Heat-related cardiovascular morbidity and mortality in Switzerland: a clinical perspective
DOI: https://doi.org/10.4414/SMW.2021.w30013
Florian
Schulteab, Martin
Röösliab, Martina S.
Ragettliab
aSwiss Tropical and Public Health Institute, University of Basel, Switzerland
bUniversity of Basel, Switzerland
Summary
AIMS: Previous studies found increased cardiovascular mortality during hot days, while emergency hospital admissions were decreasing. We explored potential underlying reasons by analysing clinically similar cardiovascular disease groups taking into account primary, underlying and immediate causes of death.
METHODS AND RESULTS: We assessed associations of daytime maximum temperature in relation to cardiovascular deaths and emergency hospital admissions between 1998 and 2016 in Switzerland. We applied conditional quasi-Poisson models with non-linear distributed lag functions to estimate relative risks (RRs) of daily cardiovascular mortality and morbidity for temperature increases from the median (22 °C) to the 98th percentile (32 °C) of the warm season temperature distribution with 10 days of lag. Cardiovascular mortality (n = 163,856) increased for total cardiovascular disease (RR 1.13, 95% confidence interval [CI] 1.08–1.19) and the disease groups hypertension (1.18, 1.02–1.38), arrhythmia (1.29, 1.08–1.55), heart failure (1.22, 1.05–1.43) and stroke of unknown origin (1.20, 1.02–1.4). In contrast, emergency hospital admissions (n = 447,577) decreased for total cardiovascular disease (0.91, 0.88–0.94), hypertension (0.72, 0.64–0.81), heart failure (0.83, 0.76–0.9) and myocardial infarction (0.88, 0.82–0.95). Opposing heat effects were most pronounced for disease groups associated with diuretic and antihypertensive drug use, with the age group ≥75 years at highest risk.
CONCLUSIONS: Volume depletion and vasodilation from heat stress plausibly explain the risk reduction of heat-related emergency hospital admissions for hypertension and heart failure. Since primary cause of death mostly refers to the underlying chronic disease, the seemingly paradoxical heat-related mortality increase can plausibly be explained by an exacerbation of heat effects by antihypertensive and diuretic drugs. Clinical guidelines should consider recommending strict therapy monitoring of such medication during heatwaves, particularly in the elderly.
Introduction
Climate change has been described as the biggest global health threat of the 21st century, partly due to more frequent and intense heatwaves [1]. Previous studies have described increasing mortality rates at high ambient temperature compared with the country- or city-specific optimal temperature range [2–9]. There is evidence that cardiovascular diseases, which are the leading cause of death globally [10], are among the main causes of heat-related deaths [11].
Heat effects on cardiovascular morbidity are less clear [12]. Previous studies reported a slight heat-related morbidity increase for cardiovascular diseases, or no effect with a tendency for a decrease [2, 13, 14]. In Switzerland, decreasing daily emergency hospital admissions for cardiovascular disease were observed during the hot summer of 2015 [15].
The reasons for different heat effects on mortality and morbidity are unclear. Some authors suggested that people die before being admitted to the hospital [14]. A deeper look into heat effects on different cardiovascular disease groups has rarely been done [2]. Existing literature reports varying effects, such as an increase in emergency hospital admissions for ischaemic heart disease or stroke, but a decrease for hypertension [16]. Furthermore, to our knowledge, previous studies took into account only the primary cause of death.
The aim of this study was to explore the reasons for different heat effects on cardiovascular mortality and morbidity to better inform clinical decisions and public health interventions during hot spells. We therefore carried out a detailed comparative analysis of heat-related mortality and emergency hospital admissions. We used clinically relevant cardiovascular disease groups and took into account primary, underlying and immediate causes of death.
Materials and methods
Health and temperature data
The Swiss Federal Statistical Office (FSO) provided us with daily mortality and emergency hospital admission data between 1998 and 2016 from the official cause of death statistics and the medical statistics of Swiss hospitals. Data included cardiovascular primary causes of death and emergency hospital admissions of Swiss residents according to the International Classification of Diseases codes, 10th Revision (ICD-10, I00-I99). The primary cause of death is defined by the FSO based on a standardised procedure evaluating information from the death certificates on underlying, immediate and contributing causes of death. The primary cause of death is for most disease groups identical to the underlying cause of death and decisive for all publications [17]. Health data were aggregated into daily deaths and daily number of emergency hospital admissions by age (<75, ≥75 years), sex, disease group and seven geographic areas across Switzerland (so-called Swiss great regions, supplementary fig. S1 in the appendix).
Before starting the data analyses, we created a diagnostic framework comprising nine cardiovascular disease groups (supplementary table S1): hypertension (I10–15), myocardial infarction (I21–22), pulmonary embolism (I26), arrhythmia (I44–49), heart failure (I50), haemorrhagic stroke (I60–62), ischaemic stroke (I63), stroke of unknown origin (I64) and deep vein thrombosis (I80). Disease groups were defined based on common clinical diagnoses that have a plausible cause-effect relationship with heat. We assumed that the following heat effects may have an influence on the diseases : volume depletion and peripheral vasodilation with subsequent reduction of cardiac pre- and afterload on hypertension and heart failure [18–20], sweating with subsequent electrolyte imbalances on cardiac arrhythmias [21, 22], and pro- and anticoagulation effects on thrombotic, infarction and bleeding events [22–24]. To support these heat sensitivity pathways and to evaluate potential other contributing effects of heat-related mortality, we also compared the primary cause of death with underlying (to describe chronic conditions) and immediate causes of death by disease group.
Daily temperature data were collected from the IDAWEB database, a service provided by MeteoSwiss, the Swiss Federal Office of Meteorology and Climatology. We restricted the analysis to the warm season (May to September).
Numbers of daily deaths and emergency hospital admissions of each great region were linked to daily daytime (6:00–18:00) maximum temperature measurements (Tmax) from a close-by representative monitoring station. A map showing the locations of the measurement stations is provided in the appendix (fig. S1).
Statistical analysis
We analysed the short-term temperature-mortality and temperature-morbidity relationships for cardiovascular disease using conditional quasi-Poisson regression models. We applied distributed lag non-linear models (DLNM) to allow for non-linear and delayed effects of temperature [25]. All regions were pooled into one dataset. To control for potential differences by year, month, weekday and region, a stratum variable was included in the model that matched the same days of the week in the same month of the same year and the same geographic region.
The model specifications were optimised for both mortality (using primary cause of death) and morbidity models using the total number of cardiovascular deaths deaths and emergency hospital admissions, and model coefficients were then estimated for the individual disease groups. For the temperature dimension of the so-called cross-basis function of the DLNM, we chose a model with a natural spline function with three internal knots at the 10th, 75th and 90th percentiles. For the lag dimension of the cross-basis, we used a natural spline function with two equally spaced internal knots on the log scale. We chose a 10-day lag period to capture delayed effects and potential harvesting. We selected the model specifications based on similar studies on the effect of heat on health [6, 26]. To validate the model, we tested different numbers and positions of knots in a sensitivity analysis, which showed similar results (figs S2 and S3).
Associations between Tmax and health outcomes were expressed as cumulative relative risks (RRs) over the whole lag period. We used the median warm season Tmax over all geographic areas and years as the reference. Cumulative and lag-specific RRs were estimated for the 98th percentile of Tmax, defined as a “hot day” (similar to previous research [4–6, 9]). To identify vulnerable population subgroups, we also assessed the effects by age (<75, ≥75 years). All analyses were conducted using the statistical software environment R (version 3.4.4) with the package dlnm [25].
Access to data and computing code
The health data used for this study are available under license from the Federal Statistical Office, Switzerland. The computing code is available on request by e-mail from the authors. All analysis was done on aggregated data, for which no ethical approval is required in Switzerland.
Results
In total, our dataset contained 163,856 cardiovascular deaths and 447,577 cardiovascular emergency hospital admissions. The age distribution was balanced for the hospital admissions (49% <75 years, 51% ≥75 years). Most deaths (80%) occurred in the population aged ≥75 years. Fifty-six percent of deaths and 70% of emergency hospital admissions were analysed in detail as part of our diagnostic framework.
For the individual disease groups, the proportion of patients ≥75 years ranged between 37% (myocardial infarction) and 74% (heart failure) for emergency hospital admissions , and between 62% (haemorrhagic stroke) and 92% (heart failure) for mortality. The detailed demographic structure of the data is shown in supplementary figures S6 (deaths) and S7 (admissions) and supplementary table S2.
Primary and underlying causes of death were the same for most disease groups from the framework (69%). The highest agreement was found for heart failure (94%) and hypertension (92%). For myocardial infarction, agreement with immediate cause of death was higher (72%, for details see table S4).
Recorded Tmax during the warm season of the years 1998–2016 in the seven geographic regions in Switzerland ranged between 3 °C and 40 °C. The median and 98th percentile of Tmax varied across monitoring stations between 19– 25 °C and 29– 34 °C, respectively; resulting in an overall median of 22 °C and 98th percentile of 32 °C.
For all cardiovascular diseases combined, an increase of Tmax from 22 °C (median) to 32 °C (98th percentile) was significantly associated with a 13% increase in deaths (95% confidence Interval [CI] 8–19%) and a 9% decrease in emergency hospital admissions (95% CI: 6–12%) . A similar pattern was observed for hypertension and heart failure (fig. 1, table 1). For arrhythmia and stroke of unknown origin, mortality increased with temperature, whereas morbidity did not. For myocardial infarction, emergency hospital admissions decreased, accompanied by a tendency for a mortality increase at very high temperatures. The remaining disease groups were smaller (<10,000 cases) and did not show significant associations between temperature and mortality or emergency hospital admissions, except a significant reduction in hospital admissions for deep vein thrombosis (fig. S6).

Figure 1 Relative risks (RRs) for emergency hospital admissions (EHA) and mortality for cardiovascular (CVD) disease groups with more than 10,000 cases by daytime maximum temperature. RRs are cumulative over the lag period of 10 days. Median daytime maximum temperature over the study period is used as reference.
Table 1Summary of results for heat-related emergency hospital admissions (EHA) and mortality, comparison with the literature and possible explanations for observed heat effects for cardiovascular disease groups >10,000 cases.
|
Disease group
a
|
Class
|
Number of cases
|
RR (95% CI)
b
|
Effect
|
Results from previous literature
|
Diagnosis involes antihypertensive drug use
|
Plausible explanations for effect in our study
|
|
Hypertension (I10–15)
|
EHA |
29,533 |
0.72 (0.64–0.81) |
↓
|
10.0% (95% CI 13.1–6.7%) risk reduction of ER visits per 10°F. NB: 12.7% (95% CI 8.3–17.4) excess risk for ER visits due to hypotension [16] |
Yes [27] |
EHA reduction: Direct heat effect,. Mortality increase: Heat-induced amplification of anti-hypertensive drug effects |
| Deaths |
17,142 |
1.18 (1.02–1.38) |
↑
|
No data found |
|
|
Heart failure (I50)
|
EHA |
57,440 |
0.83 (0.76–0.90) |
↓
|
Insignificant decrease [16] |
Yes [28] |
EHA reduction: Direct heat effect, Mortality increase: Heat-induced amplification of anti-hypertensive drug effects. Disease physiology may contribute |
| Deaths |
14,753 |
1.22 (1.05–1.43) |
↑
|
3.6% (95% CI 2.4–4.8) mortality increase per 1 °C temperature increase above region-specific heat threshold [29] |
|
|
Stroke of unknown origin (I64)
|
EHA |
11,280 |
1.04 (0.86–1.26) |
↔
|
No significant heat effect for combined stroke events [30]c
|
Yes [31, 32]d
|
Mortality increase: Heat-induced amplification of anti-hypertensive drug effects possible due to comorbidities |
| Deaths |
15,296 |
1.2 (1.02–1.4) |
↑
|
RR 1.53 (95% CI 0.9–2.15) on hot days for ischaemic and haemorrhagic stroke combinedc
|
|
|
Arrhythmia
(I44–49)
|
EHA |
49,835 |
0.97 (0.89–1.05) |
↔
|
2.8% (95% CI 0.9–4.9) increase of emergency room visits per 10 °F [16] |
Some diagnoses (e.g., atrial fibrillationc) |
Mortality increase: Heat-induced amplification of anti-hypertensive drug effects may contribute |
| Deaths |
11,323 |
1.29 (1.08–1.55) |
↑
|
5% (95% CI 3.2–6.9) mortality increase per 1 °C temperature increase above region-specific heat threshold [29] |
|
|
Myocardial infarction
(I21-22)
|
EHA |
68,861 |
0.88 (0.82–0.95) |
↓
|
RR 1.016 (95% CI 1.004–1.028) for 1 °C increase [33] |
Yesc
|
EHA reduction: cardioprotective heat effects possible |
|
|
Deaths |
20,081 |
1.01 (0.88–1.16) |
↔
|
RR 1.639 (95% CI 1.087–2.470) for a heatwave [26] |
|
|
Stratified results by age showed exposure-response associations similar to those of r the total population (figs S7 and S8, table S3). The mortality increase was greater in the age group ≥75 years, whereas the reduction in emergency hospital admissions was more prevalent in the age group <75 years. In the age group <75 years, we found a significant heat-related mortality increase for ischaemic stroke (RR 2.24), albeit with a large confidence interval (95% CI 1.07–4.73).
The time lag between high temperature and health outcomes differed between disease groups but was mostly around 0–4 days (figs. S9 and S10). For myocardial infarction and heart failure, we observed a mostly insignificant mortality reduction after an initial mortality increase.
Discussion
With increasing temperature, cardiovascular emergency hospital admissions decreased whereas mortality increased. Specifically, mortality due to hypertension, arrhythmia, heart failure and stroke of unknown origin as primary causes of death increased with temperature. Daily emergency hospital admissions decreased for hypertension, heart failure and myocardial infarction and remained unchanged for stroke of unknown origin and arrhythmia. Except for myocardial infarction, the primary cause of death mainly refers to the underlying disease and not the acute cause of death.
Both the overall effect and the results for the specific cardiovascular disease groups were in good agreement with previous work [15]. Increased heat-related mortality due to heart failure, stroke and arrhythmia, and reductions in rates of emergency hospital admissions for hypertension and heart failure have been observed previously [16, 29]. A tendency for a heat-related increase of myocardial infarction mortality was seen only at very high temperatures (>33 °C) and was thus less pronounced than in other studies. For myocardial infarction, we found a decrease in hospital admissions instead of an increase [33].
Direct heat effects can plausibly explain decreasing emergency hospital admissions due to hypertension and heart failure. Volume depletion through sweating and peripheral vasodilation reduces cardiac preload [21], cardiac afterload [23] and blood pressure [18, 35]. In volume-overloaded heart failure patients, this moves the operating point on the Frank-Starling-curve (which represents the relationship between distension of the heart and the force generated by contraction) to a more optimal location [36], similar to a targeted pharmacotherapy [28, 37]. Reduced emergency hospital admissions due to these diseases are the result.
Against this background, the heat-related mortality increases for hypertension and heart failure seem counter-intuitive. However, the primary causes of death “hypertension” and “heart failure” derive mostly from the underlying, not the acute cause of death. The corresponding immediate cause of death was mostly unspecific (table S4). Therefore, the excess mortality refers to patients dying with, not of, chronic hypertension or heart failure. Consequently, these patients did not necessarily suffer from acute hypertension or acute congestive heart failure at the time of death. They were likely taking antihypertensive or diuretic medications though, which are the standard treatment for chronic hypertension and heart failure [27, 28, 37, 38]. Antihypertensive medication can exacerbate heat effects [39–42], which has led to recommendations for a dose reduction during hot spells in some countries [43, 44]. During routine post-mortem examination, heat effects are difficult to identify [45]. Thus, if a patient dies from antihypertensive drug effects exacerbated by heat stroke, an unspecific immediate cause of death would likely be recorded. Meanwhile, in the absence of a better reason, it is likely that the patients’ known chronic disease would be recorded as the underlying, and ultimately primary, cause of death. This can show as a heat-related mortality increase for chronic diseases associated with antihypertensive drugs, as observed in our analysis (fig. 2).
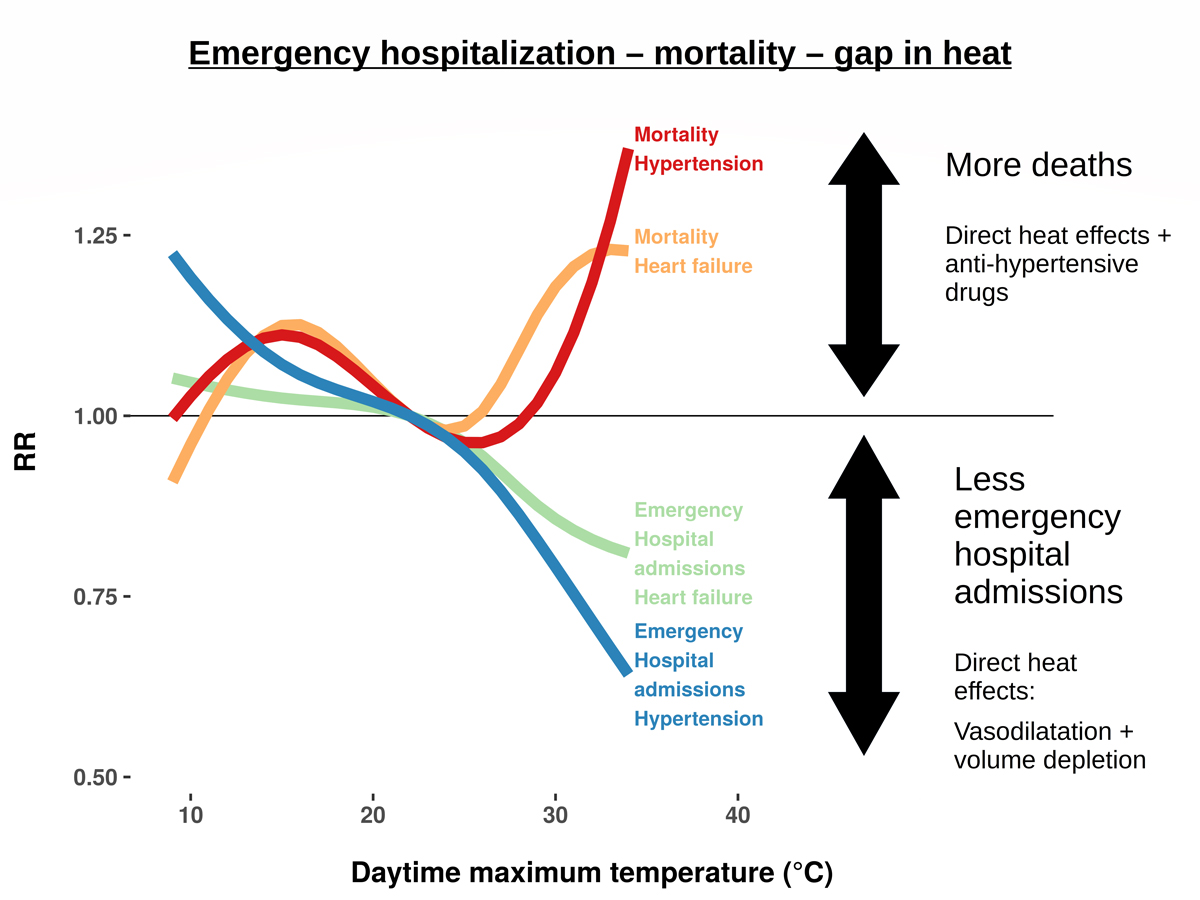
Figure 2 Heat-related “gap” between emergency hospital admissions and mortality for two key cardiovascular diseases. The reduction in emergency hospital admissions and the increase in deaths can be explained by a combined effect of heat and antihypertensive drugs. RR: relative risk
Increased heat vulnerability due to pathophysiological changes offers an alternative explanation for the mortality increase in heart failure [42], but cannot explain the corresponding decrease in emergency hospital admissions or the results for hypertension.
An exacerbation of heat effects by antihypertensive drugs can also partly explain the heat-related mortality increase in cardiac arrhythmia and stroke of unknown origin, if comorbidities are considered. For atrial fibrillation, hypertension is reported as a comorbidity in about 77% of cases [34]. For stroke, a Swiss study detected hypertension in 63.5% of the patients, and the CARes study from Texas even in 86.5% of the cases. CARes also explicitly looked for antihypertensive drug use and found it in 83% of stroke patients [31, 32]. Thus, patients with atrial fibrillation or stroke of unknown origin as primary cause of death were likely also treated with antihypertensive drugs. The largest part of the mortality increase for arrhythmia corresponds to unexplained deaths: “cardiac arrest”, which is unspecific, was the most frequent primary cause of death in the arrhythmia group (data not shown). Although the available data do not allow conclusions to be drawn about the causes for heat-related excess mortality for this subgroup, a contribution of direct heat effects or antihypertensive drugs can also not be ruled out.
A contribution of heat-induced electrolyte imbalances to the mortality increase for cardiac arrhythmia is also possible, but does not explain the absence of an increase in rates of emergency hospital admission.
Our results did not show a consistent picture regarding previously hypothesised impairment of the coagulation system by heat [22–24].
For myocardial infarction, the observed slight decrease in emergency hospital admissions may be explained by cardioprotective effects from warm weather, such as reduction of cardiac pre- and afterload or reduced oxidative stress [46]. Patients were also younger on average in this group, suggesting that their thermoregulatory control was better maintained than in older individuals [47]. Inadequate hypertension and diuretic treatment during hot days may thus be less relevant for these specific patients.
For myocardial infarction and heart failure, there was a tendency for a slight but mostly insignificant reduction in emergency hospital admissions after an initial mortality increase. Thus, an initial mortality increase may have contributed to reduced admissions and mortality rates in the subsequent days for these specific disease groups.
Strengths and limitations
To the best of our knowledge, the present study is the first comparative study on heat-related mortality and morbidity that takes into account data on primary, underlying and immediate causes of death. We analysed all cardiovascular deaths and emergency hospital admissions that occurred in the warm season between 1998 and 2016 in Switzerland without any selection bias, resulting in a large dataset of ~160,000 cardiovascular deaths and ~250,000 emergency admissions. We included only frequent, clinically relevant diagnoses with established cause-effect relationships with heat using a non-standardised framework. By doing so, we could include the majority of cases (55% of all cardiovascular emergency admissions and 70% of all cardiovascular deaths) while maintaining some coherence with the ICD-10 coding framework. However, the use of aggregated data is a limitation of this paper, and statistical power was low for some disease groups. Similar analyses in other countries are warranted to validate the results.
We used only one temperature measurement per great region and could therefore not account for within-region or altitude variability. However, relative day-to-day temperature variations are most relevant for this type of time-series analysis and are well captured by a representative meteorological station within the study area. To check, we also conducted additional analyses with data restricted to a small area around each meteorological station, which produced similar results, although with larger confidence intervals.
We focused on Tmax only and did not consider other temperature indices such as daily minimum or mean temperature. Previous work on temperature-health associations in Switzerland showed very similar results across temperature indices [5]. Other potential environmental (e.g., air pollution) and weather (e.g., humidity) risk factors were not included. The role of these variables as confounders of heat-related health effects is still debatable [48, 49]. Our framework can be used to examine such potential environmental risk factors and cumulative days of extreme temperature that may enhance the heat effect [12, 50].
Inherently, considerable uncertainty remains regarding the habits of doctors during the process of cause of death coding. Although including data on underlying and immediate causes of death offered some insights, this limitation should be addressed by future qualitative research on the death coding process in practice. Finally, a study directly examining antihypertensive treatment as a possible risk factor during periods of heat is warranted.
Conclusions
A significant mortality increase for hypertension, heart failure, stroke of unknown origin and arrhythmia was accompanied by a significant decrease in emergency hospital admissions for hypertension, heart failure and myocardial infarction in high ambient temperatures. The opposite heat effect was most prevalent for diseases routinely treated with antihypertensive drugs. Pathophysiological considerations, comorbidity patterns and the coding of chronic diseases as primary cause of death indicate that an amplification of antihypertensive drug effects by heat plausibly explains the seemingly paradoxical mortality increase. Given the projected increase of heatwaves in the future, public health programmes and clinicians should increase vigilance for signs of heat-related illness in patients on antihypertensive drugs during periods of heat.
Acknowledgements
The authors are grateful to the Swiss Federal Statistical Office for providing the health data.
Dr Martina S. Ragettli, PhD
Swiss Tropical and Public Health Institute
Socinstrasse 57
P.O. Box
CH-4002 Basel
Martina.Ragettli[at]swisstph.ch
References
1
Costello A
,
Abbas M
,
Allen A
,
Ball S
,
Bell S
,
Bellamy R
, et al.
Managing the health effects of climate change: Lancet and University College London Institute for Global Health Commission. Lancet. 2009 May;373(9676):1693–733. https://doi.org/10.1016/S0140-6736(09)60935-1
2
Bunker A
,
Wildenhain J
,
Vandenbergh A
,
Henschke N
,
Rocklöv J
,
Hajat S
, et al.
Effects of Air Temperature on Climate-Sensitive Mortality and Morbidity Outcomes in the Elderly; a Systematic Review and Meta-analysis of Epidemiological Evidence. EBioMedicine. 2016 Apr;6:258–68. https://doi.org/10.1016/j.ebiom.2016.02.034
3
Confalonieri U
,
Menne B
,
Akhtar R
,
Ebi KL
,
Hauengue M
,
Kovats RS
, et al.
Human health. In: Climate Change 2007: Impacts, Adaptation and Vulnerability Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press; 2007. p. 391–431.
4
Gasparrini A
,
Guo Y
,
Hashizume M
,
Lavigne E
,
Zanobetti A
,
Schwartz J
, et al.
Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet. 2015 Jul;386(9991):369–75. https://doi.org/10.1016/S0140-6736(14)62114-0
5
Ragettli MS
,
Vicedo-Cabrera AM
,
Schindler C
,
Röösli M
. Exploring the association between heat and mortality in Switzerland between 1995 and 2013. Environ Res. 2017 Oct;158(May):703–9. https://doi.org/10.1016/j.envres.2017.07.021
6
Vicedo-Cabrera AM
,
Sera F
,
Guo Y
,
Chung Y
,
Arbuthnott K
,
Tong S
, et al.
A multi-country analysis on potential adaptive mechanisms to cold and heat in a changing climate. Environ Int. 2018 Feb;111(111):239–46. https://doi.org/10.1016/j.envint.2017.11.006
7
Rodrigues M
,
Santana P
,
Rocha A
. Modelling of Temperature-Attributable Mortality among the Elderly in Lisbon Metropolitan Area, Portugal: A Contribution to Local Strategy for Effective Prevention Plans. J Urban Health. 2021 Apr;1–16: [cited 2021 Aug 5] https://doi.org/10.1007/s11524-021-00536-z
8
Saucy A
,
Ragettli MS
,
Vienneau D
,
de Hoogh K
,
Tangermann L
,
Schäffer B
, et al.
The role of extreme temperature in cause-specific acute cardiovascular mortality in Switzerland: A case-crossover study. Sci Total Environ. 2021 Oct;790:147958. https://doi.org/10.1016/j.scitotenv.2021.147958
9
Lee JY
,
Röösli M
,
Ragettli MS
. Estimation of Heat-Attributable Mortality Using the Cross-Validated Best Temperature Metric in Switzerland and South Korea. Int J Environ Res Public Health. 2021 Jun;18(12):6413. https://doi.org/10.3390/ijerph18126413
10
Institute for Health Metrics and Evaluation (IHME)
. GBD Compare [Internet]. 2018 [cited 2018 Oct 4]. Available from: https://vizhub.healthdata.org/gbd-compare/
11
Moghadamnia MT
,
Ardalan A
,
Mesdaghinia A
,
Keshtkar A
,
Naddafi K
,
Yekaninejad MS
. Ambient temperature and cardiovascular mortality: a systematic review and meta-analysis. PeerJ. 2017 Aug;5:e3574. https://doi.org/10.7717/peerj.3574
12
Campbell S
,
Remenyi TA
,
White CJ
,
Johnston FH
. Heatwave and health impact research: A global review. Health Place. 2018 Sep;53:210–8. https://doi.org/10.1016/j.healthplace.2018.08.017
13
Ponjoan A
,
Blanch J
,
Alves-Cabratosa L
,
Martí-Lluch R
,
Comas-Cufí M
,
Parramon D
, et al.
Effects of extreme temperatures on cardiovascular emergency hospitalizations in a Mediterranean region: a self-controlled case series study. Environmental health : a global access science source. 2017;16(1):32.
14
Turner LR
,
Barnett AG
,
Connell D
,
Tong S
. Ambient temperature and cardiorespiratory morbidity: a systematic review and meta-analysis. Epidemiology. 2012 Jul;23(4):594–606. https://doi.org/10.1097/EDE.0b013e3182572795
15
Ragettli MS
,
Vicedo-Cabrera AM
,
Flückiger B
,
Röösli M
. Impact of the warm summer 2015 on emergency hospital admissions in Switzerland. Environ Health. 2019 Aug;18(1):66. https://doi.org/10.1186/s12940-019-0507-1
16
Basu R
,
Pearson D
,
Malig B
,
Broadwin R
,
Green R
. The effect of high ambient temperature on emergency room visits. Epidemiology. 2012 Nov;23(6):813–20. https://doi.org/10.1097/EDE.0b013e31826b7f97
17
Bundesamt für Statistik
. Schweizerische Todesursachenstatistik - Richtlinien für die ärztliche Bescheinigung der Todesursachen. 1996 [cited 2020 Jun 22]. Available from: https://opendata.swiss/de/dataset/schweizerische-todesursachenstatistik
18
Nose H
,
Kamijo YI
,
Masuki S
. Interactions between body fluid homeostasis and thermoregulation in humans. Handb Clin Neurol. 2018;156:417–29. https://doi.org/10.1016/B978-0-444-63912-7.00025-4
19
Rowell LB
,
Brengelmann GL
,
Murray JA
. Cardiovascular responses to sustained high skin temperature in resting man. J Appl Physiol. 1969 Nov;27(5):673–80. https://doi.org/10.1152/jappl.1969.27.5.673
20
Schlader ZJ
,
Wilson TE
,
Crandall CG
. Mechanisms of orthostatic intolerance during heat stress. Auton Neurosci. 2016 Apr;196:37–46. https://doi.org/10.1016/j.autneu.2015.12.005
21
Heidari L
,
Winquist A
,
Klein M
,
O’Lenick C
,
Grundstein A
,
Ebelt Sarnat S
. Susceptibility to Heat-Related Fluid and Electrolyte Imbalance Emergency Department Visits in Atlanta, Georgia, USA. Int J Environ Res Public Health. 2016 Oct;13(10):E982. [cited 2020 Apr 17] Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5086721/ https://doi.org/10.3390/ijerph13100982
22
Petitti DB
,
Hondula DM
,
Yang S
,
Harlan SL
,
Chowell G
. Multiple Trigger Points for Quantifying Heat-Health Impacts: New Evidence from a Hot Climate. Environ Health Perspect. 2016 Feb;124(2):176–83. https://doi.org/10.1289/ehp.1409119
23
Borgman MA
,
Zaar M
,
Aden JK
,
Schlader ZJ
,
Gagnon D
,
Rivas E
, et al.
Hemostatic responses to exercise, dehydration, and simulated bleeding in heat-stressed humans. Am J Physiol Regul Integr Comp Physiol. 2019 Feb;316(2):R145–56. https://doi.org/10.1152/ajpregu.00223.2018
24
Meyer MA
,
Ostrowski SR
,
Overgaard A
,
Ganio MS
,
Secher NH
,
Crandall CG
, et al.
Hypercoagulability in response to elevated body temperature and central hypovolemia. J Surg Res. 2013 Dec;185(2):e93–100. https://doi.org/10.1016/j.jss.2013.06.012
25
Gasparrini A
,
Armstrong B
,
Kenward MG
. Distributed lag non-linear models. Stat Med. 2010 Sep;29(21):2224–34. https://doi.org/10.1002/sim.3940
26
Scovronick N
,
Sera F
,
Acquaotta F
,
Garzena D
,
Fratianni S
,
Wright CY
, et al.
The association between ambient temperature and mortality in South Africa: A time-series analysis. Environ Res. 2018 Feb;161:229–35. https://doi.org/10.1016/j.envres.2017.11.001
27
Whelton PK
,
Carey RM
,
Aronow WS
,
Casey DE Jr
,
Collins KJ
,
Dennison Himmelfarb C
, et al.
2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 May;71(19):e127–248. https://doi.org/10.1016/j.jacc.2017.11.006
28
Ponikowski P
,
Voors AA
,
Anker SD
,
Bueno H
,
Cleland JG
,
Coats AJ
, et al.
2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Rev Esp Cardiol (Engl Ed). 2016 Dec;69(12):1167. https://doi.org/10.1016/j.rec.2016.11.005
29
Gasparrini A
,
Armstrong B
,
Kovats S
,
Wilkinson P
. The effect of high temperatures on cause-specific mortality in England and Wales. Occup Environ Med. 2012 Jan;69(1):56–61. https://doi.org/10.1136/oem.2010.059782
30
Lian H
,
Ruan Y
,
Liang R
,
Liu X
,
Fan Z
. Short-Term Effect of Ambient Temperature and the Risk of Stroke: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2015 Jul;12(8):9068–88. https://doi.org/10.3390/ijerph120809068
31
Fischer U
,
Arnold M
,
Nedeltchev K
,
Schoenenberger RA
,
Kappeler L
,
Höllinger P
, et al.
Impact of comorbidity on ischemic stroke outcome. Acta Neurol Scand. 2006 Feb;113(2):108–13. https://doi.org/10.1111/j.1600-0404.2005.00551.x
32
Ostwald SK
,
Wasserman J
,
Davis S
. Medications, comorbidities, and medical complications in stroke survivors: the CAReS study. Rehabil Nurs. 2006 Jan-Feb;31(1):10–4. https://doi.org/10.1002/j.2048-7940.2006.tb00004.x
33
Sun Z
,
Chen C
,
Xu D
,
Li T
. Effects of ambient temperature on myocardial infarction: A systematic review and meta-analysis. Environ Pollut. 2018 Oct;241:1106–14. https://doi.org/10.1016/j.envpol.2018.06.045
34
Ruperti Repilado FJ
,
Doerig L
,
Blum S
,
Aeschbacher S
,
Krisai P
,
Ammann P
, et al.
Prevalence and predictors of atrial fibrillation type among individuals with recent onset of atrial fibrillation. Swiss Med Wkly. 2018 Sep;148(3738):w14652. [cited 2019 Dec 11] Available from: https://smw.ch/article/doi/smw.2018.14652
35
van den Hurk K
,
de Kort WL
,
Deinum J
,
Atsma F
. Higher outdoor temperatures are progressively associated with lower blood pressure: a longitudinal study in 100,000 healthy individuals. J Am Soc Hypertens. 2015 Jul;9(7):536–43. https://doi.org/10.1016/j.jash.2015.05.003
36 Ernest H. Starling. The Linacre lecture on the Law of the Heart. London: Longmans, Green and Co.; 1918 [cited 2020 May 1]. 30 p. Available from: http://handle.slv.vic.gov.au/10381/201716
37
Yancy CW
,
Jessup M
,
Bozkurt B
,
Butler J
,
Casey DE Jr
,
Drazner MH
, et al.; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines
. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013 Oct;62(16):e147–239. https://doi.org/10.1016/j.jacc.2013.05.019
38
Williams B
,
Mancia G
,
Spiering W
,
Agabiti Rosei E
,
Azizi M
,
Burnier M
, et al.; ESC Scientific Document Group
. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018 Sep;39(33):3021–104. https://doi.org/10.1093/eurheartj/ehy339
39
Fitzsimons JT
. Angiotensin, thirst, and sodium appetite. Physiol Rev. 1998 Jul;78(3):583–686. https://doi.org/10.1152/physrev.1998.78.3.583
40
Oldenburg B
,
MacDonald GJ
,
Shelley S
. Controlled trial of enalapril in patients with chronic fluid overload undergoing dialysis. Br Med J (Clin Res Ed). 1988 Apr;296(6629):1089–91. https://doi.org/10.1136/bmj.296.6629.1089
41
Hausfater P
,
Megarbane B
,
Dautheville S
,
Patzak A
,
Andronikof M
,
Santin A
, et al.
Prognostic factors in non-exertional heatstroke. Intensive Care Med. 2010 Feb;36(2):272–80. https://doi.org/10.1007/s00134-009-1694-y
42
Balmain BN
,
Sabapathy S
,
Jay O
,
Adsett J
,
Stewart GM
,
Jayasinghe R
, et al.
Heart Failure and Thermoregulatory Control: Can Patients With Heart Failure Handle the Heat? J Card Fail. 2017 Aug;23(8):621–7. https://doi.org/10.1016/j.cardfail.2017.04.003
43
Smirnova MD
,
Svirida ON
,
Ageev FT
. Protective measures of patients with cardiovascular diseases from exposure to heat waves: medicated and non-medicated. Ter Arkh. 2019 Mar;91(1):101–7. https://doi.org/10.26442/00403660.2019.01.000038
44
Tait PW
,
Allan S
,
Katelaris AL
. Preventing heat-related disease in general practice. Aust J Gen Pract. 2018 Dec;47(12):835–40. https://doi.org/10.31128/AJGP-07-18-4658
45
Sweeney KG
. Heat-related deaths. J Insur Med. 2002;34(2):114–9.
46
Yellon DM
,
Pasini E
,
Cargnoni A
,
Marber MS
,
Latchman DS
,
Ferrari R
. The protective role of heat stress in the ischaemic and reperfused rabbit myocardium. J Mol Cell Cardiol. 1992 Aug;24(8):895–907. https://doi.org/10.1016/0022-2828(92)91102-B
47
Balmain BN
,
Sabapathy S
,
Louis M
,
Morris NR
. Aging and Thermoregulatory Control: The Clinical Implications of Exercising under Heat Stress in Older Individuals. BioMed Res Int. 2018 Aug;2018:8306154. https://doi.org/10.1155/2018/8306154
48
Armstrong B
,
Sera F
,
Vicedo-Cabrera AM
,
Abrutzky R
,
Åström DO
,
Bell ML
, et al.
The Role of Humidity in Associations of High Temperature with Mortality: A Multicountry, Multicity Study. Environ Health Perspect. 2019 Sep;127(9):97007. https://doi.org/10.1289/EHP5430
49
Buckley JP
,
Samet JM
,
Richardson DB
. Commentary: does air pollution confound studies of temperature? Epidemiology. 2014 Mar;25(2):242–5. https://doi.org/10.1097/EDE.0000000000000051
50
Biondi-Zoccai G
,
Frati G
,
Gaspardone A
,
Mariano E
,
Di Giosa AD
,
Bolignano A
, et al.
Impact of environmental pollution and weather changes on the incidence of ST-elevation myocardial infarction. Eur J Prev Cardiol. 2020 Jun; :2047487320928450.
Appendix
The Appendix is
available in the PDF version of this article.

