Highly specific and reliable in vitro diagnostic analysis of memory T and B lymphocytes in a Swiss cohort of COVID-19 patients
DOI: https://doi.org/10.4414/SMW.2021.w30005
Lester
Thooa, Pierre I.
Gumowskibc, Kevin
Kammermanna, Swelia
Nusslia, Benno
Grabscheida, Oliver
Hausmannade, Ulrika
Axiusa, Werner J.
Pichlera, Daniel
Yerlya
a ADR-AC GmbH, Adverse Drug Reactions – Analysis and Consulting, Bern, Switzerland
b INRAAIC, Meyrin, Geneva, Switzerland
cClinical Immunology, Hôpital de la Tour, Meyrin, Switzerland
d Löwenpraxis Luzern, Lucerne, Switzerland
e Klinik St. Anna, Lucerne, Switzerland
Summary
The SARS-CoV-2 pandemic has claimed many lives and disrupted the quality of life of most individuals. Diagnostic tests not only serve to confirm past exposure but can provide information crucial for guiding healthcare options for patients. Current diagnostic tests for the presence of the SARS-CoV-2 virus or anti-spike protein antibodies do not address the question whether longer lasting cellular immunity is mounted in most individuals. Using an activation marker flow cytometric immunoassay (SARS-CoV-2 lymphocytes analysis), we showed that both CD4+/CD8+ T cell and B cell activation differ between naïve and infected individuals up to 11 months after infection. On the basis of the specificity of this diagnostic tool for detecting both SARS-CoV-2-experienced T and B cells, we propose that this assay could benefit immunocompromised individuals who are unable to mount sustained antibody responses, by determining cellular immunity as possible partial protection, and for studying immune correlates of protection – thereby increasing knowledge of COVID-19 in a wider range of patient groups.
Introduction
The 2019 novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pandemic has emphasised the need for efficient management of population health in the context of infectious diseases. Although physical distancing and “lockdown” measures prevented hospital saturation and mortality, these measures are not sustainable in the long term. Diagnostic tests are therefore indispensable in the strategic management of this pandemic. Currently, there are two major types of diagnostic tool, which are most informative at different stages of the disease: (1) virus detection around symptom onset (active infection) or (2) host antibody testing after symptom onset (past infection) [1, 2].
The design of vaccines for protective immunity against SARS-CoV-2 has focused on inducing neutralising antibodies against the viral spike (S) protein at its receptor-binding domain, thus blocking binding to the angiotensin converting-enzyme-2 receptor and preventing cell entry [3, 4]. The two COVID-19 vaccines currently available in Switzerland, Comirnaty® (BNT162b2; Pfizer/BioNTech) and the Moderna COVID-19 vaccine (mRNA-1273; Moderna) — approved by Swissmedic, the Swiss authorisation and supervisory authority for drugs and medical products — are both mRNA vaccines encoding the SARS-CoV-2 S protein [5]. It is encouraging that both vaccines induce robust antibody and CD4+/CD8+ T cell responses against the S protein [3, 5–7], but cellular immune responses to other viral antigens are not induced. Other viral antigens besides the S protein, such as the nucleocapsid or open reading frames, are, however, probably important for overall immunity since they are able to induce stronger CD8+ T cell responses, which help kill virus-infected cells [8]. This indicates that, although it is important to prevent entry of SARS-CoV-2 into cells via the S protein´s receptor-binding domain (which predominantly induces antibodies and CD4+ T cells), other antigens also contribute to stimulating a more complete immune response (e.g., CD8+ T cells), which improves the likelihood of persistent protection [9]. There is therefore a need to focus not just on the S protein, but also on the capacities of other viral antigens to induce immune responses, because of the inconsistencies in antigenic stimulation of immune responses [10]. Further support for the relevance of T cell responses against SARS-CoV-2 comes from the observation that the severity of COVID-19 correlates with the T cell profile of the individual, typically with higher or normal T cell counts in individuals with mild symptoms. In contrast, individuals with moderate and severe COVID-19 present with CD4+/CD8+ T cell lymphopenia [11]. Currently, there is a lack of COVID-19 diagnostic tests for memory T cells due to the need for cell culture, which requires specialised laboratory expertise and tools. However, with the growing evidence of of the relevance of T cell in COVID-19 severity and with vaccination programmes starting, it is crucial to be able to monitor an individual´s comprehensive immune profile against this disease, which is still puzzling in its presentation and severity.
Here, we sought to assess the feasibility of detecting and discerning anti-SARS-CoV-2 memory lymphocytes in an in vitro diagnostic assay starting with collection of whole blood from both COVID-19 patients and uninfected donors. To assess this, we measured upregulation of memory lymphocyte activation markers using an activation marker assay [12–14] after in vitro viral antigen stimulation. We further assessed correlations of memory lymphocyte activation with anti-SARS-CoV-2 spike antibody titres.
Aims of the study
(1) Comparison of distinguishable and lasting memory helper CD4+ and cytotoxic CD8+ T cell activation between naïve and infected individuals with an in vitro diagnostic immunoassay (SARS-CoV-2 lymphocyte analysis).
(2) Detection of activated memory T cells against other SARS-CoV-2 antigens (membrane and nucleocapsid) in addition to the S protein.
(3) Development of a diagnostic testing analysis for memory T cells against SARS-CoV-2 to complement antibody testing, hence providing an in-depth immunological profile to improve clinical understanding of immune correlates of protection, particularly for immunodeficient individuals.
Materials and methods
Cohort and ethics approval
Convalescent patients from the out-patient clinic in the Institut de recherche Appliquée en Allergologie et Immunologie Clinique (INRAAIC) or the Immunology Clinic of the Hôpital de la Tour, Meyrin, were recruited from individuals who had previously given consent to participate in observational studies and in the data bank programme of the institute. A complementary signed informed consent form explaining the purpose of the study was also obtained from all subjects. Both sexes (25 males and 15 females) of all ages (9–78 years old) were included in the study. Individuals were considered convalescent if they had past history of a positive test for SARS-CoV-2 antigen and/or antibody (within 6 weeks of symptoms). Unexposed control individuals were volunteers with no known COVID-19 symptoms nor anti-S1 IgG antibodies. Thirty millilitres of whole blood were collected for the lymphocyte analysis. Anti-S IgG antibody titres were measured using an anti-SARS-CoV-2 enzyme-linked immunosorbent assay (ELISA) directed against the S1 domain of the spike proteins (EUROIMMUN Medizinische Labordiagnostika AG, Lübeck, Germany).
In vitro culture conditions
Peripheral blood mononuclear cells (PBMCs) were isolated from heparin-treated room-temperature whole blood using Ficoll-Paque PLUS (density 1.077 g/ml; VWR) gradient centrifugation at 2000 rpm for 30 minutes at 24°C. Following two washes in 1X phophate-buffered saline (PBS; pH 7.4; Gibco), PBMCs were resuspended in Roswell Park Memorial Institute (RPMI)-1640 medium without L-glutamine (VWR) supplemented with 25 mM HEPES (VWR), 10% human serum albumin (Octapharm) and 1% GlutaMAX (Gibco). Then 1x106 PBMCs were plated into the wells of a U-bottom 96-well plate and stimulated with individual SARS-CoV-2 antigens (1 µg/ml recombinant proteins or 10 µg/ml peptide mixes); cells stimulated with recombinant proteins were cultured independently of those stimulated with peptides. The positive control for T and B cells stimulation was 1 µg/ml phytohaemagglutinin (PHA). Negative assay controls were cells cultured in medium alone or with 0.56% dimethyl sulphoxide (DMSO; solvent for peptides). Cells were kept in a 5% CO2 incubator at 37°C for 5 days. At day 5, cells were processed for flow cytometric analyses.
Design of the SARS-CoV-2 recombinant proteins and peptides
Recombinant structural S1, S2 and nucleocapsid proteins of SARS-CoV-2 produced in a human HEK293 cell line expression system were purchased from RayBiotech (Peachtree Corners, Georgia). SARS-CoV-2 peptides from S, nucleocapsid and membrane proteins (28 S peptides, 18 nucelocapsid peptides, 11 membrane peptides) were self-designed using the T Cell Epitope Prediction Tool on the Immune Epitope Database and Analysis Resource website (www.iedb.org) for fit within 11 human leucocyte antigen (HLA) supertypes (6 HLA-A and 5 HLA-B) under the three criteria important for peptide loading on HLAs: affinity to peptide groove, proteosomal processing and peptide transport into the endoplasmic reticulum. Suitable peptides were synthesised by ProImmune Ltd. (Oxford, UK).
Flow cytometry
After 5 days of in vitro stimulation, PBMCs were stained with 100 µl of fixable viability dye (ZombieYellow, BioLegend) for 15 minutes at 4°C. Following 2x wash with 1x PBS (2% FCS) at 1500 rpm for 5 minutes at 4°C, cell pellets were resuspended in 50 µl anti-human antibody mix (all from BioLegend) containing anti-CD3 (PerCP/Cyanine5.5, clone OKT3), anti-CD19 (FITC, clone HIB19), anti-CD4 (PE/Cyanine7, clone OKT4), anti-CD8 (APC/Cyanine7, clone SK1), anti-CD38 (AlexaFluor® 700, clone HIT2), anti-CD69 (APC, clone FN50), anti-CD134 (Brilliant Violet 510™, clone ACT25), and anti-CD137 (Brilliant Violet 711™, clone 4B4-1) and incubated for 45 minutes at 4°C. Following another wash, samples were resuspended in CellWASH (BD Biosciences) and analysed on an Attune NxT flow cytometer (Thermo Fisher) with the in-built Attune NxT Software.
Statistical analysis
All statistical tests were performed with GraphPad Prism5 for Windows (version 5.02). Group comparisons were analysed with the Mann Whitney t test and relationships were analysed with the Spearman correlation analysis. For all statistical analyses: * p <0.05; ** p <0.01; *** p <0.001.
Results
SARS-CoV-2 lymphocytes analysis scheme and flow cytometry gating strategy
To detect memory lymphocytes against SARS-CoV-2, isolated peripheral blood mononuclear cells (PBMCs) were isolated from individuals with confirmed past SARS-CoV-2 infection (by PCR and/or positive antibody tests within 6 weeks of symptoms). PBMCs containing lymphocytes and antigen presenting cells were then cultured with SARS-CoV-2 recombinant proteins or synthetic peptides for 5 days to stimulate CD4+ or CD8+ memory T cells (fig. 1A). CD4+ T cells recognise SARS-CoV-2 peptides processed from the recombinant proteins by antigen presenting cells whereas CD8+ T cells recognise the synthesised peptides (covering 57 specific peptides from membrane, nucleocapsid and S proteins). For the synthetic peptides, the HLA restriction covered 80% of the HLA class I haplotypes within the Caucasian population. B cells were able to directly recognise SARS-CoV-2 recombinant proteins. Upregulation of activation markers (helper CD4 T cells: CD134+ CD137+; cytotoxic CD8 T cells: CD69+ CD137+; CD19 B cells: CD38+) in SARS-CoV-2-specific memory lymphocytes were then detected by flow cytometry (fig. 1B).
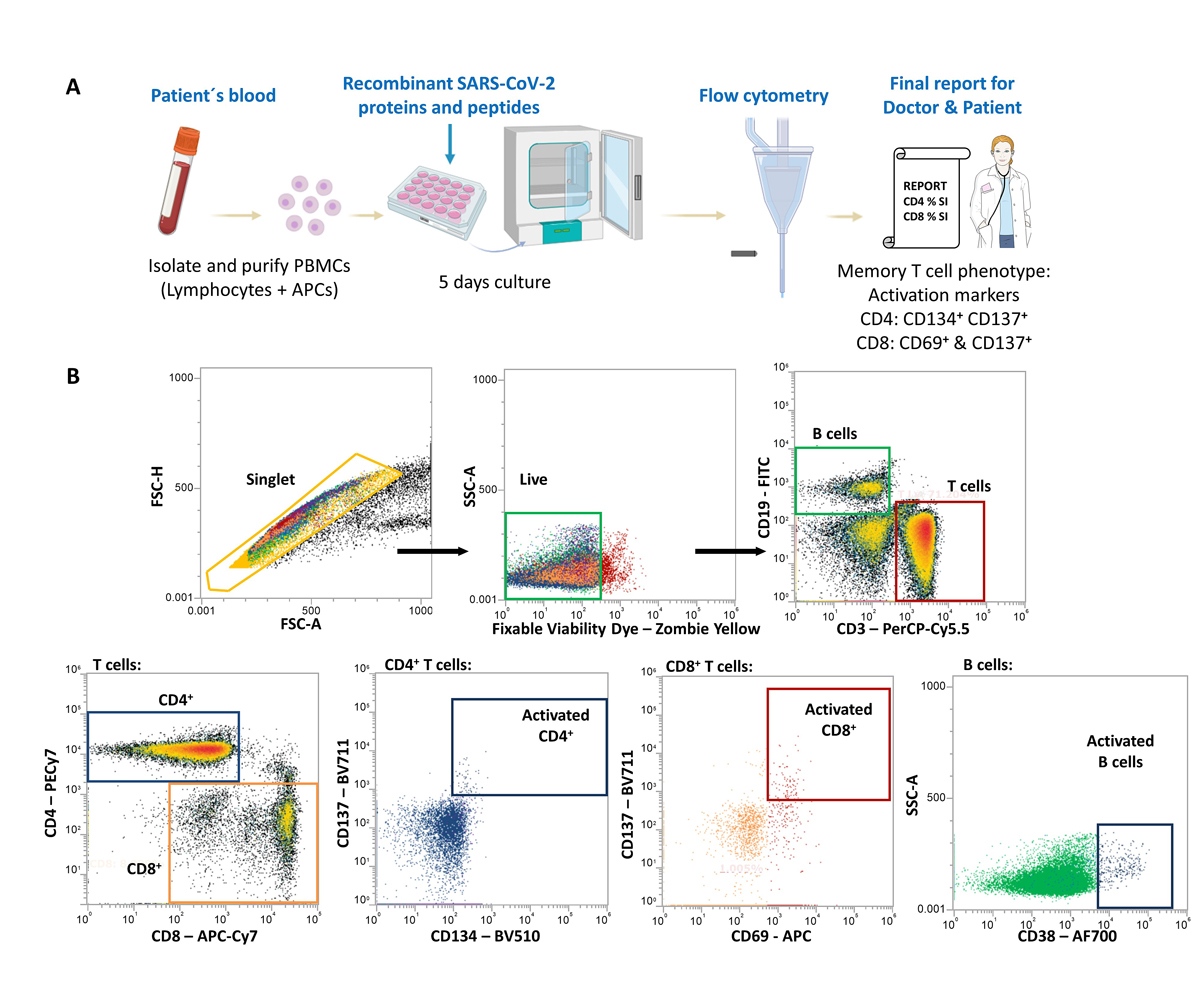
Figure 1 SARS-CoV-2 lymphocyte testing analysis scheme. (A) Flow-chart of the stages involved for the SARS-CoV-2 lymphocyte testing analysis from isolation of the patient´s peripheral blood mononuclear cells (PBMCs) to culturing conditions and flow cytometric analysis before the final report is sent to doctors and patients. (B) Flow cytometry gating strategy to identify activated T and B cells against SARS-CoV-2. APCs: antigen presenting cells.
High sensitivity and specificity for detecting memory CD4+/CD8+ T cells and CD38+ B cells in SARS-CoV-2 convalescent individuals
In a Swiss cohort of 30 COVID-19 convalescent individuals and 10 unexposed donors (table 1), memory T cell activation against all the tested antigens could be detected with overall 85% sensitivity and 75% specificity (table 2). For CD8+ cells, the maximum sensitivity value for activation by synthetic peptides was 80% due to our synthetic peptide design, which accounts for 80% of HLA class I within the Caucasian population. There was an overall >80% positive predictive value, but the overall negative predictive values ranged from 28% to 82%, depending on the antigen tested (table 2). Memory B cell activation against all tested antigens was also detectable, with overall 87% sensitivity and 90% specificity (table 2). Since B and T cell responses against particular SARS-CoV-2 antigens have been reported to be discordant [10], we used the criteria of T or B cell activation by at least one tested antigen to determine test positivity.
Table 1Cohort characteristics.
|
Variable
|
Characteristics
|
Frequency (n = 40)
|
Percent
|
|
Age (years)
|
<20 |
2 |
5 |
| 20—49 |
23 |
58 |
| ≥50 |
15 |
38 |
|
Sex and exposure status
|
Male
|
25
|
63
|
| Unexposed |
6 |
15 |
| Convalescent |
19 |
48 |
|
Female
|
15
|
38
|
| Unexposed |
4 |
10 |
| Convalescent |
11 |
28 |
Table 2Diagnostic values of the SARS-CoV-2 lymphocytes analysis. Sensitivity and specificity of the SARS-CoV-2 lymphocytes analysis against SARS-CoV-2 antigens covering the spike (S1 and S2), nucleocapsid (NC) and membrane proteins. Activation cut-off value is measured as the upregulation of CD134+ CD137+ for helper CD4 T cells, CD69+ CD137+ for cytotoxic CD8 T cells and CD38+ for B cells relative to the culture medium and DMSO negative controls.
|
Antigen
|
Activation cut-off value (relative to negative control)
|
Convalescent (n = 30)
|
Unexposed (n = 10)
|
PPV
|
NPV
|
|
+
|
—
|
Sensitivity
|
+
|
—
|
Specificity
|
| CD4+ T cells |
S1 |
2 |
26 |
4 |
87% |
1 |
9 |
90% |
96% |
69% |
| S2 |
2 |
22 |
8 |
73% |
1 |
9 |
90% |
96% |
53% |
| NC |
2 |
24 |
6 |
80% |
0 |
10 |
100% |
100% |
63% |
| Combined statistics |
2 in at least one stimuli condition |
28 |
2 |
93% |
1 |
9 |
90% |
97% |
82% |
| CD8+ T cells |
Membrane mix |
2 |
12 |
18 |
40% |
3 |
7 |
70% |
80% |
28% |
| Spike mix |
2 |
15 |
15 |
50% |
3 |
7 |
70% |
83% |
32% |
| NC mix |
2 |
17 |
13 |
57% |
1 |
9 |
90% |
94% |
41% |
| Combined statistics |
2 in at least one stimuli condition |
23 |
7 |
77% |
4 |
6 |
60% |
85% |
46% |
| CD38+ B cells |
S1 |
2 |
19 |
11 |
63% |
0 |
10 |
100% |
100% |
48% |
| S2 |
2 |
15 |
15 |
50% |
1 |
9 |
90% |
94% |
38% |
| NC |
2 |
16 |
14 |
53% |
0 |
10 |
100% |
100% |
42% |
| Combined statistics |
2 in at least one stimuli condition |
26 |
4 |
87% |
1 |
9 |
90% |
96% |
69% |
Our SARS-CoV-2 lymphocyte analysis detected both memory CD4+ (fig. 2A) and CD8+ (fig. 2B) T cells, which were significantly more activated in convalescent individuals than naïve individuals. Memory CD4+ T cells responded well to all the tested recombinant proteins (S1, S2 and nucleocapsid; fig. 2A), and memory CD8+ T cells responded well to the S and nucleocapsid peptide mixes (fig. 2B). Memory CD38+ B cells similarly responded well to all tested recombinant proteins, including to the S1/2 protein (fig. 2C), in line with the anti-SARS-CoV-2 antibody profiles being strongly directed against the S1 protein [15].
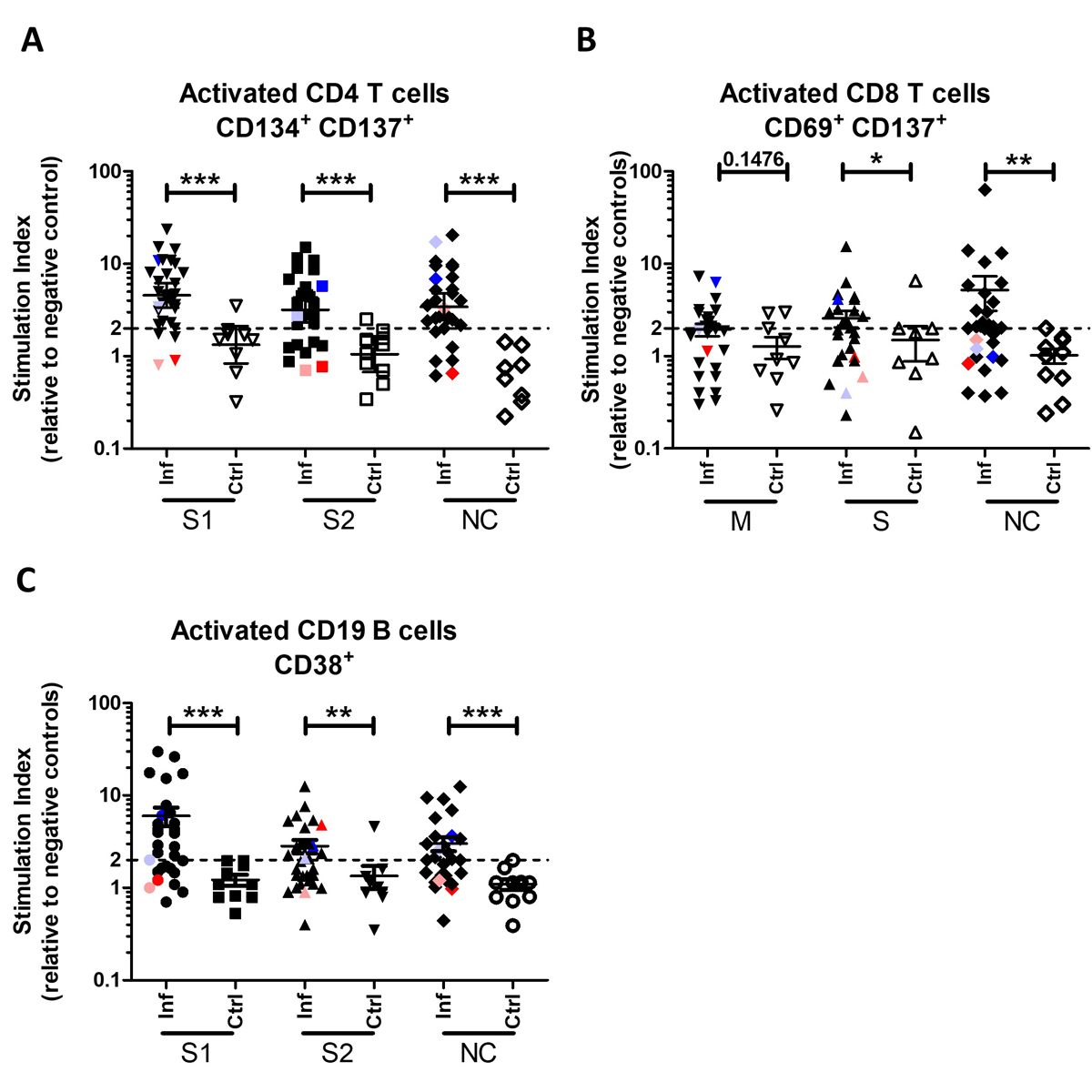
Figure 2 COVID-19 convalescent individuals were distinguishable from naïve individuals. (A) Activated CD4+ T cells (CD134+ CD137+) 5 days post-incubation with SARS-CoV-2 recombinant S1, S2 or nucelocapsid proteins. (B) Activated CD8+ T cells (CD69+ CD137+) 5 days post-incubation with mixtures of SARS-CoV-2 synthetic membrane peptides (11 total), S peptides (28 total), or nuceocapsid peptides (18 total). (C) Activated CD19+ B cells (CD38+) 5 days post-incubation with SARS-CoV-2 recombinant S1, S2 or nuceocapsid proteins. Dotted line at SI = 2 indicates activation threshold. Coloured symbols represent the four convalescent individuals with no detectable anti-S antibodies. S: spike; M: membrane; NC: nucleocapsid; Inf: infected; Ctrl: unexposed donor control. Error bars in the graph indicate mean ± SEM. Statistics: Mann Whitney t test.
Overall, this SARS-CoV-2 lymphocyte analysis is therefore able to detect specific memory T and B cells against all the tested SARS-CoV-2 antigens (table 2) and, because of the reliable detection of activation against S and nuceocapsid antigens, this assay would likely allow vaccinated individuals (good S protein-specific memory T cell responses) to be distinguished from previously (now asymptomatic) infected individuals (good S- and nuceocapsid-specific memory T cell responses).
Anti-S IgG antibody titres correlate with both memory CD4+ T cell and CD38+ B cell activation
Since CD4+ helper T cells provide support to B cells to produce antibodies, we assessed if antibody titres in convalescent individuals correlated with CD4+ T cell activation. We could detect anti-S IgG antibodies in 26/30 convalescent individuals at the time of the lymphocyte analysis, which correlated to CD4+ T cell (fig. 3A) or CD38+ B cell (fig. 3B) activation by spike proteins (S2 or S1, respectively), but not to NC recombinant SARS-CoV-2 proteins.
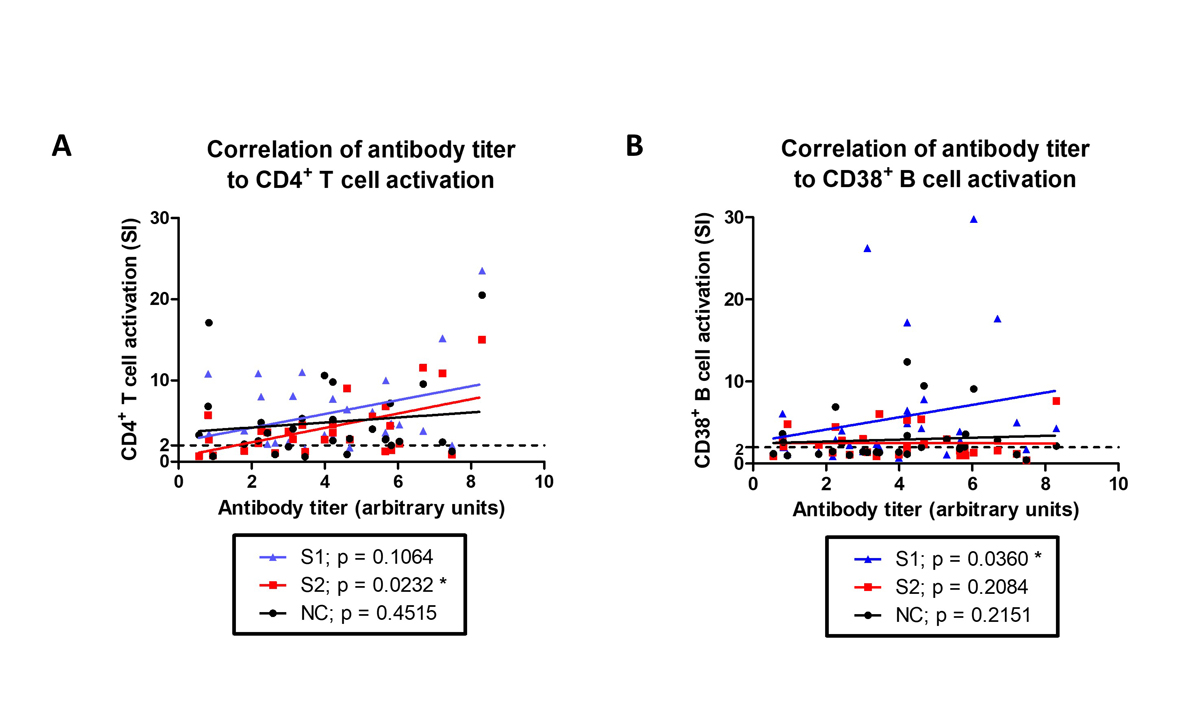
Figure 3 Both CD4+ T cell and CD38+ B cell activation are correlated to anti-S IgG antibody titres. Anti-S IgG antibody titres in convalescent individuals were correlated to their (A) CD4+ T cell or (B) CD38+ B cell activation when stimulated in vitro with S1, S2, or nuceocapsid recombinant SARS- CoV-2 proteins. Dotted line at SI = 2 indicates activation threshold. (A and B). Linear regression lines for each stimulation condition are also presented. Statistics: Spearman correlation analysis.
Of the four individuals in whom anti-SARS-CoV-2 antibodies became undetectable over time, two had increased memory CD4+/CD8+ T cell and CD19+ B cell activation (fig. 2 – blue/lilac symbols), whereas the other two remained negative in all except for B cell activation by S2 recombinant protein (fig. 2 – red/pink symbols). Although interesting, this must be followed up in more individuals to determine if memory lymphocytes may be more stably detectable than antibodies.
CD8+ T cell activation decreases at later time post-symptoms
In order to determine the longevity of recall responses against SARS-CoV-2 — specifically, if the response decreases with time after infection — we performed an analysis of the correlation of activation potential to the different times at which blood samples were obtained after symptoms (fig.4). Whereas CD4+ T cell activation remained constant irrespective of the time after symptoms (up to 11 months) or antigenic stimuli (fig. 4A), CD8+ T cell activation against the nucleocapsid peptide mix were reduced when tested at later times, albeit still mostly activated (fig. 4B and table 2). This indicates that optimal nucleocapsid-specific memory CD8+ T cell activation decreases with time after symptoms. Activated CD19+ CD38+ B cells remained constant in their potential for activation by SARS-CoV-2 recombinant proteins up to 11 months after symptoms (fig. 4C).
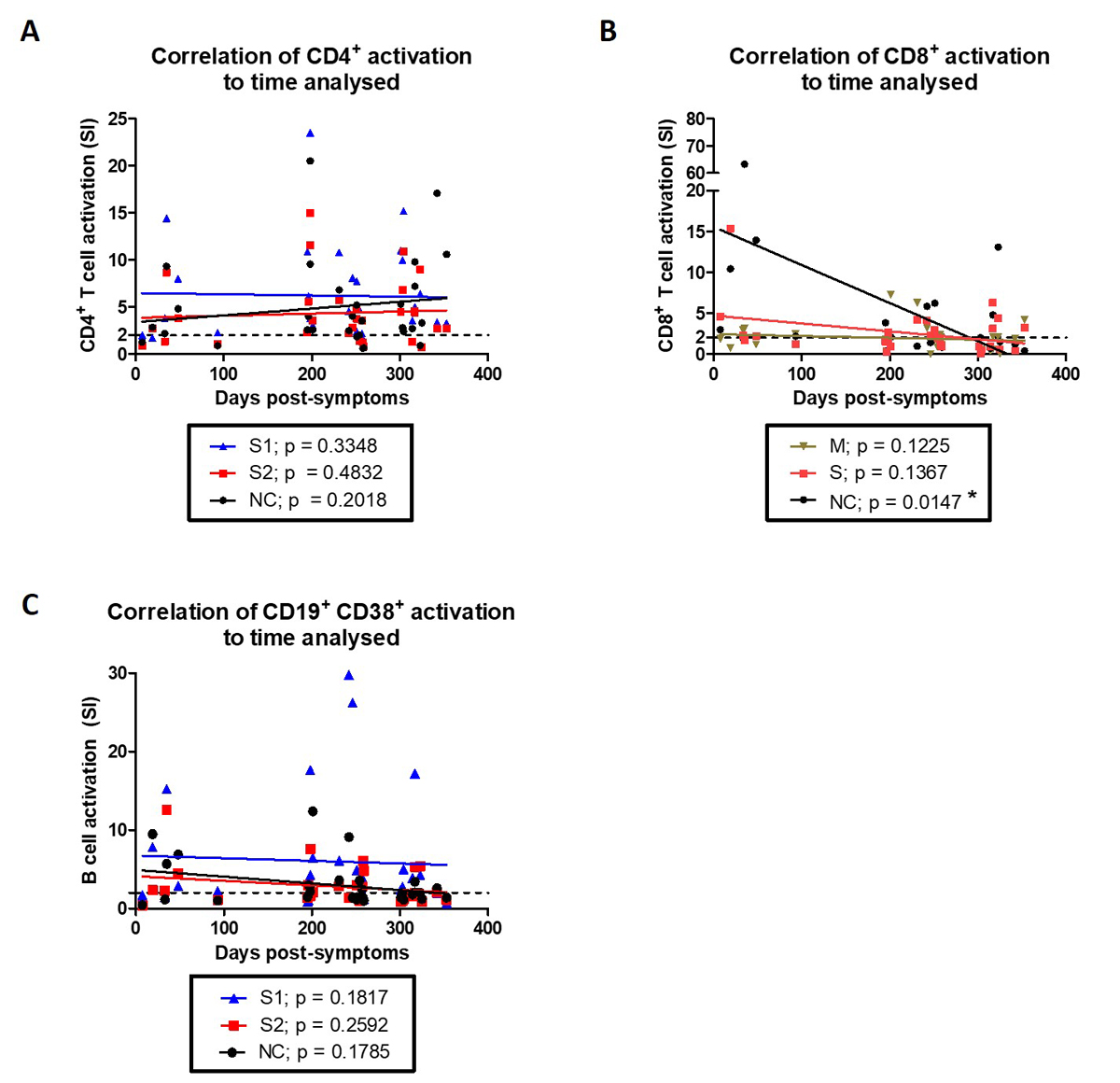
Figure 4 Decreased CD8+ memory T cell activation in later analyses. Time after symptoms in convalescent individuals was correlated to their (A) CD4+ T cell activation when stimulated in vitro with S1, S2, or nucleocapsid recombinant SARS-CoV-2 proteins or (B) CD8+ T cell activation when stimulated in vitro with peptide mixes from membrane, S or nucleocapsid SARS-CoV-2 proteins. (C) Correlation of time after symptoms in convalescent individuals to CD19+ CD38+ B cell activation when stimulated in vitro with S1, S2 or nucleocapsid recombinant SARS-CoV-2 proteins. (A—C) Linear regression lines for each stimulation condition are also presented. Statistics: Spearman correlation analysis.
Discussion and conclusion
We showed that our SARS-CoV-2 lymphocyte testing analysis can reproducibly detect memory T and B cells by activation marker upregulation against not only SARS-CoV-2 antigen from the S protein, but also against membrane and nucleocapsid proteins, which may indicate a more comprehensive and protective immunity profile of an individual. As peripheral circulating memory lymphocytes are heterogeneous in their memory marker expressions (e.g., CD45RO+ CCR7+ T central memory, CD45RO+ CCR7- T effector memory, CD45RA+ CCR7- T effector memory) [13, 14, 16–20], assessing activation markers improves detection of SARS-CoV- 2 specific lymphocytes, especially when the contribution of such specific memory subsets to COVID-19 remains undetermined.
Furthermore, by testing with various antigens other than the S protein, our test should be able to distinguish between vaccinated and previously infected individuals – this may be particularly valuable in determining asymptomatic infected individuals’ immune profiles and improving understanding of this group's relevance for immune correlates of protection.
For individuals with immunodeficiencies, be it primary (e.g., common variable immunodeficiency) or secondary due to immunosuppressive treatments for transplantation or cancer (e.g., methotrexate or rituximab), humoral antibody immune responses are significantly impaired [21]. To date, the efficacy of the two COVID-19 vaccines approved by Swissmedic and available in Switzerland has not been investigated in immunodeficient individuals, although they are a risk group for infection and severe disease. Therefore, testing for cellular immunity supports detection of vaccine responses apart from antibodies, and would provide a laboratory-measurable parameter to support clinicians decisions on how to best care for immunodeficient individuals. Ease of access to this testing service, especially geared towards a clinical environment, would also encourage clinicians to pursue clinical research into immune correlates against SARS-CoV-2.
Other applications for this test could be the clinical assessment of “long COVID” patients, especially with increasing reports of such individuals [22, 23]. Since T cells, particularly killer CD8+, are essential for the elimination of virus-infected cells, they may contribute to symptom duration in “long COVID” patients. This SARS-CoV-2 lymphocyte testing analysis could measure specific cellular immunity against SARS-CoV-2 in relation to long COVID symptoms, which might provide an improved picture of the underlying biological cause of the symptoms and differentiation of it it from post-viral syndrome or post-intensive care syndrome.
In conclusion, we describe here a useful laboratory testing analysis to characterise and quantify specific memory lymphocyte responses against SARS-CoV-2 and therefore contribute to improved screening of the population for likely protective immunity beyond the current antibody testing services.
Acknowledgements
We thank Katja Martin for help in figure design.
Daniel Yerly, PhD
ADR-AC GmbH
Holligenstrasse 91
CH-3008 Bern
daniel.yerly[at]adr-ac.ch
References
1
Weissleder R
,
Lee H
,
Ko J
,
Pittet MJ
. COVID-19 diagnostics in context. Sci Transl Med. 2020 Jun;12(546):1–7. https://doi.org/10.1126/scitranslmed.abc1931
2
Vandenberg O
,
Martiny D
,
Rochas O
,
van Belkum A
,
Kozlakidis Z
. Considerations for diagnostic COVID-19 tests. Nat Rev Microbiol. 2020; https://doi.org/10.1038/s41579-020-00461-z
3
Baden, L. R.
et al.
Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. NEJMoa2035389 (2020) doi:https://doi.org/10.1056/NEJMoa2035389.
4
Polack FP
,
Thomas SJ
,
Kitchin N
,
Absalon J
,
Gurtman A
,
Lockhart S
, et al.; C4591001 Clinical Trial Group
. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020 Dec;383(27):2603–15. https://doi.org/10.1056/NEJMoa2034577
5
Sahin U
,
Muik A
,
Derhovanessian E
,
Vogler I
,
Kranz LM
,
Vormehr M
, et al.
COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020 Oct;586(7830):594–9. https://doi.org/10.1038/s41586-020-2814-7
6
Corbett KS
,
Flynn B
,
Foulds KE
,
Francica JR
,
Boyoglu-Barnum S
,
Werner AP
, et al.
Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N Engl J Med. 2020 Oct;383(16):1544–55. https://doi.org/10.1056/NEJMoa2024671
7
Jackson LA
,
Anderson EJ
,
Rouphael NG
,
Roberts PC
,
Makhene M
,
Coler RN
, et al.; mRNA-1273 Study Group
. An mRNA Vaccine against SARS-CoV-2 — preliminary Report. N Engl J Med. 2020 Nov;383(20):1920–31. https://doi.org/10.1056/NEJMoa2022483
8
Ferretti AP
,
Kula T
,
Wang Y
,
Nguyen DM
,
Weinheimer A
,
Dunlap GS
, et al.
Unbiased Screens Show CD8+ T Cells of COVID-19 Patients Recognize Shared Epitopes in SARS-CoV-2 that Largely Reside outside the Spike Protein. Immunity. 2020 Nov;53(5):1095–1107.e3. https://doi.org/10.1016/j.immuni.2020.10.006
9
Farber DL
,
Yudanin NA
,
Restifo NP
. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol. 2014 Jan;14(1):24–35. https://doi.org/10.1038/nri3567
10
Reynolds CJ
,
Swadling L
,
Gibbons JM
,
Pade C
,
Jensen MP
,
Diniz MO
, et al.; COVIDsortium investigators; COVIDsortium immune correlates network
. Discordant neutralizing antibody and T cell responses in asymptomatic and mild SARS-CoV-2 infection. Sci Immunol. 2020 Dec;5(54):eabf3698. https://doi.org/10.1126/sciimmunol.abf3698
11
Vabret N
,
Britton GJ
,
Gruber C
,
Hegde S
,
Kim J
,
Kuksin M
, et al.; Sinai Immunology Review Project
. Immunology of COVID-19: Current State of the Science. Immunity. 2020 Jun;52(6):910–41. https://doi.org/10.1016/j.immuni.2020.05.002
12
Mateus, J.
et al.
Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science (80-.) . 370, 89–94 (2020).
13
Dan JM
,
Lindestam Arlehamn CS
,
Weiskopf D
,
da Silva Antunes R
,
Havenar-Daughton C
,
Reiss SM
, et al.
A Cytokine-Independent Approach To Identify Antigen-Specific Human Germinal Center T Follicular Helper Cells and Rare Antigen-Specific CD4+ T Cells in Blood. J Immunol. 2016 Aug;197(3):983–93. https://doi.org/10.4049/jimmunol.1600318
14
Reiss S
,
Baxter AE
,
Cirelli KM
,
Dan JM
,
Morou A
,
Daigneault A
, et al.
Comparative analysis of activation induced marker (AIM) assays for sensitive identification of antigen-specific CD4 T cells. PLoS One. 2017 Oct;12(10):e0186998. https://doi.org/10.1371/journal.pone.0186998
15
Ni L
,
Ye F
,
Cheng ML
,
Feng Y
,
Deng YQ
,
Zhao H
, et al.
Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity. 2020 Jun;52(6):971–977.e3. https://doi.org/10.1016/j.immuni.2020.04.023
16
Todryk SM
. T cell memory to vaccination. Vaccines (Basel). 2018 Dec;6(4):1–6. https://doi.org/10.3390/vaccines6040084
17
Martin MD
,
Badovinac VP
. Defining memory CD8 T cell. Front Immunol. 2018 Nov;9:2692. https://doi.org/10.3389/fimmu.2018.02692
18
Flaxman A
,
Ewer KJ
. Methods for measuring T-cell memory to vaccination: from mouse to man. Vaccines (Basel). 2018 Jul;6(3):6. https://doi.org/10.3390/vaccines6030043
19
Macallan DC
,
Borghans JA
,
Asquith B
. Human T cell memory: A dynamic view. Vaccines (Basel). 2017 Feb;5(1):5. https://doi.org/10.3390/vaccines5010005
20
Tarke A
,
Sidney J
,
Kidd CK
,
Dan JM
,
Ramirez SI
,
Yu ED
, et al.
Comprehensive analysis of T cell immunodominance and immunoprevalence of SARS-CoV-2 epitopes in COVID-19 cases. Cell Rep Med. 2021 Feb;2(2):100204. https://doi.org/10.1016/j.xcrm.2021.100204
21
Papp KA
,
Haraoui B
,
Kumar D
,
Marshall JK
,
Bissonnette R
,
Bitton A
, et al.
Vaccination Guidelines for Patients With Immune-Mediated Disorders on Immunosuppressive Therapies. J Cutan Med Surg. 2019 Jan/Feb;23(1):50–74. https://doi.org/10.1177/1203475418811335
22
Sudre CH
,
Murray B
,
Varsavsky T
,
Graham MS
,
Penfold RS
,
Bowyer RC
, et al.
Attributes and predictors of long COVID. Nat Med. 2021 Apr;27(4):626–31. https://doi.org/10.1038/s41591-021-01292-y
23
Alwan NA
. Track post-COVID sickness, not just cases and deaths. Nature. 2020;584(7820):170–170. https://doi.org/10.1038/d41586-020-02335-z



