
Figure 1 Common ocular surface diseases in atopic dermatitis patients. Stock pictures by istock and shutterstock.
DOI: https://doi.org/10.4414/SMW.2021.w30020
Eczema Area and Severity Index
Investigator Global Assessment
interleukin
ocular surface disease
scoring atopic dermatitis
A Swiss expert group of dermatologists and ophthalmologists with experience in the treatment of ocular surface diseases (OSDs) in patients with atopic dermatitis from university and cantonal hospitals reviewed data in clinical trials and real-life reports on dupilumab therapy, published case reports on the management of OSD, as well as recent national and international recommendations. Based on the observations of dupilumab-associated OSD and practical experience in identifying and treating OSDs, an algorithm has been developed that is specific to the needs in Switzerland. The level of agreement in the development of the algorithm was determined with the consent of seven of the nine experts.
Atopic dermatitis is a chronic inflammatory skin disease presenting with recurrent eczematous lesions, intense pruritus and increased risk of skin infections [1, 2]. It affects approximately 20% of all children up to the age of 6 years and 5% of adults in Western industrialised countries [1]. Because of its chronic disease course, it requires long-term treatment [1]. Atopic dermatitis is based on a genetic predisposition, which affects both the epithelial barrier and a type 2 based immune reaction [3].
Besides affecting the skin, atopic dermatitis is associated with substantial psychosocial distress as well as with other atopic diseases such as bronchial asthma, allergic rhinitis and eosinophilic oesophagitis [4–6].
Patients with atopic dermatitis have an increased risk of different ocular surface diseases with conjunctivitis as the most frequent ocular comorbidity in the atopic dermatitis population (supplementary table S1 in the appendix) [7, 8].

Figure 1 Common ocular surface diseases in atopic dermatitis patients. Stock pictures by istock and shutterstock.
Ocular surface diseases, such as allergic conjunctivitis, blepharitis and keratitis, are well-known ophthalmic comorbidities in patients with atopic dermatitis, in particular those with severe disease in whom incidence rates of 32.4–55.8% have been reported [9]. For atopic keratoconjunctivitis alone in atopic dermatitis patients, an incidence rate of 25% to 42% is given [10].
Since the clinical signs and symptoms of allergic conjunctivitis are not pathognomonic, it is essential to consider a broad spectrum of differential diagnoses (fig.1). As trigger of OSD, in particular when associated with lid dermatitis, irritant and allergic contact dermatitis have to be ruled out [11].
Red flags for immediate ophthalmological consultation are loss of transparency in the eye, vision loss, discharge and an increase in ocular pressure [12]. Patients wearing contact lenses presenting with red eyes should immediately be referred since they are at high risk of having an infectious keratitis.
Dupilumab, a monoclonal antibody, targets the shared interleukin (IL)-4/IL-13 receptor α-chain and thus blocks the action of IL-13 and IL-4 (fig. 2) [1, 13]. IL-13 in particular has been shown to play a key role in the pathogenesis of atopic dermatitis [1].
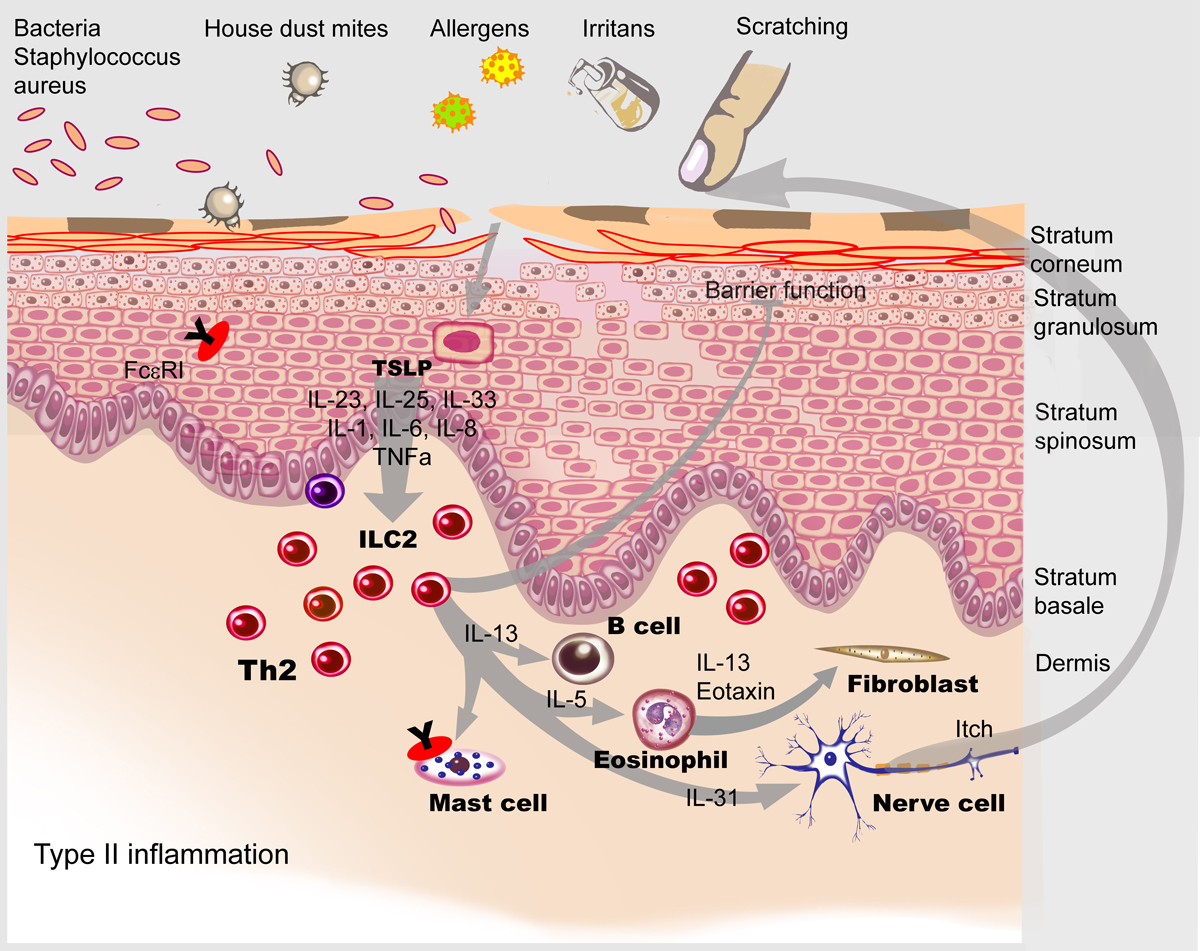
Figure 2 The targets of dupilumab in atopic dermatitis skin characterised by a type 2 immune reaction. Illustration: Aldona von Guten (modified from: Simon D, Wollenberg A, Renz H, Simon HU. Atopic Dermatitis: Collegium Internationale Allergologicum (CIA) Update 2019. Int Arch Allergy Immunol. 2019;178(3):207–18 [1], reprinted with permission from S. Karger AG, Basel).
In several clinical trials in patients with moderate-to-severe atopic dermatitis, dupilumab has been shown to exhibit an excellent and well-balanced efficacy and safety profile [14–19]. A 75% improvement of the Eczema Area and Severity Index (EASI) was achieved in 47.7% to 69% of dupilumab-treated patients versus 13.3% to 29.6% of the placebo group after 16 weeks [15, 16, 20]. More detailed information is provided in table S1, in the appendix) [14–19]. The most common adverse events observed were injection site reactions (9.6%), conjunctivitis (>1%, <10%), blepharitis (>1%, <10%) and oral herpes (>1%, <10%) [21].
In clinical trials, the diagnosis of conjunctivitis was usually reported by dermatologists and allergists, whereas no specific assessments by ophthalmologists were required for diagnosis. Therefore, a specific categorisation of conjunctivitis is not available and the frequencies reported include all cases of conjunctivitis regardless of their aetiology. The incidence of conjunctivitis was 17.9–22.1% in the dupilumab and 7.9–11.1% in the placebo group after 16 weeks (table S1). Numerical differences between placebo and dupilumab were apparent after about 4–8 weeks [9, 14–19].
In a long-term open-label extension study for patients previously enrolled in phase II and III studies, conjunctivitis was reported in only 10.7%, suggesting that it mainly occurs during the first months of dupilumab therapy and may recover [18]. In most cases, conjunctivitis was mild to moderate [18]. Overall, there were <1% of patients who discontinued dupilumab treatment because of ocular adverse events [9]. Moreover, in a long-term study, the incidence rates of 4.7% and 4.9%, respectively, were similar in both dupilumab and placebo groups at week 36, indicating that dupilumab-associated OSD is transient [9, 14–16].
Higher baseline severity of atopic dermatitis and levels of thymus and activation-regulated chemokine (TARC), total immunoglobulin E and blood eosinophil counts, as well as a history of conjunctivitis were associated with an increased risk to develop a dupilumab-associated OSD [9]. Still, the pathogenesis of dupilumab-associated OSD and the reason why it occurs almost exclusively in atopic dermatitis patients needs to be elucidated.
In atopic diseases other than atopic dermatitis, such as asthma, chronic rhinosinusitis with nasal polyposis or eosinophilic oesophagitis, dupilumab therapy was not associated with an increased risk for conjunctivitis, suggesting that the relatively high rates of conjunctivitis in atopic dermatitis trials may reflect unique effects of dupilumab on the ocular surface of these patients [9, 16, 22–25].
So far, real-world evidence data on the efficacy and safety of dupilumab in atopic dermatitis patients from the Netherlands, France and Denmark are available [26–28]. Here, conjunctivitis rates of 18.4%, 34.1% and 38.2%, respectively, have been reported (supplementary figure S1 in the appendix) [26–28]. Moreover, both severity of atopic dermatitis and the age of the patients were associated with dupilumab-associated OSD [8]. It appears that dupilumab-associated OSD in real-world evidence with unselected patients probably having more comorbidities, occurs more frequently than in clinical trials [26].
Various hypotheses have been proposed for mechanisms that drive conjunctivitis in atopic dermatitis patients treated with dupilumab. These include inflammatory and noninfectious processes such as unmasking preexisting subclinical atopic or allergic inflammatory processes [8], quantitative and qualitative tear production failures [8], the IL-4 mediated lipogenesis by Demodex mite colonisation of the Meibomian glands, an increased systemic bioavailability of free IL-4 and IL-13 causing inflammatory symptoms, a decrease of IL-13 mediated mucus production and an IL-13-related scarcity of conjunctival goblet cells [8, 29, 30].
Based on the literature and our own experiences we developed recommendations on how to manage dupilumab-associated OSD (fig. 3):
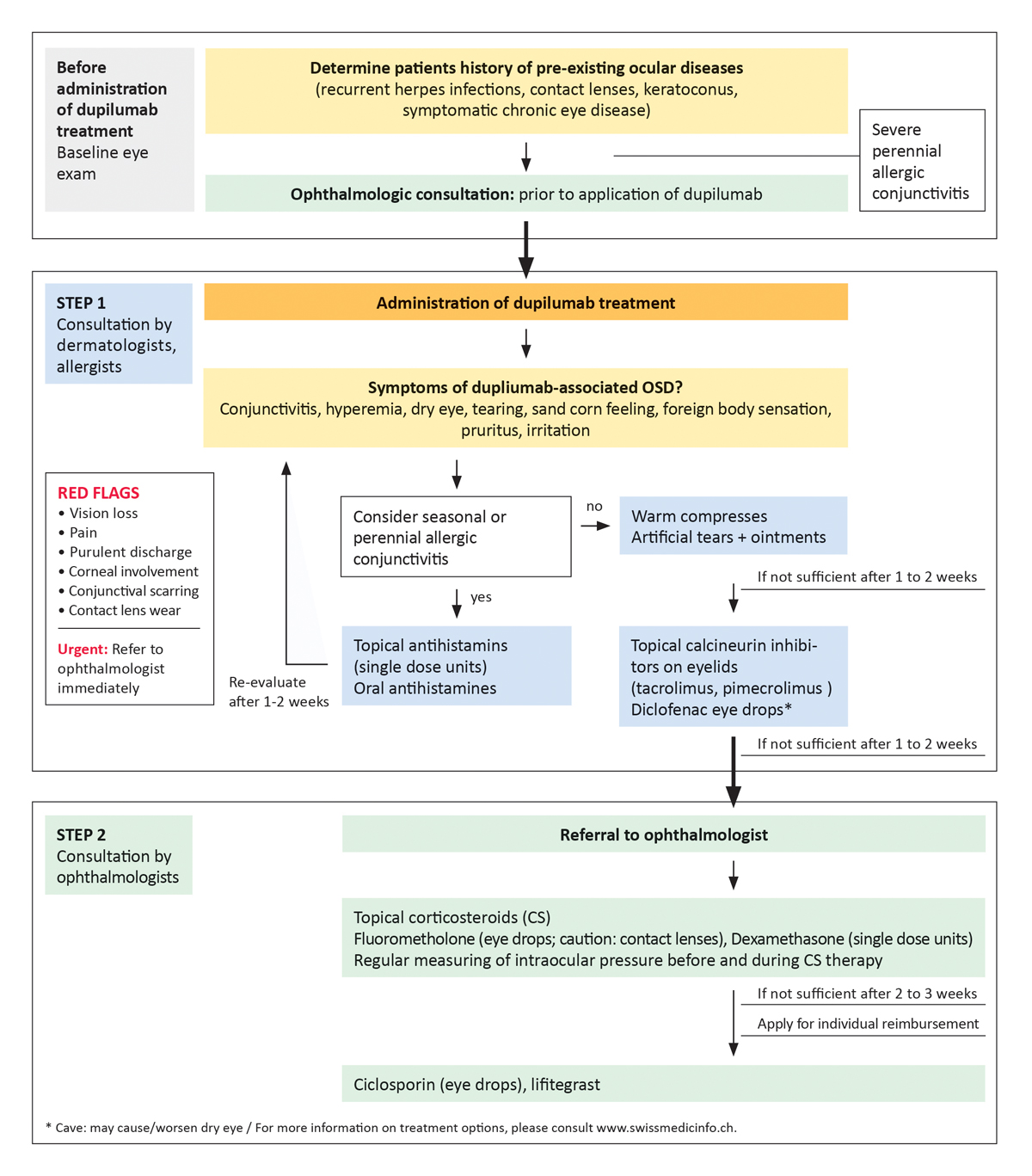
Figure 3 Algorithm for the management of dupilumab-associated ocular surface diseases in atopic dermatitis patients developed by a Swiss expert group of dermatologists and ophthalmologists.
In the spectrum of OSD, dupilumab-associated OSD presents a novel subgroup that poses a challenge for all, dermatologists, allergists and ophthalmologists. Specific challenges are
1. For the dermatologist and allergist:
(a) to diagnose dupilumab-associated OSD and distinguish it from other OSDs
(b) to evaluate the severity of OSD
(c) to manage mild forms of dupilumab-associated OSD
(d) to interact with ophthalmologists
(e) to be aware of red flags on severe forms requiring urgent ophthalmological intervention
2. For the ophthalmologist:
(a) To recognise dupilumab-associated OSD as a novel adverse effect of atopic dermatitis therapy
(b) To interact with dermatologists
(c) To manage severe forms of dupilumab-associated OSD requiring immunosuppressive or immunomodulating therapy
As result of our expert group meeting, we provide a summary of current knowledge on dupilumab-associated OSD and its management in daily practice. The discussion on and our clinical experience with dupilumab-associated OSD clearly demonstrate how important the collaboration between dermatologists, allergists and ophthalmologists is in order to achieve best therapeutic results for atopic dermatitis patients. We are aware that, as we will learn more on the pathogenesis of dupilumab-associated OSD, on how to identify patients at risk and therapeutic approaches, we will probably have to adapt and revise our recommendations in the near future.
Indication: Dupilumab (Duxipent®) was approved in Switzerland in April 2019 and is indicated for the treatment of moderate-to-severe atopic dermatitis in adult patients and adolescents aged 12 years and older when treatment with prescription topical medications does not provide adequate disease control or is not recommended. Dupixent can be used with or without topical corticosteroids [21].
Administration: Duxipent® (dupilumab) is administered in an initial dose of 600 mg by subcutaneous injection (two injections of 300 mg each), followed by a dose of 300 mg by subcutaneous injection every 2 weeks for the treatment of adult patients (from the age of 18) with severe atopic dermatitis (Investigator Global Assessment [IGA] 4, on an IGA scale of 0–4, or scoring atopic dermatitis (SCORAD) score >50 or EASI ≥21.1), if patients have had an inadequate response to intensified local treatment with prescription topical therapies (topical corticoids and/or calcineurin inhibitors) and phototherapy (if available and indicated) and systemic treatment with a conventional immunosuppressive agent (excluding systemic corticoids) for at least 1 month, or where these therapies are contraindicated or have had to be discontinued because of clinically relevant adverse events. Duxipent® is not reimbursed in combination with other systemic drugs for the treatment of atopic dermatitis. If after 16 weeks of treatment with Duxipent® no therapeutic success has been achieved, i.e., an IGA reduction of ≥2 points compared with the initial value or a ≥50% improvement of the EASI score compared with the initial value or a ≥50% improvement of the SCORAD score compared with the initial value, treatment must be discontinued. The treatment costs are reimbursed after prior consultation with a medical examiner. The diagnosis, prescription of Duxipent® and follow-up may only be made by a specialist in dermatology and venereology or a specialist in allergology and clinical immunology. After 52 weeks of uninterrupted therapy, the health insurer must again approve the costs after prior consultation with the medical advisor [25].
The contents of this practice guide were developed by Swiss experts in the therapy of atopic dermatitis and eye diseases on the occasion of an expert meeting. The expert meeting was supported by Sanofi Genzyme.
Y. Guex-Crosier: none; J. Di Lucca has served as investigator for Roche Pharma and Eli Lilly, and advisor for Sanofi Genzyme; P. Häusermann has served as a speaker, and/or advisor for AbbVie, Almirall, Amgen, Celgene, Eli Lilly, Galderma, Janssen, LEO Pharma, Novartis, Sanofi Genzyme; E. Laffitte has served as an investigator, speaker, and/or advisor from Abbvie, Amgen, Celgene, Eli Lilly, Galderma, Janssen, LEO Pharma, Novartis, Merck Sharp & Dohme, Sanofi Genzyme and Pfizer; L. Saulite: none; P. Schmid-Grendelmeier has received honoraria for advisory boards and as speaker from AbbVie, LEO Pharma, GlaxoSmithKline, Novartis, Pfizer, Roche Pharma and Sanofi Genzyme; K. Schürch: none; K. Thormann: none; D. Simon reports serving as an investigator and/or consultant for AbbVie, Astra Zeneca, Galderma, Eli Lilly, Pfizer, Roche Pharma and Sanofi Genzyme.
The authors have no conflicts of interest that are directly relevant to the content of this article.
Table S1Efficacy and safety of dupilumab in phase III clinical trials [14–19]. (Disclaimer: The only approved dosing regimen of dupilumab for the treatment of moderate-to-severe atopic dermatitis is every 2 weeks.)
| SOLO 1, 16 weeks [14] | Dupilumab 300 mg, q2w (N = 224) | Placebo (N = 224) | ||||
| Efficacy | EASI 75 | 115 (51%) | 33 (15%) | |||
| IGA Score 0 or 1 | 85 (38%) | 23 (10%) | ||||
| Safety, dupilumab 300 mg, q2w | AEs: injection-site reaction (8%), exacerbation of AD (13%), headache (9%), allergic conjunctivitis (5%), nasopharyngitis (10%), upper respiratory tract infection (3%), conjunctivitis (5%), any herpes viral infection (7%), adjudicated skin infection (6%), non-skin infection (30%) | AEs (≥1), 166 (73%) | AEs (≥1), 144 (65%) | |||
| OSD | Conjunctivits | 11 (5%) | 2 (1%) | |||
| Conjunctivitis allergic | 12 (5%) | 2 (1%) | ||||
| SOLO 2, 16 weeks [14] | Dupilumab 300 mg, q2w (N = 233) | Placebo (N = 236) | ||||
| Efficacy | EASI 75 | 103 (44%) | 28 (12%) | |||
| IGA Score 0 or 1 | 84 (36%) | 20 (8%) | ||||
| Safety, dupilumab 300 mg, q2w | AEs: injection-site reactions (14%), exacerbation of AD (14%), headache (8%), allergic conjunctivitis (1%), nasopharyngitis (20%), upper respiratory tract infection (3%), conjunctivitis (4%), any herpes viral infection (4%), adjudicated skin infection (6%), non-skin infection (25%) | AEs (≥1), 154 (65%) | AEs (≥1), 168 (72%) | |||
| OSD | Conjunctivitis | 9 (4%) | 1 (< 1%) | |||
| Conjunctivitis allergic | 2 (1%) | 2 (1%) | ||||
| LIBERTY AD CHRONOS,16 weeks, 52 weeks [15] | Dupilumab 300 mg, q2w + TCS (N = 106), week 16 | Placebo, qw + TCS (N = 315), week 16 | Dupilumab 300 mg, q2w + TCS (N = 89), week 52 | Placebo, qw + TCS (N = 264), week 52 | ||
| Efficacy | EASI 75 | 73 (69%) | 73 (23%) | 58 (65%) | 57 (22%) | |
| IGA Score 0 or 1 | 41 (39%) | 39 (12%) | 32 (36%) | 33 (13%) | ||
| Safety, dupilumab 300 mg, q2w + TCS, 52 week-period | AEs: nasopharyngitis (23%), upper respiratory tract infection (10%), sinusitits (2%), influenza (4%), eye disorders (31%), conjunctivitis (14%), atopic dermatitis (18%), injection site reaction (15%), asthma (5%), headache (5%), non-herpetic skin infections (11%), any herpes infections (7%) | AEs ≥1 97 (88%) | AEs ≥1 266 (84%) | |||
| OSD, 52 week-period | Conjunctivitis (conjunctivitis allergic, conjunctivitis bacterial, atopic keratoconjunctivitis, and conjunctivitis) | 15 (14%) | 25 (8%) | |||
| LIBERTY AD CAFÉ, 16 weeks [16] | Dupilumab 300 mg, q2w + TCS (N = 107) | Placebo + TCS (N = 108) | ||||
| Efficacy | EASI 75 | 67 (62.6%) | 32 (29.6%) | |||
| IGA | 43 (40.2%) | 15 (13.9%) | ||||
| Safety, dupilumab 300 mg, q2w + TCS | AEs: nasopharyngitis (20.6%), conjunctivitis (11.2%), oral herpes (2.8%), gastroenteritis (1.9%), upper respiratory tract infection (0.9%), pharyngitis (0.9%), herpes simplex (0.9%), atopic dermatitis (7.5%), allergic conjunctivitis (15%), lacrimation increased (0.9%), fatigue (3.7%), injection site reaction (0.9%), injection site erythema (0.9%), headache (9.3%), rhinitis allergic (6.5%), cough (3.7%), oropharyngeal pain (2.8%), asthma (0.9%), diarrhea (2.8%), back pain (0.9%), vascular disorders (3.7%), hypertension (0.9%), blood and lymphatic system disorders 3.7%, lymphadenopathy (1.9%), skin infections, excl. herpetic infections (1.9%) | AEs ≥1, 77 (72%) | AEs ≥1, 75 (69.4%) | |||
| OSD: Treat-ment-emergent conjunctivitis | Conjunctivitis | 12 (11.2%) | 3 (2.8%) | |||
| Conjunctivitis allergic | 16 (15%) | 7 (6.5%) | ||||
| Adenovirus conjunctivitis | 1 (0.9%) | 0.00% | ||||
| Conjunctivitis bacterial | 1 (0.9%) | 2 (1.9%) | ||||
| Conjunctivitis viral | 1 (0.9%) | 1 (0.9%) | ||||
| SOLO-CONTINUE, 36 weeks [17] | Dupilumab 300 mg, q8w (N = 84) | Dupilumab 300 mg, q4w (N = 86) | Dupilumab 300 mg, qw or q2w (N = 169) | Placebo (N = 83) | ||
| No./Tot No.(%) | Efficacy | EASI 75 | 45/82 (54.9%) | 49/84 (58.3%) | 116/162 (71.6%) | 24/79 (30.4%) |
| No./Tot No.(%) | IGA score 0 or 1 | 21/64 (32.8%) | 29/66 (43.9%) | 68/126 (54%) | 9/63 (14.3%) | |
| Safety, dupilumab 300 mg, wk or q2w | AEs: dermatitis atopic (20.4%), nasopharyngitis (19.2%), upper respiratory tract infection (7.8%), headache (4.8%), herpes simplex virus infection (4.2%), asthma (2.4%), back pain (3.6%), oral herpes infection (1.8%), influenza (2.4%), bronchitis (1.8%), urticaria (3.0%), arthralgia (3.0%), pharyngitis (1.8%), diarrhea (2.4%), pruritus (1.8%), sinusitis (3.6%), blood creatin phophosphokinase increased (0.6%), cough (2.4%), insomnia (2.4%), nasal congestion (2.4%), contact dermatitis (0.6%), gastroenteritis (1.8%), ligament sprain (1.2%), toothache (2.4%), contusion (0.6%), hypertension (1.2%), proteinuria (0.6%), rhinitis (0.6%), tonsillitis (0.6%), urinary tract infection (1.2%), viral infection (1.2%), ophthalmic herpes infection (0.6%), musculoskeletal pain (0.6%), vulvovaginal candidiasis (1.2%), fall (0.6%), eye disorders with preferred term conjunctivitis (5.4%), non-herpetic skin infections (2.4%), injection-site reaction (10.8%) | AEs ≥1, 63 (75%) | AEs ≥1, 64 (73.6%) | AEs ≥1, 118 (70.7%) | AEs ≥1, 67 (81.7%) | |
| OSD | Conjunctivitis: conjunctivitis, conjunctivitis bacterial, conjunctivitis viral, conjunctivitis allergic, and atopic keratoconjunctivitis | 3 (3.6%) | 4 (4.6%) | 9 (5.4%) | 4 (4.9%) | |
| OPEN LABEL EXTENSION, week 52 [8], 76 [18] and week 148 [19] | Dupilumab 300 mg, qw (N = 428), week 52 | Dupilumab 300 mg, qw (N = 284), week 76 | Dupilumab 300 mg, qw (N = 347), week 148 | |||
| n/subgroup (%) | Efficacy | EASI 75 | 346/398 (86.9%) | 220/249 (88.4%) | 56/58 (96.6%) | |
| n/subgroup (%) | IGA score 0 or 1 | 221/398 (55.5%) | 144/249 (57.8%) | 43/58 (74.1%) | ||
| Safety, 76 week-period | AEs: nasopharyngitis (20%), upper respiratory tract infection (9.5%), dermatitis atopic (8.2%), headache 7.1%), oral herpes (4.3%), blood creatin phophosphokinase increased (3.6%), bronchitis (3.2%), diarrhea (2.7%), back pain (2.7%), viral upper respiratory tract infection (2.5%), cough (2.3%), influenza (2.1%), conjunctivitis (10.7%), injection site reaction (10.1%) | AEs ≥1, 1054 (70.7%) | AEs ≥1, 2264 (84.6%) | |||
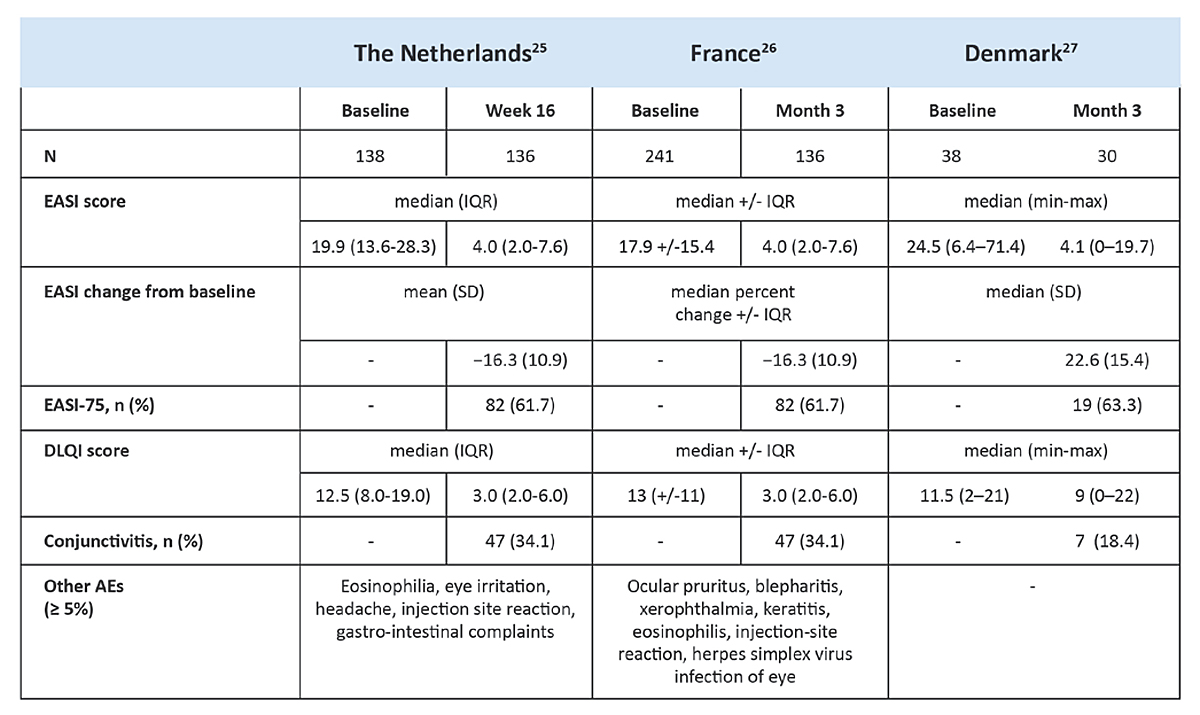
Figure S1 Real world evidence data of dupilumab collected in country-specific registries [26–28].
1. Simon D , Wollenberg A , Renz H , Simon HU . Atopic Dermatitis: Collegium Internationale Allergologicum (CIA) Update 2019. Int Arch Allergy Immunol. 2019;178(3):207–18. https://doi.org/10.1159/000497383
2. Weidinger S , Novak N . Atopic dermatitis. Lancet. 2016 Mar;387(10023):1109–22. https://doi.org/10.1016/S0140-6736(15)00149-X
3. Wohlrab J , Wollenberg A , Reimann H , Pleyer U , Werfel T . Interdisziplinäre Handlungsempfehlung bei Dupilumab-assoziierten entzündlichen Augenerkrankungen [Interdisciplinary recommendations for action in dupilumab-related inflammatory eye diseases]. Hautarzt. 2019 Jan;70(1):64–7. https://doi.org/10.1007/s00105-018-4316-1
4. Eichenfield LF , Tom WL , Chamlin SL , Feldman SR , Hanifin JM , Simpson EL , et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014 Feb;70(2):338–51. https://doi.org/10.1016/j.jaad.2013.10.010
5. Silverberg JI , Nelson DB , Yosipovitch G . Addressing treatment challenges in atopic dermatitis with novel topical therapies. J Dermatolog Treat. 2016 Nov;27(6):568–76. https://doi.org/10.1080/09546634.2016.1174765
6. Bieber T . Atopic dermatitis. N Engl J Med. 2008 Apr;358(14):1483–94. https://doi.org/10.1056/NEJMra074081
7. Thyssen JP , Toft PB , Halling-Overgaard AS , Gislason GH , Skov L , Egeberg A . Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017 Aug;77(2):280–286.e1. https://doi.org/10.1016/j.jaad.2017.03.003
8. Wohlrab J , Werfel T , Wollenberg A . Pathomechanism of dupilumab-associated inflammatory eye symptoms. J Eur Acad Dermatol Venereol. 2019 Nov;33(11):e435–6. https://doi.org/10.1111/jdv.15755
9. Akinlade B , Guttman-Yassky E , de Bruin-Weller M , Simpson EL , Blauvelt A , Cork MJ , et al. Conjunctivitis in dupilumab clinical trials. Br J Dermatol. 2019 Sep;181(3):459–73. https://doi.org/10.1111/bjd.17869
10. Bielory L . Allergic and immunologic disorders of the eye. Part II: ocular allergy. J Allergy Clin Immunol. 2000 Dec;106(6):1019–32. https://doi.org/10.1067/mai.2000.111238
11. Raffi J , Suresh R , Fishman H , Botto N , Murase JE . Investigating the role of allergic contact dermatitis in residual ocular surface disease on dupilumab (ROSDD). Int J Womens Dermatol. 2019 Nov;5(5):308–13. https://doi.org/10.1016/j.ijwd.2019.10.001
12. Cronau H , Kankanala RR , Mauger T . Diagnosis and management of red eye in primary care. Am Fam Physician. 2010 Jan;81(2):137–44.
13. Brunner PM , Guttman-Yassky E , Leung DY . The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017 Apr;139(4 4S):S65–76. https://doi.org/10.1016/j.jaci.2017.01.011
14. Simpson EL , Bieber T , Guttman-Yassky E , Beck LA , Blauvelt A , Cork MJ , et al.; SOLO 1 and SOLO 2 Investigators . SOLO 1 and SOLO 2 Investigators. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016 Dec;375(24):2335–48. https://doi.org/10.1056/NEJMoa1610020
15. Blauvelt A , de Bruin-Weller M , Gooderham M , Cather JC , Weisman J , Pariser D , et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017 Jun;389(10086):2287–303. https://doi.org/10.1016/S0140-6736(17)31191-1
16. de Bruin-Weller M , Thaçi D , Smith CH , Reich K , Cork MJ , Radin A , et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFÉ). Br J Dermatol. 2018 May;178(5):1083–101. https://doi.org/10.1111/bjd.16156
17. Worm M , Simpson EL , Thaçi D , Bissonnette R , Lacour JP , Beissert S , et al. Efficacy and Safety of Multiple Dupilumab Dose Regimens After Initial Successful Treatment in Patients With Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2020 Feb;156(2):131–43. https://doi.org/10.1001/jamadermatol.2019.3617
18. Deleuran M , Thaçi D , Beck LA , de Bruin-Weller M , Blauvelt A , Forman S , et al. Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study. J Am Acad Dermatol. 2020 Feb;82(2):377–88. https://doi.org/10.1016/j.jaad.2019.07.074
19. Beck LA , Thaçi D , Deleuran M , Blauvelt A , Bissonnette R , de Bruin-Weller M , et al. Dupilumab Provides Favorable Safety and Sustained Efficacy for up to 3 Years in an Open-Label Study of Adults with Moderate-to-Severe Atopic Dermatitis. Am J Clin Dermatol. 2020 Aug;21(4):567–77. https://doi.org/10.1007/s40257-020-00527-x
20. Thaçi D , L Simpson E , Deleuran M , Kataoka Y , Chen Z , Gadkari A , et al. Efficacy and safety of dupilumab monotherapy in adults with moderate-to-severe atopic dermatitis: a pooled analysis of two phase 3 randomized trials (LIBERTY AD SOLO 1 and LIBERTY AD SOLO 2). J Dermatol Sci. 2019 May;94(2):266–75. https://doi.org/10.1016/j.jdermsci.2019.02.002
21. Duxipent® (Dupilumab), product information for healthcare professionals, status of information: September 2020, www.swissmedicinfo.ch, last accessed: 07 January 2021.
22. Castro M , Corren J , Pavord ID , Maspero J , Wenzel S , Rabe KF , et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N Engl J Med. 2018 Jun;378(26):2486–96. https://doi.org/10.1056/NEJMoa1804092
23. Bachert C , Han JK , Desrosiers M , Hellings PW , Amin N , Lee SE , et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019 Nov;394(10209):1638–50. https://doi.org/10.1016/S0140-6736(19)31881-1
24. Hirano I , Dellon ES , Hamilton JD , Collins MH , Peterson K , Chehade M , et al. Efficacy of Dupilumab in a Phase 2 Randomized Trial of Adults With Active Eosinophilic Esophagitis. Gastroenterology. 2020 Jan;158(1):111–122.e10. https://doi.org/10.1053/j.gastro.2019.09.042
25. Ariëns LF , van der Schaft J , Bakker DS , Balak D , Romeijn ML , Kouwenhoven T , et al. Dupilumab is very effective in a large cohort of difficult-to-treat adult atopic dermatitis patients: first clinical and biomarker results from the BioDay registry. Allergy. 2020 Jan;75(1):116–26. https://doi.org/10.1111/all.14080
26. Faiz S , Giovannelli J , Podevin C , Jachiet M , Bouaziz JD , Reguiai Z , et al.; Groupe de Recherche sur l’Eczéma aTopique (GREAT), France . Effectiveness and safety of dupilumab for the treatment of atopic dermatitis in a real-life French multicenter adult cohort. J Am Acad Dermatol. 2019 Jul;81(1):143–51. https://doi.org/10.1016/j.jaad.2019.02.053
27. Olesen CM , Holm JG , Nørreslet LB , Serup JV , Thomsen SF , Agner T . Treatment of atopic dermatitis with dupilumab: experience from a tertiary referral centre. J Eur Acad Dermatol Venereol. 2019 Aug;33(8):1562–8. https://doi.org/10.1111/jdv.15609
28. Bakker DS , Ariens LF , van Luijk C , van der Schaft J , Thijs JL , Schuttelaar ML , et al. Goblet cell scarcity and conjunctival inflammation during treatment with dupilumab in patients with atopic dermatitis. Br J Dermatol. 2019 May;180(5):1248–9. https://doi.org/10.1111/bjd.17538
29. Jun I , Kim BR , Park SY , Lee H , Kim J , Kim EK , et al. Interleukin-4 stimulates lipogenesis in meibocytes by activating the STAT6/PPARγ signaling pathway. Ocul Surf. 2020 Oct;18(4):575–82. https://doi.org/10.1016/j.jtos.2020.04.015
30. Becmeur PH , Abry F , Bourcier T , Meyer N , Sauer A ; « the French Study Group for Contact Lens-Related Microbial Keratitis » . Facteurs de risque de kératites infectieuses chez les porteurs de lentilles de contact, une étude cas-témoins [Risk factors for contact lens-related microbial keratitis: A multicenter case-control study]. J Fr Ophtalmol. 2017 Mar;40(3):224–31. https://doi.org/10.1016/j.jfo.2016.10.008
31. Duxipent® (dupilumab), Federal Office of Public Health (FOPH), Spezialitätenliste. Available at: www.spezialitaetenliste.ch [last accessed: 2021 January 07].