The role of children and adolescents in the SARS-CoV-2 pandemic: a rapid review
DOI: https://doi.org/10.4414/SMW.2021.w30058
Margarethe
Wiedenmannab, Myrofora
Goutakic, Olivia
Keiserd, Silvia
Stringhinie, Marcel
Tannerabf, Nicola
Lowc
aSwiss Tropical and Public Health Institute, Basel, Switzerland
bUniversity of Basel, Switzerland
cInstitute of Social and Preventive Medicine, University of Bern, Switzerland
dUniversity of Geneva, Institute of Global Health, Geneva, Switzerland
eUniversity Hospital of Geneva, Switzerland
fSwiss Academies of Arts and Sciences
*These authors have contributed equally to this work and share last authorship.
Summary
BACKGROUND: There has been much discussion about coronavirus disease 2019 (COVID-19) and the virus that causes it, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in children and adolescents, since the pandemic was recognised in early 2020. Understanding their role in this pandemic is important for the development of appropriate prevention measures.
OBJECTIVE: To summarise evidence about three aspects of SARS-CoV-2 and COVID-19 in children and adolescents: (1) severity of SARS-CoV-2 presentation, (2) risk of SARS-CoV-2 infection and (3) risk of transmitting SARS-CoV-2.
METHODS: We searched PubMed and MedRxiv for studies on SARS-CoV-2 and COVID-19 in children and adolescents from January 2020 to 21 January 2021. The electronic search was supplemented by papers found in a manual search or suggested by experts up to 29 March 2021. We included case reports, cross-sectional studies, cohort studies, narrative reviews or viewpoints, systematic reviews and modelling studies. We synthesised the information descriptively and attempted to report findings separately for: infants and small children (0–5 years) who are mostly pre-school; school children (6–12 years) broadly covering primary school years; and adolescents (13–17 years).
RESULTS: Of 2778 screened articles, we included 63 (20 case reports, 18 cross-sectional studies, 8 cohort studies, 6 narrative reviews or viewpoints, 10 systematic reviews and 1 modelling study). Children (≤12 years of age) and adolescents (13–17 years of age) usually present with mild disease, with few requiring intensive care treatment. A minority of children of all ages (<18 years) remains asymptomatic throughout the course of infection. In serological studies, reported symptoms are similar in children with and without SARS-CoV-2 antibodies. Children and adolescents can acquire and transmit SARS-CoV-2. The risks of acquiring and transmitting SARS-CoV-2 seems to increase with age. There was limited information about SARS-CoV-2 variants of concern. Poor reporting of age groups and contextual factors such as levels of community transmission, school closures and other non-pharmaceutical interventions make synthesis of findings across studies difficult.
CONCLUSIONS: The clinical presentation and role of children and adolescents in SARS-CoV-2 susceptibility and transmission needs further investigation, particularly with regard to variants of concern. Large, prospective studies that attempt to minimise biases in design, are analysed appropriately and reported comprehensively should be conducted.
Introduction
There has been much discussion about coronavirus disease 2019 (COVID-19) and the virus that causes it, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in children and adolescents, since the pandemic was recognised in early 2020 [1–3]. Early in 2020, many countries imposed general lockdowns to limit the spread of the virus and to reduce the risk of collapse of their health systems, which included the closure of educational facilities (day care, schools and higher education institutions). The closures resulted in large numbers of children not attending school worldwide, with greater reported impacts on students from more disadvantaged socioeconomic backgrounds [4, 5].
The earliest reports of COVID-19 included small numbers of children and adolescents and commentaries suggested that children were “spared the major impact of COVID-19 virus” [6]. Their role in the transmission of SARS-CoV-2 was unclear, however, because children were tested less frequently than adults and differences in lockdown restrictions affect the contact patterns and exposure of children and adults differently [7]. In spring 2020, the Federal Office of Public Health asked the Swiss National COVID-19 Science Task Force to provide information about SARS-CoV-2 and COVID-19 in children and adolescents to inform the development of preventive measures. This request led to a rapid review of the literature, which has since been updated four times and published on the website of the Swiss National COVID-19 Science Task Force most recently on 28 April 2021 [8]. This article is based on the rapid review and aims to summarise evidence about three aspects of SARS-CoV-2 and COVID-19 in children and adolescents: (1) severity of SARS-CoV-2 presentation in children and adolescents; (2) risk of SARS-CoV-2 infection in children and adolescents; and (3) risk of children and adolescents transmitting SARS-CoV-2. In addition, we tried to identify studies about SARS-CoV-2 variants of concern, which were documented to have started to emerge in autumn 2020 [9].
Methods
This review began as a rapid review of literature selected from a systematic search, with updates conducted as the volume of publications increased. Owing to the urgent situation in early 2020, we did not write a protocol or register the review.
Search strategy
We searched for literature published since 1 January 2020 in two electronic databases (PubMed and MedRxiv). We conducted the first search on 1 May 2020 and the last search on 21 January 2021. We used the following combinations of search terms in PubMed for title and abstract: (COVID-19 OR "SARS-CoV-2" OR "novel coronavirus") AND ("attack rate" OR "secondary attack rate" OR "infectiousness" OR "transmission rate" OR "transmission") AND (children OR "pediatric patients"), and ("COVID-19" OR "SARS-CoV-2" OR "novel coronavirus") AND ("asymptomatic" OR "presymptomatic") AND ("attack rate" OR "secondary attack rate" OR "infectiousness" OR "transmission rate" OR "transmission" OR "viral shedding") and (COVID-19 OR "SARS-CoV-2" OR "novel coronavirus") AND ("attack rate" OR "secondary attack rate" OR "infectiousness" OR "transmission rate" OR "transmission") AND (children OR "pediatric patients") AND (kindergarten OR crèche OR creche OR preschool OR "elementary school" OR "secondary school" OR "high school"). In MedRxiv we searched separately for "COVID-19 and attack rate", "COVID-19 and infectiousness" and "COVID-19 and children". We supplemented the search results with a non-systematic search on 29 March 2021 of papers identified through general reading, in e-mail alerts, suggested by experts, or referenced in the aforementioned literature search. We searched, in particular, for studies that reported on variants of concern in children and adolescents. Only studies in German and English were included in the review.
Study selection
Case reports, cross-sectional studies, cohort studies, narrative and systematic reviews and modelling studies were eligible for inclusion. One reviewer (MW) screened the titles and abstracts of all identified articles and read the full text of all those potentially eligible. We did not include all identified articles that might have been relevant throughout the search period because of redundancy. For example, if many review articles reported on the same original studies, we include the more recent reviews.
Data extraction
MW extracted and recorded relevant scientific information from the selected studies. For each included study, MW and MG extracted the details of: author and publication year, country, study design, number and age range of included study participants, a summary of the study setting and, for review articles, the number of included studies.
Risk of bias
We did not use a specific tool for assessment of the risk of bias owing to the urgency of the situation at the start of the pandemic. We considered both study quality and the risk of possible biases when reading the studies included in this review, and reported these in the results section.
Data synthesis
We synthesised information from the included studies descriptively. We did not perform quantitative syntheses because, for the objectives of the review, we judged that the study designs, study settings and ages of participants would be too heterogeneous for pooling of results to be appropriate.
The findings for the three review questions might differ by age, so we tried to summarise the findings for different age categories when data were available, which would make practical sense in Switzerland. We defined these groups as: infants and small children (0–5 years) who are mostly pre-school; school children (6–12 years) broadly covering primary school years; and adolescents (13–17 years).
Results
In total, we screened 2778 studies published up to 21 January 2021, of which 29 were included. We included another 34 studies (23 published, 11 not published and/or not peer-reviewed) identified up to 29 March 2021. Of these 63 articles, there were 20 case reports, 18 cross-sectional studies, 8 cohort studies, 6 narrative reviews or viewpoints, 10 systematic reviews and 1 modelling study (fig. 1). We report findings from articles published up to 29 March 2021 in the results section. We summarise more recent developments in the discussion section.
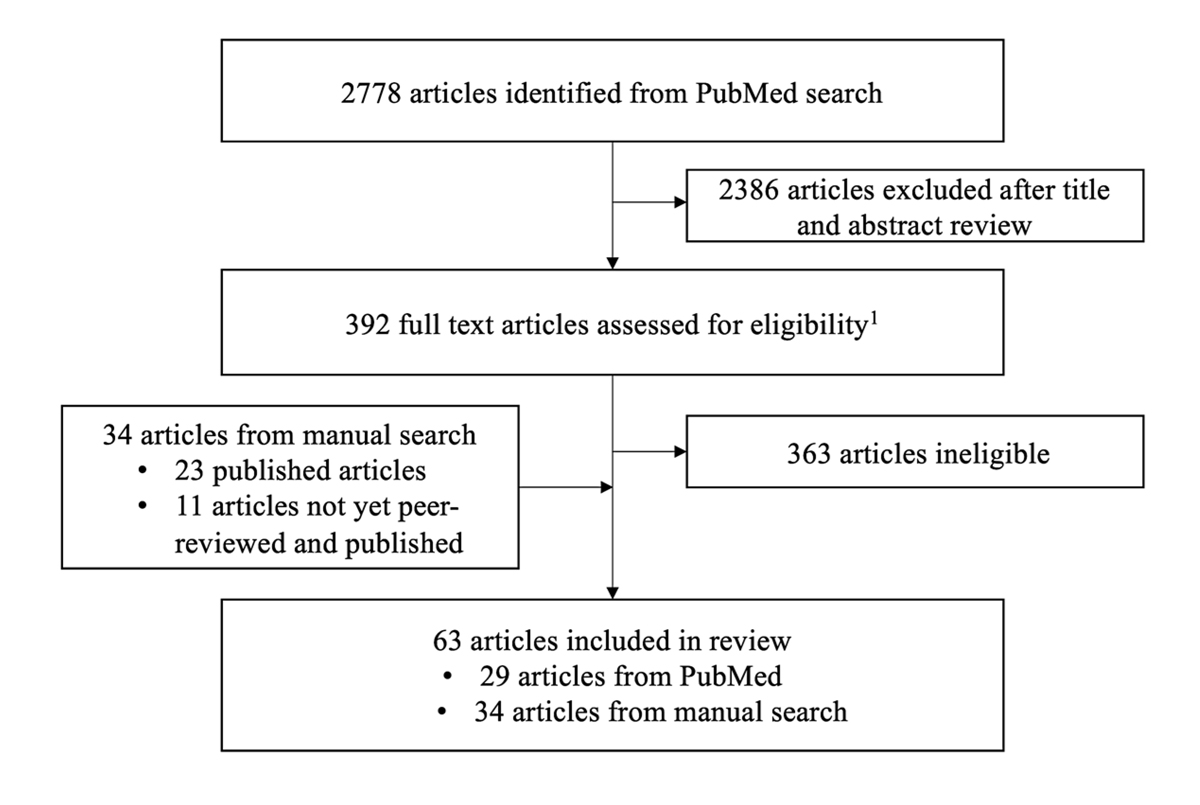
Figure 1 Study selection.
1 Eligibility: language (English, German); study design (case reports, cross-sectional studies, cohort studies, modelling studies, reviews/viewpoints; both preprints and peer-reviewed studies; study population (children and adolescents <18 years old); settings (households, schools / day care centres / camps, community)
Severity of SARS-CoV-2 presentation in children and adolescents
Paediatric SARS-CoV-2 cases were described from the early beginnings of the pandemic, but children (≤12 years of age) and adolescents (13–17 years of age) usually present with mild disease, with few requiring intensive care treatment [6, 10–15]. The reasons for the comparatively mild disease, as well as the risk factors for severe disease and potential mid-term to long-term effects on SARS-CoV-2 infection in children and partially in adolescents, are still being investigated.
Asymptomatic SARS-CoV-2 presentation in children and adolescents
An asymptomatic course of disease is relevant for public health if the infected people are infectious, go undetected and can transmit SARS-CoV-2. A systematic review of studies published up to 10 June 2020 provides information about the proportions of children of all ages, adolescents and adults who were followed through the course of infection. Most studies that distinguished participants by age were done in hospital settings, which might result in selection bias that underestimates the proportion of asymptomatic infections. The children in hospital-based studies were generally diagnosed during investigations of household contacts of confirmed cases and were hospitalised for observation. In both adults and children, the majority of individuals with SARS-CoV-2 developed symptomatic disease. The proportions with persistently asymptomatic infection were 27% in children (95% confidence interval [CI] 22–32%; 10 studies, 285 children) and 11% in adults (95% CI 6–19%; 10 studies, 3228 adults) [16]. Most studies in this review did not give detailed information about age, so it was not possible to examine differences between children of different age groups.
Asymptomatic infections can also be estimated in seroprevalence studies, although bias in recalling mild symptoms over periods of several months might result in under-recording and symptoms reported cannot be attributed to a particular infection episode. In the SEROCoV-POP study, which included randomly selected participants from a past cohort study and their household members in Geneva, Switzerland, 13% (77/590) of all participants with SARS-CoV-2 antibodies detected between 6 April and 30 June 2020 did not recall any SARS-CoV-2 symptoms since January 2020. When stratified by age, 44% (4/9) aged 4–9 years, 26% (13/50) aged 10–17 years and 10% (28/278) aged 18–49 years reported no symptoms [17]. With the exception of loss of taste or smell, symptoms were as common in seropositive as in seronegative children. In the Ciao Corona study in Zurich, a cohort study of 273 classes in 55 schools (n = 2585), 73% of all children (age range 6–16 years) experienced symptoms compatible with a SARS-CoV-2 infection between January and June 2020, with no difference between seropositive and seronegative children [18]. Between July and November 2020, when the study analysed 2831 children from 275 classes in 55 schools, only 29% (29/101) of seropositive children and 22% (420/1923) of seronegative children reported symptoms compatible with SARS-CoV-2 [19].
We did not find any studies reporting the frequency of asymptomatic paediatric SARS-CoV-2 infection caused by SARS-CoV-2 variants of concern.
Potential risk factors for severe SARS-CoV-2 disease in children and adolescents
Early reports suggested especially infants (≤1 year of age) and younger children (1–5 years of age) to be at a higher risk of severe disease than older children [13, 20]. These reports, however, have methodological limitations, such as some COVID-19 diagnoses being based on clinical symptoms alone without performing laboratory tests [13], or missing data on hospitalisation status [20]. Several narrative reviews, describing 32 different cases of neonatal SARS-CoV-2 infection in total, all show neonates mostly presenting with mild disease [21–25]. The respiratory symptoms described in some patients were generally consistent with their gestational age and could thus not be attributed to SARS-CoV-2. These findings are supported by small additional retrospective studies [26, 27] and a prospective national cohort study from the UK [28], describing mild SARS-CoV-2 disease progression in neonates. Multicentre hospital-based studies with more complete data and consistent case definitions have also been conducted. A prospective cohort study from 260 hospitals in the UK, following 651 children and young adults (225/651 <1 year old) between 17 January and 3 July 2020, found that the risk of admission to intensive care was associated with age <1 month (odds ratio [OR] 3.21, 95% CI1.36–7.66) and age 10–14 years (OR 3.23, 95% CI 1.55–6.99), compared with a baseline of 15–19 year olds [29]. Multiple small studies from around the world describe mild SARS-CoV-2 disease progression and low hospitalisation rates, even in children with severe underlying health conditions such as cancer and immunosuppression [30–36].
A hyperinflammatory syndrome in children with COVID-19, called paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in Europe and multisystem inflammatory syndrome in children (MIS-C) in the United States has been described as a serious, but rare consequence of SARS-CoV-2 infection. The first case of an infant presenting with classic Kawasaki disease who also tested positive for SARS-CoV-2 was reported in April 2020 [37]. Shortly thereafter, researchers in south-east England reported a cluster of eight children with features associated with Kawasaki disease or toxic shock syndromes, most of whom had a history of exposure to, or a positive test for SARS-CoV-2 [38]. In a report of 99 cases diagnosed with MIS-C from New York there were 31 in 0–5 year olds, 42 in 6–12 year olds and 26 in 13–20 year olds [39]. Presentation with any Kawasaki type symptoms was more common in those aged 0–12 years (31/73, 42%) than in those aged 13–20 years (3/26, 12%).
Potential mid-term and long-term effects of SARS-CoV-2 disease in children and adolescents
By the end of the search period, preliminary data from a small study in Italy found that children are at risk of long-term effects in the form of ongoing symptoms referred to as long COVID or post-acute sequelae of SARS-CoV-2 infection [40].
Risk of SARS-CoV-2 infection in children and adolescents
The risk of SARS-CoV-2 infection at different ages has been investigated in different study types. We describe the findings from population-based/cross-sectional studies, contact tracing/ household studies and seroprevalence studies separately because there are risks of bias associated with different study designs.
Population studies / cross-sectional studies
There were few population-based or cross-sectional studies available for analysis. An early study, conducted at the beginning of the COVID-19 pandemic in the town of Vo’ in Italy by Lavezzo et al. (2020) tested a majority of the population (85.9% at the first stage, 71.5% 14 days later) for SARS-CoV-2, irrespective of symptoms. There were no cases in 217 children aged 0–10 years and three cases in 250 11–20 year olds (1.2%), compared with 1.1% to 6.0% in adults in 10-year age bands [41]. This survey is at low risk of bias because nearly all residents were tested, there were no restrictions before the lockdown and children and adults lived in the same households. The COVID-19 Infection Survey (CIS), where randomly selected members of the British population aged 2 years and older submit repeated swabs for SARS-CoV-2 testing, reported greater increases in SARS-CoV-2 positivity in children aged 2 to 24 years than in older adults, coinciding with school reopenings in September 2020 [42].
Contact tracing / household studies
We identified three systematic reviews covering contact tracing / household studies. Viner et al. (2020) included 18 contact tracing studies in any setting, up to 28 July 2020. They found a lower risk of infection for younger children <10 years of age (OR 0.52, 95% CI 0.33–0.82) than for adults (reference group) but not for older children and adolescents >10 years of age (OR 0.72, 95% CI 0.46–1.10) [43]. Zhu et al. (2020) reported similar findings in 14 household studies up to 24 August 2020 (4 of which were included by Viner et al. (2020)). The risk of infection was lower among all <18-year-olds than in adults (relative risk [RR] 0.62, 95% CI 0.42–0.91) [44]. Madewell et al. (2020) analysed 15 household studies up to 19 October 2020 (7 of which were also included by Viner et al. (2020)). They compared the secondary attack rate in each study and found the summary proportion of participants with SARS-CoV-2 was higher in adult contacts (28.3%, 95% CI 20.2–37.1%) than in child and adolescent contacts of all ages (<18 years of age) (16.8%, 95% CI 12.3–21.7%) [45]. Most of the studies in these reviews were conducted during times of strict social distancing. There is a risk of bias in levels of exposure for adults and for children. Adults were at risk of infection outside their homes, for example, while shopping or at work, whilst children stayed mostly at home. Children were, however, likely in contact with adults within the families.
Some individual household studies (several of which are included in the cited systematic reviews) distinguished between narrower age groups. A cohort study analysing 392 household contacts of 105 index patients in China calculated secondary attack rates of 2.3% (1/44) for younger children (aged 0–5 years), 5.4% (3/56) for school children and adolescents (aged 6–17 years) and 17.1% (60/292) for adults [46). In Singapore, a household study described 213 children in 134 households, of whom 13 became infected with SARS-CoV-2: 0–4 years old, 1.3% (1/77); 5–9 years, 8.1% (6/68); and 10–16 years, 9.8% (6/55) [47]. In households in Israel with large numbers of children, older children and adolescents (aged 5–17 years) were found to be 61% less likely to be infected with SARS-CoV-2 than adults, with the risk for younger children 0–4 years of age being 47% lower than the risk for adults [48].
Seroprevalence studies
In a nationally representative seroprevalence survey performed during a strict national lockdown in Spain (April-May 2020), seroprevalence measured by immunoassay and point-of-care tests was lowest in the youngest age groups (both tests positive: <1 year: 0%, 95% CI 0.0–11.9%; 1–4 years: 2.6%, 95% CI 1.2–5.5% vs e.g., 20–24 years: 3.7%, 95% CI 2.7–4.9%) [49]. The SEROCoV-POP seroprevalence study in Geneva, (>5 years old) in April-May 2020 found a lower risk of antibody positivity for children <10 years (RR 0.32, 95% CI 0.11–0.65) than for adults >20 years of age. Seroprevalence among children aged 10–19 years was similar to that of >20 year olds (risk ratio 0.86, 95%-CI: 0.57–1.22) [50]. As this was a retrospective study that was conducted when Swiss schools were closed, individual sources of infection were not analysed and children might have been less exposed to SARS-CoV-2 than adult household members. Another survey round was done during a regional lockdown after the second peak of the pandemic in November-December 2020. Seroprevalence became similar for school children and adolescents (6–11 year olds: 22.8%, 95% CI 18.7–27.1%; 12–17 year olds: 23.6%, 95% CI 19.6–28.0%) and adults (25–34 years: 25.9%, 95% CI 21.8–30.2%) but was lower for children aged 0–5 years (14.9%, 95% CI 10.7–19.6%) [51].
In a study in Germany, volunteers were invited through newspaper and social media adverts and antibody levels were measured in 5042 parent-child pairs in April-May 2020 during a strict lockdown [52]. A higher proportion of parents (1.8%, 95% CI 1.3–2.4%) than children (1–5 years, 0.6%, 95% CI 0.3–1.3%) had SARS-CoV-2 antibodies. In Chile, a school outbreak of 52 reverse transcription polymerase chain reaction (RT-PCR) test positive cases was analysed and seroprevalence was measured in a random sample of students and staff 8–10 weeks after the outbreak. The index case was a staff member in the pre-school department. The authors thought it likely that the staff member infected both pre-school children at school and parents and colleagues during parent-teacher meetings. They found 16.6% (95% CI 12.1–21.9%) of staff had antibodies. A higher percentage of younger children (pre-school, 12.3%, 95% CI 7.8–18.6%) than high school students (5.7%, 95% CI 3.6–8.9%) had antibodies [53].
Risk of children and adolescents transmitting SARS-CoV-2
We found a range of relevant study designs, including laboratory-based studies, seroprevalence studies and secondary attack rate studies in households or schools/day care centres. We also analysed studies specifically looking into the paediatric transmission potential of SARS-CoV-2 variants of concern.
Laboratory-based studies measuring SARS-CoV-2
Infectious SARS-CoV-2 can be isolated from children as young as 7 days old. In Geneva, SARS-CoV-2 was successfully cultured in samples taken from 12 out of 23 children aged <16 years [54]. The same group found comparable viral load levels (inferred from cycle threshold values in PCR analyses) by age group in laboratory samples taken within 5 days of symptom onset and tested between March and May 2020 (53/405 aged <16 years) [55]. In a large laboratory-based study in Germany, researchers analysed cycle threshold values from PCR tests and found an association between increasing age and increasing viral load on one system (Roche Cobas system) and a negative association on the other (Roche LC480 system) [56]. An independent statistical analysis of the same data found that viral load increased with age [57]. A major limitation of studies based on collections of samples in diagnostic laboratories is that inclusion in a study depends on having been tested. Since testing for SARS-CoV-2 concentrates on detection of infection in people with symptoms, children who are included in these sample collections are unlikely to be representative of all those infected (selection bias).
Seroprevalence studies
A longitudinal seroprevalence study in schools in Zurich, Switzerland followed children aged 6–16 years in 55 schools and 273 classes in June/July and 275 October/November during a period when hygienic measures were in place (e.g., mask wearing for teachers and students >12 years of age). In seven classes in five schools ≥3 students had SARS-CoV-2 antibodies in periods of moderate to high community transmission when seroprevalence itself increased from 2.4% to 4.5% in schools [19]. It is not known whether the students were infected as part of an outbreak or were sporadic cases, nor whether the infections were acquired or transmitted in the school.
Secondary attack rate studies in households or schools / day care centres
A meta-analysis analysing household clusters up to 24 August 2020 reported that a child or an adolescent (<18 years) was identified as the first case in 4% (8/13) of these clusters. The paediatric index cases also led to fewer secondary cases than adult index cases (4% vs 96% of 398 secondary cases). Acknowledging the potential undercounting of children as index cases, the authors included asymptomatic children as possible index cases and the proportion of clusters with an index case <18 years increased to 19% (30/211), and the proportion of secondary cases increased to 22% (80/395 contacts) [44]. A regularly updated review that includes studies with contact tracing data up to 21 January 2021 reported very limited transmission from younger children in school settings, but found potential outbreaks in school children and adolescents (without further clarification of the respective age groups), especially during times of higher community transmission [1].
Several studies have described clusters of SARS-CoV-2 involving schools and school camps. A SARS-CoV-2 outbreak was described in a school in northern France in early 2020. In a serosurvey in one high school seroprevalence was higher in adolescents (82/205, 40% in 15–17 year olds) than adults (88/415, 21% in >18 years olds) [58]. As neither the sampling methodology is known nor were the supposed index cases laboratory-confirmed SARS-CoV-2 cases, the extent of spread in the school compared with outside is not known. Further studies describe clusters of cases in overnight camps / retreats in the US and a school in Israel [59-61]. Pray et al. (2020) [60] and Stein-Zamir et al. (2020) (61] describe outbreaks in high schools (ages 12 and older), whereas the camp outbreak described by Szabwlewski et al. (2020) [59] included children and adolescents aged 6–17 years and found similar attack rates in 6–10 year olds (51/100, 51%) and 11–17 year olds (180/409, 44%).
Other studies from schools have found limited transmission when community transmission was comparably low even in secondary education facilities. Potential protective measures in most reports were not described. Three school settings in which no child contact became infected were described in Singapore: 0/8 symptomatic contacts of an infected 12 year old; 0/34 symptomatic contacts of an infected 5 year old; and 0/77 preschool pupils where a total of 16 staff members were infected [62]. In Australia, 18 index cases (9 students and 9 teachers) in 15 different schools (primary and high schools) had close contact with 735 fellow students and 128 staff. None of the 128 staff contracted SARS-CoV-2 and the two initial cases (one student and one staff) only infected two fellow students (one in a high school and one in a primary school) [63]. In Ireland, six index cases were described in schools before pre-emptive school closures during the 2020 spring wave. Three students aged 10–15 years and three adults did not infect any of the 1001 child contacts tested (924 school contacts, 77 non-school-related contacts) including those with only mild symptoms [64]. In Germany, 137 index cases aged <19 years were described from May to August 2020. From more than 2300 contacts, 11 infections in classmates were found (3 in childcare facilities, 1 in elementary school, 4 in secondary school and 3 in vocational school). None of these secondary cases infected any other students. There were several hygienic control measures in place (e.g., reduced class size, exclusion of sick children) [65]. A low risk of infection from adult and paediatric index cases was also seen in childcare settings in Rhode Island, USA and in South Korea [66, 67].
A study from England [68] reported on 177 COVID-19-associated events in educational settings (preschool, primary and secondary) involving 130 confirmed cases in children and 213 in staff members. Most outbreaks (defined as ≥2 linked cases from the same education facility in ≤14 days, including the index case) were small (median number of cases 2, interquartile range 2–5). The maximum size of outbreaks and thus the number of secondary cases was lower when the index case was a child (median 1, maximum 6) than when the index case was a staff member (median 1, maximum 12). Despite low case numbers there was a strong association with regional incidence of reported COVID-19; for every 5 new cases per 100,000 inhabitants the risk of an outbreak increased by 72% (95% CI 28–130%). In most outbreaks, a staff member was the index case (staff to staff in 26/55 outbreaks, staff to student in 8/55 outbreaks, student to staff in 16/55 outbreaks and student to student in 5/55 outbreaks). The same pattern of index cases was seen in Georgia, USA, where nine clusters were described in elementary schools from December 2020 to January 2021. Of these nine clusters four were started by teachers, one by a student and four were unknown [69].
Studies on the paediatric transmission risks of new SARS-CoV-2 variants
The British Children’s Task and Finish Group describes an increase in infection prevalence between September and October 2020, which was most marked in the 16–24 year olds followed by 12–16 year olds [42]. The School Infection Survey conducted in November 2020 found that, despite more testing, the level of infection in schools was low even in times of high community transmission (students: 1.24%, 95% CI 0.96–1.58%; staff: 1.29%, 95% CI 0.96–1.68%) [42]. This report was written before the increased transmissibility of the alpha-variant (B.1.1.7) was confirmed and other variants such as the delta-variant (B.1.617.2) were first described as variants of concern. A modelling study predicted that under 20 year olds could have been affected more by this new SARS-CoV-2 variant than by the previous variants, but this could have resulted from increased transmission when schools were open during a lockdown period, from increased susceptibility or from increased symptoms leading to more testing [70]. We did not find any data regarding the paediatric transmission potential of other SARS-CoV-2 variants.
Discussion
This review summarises evidence from 63 studies about the potential role of children (≤12 years of age) and adolescents (13–17 years of age) in the SARS-CoV-2 pandemic. We included 63 studies, most of which were from high-income countries and were published between March 2020 and April 2021.
Strengths and limitations of the review
We summarised the literature with a broad remit, addressing both clinical and epidemiological aspects of SARS-CoV-2 infection in children and adolescents. This review was performed to provide timely evidence for policymakers in Switzerland. Owing to the time-sensitive nature of the review, we did not plan to conduct a full systematic review, so there is no written protocol or registration. There are limitations to this kind of rapid review, but we did try to reduce some of the risks of bias in traditional narrative reviews. First, we used the same systematic search up to 21 January 2021 and searched two databases to include studies published both in peer-reviewed journals and as preprints to obtain new information with a wide range of designs as soon as it became available. Second, although we did not use a published tool to assess the risk of bias in all studies, we report on methodological limitations of studies in the text, and the potential consequences for the findings. Third, we report transparently the information sources, search dates and methodology. To include more recent data, we performed a non-systematic literature search until 29 March 2021. By ending the search at 29 March 2021, the review covers studies that were done before the circulation of SARS-CoV-2 variants of concern was widely recognised. We might have missed potentially relevant literature because we only searched two databases with a comparatively basic search strategy, only one author assessed the results of the electronic search and we included only studies in English or German. However, we sought a range of expert opinions about studies to include and discussed the content and interpretation at length. We tried to describe findings in different age groups of children, but many studies did not provide enough information for a finely stratified analysis.
Interpretation
Severity of SARS-CoV-2 presentation in children and adolescents. Children and adolescents usually present with milder symptoms of SARS-CoV-2 infection than adults and are more likely to have an asymptomatic SARS-CoV-2 infection. A majority of children and adolescents in both hospital-based studies of acute infection and population-based seroprevalence studies developed symptoms of COVID-19 [16, 17]. Severe disease was uncommon in children and adolescents [6, 10–15], even amongst those with predisposing conditions such as immunosuppression or cancer [30–36]. Paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) / multisystem inflammatory syndrome in children (MIS-C) is one specific manifestation [37–39]. Our search period identified only one study of long-term symptoms after SARS-CoV-2 infection [40]. Further studies are being published, however, with researchers from Switzerland and from the UK describing a small proportion of children experiencing symptoms lasting more than 2 to 3 months [71, 72].
Risk of SARS-CoV-2 infection in children and adolescents. The risk of becoming infected with SARS-CoV-2 is a combination of susceptibility (host biological factors), external factors associated with exposure type (work, shopping, schools, etc.) and exposure intensity (level of community transmission and of preventive measures). Triangulation of evidence across study designs provides valuable information but needs to take into account differences between countries. In population-based, cross-sectional and seroprevalence studies, fewer children than adults were infected. In the first pandemic wave, when strict lockdowns were imposed [41, 42], findings might reflect, in part, children’s exposure limited to the household while parents had more contacts outside. In studies in which all household members were exposed to an infected person, the proportion becoming infected with SARS-CoV-2 also increases with age [43–47]. Household members might have more similar levels of exposure, but there are still differences in intensity of exposure, with spouses likely being more exposed to an infected adult than children [2]. As children have fewer and milder symptoms than adults, they might be less likely to be diagnosed as the index case, thus limiting the potential interpretation of this data.
Risk of children and adolescents transmitting SARS-CoV-2. Studies analysing the secondary attack rate show that children can transmit SARS-CoV-2 in households, schools and day care centres [1, 44, 58–61]. Amongst diagnosed individuals tested at the same time point after symptom onset, estimated SARS-CoV-2 viral load, in one study, was similar in children, adolescents and adults [55]. Transmission is, however, also influenced by physiological features such as lung capacity, hindering direct comparisons. As with susceptibility to infection, the relative transmissibility of SARS-CoV-2 at different ages remains uncertain, largely because of biases in the ascertainment of the challenges involved in disentangling the influences of biological, host and environmental factors [7].
The circulation of SARS-CoV-2 variants of concern, with the alpha variant becoming dominant in Switzerland in April 2021, and then being rapidly replaced by the delta variant, may change epidemiology of the infection in children and adolescents. Studies done during periods when SARS-CoV-2 variants of concern are circulating will provide new information. Studies in outbreak settings can compare the risks of infection with, and transmission of, coronavirus for children and adolescents with those of adults [73]. Large prospective longitudinal studies, with the same methodology over time, will be needed to determine whether differences between variants affect children, adolescents and adults differently.
Conclusion
Children are susceptible to SARS-CoV-2 infection and can transmit the coronavirus. Susceptibility to, and transmissibility of SARS-CoV-2 appear to be lower in young children than in adolescents and adults. Precise levels and the observed magnitude of age-related differences depend on more than biological factors, including the level of community transmission, between age-group differences in exposure and the stringency of non-pharmaceutical measures. In laboratory-based and epidemiological studies, researchers should report these factors as part of the context in which the study was done, so that the findings can be interpreted more easily. Large, prospective studies that attempt to minimise biases in design, are analysed appropriately, and are reported comprehensively should be conducted.
New studies about age-related differences in severity, susceptibility to, and transmissibility of, SARS-CoV-2 variants of concern in children and adolescents are needed. These studies are particularly important as children are a largely unvaccinated part of the population. Because of the socioeconomic costs resulting from this lack of information, more testing in schools and measures that will reduce the risk of SARS-CoV-2 transmission in schools should be considered. Research will allow monitoring of the incidence of SARS-CoV-2 infection among children in the community, including in the presence of new variants, describe long-term consequences of infection at the population level, and follow-up of vaccination uptake and effectiveness to minimise harms.
Recommendations for future research
In order to improve the significance of future studies we recommend considering the following aspects to analyse the role children and adolescents play in this pandemic:
- We recommend selecting the appropriate study design for the respective research question, for example, perform population-based studies instead of hospital-based studies to analyse the frequency of asymptomatic infection.
- We recommend studies differentiating between different paediatric age groups.
- We recommend performing prospective, longitudinal studies instead of retrospective studies (e.g., seroprevalence studies).
- We recommend performing outbreak studies based on thorough contact tracing, i.e. to trace all contacts, even those outside of the respective educational facilities or households, to properly identify the sources of infection.
Acknowledgments
This work is based on a policy brief by the public health expert group of the Swiss National COVID-19 Science Task Force [4]. All authors are/were members of the Swiss National COVID-19 Science Task Force. We would like to thank all the current and former members of the public health expert group, who contributed to multiple discussions about this document: Milo Puhan, Stefan Wolter, Suzanne Suggs, Valérie D’Acremont, Antoine Flahault, Nicole Probst-Hensch and Michael Simon. We also received very valuable input from other expert groups from the Swiss National COVID-19 Science Task Force and would like to especially thank Roman Stocker for his valuable contribution.
Author contributions: MW performed the literature search, study selection and data collection and wrote the first draft of the manuscript. MG contributed to the extraction of data. MW, MG, OK, SS, NL and MT all discussed the interpretation of the findings, reviewed and revised the manuscript with important intellectual contributions and approved the final version.
Nicola Low
Professor of Epidemiology and Public Health
Institute of Social and Preventive Medicine
University of Bern
Mittelstrasse 43
CH-3012 Bern
nicola.low[at]ispm.unibe.ch
References
1.
Boast A
,
Munro A
,
Goldstein H
. An evidence summary of Paediatric COVID-19 literature. Don't Forget the Bubbles. 2020.
2.
Goldstein E
,
Lipsitch M
,
Cevik M
. On the Effect of Age on the Transmission of SARS-CoV-2 in Households, Schools, and the Community. J Infect Dis. 2021 Feb;223(3):362–9. https://doi.org/10.1093/infdis/jiaa691
3.
Hyde Z
. COVID-19, children and schools: overlooked and at risk. Med J Aust. 2020 Nov;213(10):444–446.e1. https://doi.org/10.5694/mja2.50823
4.
Van Lancker W
,
Parolin Z
. COVID-19, school closures, and child poverty: a social crisis in the making. Lancet Public Health. 2020 May;5(5):e243–4. https://doi.org/10.1016/S2468-2667(20)30084-0
5.
Silverman M
,
Sibbald R
,
Stranges S
. Ethics of COVID-19-related school closures. Can J Public Health. 2020 Aug;111(4):462–5. https://doi.org/10.17269/s41997-020-00396-1
6.
Morand A
,
Fabre A
,
Minodier P
,
Boutin A
,
Vanel N
,
Bosdure E
, et al.
COVID-19 virus and children: what do we know? Arch Pediatr. 2020 Apr;27(3):117–8. https://doi.org/10.1016/j.arcped.2020.03.001
7.
Accorsi EK
,
Qiu X
,
Rumpler E
,
Kennedy-Shaffer L
,
Kahn R
,
Joshi K
, et al.
How to detect and reduce potential sources of biases in studies of SARS-CoV-2 and COVID-19. Eur J Epidemiol. 2021 Feb;36(2):179–96. https://doi.org/10.1007/s10654-021-00727-7
8
Swiss National COVID-19 Science Task Force
. The role of children (≤12 years of age) and adolescents (13-17 years of age) in the SARS-CoV-2 pandemic: A rapid review. 2021.
9.
World Health Organization
. COVID-19 Weekly Epidemiological Update. 2021;Edition 41.
10.
Liu W
,
Zhang Q
,
Chen J
,
Xiang R
,
Song H
,
Shu S
, et al.
Detection of Covid-19 in Children in Early January 2020 in Wuhan, China. N Engl J Med. 2020 Apr;382(14):1370–1. https://doi.org/10.1056/NEJMc2003717
11.
Mustafa NM
,
A Selim L
. Characterisation of COVID-19 Pandemic in Paediatric Age Group: A Systematic Review and Meta-Analysis. J Clin Virol. 2020 Jul;128:104395. https://doi.org/10.1016/j.jcv.2020.104395
12.
Lu X
,
Zhang L
,
Du H
,
Zhang J
,
Li YY
,
Qu J
, et al.; Chinese Pediatric Novel Coronavirus Study Team
. SARS-CoV-2 Infection in Children. N Engl J Med. 2020 Apr;382(17):1663–5. https://doi.org/10.1056/NEJMc2005073
13.
Dong Y
,
Mo X
,
Hu Y
,
Qi X
,
Jiang F
,
Jiang Z
, et al.
Epidemiology of COVID-19 Among Children in China. Pediatrics. 2020 Jun;145(6):e20200702. https://doi.org/10.1542/peds.2020-0702
14.
Castagnoli R
,
Votto M
,
Licari A
,
Brambilla I
,
Bruno R
,
Perlini S
, et al.
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in Children and Adolescents: A Systematic Review. JAMA Pediatr. 2020 Sep;174(9):882–9. https://doi.org/10.1001/jamapediatrics.2020.1467
15.
Hoang A
,
Chorath K
,
Moreira A
,
Evans M
,
Burmeister-Morton F
,
Burmeister F
, et al.
COVID-19 in 7780 pediatric patients: A systematic review. EClinicalMedicine. 2020 Jun;24:100433. https://doi.org/10.1016/j.eclinm.2020.100433
16.
Buitrago-Garcia D
,
Egli-Gany D
,
Counotte MJ
,
Hossmann S
,
Imeri H
,
Ipekci AM
, et al.
Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: A living systematic review and meta-analysis. PLoS Med. 2020 Sep;17(9):e1003346. https://doi.org/10.1371/journal.pmed.1003346
17.
Richard A
,
Wisniak A
,
Perez-Saez J
,
Garrison-Desany H
,
Petrovic D
,
Piumatti G
, et al.
Seroprevalence of anti-SARS-CoV-2 IgG antibodies, risk factors for infection and associated symptoms in Geneva, Switzerland: a population-based study. medRxiv. 2020.
18.
Ulyte A
,
Radtke T
,
Abela IA
,
Haile SR
,
Blankenberger J
,
Jung R
, et al.
Variation in SARS-CoV-2 seroprevalence across districts, schools and classes: baseline measurements from a cohort of primary and secondary school children in Switzerland. BMJ Open. 2021 Jul;11(7):e047483. https://doi.org/10.1136/bmjopen-2020-047483
19.
Ulyte A
,
Radtke T
,
Abela IA
,
Haile SR
,
Berger C
,
Huber M
, et al.
Clustering and longitudinal change in SARS-CoV-2 seroprevalence in school children in the canton of Zurich, Switzerland: prospective cohort study of 55 schools. BMJ. 2021 Mar;372(616):n616. https://doi.org/10.1136/bmj.n616
20.
Bialek S
,
Gierke R
,
Hughes M
,
McNamara LA
,
Pilishvili T
,
Skoff T
; CDC COVID-19 Response Team
. Coronavirus Disease 2019 in Children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020 Apr;69(14):422–6. https://doi.org/10.15585/mmwr.mm6914e4
21.
Gordon M
,
Kagalwala T
,
Rezk K
,
Rawlingson C
,
Ahmed MI
,
Guleri A
. Rapid systematic review of neonatal COVID-19 including a case of presumed vertical transmission. BMJ Paediatr Open. 2020 May;4(1):e000718. https://doi.org/10.1136/bmjpo-2020-000718
22.
Dumpa V
,
Kamity R
,
Vinci AN
,
Noyola E
,
Noor A
. Neonatal Coronavirus 2019 (COVID-19) Infection: A Case Report and Review of Literature. Cureus. 2020 May;12(5):e8165.
23.
Sheth S
,
Shah N
,
Bhandari V
. Outcomes in COVID-19 Positive Neonates and Possibility of Viral Vertical Transmission: A Narrative Review. Am J Perinatol. 2020 Oct;37(12):1208–16. https://doi.org/10.1055/s-0040-1714719
24.
Kyle MH
,
Glassman ME
,
Khan A
,
Fernández CR
,
Hanft E
,
Emeruwa UN
, et al.
A review of newborn outcomes during the COVID-19 pandemic. Semin Perinatol. 2020 Nov;44(7):151286. https://doi.org/10.1016/j.semperi.2020.151286
25.
Vardhelli V
,
Pandita A
,
Pillai A
,
Badatya SK
. Perinatal COVID-19: review of current evidence and practical approach towards prevention and management. Eur J Pediatr. 2021 Apr;180(4):1009–31. https://doi.org/10.1007/s00431-020-03866-3
26.
Wei M
,
Yuan J
,
Liu Y
,
Fu T
,
Yu X
,
Zhang ZJ
. Novel Coronavirus Infection in Hospitalized Infants Under 1 Year of Age in China. JAMA. 2020 Apr;323(13):1313–4. https://doi.org/10.1001/jama.2020.2131
27.
Zhang ZJ
,
Yu XJ
,
Fu T
,
Liu Y
,
Jiang Y
,
Yang BX
, et al.
Novel coronavirus infection in newborn babies aged <28 days in China. Eur Respir J. 2020 Jun;55(6):2000697. https://doi.org/10.1183/13993003.00697-2020
28.
Gale C
,
Quigley MA
,
Placzek A
,
Knight M
,
Ladhani S
,
Draper ES
, et al.
Characteristics and outcomes of neonatal SARS-CoV-2 infection in the UK: a prospective national cohort study using active surveillance. Lancet Child Adolesc Health. 2021 Feb;5(2):113–21. https://doi.org/10.1016/S2352-4642(20)30342-4
29.
Swann OV
,
Holden KA
,
Turtle L
,
Pollock L
,
Fairfield CJ
,
Drake TM
, et al.; ISARIC4C Investigators
. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020 Aug;370:m3249. https://doi.org/10.1136/bmj.m3249
30.
Balduzzi A
,
Brivio E
,
Rovelli A
,
Rizzari C
,
Gasperini S
,
Melzi ML
, et al.
Lessons after the early management of the COVID-19 outbreak in a pediatric transplant and hemato-oncology center embedded within a COVID-19 dedicated hospital in Lombardia, Italy. Estote parati. Bone Marrow Transplant. 2020 Oct;55(10):1900–5. https://doi.org/10.1038/s41409-020-0895-4
31.
Ferrari A
,
Zecca M
,
Rizzari C
,
Porta F
,
Provenzi M
,
Marinoni M
, et al.
Children with cancer in the time of COVID-19: An 8-week report from the six pediatric onco-hematology centers in Lombardia, Italy. Pediatr Blood Cancer. 2020 Aug;67(8):e28410. https://doi.org/10.1002/pbc.28410
32.
Marlais M
,
Wlodkowski T
,
Vivarelli M
,
Pape L
,
Tönshoff B
,
Schaefer F
, et al.
The severity of COVID-19 in children on immunosuppressive medication. Lancet Child Adolesc Health. 2020 Jul;4(7):e17–8. https://doi.org/10.1016/S2352-4642(20)30145-0
33.
Boulad F
,
Kamboj M
,
Bouvier N
,
Mauguen A
,
Kung AL
. COVID-19 in Children With Cancer in New York City. JAMA Oncol. 2020 Sep;6(9):1459–60. https://doi.org/10.1001/jamaoncol.2020.2028
34.
Hrusak O
,
Kalina T
,
Wolf J
,
Balduzzi A
,
Provenzi M
,
Rizzari C
, et al.
Flash survey on severe acute respiratory syndrome coronavirus-2 infections in paediatric patients on anticancer treatment. Eur J Cancer. 2020 Jun;132:11–6. https://doi.org/10.1016/j.ejca.2020.03.021
35.
Minotti C
,
Tirelli F
,
Barbieri E
,
Giaquinto C
,
Donà D
. How is immunosuppressive status affecting children and adults in SARS-CoV-2 infection? A systematic review. J Infect. 2020 Jul;81(1):e61–6. https://doi.org/10.1016/j.jinf.2020.04.026
36.
Rawson A
,
Wilson AC
,
Schwaderer AL
,
Spiwak E
,
Johnston B
,
Anderson S
, et al.
Coronavirus disease 2019 (COVID-19) in two pediatric patients with kidney disease on chronic immunosuppression: A case series. Hemodial Int. 2021 Jan;25(1):E1–5. https://doi.org/10.1111/hdi.12876
37.
Jones VG
,
Mills M
,
Suarez D
,
Hogan CA
,
Yeh D
,
Segal JB
, et al.
COVID-19 and Kawasaki Disease: Novel Virus and Novel Case. Hosp Pediatr. 2020 Jun;10(6):537–40. https://doi.org/10.1542/hpeds.2020-0123
38.
Riphagen S
,
Gomez X
,
Gonzalez-Martinez C
,
Wilkinson N
,
Theocharis P
. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020 May;395(10237):1607–8. https://doi.org/10.1016/S0140-6736(20)31094-1
39.
Dufort EM
,
Koumans EH
,
Chow EJ
,
Rosenthal EM
,
Muse A
,
Rowlands J
, et al.; New York State and Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome in Children Investigation Team
. Multisystem Inflammatory Syndrome in Children in New York State. N Engl J Med. 2020 Jul;383(4):347–58. https://doi.org/10.1056/NEJMoa2021756
40.
Buonsenso D
,
Munblit D
,
De Rose C
,
Sinatti D
,
Ricchiuto A
,
Carfi A
, et al.
Preliminary evidence on long COVID in children. Acta Paediatr. 2021 Jul;110(7):2208–11. https://doi.org/10.1111/apa.15870
41.
Lavezzo E
,
Franchin E
,
Ciavarella C
,
Cuomo-Dannenburg G
,
Barzon L
,
Del Vecchio C
, et al.; Imperial College COVID-19 Response Team; Imperial College COVID-19 Response Team
. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature. 2020 Aug;584(7821):425–9. https://doi.org/10.1038/s41586-020-2488-1
42
Children’s Task and Finish Group. Children’s Task and Finish Group
: update to 4th Nov 2020 paper on children, schools and transmission. 2020.
43.
Viner RM
,
Mytton OT
,
Bonell C
,
Melendez-Torres GJ
,
Ward J
,
Hudson L
, et al.
Susceptibility to SARS-CoV-2 Infection Among Children and Adolescents Compared With Adults: A Systematic Review and Meta-analysis. JAMA Pediatr. 2021 Feb;175(2):143–56. https://doi.org/10.1001/jamapediatrics.2020.4573
44.
Zhu Y
,
Bloxham CJ
,
Hulme KD
,
Sinclair JE
,
Tong ZW
,
Steele LE
, et al.
A meta-analysis on the role of children in SARS-CoV-2 in household transmission clusters. Clin Infect Dis. 2020.
45.
Madewell ZJ
,
Yang Y
,
Longini IM Jr
,
Halloran ME
,
Dean NE
. Household Transmission of SARS-CoV-2: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020 Dec;3(12):e2031756. https://doi.org/10.1001/jamanetworkopen.2020.31756
46.
Li W
,
Zhang B
,
Lu J
,
Liu S
,
Chang Z
,
Peng C
, et al.
Characteristics of Household Transmission of COVID-19. Clin Infect Dis. 2020 Nov;71(8):1943–6. https://doi.org/10.1093/cid/ciaa450
47.
Yung CF
,
Kam KQ
,
Chong CY
,
Nadua KD
,
Li J
,
Tan NW
, et al.
Household Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 from Adults to Children. J Pediatr. 2020 Oct;225:249–51. https://doi.org/10.1016/j.jpeds.2020.07.009
48.
Somekh E
,
Gleyzer A
,
Heller E
,
Lopian M
,
Kashani-Ligumski L
,
Czeiger S
, et al.
The Role of Children in the Dynamics of Intra Family Coronavirus 2019 Spread in Densely Populated Area. Pediatr Infect Dis J. 2020 Aug;39(8):e202–4. https://doi.org/10.1097/INF.0000000000002783
49.
Pollán M
,
Pérez-Gómez B
,
Pastor-Barriuso R
,
Oteo J
,
Hernán MA
,
Pérez-Olmeda M
, et al.; ENE-COVID Study Group
. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020 Aug;396(10250):535–44. https://doi.org/10.1016/S0140-6736(20)31483-5
50.
Stringhini S
,
Wisniak A
,
Piumatti G
,
Azman AS
,
Lauer SA
,
Baysson H
, et al.
Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020 Aug;396(10247):313–9. https://doi.org/10.1016/S0140-6736(20)31304-0
51.
Stringhini S
,
Zaballa ME
,
Perez-Saez J
,
Pullen N
,
de Mestral C
,
Picazio A
, et al.; Specchio-COVID19 Study Group
. Seroprevalence of anti-SARS-CoV-2 antibodies after the second pandemic peak. Lancet Infect Dis. 2021 May;21(5):600–1. https://doi.org/10.1016/S1473-3099(21)00054-2
52
Debatin K-M
,
Henneke P
,
Hoffmann GF
,
Kräusslich H-G
. Prevalence of COVID-19 in children in Baden-Württemberg - Preliminary study report. 2020.
53.
Torres JP
,
Pinera C
,
De La Maza V
,
Lagomarcino AJ
,
Simian D
,
Torres B
, et al.
SARS-CoV-2 antibody prevalence in blood in a large school community subject to a Covid-19 outbreak: a cross-sectional study. Clin Infect Dis. 2021;73(2):e458–65. https://doi.org/10.1093/cid/ciaa955
54.
L’Huillier AG
,
Torriani G
,
Pigny F
,
Kaiser L
,
Eckerle I
. Culture-Competent SARS-CoV-2 in Nasopharynx of Symptomatic Neonates, Children, and Adolescents. Emerg Infect Dis. 2020 Oct;26(10):2494–7. https://doi.org/10.3201/eid2610.202403
55.
Baggio S
,
L’Huillier AG
,
Yerly S
,
Bellon M
,
Wagner N
,
Rohr M
, et al.
SARS-CoV-2 viral load in the upper respiratory tract of children and adults with early acute COVID-19. Clin Infect Dis. 2020.
56.
Jones TC
,
Mühlemann B
,
Veith T
,
Biele G
,
Zuchowski M
,
Hofmann J
, et al.
An analysis of SARS-CoV-2 viral load by patient age. medRxiv. 2020:2020.06.08.20125484. https://doi.org/10.1101/2020.06.08.20125484
57
Held L.
A discussion and reanalysis of the results reported in Jones et al. (2020). 2020.
58.
Fontanet A
,
Tondeur L
,
Madec Y
,
Grant R
,
Besombes C
,
Jolly N
, et al.
Cluster of COVID-19 in northern France: A retrospective closed cohort study. medRxiv. 2020:2020.04.18.20071134.
59.
Szablewski CM
,
Chang KT
,
Brown MM
,
Chu VT
,
Yousaf AR
,
Anyalechi N
, et al.
SARS-CoV-2 Transmission and Infection Among Attendees of an Overnight Camp - Georgia, June 2020. MMWR Morb Mortal Wkly Rep. 2020 Aug;69(31):1023–5. https://doi.org/10.15585/mmwr.mm6931e1
60.
Pray IW
,
Gibbons-Burgener SN
,
Rosenberg AZ
,
Cole D
,
Borenstein S
,
Bateman A
, et al.
COVID-19 Outbreak at an Overnight Summer School Retreat - Wisconsin, July-August 2020. MMWR Morb Mortal Wkly Rep. 2020 Oct;69(43):1600–4. https://doi.org/10.15585/mmwr.mm6943a4
61.
Stein-Zamir C
,
Abramson N
,
Shoob H
,
Libal E
,
Bitan M
,
Cardash T
, et al.
A large COVID-19 outbreak in a high school 10 days after schools’ reopening, Israel, May 2020. Euro Surveill. 2020 Jul;25(29). https://doi.org/10.2807/1560-7917.ES.2020.25.29.2001352
62.
Yung CF
,
Kam KQ
,
Nadua KD
,
Chong CY
,
Tan NW
,
Li J
, et al.
Novel Coronavirus 2019 Transmission Risk in Educational Settings. Clin Infect Dis. 2021 Mar;72(6):1055–8. https://doi.org/10.1093/cid/ciaa794
63
The National Centre for Immunisation Research and Surveillance (NCIRS)
. COVID-19 in schools – the experience in NSW. 2020.
64.
Heavey L
,
Casey G
,
Kelly C
,
Kelly D
,
McDarby G
. No evidence of secondary transmission of COVID-19 from children attending school in Ireland, 2020. Euro Surveill. 2020 May;25(21). https://doi.org/10.2807/1560-7917.ES.2020.25.21.2000903
65.
Ehrhardt J
,
Ekinci A
,
Krehl H
,
Meincke M
,
Finci I
,
Klein J
, et al.
Transmission of SARS-CoV-2 in children aged 0 to 19 years in childcare facilities and schools after their reopening in May 2020, Baden-Württemberg, Germany. Euro Surveill. 2020 Sep;25(36). https://doi.org/10.2807/1560-7917.ES.2020.25.36.2001587
66.
Link-Gelles R
,
DellaGrotta AL
,
Molina C
,
Clyne A
,
Campagna K
,
Lanzieri TM
, et al.
Limited Secondary Transmission of SARS-CoV-2 in Child Care Programs - Rhode Island, June 1-July 31, 2020. MMWR Morb Mortal Wkly Rep. 2020 Aug;69(34):1170–2. https://doi.org/10.15585/mmwr.mm6934e2
67.
Yoon Y
,
Choi GJ
,
Kim JY
,
Kim KR
,
Park H
,
Chun JK
, et al.
Childcare Exposure to Severe Acute Respiratory Syndrome Coronavirus 2 for 4-Year-Old Presymptomatic Child, South Korea. Emerg Infect Dis. 2021 Feb;27(2):341–7. https://doi.org/10.3201/eid2702.203189
68.
Ismail SA
,
Saliba V
,
Lopez Bernal J
,
Ramsay ME
,
Ladhani SN
. SARS-CoV-2 infection and transmission in educational settings: a prospective, cross-sectional analysis of infection clusters and outbreaks in England. Lancet Infect Dis. 2021 Mar;21(3):344–53. https://doi.org/10.1016/S1473-3099(20)30882-3
69.
Gold JA
,
Gettings JR
,
Kimball A
,
Franklin R
,
Rivera G
,
Morris E
, et al.; Georgia K–12 School COVID-19 Investigation Team
. Clusters of SARS-CoV-2 Infection Among Elementary School Educators and Students in One School District - Georgia, December 2020-January 2021. MMWR Morb Mortal Wkly Rep. 2021 Feb;70(8):289–92. https://doi.org/10.15585/mmwr.mm7008e4
70.
Volz E
,
Mishra S
,
Chand M
,
Barrett JC
,
Johnson R
,
Geidelberg L
, et al.
Transmission of SARS-CoV-2 Lineage B.1.1.7 in England: Insights from linking epidemiological and genetic data. medRxiv. 2021:2020.12.30.20249034.
71.
Radtke T
,
Ulyte A
,
Puhan MA
,
Kriemler S
. Long-term Symptoms After SARS-CoV-2 Infection in Children and Adolescents. JAMA. 2021 Jul. https://doi.org/10.1001/jama.2021.11880
72.
Molteni E
,
Sudre CH
,
Canas LS
,
Bhopal SS
,
Hughes RC
,
Antonelli M
, et al.
Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2. Lancet Child Adolesc Health. 2021 Aug;S2352-4642(21)00198-X.
73.
Loenenbach A
,
Markus I
,
Lehfeld AS
,
An der Heiden M
,
Haas W
,
Kiegele M
, et al.
SARS-CoV-2 variant B.1.1.7 susceptibility and infectiousness of children and adults deduced from investigations of childcare centre outbreaks, Germany, 2021. Euro Surveill. 2021 May;26(21). https://doi.org/10.2807/1560-7917.ES.2021.26.21.2100433
Appendix
Table S1Summary of characteristics of studies included in the review.
|
Author
|
Study type
|
Country
|
Number of study participants
|
Age range of included participants
|
Number of studies (in reviews)
|
Comments
|
| Baggio, S. et al. (2020) |
Cohort study |
Switzerland |
53 <16 y old, 352 ≥16 y old |
0-82y |
n/a |
Laboratory-based study among symptomatic children and adults |
| Balduzzi, A. et al. (2020) |
Case report |
Italy |
5 |
not reported |
n/a |
Observational report in a paediatric haemato-oncology department |
| Boast, A. et al. (2021) |
Systematic review |
n/a |
n/a |
n/a |
not reported |
Rapid literature review of paediatric literature |
| Boulad, F. et al. (2020) |
Cross-sectional study |
USA |
20 children, 13 caregivers |
not reported |
n/a |
Observational study in a paediatric hemato-oncology department |
| Buitrago-Garcia, D. et al. (2020) |
Systematic Review |
n/a |
n/a |
n/a |
94 |
Living systematic review and meta-analysis on asymptomatic/presymptomatic infections |
| Buonsenso, D. et al. (2020) |
Cross-sectional study |
Italy |
129 children |
mean 11 ± 4.4y |
n/a |
Observational study in children with confirmed SARS-CoV-2 infection |
| Castagnoli, R. et al. (2020) |
Systematic Review |
n/a |
n/a |
n/a |
18 |
Systematic review of studies on children with confirmed SARS-CoV-2 infection |
| CDC Covid-Response Team (2020) |
Cross-sectional study |
USA |
2572 |
0-17y |
n/a |
National observational study in children with SARS-CoV-2 infection |
| Children’s Task and Finish Group (2020) |
Narrative Review/Viewpoint |
UK |
n/a |
n/a |
n/a |
Review of available data on children, schools and transmission in UK |
| Debatin, K.-M. et al. (2020) |
Cross-sectional study |
Germany |
2466 children, 2466 corresponding parents |
0-10y for children |
n/a |
Seroprevalence study in children and parents (interim analysis) |
| Dong, Y. et al. (2020) |
Cross-sectional study |
China |
2135 |
n/a |
n/a |
Nationwide observational study of confirmed and suspected paediatric cases |
| Dufort, E. M. et al. (2020) |
Case report |
USA |
99 |
0-20y |
n/a |
Observational report of confirmed and suspected paediatric cases |
| Dumpa, V. et al. (2020) |
Narrative Review/Case report |
n/a |
n/a |
n/a |
8 |
Case report and narrative review on SARS-CoV-2 infection in neonates |
| Ehrhardt, J. et al. (2020) |
Cross-sectional study |
Germany |
137 index cases, >2300 contacts |
0-19y |
n/a |
Observational study in SARS-CoV-2 infected children attending daycare/school and their contacts to evaluate transmission |
| Ferrari, A. et al. (2020) |
Case report |
Italy |
21 |
0-18y |
n/a |
Observational report in a paediatric haemato-oncology departments |
| Fontanet, A. et al. (2020) |
Cohort study |
France |
326 adolescents and school staff, 345 parents and siblings |
not reported |
n/a |
Retrospective cohort of high-school students, their families and school staff (COVID19 cluster) |
| Gale, C. et al. (2020) |
Cohort study |
UK |
66 |
0-30 days |
n/a |
National observational study in neonates with SARS-CoV-2 infection |
| Gold, J. A. W. et al. (2020) |
Case report |
USA |
32 children, 13 educators |
not reported |
n/a |
Observational report of SARS-CoV-2 infections in students and teachers (COVID19 clusters) |
| Gordon, M. et al. (2020) |
Systematic Review |
n/a |
n/a |
n/a |
8 |
Rapid literature review of neonatal SARS-CoV-2 infections |
| Heavey, L. et al. (2020) |
Case report |
Ireland |
6 index cases (3 children and 3 staff members), 1025 contacts |
not reported |
n/a |
Observational study in SARS-CoV-2 infected children attending school and school staff to evaluate transmission |
| Held, L. (2020) |
Narrative Review/Viewpoint |
n/a |
n/a |
n/a |
n/a |
Reanalysis of data presented in ref Jones, TC et al (2020) |
| Hoang, A. et al. (2020) |
Systematic Review |
n/a |
7780 children |
n/a |
131 |
Systematic review of studies on children with confirmed SARS-CoV-2 infection |
| Hrusak, O. et al. (2020) |
Case report |
25 countries |
200 children |
not reported |
n/a |
Multinational survey in a paediatric hemato-oncology departments |
| Ismail, S. A. et al. (2020) |
Cohort study |
UK |
928 000 (median student number) |
0-18y |
n/a |
Observational study of SARS-CoV-2 infection clusters and outbreaks in schools |
| Jones, T.C. et al. (2020) |
Case report |
Germany |
5524 children tested |
0-19y |
n/a |
Laboratory study of routine testing samples for SARDCoV2 |
| Jones, V.G. et al. (2020) |
Case report |
USA |
1 |
0.5y |
n/a |
Case report describing Kawasaki-like syndrome in an infant |
| Kyle, M.H. et al. (2020) |
Narrative Review/Viewpoint |
n/a |
35 |
newborns |
17 |
Review of studies reporting on children born by mothers with SARS-CoV-2 infection |
| L‘Huillier, A.G. et al. (2020) |
Case report |
Switzerland |
23 |
median 2y (IQR 3.8–14.5y) |
n/a |
Laboratory based study on children infected by SARS-CoV-2 |
| Lavezzo, E. et al. (2020) |
Cross-sectional study |
Italy |
234 children 0-10 y, 28907 overall |
all age groups |
n/a |
Population based study |
| Li, W.B. et al. (2020) |
Case report |
China |
105 index cases, 392 household contacts |
median 51y (IQR 39-60y) |
n/a |
Observational study to evaluate household transmission |
| Link-Gelles, R. et al. (2020) |
Cohort study |
USA |
30 pediatric cases, 22 adult cases |
not reported |
n/a |
Statewide observational program in child care programs to identify SARS-CoV-2 infections |
| Liu, W. et al. (2020) |
Cross-sectional study |
China |
366 |
0-16y |
n/a |
Observational report in children with SARS-CoV-2 infection |
| Lu, X. et al. (2020) |
Case report |
China |
171 |
0-16y |
n/a |
Observational report in children with SARS-CoV-2 infection |
| Madewell et al. (2020) |
Systematic Review |
n/a |
77758 |
Children, <18 y Adults, ≥18y |
54 |
Systematic review of studies on household transmission |
| Marlais et al. (2020) |
Cross-sectional study |
11 countries |
18 |
6-14y |
n/a |
Observational study in immunosuppressed children with SARS-CoV-2 infections |
| Minotti et al. (2020) |
Systematic Review |
n/a |
4 children, 106 adults |
not reported |
16 |
Systematic review of studies in immunosuppressed children and adults with SARS-CoV-2 infection |
| Morand et al. (2020) |
Narrative review/viewpoint |
n/a |
n/a |
n/a |
n/a |
Narrative review about SARS-CoV-2 infection in children |
| Mustafa, N. M. et al. (2020) |
Systematic review |
n/a |
n/a |
n/a |
17 |
Systematic review of studies describing clinical picture and transmission of SARS-CoV-2 in children |
| National Centre for Immunisation Research and Surveillance (NCIRS) |
Case report |
Australia |
863 |
not reported |
n/a |
Observational study evaluating transmission between SARS-CoV-2 infected school children and staff and their contacts |
| Pollan, M. et al. (2020) |
Cross-sectional study |
Spain |
6627 children, 45331 adults |
all age groups |
n/a |
Nationwide population based seroprevalence study |
| Pray, I. W. et al. (2020) |
Cross-sectional study |
USA |
127 children and 25 staff members |
14-45y |
n/a |
Observational study about a summer school outbreak |
| Rawson, A. et al. (2020) |
Case report |
USA |
2 |
13y and 18y |
n/a |
Observational report of immunosuppressed children with SARS-CoV-2 infections |
| Richard, A. et al. (2020) |
Cross-sectional study |
Switzerland |
902 children, 7442 adults |
5-94y |
n/a |
Population based seroprevalence survey |
| Riphagen, S. et al. (2020) |
Case report |
UK |
8 |
6-14y |
n/a |
Observation report of Kawasaki-like syndrome in children with SARS-CoV-2 infection |
| Sheth, S. et al. (2020) |
Narrative Review/Viewpoint |
n/a |
326 mothers-children pairs |
newborns |
39 |
Review of studies reporting on children born by mothers with SARS-CoV-2 infection |
| Somekh, E. et al. (2020) |
Case report |
Israel |
58 children, 36 adults |
6-48y |
n/a |
Observational study on identified family clusters of SARS-CoV-2 infection |
| Stein-Zamir, C. et al. (2020) |
Cross-sectional study |
Israel |
1164 students, 152 staff |
≥12y |
n/a |
Observational study about a high school outbreak |
| Stringhini, S. et al. (2020) |
Cross-sectional study |
Switzerland |
455 children, 2311 adults |
≥5y |
n/a |
Population based seroprevalence study |
| Stringhini, S. et al. (2021) |
Cross-sectional study |
Switzerland |
1014 children, 2986 adults |
all age groups |
n/a |
Population based seroprevalence study |
| Swann, O.V. et al. (2020) |
Cohort study |
UK |
651 |
median 4.6y (IQR 0.3-13.7y) |
n/a |
Observational cohort study in 260 hospitals describing characteristics of hospitalised children infected with SARS-CoV-2 |
| Szablewski, C.M. et al. (2020) |
Cross-sectional study |
USA |
344 children and staff |
6-19y for campers, 14-59y for staff |
n/a |
Observational study about a camp outbreak |
| Torres, J.P. et al. (2020) |
Cross-sectional study |
Chile |
1009 students, 235 staff |
Mean 10.8y (SD 4.1) for students, mean 42.8y (SD 10.4) for staff |
n/a |
Observational study about a school outbreak |
| Ulyte, A. et al. (2021) |
Cohort study |
Switzerland |
2831 students |
6-16y |
n/a |
Seroprevalence study in school children |
| Ulyte, A. et al. (2021) |
Cohort study |
Switzerland |
2585 students |
6-16y |
n/a |
Seroprevalence study in school children |
| Vardhelli, V. et al. (2020) |
Systematic review |
n/a |
793 neonates |
newborns |
45 |
Systematic review of studies reporting on children born by mothers with SARS-CoV-2 infection |
| Viner, R. M. et al. (2020) |
Systematic review |
n/a |
41640 |
n/a |
32 |
Systematic review on susceptibility and transmission of children to SARS-CoV-2 compared to adults |
| Volz, E. et al. (2020) |
Modelling study |
UK |
n/a |
n/a |
n/a |
Modelling study about the B 1.1.7 variant of SARS-CoV-2 |
| Wei, M. et al. (2020) |
Cross-sectional study |
China |
9 |
0-1y |
n/a |
Observational study in hospitalised infants with SARS-CoV-2 infection |
| Yoon, Y. et al. (2020) |
Case report |
South Korea |
1 index case, 190 contacts |
4y |
n/a |
Observational study examining transmission of SARS-CoV-2 from infected toddler to contacts |
| Yung, C.F. et al. (2020) |
Case report |
Singapore |
223 adult index patients, 213 child contacts |
0-16y for children |
n/a |
Observational study examining household transmission of SARS-CoV-2 from adults to children |
| Yung, C.F. et al. (2021) |
Case report |
Singapore |
3 index cases (2 pediatric, 1 adult), 119 contacts |
5y, 12y |
n/a |
Nationwide contact surveillance and contact tracing study on SARS-CoV-2 infections in schools |
| Zhang, Z.J. et al. (2020) |
Case report |
China |
4 |
0-28 days |
n/a |
Observational report of neonates with SARS-CoV-2 infection |
| Zhu et al. (2020) |
Meta-analysis |
12 countries |
213 clusters |
n/a |
57 |
Meta-analysis of studies describing household transmission clusters of SARS-CoV-2 |
