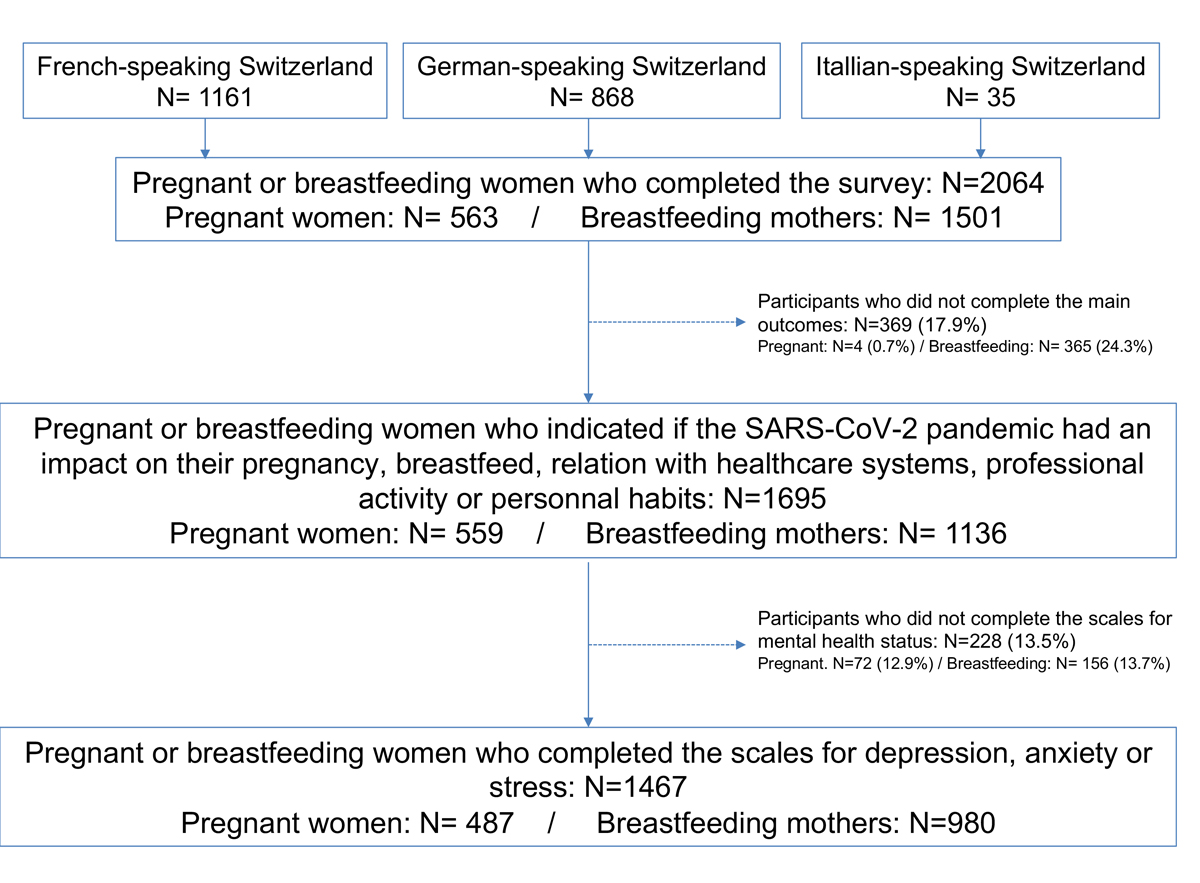
Figure 1 Flow chart.
DOI: https://doi.org/10.4414/SMW.2021.w30009
Since 11 March 2020, new virus SARS-CoV-2 expansion from China has been designated as a global pandemic by the World Health Organization (WHO) [1]. There have been over 108.2 million cases of the disease related to this virus (COVID-19) over 2.3 million deaths worldwide as of 19 February 2021 [2]. Certain categories of the population are more vulnerable to this virus, including pregnant women which have been described as a population at risk of severe outcomes [3–6]. To slow down the transmission of the virus, drastic and unprecedented measures were taken in many countries: containment, quarantine, and closure of schools, workplaces and stores [7]. In Switzerland, these measures were introduced during the first wave of the pandemic, from the middle of March to the end of May 2020 [8].
In addition to professional and financial concerns, pregnant and breastfeeding women may have been affected in their relationship with healthcare professionals and in their perinatal care, owing to restricted access to hospitals and postponement of non-urgent care. Furthermore, lack of information about this new virus may have generated concerns about maternal and fetal outcomes in the case of infection and could have influenced maternal choices with regard to personal restrictions, home birthing and breastfeeding. Such occurrences could have led to psychological distress, particularly because this specific population is vulnerable to mental health problems [9, 10]. According to the WHO, about 10% of women will experience symptoms of depression during pregnancy or post-partum independently of the COVID-19 pandemic [11]. Risks factors known to contribute to antenatal depression are lack of a partner or social support, unplanned pregnancy, comorbidities and a history of mental illness, and adverse/unplanned events modifying life habits [12]. Previous studies have indicated increased rates of depressive symptoms and anxiety among pregnant and breastfeeding women during the early stages of the COVID-19 pandemic and during quarantine [9, 13, 14]. However, the extent to which their experience of pregnancy and breastfeeding and their mental health have been affected by the pandemic remains poorly described. Furthermore, direct (exposure, hospitalisation, adverse outcomes) and indirect (alteration in personal and professional habits, access to prenatal care, fears) factors associated with mental health symptoms during the pandemic have not been clearly identified.
The first aim of this study was to describe the potential association between the changes in the pregnancy/breastfeeding experience in the COVID-19 pandemic and the changes related to the healthcare system and life and professional habits. The second aim was to describe pregnant and breastfeeding women’s mental health symptoms during the first wave of the pandemic, as well as sociodemographic, health, exposure and indirect factors contributing to such mental health symptoms.
This Swiss cross-sectional online study is part of a European multicentre study led by the University of Leuven and conducted in several countries (Belgium, Ireland, Norway, the Netherlands, the United Kingdom and Switzerland), with a questionnaire available in German, French and Italian used in Switzerland. In 2019, 62.1% of Swiss population spoke German as their main language, 22.8% French and 8.0% Italian [15]. The multicentre study aimed to examine the impact of the COVID-19 pandemic on pregnant and breastfeeding women from a broad perspective (on pregnancy/breastfeeding experience, relationship with the healthcare system, life and professional habits, personal fears, as well as mental health) [16]. In Switzerland, the questionnaire link was accessible from 18 June to 12 July 2020 through websites and forums dedicated to pregnant and breastfeeding women: www.letsfamily.ch (German and French),www.swissmom.ch (German and French), www.medela.ch (German, French and Italian), hospital website: www.chuv.ch (French), and social media, in the form of an advertisement inviting them to participate. All data were stored and handled anonymously.
Women aged 18 years or more, pregnant at the time of survey or who had breastfed during the previous 3 months, were eligible. The questionnaire was available in the three predominant national languages in Switzerland and women from each linguistic region had the opportunity to be represented.
The questionnaire was divided in several parts. The first part collected information on the current pregnancy (gravidity, parity, previous children, pregnancy planning status and gestational age) or on the infant and breastfeeding status (age of the infant, admission to a neonatal intensive care unit [NICU], change or cessation of breastfeeding because of SARS-CoV-2, precautions taken during breastfeeding) according to the situation of the participant. Information on how the pandemic influenced their pregnancy/breastfeeding experience compared with a previous experience and on how their relationship with healthcare services had been affected were also collected.
The second part recorded the exposure to COVID-19 using questions on potential symptoms, testing (reverse transcription polymerase chain reaction [RT-PCR], serology and/or computed tomography) and COVID-19 related hospitalisation. Fears and beliefs related to the infection were also gathered (fetal adverse outcomes, willingness to consider abortion in case of early infection).
The third part evaluated personal restrictions and mental health symptoms using validated screening tests: the Edinburgh Postnatal Depression Scale for depression [17, 18], the Generalized Anxiety Disorder 7-item Scale for anxiety [19] and the Perceived Stress Scale for stress [20, 21] .
Finally, information on sociodemographic and medical history was gathered, including country of residence, age, language, marital status, working status, education level, smoking during pregnancy, comorbidities, professional and financial situation during the pandemic, as well as information on people living in the same home (number, age and whether they presented COVID-19 symptoms or had been tested).
The impact of the pandemic on pregnancy or breastfeeding experience was evaluated through participants’ graded answers: “had a great impact”, “had rather an impact”, “had little impact” or “had no impact”. Open answers were also analysed and grouped according to frequently discussed themes, to illustrate more precisely how the pandemic and the restrictive measures had had an impact on these pregnancy and breastfeeding experiences.
The impact of the pandemic on the relationship with healthcare systems, on personal and professional habits, on fears of adverse maternal and fetal/neonatal outcomes were also assessed through graduated answers.
Women’s mental health was assessed through the validated screening instruments listed above and was interpreted as follows:
The prevalence of baseline characteristics and exposure (COVID-19 testing, symptoms and hospitalisation) was presented for both pregnant and breastfeeding women. The impact on their pregnancy and breastfeeding experience was presented as descriptive statistics. Fears, relationships with healthcare systems and mental health symptoms were also presented as descriptive statistics. The scores for depression, anxiety and perceived stress were presented as minimal/low, mild, moderate and severe/high according to the definition above.
A nested case-control study was conducted to compare sociodemographic and health characteristics, exposure parameters and impacts on personal and professional habits of pregnant and breastfeeding women, taken together, with high levels of mental health symptoms, considered as cases, with those of women with low levels of mental health symptoms, considered as controls. Having symptoms of severe depression score (EPDS ≥13), having a severe anxiety score (GAD-7 ≥15) or experiencing a high perceived stress score (PSS ≥7) were combined into a unique “poor mental health” variable for this analysis. These associations were estimated by univariable and multivariable logistic regression and were presented as crude odds radio (ORs) and adjusted odds ratios (aORs) with 95% confidence intervals (95% CIs). Variables were not considered for the multivariable model if p >0.10 in the univariable analysis. The association between variables specific to pregnant or breastfeeding women (gravidity, parity, pregnancy planning and current gestational age were asked only for pregnant women whereas hospitalisation of the neonate in an NICU was asked only for breastfeeding mothers) and “poor mental health” was estimated in additional univariable and multivariable models including only pregnant and breastfeeding women.
Maternal comorbidities were considered as absent if not reported, based on the assumption that severe comorbidities are normally well documented. Based on the hypothesis of missing variables completely at random (MCAR), multiple imputations (chained equations) were performed to increase the power of comparisons for missing covariates.
A total of 2064 respondents participated in the survey (1161 using the French, 868 the German and 35 the Italian questionnaires), including 1501 breastfeeding and 563 pregnant women. A total of 369 (17.9%) women did not complete the questions on the impact of the pandemic on pregnancy or breastfeeding experience. Therefore, 1695 (82.1%) contributed to the analyses addressing the primary aim of the study (1136 breastfeeding mothers and 559 pregnant women). Of these, 1467 (86.5%) patients completed the scales for depression, anxiety or stress, including 980 breastfeeding women and 487 pregnant women (fig. 1).

Figure 1 Flow chart.
Baseline characteristics are presented in table 1. The median age of responders was 33 years and the majority were married or cohabiting (73.0%). A significant proportion of women were healthcare providers (18.7%) or housewives (8.2%). The educational level was high (42.5% had an education level higher than high school) and 12.9% reported smoking during pregnancy and/or breastfeeding. Less than 5% reported a language different from the official ones. In total, 8.9% of women reported comorbidities. Among pregnant women, 94.1% of pregnancies were planned and the median gestational age at the time the women participated in the survey was 28 weeks. In total, 46.5% were pregnant for the first time. Among the multigravida participants, 74.2% had one previous infant and 18.4% had more than one infant.
Table 1Baseline and pregnancy characteristics, maternal comorbidities and SARS-CoV-2 exposure among 1695 participants.
| Pregnant women (n = 559) | Breastfeeding mothers (n = 1136) | Total ( n = 1695) | |||||
| Baseline characteristics | |||||||
| Maternal age (years), median (IQR) | 33 | (31–35) | 34 | (31–36) | 33 | (31–36) | |
| Marital status | Married / cohabiting | 422 | (75.5) | 815 | (71.7) | 1237 | (73.0) |
| Single / divorced / other | 4 | (0.7) | 9 | (0.8) | 13 | (0.8) | |
| Unspecified | 133 | (23.8) | 312 | (27.5) | 445 | (26.2) | |
| Working status | Healthcare provider | 122 | (21.8) | 195 | (17.2) | 317 | (18.7) |
| Employed other than HCP | 257 | (46.0) | 465 | (40.9) | 722 | (45.6) | |
| Student | 3 | (0.5) | 7 | (0.6) | 10 | (0.6) | |
| Housewife | 21 | (3.8) | 118 | (10.4) | 139 | (8.2) | |
| Job seeker | 12 | (2.2) | 23 | (2.0) | 35 | (2.1) | |
| Other, unspecified | 144 | (25.8) | 328 | (28.9) | 472 | (27.8) | |
| Educational level | Less than high school | 9 | (1.6) | 20 | (1.8) | 29 | (1.7) |
| High school | 75 | (13.4) | 212 | (18.7) | 287 | (16.9) | |
| More than high school | 257 | (46.0) | 464 | (40.9) | 721 | (42.5) | |
| Other, unspecified | 218 | (39.0) | 440 | (38.7) | 658 | (38.8) | |
| Language other than the official ones | 18 | (3.2) | 61 | (5.4) | 79 | (4.7) | |
| Maternal comorbidities | |||||||
| Any comorbidity | 51 | (9.1) | 100 | (8.8) | 151 | (8.9) | |
| Pulmonary | 14 | (2.5) | 28 | (2.5) | 42 | (2.5) | |
| Cardiovascular | 6 | (1.1) | 11 | (1.0) | 17 | (1.0) | |
| Pregestational diabetes | 5 | (0.9) | 9 | (0.8) | 14 | (0.8) | |
| Thyroid dysfunction | 12 | (2.1) | 27 | (2.4) | 39 | (2.3) | |
| Oncological | 1 | (0.2) | 2 | (0.2) | 3 | (0.2) | |
| Haematological | 2 | (0.4) | 0 | (0.0) | 2 | (0.1) | |
| Autoimmune | 2 | (0.4) | 4 | (0.4) | 6 | (0.4) | |
| Neurological | 3 | (0.5) | 4 | (0.4) | 7 | (0.4) | |
| Psychiatric | 3 | (0.5) | 6 | (0.5) | 9 | (0.5) | |
| Digestive | 3 | (0.5) | 7 | (0.6) | 10 | (0.6) | |
| Urogenital tract | 6 | (1.1) | 15 | (1.3) | 21 | (1.2) | |
| Cutaneous | 2 | (0.4) | 4 | (0.4) | 6 | (0.4) | |
| Ear, nose and throat | 0 | (0.0) | 1 | (0.1) | 1 | (0.1) | |
| Smoking | 69 | (12.3) | 149 | (13.1) | 218 | (12.9) | |
| Current pregnancy | |||||||
| Gravidity | 1 | 266 | (46.5) | ||||
| >1 | 299 | (53.5) | |||||
| Parity | 0 | 22/298 | (7.4) | ||||
| 1 | 221/298 | (74.2) | |||||
| >1 | 55/298 | (18.4) | |||||
| Planned pregnancy | 526 | (94.1) | |||||
| Getational age (weeks), median (IQR) | 28 | (18-34) | |||||
| SARS-CoV-2 exposure | |||||||
| Symptoms | 311 | (55.6) | 566 | (49.8) | 877 | (51.7) | |
| Hospitalized | 2 | (0.4) | 7 | (0.6) | 9 | (0.5) | |
| Tested for SARS-CoV-2 infection | 48 | (8.6) | 122 | (10.7) | 170 | (10.0) | |
| PCR on nasopharyngeal swab | 42 | (7.5) | 114 | (10.0) | 156 | (9.2) | |
| Positive | 7/42 | (16.7) | 6/114 | (5.3) | 13/156 | (8.3) | |
| Negative | 34/42 | (81.0) | 104/114 | (91.2) | 138/156 | (88.5) | |
| Unknown | 1/42 | (2.3) | 4/114 | (3.5) | 5/156 | (3.2) | |
| Serology | 10 | (1.8) | 21 | (1.8) | 31 | (1.8) | |
| Positive | 4/10 | (40.0) | 2/21 | (9.5) | 6/31 | (19.4) | |
| Negative | 5/10 | (50.0) | 16/21 | (76.2) | 21/31 | (67.7) | |
| Unknown | 1/10 | (10.0) | 3/21 | (14.3) | 4/31 | (12.9) | |
| CT scan | 2 | (0.4) | 2 | (0.2) | 4 | (2.4) | |
| Positive | 2/2 | (100.0) | 0/2 | (0.0) | 2/4 | (50.0) | |
| Negative | 0/2 | (0.0) | 2/2 | (100.0) | 2/4 | (50.0) | |
| Living with someone with symptoms | 82 | (14.7) | 220 | (19.4) | 302 | (17.8) | |
| Living with someone tested positive | 4 | (0.7) | 10 | (0.9) | 14 | (0.8) | |
CT: computed tomography; HCP: healthcare provider; PCR: polymerase chain reaction test
Data are presented as n (%) or median (inter-quartile range [IQR])
Overall, 51.7% (877/1695) of participants experienced symptoms potentially related to COVID-19 in the previous weeks but only 10.0% (170/1695) were tested and 0.5% (9/1695) were hospitalized. A total of 156 women were tested with PCR on a nasopharyngeal swab, among them 13 (8.3%) were positive. A serology test was performed in 31 participants and 6 (19.4%) were positive (some participants received both PCR and serology test). Only 4 women had a computed tomography scan and 2 (50.0%) were indicative of COVID-19-related pneumonia. Overall, 302 (17.8%) women indicated living with someone presenting symptoms potentially related to COVID-19 and only 14 (0.8%) were living with someone tested positive (table 1).
Overall, 36.8% (110/299) of pregnant women and 8.2% (46/555) of breastfeeding women who answered the question indicated that the pandemic had “rather an impact” (27.1% and 4.1%, respectively) or “had a great impact” (9.7% and 4.1%, respectively) on their current pregnancy experience or breastfeeding experience. Among pregnant women who specified how the pandemic impacted their pregnancy, 29/168 (17.3%) and 21/168 (12.5%) indicated they had “concerns about precautions” to take and experienced “some anxiety during social contact”, respectively. Among breastfeeding women who specified the impact of the pandemic in the open question, 9/30 (30.0%) indicated their breastfeeding experience was an “additional stress factor” during the pandemic. Among women experiencing symptoms potentially related to COVID-19, 16.4% (142/869) indicated that it had directly impaired their mental health during pregnancy or breastfeeding. Among pregnant women, 84.0% (432/515) believed maternal COVID-19 can affect the development of the unborn child, but only 3.7% (19/515) would have considered abortion in the event of maternal infection in early pregnancy. Among breastfeeding women, only 2.1% (22/1066) had already considered stopping breastfeeding because of SARS-Cov-2 and 16/172 (9.3%) specified in the open-ended question they would prefer to “stop earlier to avoid taking risks” (fig. 2.).
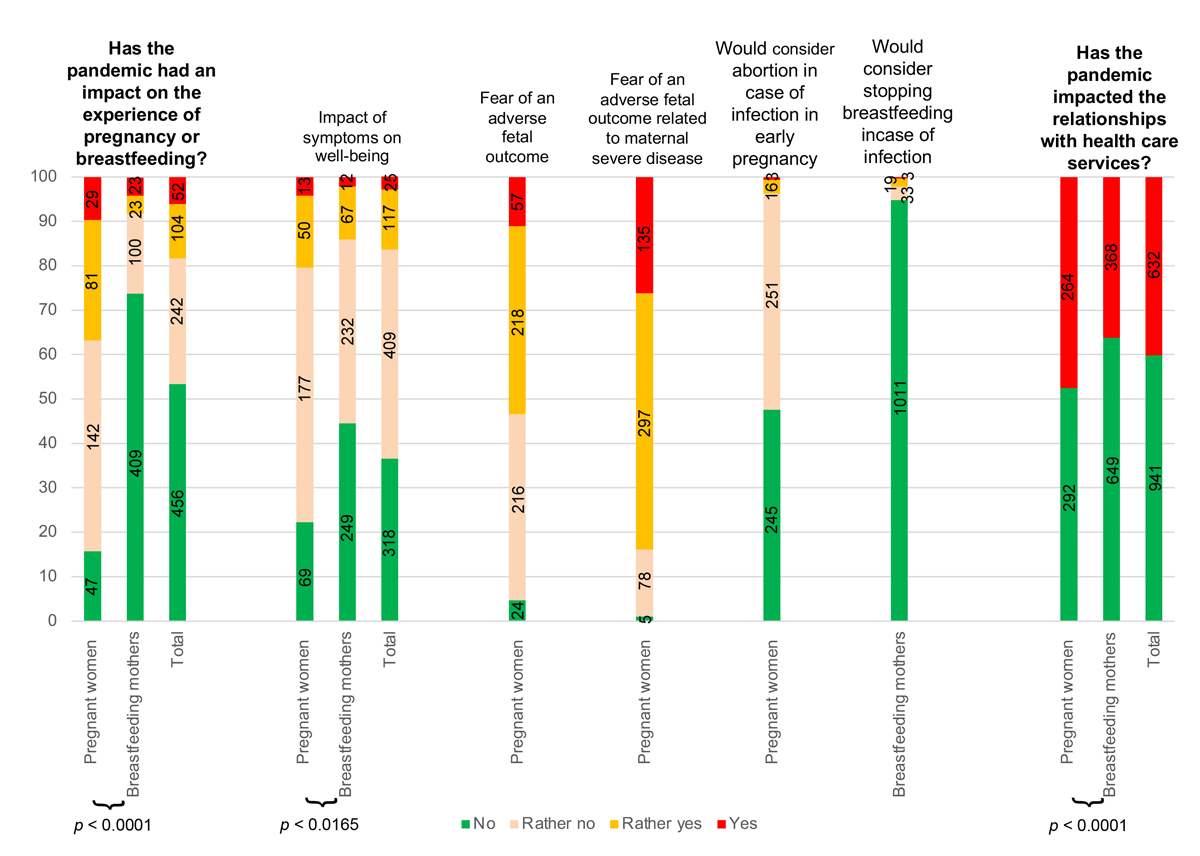
Figure 2 Impact of the COVID-19 pandemic on pregnancies, breastfeeding and relationships with healthcare services
A large majority (69.2%; 1050/1518) of both pregnant and breastfeeding women indicated that the pandemic restricted their life habits, such as self-imposed strict confinement because of “fear of catching the virus and that it will endanger breastfeeding”, as an example. A large majority of pregnant (71,1%; 295/415) and breastfeeding women (55%; 394/716) mentioned that the COVID-19 pandemic had an impact on their professional life, with negative (30/42, 71.4%) or positive (12/42, 28.6%) aspects, such as “positive impact of working from home with less stress and fatigue from the commuting, and more time to rest” but also “working from home and no daycare for my daughter, so it's hard to manage both and enjoy my pregnancy at the same time (e.g., shopping for baby)."
A substantial proportion of pregnant (47.5%; 264/556) and breastfeeding women (36.2%; 368/1017) indicated that the pandemic affected their interaction with healthcare services (fig. 2). Among pregnant women, “restricted visits to the medical office”, “absence of the father at the medical office or ultrasounds” and “the absence of care related to the comfort of pregnancy” were repeated concerns in 42/168 (25.0%), 70/168 (41.7%) and 16/168 (9.5%) women who answered the open-ended question, respectively. Among 172 breastfeeding women, half of them (51.7%, 89/172) reported they received less support during the pandemic.
Perinatal mental health measures among pregnant and breastfeeding women are presented in table 2. A total of 1467 patients completed the EPDS score (487 pregnant women and 980 breastfeeding women). Results of EPDS were comparable between pregnant and breastfeeding women with, in total, 75.8% (1112/1467) reporting no symptoms of depression, 13.7% (201/1467) symptoms of moderate dysphoria and 10.5% (154/1467) elevated symptoms of depression. Overall, 1422 women completed the GAD-7 score (468 pregnant women and 954 breastfeeding women). Results were similar between both groups, with 63.1% (897/1422) of all respondents having a score compatible with minimal anxiety, 29.6% (421/1422) mild anxiety, 5.7% (81/1422) moderate anxiety and 1.6% (23/1422) severe anxiety symptoms. Finally, 1332 participants completed the PSS with 45.4% (606/1332) receiving a score compatible with low perceived stress, 51.3% (683/1332) moderate perceived stress and 3.2% (43/1332) high perceived stress.
Table 2EPDS (Edinburgh Depression Scale), GAD (General Anxiety Disorder) and PSS (Perceived Stress Scale) scores in 1467 patients.
| Pregnant women (n = 487) | Breastfeeding mothers (n = 980) | p-value | ||||
| EPDS | Minimal (<10) | 362 | (74.3) | 750 | (76.5) | 0.3546 |
| Moderate (10–12) | 74 | (15.2) | 127 | (13.0) | 0.2409 | |
| Elevated (≥13) | 51 | (10.5) | 103 | (10.5) | 0.9822 | |
| GAD | Minimal (0–4) | 295/468 | (63.0) | 602/954 | (63.1) | 0.9799 |
| Mild (5–9) | 146/468 | (31.2) | 275/954 | (28.8) | 0.3575 | |
| Moderate (10–14) | 21/468 | (4.5) | 60/954 | (6.3) | 0.1683 | |
| Severe (15–21) | 6/468 | (1.3) | 17/954 | (1.8) | 0.4825 | |
| PSS | Low (<14) | 203/440 | (46.1) | 403/892 | (45.2) | 0.7415 |
| Moderate (14–26) | 228/440 | (51.8) | 455/892 | (51.0) | 0.7811 | |
| High (>26) | 9/440 | (2.0) | 34/892 | (3.8) | 0.0863 | |
Data are presented as n (%); p-values estimated using chi2 tests
The results of the case-control study that evaluated the association between women presenting a poor perinatal mental health status (elevated symptoms of depression, severe anxiety or high perceived stress) and several known risk factors are presented in table 3. In total, 170 (11.6%) women (pregnant and breastfeeding together) presented poor mental health symptoms out of 1467 respondents and were considered as cases. In the multivariate analysis, being a housewife (aOR 1.89, 95% CI 1.05–3.43), having maternal comorbidity (aOR 1.73, 95% CI 1.00–3.01), presenting symptoms potentially related to COVID-19 (aOR 1.69, 95% CI 1.06–2.69), being hospitalized (aOR 5.8, 95% CI 1.41–23.84), living with someone who presented symptoms (aOR 1.71, 95% CI 1.08–2.70), having personal habits affected (aOR 2.37, 95% CI 1.37–4.10), or having restricted access to healthcare services (aOR 2.00, 95% CI 1.30–3.09) were associated with a higher risk of psychological distress / mental health impairment. Among breastfeeding women, having a neonate admitted to a NICU was associated with a higher risk of psychological distress / mental health impairment (OR 3.09, 95% CI 1.33–7.19). Protective factors among pregnant and breastfeeding women were having a high level of education (aOR 0.56, 95% CI 0.36–0.89), and being married or cohabiting with a partner (aOR 0.20, 95% CI0.04–1.12) (table 3).
Table 3Factors associated with an altered mental health status in 1467 patients.
| Baseline characteristics | Women with altered mental health status | Women with normal mental health status | OR | (95% CI) | aOR d | (95% CI) | |||
| n = 170 | 11.6% | n = 1297 | 88.4% | ||||||
| Baseline characteristics | |||||||||
| Maternal age >40 years | 10 | (5.9) | 72 | (5.6) | 1.06 | (0.54–2.10) | |||
| Married or cohabiting | 142/146a | (97.3) | 1095/1104a | (99.2) | 0.29a | (0.08–0.96) | 0.20 | (0.04–1.12) | |
| Healthcare provider | 33 | (19.4) | 284 | (21.9) | 0.85 | (0.57–1.28) | |||
| Housewife | 26 | (15.3) | 113 | (8.7) | 1.89 | (1.19–2.99) | 1.89 | (1.05–3.43) | |
| Educational level >high school | 70/117* | (59.8) | 651/920* | (70.8) | 0.61 | (0.41–0.94) | 0.56 | (0.36–0.89) | |
| Language other than the official ones | 9/146* | (6.2) | 70/1104 | (6.3) | 0.97 | (0.47–1.99) | |||
| Any maternal comorbidity | 28 | (16.5) | 123 | (9.5) | 1.88 | ||||
| Smoking | 32 | (18.8) | 186 | (14.3) | 1.38 | (1.21–2.94) | 1.73 | (1.00–3.01) | |
| Current pregnancy | |||||||||
| First pregnancy | 27/53b | (50.9) | 202/434b | (46.5) | 1.19 | (0.67–2.11) | |||
| Previous children | 51/53b | (96.2) | 417/434b | (96.1) | 1.03 | (0.23–4.63) | |||
| Planned pregnancy | 49/53b | (92.5) | 408/434b | (94.0) | 0.78 | (0.26–2.33) | |||
| Gestational age | 1st trimester | 9/53b | (17.0) | 65/434b | (15.0) | 1.11 | (0.45–2.43) | ||
| 2nd trimester | 20/53b | (37.7) | 166/434b | (38.2) | 0.85 | (0.37–1.98) | |||
| 3rd trimester | 24/53b | (45.3) | 203/434b | (46.8) | 0.84 | (0.37–1.90) | |||
| Neonate admitted in NICU | 8/117c | (6.8) | 20/863c | (2.3) | 3.09 | (1.33–7.19) | |||
| SARS-COV-2 exposure | |||||||||
| Symptoms | 114 | (67.1) | 692 | (53.4) | 1.78 | (1.27–2.50) | 1.69 | (1.06–2.69) | |
| Hospitalized for COVID-19 | 5 | (2.9) | 4 | (0.3) | 9.80 | (2.60–38.84) | 5.80 | (1.41–23.84) | |
| Tested positive for SARS-COV-2 on RT-PCR | 1 | (0.6) | 11 | (0.8) | 0.69 | (0.09–5.39) | |||
| Living with someone with symptoms | 48 | (28.2) | 254 | (19.6) | 1.61 | (1.12–2.31) | 1.71 | (1.08–2.70) | |
| Living with someone tested positive | 1 | (0.6) | 13 | (1.0) | 0.58 | (0.08–4.50) | |||
| Impact of the SARS-COV-2 pandemic on | |||||||||
| Professional situation | 77/128a | (60.2) | 612/1003a | (61.0) | 0.96 | (0.66–1.40) | |||
| Life habits | 143 | (84.1) | 875 | (67.5) | 2.55 | (1.67–3.92) | 2.37 | (1.37–4.10) | |
| Relationship with healthcare systems | 88/159a | (55.3) | 473/1240a | (38.1) | 2.00 | (1.44–2.80) | 2.00 | (1.30–3.09) | |
aOR: adjusted odds ratio; CI: confidence interval; OR: odds ratio; RT-PCR: reverse transcription polymerase chain reaction
a Multiple imputations were performed on missing values, for variables with a denominator different from the overall denominator
b Tested only in pregnant women
c Tested only in breastfeeding women
d Only significant risk factors have been included in the multivariable analysis
The first wave of the COVID-19 pandemic had a significant impact on the current pregnancy or breastfeeding experiences of one-fifth of the sample of Swiss women. Two thirds of pregnant and breastfeeding women restricted their life habits during the first containment period in Switzerland in Spring 2020. The pandemic significantly altered their relationship with healthcare services according to half of the pregnant women and one third of the breastfeeding women. More than half of the breastfeeding women felt less supported during this period. Moreover, 11.6% of participants reported significant psychological distress (symptoms of severe depression, severe anxiety or high perceived stress). Potential risk factors associated with poor mental health status were being a housewife, having comorbidities, presenting symptoms potentially related to COVID-19, being hospitalized, living with someone presenting symptoms, having personal habits affected and having restricted access to healthcare services. Potential protective factors associated with fewer symptoms of potential mental health problems were a high level of education and living with a partner.
The prevalence of symptoms potentially compatible with COVID-19 among respondents seems to be high in our sample (54.9%; 806/1467). Some of the symptomatic participants may have had other infections indistinguishable from COVID-19. Surprisingly, a very low proportion of the participants reported having been tested (10.0%; 170/1695) despite the high prevalence of reported symptoms. This might reflect the testing policy at the beginning of the pandemic, the limited availability of nasopharyngeal RT-PCR or the reluctance to visit a health facility at the time of the first wave.
Overall, the rate of Swiss pregnant and breastfeeding women who reported poor mental health in our survey (11.6%) is comparable to the 10% of pregnant women experiencing clinically significant mental health problems worldwide before the COVID-19 pandemic, according to the WHO [11] . This rate is also similar to the 11.7% of the general Swiss population surveyed between 11 May and 1 June 2020, as described by Quervain et al. They noticed an increase in the prevalence of moderate and severe depressive symptoms compared with the pre-pandemic period (3.4%) [25]. However, a few previous studies have shown that middle- and lower-income countries have a higher prevalence of poor mental health in the perinatal period compared with western countries [26, 27] . In high-income countries such as Switzerland, 9.5% of women of childbearing age had moderate or severe major depression in 2017 [28]. Moreover, we selected only women presenting severe mental health symptoms, and therefore have underestimated the impact of moderate psychological distress, as found in a study by Wu et al. [29].
In the literature, several studies highlighted similar results on the impact of the COVID-19 pandemic on perinatal mental health of women living in different countries [10, 13, 29–34]. Most of them focused on pregnant women who experienced a range of disorders, such as anxiety, symptoms of depression and sleep disturbances during the COVID-19 pandemic. Extreme changes in daily life have been associated with an increased risk of depression, in line with our findings [30] . However, it is interesting to note that in our results, a quarter of the women indicating that the pandemic has had an impact on their daily lives described a positive impact, particularly the implementation of “working from home”, which reduced their stress and fatigue. Low education level and working part-time or less were risk factors for depression according to Wu et al. [29]. This supports our finding of a high educational level as a protective factor for psychological distress and being a housewife as a risk factor. Low familial support could participate in developing a depression [29], just as having good social support seems protective against stress [10, 35]. This is in line with our finding of a possible protective effect of being married / cohabiting. Changes in prenatal care during the confinement period has been depicted as a stress factor by Preis et al. [36] which seems in accordance with the impact of restricted access to healthcare services on perinatal mental health in our study. Among the general Chinese population, having confirmed or suspected COVID-19 or having relatives with confirmed or suspected COVID-19 were factors associated with poor mental health according to Shi et al. [37] , in line with our findings. Future studies might investigate the role of other protective factors against stress and depression, such as adequate sleep duration [10] and regular physical activity [29, 36] .
During the pandemic, a higher percentage of women with thoughts of self-harm has been found in China [29]. Moreover, mental health issues have a severe impact on quality of life, social and partner relationships and on child development [38, 39]. Therefore, future searches for effective interventions to manage potential psychological distress among pregnant and breastfeeding women are urgently needed. Psychological first aid and telehealth could be appropriate tools to support pregnant and breastfeeding women with mental health problems during outbreaks [40].
The large number of participants and the fact that the survey was performed in three official languages (women from different parts of Switzerland were reached) are important strengths of the present study. The content analysis of the open-ended quotations provides a good overview of the reality of these women during this outbreak.
However, sociodemographic characteristics of the participants differ from those presented by the official Swiss statistics (appendix), particularly regarding age distribution, professional activity and education. Women who participated in our survey seem to be more representative of a population over 26 years of age, with a high level of education and a high activity rate, preventing the generalisation of these results to the general population of Swiss pregnant and breastfeeding women. Although Swiss women do have good internet access (95%) [15], selection bias in favour of more motivated or concerned women could have occurred, as the survey was online. Women seeking information about their pregnancy or breastfeeding were more likely to encounter the online survey than less worried women. They may represent a more educated population of website users. The high proportion of healthcare providers participating in this survey (18.7%) may also represent a more educated population, leading to a potential selection bias for a protective factor for mental health, which could underestimate the prevalence of psychological distress. Severely ill women were most probably not included as they would not have the capacity to participate. In our survey, 4.7% of respondents indicated speaking another language as main language, whereas a higher proportion (17.7%) of people living in Switzerland report speaking language other than the official ones [28] . Therefore, it is possible that the immigrant population was underrepresented. The high proportion of French-speaking answers compared with the proportion of Swiss people who speak French as their primary language (23%) could reflect a selection bias, partly explained by the fact that the distribution of the survey was initiated by the CHUV (Centre Hospitalier Universitaire Vaudois, university hospital of the biggest French-speaking canton) [15]. Another explanation may be that the French-speaking part of Switzerland has been more seriously affected by the pandemic than the German-speaking part [41]. This may have led to an overrepresentation of one of the linguistic regions.
A limitation could be the fact that symptoms could not specifically be assigned to SARS-CoV-2 infection.
The mental health scores only measure depressive symptoms, anxiety and stress experienced over the last 4 weeks, preventing us from drawing any conclusions on mental health status during the peak of the first pandemic wave.
The study was a cross-sectional study and no information was collected on the long-term impact of the pandemic on mental health and perinatal experiences.
The drop-out from the survey (17.9%) could be attributed to the length of the questionnaire, with a certain proportion of open-ended questions. Some questions might have also triggered emotions (fear, irritability or feeling misunderstood, for example) and might thus have been skipped by participants, selecting women with a lower risk of psychological distress, thus violating the assumption of information missing at random. Finally, recall bias as a result of better recall among women with stronger negative experiences due to the pandemic cannot be excluded.
Our findings suggest that the first wave of the COVID-19 pandemic may have had a severe impact on the mental health and perinatal experiences of pregnant and breastfeeding women in Switzerland, both directly through exposure to SARS-CoV-2, and indirectly through the impact on their life habits and contact with healthcare facilities. Prevention and support strategies should be set-up to counter these consequences of the COVID-19 epidemic for maternal-child health.
All authors declare no support from any organisation for the submitted work, no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years, no other relationships or activities that could appear to have influenced the submitted work.
1. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020 [Internet]. [cited 2020 Apr 9]. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
2. Coronavirus Disease (COVID-19) Situation Reports [Internet]. [cited 2020 Sep 23]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
3. Allotey J , Stallings E , Bonet M , Yap M , Chatterjee S , Kew T , et al.; for PregCOV-19 Living Systematic Review Consortium . Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020 Sep;370:m3320. https://doi.org/10.1136/bmj.m3320
4. Martinez‐Portilla RJ , Sotiriadis A , Chatzakis C , Torres‐Torres J , Sosa SE , Sandoval‐Mandujano K , et al. Pregnant women with SARS-CoV-2 infection are at higher risk of death and severe pneumonia: propensity score-matched analysis of a nationwide prospective cohort study (COV19Mx). Ultrasound in Obstetrics & Gynecology [Internet]. [cited 2020 Dec 27];n/a(n/a). Available from: https://obgyn.onlinelibrary.wiley.com/doi/abs/10.1002/uog.23575
5. Zambrano LD , Ellington S , Strid P , Galang RR , Oduyebo T , Tong VT , et al.; CDC COVID-19 Response Pregnancy and Infant Linked Outcomes Team . Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020 Nov;69(44):1641–7. https://doi.org/10.15585/mmwr.mm6944e3
6. Jering KS , Claggett BL , Cunningham JW , Rosenthal N , Vardeny O , Greene MF , et al. Clinical Characteristics and Outcomes of Hospitalized Women Giving Birth With and Without COVID-19. JAMA Intern Med [Internet]. 2021 Jan 15 [cited 2021 Feb 16]; Available from: https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2775396
7. Flaxman S , Mishra S , Gandy A , Unwin HJ , Mellan TA , Coupland H , et al.; Imperial College COVID-19 Response Team . Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature. 2020 Aug;584(7820):257–61. https://doi.org/10.1038/s41586-020-2405-7
8. fédéral LC. Page d’accueil [Internet]. [cited 2021 Feb 15]. Available from: https://www.admin.ch/gov/fr/start.html
9. Brooks SK , Webster RK , Smith LE , Woodland L , Wessely S , Greenberg N , et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020 Mar;395(10227):912–20. https://doi.org/10.1016/S0140-6736(20)30460-8
10. Lebel C , MacKinnon A , Bagshawe M , Tomfohr-Madsen L , Giesbrecht G . Elevated depression and anxiety among pregnant individuals during the COVID-19 pandemic [Internet]. PsyArXiv; 2020 Apr [cited 2020 Sep 22]. Available from: https://osf.io/gdhkt https://doi.org/10.31234/osf.io/gdhkt
11. Maternal mental health [Internet]. [cited 2020 Dec 27]. Available from: https://www.who.int/teams/maternal-newborn-child-adolescent-health-and-ageing/maternal-health/about/mental-health-and-substances-use
12. Biaggi A , Conroy S , Pawlby S , Pariante CM . Identifying the women at risk of antenatal anxiety and depression: A systematic review. J Affect Disord. 2016 Feb;191:62–77. https://doi.org/10.1016/j.jad.2015.11.014
13. Ceulemans M , Hompes T , Foulon V . Mental health status of pregnant and breastfeeding women during the COVID-19 pandemic: A call for action. Int J Gynaecol Obstet. 2020 Oct;151(1):146–7. https://doi.org/10.1002/ijgo.13295
14. Liu X , Chen M , Wang Y , Sun L , Zhang J , Shi Y , et al. Prenatal anxiety and obstetric decisions among pregnant women in Wuhan and Chongqing during the COVID-19 outbreak: a cross-sectional study. BJOG. 2020 Sep;127(10):1229–40. https://doi.org/10.1111/1471-0528.16381
15. Office FS . Federal Statistical Office [Internet]. [cited 2021 Feb 20]. Available from: https://www.bfs.admin.ch/bfs/en/home.html
16. Ceulemans M , Foulon V , Ngo E , Panchaud A , Winterfeld U , Pomar L , et al. Mental health status of pregnant and breastfeeding women during the COVID-19 pandemic-A multinational cross-sectional study. Acta Obstet Gynecol Scand. 2021 Jul;100(7):1219–29. https://doi.org/10.1111/aogs.14092
17. Cox JL , Holden JM , Sagovsky R . Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987 Jun;150(6):782–6. https://doi.org/10.1192/bjp.150.6.782
18. Bergink V , Kooistra L , Lambregtse-van den Berg MP , Wijnen H , Bunevicius R , van Baar A , et al. Validation of the Edinburgh Depression Scale during pregnancy. J Psychosom Res. 2011 Apr;70(4):385–9. https://doi.org/10.1016/j.jpsychores.2010.07.008
19. Spitzer RL , Kroenke K , Williams JB , Löwe B . A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006 May;166(10):1092–7. https://doi.org/10.1001/archinte.166.10.1092
20. Cohen S , Kamarck T , Mermelstein R . A global measure of perceived stress. J Health Soc Behav. 1983 Dec;24(4):385–96. https://doi.org/10.2307/2136404
21. Taylor JM . Psychometric analysis of the Ten-Item Perceived Stress Scale. - PsycNET [Internet]. [cited 2020 Dec 28]. Available from: https://content.apa.org/record/2014-44666-001
22. Department of Health, Government of Western Australia . District of Columbia’s HealthCheck Provider Education System - EPDS Translations (Govt Western Australia).pdf [Internet]. 2006 [cited 2021 Feb 22]. Available from: https://www.dchealthcheck.net/
23. Kozinszky Z , Dudas RB . Validation studies of the Edinburgh Postnatal Depression Scale for the antenatal period. J Affect Disord. 2015 May;176:95–105. https://doi.org/10.1016/j.jad.2015.01.044
24. Teissèdre F , Chabrol H . Detecting women at risk for postnatal depression using the Edinburgh Postnatal Depression Scale at 2 to 3 days postpartum. Can J Psychiatry. 2004 Jan;49(1):51–4. https://doi.org/10.1177/070674370404900108
25. de Quervain D , Aerni A , Amini E , Bentz D , Coynel D , Gerhards C , et al. The Swiss Corona Stress Study [Internet]. OSF Preprints; 2020 [cited 2021 May 15]. Available from: https://osf.io/jqw6a/
26. Fisher J , Cabral de Mello M , Patel V , Rahman A , Tran T , Holton S , et al. Prevalence and determinants of common perinatal mental disorders in women in low- and lower-middle-income countries: a systematic review. Bull World Health Organ. 2012 Feb;90(2):139G–49G. https://doi.org/10.2471/BLT.11.091850
27. Sawyer A , Ayers S , Smith H . Pre- and postnatal psychological wellbeing in Africa: a systematic review. J Affect Disord. 2010 Jun;123(1-3):17–29. https://doi.org/10.1016/j.jad.2009.06.027
28. statistique O fédéral de la. Office fédéral de la statistique [Internet]. [cited 2021 Jan 24]. Available from: https://www.bfs.admin.ch/bfs/fr/home.html
29. Wu Y , Zhang C , Liu H , Duan C , Li C , Fan J , et al. Perinatal depressive and anxiety symptoms of pregnant women during the coronavirus disease 2019 outbreak in China. Am J Obstet Gynecol. 2020 Aug;223(2):240.e1–9. https://doi.org/10.1016/j.ajog.2020.05.009
30. Saccone G , Florio A , Aiello F , Venturella R , De Angelis MC , Locci M , et al. Psychological impact of coronavirus disease 2019 in pregnant women. Am J Obstet Gynecol. 2020 Aug;223(2):293–5. https://doi.org/10.1016/j.ajog.2020.05.003
31. Taubman-Ben-Ari O , Chasson M , Abu Sharkia S , Weiss E . Distress and anxiety associated with COVID-19 among Jewish and Arab pregnant women in Israel. J Reprod Infant Psychol. 2020 Jul;38(3):340–8. https://doi.org/10.1080/02646838.2020.1786037
32. Corbett GA , Milne SJ , Hehir MP , Lindow SW , O’connell MP . Health anxiety and behavioural changes of pregnant women during the COVID-19 pandemic. Eur J Obstet Gynecol Reprod Biol. 2020 Jun;249:96–7. https://doi.org/10.1016/j.ejogrb.2020.04.022
33. Nanjundaswamy MH , Shiva L , Desai G , Ganjekar S , Kishore T , Ram U , et al. COVID-19-related anxiety and concerns expressed by pregnant and postpartum women-a survey among obstetricians. Arch Womens Ment Health. 2020 Aug 25;
34. Stepowicz A , Wencka B , Bieńkiewicz J , Horzelski W , Grzesiak M . Stress and Anxiety Levels in Pregnant and Post-Partum Women during the COVID-19 Pandemic. Int J Environ Res Public Health. 2020 Dec;17(24):9450. https://doi.org/10.3390/ijerph17249450
35. Reid KM , Taylor MG . Social support, stress, and maternal postpartum depression: A comparison of supportive relationships. Soc Sci Res. 2015 Nov;54:246–62. https://doi.org/10.1016/j.ssresearch.2015.08.009
36. Preis H , Mahaffey B , Heiselman C , Lobel M . Vulnerability and resilience to pandemic-related stress among U.S. women pregnant at the start of the COVID-19 pandemic. Soc Sci Med. 2020 Dec;266:113348. https://doi.org/10.1016/j.socscimed.2020.113348
37. Shi L , Lu ZA , Que JY , Huang XL , Liu L , Ran MS , et al. Prevalence of and Risk Factors Associated With Mental Health Symptoms Among the General Population in China During the Coronavirus Disease 2019 Pandemic. JAMA Netw Open. 2020 Jul;3(7):e2014053. https://doi.org/10.1001/jamanetworkopen.2020.14053
38. Slomian J , Honvo G , Emonts P , Reginster JY , Bruyère O . Consequences of maternal postpartum depression: A systematic review of maternal and infant outcomes. Womens Health (Lond) [Internet]. 2019 Apr 29 [cited 2021 Feb 20];15. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6492376/
39. Brummelte S , Galea LA . Postpartum depression: Etiology, treatment and consequences for maternal care. Horm Behav. 2016 Jan;77:153–66. https://doi.org/10.1016/j.yhbeh.2015.08.008
40. Antenatal and postnatal mental health: clinical management and service guidance. NICE 2021. Available from: https://www.nice.org.uk/guidance/cg192. Last updated 11 February 2020.
41. COVID-19 Suisse | Coronavirus [Internet]. [cited 2021 Jan 24]. Available from: https://www.covid19.admin.ch/fr/overview
Table S1Comparison between the general birthing population and the study sample of pregnant and breastfeeding women in Switzerland.
| General population* | Pregnant (n = 563) | Lactation (n = 1193) | ||
| Maternal age (years) | 18–25 | 8.1 | 2.8 | 1.7 |
| 26–30 | 26.4 | 25.1 | 20.5 | |
| 31–35 | 39.3 | 47.5 | 49.7 | |
| 36–40 | 21.8 | 22.0 | 23.1 | |
| >40 | 4.4 | 2.6 | 4.9 | |
| Parity | Nulliparous | 45.3 | 51.1 | N/A |
| Multiparous | 54.7 | 48.9 | N/A | |
| Professional status | Professionally active | 83.0 | 91.0 | 81.7 |
| Highest education level | Low | 42.7 | 25.7 | 33.1 |
| Medium | 12.0 | 23.4 | 17.8 | |
| High | 45.3 | 50.9 | 49.1 | |
| Smoking in pregnancy | Yes | 7.0 | 5.9 | N/A |
| No | 93.0 | 94.1 | N/A |
Data are presented as %. N/A: not available
* Statistics for the general birthing population in Switzerland were retrieved from the Federal Statistical Office (FSO) in 2019 for maternal age, professional status (aged 25–54 years), education level (aged 25–34 years) and 2018 for parity (aged 25–44 years) (https://www.bfs.admin.ch). Smoking in pregnancy in Switzerland has been monitored on behalf of FSO between 2011 and 2016 (https://www.infoset.ch/fr/tabac.html ).