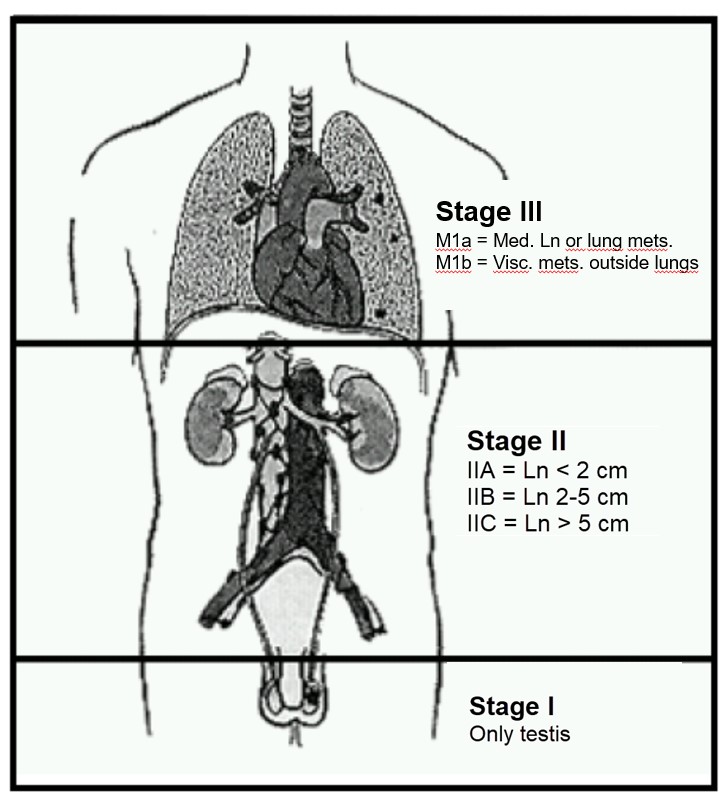
Figure 1 Anatomical Staging of testis cancer. Ln = lymp hnodes; Med. = mediastinal; Mets. = metastases; Visc. = visceral
DOI: https://doi.org/10.4414/SMW.2021.w30023
Although infrequent overall, germ cell cancer (GCC) is the most common cancer in men between puberty and the age of around 40 years [1–3]. Accepted risk factors for the development of GCC are maldescended testis, small testicular volume, family history and infertility. Three percent of GCCs are primarily extragonadal. Hence, a high index of suspicion should be maintained not only in men with symptoms such as testicular swelling, nodules or pain, but in all men in the relevant age group with abdominal or mediastinal masses, as well as supraclavicular lymph node enlargement. It is important not to confuse extragonadal GCC with, for example, lymphoma or sarcoma.
Past medical history as well as testicular palpation and ultrasound remain the mainstay of the clinical diagnosis, which is supplemented by measurements of alpha-fetoprotein (AFP), human chorionic gonadotropin (hCG) and lactate dehydrogenase (LDH) levels in the serum. Further staging investigations include a computed tomography (CT) scan of the thorax, abdomen and pelvis, which should be performed prior to orchiectomy. Positron-emission tomography (PET)-CT scanning has no role in the routine staging of GCC (appendix 1 vote F4).
Men with testicular GCC should undergo initial orchiectomy, but the procedure must be delayed until completion of chemotherapy in men with widely metastatic, high volume and life-threatening disease (appendix 1 vote F1). Patients with history of maldescended testis, a testicular volume less 12 ml or infertility should be offered a biopsy of the contralateral “healthy” testis to identify “germ cell neoplasia in situ”, which predisposes them to a second contralateral cancer (appendix 1 vote F2). Men with extragonadal GCC can be identified by unequivocal marker elevations alone or undergo additional biopsy for histological confirmation (appendix 1 vote F3).
Staging is performed according to the American Joint Committee on Cancer (AJCC) classification [4]. However, all patients with metastases should also be classified according to the International Germ Cell Cancer Cooperative Group (IGCCCG) classification (table 1) [5]. Magnetic resonance imaging of the brain should be performed in all patients with metastases with an “intermediate prognosis” according to the IGCCCG if multiple lung metastases are present, in all “poor prognosis” patients, even if asymptomatic, as well as in all symptomatic patients (appendix 1 vote F5).
Table 1Prognostic categories according to the original IGCCCG classification [5]
| Good prognosis: about 70% of patients with metastases | |
| Overall survival ~90% (original IGCCCG) and ~95% (IGCCCG contemporary series)* | |
| Nonseminoma† | Seminoma |
| testicular or retroperitoneal primary tumour | any primary site |
| and | and |
| no non-pulmonary visceral metastases | no non-pulmonary visceral metastases |
| and | and |
| good prognosis markers, all of: | normal AFP |
| AFP <1000 ng/ml | any hCG |
| hCG <5000 U/l | any LDH [4] |
| LDH <1.5 × upper limit of normal‡ | |
| Intermediate prognosis: about 20% of patients with metastases | |
| Overall survival ~75% (original IGCCCG) and ~91% (IGCCCG contemporary series)* | |
| Nonseminoma† | Seminoma |
| testicular or retroperitoneal primary tumour | Any primary site |
| and | and |
| no non-pulmonary visceral metastases | non-pulmonary visceral metastases |
| and | and |
| intermediate prognosis markers, any of: | any hCG Normal AFP |
| AFP 1000–10,000 ng/ml | any LDH |
| hCG 5000–50,000 U/l | |
| LDH 1.5–10 × upper limit of normal | |
| Poor prognosis: about 10% of patients with metastases Overall survival ~45% (original IGCCCG) and ~65% (IGCCCG contemporary series)* | |
| Nonseminoma† | Seminoma |
| Mediastinal primary tumour | No patients classified as poor prognosis |
| or | |
| Non-pulmonary visceral metastases | |
| or | |
| Poor prognosis markers, any of | |
| AFP >10,000 ng/ml | |
| hCG >50,000 U/l | |
| LDH >10 × upper limit-of normal | |
AFP = alpha-fetoprotein; hCG = human chorionic gonadotropin; IGCCCG = International Germ-Cell Classification Cooperative Group; LDH = lactate dehydrogenase
* Survival probabilities have increased in recent cohorts as compared with the publication of the original IGCCCG classification [5–8]
† Age and presence of pulmonary metastases are additional risk factors in nonseminoma according to the IGCCCG update analysis [7]
‡ LDH >2.5 × upper limit of normal as risk factor in good prognosis nonseminoma and seminoma patients according to the IGCCCG update analysis [6, 7]
Expert histology of the primary tumour determines further treatment algorithms and identifies potential risk factors for occult metastases in stage I seminoma and nonseminoma as well as any teratoma or rare non-germinal cancer elements such as adenocarcinoma or sarcoma (table 2). Mixed germ cell tumours containing a nonseminomatous component and patients with elevated AFP are treated according to nonseminoma recommendations.
Table 2 Obligatory elements of a histopathological report.
| Localisation | |
| Size | |
| Multiplicity | |
| Extension of tumour | Rete testis involvement |
| Lymphovascular invasion | |
| pT category (according to the UICC) | |
| Histopathological types and subtypes (according to WHO) | |
| In mixed tumours, description of each individual component | |
| Presence of syncytiotrophoblasts in seminoma | |
| Presence of germ-cell neoplasia in situ (GCNIS) | |
| Completeness of resection (R-status) | |
pT = primary tumour; UICC = Union Internationale Contre le Cancer; WHO = World Health Organization
In Switzerland, stage I disease is the most frequent presentation in up to 80% of seminoma patients, and is defined as disease limited to the testis without radiological evidence of metastatic disease, as well as normal post-orchiectomy serum tumour markers (fig. 1) [9]. Active surveillance is the standard of care, as only approximately 10–20% patients will eventually relapse during active surveillance and are virtually all cured by subsequent therapy (appendix 1 vote F7). Risk factors for occult metastases and thus for the risk of relapse have been identified. However, the clinical usefulness of these risk factors is debated (appendix 1 vote F6). One cycle of adjuvant carboplatin at a dose of Area under the Curve (AUC) 7 is usually well tolerated and will reduce the risk of relapse to about 5%, but represents an overtreatment for the majority of patients (appendix 1 vote F9). Adjuvant radiation is no longer recommended (appendix 1 vote F8).

Figure 1 Anatomical Staging of testis cancer. Ln = lymp hnodes; Med. = mediastinal; Mets. = metastases; Visc. = visceral
Stage II A/B is infrequent and poses particular challenges. Patients with equivocal lymph node enlargement in the ipsilateral para-aortic or paracaval infrahilar region (“the primary landing zone”) should be followed up until unequivocal progression. In patients with larger lymph nodes, CT-guided biopsy might be feasible (appendix 1 vote F13). In patients with unequivocal or histologically proven stage IIA seminoma, radiotherapy is favoured over combination chemotherapy, whereas in stage IIB patients, cisplatin-based combination chemotherapy is the treatment of choice (appendix 1 votes F14 and F15). At the present time, patients with seminoma stage IIA/B should preferably be treated in the ongoing SAKK 01/18 trial investigating stage-adapted treatment with one infusion of carboplatin followed by 12 × 2 Gy involved-node radiation therapy for stage IIA seminoma, and one cycle of etoposide and cisplatin followed by 15 × 2 Gy involved-node radiation therapy for stage IIB seminoma. Retroperitoneal lymph node dissection (RPLND) should not be offered for stage IIA/B seminoma outside clinical trials (appendix 1 vote F16).
All seminoma patients with large abdominal masses beyond stage II A/B or more widely metastatic disease must receive cisplatin-based combination chemotherapy. Three cycles of cisplatin, etoposide and bleomycin (BEP) is the recommended combination in the majority of “good prognosis” patients according to the IGCCCG classification (appendix 1 vote F17). Four cycles of cisplatin and etoposide (PE) have equivalent antitumour activity to three cycles BEP and should be administered in the case of contraindications to bleomycin (table 3). Infrequently, patients present with extrapulmonary metastases in the liver, bone or, even more rarely, in the brain. The prognosis of these men is classified as “intermediate prognosis” and they must receive four cycles of BEP, or four cycles of cisplatin and etoposide in combination with ifosfamide (VIP) in the case of contraindications to bleomycin (appendix 1 vote F19). In a recent analysis, an elevated LDH above 2.5 × the upper limit of normal was identified as an adverse prognostic marker in metastatic seminoma, but is not yet uniformly accepted as a trigger for treatment decisions (appendix 1 vote F18) [6].
Table 3First-line treatment protocols.
| BEP | (repeated day 22) | 3–4 cycles* |
| Cisplatin | 20 mg/m2 BSA | Days 1–5 |
| Etoposide | 100 mg/m2 BSA | Days 1–5 |
| Bleomycin | 30 mg absolute dose | Days 1, 8, 15 |
| PE | (repeated day 22) | 4 cycles† |
| Cisplatin | 20 mg/m2 BSA | Days 1–5 |
| Etoposide | 100 mg/m2 BSA | Days 1–5 |
| VIP | (repeated day 22) | 4 cycles‡ |
| Cisplatin | 20 mg/m2 BSA | Days 1–5 |
| Etoposide | 75–100 mg/m2 BSA | Days 1-5 |
| Ifosfamide | 1.2 g/m2 BSA | Days 1–5 |
BSA = body surface area (dose capping, for example at 2.0 m2, is not recommended)
* Three cycles in “good prognosis” patients, four cycles in “intermediate prognosis” patients
† In “good prognosis” patients with contraindications to bleomycin
‡ In “intermediate prognosis” and “poor prognosis” patients with contraindications to bleomycin
Residual masses after chemotherapy are frequent in metastatic seminoma and should not be resected irrespective of size. Masses below 3 cm usually contain necrotic tissue only and patients can directly enter standard follow up. For patients with residual masses >3cm PET-CT scanning may be helpful if negative, to identify patients for follow up in whom active residual tumour is unlikely (appendix 1 vote F20). On the other hand, false-positive PET-CT scans are frequent and should not trigger immediate further treatment [10]. No consensus could be achieved concerning the management of seminoma patients with a positive PET-CT after chemotherapy, but the majority of experts believe that these patients should be closely followed and receive salvage treatment in the case of unequivocal progression (appendix 1 vote F21).
Stage I nonseminoma also is defined as disease limited to the testis without radiological evidence of metastatic disease as well as normal serum tumour markers, which may however take several weeks to normalise after orchiectomy. Lymphovascular invasion is the only accepted risk factor for occult metastases. In patients on active surveillance, the risk of relapse is about 14% if lymphovascular invasion is absent, but increases to about 50% if it is present. One cycle of adjuvant BEP can reduce this risk to about 1–2%, but represents an overtreatment in the majority of patients (appendix 1 vote F23). Adjuvant primary nerve-sparing RPLND is not a standard in Switzerland; according to some Swiss experts it may be an alternative to active surveillance in patients with pure teratoma, but should only be performed in expert centres with adequate experience in this surgical procedure (appendix 1 vote F25). As relapses from stage I nonseminoma can be cured by combination chemotherapy with or without post-chemotherapy RPLND, Swiss experts agree that the risks and benefits of management strategies have to be discussed with an individual patient and his personal preferences respected.
Three cycles of BEP in “good prognosis” and four cycles of BEP in “intermediate prognosis” and “poor prognosis” patients is the recommended treatment according to the IGCCCG classification (appendix 1 votes F27 and F29). Four cycles of cisplatin and etoposide serve as an alternative to BEP in “good prognosis” patients. In “intermediate prognosis” and “poor prognosis” patients bleomycin can be replaced by ifosfamide in the case of contraindications (appendix 1 vote F30). For the treatment of intermediate and poor prognosis patients, referral to an expert centre is strongly recommend, since outcomes significantly depend on expertise and case load. Levels of serum tumour marker before chemotherapy, as opposed to pre-orchiectomy, should be used for IGCCCG risk classification [11]. Recently, age, the presence of lung metastases and an elevated LDH above 2.5 × the upper limit of normal have been reported as additional risk factors, but still need to be evaluated in prospective trials prior to their routine use and should therefore not guide clinical treatment decisions at present [7]. The first-line treatment of patients with liver, bone or brain metastases, as well as of all patients with extragonadal primary mediastinal nonseminoma and of those with an inadequate decline of serum tumour markers after their initial treatment cycle, should be intensified, as four cycles of BEP will achieve unsatisfactory results (appendix 1 vote F31). Rare patients with very high volume disease and/or impeding organ dysfunction should receive an additional dose-reduced pre-treatment cycle (appendix 1 vote F52).
Residual masses after chemotherapy are also frequent in metastatic nonseminoma and – in contrast to seminoma – must be resected if larger than 1 cm in the short axis diameter, as viable cancer is much more frequent than in seminoma and because of the risk of residual teratoma (appendix 1 votes F38). Post-chemotherapy surgery should be performed at an expert surgical centre as soon as possible after chemotherapy, but not later than 12 weeks after the last treatment (appendix 1 vote F40). PET-CT scanning is not helpful in nonseminoma patients with residual tumours after chemotherapy.
Relapses from stage I in patients with seminoma and nonseminoma should be treated in the same way as those with de novo metastatic disease. In all other patients, first-salvage options include four cycles of conventional-dose salvage chemotherapy or sequential high-dose chemotherapy with autologous stem-cell support (appendix 1 vote F43). In patients with multiple relapses, sequential high-dose chemotherapy can still achieve long-term remissions.
Late relapses more than 2 years after the last cisplatin-based chemotherapy represent another challenge. Treatments in late-relapsing patients need to be individualised. Patients with resectable disease and slow marker increase should undergo upfront resection (appendix 1 vote F44). Those with unresectable disease, or very high or rapidly increasing serum tumour markers will benefit from induction salvage chemotherapy prior to resection (appendix 1 votes F45 and F46).
Expert supportive care will contribute to the favourable outcomes, which have substantially improved in recent years, particularly in metastatic “intermediate prognosis” and “poor prognosis” patients according to the IGCCCG classification [8]. Cisplatin remains the treatment backbone in all patients with metastases and cannot be replaced by conventional-dose carboplatin without loss of efficacy or even the prospect of cure. Similarly, doses of cytostatic drugs should not be capped in patients with a large body surface above 2.0 m2 (appendix 1 vote F48). Most experts recommended avoiding central venous catheters whenever feasible to reduce the risk of venous thromboembolism, which is increased in testis cancer patients receiving cisplatin-based chemotherapy (appendix 1 vote F47) [12].
Central venous catheters as well as risk factors such as a high Khorana score or a large abdominal mass >5 cm should prompt prophylactic anticoagulation with low-molecular weight heparin or a new direct oral anticoagulant from the initiation of cisplatin-based chemotherapy until about 4 weeks after the last cisplatin dose (appendix 1 votes F49, F50 and F51). About 50% of Swiss experts even believe that all metastatic GCC patients undergoing cisplatin-based chemotherapy should receive prophylactic anticoagulation (appendix 1 vote F51). However, owing to the lack of prospective data, discussions were controversial and no consensus could be achieved with respect to patient selection, start and duration of prophylactic anticoagulation, as well as choice and dose of an individual agent.
High cure rates can be achieved in testis cancer even in patients with widely metastatic disease. However, the majority of patients in Switzerland present with early stage I disease and are at risk of overtreatment with its associated long-term toxicities. Consultations for expert opinion as well as treatment at expert centres offer the best chances of success and should become part of routine care of GCC patients in Switzerland.
AL received speaker honoraria from the non-commercial organisations SAMO (Swiss Academy of Medical Oncology) and ESMO (European Society of Medical Oncology). She was also a member of the S3 guideline committee on germ-cell cancers from the (German Working Group of Scientific Medical Societies) (AWMF). SG reports speaker honoraria and participation on Advisory Boards, outside the submitted manuscript. The other authors report no conflict of interest related to the content of the manuscript.
The appendix is provided as a separate PDF file for download.
1. Gilligan T , Lin DW , Aggarwal R , Chism D , Cost N , Derweesh IH , et al. Testicular cancer, version 2.2020. J Natl Compr Canc Netw. 2019 Dec;17(12):1529–54. https://doi.org/10.6004/jnccn.2019.0058
2. Honecker F , Aparicio J , Berney D , Beyer J , Bokemeyer C , Cathomas R , et al. ESMO Consensus Conference on testicular germ cell cancer: diagnosis, treatment and follow-up. Ann Oncol. 2018 Aug;29(8):1658–86. https://doi.org/10.1093/annonc/mdy217
3. Albers P , Albrecht W , Algaba F , Bokemeyer C , Cohn-Cedermark G , Fizazi K , et al.; European Association of Urology . Guidelines on Testicular Cancer: 2015 Update. Eur Urol. 2015 Dec;68(6):1054–68. https://doi.org/10.1016/j.eururo.2015.07.044
4. AJCC . AJCC Cancer Staging Manual. Berlin: Springer International Publishing; 2017.
5. Mead GM ; International Germ Cell Cancer Collaborative Group . International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol. 1997 Feb;15(2):594–603. https://doi.org/10.1200/JCO.1997.15.2.594
6. Beyer J , Collette L , Sauvé N , Daugaard G , Feldman DR , Tandstad T , et al.; International Germ Cell Cancer Classification Update Consortium . Survival and New Prognosticators in Metastatic Seminoma: Results From the IGCCCG-Update Consortium. J Clin Oncol. 2021 May;39(14):1553–62. https://doi.org/10.1200/JCO.20.03292
7. Gillessen S , Sauvé N , Collette L , Daugaard G , de Wit R , Albany C , et al.; International Germ Cell Cancer Classification Update Consortium . Predicting outcomes in men with metastatic nonseminomatous germ-cell tumors (NSGCT): Results from the IGCCCG-Update Consortium. J Clin Oncol. 2021 May;39(14):1563–74. https://doi.org/10.1200/JCO.20.03296
8. Fankhauser CD , Sander S , Roth L , Beyer J , Hermanns T . Improved survival in metastatic germ-cell cancer. Ann Oncol. 2018 Feb;29(2):347–51. https://doi.org/10.1093/annonc/mdx741
9. Rothermundt C , Thurneysen C , Cathomas R , Müller B , Mingrone W , Hirschi-Blickenstorfer A , et al. Baseline characteristics and patterns of care in testicular cancer patients: first data from the Swiss Austrian German Testicular Cancer Cohort Study (SAG TCCS). Swiss Med Wkly. 2018 Jul;148:w14640. https://doi.org/10.4414/smw.2018.14640
10. Cathomas R , Klingbiel D , Bernard B , Lorch A , Garcia Del Muro X , Morelli F , et al. Questioning the Value of Fluorodeoxyglucose Positron Emission Tomography for Residual Lesions After Chemotherapy for Metastatic Seminoma: Results of an International Global Germ Cell Cancer Group Registry. J Clin Oncol. 2018 Oct;36(34):JCO1800210. https://doi.org/10.1200/JCO.18.00210
11. Fankhauser CD , Gerke TA , Roth L , Sander S , Grossmann NC , Kranzbühler B , et al. Pre-orchiectomy tumor marker levels should not be used for International Germ Cell Consensus Classification (IGCCCG) risk group assignment. J Cancer Res Clin Oncol. 2019 Mar;145(3):781–5. https://doi.org/10.1007/s00432-019-02844-z
12. Lauritsen J , Hansen MK , Bandak M , Kreiberg MB , Skøtt JW , Wagner T , et al. Cardiovascular risk factors and disease after male germ cell cancer. J Clin Oncol. 2020 Feb;38(6):584–92. https://doi.org/10.1200/JCO.19.01180