Team) network
. Incidence, clinical, risk factors and outcomes of Guillain-Barré in Covid-19. Ann Neurol. 2021 Mar;89(3):598–603. https://doi.org/10.1002/ana.25987
13.
Keddie S
,
Pakpoor J
,
Mousele C
,
Pipis M
,
Machado PM
,
Foster M
, et al.
Epidemiological and cohort study finds no association between COVID-19 and Guillain-Barré syndrome. Brain. 2021 Mar;144(2):682–93. https://doi.org/10.1093/brain/awaa433
14.
Awong IE
,
Dandurand KR
,
Keeys CA
,
Maung-Gyi FA
. Drug-associated Guillain-Barré syndrome: a literature review. Ann Pharmacother. 1996 Feb;30(2):173–80. https://doi.org/10.1177/106002809603000212
15.
Ali AK
. Peripheral neuropathy and Guillain-Barré syndrome risks associated with exposure to systemic fluoroquinolones: a pharmacovigilance analysis. Ann Epidemiol. 2014 Apr;24(4):279–85. https://doi.org/10.1016/j.annepidem.2013.12.009


