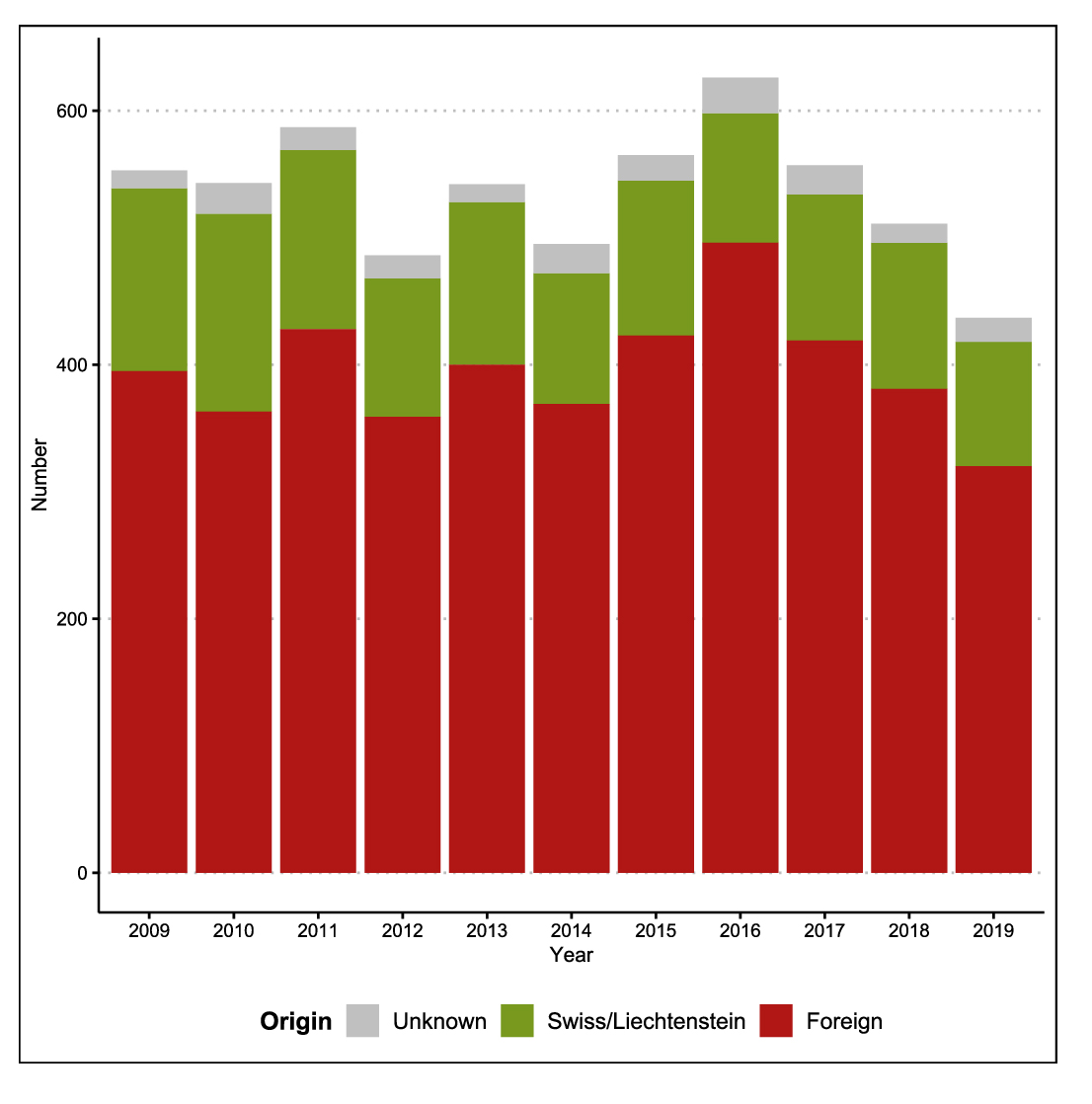
Figure 1 Annual numbers of tuberculosis, by origin, in Switzerland and Liechtenstein 2009–2019.
The Swiss case definition for tuberculosis is met if laboratory or clinical criteria are met. For details see ‘Materials and methods’.
DOI: https://doi.org/10.4414/SMW.2021.w30032
Tuberculosis is a communicable disease caused by pathogenic species of the Mycobacterium tuberculosis complex. It is largely transmitted by the airborne route via droplets produced by respiratory manoeuvres (predominantly coughing). Its manifestations result in tuberculosis that may involve any extrapulmonary site in addition to the predominant pulmonary form. Houben and Dodd estimated that about one quarter of the world’s population is latently infected with M. tuberculosis and thus at risk of developing tuberculosis [1]. With early diagnosis and correct anti-tuberculosis treatment, virtually all cases are curable, and transmission can be arrested promptly. The World Health Organization (WHO) estimated a global incidence of 130 per 100,000 inhabitants for 2019, a 9% reduction since 2015 [2].
Tuberculosis and M. tuberculosis isolation are notifiable in Switzerland, whereas latent infection with M. tuberculosis is not. Since 1988, the Swiss Federal Office of Public Health (FOPH) systematically collects tuberculosis data in the national infectious disease surveillance system. Information on drug susceptibility data is available since 1995. WHO treatment outcome monitoring was implemented in 2016.
In this paper, we describe:
Physicians and laboratories are legally mandated to report a defined set of diseases and pathogens. Physicians report to the cantonal medical service (sub-national level), which forwards the reports to the FOPH (national level). Laboratories notify the cantonal medical service and in parallel the FOPH [3]. All information on a disease or pathogen is centrally linked and consolidated in a single database [4].
Physicians have to report information on age, gender, country of birth, nationality, affected organs, prior tuberculosis diagnosis and treatment, treatment start, and drugs used, as well as the type of applied diagnostics, (microscopy, nucleic acid amplification testing and culture). Since 2016, physicians have to report treatment outcomes according to the WHO recommendation [5].
Laboratories have to notify results from microscopy, nucleic acid amplification tests (such as the commercially available Xpert® MTB/RIF assay or others), culture, species and susceptibility/resistance for isoniazid, rifampicin, pyrazinamide and ethambutol. In addition to the mandatory notification, they must forward all rifampicin-resistant strains to the Swiss National Reference Centre for Mycobacteria. The National Reference Centre analyses all rifampicin-resistant isolates and determines the susceptibility pattern in these for first- and second-line drugs. It also organises national quality assurance assessment schemes for microscopy, culture and first-line drug susceptibility testing.
Upon request, the Swiss Lung Association and its cantonal branches support the cantonal medical service. The Association also supports the FOPH by compiling and updating tuberculosis guidelines in collaboration with the professional medical organisations and compiles the data on contact tracing.
The Swiss case definition for tuberculosis is met if either laboratory or clinical confirmation (or both) are present. A case of tuberculosis is laboratory-confirmed if: (1) at least one culture is positive for a pathogenic species of the M. tuberculosis complex, or (2) if there is evidence for specific genetic components (from nucleic acid amplification tests such as the WHO recommended Xpert® MTB/RIF assay or others) in conjunction with microscopic evidence of mycobacteria in a respiratory sample.
A case is clinically confirmed if a physician decides to treat a patient with at least three anti-tuberculosis drugs.
Repeat cases are defined as a new episode of tuberculosis occurring more than 12 months after the initial notification.
Origin refers to the country of birth if known. If country of birth is unknown, nationality is used instead. If both are missing, origin is missing as well. This results in the following categories: Swiss (including the Principality of Liechtenstein), foreign and unknown origin. The FOPH defines world regions according to the Swiss Federal Statistical Office [6].
In this report, ‘year’ refers to the year of treatment start; if this information is missing, the year of first laboratory evidence is used instead. This approach differs from earlier publications [3], where the reporting year was used [7]. This leads to minor differences when comparing data from earlier publications [3].
Resistance to both isoniazid and rifampicin defines multidrug-resistant (MDR) tuberculosis. MDR strains are termed extensively drug-resistant (XDR) if there is additional resistance to both a fluoroquinolone and at least one of the injectable second-line drugs. The present analysis focuses on rifampicin resistance [5], because this resistant pattern is relevant for the choice of treatment, it is easy and quick to diagnose by means of the broad application of molecular technics (e.g., GenXpert), and about 80 per cent of rifampicin resistant strains are also MDR.
The purpose of treatment outcome monitoring is to evaluate national tuberculosis programmes. Treatment outcomes are defined in accordance with WHO guidelines [5].
Cured: A pulmonary tuberculosis patient with bacteriologically confirmed tuberculosis at the beginning of treatment who was smear- or culture-negative in the last month of treatment and on at least one previous occasion.
Treatment completed: A tuberculosis patient who completed treatment without evidence of failure but with no record to show that sputum smear or culture results in the last month of treatment and on at least one previous occasion were negative, either because tests were not done or because results are unavailable.
Treatment failed: A tuberculosis patient whose sputum smear or culture is positive at month 5 or later during treatment.
Died: A tuberculosis patient who dies for any reason before starting or during the course of treatment.
Lost to follow-up: A tuberculosis patient who did not start treatment or whose treatment was interrupted for 2 consecutive months or more.
Not evaluated: A tuberculosis patient for whom no treatment outcome is assigned. This includes cases “transferred out” to another treatment unit as well as cases for whom the treatment outcome is unknown to the reporting unit.
Treatment success: The sum of ‘cured’ and ‘treatment completed’.
In this publication we report the WHO combined outcome of ‘treatment success’. The FOPH collects treatment outcome data at 12 months after start of treatment in non-MDR and after 24 months in MDR tuberculosis cases. Therefore, based on data up to 31 December 2019, our analysis of treatment outcomes is limited to the 3-year period from 2016 to 2018 among non-MDR tuberculosis cases. The FOPH has published on MDR tuberculosis elsewhere [8]. In this publication we summarise the outcome data on rifampicin-susceptible tuberculosis since susceptibility to rifampicin is the key determinant for the efficacy of the first-line standard treatment regimen [9]. We further defined a second composite outcome called ‘programmatically unsuccessful treatment’ as the sum of ‘treatment failed’, ‘lost to follow-up’ and ‘not evaluated’ but excluding ‘died’ from the numerator.
The statistical methods include the determination of numbers and proportions, medians and quartiles. Linear trends in the annual number of cases were estimated by generalised linear models with the annual case numbers as response and the reporting year as predictor. The results are presented as the change in percent per year. One was subtracted from the exponentiated regression coefficient, and this difference was multiplied by 100 in order to express the change in percent per year over time. Ninety-five percent confidence intervals (CIs) were estimated by profiling the log-likelihood. The change in the proportion of tuberculosis cases of male gender was estimated by a simple linear regression model with the proportion of men among cases as response and the reporting year as predictor. CIs were estimated by standard least square methods expressing the change over time in percent points. This result was validated by the standard test for trend in proportion made available by the prop.trend.test in the stats library in R. Risk factors for Rifampicin resistance were estimated using univariable and multivariable logistic regression, considering origin and prior treatment as primary risk factors. In the basic model we used origin as a binary variable (Swiss vs foreign) and added an interaction term of prior treatment and origin. In the extended model we used origin as a country-specific variable, with Switzerland as reference. Statistical inference was done by means of 95% confidence limits. Notched boxplots were compiled for the age distribution [10]. All analyses were done with R 4.0.2 under Windows [11].
The Swiss epidemic law (EpidA SR 818.101, EpidO SR 818.101.1 FPHA-ORD SR 818.101.126), forms the legal framework for collecting, analysing and reporting notification data in an anonymized format. Ethics clearance was not required.
The annual number of tuberculosis cases decreased from 553 in 2009 to 437 in 2019 (table 1 and fig. 1), corresponding to annual incidences of 7.1 and 5.1 cases per 100,000 inhabitants, respectively. The Swiss case definition for tuberculosis is met if laboratory or clinical criteria are met. For details see ‘Materials and methods’.

Figure 1 Annual numbers of tuberculosis, by origin, in Switzerland and Liechtenstein 2009–2019.
The Swiss case definition for tuberculosis is met if laboratory or clinical criteria are met. For details see ‘Materials and methods’.
The average decline of overall annual case numbers was −0.84% (95% CI −1.63% to −0.03%). The overall decline was mainly attributable to persons of Swiss origin (average decline of annual number −3.63%, 95% CI −5.26 to −1.98%), whereas the number of cases involving persons of foreign origin remained fairly stable over the observation period (average decline of annual number −0.08%, 95% CI −1.01 to +0.87%), peaking in 2016 with respect to people originating from high-incidence countries (particularly Eritrea). The declining trend among persons of Swiss origin becomes much clearer when previous years, 1995–2008, are included in addition to our selected time frame 2009–2019 (supplementary fig. S1 in the appendix).
The large majority (73.8%) of tuberculosis cases in Switzerland was acquired abroad, in immigrants from countries with a high incidence of tuberculosis. Most foreign tuberculosis patients immigrated from Eastern Africa (28%), South-East Europe (12%) and Southern Asia (9%) (fig. 2). When cases with missing information were excluded, patients from these three world regions made up 52% of all with foreign origin. The eight most affected countries were Eritrea (n = 685), Somalia (n = 420), Portugal (n = 232), Kosovo (n = 179), Tibet (China) (n = 156), India (n = 140), Sri Lanka (n = 128) and Ethiopia (n = 110). These eight contribute 47.4% of notifications among those of foreign origin with known provenance.
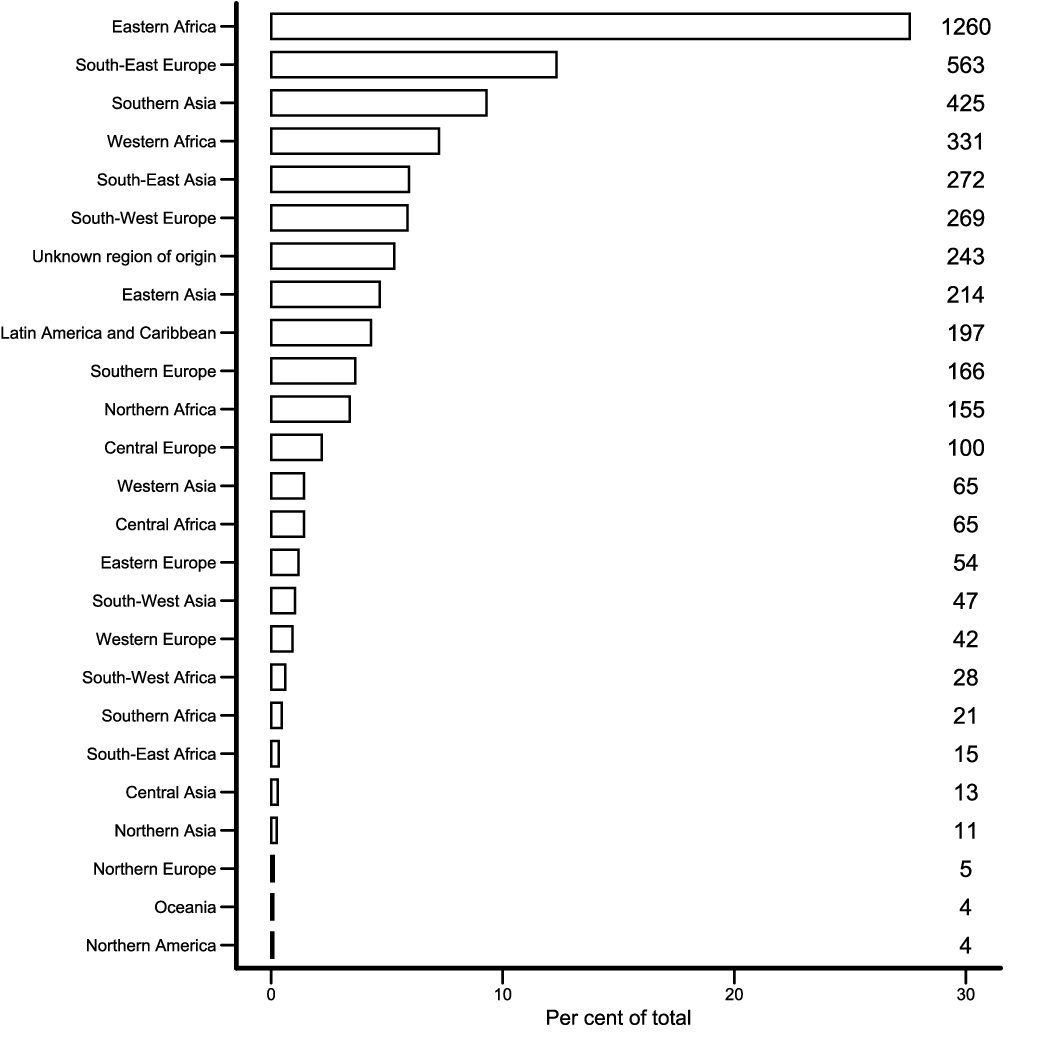
Figure 2 Distribution of foreign regions of origin among tuberculosis cases in Switzerland and Liechtenstein, 2009–2019.
Eastern Africa includes Eritrea, Somalia, Ethiopia, Kenya, and Tanzania; South-East Europe Kosovo, Serbia, Montenegro, Bosnia and Herzegovina, Macedonia, Romania, Albania, Croatia, Bulgaria, and Slovenia; Southern Asia India, Sri Lanka, Afghanistan, Pakistan, Nepal, and Bangladesh. The country of origin was missing in 216 cases (see table 1), and in 27 cases origin was reportedly non-Swiss, but without specifying the country.
The proportion of men slightly declined from 57% in 2009 to 54% in 2019 (annual average linear decline in per cent points −0.61%, 95% CI −1.16 to −0.06%), whereas that of women slightly increased from 43% to 46% (p for trend <0.01). On average, there were more male (58%) than female cases (42%).
The median age undulated between 37 and 38 years over the observation period. Fifty percent were aged between 26 and 60 years (table 1). The age distribution over the last 11 years mirrors the fact that young men dominate the demographics in the migrating population (fig. 3). The age distribution was dominated by young adult men in persons of foreign origin. Elderly adults dominated the age distribution in persons of Swiss origin. The difference between men and women was less marked. The number of notified cases in children of Swiss origin below the age of 5 years (n = 89) is attributable to our definition of origin, and mostly concerns children of immigrants from high-incidence countries.
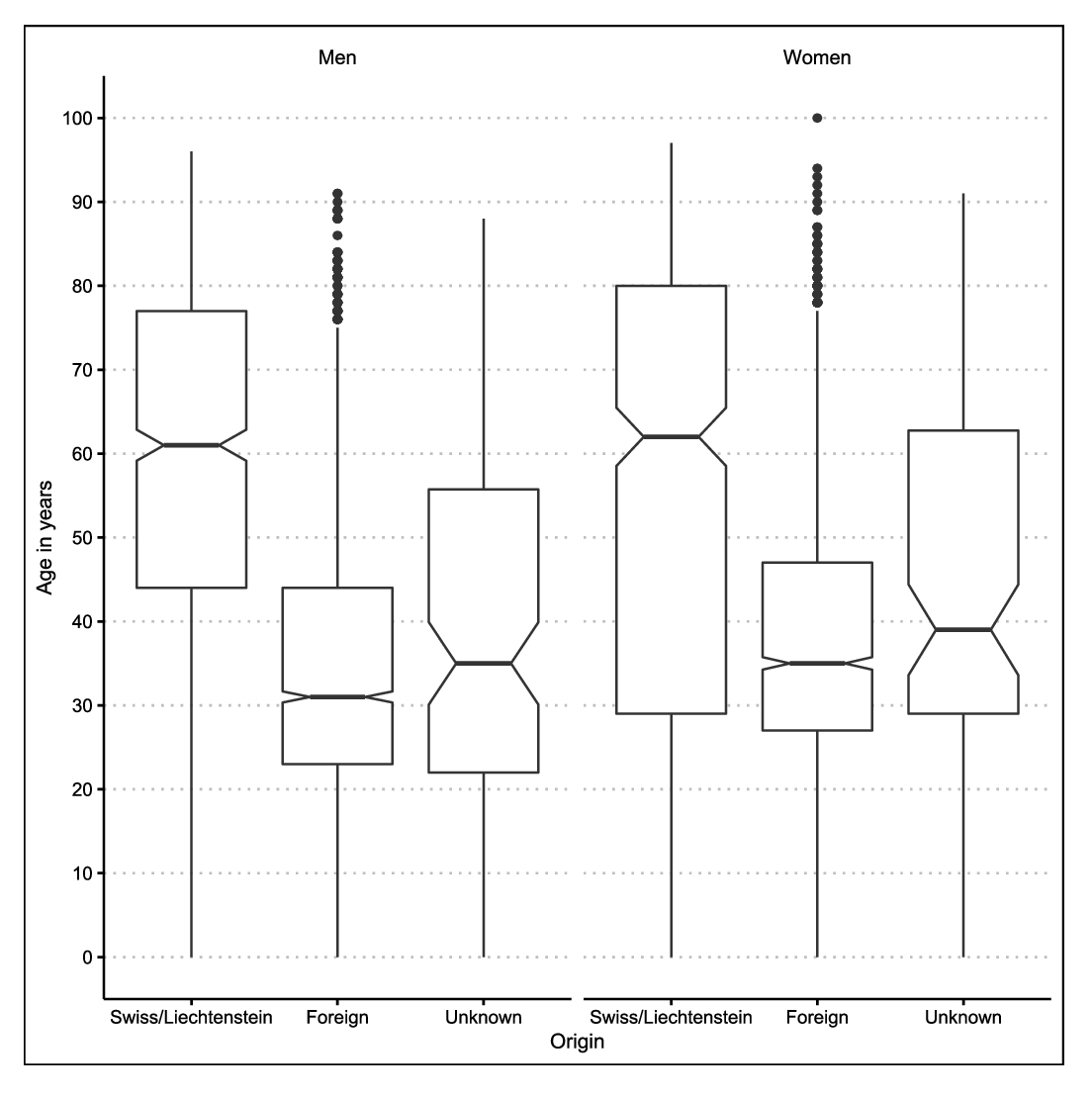
Figure 3 Age distribution, by sex and origin, of tuberculosis cases in Switzerland and Liechtenstein, 2009–2019.
As expected, cantons with the largest populations also reported most of the cases (table 1).
Table 1Summary of tuberculosis cases in Switzerland and Liechtenstein, 2009–2019.
| 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | Total | Percent | |
| Overall cases (Swiss case definition*) | 553 | 543 | 587 | 486 | 542 | 495 | 565 | 626 | 557 | 511 | 437 | 5902 | 100.0 |
| – Clinically confirmed | 529 | 509 | 548 | 456 | 516 | 448 | 523 | 584 | 510 | 479 | 409 | 5511 | 93.4 |
| – Laboratory-confirmed | 460 | 457 | 484 | 411 | 464 | 434 | 478 | 537 | 474 | 440 | 373 | 5012 | 84.9 |
| – Both | 436 | 423 | 446 | 381 | 438 | 387 | 436 | 495 | 428 | 408 | 346 | 4624 | 78.3 |
| – Repeat case | 6 | 7 | 12 | 14 | 16 | 12 | 12 | 12 | 11 | 14 | 10 | 126 | 2.1 |
| Age | |||||||||||||
| Median | 37 | 36 | 38 | 37 | 37 | 37 | 35 | 31 | 34 | 36 | 38 | 36 | n.a. |
| Lower Quartile | 26 | 27 | 26 | 28 | 27 | 26 | 24 | 22 | 23 | 25 | 27 | 25 | n.a. |
| Upper Quartile | 58 | 56 | 54 | 59 | 55 | 52 | 53 | 47 | 51 | 56 | 58 | 54 | n.a. |
| Gender | |||||||||||||
| Male | 313 | 296 | 350 | 265 | 291 | 312 | 340 | 401 | 352 | 308 | 238 | 3466 | 58.7 |
| Female | 240 | 247 | 237 | 221 | 251 | 183 | 225 | 225 | 205 | 203 | 199 | 2436 | 41.3 |
| Origin | |||||||||||||
| Switzerland/Liechtenstein | 144 | 156 | 141 | 109 | 128 | 103 | 122 | 102 | 115 | 115 | 98 | 1333 | 22.6 |
| Foreign | 395 | 363 | 428 | 359 | 400 | 369 | 423 | 496 | 419 | 381 | 320 | 4353 | 73.8 |
| Unknown | 14 | 24 | 18 | 18 | 14 | 23 | 20 | 28 | 23 | 15 | 19 | 216 | 3.7 |
| Canton | |||||||||||||
| AG | 29 | 33 | 32 | 20 | 25 | 22 | 39 | 48 | 41 | 34 | 35 | 358 | 6.1 |
| AR | 4 | 5 | 4 | 2 | 1 | 3 | 2 | 1 | 22 | 0.4 | |||
| BE | 53 | 49 | 69 | 40 | 59 | 56 | 55 | 66 | 55 | 48 | 35 | 585 | 9.9 |
| BL | 17 | 9 | 13 | 11 | 15 | 8 | 10 | 15 | 19 | 16 | 8 | 141 | 2.4 |
| BS | 22 | 25 | 26 | 25 | 27 | 17 | 21 | 21 | 17 | 21 | 15 | 237 | 4.0 |
| FR | 18 | 16 | 8 | 18 | 12 | 10 | 15 | 13 | 14 | 19 | 12 | 155 | 2.6 |
| GE | 64 | 48 | 66 | 54 | 60 | 53 | 68 | 74 | 54 | 47 | 47 | 635 | 10.8 |
| GL | 2 | 3 | 3 | 1 | 1 | 1 | 1 | 2 | 14 | 0.2 | |||
| GR | 5 | 11 | 4 | 8 | 14 | 8 | 17 | 15 | 10 | 9 | 10 | 111 | 1.9 |
| JU | 2 | 3 | 11 | 2 | 2 | 10 | 9 | 5 | 5 | 7 | 5 | 61 | 1.0 |
| LU | 29 | 22 | 26 | 12 | 20 | 23 | 23 | 15 | 27 | 22 | 17 | 236 | 4.0 |
| NE | 14 | 11 | 12 | 8 | 7 | 13 | 14 | 14 | 16 | 11 | 8 | 128 | 2.2 |
| NW | 2 | 2 | 1 | 1 | 1 | 2 | 2 | 11 | 0.2 | ||||
| OW | 1 | 5 | 2 | 2 | 1 | 1 | 1 | 2 | 1 | 2 | 18 | 0.3 | |
| SG | 24 | 23 | 32 | 32 | 17 | 27 | 18 | 34 | 28 | 13 | 21 | 269 | 4.6 |
| SH | 4 | 3 | 7 | 3 | 6 | 7 | 4 | 5 | 4 | 9 | 8 | 60 | 1.0 |
| SO | 17 | 17 | 14 | 7 | 9 | 13 | 15 | 14 | 15 | 14 | 10 | 145 | 2.5 |
| SZ | 10 | 11 | 5 | 6 | 5 | 10 | 6 | 7 | 7 | 7 | 4 | 78 | 1.3 |
| TG | 11 | 15 | 15 | 11 | 17 | 13 | 11 | 17 | 8 | 9 | 10 | 137 | 2.3 |
| TI | 23 | 24 | 22 | 16 | 18 | 25 | 40 | 51 | 33 | 29 | 9 | 290 | 4.9 |
| VD | 65 | 77 | 93 | 70 | 83 | 55 | 76 | 60 | 76 | 72 | 52 | 779 | 13.2 |
| VS | 20 | 12 | 12 | 17 | 27 | 22 | 13 | 15 | 20 | 19 | 14 | 191 | 3.2 |
| ZG | 15 | 11 | 7 | 5 | 7 | 7 | 8 | 5 | 6 | 4 | 5 | 80 | 1.4 |
| ZH | 100 | 112 | 101 | 107 | 106 | 87 | 92 | 123 | 97 | 94 | 105 | 1124 | 19.0 |
| AI | 1 | 1 | 1 | 2 | 1 | 6 | 0.1 | ||||||
| UR | 2 | 2 | 1 | 2 | 3 | 1 | 1 | 1 | 2 | 1 | 16 | 0.3 | |
| Principality of Liechtenstein | 2 | 2 | 3 | 1 | 2 | 2 | 1 | 1 | 1 | 15 | 0.2 | ||
| Site of disease | |||||||||||||
| Pulmonary only | 274 | 289 | 299 | 256 | 286 | 262 | 278 | 304 | 265 | 253 | 207 | 2973 | 50.4 |
| Pulmonary and extrapulmonary | 108 | 105 | 109 | 89 | 105 | 88 | 135 | 139 | 125 | 114 | 97 | 1214 | 20.6 |
| Extrapulmonary only | 166 | 141 | 172 | 136 | 143 | 141 | 143 | 173 | 161 | 138 | 126 | 1640 | 27.8 |
| Unknown | 5 | 8 | 7 | 5 | 8 | 4 | 9 | 10 | 6 | 6 | 7 | 75 | 1.3 |
| All pulmonary cases | 382 | 394 | 408 | 345 | 391 | 350 | 413 | 443 | 390 | 367 | 304 | 4187 | 100.0 |
| – Culture confirmed | 327 | 337 | 333 | 303 | 340 | 310 | 345 | 384 | 329 | 313 | 257 | 3578 | 85.5 |
| – Culture confirmed rifampicin susceptible cases | 317 | 326 | 319 | 293 | 322 | 288 | 326 | 341 | 307 | 304 | 244 | 3387 | 80.9 |
| Drug resistance (cases tested for rifampicin)‡ | 437 | 426 | 449 | 383 | 440 | 402 | 439 | 471 | 427 | 407 | 344 | 4625 | 100.0 |
| – Rifampicin-susceptible | 430 | 415 | 438 | 375 | 423 | 385 | 423 | 454 | 415 | 399 | 334 | 4491 | 97.1 |
| – Rifampicin-resistant, foreign origin | 7 | 11 | 10 | 7 | 15 | 14 | 14 | 16 | 12 | 8 | 9 | 123 | 2.7 |
| – Rifampicin-resistant, Swiss origin | 0 | 0 | 1 | 1 | 2 | 3 | 2 | 1 | 0 | 0 | 1 | 11 | 0.2 |
AG: Aargau; AR: Appenzell Ausserrhoden; BE: Bern; BL: Basel-Landschaft; BS: Basel-Stadt; FR: Fribourg; GE: Geneva; GL: Glarus; GR: Graubünden; JU; Jura; LU: Lucerne; NE: Neuchâtel; NW: Nidwalden; OW: Obwalden; SG: St Gallen; SH: Schaffhausen; SO: Solothurn; SZ: Schwyz ; TG: Thurgau; TI: Ticino; VD: Vaud; VS: Valais; ZG: Zug; ZH: Zurich; AI: Appenzell Innerrhoden; UR: Uri
* The Swiss case definition for tuberculosis is met if laboratory or clinical criteria are met. For details see ‘Materials and methods’. ‡ only cases with known origin and rifampicin susceptibility test result
Seventy-one percent of all cases manifested as pulmonary tuberculosis (50% exclusively pulmonary, 21% pulmonary and extrapulmonary), in 28% the manifestation was exclusively extrapulmonary, and in 1%, the manifestation site was unknown (tables 1 2). The most frequently notified extrapulmonary sites were extra-thoracic lymphatic (n = 987, 16.7%), intrathoracic lymphatic (n = 838, 14.2%) and pleural (n = 535, 9.1%). Disseminated tuberculosis occurred in 414 cases (7.0%) and the central nervous system was involved in 111 cases (1.9%) – both disease manifestations are considered clinically particularly problematic. At least one of those two problematic manifestations was present in 479 cases (8.1%).
Table 2Site of disease, tuberculosis cases in Switzerland and Liechtenstein, 2009–2019.
| Site of disease | N | Percent a |
| Pulmonary | 4187 | 70.9 |
| Pleural | 535 | 9.1 |
| Lymphatic, intrathoracic | 838 | 14.2 |
| Lymphatic, extrathoracic | 987 | 16.7 |
| Osteo-articular, spine | 188 | 3.2 |
| Osteo-articular, other | 109 | 1.9 |
| Central nervous system, meningeal | 64 | 1.1 |
| Central nervous system, other | 61 | 1.0 |
| Central nervous system, allb | 111 | 1.9 |
| Disseminatedc | 414 | 7.0 |
| Other sites | 313 | 5.3 |
| Site not specified | 75 | 1.3 |
a Percentages do not add up to 100 owing to overlapping categories. The denominator is n = 5902.
b Numbers and percent are not the sum of meningeal and central nervous system involvement owing to overlapping categories.
c Includes acute and protracted, i.e. miliary, mycobacteraemia, and polytopic (three or more sites affected)
Since 1995, at least one isolate from each culture-positive case was subject to phenotypic drug susceptibility testing for at least isoniazid and rifampicin. Genotypic drug susceptibility testing for rifampicin has become more widely available since 2010 [12]. The annual number of rifampicin-resistant tuberculosis cases varied between 7 and 16. From 2009 to 2019, 134 cases of rifampicin resistance with known origin were reported (table 1). This corresponds to an overall rifampicin resistance rate of 2.9% (134 out of 4625). Eleven cases were persons of Swiss origin corresponding to 1.1% out of 1045 Swiss cases tested. Main risk factors were foreign origin (n = 123 out of 3994, 3.4%) and prior anti-tuberculosis treatment (n = 41 out of 341, 10.7%).
Origin and prior anti-tuberculosis treatment were independent risk factors in the logistic regression, i.e. the interaction term showed an adjusted odds ratio (aOR) of 1.5, but with a large 95% CI of 0.4 to 7.2) and therefore attributable to chance alone, which is why we estimated the model without interaction term (table 3). The aOR comparing foreign versus Swiss origin was 3.6 (95% CI 2.7–7.0) and prior anti-tuberculosis treatment versus no or unknown treatment status 5.5 (3.7–8.1). Persons from Somalia (n = 27 out of 355, 7.6%), Eritrea (n = 13 out of 561, 2.3%), Tibet (China) (n = 11 out of 136, 8.1%) und Georgia (n = 9 out of 32, 28.1%) accounted for 60 out of 134 cases (49.0%). In the extended model using Swiss origin as reference and keeping prior anti-tuberculosis treatment as confounder (aOR 4.5, 95% CI 3.0–6.7), we obtained the following aORs (95% CI): Georgia 10.0 (4.0–23.1), Ethiopia 9.4 (3.5–24.2), Tibet (China) 6.9 (2.9–16.6), Somalia 8.1 (4.0–17.2), Eritrea 2.6 (1.1–5.9,) sorted by decreasing order. The remaining countries show an increased aOR of 2.3 with a 95% CI of 1.2–4.7.
Overall, 117 out of 134 cases (87%) were MDR, and 4 of the MDR cases were XDR. All four XDR cases were among persons of foreign origin: Moldova, Morocco, China, Georgia. The annual number of MDR cases varied between four and fourteen cases in the observation period without a clear trend.
Tabler 3Rifampicin resistance risk factor models, Switzerland and Liechtenstein, 2009–2019.
| Univariable | Multivariable | Multivariable | ||||||||
| OR | 2.5% | 97.5% | aOR | 2.5% | 97.5% | aOR | 2.5% | 97.5% | ||
| Basic Model | ||||||||||
| Prior treatment | No | Ref. | Ref. | Ref. | ||||||
| Yes | 5.4 | 3.6 | 7.8 | 3.9 | 0.8 | 13.6 | 5.5 | 3.7 | 8.1 | |
| Origin | Swiss | Ref. | Ref. | Ref. | ||||||
| Foreign | 3.4 | 1.9 | 6.7 | 3.2 | 1.6 | 7.1 | 3.6 | 2.0 | 7.0 | |
| Interaction term | 1.5 | 0.4 | 7.2 | |||||||
| Extended Model | ||||||||||
| Prior treatment | No | Ref. | Ref. | |||||||
| Yes | 5.4 | 3.6 | 7.8 | 4.7 | 3.1 | 7.4 | ||||
| Origin | Swiss | Ref. | Ref. | |||||||
| Somalia | 7.7 | 3.9 | 16.4 | 8.1 | 4.0 | 17.2 | ||||
| Eritrea | 2.2 | 1.0 | 5.1 | 2.6 | 1.1 | 5.9 | ||||
| Ethiopia | 9.3 | 3.5 | 23.6 | 9.4 | 3.5 | 24.2 | ||||
| Tibet (China) | 8.3 | 3.5 | 19.7 | 6.9 | 2.9 | 16.6 | ||||
| Georgia | 16.6 | 7.0 | 36.4 | 10.0 | 4.0 | 23.1 | ||||
| Other | 2.2 | 1.2 | 4.5 | 2.3 | 1.2 | 4.7 | ||||
Data on treatment outcome monitoring were available for the 3 years 2016 to 2018. Treatment success (cured or treatment completed) increased slightly from 77% to 80% among laboratory-confirmed pulmonary, rifampicin-susceptible tuberculosis cases (table 4). Adolescents and elderly patients were less likely to achieve treatment success (table 5). With the possible exception of adolescents, women had a slightly higher proportions of treatment success than men. Death (of any cause) was rare (4.4%). The yearly proportion of patients with programmatically unsuccessful treatment slightly decreased from 20.2% in 2016 to 15.5% in 2018. The highest single contributor to this composite outcome was the proportion of ‘not evaluated’ at 80.8% of unsuccessful treatments. This suggests that motivating clinicians to fill in notification forms more completely might already lead to meeting WHO targets.
Table 4Treatment outcome monitoring according to WHO standards among pulmonary culture-positive, rifampicin-susceptible tuberculosis cases, Switzerland and Liechtenstein, 2016–2018.
| 2016 | 2017 | 2018 | Total | Percent | |
| Cured | 120 | 125 | 130 | 375 | 39.4 |
| Treatment completed | 143 | 111 | 114 | 368 | 38.7 |
| Treatment failure | 2 | 0 | 0 | 2 | 0.2 |
| Lost to follow up | 13 | 13 | 4 | 30 | 3.2 |
| Not evaluated | 54 | 38 | 43 | 135 | 14.2 |
| Died | 9 | 20 | 13 | 42 | 4.4 |
| Total | 341 | 307 | 304 | 952 | 100.0 |
| Treatment success (WHO): Sum of Cured and Treatment completed | |||||
| N | 263 | 236 | 244 | 743 | 78.0 |
| Percent | 77.1 | 76.9 | 80.3 | ||
| Programmatically unsuccessful: Sum of Treatment failure, Lost to follow-up and Not evaluated | |||||
| N | 69 | 51 | 47 | 167 | 17.5 |
| Percent | 20.2 | 16.6 | 15.5 | ||
Table 5Treatment success according to the WHO (cured or treatment completed) among pulmonary culture-positive rifampicin-susceptible tuberculosis cases, Switzerland and Liechtenstein, 2016–2018.
| Male | Female | Overall | ||||
| Age Groups | Success/total | Percent | Success/total | Percent | Success/total | Percent |
| 0–14 | 18/22 | 81.8 | 13/15 | 86.7 | 31/37 | 83.8 |
| 15–19 | 57/83 | 68.7 | 11/20 | 55.0 | 68/103 | 66.0 |
| 20–39 | 224/279 | 80.3 | 143/166 | 86.1 | 367/445 | 82.5 |
| 40–64 | 118/147 | 80.3 | 71/80 | 88.8 | 189/227 | 83.3 |
| 65+ years | 49/81 | 60.5 | 39/59 | 66.1 | 88/140 | 62.9 |
| Total | 466/612 | 76.1 | 277/340 | 81.5 | 743/952 | 78.0 |
Over the past 11 years 2009–2019, tuberculosis notifications in Switzerland and Liechtenstein have decreased to an incidence of 5.1 cases per 100,000 in 2019. Most cases are found among immigrants from countries with a high incidence of tuberculosis, particularly Eritrea, Somalia and Ethiopia. Within Europe, Portugal and Kosovo are the most important countries of origin of tuberculosis patients in Switzerland. Previous analyses of tuberculosis in Switzerland had shown a similar trend [13].
The decreasing trend in the total number of notified tuberculosis cases fits with that observed most notably in other industrialised countries [2, 14–16]. In contrast to this favourable development, trends in lower income countries have been declining too slowly to achieve the targets of the WHO “End TB strategy” in a timely fashion [17].
The age distribution mirrors the demography of patients of foreign origin as observed in previous analyses [3]. Characteristically, migrants with tuberculosis entering Switzerland are nowadays young adult men from Eritrea and Somalia (largely refugees and asylum seekers) and from Portugal (directly joining the workforce). The FOPH offers a web-based platform with 10 key questions about tuberculosis contextual factors (www.tb-screen.ch). This online tool is available in the 34 languages most relevant to asylum seekers in Switzerland and has been validated for supporting clinicians in taking a tuberculosis-specific medical history [18]. Unfortunately, data on immigrant screening are no longer available since the revision of the Epidemic Act in 2016. Therefore, we cannot anymore distinguish between asylum seekers and other groups of foreign origin.
Drug resistance to rifampicin – relevant to the indication for second-line drugs – has fortunately remained rare in Switzerland [3]. The two most important risk factors for drug resistance are foreign origin, particularly Georgia, Tibet (China), Somalia, Ethiopia and Eritrea, as well as a history of previous anti-tuberculosis treatment [3, 4]. A proper history of a pervious treatment is crucial for the choice of an appropriate initial treatment regimen [19] and must be accompanied by prompt drug susceptibility testing. Since the introduction of genotyping methods, which became widely available in 2010 [12], drug susceptibility testing of rifampicin has been greatly applied, and results are theoretically available within one working day [3]. It has to be stressed here, that treating cases of rifampicin-resistant tuberculosis requires specific medical training. The Swiss Lung Association offers support by experienced physicians and a closed user group for an anonymised discussion of specific cases. Rifampicin-susceptible tuberculosis without evidence of prior anti-tuberculosis treatment may be treated by general practitioners if they comply with the Swiss recommendations or under the supervision of a well-trained colleague [9].
The FOPH included treatment outcome monitoring according to WHO standards in the national notification system with the revision of the Epidemics Act in 2016. The overall treatment success in culture-confirmed, rifampicin-susceptible, pulmonary tuberculosis was 78% (among the 952 cases with the relevant information), and thus below the WHO target of 85%, largely because of missing data (called ‘not evaluated’ in the WHO definitions). The Swiss surveillance system does not record directly observed treatment. Poor treatment adherence and high proportions of loss to follow-up may be common in male adolescents, and both has been described specifically for tuberculosis treatment outcomes in Haiti and in South Africa [20, 21]. Immigrant adolescents with tuberculosis often come unaccompanied from a cultural background very different from the Swiss context and may benefit from specific support regarding adherence to medication.
The overall treatment success was similar in 1996, with a success of 79% [22]; however, that article had been published prior to the WHO recommendations in 2013 with the 2020 revision [5], limiting direct comparison. With regard to the current data, the proportion of patients with treatment success may have slightly improved from 77% in 2016 and 2017 to 80% in 2018. WHO reporting uses an intention-to-treat approach. We propose using an additional composite outcome ‘programmatically unsuccessful’ as defined above and shown at the bottom of table 4, excluding patients who died during the observation period from the numerator (but not the denominator). This additional indicator might better reflect the performance of national tuberculosis programme in countries where tuberculosis patients rarely die of tuberculosis, but rather of other causes. We observed a small but steady decline in this composite outcome over the 3 years of observation. This was paralleled by a decrease of missing information about treatment outcome, showing slowly improved adherence to notification guidelines. However, asylum seekers diagnosed with tuberculosis in national registration centres and subsequently transferred to a specific canton that is different from the canton of the registration centre often result in ‘loss to follow-up’.
With respect to tuberculosis notification rates 2015–19, Switzerland is very similar to countries of the Western sub-region of WHO Europe [23]; however, the estimated incidence in Switzerland was lower than the European Economic Area average (5.1 versus 10.6 per 100,000 in 2019) [23]. Also the Swiss gender distribution is in accordance with European data, with more men than women reported in 2019. The percentage of foreign-born tuberculosis cases is in line with comparable high-income immigration countries such as Sweden, Norway or Denmark. Drug resistance is a problem in the broad WHO European Region (17.0% in 2019) but is not in Switzerland (1.1%) [23, p42]. As in Switzerland, previous treatment is a risk factor for drug resistance. This fact is confirmed by our logistic regression models (see table 3).
Switzerland is not alone in failing to meet the WHO ‘treatment success’ target of 85%. This is explained by the large number of ‘not evaluated’ cases, and there are countries in the European region that are performing even worse than Switzerland such as Finland, France and Denmark – strongly suggesting that compiling treatment outcome data in routine surveillance is an issue in Switzerland as well as in other European countries.
A possible limitation to our analysis is a potential bias that might be introduced by underreporting – a concern of every notification system. However, no national studies exist addressing this specific problem. The dual system mandating both physicians and laboratories to report on tuberculosis gives some assurance that at least the notification of laboratory-confirmed tuberculosis is likely to be a good proxy for the incidence of tuberculosis diagnoses. Because tuberculosis notification uses the patient’s full name, the FOPH cannot collect potentially stigmatising medical risk factors such as human immunodeficiency virus infection, alcoholism, or diabetes.
Tuberculosis has become a rare disease in Switzerland, and resistance to the key drug rifampicin remains fortunately rare among previously untreated patients. Twenty-eight percent of tuberculosis cases exclusively concern extrapulmonary manifestations, therefore a normal chest radiograph or negative cultures from respiratory samples do not definitively exclude tuberculosis. In Switzerland, the most important risk group for tuberculosis, both drug-susceptible and -resistant, are migrants from the Horn of Africa, but also from South-East and South-West Europe. A careful history of migration and previous treatment with anti-tuberculosis drugs is essential for the appropriate choice of the treatment regimen.
No financial support and no potential conflict of interest relevant to this article was reported.
1. Houben RM , Dodd PJ . The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling. PLoS Med. 2016 Oct;13(10):e1002152. https://doi.org/10.1371/journal.pmed.1002152
2. World Health Organization . Global tuberculosis report 2020. Geneva: WHO; 2020. Available from: https://www.who.int/publications/i/item/9789240013131
3. Altpeter E , Schoch O , Helbling P . Tuberkulose in der Schweiz: selten, und manchmal kompliziert. Swiss Med Forum 2015;15:925-30.
4. Helbling P , Altpeter E , Raeber PA , Pfyffer GE , Zellweger JP . Surveillance of antituberculosis drug resistance in Switzerland 1995-1997: the central link. Eur Respir J. 2000 Aug;16(2):200–2. https://doi.org/10.1034/j.1399-3003.2000.16b03.x
5. World Health Organization . Definitions and reporting framework for tuberculosis—2013 revision (updated December 2014 and January 2020). Geneva: WHO; 2013. Report No.: WHO/HTM/TB/2013.2.
6. Bundesamt für Statistik / Office fédéral de la statistique. Verzeichnis der Staaten und Gebiete. Neuchâtel: BFS / OFS; 2019. Report No.: be-b-00.04-sg-01. Available from: https://www.bfs.admin.ch/bfs/de/home/grundlagen/stgb.html
7. International Organization for Standardization . Date and time — Representations for information interchange — Part 1: Basic rules. Geneva: ISO; 2016. Report No.: ISO 8601-1:2019. Available from: https://www.iso.org/standard/70907.html
8. Helbling P , Altpeter E , Egger JM , Zellweger JP . Treatment outcomes of multidrug-resistant tuberculosis in Switzerland. Swiss Med Wkly. 2014 Dec;144:w14053. https://doi.org/10.4414/smw.2014.14053
9. Swiss Lung Association, Federal Office of Public Health . Tuberculosis in Switzerland. Guidance for healthcare professionals. Bern Swiss Lung Association; 2019.
10. McGill R , Tukey JW , Larsen WA . Variations of Box Plots. Am Stat. 1978;32:12–6.
11. R Core Team . R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2020.
12. Boehme CC , Nabeta P , Hillemann D , Nicol MP , Shenai S , Krapp F , et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010 Sep;363(11):1005–15. https://doi.org/10.1056/NEJMoa0907847
13. Bundesamt für Gesundheit / Office Fédéral de la Santé Publique . Tuberkulose in der Schweiz. Bulletin. 1995;37:10–1.
14. European Centre for Disease Prevention and Control, World Health Organization Regional Office for Europe . Tuberculosis surveillance and monitoring in Europe 2019 (2017 data). Stockholm: ECDC, WHO Regional Office for Europe; 2019.
15. Ota M , Uchimura K . Trends of tuberculosis rates before and after the declaration as a public health emergency in Japan, 1992-2006. Int J Tuberc Lung Dis. 2019 Sep;23(9):1000–4. https://doi.org/10.5588/ijtld.18.0650
16. Schwartz NG , Price SF , Pratt RH , Langer AJ . Tuberculosis - United States, 2019. MMWR Morb Mortal Wkly Rep. 2020 Mar;69(11):286–9. https://doi.org/10.15585/mmwr.mm6911a3
17. Sahu S , Ditiu L , Lawson L , Ntoumi F , Arakaki D , Zumla A . UN General Assembly tuberculosis targets: are we on track? Lancet. 2020 Mar;395(10228):928–30. https://doi.org/10.1016/S0140-6736(20)30565-1
18. Schneeberger Geisler S , Helbling P , Zellweger JP , Altpeter ES . Screening for tuberculosis in asylum seekers: comparison of chest radiography with an interview-based system. Int J Tuberc Lung Dis. 2010 Nov;14(11):1388–94.
19. Rieder HL . Drug-resistant tuberculosis: issues in epidemiology and challenges for public health. Tuber Lung Dis. 1994 Oct;75(5):321–3. https://doi.org/10.1016/0962-8479(94)90075-2
20. Reif LK , Rivera V , Bertrand R , Rouzier V , Kutscher E , Walsh K , et al. Outcomes across the tuberculosis care continuum among adolescents in Haiti. Public Health Action. 2018 Sep;8(3):103–9. https://doi.org/10.5588/pha.18.0021
21. Berry KM , Rodriguez CA , Berhanu RH , Ismail N , Mvusi L , Long L , et al. Treatment outcomes among children, adolescents, and adults on treatment for tuberculosis in two metropolitan municipalities in Gauteng Province, South Africa. BMC Public Health. 2019 Jul;19(1):973. https://doi.org/10.1186/s12889-019-7257-4
22. Helbling P , Medinger C , Altpeter E , Raeber PA , Beeli D , Zellweger JP . Outcome of treatment of pulmonary tuberculosis in Switzerland in 1996. Swiss Med Wkly. 2002 Sep;132(35-36):517–22.
23. European Centre for Disease Prevention and Control, WHO Regional Office for Europe . Tuberculosis surveillance and monitoring in Europe 2021 – 2019 data. Copenhagen: WHO Regional Office for Europe; 2021.
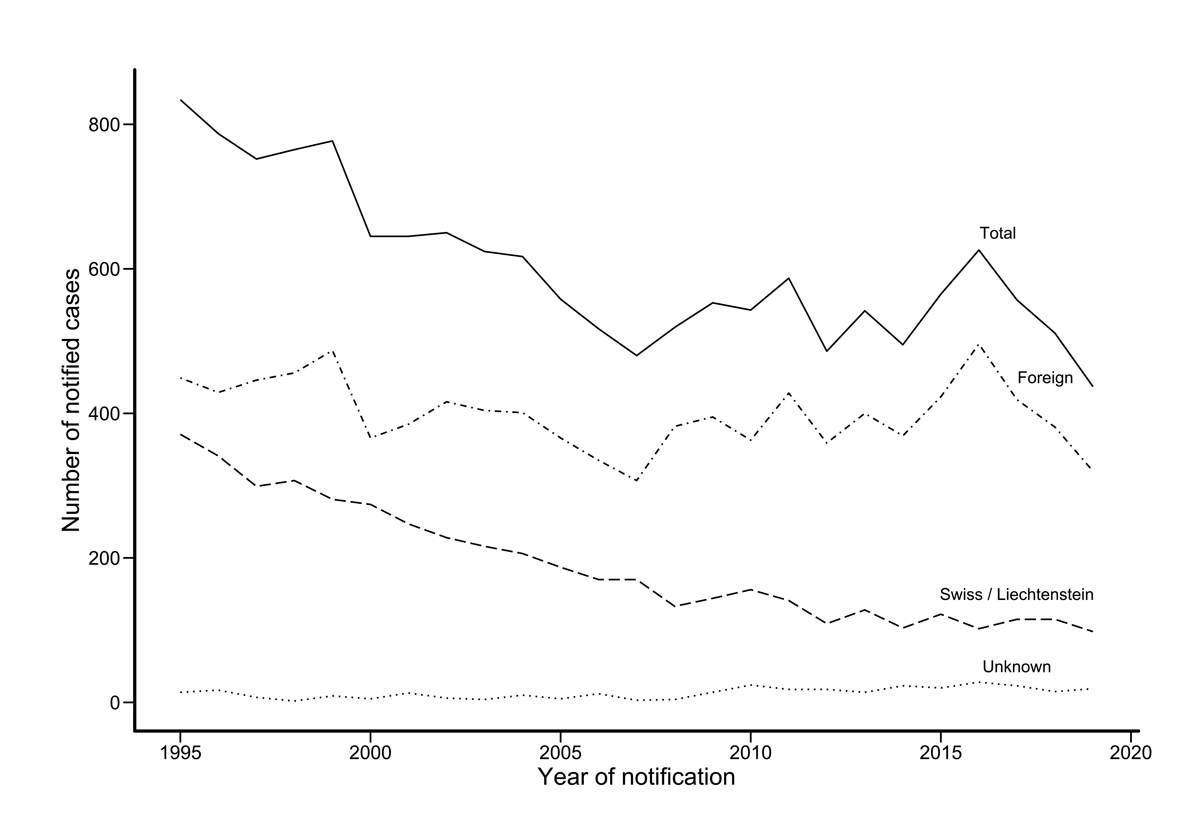
Figure S1 Annual numbers of tuberculosis, by origin, in Switzerland and Liechtenstein 1995–2019. The Swiss case definition for tuberculosis is met if laboratory or clinical criteria are met. For details see ‘Materials and methods’.