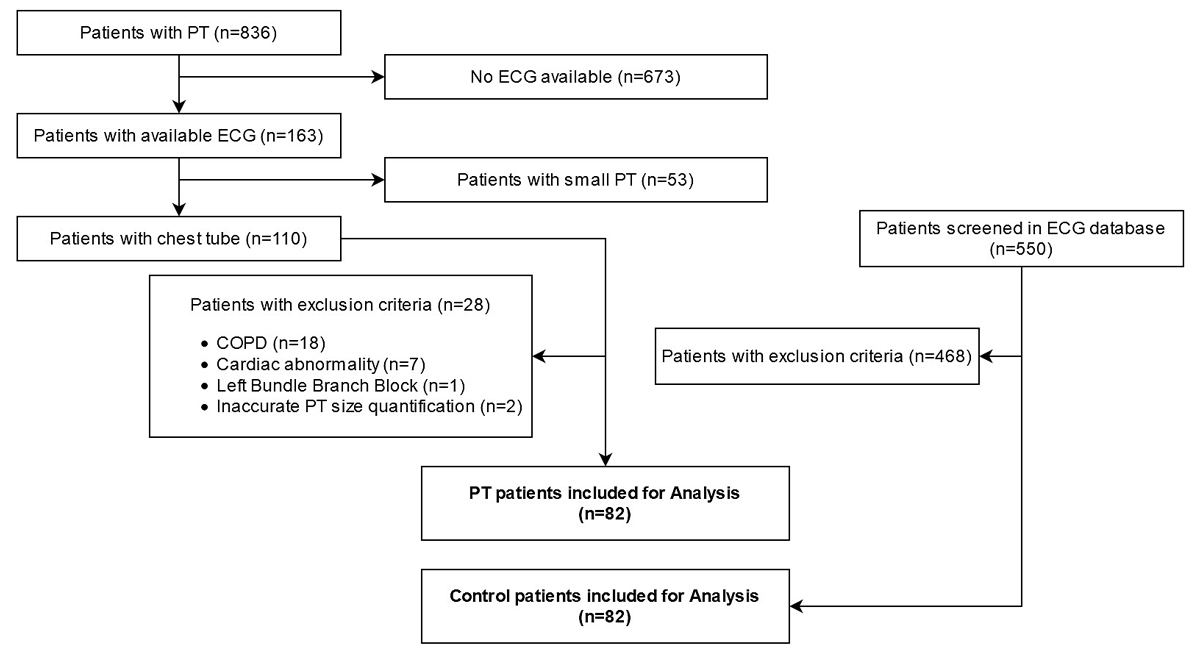
Figure 1 Flowchart of patient selection for the pneumothorax (PT) group. COPD = chronic obstructive pulmonary disease.
DOI: https://doi.org/10.4414/SMW.2021.w30041
Pneumothorax is defined as collapse of the lung due to a collection of air in the pleural cavity between the parietal pleura and the lung. It may be spontaneous or traumatic. Pathological findings as to the cause on conventional chest radiography are absent in primary spontaneous pneumothorax, although small blebs and bullae are often found on computed tomography (CT). Underlying pathological findings in the lungs are found in secondary spontaneous pneumothorax. Traumatic pneumothorax is caused by lesions of the thoracic wall, or lesions of the lung or the pleura via the airways. The incidence of spontaneous pneumothorax ranges from 14.1–22.7/100,000 with a male to female ratio of 3.3:1 [1, 2]. Traumatic pneumothorax is found in about 20% of all chest injuries [3].
The diagnosis is confirmed by imaging. The electrocardiogram (ECG) as a diagnostic tool is not mentioned in the diagnostic algorithms for pneumothorax in any guidelines [4]. However, it is mentioned in “chest pain” guidelines [5] and a door-to-ECG time of 10 minutes is recommended by the American Heart Association [6].
Numerous ECG alterations due to pneumothorax have been reported over the last century. One of the first case series on ECG alterations was published in 1928 and showed changes of the QRS axis with nonspecific alterations in repolarisation [7]. Subsequently, many other alterations were reported, mostly in case reports. Defining and recognising these signs in patients entering the emergency department (ED) with chest pain or dyspnoea could lead to immediate imaging, potentially avoiding delayed treatment of the pneumothorax.
The objective of this study was to collect all ECG alterations in pneumothorax patients and perform a systematic literature review to establish the diagnostic accuracy of these alterations in a large case-control series of patients, retrospectively assessed from our hospital database.
A literature search of the electronic PubMed/MEDLINE and EMBASE databases was carried out from inception to 18 September 2019 to identify literature concerning ECG alterations and pneumothorax. A search algorithm was established using a combination of the following terms: pneumothorax AND (ECG OR electrocardiogram OR electrocardiographic) (see the appendix). The local ethics committee approved the study (EKOS 16/093).
The electronic medical records and ECG database at our institution were retrospectively searched for patients leaving the emergency department (ED) with a final diagnosis of pneumothorax (cases) between 01 January 2011 and 31 December 2015. All patients treated with chest tube insertion and an ICD-code of spontaneous tension pneumothorax (J93.0), other spontaneous pneumothorax (J93.1), other pneumothorax and air leak (J93.8), pneumothorax unspecified (J93.9) or traumatic pneumothorax (S27.0) were included if they had a 12-lead ECG recorded at ED admission. Exclusion criteria were: patients without placement of a chest tube, patients with an imaging modality not allowing quantification of pneumothorax size (ultrasound diagnosis, chest X-ray in the supine position, or a chest CT scan with large slices), patients with known or acute heart or lung diseases, phrenic nerve palsy, chest deformities, prior lung resection, left bundle branch block, severe arrhythmias and pacemakers. Control patients were retrieved as follows: for each pneumothorax patient, we searched on the ECG database of the hospital for a sex- and age-matched patient in the same year. The age match started from 1 January of the year of birth of the pneumothorax patient, continuing sequentially to subsequent days until a patient with an ECG in the same year was found. This patient was finally screened using the same exclusion criteria as for the pneumothorax cohort. We defined a single control patient for each pneumothorax patient only – if a control patient was already included by the matching process, the next one identified following the same rules was chosen. In addition, we searched for follow-up ECGs in all pneumothorax patients after recovery of the pneumothorax. All ECGs were taken immediately after ED admission before chest tube insertion.
ECGs of all case and control patients were systematically examined by one emergency physician (BM) and an experienced electrophysiologist (PA) unaware of the clinical diagnosis of the patient. Every ECG was assessed for the presence of the ECG alterations retrieved by the literature search. From this review we defined 17 ECG signs to be analysed (see supplementary table A3 in the appendix). In the case of different interpretations of an ECG, the final diagnosis was made by consensus between both physicians.
The diagnosis of pneumothoraxwas based on chest X-ray or chest CT. The size of the pneumothorax on the chest X-ray was calculated using the Collin’s formula [8]. The size of pneumothorax on the chest CT scan was calculated by radiologists unaware of the ECG findings using volumetry per manual segmentation of the slices. The size was calculated as a percentage of the hemithorax volume.
Sensitivity and specificity for pneumothorax were calculated for each ECG sign. Subgroup analyses were made for the location, gender, aetiology (trauma vs non-trauma) and size of the pneumothorax. A separate subgroup consisting of pneumothorax patients with follow-up ECGs was analysed to describe the evolution of ECG changes. The McNemar test was used to establish statistically significant differences between proportions in the paired data, and the chi-square test was used for unpaired data. Continuous paired variables were compared using the Wilcoxon signed-rank test or paired t-test as appropriate. Continuous unpaired data were compared using Student’s t-test or the Mann-Whitney test as appropriate. To compare the prevalence of the ECG signs in the subgroups, the Z score was used. We defined a level of significance of 0.05. Point biserial correlation was used to assess any correlation between the presence/absence of ECG signs and pneumothorax size. Cohen’s kappa was used to test interobserver agreement of the ECG signs. Continuous data are presented as mean ± standard deviation or median with interquartile range as appropriate. Categorical data are presented as percentages. The IBM SPSS Version 25.0 software package was used for the statistical calculation.
We identified 836 patients with a final diagnosis of pneumothorax, of whom 82 included for the analysis. Ultimately, 82 age- and sex-matched controls were retrieved. The patient selection pathway is presented in figure 1.

Figure 1 Flowchart of patient selection for the pneumothorax (PT) group. COPD = chronic obstructive pulmonary disease.
Baseline characteristics of the pneumothoraxpatients and controls are presented in table 1. There was no case of tension pneumothoraxor documented cardiac contusion/lesion in traumatic pneumothorax patients included into the study.
Table 1Baseline characteristics of the study population.
| Cases (n = 82) | Controls (n = 82) | |
| Age (years), median (IQR) | 53 (29.25–69.75) | 53 (29.25–69.75) |
| Sex, n (%) | ||
| – Male | 58 (69.9) | 58 69.9) |
| – Female | 24 (29.3) | 24 (29.3) |
| Site of pneumothorax, n (%) | ||
| – Left | 36 (43.9) | – |
| – Right | 46 (56.1) | – |
| Clinical characteristics of PT, n (%) | ||
| – Spontaneous | 35 (42.7) | – |
| – Traumatic | 47 (57.3) | – |
| Size, % | 37 ± 32 | – |
IQR = interquartile range; PT = pneumothorax
The prevalence (sensitivity) and specificity according to the different ECG signs are given in table 2.
Table 2Sensitivity and specificity for the presence of pneumothorax for different ECG signs and point biserial correlation with pneumothorax and size.
| ECG sign | Prevalence in cases / sensitivity (%, n = 82) | Prevalence in controls (%, n = 82) | Specificity (%, n = 82) | p-value for difference in proportions | Point biserial correlation on PT size (Rpb) | Interobserver agreement (Cohen’s kappa) |
| Phasic QRS voltage | 25.6 | 1.2 | 98.8 | <0.001 | +0.3, p = 0.01 | 1.0 |
| Right axis deviation | 14.6 | 3.6 | 96.3 | 0.039 | 0, p = 0.57 | 1.0 |
| Left axis deviation | 7.3 | 4.9 | 95.1 | 0.683 | −0.1, p = 0.24 | 1.0 |
| Incomplete RBBB | 13.4 | 11.0 | 89.0 | 0.803 | −0.1, p = 0.43 | 0.8 |
| RBBB | 4.9 | 0 | 98.8 | 0.371 | 0, p = 0.80 | 1.0 |
| P pulmonale | 14.6 | 0 | 100 | 0.001 | +0.3, p <0.01 | 0.9 |
| P-wave inversion in lead I | 15.8 | 0 | 100 | <0.001 | +0.5, p <0.01 | 1.0 |
| Baseline shift with P pulmonale | 17.1 | 0 | 100 | <0.001 | +0.4, p <0.01 | 0.9 |
| T-wave inversion | 31.7 | 2.4 | 97.6 | <0.001 | +0.4, p <0.01 | 0.9 |
| Low QRS voltage | 8.5 | 4.9 | 95.1 | 0.547 | 0, p = 0.97 | 0.8 |
| QRS voltage ratio aVF/I >2 | 41.5 | 22.0 | 79.3 | 0.015 | +0.2, p = 0.03 | 1.0 |
| ST segment elevation | 18.3 | 14.6 | 85.4 | 0.628 | +0.2, p = 0.16 | 0.9 |
| ST segment depression | 1.2 | 0 | 100 | 1.000 | −0.1, p = 0.25 | 1.0 |
| S <1.2 mV in V2 | 57.3 | 62.2 | 37.8 | 0.596 | −0.1, p = 0.54 | 1.0 |
| S <0.9 mV in V3 | 46.3 | 53.7 | 46.3 | 0.511 | 0, p = 0.74 | 1.0 |
| Prolonged QTc | 11.0 | 2.4 | 97.6 | 0.046 | −0.1, p = 0.28 | 1.0 |
| Baseline shift with ST elevation (“spiked helmet sign”) | 0 | 0 | 100 | Not available | Not available | 1.0 |
CI = confidence interval; ECG = electrocardiogram; PT = pneumothorax; RBBB= right bundle branch block (Brugada pattern was searched for and not found in any of the cases).
The p-value for difference in proportions is given by McNemar (odds-ratio). Interobserver agreement is given with Cohen’s kappa.
The most frequent ECG signs seen in pneumothorax patients were S reduction in V2 or V3 and T-wave inversion, which was most often visible in lead aVL (19 of 26 cases, 73.1%). The prevalence of these ECG signs was similar in the control patients. Seven of the 17 analysed ECG signs had a specificity of >98%, although sensitivity was low for these signs. Prevalence differed significantly between the cases and controls for phasic QRS voltage, T-wave inversion, right axis deviation and aVF/I QRS voltage ratio >2 (table 2). Four good examples of ECG signs associated with pneumothorax with resolution in a follow-up ECG are shown in figure 2.
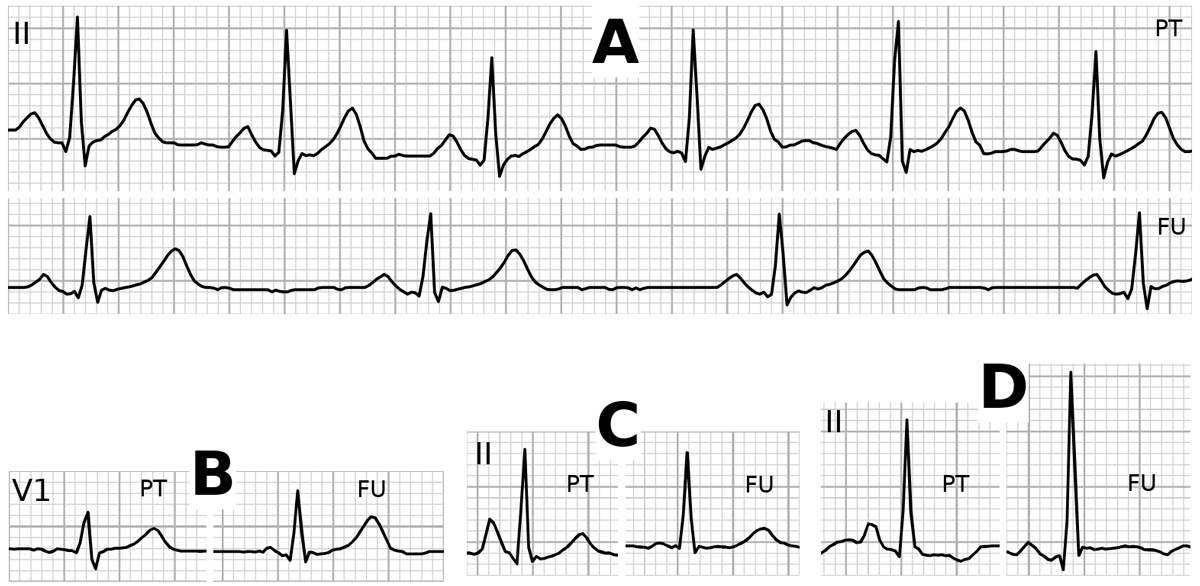
Figure 2 Examples of positive ECG signs by pneumothorax (PT) with resolution at follow-up (FU) in the same patient. A. Lead II with phasic QRS voltage changes. B. Lead V1 with P-wave inversion. C. Lead II with P pulmonale. D. Lead II with baseline shift before the P-wave onset.
P-wave inversion in lead I was more frequent in right-sided pneumothorax (25.0 vs 5.6%, p = 0.024), whereas a baseline shift with P pulmonale (27.8 vs 9.1%, p = 0.023), the S <1.2 mV in V2 (72.2 vs 43.2%, p = 0.016), and the S <0.9 mV in V3 (61.1 vs 36.9%, p = 0.030) were more frequent with left-sided pneumothorax. Prevalence did not differ significantly between left- and right-sided pneumothorax for any of the other signs.
Incomplete right bundle branch block was significantly more frequent in males (18.9 vs 0%, p = 0.022), whereas P pulmonale was more frequent in females (20.8 vs 12.1%, p <0.001).
There were several differences between trauma vs non-trauma patients. Phasic voltage ECG (p = 0.01), P pulmonale (p = 0.028), P inversion in I (p = 0.035), baseline shift with P pulmonale (p = 0.003), T-wave inversion (p = 0.005), aVF/I >2 (p <0.001), and ST elevation (p = 0.008) were more frequent with spontaneous pneumothorax, whereas left axis shift (p = 0.028) and long QTc (p = 0.423) were more frequent in traumatic pneumothorax. Mean size of the pneumothorax in the non-trauma group was 56.7% vs 22.8% in the trauma group (p <0.001).
The prevalence of a positive ECG sign correlated statistically significantly with pneumothorax size for phasic QRS voltage, aVF/I QRS voltage ratio >2, T wave inversion, P pulmonale, baseline shift with P pulmonale, and P inversion in lead I.
Twenty-one pneumothorax patients had a follow-up ECG a median of 87 days (IQR 19.5–571.5) after the initial ECG. The prevalence, evolution of an initial positive EGC sign and the specificity in this subgroup is presented in figure 3. In patients with a left-sided pneumothorax, there was a significant increase in R-wave amplitude in left-sided ECG leads after resolution of the pneumothorax (p = 0.0025 for V5 and p = 0.036 for V6), whereas the increase in R-wave amplitude in these leads was not statistically significant in patients with right-sided pneumothorax (p = 0.94 for V5 and p = 0.26 for V6).
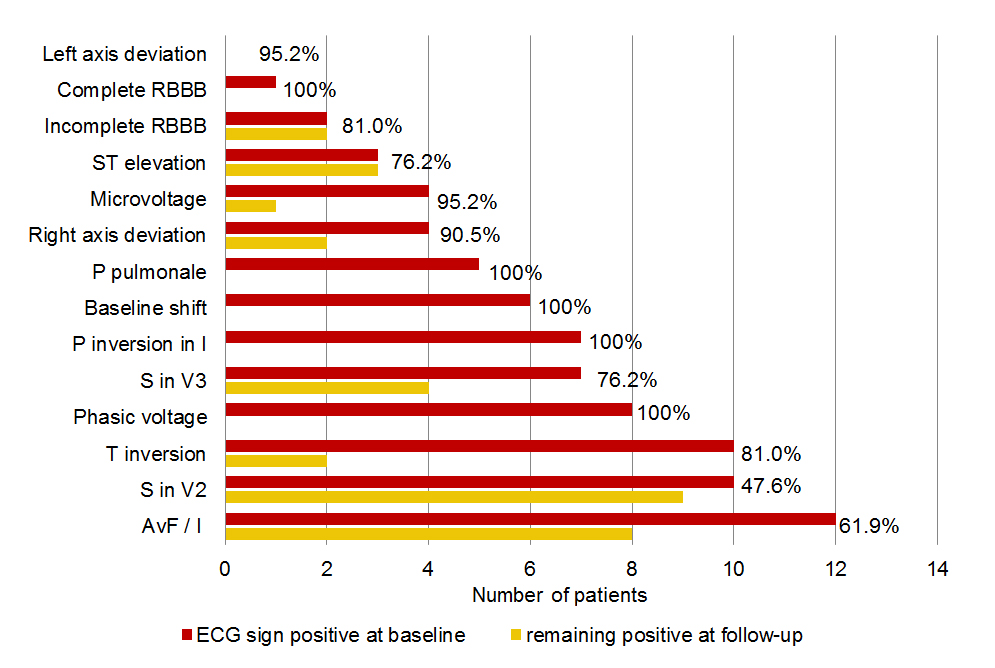
Figure 3 Prevalence (number of patients) and evolution of positive ECG signs in 21 patients with pneumothorax and a follow-up ECG. The number at the end of the bars denotes the specificity for the ECG sign in this subgroup of 21 patients.
Different ECG alterations in acute pneumothorax have been published over past decades. QRS phasic voltage, T-wave inversion, QRS right axis deviation, aVF/I QRS voltage ratio >2 and QTc prolongation were the ECG signs that were significantly more frequent in our pneumothorax patients than in controls.
Only one third of our pneumothorax patients showed T-wave inversion on the ECG. However, this finding showed the strongest prevalence with pneumothorax. In contrast to our findings, in a study published by Krenke et al. [9], only 12.2% of pneumothorax patients showed T-wave inversions, 6.1% of patients in V2 to V6 (mostly V2), 3.6% in lead III, and only one patient (2.5%) in lead aVL. This is in contrast to a study by Kurisu et al., who analysed 10 patients with left-sided pneumothorax [10]. None of this group had T-wave inversion in the precordial leads. Unfortunately Kurisu did not analyse T-wave inversions in the limb leads. One of the ECG alterations most specific for pneumothorax in our study was phasic QRS voltage (or electrical alternans), which is associated with pericardial effusion and atrioventricular junctional tachycardias with an accessory pathways also [11]. Unfortunately, there is not a clear definition of “alternans” in the literature. Kurisu defined it as a variation of the R wave amplitude of at least 5 mm in at least one lead, whereas Huang defined it as phasic voltage variations of the R wave of over 2 mm in lead II [12]. Other authors described only the pattern without a metrical definition. In our cohort, we decided to use a variation in the R wave amplitude of at least 3 mm in at least one lead, as the best compromise between sensitivity and specificity.
The prevalence of the QRS voltage ratio avF/I >2 in our study was only 41.5%, as opposed to the 100% found by Kurisu. The reason for this difference is unclear.
In a series described by Huang, the combination of three signs (reduction in S-wave in V2 and V3, and phasic QRS voltage) was found only in a group with large pneumothoraxes (light index >20%). Krenke et al. also showed that the number of ECG signs predicting pneumothorax was associated with the size of the pneumothorax. The number of positive ECG signs and pneumothorax size were not directly compared in all of these studies.
QTc prolongation was reported by Athanasopoulos et al. [13], who described QTc prolongation in 11 pneumothorax patients, although without findings for the prevalence of this sign.
The mechanisms of ECG alterations are discussed in many studies and seem to have multiple explanations. Cardiac displacement (downward and posterior) and (clockwise) rotation around the long axis seem to be responsible for many ECG alterations [12]. In addition, Feldmann [14] and Littmann [15] described T-wave inversion and QRS depression in pneumothorax patients in the supine position, demonstrating a “geometrical” relation between a pneumothorax and ECG alterations. They postulated an “insulation effect” of retrosternal free air, which might contribute to these changes. It is believed that phasic QRS voltage alteration is caused by the same displacement (in particular downward), emphasising the respirophasic pendular motion of heart [16]. Additionally, Huang et al. [12] hypothesised a lack of the parenchymal lung support in pneumothorax, which lets the heart “swing” within the respiratory cycle. Furthermore, right axis deviation is associated with right ventricular strain due to the collapsed lung, resulting in increased pulmonary resistance [17, 18]. Consequently reduced venous return and hypotension causing tachycardia and ischaemia might be another potential mechanism of ST segment alterations [19, 20].
Athanasopoulos et al. [13] hypothesised that autonomic imbalance could play a role in QTc prolongation and that epicardial stretch might result in the “spiked helmet sign” [21, 22]. In addition, takotsubo cardiomyopathy due to acute pneumothorax has been described in the literature, and increased sympathetic activation is discussed as an underlying pathophysiological concept, which again might cause ECG changes (e.g., tachycardia, QTc prolongation and ST segment alterations) seen in pneumothorax patients[23]. The mechanisms leading to T-wave inversion in patients with a pneumothorax are unknown. However, it is tempting to speculate as to whether a pneumothorax might cause ischaemia of the inner layers of the myocardium due to intrathoracic pressure shifts or catecholaminergic stress. The fact that so-called “false positive” elevation of cardiac troponins has been observed in pneumothorax patients [24] and that T-wave changes are more frequent in more severe pneumothorax might support this hypothesis.
Our study has several limitations. Because of the retrospective design, we were not able to test the published ECG alterations in our individual pneumothorax patients before and after treatment of pneumothorax, because most of the patients had no follow-up ECG. Analysis of pneumothorax in patients without lung and/or heart pathologies resulted in patients with traumatic or primary spontaneous pneumothorax only. However, it is possible that some patients had unknown heart/lung pathologies. This might reduce the power of ECG alterations for pneumothorax diagnosis only, and might somewhat reduce their specificity. Many patients with pneumothorax had to be excluded because no ECG has been recorded before pneumothorax treatment for a variety of reasons, but most possibly because an ECG has not been established as a tool to search for pneumothorax routinely. The study period goes back to 2015 based on an old database search. Because of the results presented, we decided to not update the study period, because we are sure that this would not have changed our results substantially. The choice of selecting only patients with chest tube insertion was also made because this was initially defined as an indirect measure of a clinically relevant pneumothorax size. Since cardiac troponins were not measured routinely in all pneumothorax patients, we cannot exclude that ECG alterations in traumatic pneumothorax could in part be due to heart contusion too. The small collective may have also contributed to statistical bias.
Taken together, we showed that the specificity for many of the listed ECG abnormalities is high, whereas the sensitivity is low. Thus, ECG signs are not suitable as a screening tool for pneumothorax. However, the presence of positive ECG signs discussed above might raise a suspicion of pneumothorax in patients presenting with dyspnoea or unclear chest discomfort in the ED, leading to early imaging and, in the case of confirmation, early treatment of pneumothorax.
This research received no external funding.
The authors declare that there is no conflict of interest regarding the publication of this paper.
1. Bobbio A , Dechartres A , Bouam S , Damotte D , Rabbat A , Régnard JF , et al. Epidemiology of spontaneous pneumothorax: gender-related differences. Thorax. 2015 Jul;70(7):653–8. https://doi.org/10.1136/thoraxjnl-2014-206577
2. Hallifax RJ , Goldacre R , Landray MJ , Rahman NM , Goldacre MJ . Trends in the Incidence and Recurrence of Inpatient-Treated Spontaneous Pneumothorax, 1968-2016. JAMA. 2018 Oct;320(14):1471–80. https://doi.org/10.1001/jama.2018.14299
3. Kulshrestha P, Munshi I, Wait R. Profile of chest trauma in a level I trauma center. J Trauma. 2004 Sep;57(3):576–81.
4. MacDuff A, Arnold A, Harvey J, BTS Pleural Disease Guideline Group. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010 Aug;65 Suppl 2:ii18-31.
5. Smeeth L, Skinner JS, Ashcroft J, Hemingway H, Timmis A. NICE clinical guideline: chest pain of recent onset. Br J Gen Pract. 2010 Aug 1;60(577):607–10.
6. Atzema CL, Austin PC, Tu JV, Schull MJ. Effect of time to electrocardiogram on time from electrocardiogram to fibrinolysis in acute myocardial infarction patients. CJEM. 2011 Mar;13(2):79–89.
7. Master AM. The electrocardiographic changes in pneumothorax in which the heart has been rotated: The similarity of some of these changes to those indicating myocardial involvement. American Heart Journal. 1928 Apr 1;3(4):472–83.
8. Collins CD, Lopez A, Mathie A, Wood V, Jackson JE, Roddie ME. Quantification of pneumothorax size on chest radiographs using interpleural distances: regression analysis based on volume measurements from helical CT. American Journal of Roentgenology. 1995 Nov 1;165(5):1127–30.
9. Krenke R, Nasilowski J, Przybylowski T, Chazan R. Electrocardiographic changes in patients with spontaneous pneumothorax. J. Physiol. Pharmacol. 2008 Dec;59 Suppl 6:361–73.
10. Kurisu S, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Nakama Y. Electrocardiographic findings in left-sided pneumothorax. Am J Emerg Med. 2008 Oct;26(8):959–62.
11. Goyal M, Woods KM, Atwood JE. Electrical alternans: a sign, not a diagnosis. South. Med. J. 2013 Aug;106(8):485–9.
12. Huang S-C, Lin G-M, Li Y-H, Lin C-S, Kao H-W, Han C-L. Abnormal Changes of a 12-Lead Electrocardiogram in Male Patients with Left Primary Spontaneous Pneumothorax. Acta Cardiol Sin. 2014 Mar;30(2):157–64.
13. Athanasopoulos C, Childers R. Q-T prolongation in acute pneumothorax. Acta Cardiol. 1979;34(2):85–93.
14. Feldman T, January CT. ECG changes in pneumothorax. A unique finding and proposed mechanism. Chest. 1984 Jul;86(1):143–5.
15. Littmann D. Electrocardiographic phenomena associated with spontaneous pneumothorax and mediastinal emphysema. Am. J. Med. Sci. 1946 Dec;212(6):682–90.
16. Silverberg C, Kingsland R, Feldman D. Electrocardiographic changes in pulmonary collapse; artificial and spontaneous left-sided pneumothorax studied by conventional and unipolar methods. Dis Chest. 1950 Feb;17(2):181–9.
17. Ruhela M, Khandelwal G, Gupta S, Bansal A. Acute right bundle branch block due to pneumothorax. J Family Med Prim Care. 2018 Oct;7(5):1126–8.
18. Saks MA, Griswold-Theodorson S, Shinaishin F, Demangone D. Subacute tension hemopneumothorax with novel electrocardiogram findings. West J Emerg Med. 2010 Feb;11(1):86–9. 19. Strizik B, Forman R. New ECG changes associated with a tension pneumothorax: a case report. Chest. 1999 Jun;115(6):1742–4.
20. Johnson P, Paccione R, Burwell J, Lo B. LBBB ASSOCIATED WITH SPONTANEOUS PNEUMOTHORAX: A CASE REPORT. Journal of Investigative Medicine. 2014 Feb 1;62:448–448.
21. Littmann L, Proctor P. Real time recognition of the electrocardiographic “spiked helmet” sign in a critically ill patient with pneumothorax. Int. J. Cardiol. 2014 May 15;173(3):e51-52.
22. Tomcsányi J, Frész T, Proctor P, Littmann L. Emergence and resolution of the electrocardiographic spiked helmet sign in acute noncardiac conditions. Am J Emerg Med. 2015 Jan;33(1):127.e5-7.
23. Abu Ghanimeh M, Bhardwaj B, Aly A, Baweja P. Takotsubo cardiomyopathy secondary to spontaneous right-sided pneumothorax. BMJ Case Rep. 2017 Mar 1;2017.
24. Chan W-H, Lin C-S, Yang S-P, Cheng S-M. ECG changes with elevated troponin I in a patient with tension pneumothorax. South. Med. J. 2009 Sep;102(9):969–71.
The appendix is available in the PDF version of this article.