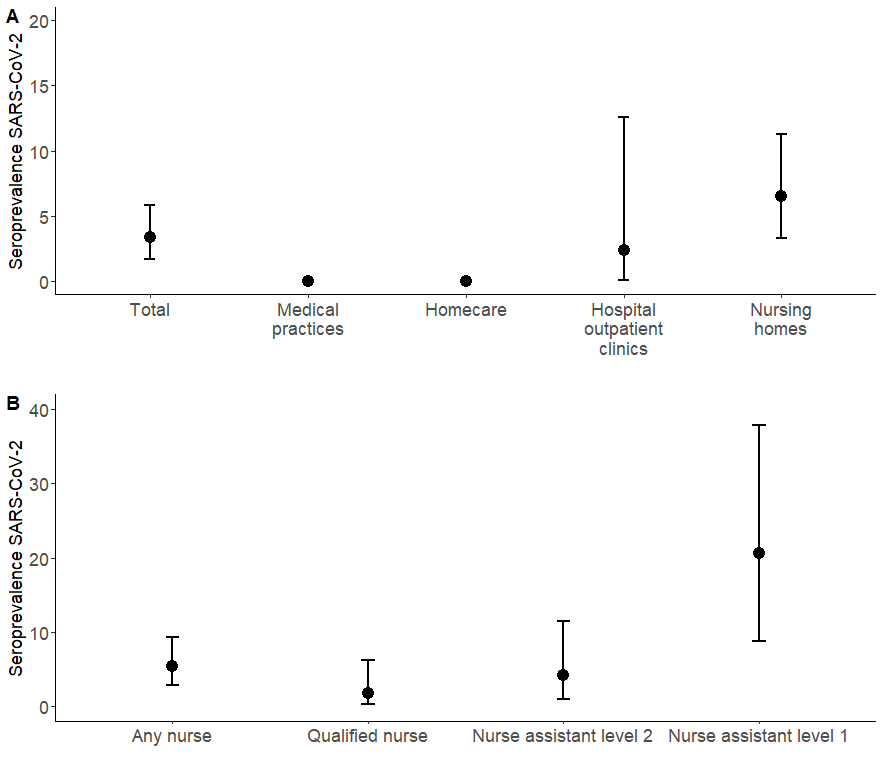
Figure 1 eSARS-coV-2 seroprevalence by (A) workplace and (B) among nurses (homecare, outpatient departments of the hospital, nursing homes). There were no events in the categories “medical practices” and “homecare”.
DOI: https://doi.org/10.4414/SMW.2021.w30021
The emergence and rapid spread of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19) has achieved a pandemic scale. In Switzerland, as of 1 June 2020, more than 32,092 people had tested positive for SARS-CoV-2, and 1728 people had died from COVID-19 [1].
The primary mode of infection of SARS-CoV-2 involves the respiratory transmission of mouth and nose secretions via direct and indirect contact with infected people; touching the face after touching contaminated surfaces is a secondary mode of transmission. Healthcare workers have an increased risk of SARS-CoV-2 infection because of their close contact with highly infectious patients and their exposure to undiagnosed or subclinical infectious persons. In Italy, 8% of all healthcare workers in hospital settings had a positive serological test; a corresponding figure in Spain was 11% and in southern Switzerland 10% during the first wave [2–5]. The high prevalence among healthcare workers in Italy, Spain or southern Switzerland might be because these regions were initially overwhelmed by the pandemic early during the first wave when the pathogen was less well described.
The current state-of-the-art for diagnosing acute SARS-CoV-2 infection uses a real-time reverse transcriptase polymerase chain reaction (rRT-PCR) to detect viral genetic material on nasopharyngeal swabs [6]. Serological tests, in contrast, detect antibodies against the virus, which persist after the infection [7, 8]. However, both methods are time-dependent. rPCR reliably detects viral DNA early, during the first week after infection, whereas serological antibody tests produce positive results only 5 to 7 days after infection [9, 10], rendering them unreliable for diagnosing acute infection. A key feature of serological tests is that they can detect prior infection for a longer time period [11].
The Canton of Solothurn is a mid-sized canton in Switzerland, which was moderately affected by the first wave (01 January to 30 June 2020) of the COVID-19 pandemic. It is unknown how well healthcare workers were able to protect themselves during the first wave of the epidemic. We studied the seroprevalence of SARS-CoV-2 in Switzerland among healthcare workers in the Canton of Solothurn's outpatient facilities and retirement or nursing homes and among their close contacts.
This was a longitudinal seroprevalence study among healthcare workers in outpatient facilities and retirement or nursing homes in the Canton of Solothurn, Switzerland, with two examination time-points (baseline and 2 months later). The cohort consisted of healthcare workers with a minimum workload of ≥50% who worked in the outpatient clinic at a hospital, medical practice, retirement or nursing home, or in home care, who were at least 18 years old and had contact with patients before and during the first SARS-CoV-2 epidemic wave from 1 January to 30 June 2020. The degree of patient contact depends on the workplace. At baseline (time point 0, which was between 1 June and 1 July 2020), participants completed a questionnaire and a blood sample was taken by a healthcare professional at the facility; if the result was positive another blood sample was taken 2 months later.
In addition, persons in close contact from the same household as the HCW who tested positive for SARS-CoV-2 by serology, were also invited to participate in this study. These close contacts also completed the questionnaire and were invited to have a blood sample taken.
To encompass the spectrum of outpatient care facilities in the Canton of Solothurn, we included outpatient clinics at the hospitals, medical practices and home care organisations in this study. In addition, we included retirement or nursing homes. These sites included a secondary referral hospital in the canton, where COVID-19 patients are treated. We included all of the medical practices that performed SARS-CoV-2 testing, a sample of medical practices that did not test for SARS-CoV-2 and a convenience sample of paediatric practices. We chose a representative sample of retirement or nursing homes by size (small and large) and location. Similar sampling strategies were applied for the homecare organisations. For each of the participating outpatient care facilities and retirement or nursing homes, we invited all healthcare workers to participate in the study according to the inclusion criteria.
We developed and pilot-tested a questionnaire in collaboration with the participants. The paper-based questionnaire had three sections: (i) basic information such as sex, age, profession, workload, healthcare facility; (ii) episodes of illness including date, symptoms, testing for SARS-CoV-2, self-isolation and quarantine; (iii) travel including date and destination. Questionnaire data were entered into REDCap by a single person [12, 13].
A blood sample was taken from each participant and centrally processed and stored at the Institute of Laboratory Medicine in Olten. Analyses were made at the Institute for Infectious Diseases (IFIK) of the University of Bern. Blood samples were run on the Abbott ARCHITECT i2000 instrument using the Abbott SARS-CoV-2 IgG assay (Abbott Diagnostics, Chigago, US) and the Liaison SARS-CoV-2 S1/S2 IgG assay (DiaSorin, Saluggia, Italy) following the manufacturer’s instructions. The Abbott assay is a chemiluminescent microparticle immunoassay for qualitative detection of IgG in human serum or plasma against the SARS-CoV-2 nucleoprotein [8]. The Liaison SARS-CoV-2 S1/S2 is a chemiluminescence assay consisting of paramagnetic microparticles coated with S1 and S2 fragments of the viral surface spike protein. It is used for the qualitative detection of IgG in human serum or plasma against the SARS-CoV-2 [7].
We defined COVID-19 seropositivity as having a positive Abbott SARS-CoV-2 IgG or Liaison SARS-CoV-2 S1/S2 IgG assay, to increase the sensitivity. The two assays detect antibodies against two different components of the virus. The nursing profession in Switzerland includes three groups of HCW. The level 1 nurse assistant is a nurse with a short education on basic care. The level 2 nurse assistant is a nurse with 3 years of education who works under the direction of a qualified nurse. The qualified nurse is a nurse with 4 years of education at a university of applied science.
We used descriptive statistics to characterise the study population by seroprevalence. Differences between groups were assessed using chi‐square, Fisher's exact, or Wilcoxon rank‐sum tests. We calculated the seroprevalence with the corresponding 95% confidence interval (CI). All analyses were done in Stata (version 15.1, College Station, TX, USA).
The Cantonal Ethics Committee Nordwestschweiz (Switzerland) approved this study (project ID no. 2020-01004). Written informed consent was obtained from all participants.
We drew upon 26 healthcare facilities: 12 medical practices, 11 retirement or nursing homes, one hospital outpatient clinic, and two homecare organisations (see supplementary table S1 in the appendix). More information about the health care facilities can be found in table S1.
Full-time equivalents were lower for homecare than retirement or nursing homes (67.5% vs 90%). Overall, median contact with patients among the different healthcare facilities was around 30 hours per week and 23 hours per week for home care.
We included 357 healthcare workers. Their median age was 43 years (interquartile range [IQR] 29–54), and 315 were women (88.2%, table 1). Across all healthcare workers, 169 (47.3%) were based in retirement or nursing homes, 118 (33.1%) in medical practices, 42 (11.8%) at the hospital, and 28 (7.8%) in home care. A majority of nurses worked in homecare or retirement or nursing homes, whereas the physicians and medical assistants worked at medical practices or the hospital (table 1, supplementary table S2).
Overall, the uptake of the 2019/20 seasonal influenza vaccination was 33.3% (119/357). Influenza vaccine uptake was high among physicians, at 85.7% (42/49), and lowest among nurses, at 21.7% (48/221, table 1).
Table 1Characteristics of study participants by profession (n = 357).
| Total | Physician | Practice assistant | Nurse | ||||
| Any nurse | Qualified nurse | Nurse assistant level 2 | Nurse assistant level 1 | ||||
| n = 357 | n = 49 | n = 87 | n = 221 | n = 114 | n = 73 | n = 34 | |
| Sex | |||||||
| Male | 42 (11.8) | 22 (44.9) | 2 (2.3) | 18 (8.1) | 7 (6.1) | 7 (9.6) | 4 (11.8) |
| Female | 315 (88.2) | 27 (55.1) | 85 (97.7) | 203 (91.9) | 107 (93.9) | 66 (90.4) | 30 (88.2) |
| Age (years), median (IQR) | 43 (29–54) | 44 (38–49) | 32 (25–47) | 51.5 (39–59) | 30 (22–49) | 48 (38–57) | |
| Number of observations | 357 | 49 | 87 | 221 | 114 | 73 | 34 |
| Healthcare facilities | |||||||
| Hospital | 42 (11.8) | 7 (14.3) | 16 (18.4) | 19 (8.6) | 13 (11.4) | 4 (5.5) | 2 (5.9) |
| Medical practice | 118 (33.1) | 42 (85.7) | 69 (79.3) | 7 (3.2) | 7 (6.1) | 0 | 0 |
| Retirement or nursing home | 169 (47.3) | 0 | 1 (1.1) | 168 (76.0) | 82 (71.9) | 59 (80.8) | 27 (79.4) |
| Homecare | 28 (7.8) | 0 | 1 (1.1) | 27 (12.2) | 12 (10.5) | 10 (13.7) | 5 (14.7) |
| Illness | |||||||
| Yes | 150 (42.0) | 24 (49.0) | 29 (33.3) | 97 (43.9) | 48 (42.1) | 33 (45.2) | 16 (47.1) |
| No | 207 (58.0) | 25 (51.9) | 58 (66.7) | 124 (56.1) | 66 (57.9) | 40 (54.8) | 18 (52.9) |
| Self-reported symptoms | |||||||
| Yes | 150 (42.0) | 24 (49.0) | 29 (33.3) | 97 (43.9) | 48 (42.1) | 33 (45.2) | 16 (47.1) |
| Fever | 42 (11.8) | 2 (4.1) | 8 (9.2) | 32 (14.5) | 13 (11.4) | 12 (16.4) | 7 (20.6) |
| Cough | 84 (23.5) | 15 (30.6) | 16 (18.4) | 53 (24.0) | 27 (23.7) | 20 (27.4) | 6 (17.6) |
| Shortness of breath or difficulty breathing | 26 (7.3) | 1 (2.0) | 0 | 25 (11.3) | 10 (8.8) | 12 (16.4) | 3 (8.8) |
| Muscle or body aches | 51 (14.3) | 8 (16.3) | 9 (10.3) | 34 (15.4) | 16 (14.0) | 11 (15.1) | 7 (20.6) |
| Loss of taste or smell | 7 (1.9) | 0 | 1 (1,1) | 6 (2.7) | 2 (1.8) | 3 (4.1) | 1 (2.9) |
| Headache | 70 (19.6) | 9 (18.4) | 10 (11.5) | 51 (23.1) | 27 (23.7) | 18 (24.7) | 6 (17.6) |
| Congestion or runny nose | 68 (19.0) | 18 (36.7) | 14 (16.1) | 36 (16.3) | 20 (17.5) | 12 (16.4) | 4 (11.8) |
| Diarrhoea | 7 (1.9) | 1 (2.0) | 1 (1.1) | 5 (2.3) | 1 (0.9) | 2 (2.7) | 2 (5.9) |
| Sore throat | 12 (3.4) | 1 (2.0) | 1 (1.1) | 10 (4.5) | 4 (3.5) | 3 (4.1) | 3 (8.8) |
| No | 207 (58.0) | 25 (51.0) | 58 (66.7) | 124 (56.1) | 66 (57.9) | 40 (54.8) | 18 (52.9) |
| Influenza vaccination 2019/20 | |||||||
| Yes | 119 (33.3) | 42 (85.7) | 29 (33.3) | 48 (21.7) | 27 (23.7) | 10 (13.7) | 11 (32.4) |
| No | 237 (66.4) | 7 (14.3) | 58 (66.7) | 172 (77.8) | 86 (75.4) | 63 (86.3) | 23 (67.7) |
| Unknown | 1 (0.3) | 0 | 0 | 1 (0.5) | 1 (0.9) | 0 | 0 |
| Quarantine | |||||||
| Yes | 8 (2.2) | 2 (4.1) | 0 | 5 (2.3) | 0 | 4 (5.5) | 1 (2.9) |
| No | 349 (97.8) | 47 (95.9) | 0 | 216 (97.7) | 114 (100) | 69 (94.5) | 33 (97.1) |
| Self-isolation | |||||||
| Yes | 14 (3.9) | 1 (2.0) | 2 (2.3) | 11 (5.0) | 2 (1.7) | 5 (6.8) | 4 (11.8) |
| No | 343 (96.1) | 48 (98.0) | 85 (97.7) | 210 (95.0) | 112 (98.3) | 68 (93.2) | 30 (88.2) |
| Seroprevalence | |||||||
| Number of seropositive persons | 12 | 0 | 0 | 12 | 2 | 3 | 7 |
| Percent (95% Cl) | 3.4 (1.7–5.8) | 0 | 0 | 5.4 (2.8–9.3) | 1.7 (0.2–6.2) | 4.1 (0.9–11.5) | 20.6 (8.7–37.9) |
CI = confidence interval; IQR = interquartile rangeData are n (%) unless otherwise indicated.
We identified 12 seropositive persons among the 357 healthcare workers (3.4%, 95% Cl 1.7–5.8%). All were among the 221 nursing staff, giving a respective seroprevalence of 5.4% (95% CI 2.8–9.3%, table 2, fig. 1A). The median age of the seropositive healthcare workers was 43 years (IQR 29–54), and all were women (table 2). Seven out of 34 level 1 nurse assistants (20.6%) were seropositive, as were three out of 73 level 2 nurse assistants (4.1%), and two out of 117 qualified nurses (1.7%) (table 1, fig. 1B). Eleven of the 12 seropositive healthcare workers worked at a retirement or nursing home, a seroprevalence of 6.5% (11/169, 95% Cl 3.3–11.3%). The other seropositive HCW worked at the hospital, a seroprevalence of 2.4% (1/42, 95% Cl 0.1–12.6%) among hospital participants (fig.1B, table S2). Three of the seropositive healthcare workers were asymptomatic and nine symptomatic (tables2 and 3). Among the nine reporting symptoms, the most frequently mentioned symptoms were fever 6/9 (66.7%), 6/9 (66.7%) headache, 5/9 (55.6%) muscle and body aches, 4/9 (44.4%) shortness of breath and 3/9 (33.3%) cough. In addition, loss of smell or taste were mentioned by 2/9, (22.2%) of healthcare workers (table 3). Symptoms such as loss of smell or taste, or shortness of breath were more prevalent among seropositive healthcare workers than among seronegative healthcare workers (p <0.05). Only two of the 12 healthcare workers were tested for SARS-CoV-2 by PCR, one with a positive and one with a negative result. Half of seropositive healthcare workers (6/12, 50.0%) reported having been self-isolating and 16.7% (2/12) were in self-quarantine. Seropositive healthcare workers reported more frequently having been in self-isolation or self-quarantine than seronegative healthcare workers (self-isolation 50.0% vs 2.3%, p <0.001; self-quarantine 1.7% vs 16.7%, p <0.001, table 2).

Figure 1 eSARS-coV-2 seroprevalence by (A) workplace and (B) among nurses (homecare, outpatient departments of the hospital, nursing homes). There were no events in the categories “medical practices” and “homecare”.
Table 2Characteristics by serology status.
| Total | Seronegative | Seropositive | p-value | |
| n = 357 | n = 345 | n = 12 | ||
| Sex | 0.37 | |||
| Male | 42 (11.8) | 42 (12.2) | 0 | |
| Female | 315 (88.2) | 303 (87.8) | 12 (100) | |
| Age (years) median (IQR) | 43 (29-54) | 48 (26.5-61.5) | 43 (29-54) | 0.47 |
| Profession | <0.001 | |||
| Physician | 49 (13.7) | 49 (14.2) | 0 | |
| Any nurse | 221 (61.9) | 209 (60.6) | 12 (100) | |
| Qualified nurse | 114 (31.9) | 112 (32.5) | 2 (16.7) | |
| Nurse assistance level 2 | 73 (20.5) | 70 (20.3) | 3 (25.0) | |
| Nurse assistance level 1 | 34 (9.5) | 27 (7.8) | 7 (58.3) | |
| Practice assistant | 87 (24.4) | 87 (25.2) | 0 | |
| Institution | 0.012 | |||
| Hospital | 42 (11.8) | 41 (11.9) | 1 (8.3) | |
| Medical practice | 118 (33.1) | 118 (34.2) | 0 | |
| Retirement or nursing home | 169 (47.3) | 158 (45.8) | 11 (91.7) | |
| Homecare | 28 (7.8) | 28 (8.1) | 0 | |
| Illnesses, self-reported since 1 Jan. 2020 | 0.03 | |||
| Yes | 150 (42.0) | 141 (40.9) | 9 (75.0) | |
| No | 207 (58.0) | 204 (59.1) | 3 (25.0) | |
| Symptoms | 0.03 | |||
| Yes | 150 (42.0) | 141 (40.9) | 9 (75.0) | |
| Fever | 42 (11.8) | 36 (10.4) | 6 (50.0) | 0.08 |
| Cough | 84 (23.5) | 81 (23.5) | 3 (25.0) | 0.16 |
| Shortness of breath or difficulty breathing | 26 (7.3) | 22 (62.9) | 4 (33.3) | 0.03 |
| Muscle or body aches | 51 (14.3) | 47 (13.6) | 5 (41.7) | 0.50 |
| Loss of taste or smell | 7 (1.9) | 5 (1.4) | 2 (16.7) | 0.01 |
| Headache | 70 (19.6) | 64 (18.6) | 6 (50.0) | 0.22 |
| Congestion or runny nose | 68 (19.0) | 65 (18.8) | 3 (25.0) | 0.46 |
| Diarrhoea | 7 (1.9) | 7 (2.0) | 0 | 0.49 |
| Sore throat | 12 (3.4) | 12 (3.5) | 0 | 0.36 |
| No | 207 (58.0) | 204 (59.1) | 3 (25.0) | |
| Vaccination against influenza 2019/20 | 0.99 | |||
| Yes | 119 (33.3) | 115 (33.3) | 4 (33.3) | |
| No | 237 (66.4) | 229 (66.4) | 8 (66.7) | |
| Unknown | 1 (0.3) | 1 (0.3) | 0 | |
| Self-quarantine | <0.001 | |||
| Yes | 8 (2.2) | 6 (1.7) | 2 (16.7) | |
| No | 349 (97.8) | 339 (98.3) | 10 (83.3) | |
| Self-isolation | <0.001 | |||
| Yes | 14 (3.9) | 8 (2.3) | 6 (50.0) | |
| No | 343 (96.1) | 337 (97.7) | 6 (50.0) |
IQR = interquartile rangeData are n (%) unless otherwise indicated.
Among the 12 seropositive healthcare workers, seven (58.3%) had both serological tests (Abbott ARCHITECT i2000 and Liaison SARS-CoV-2 S1/S2 IgG) positive, three (25.0%) only the liaison SARS-CoV-2 S1/S2 IgG and the remaining two (16.7%) only the Abbott ARCHITECT i2000 in the first blood sample. In the second blood sample, one (8.3%) HCW had both serological tests positive, three (25.0%) only the liaison SARS-CoV-2 S1/S2 IgG, two (16.7%) only the Abbott ARCHITECT i2000 and the remaining six (50.0%) did not provide a second sample. In healthcare workers with two samples, we observed a decline of the signal (index) in the ARCHITECT nucleocapsid targeting assay in five. Both assays (S1/S2 and N) were congruently positive above 93 relative units (Liason; S1/S2) rand above an index of 4.8 (ARCHITECT; N). Details on the serology results from the first and second blood samples can be found in table 3.
Among the 12 seropositive healthcare workers, six lived alone and reported no close contacts in a home setting, while the remaining six had one to three close contacts at home for a total of ten close contacts. None of them had a positive serology result during this study. Among the ten close contacts three (30.0%) self-quarantined.
Table 3Characteristics of the twelve study participants seropositive for SARS-CoV-2.
| Characteristics of participants tested positive for antibodies | PCR | First blood sample | Second blood sample | ||||||||||||
| No | Sex | Age in years | Healthcare facility | Profession | Illness | Symptoms | SARS-coV-2 | COVID Architect | COVID Liaison | COVID Architect | COVID Liaison | ||||
| Value | Inter. | Value | Inter. | Value | Inter. | Value | Inter | ||||||||
| 1 | F | 61 | Retirement or nursing home | Qualified nurse | No | – | 0.02 | Negative | 22.5 | Positive | |||||
| 2 | F | 55 | Retirement or nursing home | Qualified nurse | Yes | Fever, cough, shortness of breath difficulty breathing, muscle or body aches, headache | 4.64 | Positive | 105 | Positive | |||||
| 3 | F | 18 | Retirement or nursing home | Nurse assistant level 2 | Yes | Fever, cough, muscle or body aches, loss of taste or smell, headache, congestion or runny nose | 2.10 | Positive | 54.5 | Positive | |||||
| 4 | F | 30 | Retirement or nursing home | Nurse assistant level 2 | Yes | Fever, shortness of breath or difficulty breathing, headache | Negative | 1.87 | Positive | 3.8 | Negative | 1.82 | Positive | 3.8 | Negative |
| 5 | F | 21 | Retirement or nursing home | Nurse assistant level 2 | Yes | Shortness of breath or difficulty breathing, loss of taste or smell, headache | 0.71 | Negative | 55.4 | Positive | |||||
| 6 | F | 40 | Retirement or nursing home | Nurse assistant level 1 | Yes | Muscle or body aches, headache | 7.13 | Positive | 68.4 | Positive | 6.06 | Positive | 146 | Positive | |
| 7 | F | 41 | Hospital | Nurse assistant level 1 | Yes | Fever | 3.88 | Positive | 10.3 | Negative | 2.95 | Positive | 5.7 | Negative | |
| 8 | F | 62 | Retirement or nursing home | Nurse assistant level 1 | Yes | Fever, shortness of breath or difficulty breathing, muscle or body aches | 1.57 | Positive | 27.6 | Positive | 0.71 | Negative | 63.1 | Positive | |
| 9 | F | 64 | Retirement or nursing home | Nurse assistant level 1 | Yes | Fever, cough, congestion or runny nose | 1.11 | Negative | 44 | Positive | |||||
| 10 | F | 56 | Retirement or nursing home | Nurse assistant level 1 | Yes | Headache, congestion or runny nose, sore throat | Positive | 2.86 | Positive | 60.4 | Positive | 0.96 | Negative | 70.1 | Positive |
| 11 | F | 64 | Retirement or nursing home | Nurse assistant level 1 | No | – | 2.15 | Positive | 50.1 | Positive | 1.17 | Negative | 78.9 | Positive | |
| 12 | F | 23 | Retirement or nursing home | Nurse assistant level 1 | No | – | 1.87 | Positive | 20.4 | Positive | |||||
Inter. = interpretation; PCR = polymerase chain-reaction test
The overall seroprevalence among healthcare workers in outpatient settings and retirement or nursing homes in the Canton of Solothurn, Switzerland, was low at 3.4%. However, seroprevalence among these healthcare workers has interesting characteristics. All 12 of the seropositive healthcare workers were nursing staff, 11 of whom worked in retirement or nursing homes. Among these seropositive nursing participants, prevalence fell with level increasing nursing skills and associated character of patient involvement. At 21%, seroprevalence was highest among the level 1 nurse assistants, followed by a prevalence of 4% among the level 2 nurse assistants and 1.7% among the qualified nurses. As expected, the characteristic symptoms, such as loss of smell or taste, shortness of breath, and fever, were more common among seropositive than seronegative healthcare workers, indicating the serological results of true positive, previously undiagnosed COVID-19 infections.
At the beginning of the pandemic, the supply of personal protective equipment (PPE), such as protective clothing, masks, and gloves, was limited in Switzerland. PPE was primarily provided to hospitals and medical practices treating confirmed COVID-19 patients or performing COVID-19 testing. The supply of PPE was often insufficient at retirement or nursing homes. This was also the case in other countries where COVID-19 outbreaks were reported at retirement or nursing homes. These studies reported attack rates of 40% to 72% among nursing home residents [14–18]. Infection rates among staff members ranged from 1.5% to 5.9% in studies where all staff were routinely tested during an outbreak [17, 19, 20].
Imperfect use of PPE and infection prevention and control measures (IPCs) among healthcare workers increases their risk of infection and potential transmission of the virus through daily interactions with patients and staff [16, 21]. This might particularly be true in for nursing homes and home care organisations, where IPC expertise is generally lower than in the hospital setting or medical practices, and where balancing the preservation of a home-like environment and the adoption of IPC measures is challenging [22]. A Swiss study in retirement or nursing homes showed that only 52% of the institutions provided regular hand hygiene training [23]. On the other hand, patient contact is likely to be longer and more intensive among healthcare workers in nursing homes. These factors might have contributed to the higher seroprevalence at retirement or nursing homes compared with medical practices. With clear communication, a strong safety climate, access to PPE, regular staff training and involvement of all staff members in the implementation, the adherence to IPCs is increased [24, 25]. In the Canton of Solothurn, major efforts were undertaken during the first wave to train and support nursing homes in prevention measures, including online tutorials and webinars, counselling by IPC experts and supply of PPE.
Several studies have shown that healthcare workers, especially nurses, have a higher seroprevalence than the general population [26, 27]. In our study, only nurses were seropositive and seroprevalence declined with higher levels of nursing education. Our findings are in line with a large, hospital-based study in Sweden, which found the highest seroprevalence among assistant nurses, followed by qualified nurses and medical doctors [28]. In Switzerland, nurse assistants at levels 1 and 2 are mainly responsible for patients’ personal care, such as washing, going to the toilet or getting dressed. In contrast, qualified nurses have different responsibilities, such as preparing medications for patients and being more involved in administrative tasks.
Asymptomatic healthcare workers might play a role in transmitting SARS-CoV-2 to residents, patients or family members [29]. In our study, 25% of the seropositive healthcare workers were asymptomatic. A study in a large teaching hospital in Wuhan found that infections were asymptomatic in 9.7% of healthcare workers [30, 31].
Of particular interest, in addition to the finding of an increased seroprevalence in nurses with the closest and most prolonged contact with patients, was the finding of no seropositive front-line healthcare workers treating patients with respiratory symptoms and taking nasopharyngeal swabs from suspected cases. These findings support the conclusion that the use of PPE renders sufficient protection during contact limited in terms of time and physical distance with a suspected SARS-CoV-2 index case. A previous study in the Canton Solothurn has shown that even in the absence of PPE, limited contact with an oligosymptomatic index case resulted in no detectable secondary cases [32]. Therefore the cumulative exposure time and the intensity of physical contact, in combination with consequent PPE, seem to be the most prominent factors, rendering short contacts in the ambulatory healthcare sector safe even when undertaking diagnostic procedures in COVID-19 suspects.
This study has several limitations. The number of seropositive healthcare workers was low, and therefore, we could not use analytical statistics to examine risk factors. Participation in this study was voluntary, and we do not know the serological status of those who chose not to participate. Another limitation pertains to the fact that serological tests may show cross-reactivity with other coronaviruses. Finally, our study may underestimate or overestimate the seroprevalence among healthcare workers. Even though we can not be sure how representative of the true seroprevalence our results are, this study’s strength is that outpatient and long-term care facilities included in the survey are representative of the Canton of Solothurn.
The overall seroprevalence among healthcare workers after the first wave was low in outpatient and nursing home settings in Switzerland. However, HCW seropositivity was increased in retirement or nursing homes, and furthermore increased with the nurse assistants’ responsibilities, possibly due to increased patient contact. Healthcare workers at private practices could protect themselves sufficiently during the first wave of the COVID-19 pandemic.
No financial support and potential conflict of interest relevant to this article was reported.
We would like to thank all the participating healthcare workers. In addition, we would like to thank Christopher Ritter for editorial assistance.
Author contributions: Conception and design LF, KZ, CM, ME. Study coordination LF. Development of the questionnaire: LF, MH, KZ. Data collection and entry: PW, SM. Laboratory work: FS,. Data analysis: KZ. KZ, CM, LF wrote the first draft of the paper and revised it based on comments from all authors (KZ, CM, FS, PK, PW, ME, MH, LF). All authors reviewed and approved the final version of the manuscript.
1. Federal Office of Public Health (FOPH) . Coronavirus: Situation in Switzerland Bern: Federal Office of Public Health (FOPH); 2020 Available from: https://www.bag.admin.ch/coronavirus-situation-schweiz
2. Garcia-Basteiro AL , Moncunill G , Tortajada M , Vidal M , Guinovart C , Jiménez A , et al. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun. 2020 Jul;11(1):3500. https://doi.org/10.1038/s41467-020-17318-x
3. Folgueira MD , Muñoz-Ruipérez C , Alonso-López MÁ , Delgado R . SARS-CoV-2 infection in Health Care Workers in a large public hospital in Madrid, Spain, during March 2020. medRxiv. 2020:20055723. doi:https://doi.org/https://doi.org/10.1101/2020.04.07.20055723
4. Lombardi A , Mangioni D , Consonni D , Cariani L , Bono P , Cantù AP , et al. Seroprevalence of anti-SARS-CoV-2 IgG among healthcare workers of a large university hospital in Milan, Lombardy, Italy: a cross-sectional study. BMJ Open. 2021 Feb;11(2):e047216. https://doi.org/10.1136/bmjopen-2020-047216
5. Piccoli L , Ferrari P , Piumatti G , Jovic S , Rodriguez BF , Mele F , et al. Risk assessment and seroprevalence of SARS-CoV-2 infection in healthcare workers of COVID-19 and non-COVID-19 hospitals in Southern Switzerland. Lancet Reg Health Eur. 2021 Feb;1:100013. https://doi.org/10.1016/j.lanepe.2020.100013
6. Corman VM , Landt O , Kaiser M , Molenkamp R , Meijer A , Chu DK , et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020 Jan;25(3): https://doi.org/10.2807/1560-7917.ES.2020.25.3.2000045
7. Bonelli F , Sarasini A , Zierold C , Calleri M , Bonetti A , Vismara C , et al. Clinical and Analytical Performance of an Automated Serological Test That Identifies S1/S2-Neutralizing IgG in COVID-19 Patients Semiquantitatively. J Clin Microbiol. 2020 Aug;58(9):e01224–20. https://doi.org/10.1128/JCM.01224-20
8. Bryan A , Pepper G , Wener MH , Fink SL , Morishima C , Chaudhary A , et al. Performance Characteristics of the Abbott Architect SARS-CoV-2 IgG Assay and Seroprevalence in Boise, Idaho. J Clin Microbiol. 2020 Jul;58(8):e00941–20. https://doi.org/10.1128/JCM.00941-20
9. Guo L , Ren L , Yang S , Xiao M , Chang D , Yang F , et al. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin Infect Dis. 2020 Jul;71(15):778–85. https://doi.org/10.1093/cid/ciaa310
10 Roche. Elecsys® Anti-SARS-CoV-2 Immunoassay for the quantitative determination of antibodies to the SARS-CoV-2 spike protein Switzerland. Roche Diagnostics International Ltd; 2020. Available from: file:///C:/Users/KZUERC~1/AppData/Local/Temp/Elecsys-Anti-SARS-CoV-2-S-factsheet-SEPT-2020-2-1.pdf.
11. Choe PG , Kim KH , Kang CK , Suh HJ , Kang E , Lee SY , et al. Antibody Responses 8 Months after Asymptomatic or Mild SARS-CoV-2 Infection. Emerg Infect Dis. 2021 Mar;27(3):928–31. https://doi.org/10.3201/eid2703.204543
12. Harris PA , Taylor R , Thielke R , Payne J , Gonzalez N , Conde JG . Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–81. https://doi.org/10.1016/j.jbi.2008.08.010
13. Harris PA , Taylor R , Minor BL , Elliott V , Fernandez M , O’Neal L , et al.; REDCap Consortium . The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019 Jul;95:103208. https://doi.org/10.1016/j.jbi.2019.103208
14. Arons MM , Hatfield KM , Reddy SC , Kimball A , James A , Jacobs JR , et al.; Public Health–Seattle and King County and CDC COVID-19 Investigation Team . Presymptomatic SARS-CoV-2 Infections and Transmission in a Skilled Nursing Facility. N Engl J Med. 2020 May;382(22):2081–90. https://doi.org/10.1056/NEJMoa2008457
15. Graham NS , Junghans C , Downes R , Sendall C , Lai H , McKirdy A , et al. SARS-CoV-2 infection, clinical features and outcome of COVID-19 in United Kingdom nursing homes. J Infect. 2020 Sep;81(3):411–9. https://doi.org/10.1016/j.jinf.2020.05.073
16. McMichael TM , Currie DW , Clark S , Pogosjans S , Kay M , Schwartz NG , et al.; Public Health–Seattle and King County, EvergreenHealth, and CDC COVID-19 Investigation Team . Epidemiology of Covid-19 in a Long-Term Care Facility in King County, Washington. N Engl J Med. 2020 May;382(21):2005–11. https://doi.org/10.1056/NEJMoa2005412
17 Geschwindner H , Bieri-Brüning G. Covid-19: prevention and outbreak management in nursing homes: experiences from the nursing homes of the city of Zurich. Journal of Nursing Home Research. 2020.
18. Goldberg SA , Pu CT , Thompson RW , Mark E , Sequist TD , Grabowski DC . Asymptomatic Spread of COVID-19 in 97 Patients at a Skilled Nursing Facility. J Am Med Dir Assoc. 2020 Jul;21(7):980–1. https://doi.org/10.1016/j.jamda.2020.05.040
19. Guery R , Delaye C , Brule N , Nael V , Castain L , Raffi F , et al. Limited effectiveness of systematic screening by nasopharyngeal RT-PCR of medicalized nursing home staff after a first case of COVID-19 in a resident. Med Mal Infect. 2020 Nov;50(8):748–50. https://doi.org/10.1016/j.medmal.2020.04.020
20. Roxby AC , Greninger AL , Hatfield KM , Lynch JB , Dellit TH , James A , et al. Detection of SARS-CoV-2 Among Residents and Staff Members of an Independent and Assisted Living Community for Older Adults - Seattle, Washington, 2020. MMWR Morb Mortal Wkly Rep. 2020 Apr;69(14):416–8. https://doi.org/10.15585/mmwr.mm6914e2
21. World Health Organization . Preventing and managing COVID-19 across long-term care services: policy brief. Geneva: World Health Organization; 2020.
22. Smith PW , Bennett G , Bradley S , Drinka P , Lautenbach E , Marx J , et al.; SHEA; APIC . SHEA/APIC guideline: infection prevention and control in the long-term care facility, July 2008. Infect Control Hosp Epidemiol. 2008 Sep;29(9):785–814. https://doi.org/10.1086/592416
23 Zúñiga F , Favez L. SHURP 2018 Infektionskontrolle - Resultate zu Handen Strategie NOSO. 2020.
24. Houghton C , Meskell P , Delaney H , Smalle M , Glenton C , Booth A , et al. Barriers and facilitators to healthcare workers’ adherence with infection prevention and control (IPC) guidelines for respiratory infectious diseases: a rapid qualitative evidence synthesis. Cochrane Database Syst Rev. 2020 Apr;4(4):CD013582.
25. Leiss JK . Safety climate and use of personal protective equipment and safety medical devices among home care and hospice nurses. Ind Health. 2014;52(6):492–7. https://doi.org/10.2486/indhealth.2014-0074
26. Gómez-Ochoa SA , Franco OH , Rojas LZ , Raguindin PF , Roa-Díaz ZM , Wyssmann BM , et al. COVID-19 in Health-Care Workers: A Living Systematic Review and Meta-Analysis of Prevalence, Risk Factors, Clinical Characteristics, and Outcomes. Am J Epidemiol. 2021 Jan;190(1):161–75. https://doi.org/10.1093/aje/kwaa191
27. Rostami A , Sepidarkish M , Leeflang MM , Riahi SM , Nourollahpour Shiadeh M , Esfandyari S , et al. SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis. Clin Microbiol Infect. 2021 Mar;27(3):331–40. https://doi.org/10.1016/j.cmi.2020.10.020
28. Rudberg AS , Havervall S , Månberg A , Jernbom Falk A , Aguilera K , Ng H , et al. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat Commun. 2020 Oct;11(1):5064. https://doi.org/10.1038/s41467-020-18848-0
29. Thompson HA , Mousa A , Dighe A , Fu H , Arnedo-Pena A , Barrett P , et al. SARS-CoV-2 setting-specific transmission rates: a systematic review and meta-analysis. Clin Infect Dis. 2021 Aug;73(3):e754–64. https://doi.org/10.1093/cid/ciab100
30. Zhao D , Wang M , Wang M , Zhao Y , Zheng Z , Li X , et al. Asymptomatic infection by SARS-CoV-2 in healthcare workers: A study in a large teaching hospital in Wuhan, China. Int J Infect Dis. 2020 Oct;99:219–25. https://doi.org/10.1016/j.ijid.2020.07.082
31. Buitrago-Garcia D , Egli-Gany D , Counotte MJ , Hossmann S , Imeri H , Ipekci AM , et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: A living systematic review and meta-analysis. PLoS Med. 2020 Sep;17(9):e1003346. https://doi.org/10.1371/journal.pmed.1003346
32. Canova V , Lederer Schläpfer H , Piso RJ , Droll A , Fenner L , Hoffmann T , et al. Transmission risk of SARS-CoV-2 to healthcare workers -observational results of a primary care hospital contact tracing. Swiss Med Wkly. 2020 Apr;150:w20257. https://doi.org/10.4414/smw.2020.20257
Table S1Capacity of the individual healthcare facilities participating in the study (n = 26) during first wave of the COVID-19 pandemic.
| Median (IQR) | |
| Medical practice, n | 12 |
| Number of patients per week | 150 (50–410) |
| Number of practise assistant | 7 (5–12) |
| Number of physicians | 5 (3–6) |
| Workload | 80 (60–100) |
| Patient contact in hours per week | 32.5 (22.5–40) |
| Retirement or nursing home, n | 11 |
| Number of residents | 63 (24–82) |
| Number of health workers | 47 (26–80) |
| Number of other employees | 37 (11–43) |
| Workload | 82.5 (75–100) |
| Patient contact in hours per week | 32 (25–40) |
| Homecare organisation, n | 2 |
| Number of people cared by organisation | 106 (50–159) |
| Number of health care professionals per organisation | 23 (11–29) |
| Number of people cared by nurse per week | 19 (15–20) |
| Workload | 67.5 (60–85) |
| Patient contact in hours per week | 23 (18–29) |
| Hospital outpatient clinic, n | 1 |
| Number of patients per week | 55 (31–100) |
| Number of medical doctors in the clinic | 6 (6–9) |
| Number of nurses in the clinic | 15 (12–15) |
| Workload | 80 (60–100) |
| Patient contact in hours per week | 30 (18–35) |
IQR = interquartile range
Table S2Characteristics by healthcare facility (n = 357).
| Total | Hospital | Medical practice | Retirement or nursing home | Homecare | |
| n = 357 | n = 42 | n = 118 | n = 169 | n = 28 | |
| Sex | |||||
| Male | 42 (11.8) | 5 (11.9) | 20 (16.9) | 17 (10.1) | 0 |
| Female | 315 (88.2) | 37 (88.1) | 98 (83.1) | 152 (89.9) | 28 (100) |
| Age (years), median (IQR) | 43 (29–54) | 39 (32–48) | 39 (28–49) | 46 (28–57) | 49 (38.5–55.5) |
| Profession | |||||
| Physician | 49 (13.7) | 7 (16.7) | 42 (35.6) | 0 | 0 |
| Adults | 40 (11.2) | 7 (16.7) | 33 (28.0) | 0 | 0 |
| Children | 9 (2.5) | 0 | 9 (7.6) | 0 | 0 |
| Any nurse | 221 (61.9) | 19 (45.2) | 7 (5.9) | 168 (99.4) | 27 (96.4) |
| Qualified Nurse | 114 (31.9) | 13 (31.0) | 7 (5.9) | 82 (48.5) | 12 (42.9) |
| Nurse assistance level 2 | 73 (20.5) | 4 (9.5) | 0 | 59 (34.9) | 10 (35.7) |
| Nurse assistance level 1 | 34 (9.5) | 2 (4.8) | 0 | 27 (16.0) | 5 (17.9) |
| Practice assistant | 87 (24.4) | 16 (38.1) | 69 (58.5) | 1 (0.6) | 1 (3.6) |
| Employment (percentage of FTE) | 80 (60–100) | 80 (60–100) | 80 (60–100) | 90 (80–100) | 67.5 (60–85) |
| Number of observations | 356 | 41 | 118 | 169 | 28 |
| Contact with patients (hours/week), median (IQR) | 30 (22–40) | 30 (17–35.5) | 33 (23–40) | 32 (25–40) | 23 (18–26) |
| Number of observations | 311 | 40 | 111 | 137 | 23 |
| Seroprevalence | |||||
| Number of seropositive persons | 12 | 1 | 0 | 11 | 0 |
| Percent, 95% Cl | 3.4 (1.7–5.8) | 2.4 (0.1–12.6) | 0 | 6.5 (3.3–11.3) | 0 |
CI = confidence interval; FTE = full time equivalent; IQR = interquartile rangeData are n (%) unless otherwise indicated.