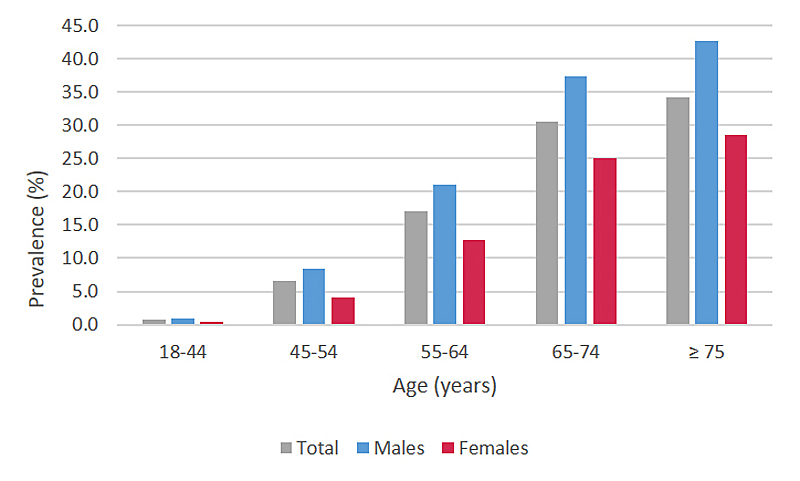
Figure 1 Prevalence of patients with ≥ 1 filled prescription of any lipid-lowering drug in the year 2019 (n= 841682), by age and sex, extrapolated to the Swiss population level.
DOI: https://doi.org/10.4414/SMW.2021.w30018
Cardiovascular diseases (CVD) are the leading cause of death in Switzerland, accounting for almost a third of all deaths [1]. They are responsible for 15.6% and 9.1% of potential years of life lost in males and females, respectively [2]. However, the burden of CVD is not only a health issue, but also an increasing economic challenge to the healthcare system. It has been estimated that up to 19% of total healthcare expenditure in selected high-income European countries is attributable to CVD [3].
Numerous genetic, epidemiological and clinical studies provide compelling evidence that high plasma levels of low-density lipoprotein cholesterol (LDL-C) cause the development of atherosclerotic CVD, and that any mechanism of lowering LDL-C should reduce the risk of cardiovascular events [4].
To reach the recommended LDL-C target values, along with lifestyle modifications, most patients at high cardiovascular risk need lipid-lowering drugs. Primarily three drug classes with different mechanisms of action are used: statins (inhibition of cholesterol synthesis in the liver), ezetimibe (inhibition of intestinal cholesterol absorption), and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors (promotion of cholesterol disposal via LDL-C receptors in the liver). If, besides LCL-C, triglycerides also are elevated, in selected cases the prescription of fibrates can be reasonable (regulation of lipid metabolism-related gene expression) [5].
Despite their widespread use, to date little is known about utilisation patterns and related costs of lipid-lowering drugs in Switzerland.
The aim of this study was to characterise patients receiving a lipid-lowering therapy and to analyse filled prescriptions and costs of different lipid-lowering agents over time based on administrative claims data provided by the Swiss health insurance company Helsana.
We conducted a retrospective descriptive study using administrative claims data of adult persons (age ≥18 years) enrolled with the Swiss health insurance company Helsana.
In Switzerland, basic health insurance is compulsory for all residents, and insurers are obliged to accept every applicant. Insurance companies are private, but they all must offer the same benefits covered under the basic insurance policy. The Helsana group is one of Switzerland’s leading health insurers, providing some 1.2 million residents across all 26 cantons with basic insurance (approximately 14% of the overall Swiss population, year 2019).
Helsana’s administrative claims data are considered complete and reliable, and have been widely used in health research. The recorded variables include patient demographics, postal codes and all claims sent to Helsana for reimbursement, such as diagnostic assessments, medical treatments and drug prescriptions (labelled according to the anatomical therapeutic chemical [ATC] classification system). Hospital diagnoses are also available as diagnosis-related groups (DRGs).
The database is located at Helsana and access was granted to an anonymised dataset containing the relevant parameters for this study.
We identified prescriptions of lipid-lowering drugs filled between 1 January 2013 and 31 December 2019 in the outpatient sector (public pharmacies, medical practices, outpatient clinics) using ATC codes (table 1). The time period was selected based on data availability.
Table 1Lipid-lowering drug classes and active substances with anatomical therapeutic chemical (ATC) classification system codes.
| Drug class | Active substance | ATC code | Combinations approved in Switzerland | ATC code |
| Statins | Atorvastatin | C10AA05 | Atorvastatin + ezetimibe | C10BA05 |
| Atorvastatin + amlodipine | C10BX03 | |||
| Atorvastatin + perindopril | C10BX15 | |||
| Atorvastatin + amlodipine + perindopril | C10BX11 | |||
| Rosuvastatin | C10AA07 | Rosuvastatin + ezetimibe | C10BA06 | |
| Simvastatin | C10AA01 | Simvastatin + ezetimib | C10BA02 | |
| Simvastatin + fenofibrate | C10BA04 | |||
| Pravastatin | C10AA03 | |||
| Fluvastatin | C10AA04 | |||
| Pitavastatin | C10AA08 | |||
| Ezetimibe | Ezetimibe | C10AX09 | Ezetimibe + atorvastatin | C10BA05 |
| Ezetimibe + rosuvastatin | C10BA06 | |||
| Ezetimibe + simvastatin | C10BA02 | |||
| PCSK9 inhibitors | Evolocumab | C10AX13 | ||
| Alirocumab | C10AX14 | |||
| Fibrates | Bezafibrate | C10AB02 | ||
| Fenofibrate | C10AB05 | Fenofibrate + simvastatin | C10BA04 | |
| Gemfibrozil | C10AB04 |
PCSK9 = proprotein convertase subtilisin/kexin type 9
We restricted our analyses to statins, ezetimibe, PCSK9 inhibitors and fibrates. Other lipid-lowering agents such as bile acid sequestrants and nicotinic acid were not assessed, since they no longer play a relevant role in the treatment of dyslipidaemias owing to their limited efficacy and tolerability.
We defined prescriptions of atorvastatin ≥40 mg or rosuvastatin ≥20 mg as high-intensity statin therapy [6].
We assessed the number and prevalence of patients with filled prescriptions of lipid-lowering drugs, the number of filled prescriptions of lipid-lowering drugs and the resulting total and per capita costs (in Swiss francs [CHF]). Costs were not adjusted for inflation, since inflation was negligible in Switzerland over the study period.
Depending on the analysis, we stratified by drug class, active substance, year of prescription, sex, age group and the seven major statistical regions of Switzerland (Lake Geneva region, Espace Mittelland, North-West Switzerland, Zurich region, Eastern Switzerland, Central Switzerland, and Ticino).
We identified patients who filled a statin prescription for the first time in the year 2017 and who were continuously enrolled with Helsana from at least 12 months before until 24 months after their first-time statin prescription. This minimum period of enrolment was required to increase the likelihood of including incident (first-time) rather than prevalent statin users, to enable applying the definitions of therapy switching, augmentation and discontinuation as described in table 2, and to assess the presence or absence of cardiovascular events within a reasonable time frame before and after the first-time statin prescription (see below). For this analysis, we did not consider patients with a first-time statin prescription in earlier years, since their options for therapy switching and augmentation were not comparable (PCSK9 inhibitors became available in Switzerland in 2016).
Table 2Definitions of therapy switching, augmentation, and discontinuation.
| Definition | |
| Therapy switching | Prescription of another lipid-lowering drug (other statin or other drug class) without a following prescription of the initial statin (switching to another statin) or any statin (switching to another drug class) within 6 months |
| Therapy augmentation | Prescription of an additional lipid-lowering drug of another drug class (within 6 months after the first-time prescription of another lipid-lowering drug, at least one further prescription of any statin was required) |
| Therapy discontinuation | No further prescription of any lipid-lowering drug within 6 months after the first-time statin prescription |
We excluded patients who received another lipid-lowering drug (ezetimibe, a PCSK9 inhibitor or a fibrate) within 12 months before or on the day of the first-time statin prescription.
During a follow-up period of 18 months, we determined the proportions of patients who underwent switching, augmentation or discontinuation of the lipid-lowering treatment (see definitions provided in table 2). Furthermore, we assessed the average number of prescriptions of statins and lipid-lowering drugs overall per patient as well as the number of different lipid-lowering substances and drug classes used until the therapy was changed or discontinued.
We stratified the analyses by the presence or absence of a cardiovascular event within 12 months before or 18 months after the first-time statin prescription. Using SwissDRG flat-fee reimbursement codes, cardiovascular events were defined as transient ischaemic attack/stroke (B39A-C; B69A-D; B70A-K) and myocardial infarction (F41A-B; F60A-B) [7].
We identified patients who filled a PCSK9 inhibitor prescription for the first time in the year 2018 and who were continuously enrolled with Helsana from at least 5 years before until 12 months after their first-time PCSK9 inhibitor prescription. Before 2018, there was only a small number of patients with a first-time PCSK9 inhibitor prescription on the database. The minimum period of enrolment was required to increase the likelihood of including incident (first-time) rather than prevalent PCSK9 inhibitor users and to allow for an adequate observation time of pre-treatment, follow-up treatment and presence or absence of cardiovascular events.
During the observational period, we examined the entire lipid-lowering drug therapy and assessed cardiovascular events (definition see above; because of the small number of patients, we did not perform stratified analyses).
We applied descriptive statistics and reported results as counts and proportions. Where applicable, we tested differences between groups for statistical significance at the alpha level of 0.05 using chi-square tests.
To enable statements at the Swiss population level, we extrapolated the number of patients with filled prescriptions, the number of filled prescriptions and costs of lipid-lowering drugs according to age, sex, and canton of residence using data on balancing of risks provided by the Gemeinsame Einrichtung KVG (Joint Institution under the Federal Health Insurance Act). These annually published data are based on the entire pool of insured persons (including the offered benefits) from all health insurance companies in Switzerland [8].
The analyses on therapy switching, augmentation and discontinuation solely refer to the described subgroups of the Helsana population (no extrapolation).
We performed all analyses with SAS 9.4 software (SAS Institute, Cary, NC).
Table 3 displays the number of patients with filled prescriptions, the number of filled prescriptions, total costs and per capita costs of lipid-lowering drugs by drug class between the years 2013 and 2019.
Table 3Patients with filled prescriptions, filled prescriptions, and costs of lipid-lowering drugs between the years 2013 and 2019, by drug class, extrapolated to the Swiss population level.
| 2013 | 2016 | 2017 | 2018 | 2019 | Change since 2013** (%) | |
| Overall | ||||||
| Patients (n) | 736,174 | 810,510 | 822,470 | 828,505 | 841,682 | +14.3 |
| Prescriptions (n) | 2,145,969 | 2,444,393 | 2,495,575 | 2,615,894 | 2,694,247 | +25.5 |
| Total costs (CHF) | 222,172,326 | 251,912,823 | 251,094,595 | 224,390,672 | 230,040,365 | +3.5 |
| Per capita costs (CHF) | 302 | 311 | 305 | 271 | 273 | −9.4 |
| Statins | ||||||
| Patients (n) | 717,894 | 789,464 | 799,894 | 804,759 | 817,521 | +13.9 |
| Prescriptions (n) | 2,030,703 | 2,288,759 | 2,318,124 | 2,426,592 | 2,494,695 | +22.8 |
| Total costs (CHF) | 207,334,621 | 228,442,516 | 219,493,810 | 193,404,439 | 195,306,795 | −5.8 |
| Per capita costs (CHF) | 289 | 289 | 274 | 240 | 239 | −17.3 |
| Ezetimibe | ||||||
| Patients (n) | 54,762 | 72,835 | 84,446 | 99,598 | 118,237 | +115.9 |
| Prescriptions (n) | 157,058 | 210,664 | 240,221 | 295,125 | 357,485 | +127.6 |
| Total costs (CHF) | 32,615,892 | 42,901,445 | 47,307,671 | 45,140,417 | 50,763,128 | +55.6 |
| Per capita costs (CHF) | 596 | 589 | 560 | 453 | 429 | −27.9 |
| PCSK9 inhibitors* | ||||||
| Patients (n) | - | 692 | 1760 | 2631 | 3729 | +439.2 |
| Prescriptions (n) | - | 2262 | 8323 | 13,855 | 21,139 | +834.7 |
| Total costs (CHF) | - | 2,019,598 | 7,781,225 | 12,593,322 | 17,670,522 | +775.0 |
| Per capita costs (CHF) | - | 2920 | 4422 | 4787 | 4739 | +62.3 |
| Fibrates | ||||||
| Patients (n) | 18,781 | 19,203 | 18,620 | 17,221 | 16,619 | −11.5 |
| Prescriptions (n) | 51,989 | 52,468 | 51,247 | 48,193 | 46,456 | −10.6 |
| Total costs (CHF) | 3,508,667 | 3,236,380 | 3,158,290 | 2,920,252 | 2,762,876 | −21.3 |
| Per capita costs (CHF) | 187 | 169 | 170 | 170 | 166 | −11.0 |
CHF = Swiss francs; PCSK9 = proprotein convertase subtilisin/kexin type 9
*Approved in Switzerland since 2016; ** PCSK9 inhibitors since 2016
In 2019, 841,682 adults received a lipid-lowering drug (extrapolated to the Swiss population level). This corresponds to a treatment prevalence with lipid-lowering drugs of 11.6% (males: 13.7%; females: 9.6%; p <0.0001). Among individuals aged 65 years or older, more than every third person was treated with a lipid-lowering drug (fig. 1). Regional differences in the prevalence of patients under therapy with lipid-lowering drugs are depicted in figure 2. We found the highest treatment prevalence in Ticino, the lowest in the Zurich region.

Figure 1 Prevalence of patients with ≥ 1 filled prescription of any lipid-lowering drug in the year 2019 (n= 841682), by age and sex, extrapolated to the Swiss population level.
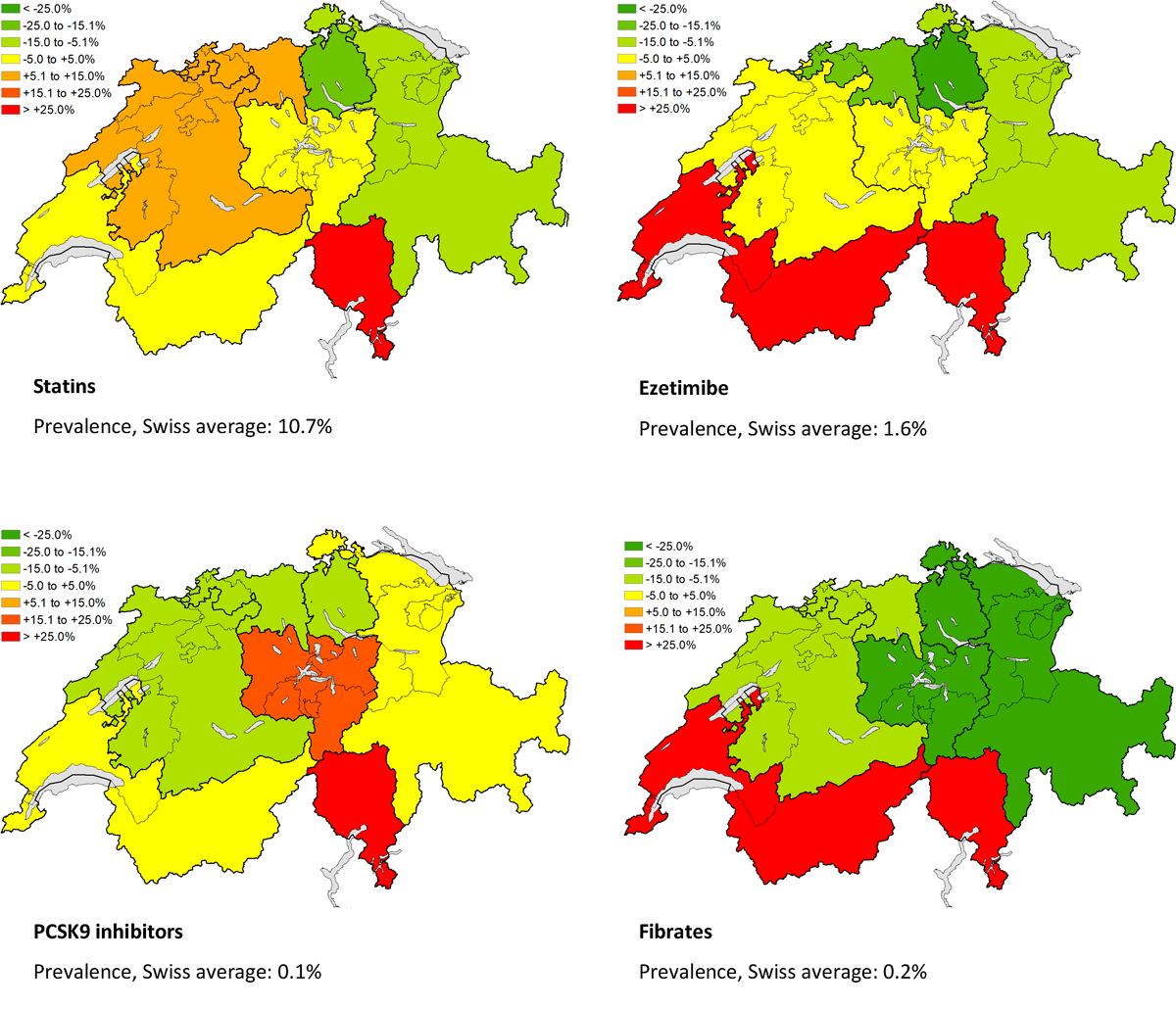
Figure 2 Relative deviation of the prevalence of patients with ≥1 filled prescription of a lipid-lowering drug from the Swiss average in the year 2019, by major statistical region and drug class, extrapolated to the Swiss population level. PCSK9 = proprotein convertase subtilisin/kexin type 9
The prevalence of patients taking lipid-lowering drugs increased between 2013 and 2019, in particular between 2018 and 2019 (2013: 8.9%; 2018: 9.4%; 2019: 11.6%; p <0.0001). The average age of these patients rose from 67.5 years (SD [standard deviation] 29.5) in 2013 to 68.6 years (SD 30.5) in 2019. The large majority used statins, with a percentage growth of +13.9% over the study period. The percentage growth of patients on ezetimibe (+115.9% between 2013 and 2019) and PSCK9 inhibitors (+439.2% between 2016 and 2019) was markedly higher; however absolute numbers were still at a low level in 2019 compared with statins. A decrease in the number of patients was seen for fibrates (−11.5% between 2013 and 2019) (table 3).
In parallel with the number of patients, the number of filled prescriptions of lipid-lowering drugs continuously rose between 2013 and 2019 (+25.5%). Throughout the study period, statins were by far the most commonly used drug class (>90% of prescriptions), followed by ezetimibe, fibrates and PCSK9 inhibitors (see table 3). At the level of active substances, we observed a trend towards the prescription of more potent statins (atorvastatin, rosuvastatin) in recent years (fig. 3).

Figure 3 Filled prescriptions of statins in the years 2013 (n = 2,030,703) and 2019 (n = 2,494,695), by active substance, extrapolated to the Swiss population level.
Relative to all statin prescriptions (originator and generic products), the proportion of generic statins increased from 55.0% in 2013 to 81.6% in 2019. Generics of ezetimibe became available in 2017 and accounted for 23.9% of all ezetimibe prescriptions in 2019. There were no generics of PCSK9 inhibitors or fibrates on the market.
In 2019, total costs of lipid-lowering drugs amounted to CHF 230,040,365, which corresponds to 3.0% of overall drug costs covered by the basic health insurance in the outpatient sector.
The annual per capita costs of lipid-lowering drugs decreased from CHF 302 in 2013 to CHF 273 in 2019 (−9.4%). As a consequence, the total costs only slightly increased between 2013 and 2019 (+3.5%), despite the marked growth in prescriptions including the high-priced PSCK9 inhibitors (table 3).
We included 16,668 patients in the analysis who filled a statin prescription for the first time in the year 2017. The average age was 66.1 years (SD 12.5), and 51.8% were male. During the 12 months before and 18 months after the first-time statin prescription, 8.5% experienced a cardiovascular event.
Within 18 months after the first-time statin prescription, 11.1% switched to another statin and 1.8% to another lipid-lowering drug class (mostly ezetimibe); 3.6% received an additional lipid-lowering drug (mostly ezetimibe) and 22.9% discontinued their lipid-lowering therapy.
On average, therapy switching to another drug class and therapy augmentation occurred after 2.4 (SD 1.7) and 2.9 (SD 1.8) statin prescriptions (all substances), respectively; therapy discontinuation was observed after 1.5 prescriptions (SD 0.8) of any lipid-lowering agent. Before therapy switching to another drug class, augmentation or discontinuation, fewer than two different statins (different substances) were used.
Compared with patients without a cardiovascular event, therapy augmentation was significantly more frequent, and discontinuation significantly less frequent among patients with a cardiovascular event. The latter also significantly more often switched to another statin, but not to another lipid-lowering drug class (table 4).
Table 4Switching, augmentation and discontinuation of the lipid-lowering therapy among patients in the Helsana population with a first-time statin prescription in the year 2017 during a follow-up period of 18 months.
| Patients overall (n = 16,668) | Patients without a cardiovascular event* (n = 15,243) | Patients with a cardiovascular event* (n = 1425) | p-value | |
| Therapy switching | ||||
| Switch to another statin | ||||
| Total, n (%) | 1845 (11.1) | 1627 (10.7) | 218 (15.3) | <0.0001 |
| Statin prescriptions until 1st switch, mean (SD) | 2.0 (1.4) | 2.0 (1.3) | 2.3 (1.6) | |
| Switch to another drug class | ||||
| Total, n (%) | 301 (1.8) | 284 (1.9) | 17 (1.2) | 0.07 |
| Switch to ezetimibe, n (%) | 244 (1.5) | 229 (1.5) | 15 (1.1) | |
| Switch to PCSK9 inhibitor, n (%) | 24 (0.1) | 23 (0.2) | 1 (0.1) | |
| Switch to fibrate, n (%) | 55 (0.3) | 53 (0.4) | 2 (0.1) | |
| Different statins until 1st switch, mean (SD) | 1.3 (0.6) | 1.3 (0.6) | 1.9 (1.0) | |
| Statin prescriptions (all substances) until 1st switch, mean (SD) | 2.4 (1.7) | 2.4 (1.7) | 3.1 (1.7) | |
| Therapy augmentation | ||||
| Total, n (%) | 605 (3.6) | 515 (3.4) | 90 (6.3) | <0.0001 |
| Addition of ezetimibe, n (%) | 585 (3.5) | 497 (3.3) | 88 (6.2) | |
| Addition of PCSK9 inhibitor, n (%) | 7 (0.0) | 5 (0.0) | 2 (0.1) | |
| Addition of fibrate, n (%) | 26 (0.2) | 24 (0.2) | 2 (0.1) | |
| Different statins until augmentation, mean (SD) | 1.2 (0.4) | 1.2 (0.4) | 1.2 (0.4) | |
| Statin prescriptions (all substances) until augmentation, mean (SD) | 2.9 (1.8) | 2.8 (1.7) | 3.4 (2.0) | |
| Therapy discontinuation | ||||
| Total, n (%) | 3823 (22.9) | 3599 (23.6) | 224 (15.7) | <0.0001 |
| Different statins until discontinuation, mean (SD) | 1.0 (0.2) | 1.0 (0.2) | 1.1 (0.3) | |
| Different classes of lipid-lowering drugs until discontinuation, mean (SD) | 1.0 (0.1) | 1.0 (0.1) | 1.0 (0.0) | |
| Lipid-lowering drug prescriptions (all drug classes) until discontinuation, mean (SD) | 1.5 (0.8) | 1.4 (0.8) | 1.8 (0.9) | |
PCSK9 = proprotein convertase subtilisin/kexin type 9; SD = standard deviation
* Observation period: 12 months before until 18 months after first-time statin prescription
We included 130 patients in the analysis who filled a PCSK9 inhibitor prescription for the first time in the year 2018. The average age was 63.7 years (SD 10.4), and 60.0% were male. During the 5 years before and 12 months after the first-time PCSK9 inhibitor prescription, two patients experienced a cardiovascular event.
Most patients (93.1%) were previously treated with a statin (on average 10.8 [SD 7.4] prescriptions of 2 [SD 1.1] different active substances). However only 55.4% of these received a high-intensity statin therapy (atorvastatin ≥40 mg or rosuvastatin ≥20 mg). Ezetimibe was used as a pre-treatment in 64.6% of all patients (on average 4.1 [SD 5.6] prescriptions); 60.8% were on both a statin and ezetimibe before the first PCSK9 inhibitor prescription.
After initiation of the PCSK9 inhibitor, only a minority continued the previous statin/ezetimibe therapy (statin: 39.2%; ezetimibe: 23.1%; statin plus ezetimibe: 11.5%).
Within 12 months after the first-time PCSK9 inhibitor prescription, on average 7.8 (SD 4.3) further prescriptions of this drug class followed. After 3, 6 and 9 months, 86.2%, 80.8% and 75.4%, respectively, were still under treatment with a PCSK9 inhibitor. Five patients switched to another PCSK9 inhibitor (other active substance).
In the present study, we analysed utilisation patterns of lipid-lowering drugs and related costs in Switzerland between the years 2013 and 2019.
The prevalence of lipid-lowering drug use in the Swiss adult population rose from 8.9% in 2013 to 11.6% in 2019. Also in other countries, the use of lipid-lowering drugs increased over time [9-15]. Factors that could have influenced the growth in lipid-lowering drug utilisation include demographic changes such as population ageing, pharmaceutical industry marketing, health authority programmes and the release of new guidelines promoting lower LDL-C target values and thereby extending the number of patients eligible for a lipid lowering therapy [5, 9].
Of note, in international comparisons, the prevalence of lipid-lowering drug use is still relatively low in Switzerland. In Germany, 9.9% of 18–79-year-olds were treated with lipid-lowering drugs between 2008 and 2011 [13]. In Ireland (2009–2011) [16], the United States (2011–2012/2004–2013) [14, 15], and Australia (2016) [10], more than 40% of the population aged 65 years or older received lipid-lowering drugs, whereas this proportion was around one third in Switzerland according to our study.
The prevalence of lipid-lowering drug use was lower in females than males across all age groups and observation years, which was likewise seen in several other studies [10, 11, 13, 15-18]. This may indicate undertreatment of females, given that prevalences of hypercholesterolaemia and other cardiovascular risk factors are comparable for both sexes [19]. Although awareness about CVD prevention in women has risen over the last decades, sex-related disparities in cardiovascular health care still persist and need to be further addressed in the future [20, 21]. Beyond that, adverse side effects of statins are more common in females and can lead to discontinuation of therapy [22].
The average age of patients taking lipid-lowering drugs steadily increased between 2013 and 2019. Whereas in the past there was uncertainty about the efficacy and safety of statins among older people, recent evidence speaks in favour of a benefit irrespective of age, if occlusive vascular disease is present (i.e., secondary prevention). In patients without vascular disease (i.e., primary prevention) there is less evidence of benefit [23].
Relative to the Swiss average, we found a higher prevalence of use of most lipid-lowering drug classes in the Italian-speaking and French-speaking parts of Switzerland (Ticino and Lake Geneva region), and a lower prevalence in the Zurich region and Eastern Switzerland. This is in line with findings from the Swiss Health Survey 2007, which reported higher and lower dyslipidaemia screening and treatment rates in the mentioned regions, respectively. The regional differences cannot be accounted for by differences in population characteristics such as sex, age and educational level, but may partly be due to differing local habits and health policies [24].
The frequencies of use of different lipid-lowering drugs reflect valid therapeutic guidelines [5, 25, 26]. As first-line treatments, statins were by far the most commonly invoiced drug class. Prescriptions of ezetimibe (second-line treatment) and PCSK9 inhibitors (third-line treatment) were at a comparatively low level, but showed a marked relative increase over the study period. In 2015, results from the IMPROVE-IT trial were published, providing evidence that adding ezetimibe to a statin leads to incremental lowering of LDL-C and significantly improves cardiovascular outcomes compared with a statin alone [27]. This supports the use of ezetimibe not only in patients with statin intolerance, but also as an adjunct therapy in patients who cannot reach their LDL-C target values with a statin monotherapy. In 2016, PCSK9 inhibitors came onto the market; these are generally well tolerated and highly effective. However, because of the high costs, their use is currently limited to selected patients. Fibrates are only recommended in specific situations in patients with hypertriglyceridaemia and became less important over time [5, 26].
Although there was a trend towards the prescription of more potent statins in recent years, still too few patients appear to receive a high-intensity statin therapy. In a study based on data from Swiss general practices, only 49.6% of statin-treated patients achieved the recommended LDL-C target values according to their assigned cardiovascular risk category (statin treatment intensity was high for 39.0%) [28].
Within 18 months after the first-time statin prescription, about every tenth patient in the Helsana population switched to another statin, and every fifth patient discontinued the lipid-lowering therapy. Common reasons for statin switching or discontinuation are drug-drug interactions and adverse effects, particularly muscle symptoms, which affect 7–29% of patients on statins [29]. Alternative explanations for the relatively high discontinuation rate could be poor understanding of the necessity of therapy from the patients’ side or initial treatment indications that were subsequently not confirmed. Indicative of this might be the facts that patients had on average only 1.5 prescriptions of a single lipid-lowering drug until discontinuation, and only a minority was switched to an alternative drug class as suggested by therapeutic guidelines in the case of statin intolerance [5, 26]. Therapy augmentation with an additional lipid-lowering drug was implemented in 3.6% of patients. Bearing in mind that around half of statin-treated patients in Switzerland do not achieve LDL-C target values [28], this option does not seem to be sufficiently considered.
As required by the Swiss Federal Office of Public Health [30], the large majority of patients insured with Helsana tried two different statins before the first-time prescription of a PCSK9 inhibitor. However, just over half obtained a high-intensity statin therapy. One could therefore conclude that pre-treatment with statins was often not adequate, but it should be noted that in a number of patients, intolerance to high-dose statins may have been the explicit reason for prescribing a PCSK9 inhibitor. Statin intolerance might also explain why – contrary to the ESC/EAS recommendations [5, 25, 26]– statins were not continued in most patients after starting a PCSK9 inhibitor. Nine months after the first-time PCSK9 inhibitor prescription, a quarter of patients had stopped the drug again, likely due to non-achievement of defined treatment targets [30]. The occurrence of adverse drug effects or discomfort with subcutaneous drug administration might be alternative explanations for therapy discontinuation in individual patients.
Total costs of lipid-lowering drugs rose only slightly over past years, despite the marked increase in the number of patients and prescriptions and the new approval of the high-priced PCSK9 inhibitors. This is attributed to the increasing use of lower-cost generic drugs. Nevertheless, the potential for cost savings through generics is not yet fully exploited; the prescription of generics could be further fostered.
Our analyses rely on administrative claims data and have a number of limitations. Recorded drug prescriptions do not confirm that the patients have actually taken the drugs. Moreover, the invoiced dose strengths of statins do not necessarily correspond to the dose strengths used, since sometimes half tablets are prescribed to save costs. Hence, the proportion of patients on high-intensity statin therapy was possibly overestimated. We could not assess drugs dispensed in the inpatient sector (owing to flat-fee reimbursement of inpatient episodes) and drugs paid for out-of-pocket. However, the latter is unlikely to be relevant, because lipid-lowering drugs are not available over-the-counter, usually prescribed for the long-term and fully covered by the mandatory health insurance. As therapy indications and LDL-C values are not captured in the Helsana database, it was not possible to evaluate the appropriateness of prescriptions and therapeutic outcomes on an individual level. Information on socio-economic status was not available, and it is therefore difficult to estimate whether our study population is representative of the Swiss population in this respect.
In conclusion, lipid-lowering drugs make a major contribution to prevent cardiovascular events. Their increasing use reflects current therapeutic guidelines, but results in high costs for the health care system.
The authors have no relevant personal or financial conflicts of interest to declare.
The project was funded by Helsana Group, Zurich, Switzerland. The funder engaged neither in the development of the study design nor in the data analysis.
1. Swiss Federal Statistical Office . 2017. Causes of deaths statistics 2017 (available from https://www.bfs.admin.ch/bfs/en/home/news/whats-new.assetdetail.11227248.html [accessed on 23 October 2020]).
2. Swiss Federal Statistical Office . 2017. Specific causes of death (available from https://www.bfs.admin.ch/bfs/en/home/statistics/health/state-health/mortality-causes-death/specific.html [accessed on 23 October 2020]).
3. Timmis A , Townsend N , Gale CP , Torbica A , Lettino M , Petersen SE , et al.; European Society of Cardiology . European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur Heart J. 2020 Jan;41(1):12–85. https://doi.org/10.1093/eurheartj/ehz859
4. Ference BA , Ginsberg HN , Graham I , Ray KK , Packard CJ , Bruckert E , et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017 Aug;38(32):2459–72. https://doi.org/10.1093/eurheartj/ehx144
5. Mach F , Baigent C , Catapano AL , Koskinas KC , Casula M , Badimon L , et al.; ESC Scientific Document Group . 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020 Jan;41(1):111–88. https://doi.org/10.1093/eurheartj/ehz455
6. Grundy SM , Stone NJ , Bailey AL , Beam C , Birtcher KK , Blumenthal RS , et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019 Jun;73(24):3168–209. https://doi.org/10.1016/j.jacc.2018.11.002
7. Swiss DRG AG . Fallpauschalen-Katalog SwissDRG-Version 9.0 Abrechnungsversion (2020/2020) (available from: https://www.swissdrg.org/application/files/2615/7650/6488/SwissDRG-Version_9.0_Fallpauschalenkatalog_AV_2020_2020.pdf [accessed on 2 July 2020]).
8. Gemeinsame Einrichtung KVG . Statistics (available from https://www.kvg.org/de/statistik-_content---1--1052.html [accessed on 18 November 2020]).
9. Vancheri F , Backlund L , Strender LE , Godman B , Wettermark B . Time trends in statin utilisation and coronary mortality in Western European countries. BMJ Open. 2016 Mar;6(3):e010500. https://doi.org/10.1136/bmjopen-2015-010500
10. Ofori-Asenso R , Ilomäki J , Zomer E , Curtis AJ , Zoungas S , Liew D . A 10-Year Trend in Statin Use Among Older Adults in Australia: an Analysis Using National Pharmacy Claims Data. Cardiovasc Drugs Ther. 2018 Jun;32(3):265–72. https://doi.org/10.1007/s10557-018-6794-x
11. O’Keeffe AG , Nazareth I , Petersen I . Time trends in the prescription of statins for the primary prevention of cardiovascular disease in the United Kingdom: a cohort study using The Health Improvement Network primary care data. Clin Epidemiol. 2016 May;8:123–32. https://doi.org/10.2147/CLEP.S104258
12. Mortensen MB , Falk E , Schmidt M . Twenty-Year Nationwide Trends in Statin Utilization and Expenditure in Denmark. Circ Cardiovasc Qual Outcomes. 2017 Jul;10(7):e003811. https://doi.org/10.1161/CIRCOUTCOMES.117.003811
13. Knopf HC , Busch MA , Du Y , Truthmann J , Schienkiewitz A , Scheidt-Nave C . [Changes in the prevalence of statin use in Germany - findings from national health interview and examination surveys 1997-1999 and 2008-2011]. Z Evid Fortbild Qual Gesundhwes. 2017 May;122:22–31. https://doi.org/10.1016/j.zefq.2017.04.001
14. Gu Q , Paulose-Ram R , Burt VL , Kit BK . Prescription cholesterol-lowering medication use in adults aged 40 and over: united States, 2003-2012. NCHS Data Brief. 2014 Dec;(177):1–8.
15. Salami JA , Warraich H , Valero-Elizondo J , Spatz ES , Desai NR , Rana JS , et al. National Trends in Statin Use and Expenditures in the US Adult Population From 2002 to 2013: Insights From the Medical Expenditure Panel Survey. JAMA Cardiol. 2017 Jan;2(1):56–65. https://doi.org/10.1001/jamacardio.2016.4700
16. Byrne P , Cullinan J , Murphy C , Smith SM . Cross-sectional analysis of the prevalence and predictors of statin utilisation in Ireland with a focus on primary prevention of cardiovascular disease. BMJ Open. 2018 Feb;8(2):e018524. https://doi.org/10.1136/bmjopen-2017-018524
17. Rodriguez F , Olufade TO , Ramey DR , Friedman HS , Navaratnam P , Heithoff K , et al. Gender Disparities in Lipid-Lowering Therapy in Cardiovascular Disease: Insights from a Managed Care Population. J Womens Health (Larchmt). 2016 Jul;25(7):697–706. https://doi.org/10.1089/jwh.2015.5282
18. Reiner Ž , De Backer G , Fras Z , Kotseva K , Tokgözoglu L , Wood D , et al.; EUROASPIRE Investigators . Lipid lowering drug therapy in patients with coronary heart disease from 24 European countries—findings from the EUROASPIRE IV survey. Atherosclerosis. 2016 Mar;246:243–50. https://doi.org/10.1016/j.atherosclerosis.2016.01.018
19. Appelman Y , van Rijn BB , Ten Haaf ME , Boersma E , Peters SA . Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis. 2015 Jul;241(1):211–8. https://doi.org/10.1016/j.atherosclerosis.2015.01.027
20. Mosca L , Barrett-Connor E , Wenger NK . Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011 Nov;124(19):2145–54. https://doi.org/10.1161/CIRCULATIONAHA.110.968792
21. Lee MT , Mahtta D , Ramsey DJ , Liu J , Misra A , Nasir K , et al. Sex-Related Disparities in Cardiovascular Health Care Among Patients With Premature Atherosclerotic Cardiovascular Disease. JAMA Cardiol. 2021 Jul;6(7):782–90. https://doi.org/10.1001/jamacardio.2021.0683
22. Karalis DG , Wild RA , Maki KC , Gaskins R , Jacobson TA , Sponseller CA , et al. Gender differences in side effects and attitudes regarding statin use in the Understanding Statin Use in America and Gaps in Patient Education (USAGE) study. J Clin Lipidol. 2016 Jul-Aug;10(4):833–41. https://doi.org/10.1016/j.jacl.2016.02.016
23. Cholesterol Treatment Trialists’ Collaboration . Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019 Feb;393(10170):407–15. https://doi.org/10.1016/S0140-6736(18)31942-1
24. Marques-Vidal P , Paccaud F . Regional differences in self-reported screening, prevalence and management of cardiovascular risk factors in Switzerland. BMC Public Health. 2012 Mar;12(1):246. https://doi.org/10.1186/1471-2458-12-246
25. Reiner Z , Catapano AL , De Backer G , Graham I , Taskinen MR , Wiklund O , et al.; European Association for Cardiovascular Prevention & Rehabilitation; ESC Committee for Practice Guidelines (CPG) 2008-2010 and 2010-2012 Committees . ESC/EAS Guidelines for the management of dyslipidaemias. Eur Heart J. 2011 Jul;32(14):1769–818. https://doi.org/10.1093/eurheartj/ehr158
26. Catapano AL , Graham I , De Backer G , Wiklund O , Chapman MJ , Drexel H , et al.; ESC Scientific Document Group . 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J. 2016 Oct;37(39):2999–3058. https://doi.org/10.1093/eurheartj/ehw272
27. Cannon CP , Blazing MA , Giugliano RP , McCagg A , White JA , Theroux P , et al.; IMPROVE-IT Investigators . Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med. 2015 Jun;372(25):2387–97. https://doi.org/10.1056/NEJMoa1410489
28. Rachamin Y , Meier R , Rosemann T , Langenegger S , Markun S . Statin treatment and LDL target value achievement in Swiss general practice - a retrospective observational study. Swiss Med Wkly. 2020 May;150:w20244.
29. Stroes ES , Thompson PD , Corsini A , Vladutiu GD , Raal FJ , Ray KK , et al.; European Atherosclerosis Society Consensus Panel . Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015 May;36(17):1012–22. https://doi.org/10.1093/eurheartj/ehv043
30. Swiss Federal Office of Public Health . Spezialitätenliste 2020 (available from http://www.spezialitätenliste.ch [accessed on 18 November 2020]).