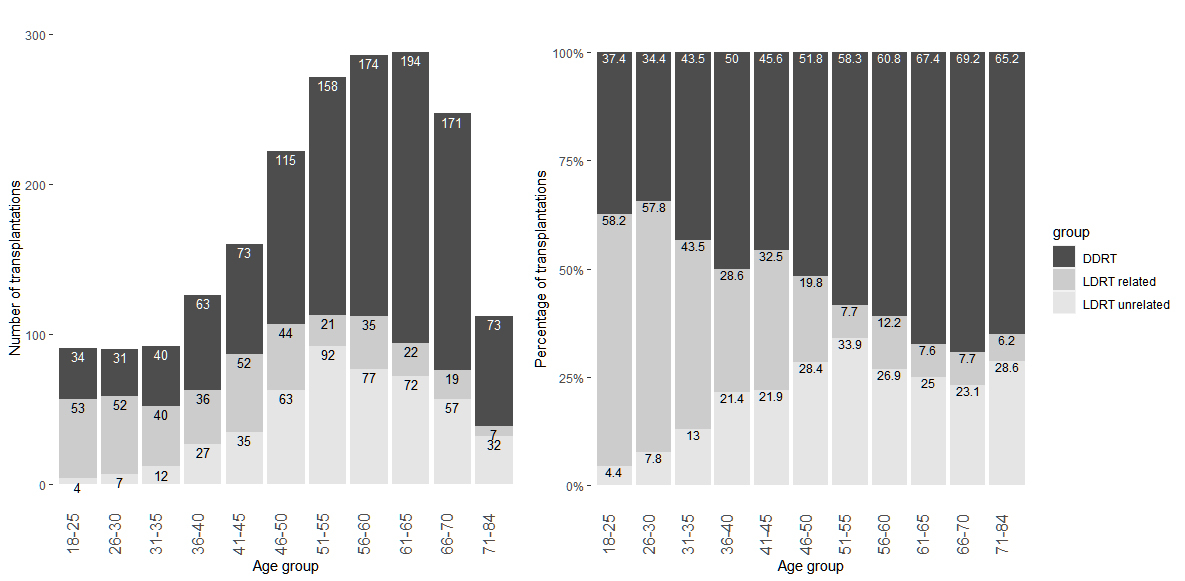
Figure 1 Number (left) and percentage (right) of deceased and living donors by age group of recipients, n = 1985. The group of living donors is further split into donor related (first-degree relatives) and unrelated donors.
DOI: https://doi.org/10.4414/smw.2021.20532
Living donor renal transplantation (LDRT) was introduced in Switzerland in the 1960s [1] and accounted for 32% of all renal transplantations in 2018; 47% of all kidney donors in 2018 were living donors [2], corresponding to 11.3 donations per million population (pmp) [3]. Northern European countries and the United Kingdom have similar rates, the Netherlands is much higher at 29.0 ppm, but most other European countries have lower rates of between 5 and 10 pmp [3]. LDRT has been shown to result in a lower allograft failure rate than transplantation from deceased donors (DDRT) [4–6] and a long-term systematic follow-up of donors from the Swiss Living Donor Health Registry [7] showed that the risks for a qualified donor are acceptable [1, 8]. The latter result is also supported by a British study that showed that medium-term morbidity and mortality outcomes of live donors in comparison with a healthy cohort suggest that live donation is not associated with excess mortality, end-stage renal disease or morbidity in at least 10 years of follow-up [9].
According to the Swiss transplantation law, living donors must fulfil the following criteria: at least 18 years old, fully informed, free of coercion and capable of giving written consent. Furthermore, a thorough somatic and psychological health assessment must show no serious health risk and a global health assessment compatible with kidney donation. There is no constraint in the law about the relationship between the living donor and the transplant candidate. The principles dealing with various issues of living donation assessment are discussed in depth by the Swiss Academy of Medical Science and published as medical guidelines [10]. These guidelines are binding for certified doctors in Switzerland. The law forbids any financial gain through living donation. However, the costs for the donors’ investigations before donation and for the health follow up after donation are covered by the mandatory health insurance of the recipient.
Transplant candidates who qualify as potential kidney recipients have to be registered on a national waiting list. Transplant candidates without a suitable living donor have to wait for a deceased donation. In 2018, median waiting time was 896 days, the annual mortality of candidates on the waiting list was 1.6% [2]. The waiting list encompassed 1518 candidates, 4.3 times the number of kidney transplantations performed in 2018 (n = 352). If more candidates could benefit from a living donor, the waiting list and waiting time could be reduced. Therefore analysing the psychosocial and demographic characteristics of transplant candidates who have not undergone LDRT in Switzerland could bring insight into the access to LDRT.
Several studies showed that candidates of older age [8, 11–13], lower education [11, 14, 15], lower income [11], not living in a committed relationship [14, 16] and not working [17] are less likely to undergo LDRT. These results do not necessarily hold for Switzerland as they possibly depend on the healthcare system, cultural beliefs and organisation of transplantation at a national level. The aim of this study was to verify whether these findings also apply to Switzerland. In addition, we examined whether further characteristics collected within the Swiss Transplant Cohort Study [18], a nationwide mandatory register for all solid organ transplantations in Switzerland, were associated with a lower incidence of LDRT. This includes the region of the transplant centre as a proxy for cultural attitudes towards LDRT and the way healthcare professionals in- and outside the transplant centres advocate living transplantation and the health status of the transplant candidate at the time of registering on the waiting list operationalized as quality of life and symptoms of depression.
The present study was a nationwide multi-centre study nested within the Swiss Transplant Cohort Study (STCS). Since May 2008, the STCS prospectively has enrolled and followed up all solid organ recipients undergoing transplants in Switzerland. For consenting patients, the STCS collects a large variety of clinical, psychosocial, infectious disease and bio-banking data. For non-consenting recipients, the STCS collects a minimal set of transplant outcome data by legal mandate. Recipients are prospectively followed up until death or drop out. Further details regarding data collection and cohort design can be found in Koller et al. [18, 19]. For the present study, 1985 patients with any first single or double kidney transplantation performed between May 2008 and December 2017 were eligible. Patients were excluded if they had transplantation of any organ other than kidney, a re-transplantation within the observation period or were aged less than 18 years. A total of 176 patients who met the inclusion criteria could not be included because they did not consent.
Transplant candidates are asked to fill in the STCS psychosocial questionnaire (PSQ) before transplantation, usually at the time of registering on the waiting list. The PSQ covers the EuroQuol EQ-5D-3L questionnaire [20], quality of life measured by visual analogue scale (EQ-VAS) [20], seven items of the hospital anxiety and depression scale related to symptoms of depression (HADS-Depression) [21] and further questions about education, relationship status, work capacity and, since August 2012, household income in CHF per month. These variables are further described by De Geest et al. [22]. Scores for EQ-5D-3L were calculated based on the time-trade-off (TTO) value set of France and on the EQ-5D VAS value set of Europe, as there was no value set available for Switzerland. Individuals showing depressive symptoms were identified based on the cut off values defined in the HADS-Depression scale manual [21]. Higher education was defined as having a qualification to enter university. Additional information such as the cause of the native kidney disease, comorbidities and results of standard clinical examinations were collected at the time of transplantation. For the region, the geographical location of the transplant centre was taken, thus four regions (German 1–4) represented the German- and two regions (French 1, 2) the French-speaking parts of Switzerland. There is no transplant centre in the Italian-speaking region of Switzerland, an area that accounts for 4.2% of the Swiss population. Thus candidates from the Italian region are sent to any of the German or French regions. Reference level for the analysis was set to German 1, the centre with the highest number of total renal transplantations.
The data collection and their further use for research purposes is covered by the Ethics approval of the STCS by the ‘Ethik Kommission beider Basel’ (EKBB 351/07).
The primary outcome of the study was the type of donation operationalised as living or deceased. Logistic regression was performed to find associations between a set of predefined covariates. This predefined set was then further reduced based on likelihood ratio chi-square tests, and an interaction term between covariate age and work capacity and a transformation of age to a categorical variable was assessed. Multi-collinearity was assessed by inspecting the design matrix and variance inflation factor GVIF(1/2Df) adjusted for the degree of freedom [23]. Confidence intervals (CIs) are based on the profile likelihood method and were compared with bootstrapped intervals (sample size 80% of total number of observations, sampling with replacement). Deviance residuals were inspected visually (R-library arm::binnedplot).
As 428 (21.6%) patients had at least one missing covariate, we used multiple imputation assuming a missing at random mechanism. Pooled estimates from 10 imputed data sets were generated using multivariate imputation by chained equation algorithm (R-library::mice) and Rubin’s method and compared with estimates using the complete dataset.
Some patients completed the PSQ questionnaire close to the transplant event. In some LDRT candidates, the transplant event might have already been scheduled at that time, which could influence the self-assessment of EQ-VAS or HADS-Depression score. Therefore, to assess reliability of the results, we repeated the logistic regression on two subgroups: one with candidates who had completed the questionnaire at least 6 months before transplantation and a second group with candidates who had completed the questionnaire at least 30 days before transplantation. In another sensitivity analysis, we examined whether the estimates for the covariate work capacity change when only working-age individuals were included, hence restricting the analysis to candidates of age between 18 and 65 years.
All analysis were performed using R Statistical Software Version 3.3.3 [24].
In Switzerland, between May 2008 and December 2017, 2161 adults of age 18 or older underwent a first kidney only transplantation. A total of 1985 patients (91.9%) gave informed consent and were included in the present study. Of those, 1126 (56.7%) kidney transplants were from a deceased donor and 859 (43.3%) from a living donor (table 1, fig. 1). Among living donors, unrelated donors (not first-degree relatives) were slightly overrepresented (55.6%) compared with first-degree related donors (44.4%).
Table 1 Socioeconomic, demographic and health characteristics of renal transplant candidates by deceased (DDRT) and living (LDRT) donation.
| DDRT | LDRT | |
|---|---|---|
| Number of recipients | 1126 | 859 |
| Number of males (%) | 694 (61.6%) | 579 (67.4%) |
| Age at transplantation in years, median (IQR) | 58 (48–65.75) | 52 (40–61) |
| Region | ||
| German 1 | 344 (64.5%) | 189 (35.5%) |
| German 2 | 143 (60.3%) | 94 (39.7%) |
| German 3 | 101 (65.2%) | 54 (34.8%) |
| German 4 | 255 (51.6%) | 239 (48.4%) |
| French 1 | 174 (49.7%) | 176 (50.3%) |
| French 2 | 109 (50.5%) | 107 (49.5%) |
| Quality of life | ||
| Visual analogue scale (EQ-VAS), mean ± SD | 62.8 ± 20.73 | 62.04 ± 20.28 |
| EQ-5D-3L index score (TTO France), mean ± SD | 72.29 ± 28.57 | 76.11 ± 25.8 |
| EQ-5D-3L index score (VAS Europe), mean ± SD | 67.09 ± 20.77 | 70.46 ± 18.61 |
| HADS-Depression score, mean ± SD | 4.71 ± 3.83 | 3.85 ± 3.06 |
| No cases (score 0–7) | 777 (69%) | 653 (76%) |
| Mild cases (score 8–10) | 121 (10.7%) | 57 (6.6%) |
| Moderate cases (score 11–14) | 63 (5.6%) | 25 (2.9%) |
| Severe cases (score 15–21) | 24 (2.1%) | 4 (0.5%) |
| Missing | 141 (12.5%) | 120 (14%) |
| Higher education | ||
| Yes | 206 (18.3%) | 300 (34.9%) |
| No | 811 (72%) | 456 (53.1%) |
| Unknown | 109 (9.7%) | 103 (12%) |
| Relationship status | ||
| Living in a relationship | 634 (56.3%) | 574 (66.8%) |
| Formerly married | 209 (18.6%) | 71 (8.3%) |
| Single | 180 (16%) | 116 (13.5%) |
| Unknown | 103 (9.1%) | 98 (11.4%) |
| Income (CHF) | ||
| >9000 | 31 (2.8%) | 72 (8.4%) |
| 6001–9000 | 52 (4.6%) | 68 (7.9%) |
| 4501–6000 | 123 (10.9%) | 104 (12.1%) |
| <4500 | 301 (26.7%) | 132 (15.4%) |
| Refused | 78 (6.9%) | 60 (7%) |
| Unknown | 73 (6.5%) | 54 (6.3%) |
| Not recorded* | 468 (41.6%) | 369 (43%) |
| Work capacity of individuals aged 18-65 years | ||
| Number of individuals (% of all patients) | 828 (73.5%) | 721 (83.9%) |
| – Not working | 322 (28.6%) | 196 (22.8%) |
| – 1–50% | 207 (18.4%) | 126 (14.7%) |
| – >50 | 205 (18.2%) | 300 (34.9%) |
| – Missing | 94 (8.3%) | 99 (11.5%) |
| Reason for not working | ||
| – Housewife/man | 49 (15.2%) | 40 (20.4%) |
| – Illness/invalidity | 188 (58.4%) | 104 (53.1%) |
| – Other | 61 (18.9%) | 30 (15.3%) |
HADS = hospital anxiety and depression scale; IQR = interquartile range; SD = standard deviation * Income was recorded only since August 2012.

Figure 1 Number (left) and percentage (right) of deceased and living donors by age group of recipients, n = 1985. The group of living donors is further split into donor related (first-degree relatives) and unrelated donors.
Older candidates were less likely to undergo LDRT. In the multivariable logistic regression model including 1557 candidates with no missing values, the odds ratio (OR) for age per 10 years increase was 0.67 (95% CI 0.60–0.74, p <0.0001) (table 2). The proportion of LDRT recipients was highest in the age group 26–30 years (65.6%) and decreased steadily with increasing age (fig. 1). At younger age, living donors were most often first-degree relatives, whereas unrelated living donors were more prevalent in older patients. No difference was observed for gender (OR 1.06, 95% CI 0.83–1.35). The proportion of living donation recipients by region varied between 34.8% and 50.3%. In the logistic regression, the overall p-value for the covariate region was <0.0001. Odds ratios for the French regions and one German region, the transplant centres with the highest proportion of living donor transplantations, ranged between 1.86 and 1.96, compared with the reference category, German 1, the region with the highest number of transplantations.
Table 2 Odds ratios, 95% confidence intervals (CIs) of multivariable and univariate logistic regression with outcome living donor renal transplant on complete data set (n = 1558).
| Covariate | Level | Multivariable | Univariate | |
|---|---|---|---|---|
| Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | ||
| Intercept | 70.28 (29.25–172.52) | <0.0001 | ||
| Age at time of transplant (per 10 years) | 0.67 (0.60–0.74) | <0.0001 | 0.73 (0.67–0.79) | |
| Gender | Male vs female | 1.06 (0.83–1.35) | 0.6442 | 1.27 (1.03–1.57) |
| Relationship status | Formerly married vs living relationship | 0.37 (0.26–0.53) | <0.0001 | 0.37 (0.26–0.5) |
| Single vs living relationship | 0.38 (0.26–0.53) | <0.0001 | 0.73 (0.55–0.96) | |
| Higher education | No vs yes | 0.46 (0.36–0.59) | <0.0001 | 0.41 (0.33–0.52) |
| Working capacity | 1–50% vs >50% | 0.48 (0.35–0.66) | <0.0001 | 0.43 (0.32–0.57) |
| 0% vs >50% | 0.51 (0.38–0.67) | <0.0001 | 0.39 (0.31–0.5) | |
| Income CHF >6000 per month | No vs yes | 0.81 (0.57–1.14) | 0.2261 | 0.66 (0.49–0.9) |
| Unknown / not recorded vs yes | 0.87 (0.62–1.23) | 0.4269 | 0.74 (0.54–1) | |
| HADS-Depression score (per 5 points) | 0.61 (0.50–0.74) | <0.0001 | 0.68 (0.59–0.79) | |
| EQ-VAS (per 10 points) | 0.87 (0.81–0.93) | <0.0001 | 0.98 (0.93–1.03) | |
| Cardiopulmonary disease | Yes vs no | 0.95 (0.74–1.21) | 0.6572 | 0.61 (0.5–0.74) |
| Diabetes | Yes vs no | 0.98 (0.7–1.36) | 0.8829 | 0.64 (0.48–0.86) |
| Region | <0.0001 | |||
| German 2 vs German 1 | 1.28 (0.84–1.95) | 1.01 (0.69–1.47) | ||
| German 3 vs German 1 | 1.07 (0.69–1.66) | 1.02 (0.68–1.52) | ||
| German 4 vs German 1 | 1.96 (1.43–2.69) | 1.73 (1.31–2.28) | ||
| French 1 vs German 1 | 1.86 (1.32–2.62) | 1.66 (1.22–2.27) | ||
| French 2 vs German 1 | 1.89 (1.25–2.86) | 1.73 (1.2–2.49) | ||
HADS = hospital anxiety and depression scale For the multivariable model p-values are provided, for the categorical covariate region only the overall p-value.
The relationship status was a significant factor for having a LDRT. Not living in a relationship compared with living in a relationship decreased the odds for LDRT, for recipients formerly married to 0.37 (95% CI 0.26–0.53, p <0.0001) and for singles to 0.38 (95% CI 0.26–0.53, p <0.0001). Recipients without higher education, defined as not having a qualification to enter university, were less likely to undergo living donor transplantation (OR 0.46, 95% CI 0.36-0.59; p <0.0001). Work capacity was also a significant factor, recipients not working or working less than 50% were less likely to undergo living donor transplantation compared with recipients working more than 50% (OR 0.51, 95% CI 0.38–0.67; p <0.0001 and OR 0.48, 95% CI 0.35–0.66, respectively, p <0.0001). When the analysis was restricted to working-age recipients between the ages of 18 and 65, the OR decreased only slightly (ORs of 0.41 and 0.45, respectively) (supplementary table S1 in the appendix). Lower household income below CHF 6000 per month was not significantly associated with the likelihood of a LDRT (OR 0.81, 95% CI 0.57–1.14, p = 0.4269).
An elevated level of symptoms of depression, defined as a score above 7 on the HADS-Depression scale, was observed in 20.2% of DDRT recipients who answered the questionnaire, but only in 11.5% of the LDRT recipients (table 1); the median and interquartile range were correspondingly higher (fig. 2). In the multivariable logistic regression, the score was associated with a lower chance of having a living donor, the OR per 5 point increase was 0.61 (95% CI 0.50–0.74, p <0.0001).
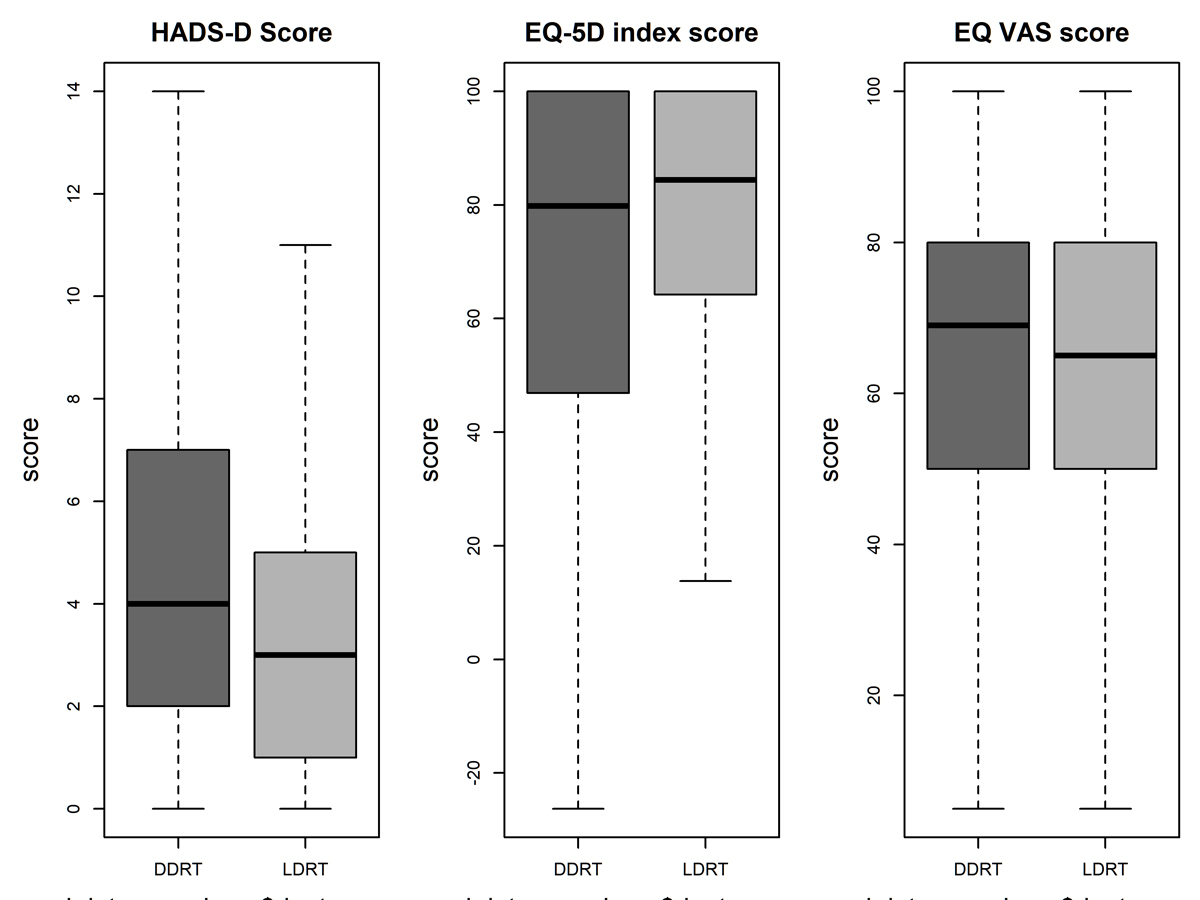
Figure 2 Boxplot for HADS–Depression score, quality of life EQ-5D-3L index score (value set time-trade-off France) and self-perceived quality of life measured by visual analogue scale EQ-VAS for DDRT and LDRT. The solid line represents the median, the hinges of the box the inter-quartile range.
HADS-Depression 0: no problems. EQ-5D and EQ VAS score 100: best possible state of health. DDRT = deceased donor renal transplant; HADS = hospital anxiety and depression scale; LDRT = living donor renal transplant
Self-perceived health measured by visual analogue scale (EQ-VAS) was similar in both groups, with a mean value of 62.04 (standard deviation [SD] 20.28) for LDRT and 62.80 (SD 20.73) for DDRT candidates (fig. 2). A value of 100 indicates ‘Best imaginable health state’ and 0 ‘Worst imaginable health state’. In the multivariable logistic regression, a higher EQ-VAS was associated with a lower chance of LDRT, the OR per 10 points increase was 0.87 (95%, CI 0.81–0.93, p <0.0001). However, this association was not significant when restricted to candidates who filled in the PSQ at least 30 days before the transplant (table S1), or after removal of HADS-Depression score and work capacity from the logistic regression model. An alternative measurement for the state of health, the EQ-5D index score based on the French time-trade-off value set, showed higher mean score for LDRT (76.11, SD 25.08) compared with DDRT (72.29, SD 28.57), mainly due to the fact that LDRT recipients reported less mobility problems (fig. 3). The fit of the logistic regression model did not improve when the EQ-5D index score was included, therefore this score was not included in the final model. Regarding other indicators that reflect the state of health, the proportion of candidates with diabetes or cardiopulmonary disease was lower in the LDRT group (12.1% vs 17.9%; 45.3% vs 56.0%), but not significantly in the multivariable analysis (table 2).
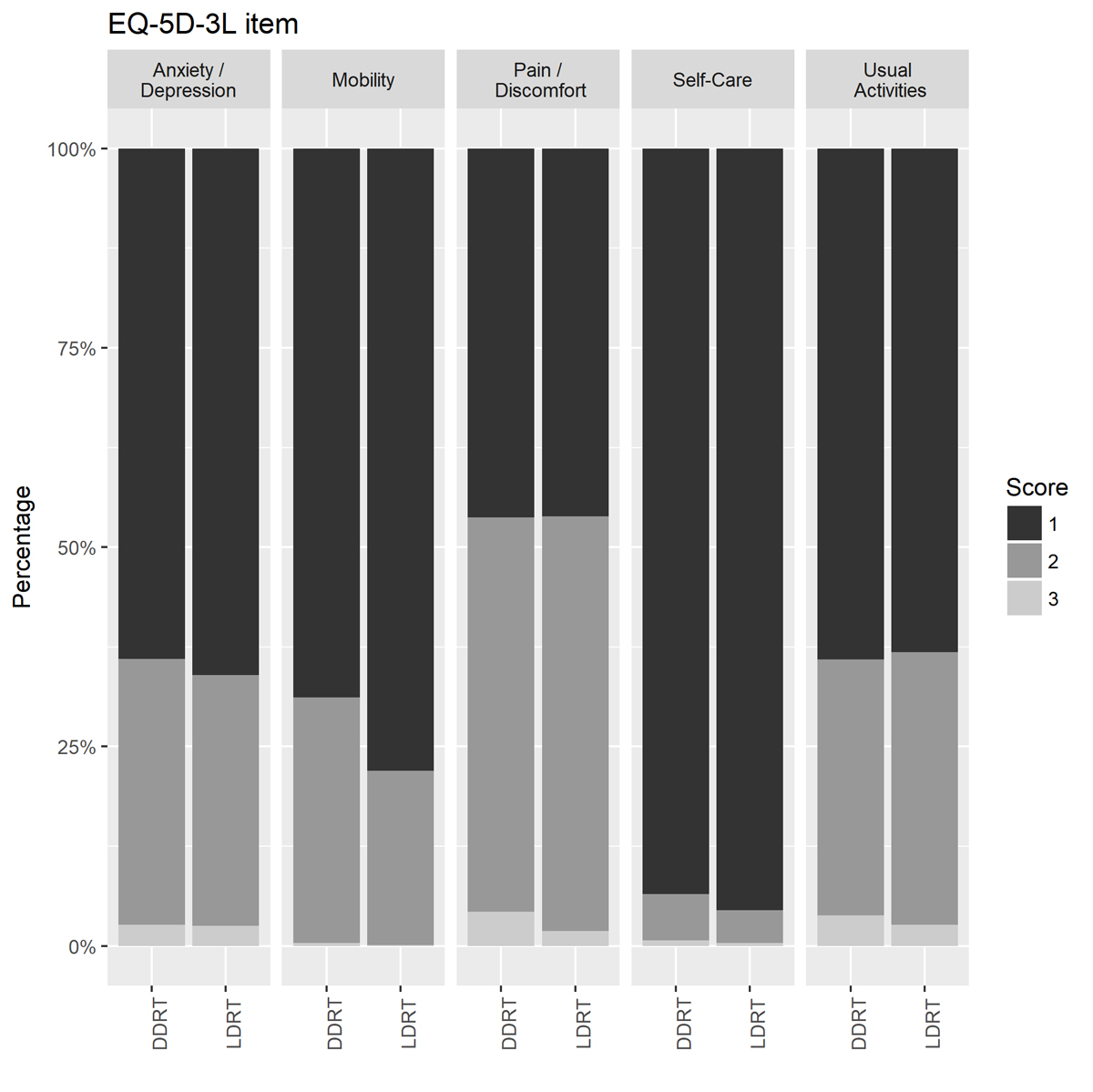
Figure 3 Bar plot of percentages of transplant recipients of EQ-5D-3L scores by item for 1780 DDRT and LDRT transplant candidates before transplantation. For 205 recipients response was missing. Score 1 = no problems, 2 = some problems, 3 = severe problems. DDRT = deceased donor renal transplant; LDRT = living donor renal transplant
Living donors were rarely more than 20 years younger than the recipient (3.4%), most donors were within 10 years (63.2%) or between 20 and 40 years older (18.5%). In the DDRT group 20.1% of donors were at least 20 years younger than the recipient, 44.4% at a similar age and 8.1% at least 20 years older. Regarding gender, 37.0% of LDRT donors were males, 40.1% of the related and 34.5% of the unrelated donors. In the DDRT group, male donors were more prevalent with 56.2%.
Accuracy for the final model rose from 56.5% for a model with no covariates to 70.2% after inclusion of the covariates, the area under the curve (c-index) was 74.5. Neither tests for multicollinearity of the covariates nor the binned Pearson residual plots to assess the assumptions for the logistic regression indicated a problem with the estimators of the model.
LDRT recipients filled in the PSQ much closer in time to the transplant event; the median was 15 days (IQR 3–157) versus 573 days (IQR 158–1091) for DDRT recipients. To assess whether this circumstance influenced the estimators, we applied the multivariable logistic regression on a subgroup of candidates who filled in the questionnaire at least 30 days or at least 180 days before transplantation. Results were similar except for EQ-VAS with OR close to 1 (fig. S3, table S1). Regarding missing information, the results based on an imputed dataset (table S1) did not contradict our findings on the complete dataset.
Transplant candidates who were older, less educated, less able to work and not in a committed relationship were less likely to undergo LDRT. Also, regional differences were observed. Income and gender were not associated with the type of donor. Candidates with higher symptoms of depression (HADS-Depression) or, unexpectedly, higher quality of life (EQ-VAS) were less likely to undergo LDRT.
Living donation is less frequent in recipients of older age, as already shown by several studies [11, 12, 14, 17]. With increasing age, potential donors such as siblings, parents and partners become older and their health decreases, thus fewer meet the criteria to donate. In addition, older transplant candidates are less in favour of accepting living donation [25], in particular from much younger donors such as their children [26]. This attitude is reflected in our data, as only 2.9% of living donors were more than 20 years younger than the recipient.
Since partners account for 33% of the living donations [7], living in a stable relationship increases the chance of having a living donor, as confirmed by our study. It also explains why females were more prominent among living donors (table 3). Most often, female spouses or partners are the living donors of male candidates and males are more frequent in the LDRT group (67.4%). Furthermore, HLA incompatibility can arise when a male living donor wants to donate to a female partner/spouse, as previous pregnancies may have immunised the female transplant candidate, further skewing the gender distribution. Female donors were also more common among first-degree relatives (58.8%), hence donation to a sibling, child or parent. However, the gender of the transplant candidates was not associated with LDRT; men were not more likely to receive a transplant from a living donor.
Table 3 Characteristics related to the transplant event of recipients by deceased (DDRT) and living (LDRT) donation.
| DDRT | LDRT | |
|---|---|---|
| Graft function | ||
| Delayed graft function | 209 (18.6%) | 15 (1.75%) |
| Primary non function | 13 (1.2%) | 0 (0%) |
| Dialysis | ||
| Days on dialysis, median (IQR) | 1238 (794–1794) | 377 (182–716) |
| Dialysis type | ||
| – Haemodialysis | 903 (80.2%) | 433 (50.41%) |
| – Peritoneal dialysis | 163 (14.5%) | 101 (11.76%) |
| – Pre-emptive | 58 (5.2%) | 324 (37.72%) |
| – Unknown | 0 (0%) | 0 (0%) |
| Donor | ||
| Number of mismatches, mean (IQR) | 3.97 (3–5) | 3.66 (3–5) |
| Number of ABO compatible patients (%) | 1126 (100%) | 719 (83.7%) |
| Age years, median (IQR) | 56 (43–65) | 54 (46, 61) |
| Number of males (%) | 633 (56.2%) | 318 (37.02%) |
| Number of patients with same sex as donor, (%) | 607 (53.9%) | 268 (31.2%) |
| Physiology | ||
| Systolic blood pressure (mmHg), mean ± SD | 142.01 ± 21.66 | 139 ± 19.51 |
| Diastolic blood pressure (mm Hg), mean ± SD | 80.89 ± 13.71 | 81.73 ± 13.03 |
| LDL cholesterol (mmol/l), mean ± SD | 2.36 ± 0.99 | 2.35 ± 1.03 |
| HDL cholesterol (mmol/l), mean ± SD | 1.31 ± 0.55 | 1.33 ± 0.55 |
| BMI (kg/m2), mean ± SD | 27.55 ± 19.43 | 26.36 ± 15.53 |
| Aetiology | ||
| Glomerulonephritis/vasculitis | 235 (20.9%) | 238 (27.7%) |
| Polycystic kidney disease | 230 (20.4%) | 195 (22.7%) |
| Hypertensive/renovascular nephrosclerosis | 164 (14.6%) | 97 (11.3%) |
| Diabetic nephropathy | 122 (10.8%) | 56 (6.5%) |
| Obstructive nephropathy / reflux / pyelonephritis | 45 (4%) | 47 (5.5%) |
| Hereditary kidney disease other than polycystic kidney disease | 33 (2.9%) | 34 (4%) |
| Interstitial nephritis, not hereditary | 41 (3.6%) | 32 (3.7%) |
| Congenital disease/malformation | 19 (1.7%) | 23 (2.7%) |
| Other | 233 (20.7%) | 137 (15.9%) |
| Comorbidity/diseases at time of transplantation* | ||
| Cancer other than skin | 131 (11.6%) | 90 (10.5%) |
| Cardiopulmonary diseases | 630 (56%) | 389 (45.3%) |
| Infectious diseases | 281 (25%) | 176 (20.5%) |
| Metabolic, endocrine or kidney diseases | 1115 (99%) | 850 (99%) |
| – Diabetes | 202 (17.9%) | 104 (12.1%) |
| Other events and diseases | 348 (30.9%) | 234 (27.2%) |
| Skin cancer | 46 (4.1%) | 33 (3.8%) |
HDL = high-density lipoprotein; IQR = interquartile range; LDL = low-density lipoprotein; SD = standard deviation * Multiple answers possible.
Recipients with lower education were less likely to undergo living transplantation. Although transplant candidates are informed about the possibility of a living donation, we speculate that recipients with a higher education appraise the benefit of a living donation and the risk for the donor differently from candidates with lower education. Hence, recipients with higher education might be less reluctant to initiate a conversation about live donor kidney transplantation. Not knowing how to initiate a conversation about living donation or being embarrassed to ask others for a donation was identified by several studies [27–29] as a major obstacle to finding a donor.
Transplant candidates were not overly depressed prior to transplantation. The mean score for both groups (4.71 DDRT, 3.85 LDRT) was comparable to the mean HADS-Depression score of 3.68 from a normative sample of the adult British population [30], three samples from the Dutch population with a mean score between 3.4 and 4.6 [31] and the German population with a mean score of 4.8 for men and 5.1 for women [32]. However, recipients with a higher HADS-Depression score were less likely to undergo living donor transplantation (table 2). Like lower education, this could be related to the ability to actively seek a living donor. However, it cannot be ruled out that the higher HADS-Depression score of the DDRT recipients could also be a consequence of not finding a living donor and being on dialysis for a long time, as described by other studies [33, 34].
In terms of physical health, we have little evidence of differences between the LDRT and DDRT. Although EQ-VAS was associated with the type of donor, this association disappeared when the analysis was restricted to recipients who filled in the PSQ questionnaire at least 30 days before the transplant event. The EQ-5D index score, another instrument measuring health-related quality of life, did not differ significantly between LDRT and DDRT. Cardiopulmonary disease or diabetes as comorbidities occurred more frequently in the DDRT group but were not significant factors in the multivariable model. Health parameters such as blood pressure, body mass index (BMI) or cholesterol measured at the time of transplantation were similar in both groups (table 3).
Individuals working more than 50% had higher odds for having a living donor. When the analysis was restricted to candidates aged 20 to 65 and pre-emptive transplantations were excluded the results did not change significantly. A possible explanation for our finding is that candidates fully integrated into the labour market do not want to impair their work and therefore have a higher motivation to search actively for a living donor. To remain on dialysis treatment can limit the ability and flexibility to work and increases the risk of dropping out of the labour market. Also, candidates integrated into the labour market usually have a more active social net that fosters living transplantation.
Higher household income of the recipient was not associated with a living donation in the logistic regression model. This result is in line with our expectations, as healthcare costs related to dialysis and transplantations for donor and recipient are covered by health insurance, which is mandatory in Switzerland. Although donor income was not part of the information collected, we expect only a marginal influence on the decision to donate. Health insurance compensates for any loss of income or other unavoidable expenses resulting from kidney donation [35]. Other studies found that economic factors such as the household income of the donor [36], or car or house ownership as a proxy for household income [14] are associated with the decision to donate. In this context, a country’s healthcare system can influence living donation and studies may come to a different conclusion regarding income. Also, income may increase with age, education and ability to work; study results depend on whether the analysis has been adjusted for such confounders. Our results may also be biased by the high number of missing values. Household income has only been recorded since 2012.
The two centres located in the French-speaking part of Switzerland had a higher proportion of LDRT than the centres located in the German-speaking area, with one exception. According to a 2015 survey [37], the French-speaking regions have a higher proportion of respondents with a positive attitude towards organ donation after death (99%) compared with respondents in the German-speaking region (89%), and therefore presumably also towards living donation [37]. However, one centre in the German-speaking part with a long history of performing and promoting living kidney transplantations had a similarly high proportion of LDRT. We assume that the way healthcare professionals in and outside the transplant centres advocate living transplantation, as well as experiences shared between patients, also influence the willingness of living donations.
The present study includes almost all (91.9%) first kidney only transplant recipients of Switzerland within the observation period. Therefore, our analysis does not suffer from selection bias regarding the population of transplanted patients. As only candidates effectively transplanted were included, the transplant event is not hypothetical. However, we cannot exclude any selection bias for being accepted on the waiting list, a prerequisite for both LDRT and DDRT candidates. Although only candidates effectively transplanted are included, the information was collected at the time of registering on the waiting list, hence before the transplant event was assured. The strength of our study is that we collected characteristics of LDRT and DDRT recipients before transplantation, unlike many other studies that compare characteristics between LDRT and DDRT recipients after transplantation, which may be influenced by the transplant event.
Our model explains only moderately which candidate characteristics were associated with having a living donor; accuracy of the statistical model rose from 56.5% to 70.2%.To gain more insight into the driving factors of living donation, further research is needed. In particular, whether DDRT candidates had potential living donors who were not accepted and if so what were the reasons for declining, about candidate’s beliefs and knowledge regarding living donation and most importantly understand difficulties about looking for a living donor. The challenge to undertake is to promote living donation among older transplant candidates, among those without higher education, not living in a committed relationship and not fully integrated into the labour market, as these candidates are currently underserved. Also paired donation approved by the Swiss transplantation law 2019 has become an option and may increase access to living donation by rescuing living donor-transplant candidate pairs which previously would not have been accepted at least due to HLA incompatibility.
The members of the Psychosocial Interest Group are: Sabina De Geest, Kris Denhaerynck, Remon Helmy, Lynn Leppla, Oliver Mauthner, Marian Struker (University of Basel); Annette Boehler, Sabine Gerull, Michael Koller, Lut Berben (University Hospital Basel); Uyen Huynh-Do (University Hospital Inselspital Bern); Emmanuelle Catana (University Hospital Lausanne); Annina Seiler, Richard Klaghofer (University Hospital Zurich); Isabelle Binet (Cantonal Hospital St. Gallen); Patrizia Künzler-Heule (University of Basel, Cantonal Hospital St. Gallen); Hanna Burkhalter (Sleepmed Hirslanden), Sonja Beckmann (University of Basel, University Hospital Zurich)
The members of the Swiss Transplant Cohort Study are: Rita Achermann, Patrizia Amico, John-David Aubert, Vanessa Banz, Guido Beldi, Christian Benden, Christoph Berger, Isabelle Binet, Pierre-Yves Bochud, Heiner Bucher, Thierry Carell, Emmanuelle Catana, Yves Chalandon, Sabina de Geest, Olivier de Rougemont, Michael Dickenmann, Michel Duchosal, Laure Elkrief, Thomas Fehr, Sylvie Ferrari-Lacraz, Christian Garzoni, Paola Gasche Soccal, Christophe Gaudet, Emiliano Giostra, Déla Golshayan, Karine Hadaya, Jörg Halter, Dominik Heim, Christoph Hess, Sven Hillinger, Hans H. Hirsch, Günther Hofbauer, Uyen Huynh-Do, Franz Immer, Richard Klaghofer, Michael Koller (Head of the data center), Bettina Laesser, Roger Lehmann, Christian Lovis, Pietro Majno; Oriol Manuel, Hans-Peter Marti, Pierre Yves Martin, Pascal Meylan, (Head, Biological samples management group), Paul Mohacsi, Philippe Morel, Ulrike Mueller, Nicolas J Mueller (Chairman Scientific Committee), Helen Mueller-McKenna (Head of local data management), Antonia Müller, Thomas Müller, Beat Müllhaupt, David Nadal, Manuel Pascual (Executive office), Jakob Passweg, Juliane Rick, Eddy Roosnek, Anne Rosselet, Silvia Rothlin, Frank Ruschitzka, Urs Schanz, Stefan Schaub, Aurelia Schnyder, Christian Seiler, Susanne Stampf, Jürg Steiger (Head, Executive Office), Guido Stirnimann, Christian Toso, Christian Van Delden (Executive office), Jean-Pierre Venetz, Jean Villard, Madeleine Wick (STCS coordinator), Markus Wilhelm, Patrick Yerly.
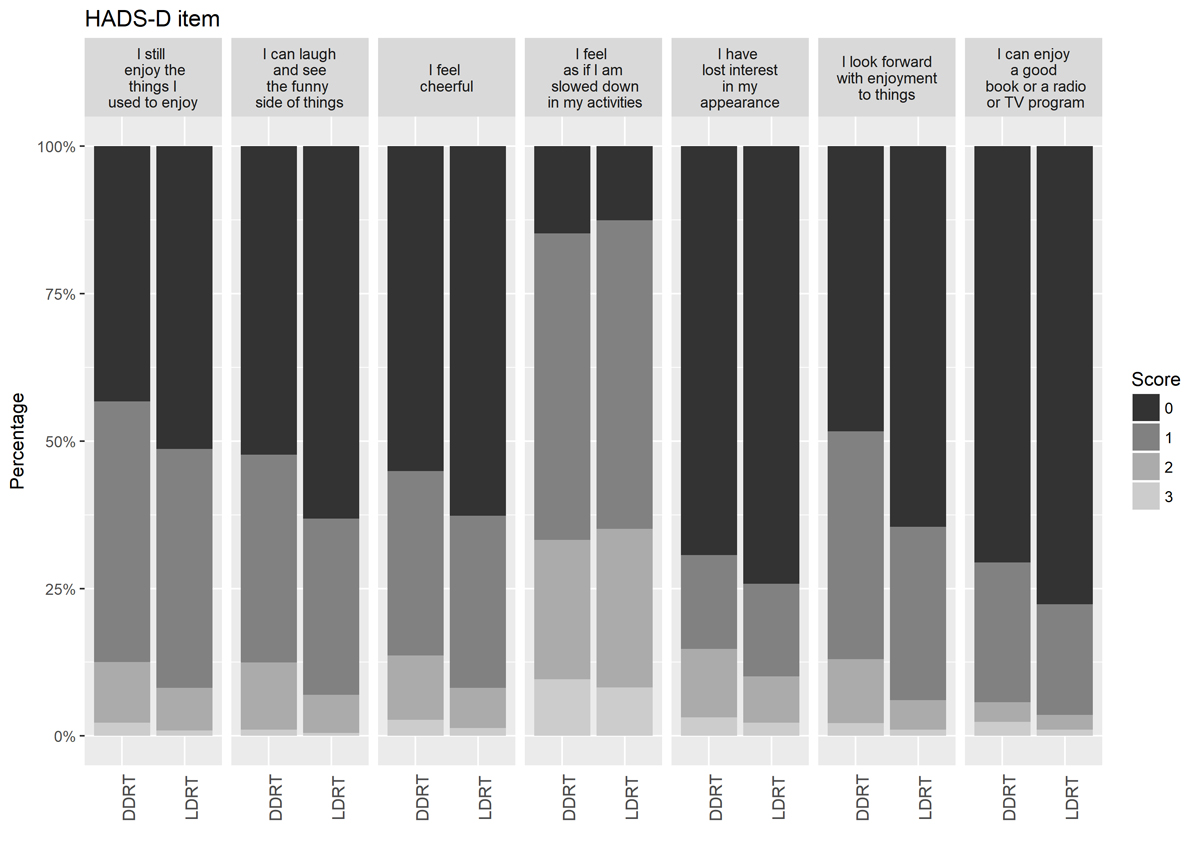
Figure S1 Bar plot of percentages of scores for hospital anxiety and depression score by DDRT and LDRT renal transplant candidates for items related to depression. n = 1766. Score 0 indicates no impairment, score 3 severe impairment. DDRT = deceased donor renal transplant; HADS = hospital anxiety and depression scale; LDRT = living donor renal transplant
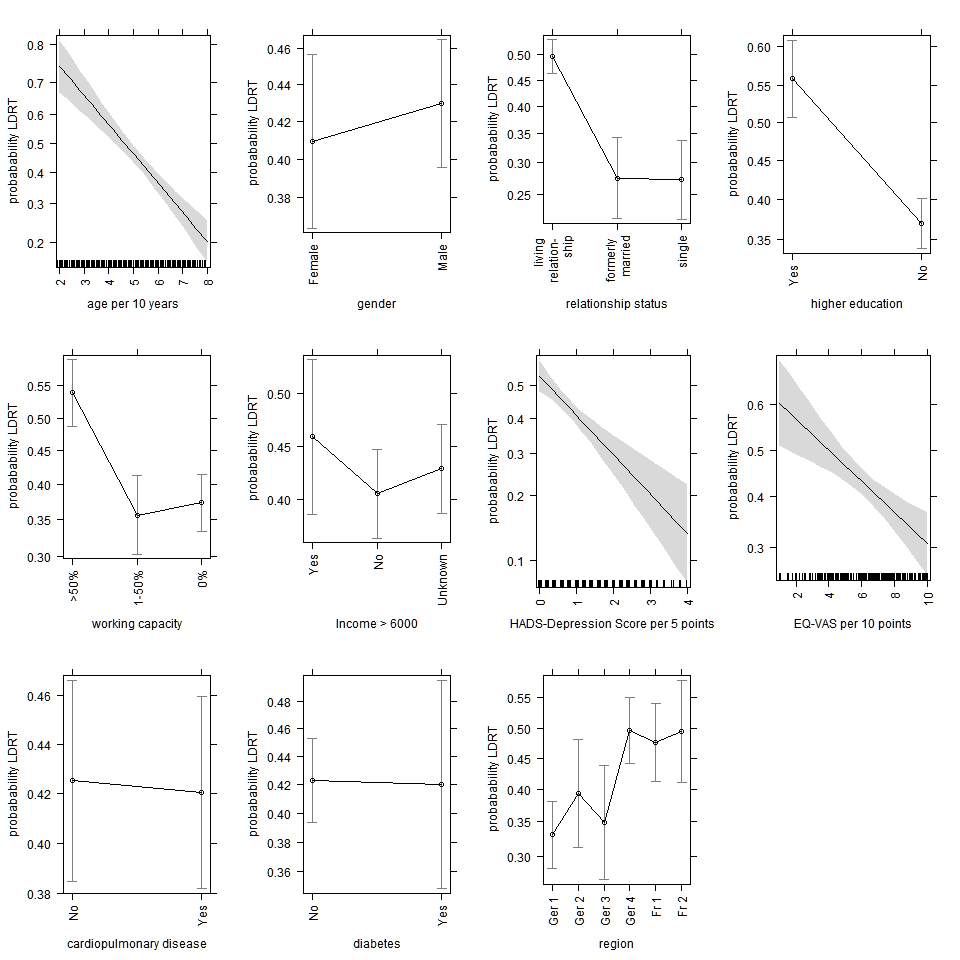
Figure S2 Effect plots for the estimates of the covariates of the logistic regression for outcome live donor renal transplant (LDRT). Covariates not shown in a single graph are standardised as described by Fox [38].
We assessed whether multi-collinearity of the covariates influenced estimates of the regression coefficients. Correlations above 0.4 were observed between age and relationship status single (-0.43) and between HADS-Depression and EQ-VAS (-0.53). However, the variance inflation factors (GVIF 1/(2*df) [23]) was below the critical threshold of 4 for all covariates, and therefore should not affect the estimates on a larger scale.
Restricting the analysis to transplant candidates who filled in the PSQ at least 30 days or 180 days before transplantation did not contradict our findings (fig. S3 and table S1) except for EQ-VAS. For EQ-VAS the odds ratio was around 1 indicating no difference when restricting the analysis to candidates who filled in the questionnaire at least 30 respectively 180 days before transplantation.
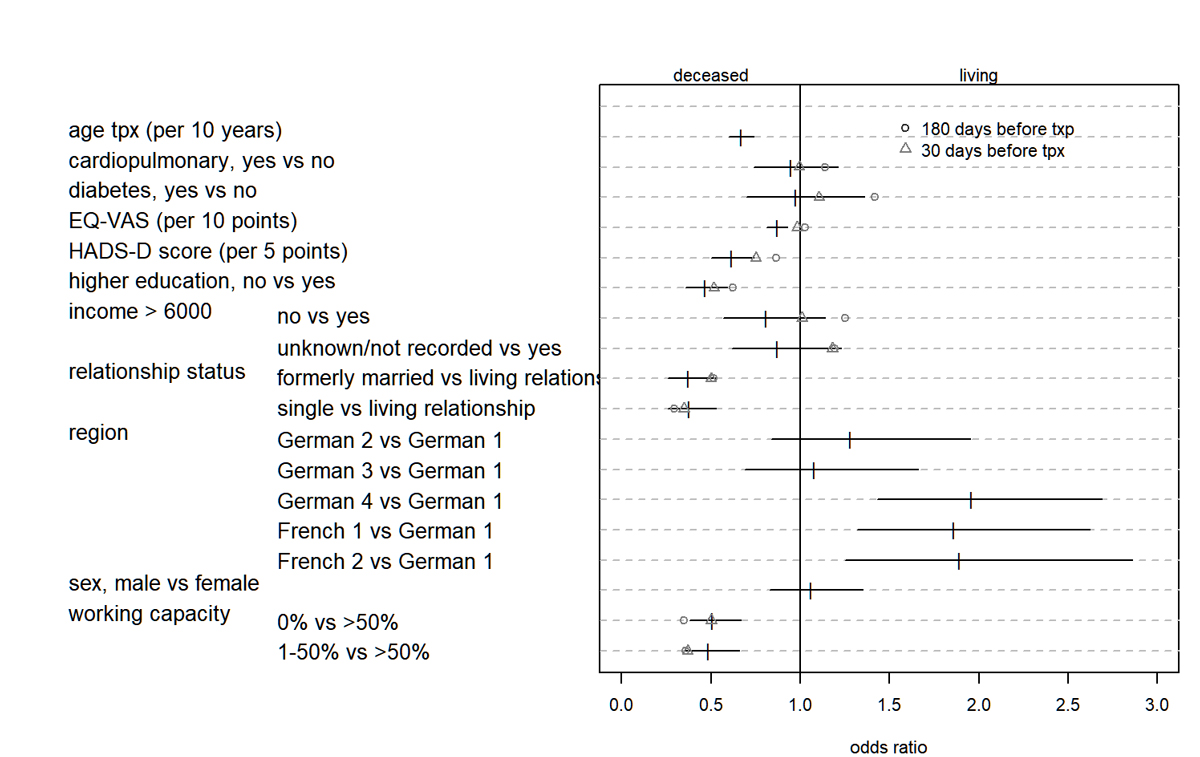
Figure S3 Odds ratio and 95% confidence intervals for covariates for the logistic regression model for outcome LDRT. In addition, regression coefficients for two subgroups are shown: Δ with candidates filled in PSQ at least 30 days (n: DDRT 867, LDRT 315); O with candidates filled in PSQ at least 180 days (n: DDRT 767, LDRT 166) before transplant event. Only estimates for covariates that might change over time are shown for the subgroup analysis. DDRT = deceased donor renal transplant; HADS = hospital anxiety and depression scale; LDRT = living donor renal transplant; PSQ = Swiss Transplant Cohort Study psychosocial questionnaire
Table S1 Results of the logistic regression model for the imputed data set, subgroup restricted to recipients of working age 18–65, and subgroup of recipients who filled in the PSQ questionnaire at least 30 days or at least 180 days before the transplant event.
| Logistic regression model with imputed data set | ||||
| Covariate | Estimate | p-value | 0.025 | 0.975 |
| (Intercept) | 44.48 | <0.0001 | 20.42 | 96.89 |
| Age at time of transplant (per 10 years) | 0.69 | <0.0001 | 0.63 | 0.75 |
| Gender male vs female | 1.06 | 0.5686 | 0.86 | 1.32 |
| Relationship status, formerly married vs living relationship | 0.40 | <0.0001 | 0.29 | 0.55 |
| Relationship status, formerly married vs single | 0.38 | <0.0001 | 0.28 | 0.53 |
| Higher education | 0.44 | <0.0001 | 0.35 | 0.56 |
| Working capacity 0% vs >50% | 0.48 | <0.0001 | 0.36 | 0.65 |
| Working capacity 1–50% vs >50% | 0.53 | <0.0001 | 0.41 | 0.69 |
| Income CHF >6000 per month, no vs yes | 0.84 | 0.287 | 0.61 | 1.16 |
| Income unknown vs high income | 0.95 | 0.7285 | 0.70 | 1.29 |
| HADS-Depression score (per 5 points) | 0.68 | <0.0001 | 0.57 | 0.81 |
| EQ-VAS (per 10 points) | 0.89 | 0.0002 | 0.83 | 0.94 |
| Cardiopulmonary disease, no vs yes | 1.01 | 0.9214 | 0.81 | 1.25 |
| Diabetes | 0.89 | 0.4425 | 0.67 | 1.19 |
| German 2 vs German 1 | 1.48 | 0.0266 | 1.05 | 2.09 |
| German 3 vs German 1 | 1.02 | 0.9401 | 0.68 | 1.53 |
| German 4 vs German 1 | 2.01 | <0.0001 | 1.52 | 2.66 |
| French 1 vs German 1 | 2.02 | <0.0001 | 1.50 | 2.73 |
| French 2 vs German 1 | 1.88 | 0.0006 | 1.31 | 2.69 |
| Logistic regression model restricted to patients of working age (18 to 65 years) | ||||
| Covariate | Estimate | p-value | 0.025 | 0.975 |
| (Intercept) | 101.74 | <0.0001 | 36.51 | 292.19 |
| Age at time of transplant (per 10 years) | 0.63 | <0.0001 | 0.55 | 0.71 |
| Gender male vs female | 0.99 | 0.9598 | 0.76 | 1.30 |
| Relationship status, formerly married vs living relationship | 0.37 | <0.0001 | 0.25 | 0.55 |
| Relationship status, formerly married vs single | 0.34 | <0.0001 | 0.23 | 0.50 |
| Higher education | 0.43 | <0.0001 | 0.32 | 0.56 |
| Working capacity 0% vs >50% | 0.45 | <0.0001 | 0.32 | 0.62 |
| Working capacity 1–50% vs >50% | 0.41 | <0.0001 | 0.30 | 0.57 |
| Income CHF >6000 per month, no vs yes | 0.84 | 0.3876 | 0.57 | 1.24 |
| Income unknown vs high income | 0.92 | 0.6661 | 0.62 | 1.35 |
| HADS-Depression score (per 5 points) | 0.67 | 0.0002 | 0.54 | 0.82 |
| EQ-VAS (per 10 points) | 0.86 | 0.0002 | 0.80 | 0.93 |
| Cardiopulmonary disease, no vs yes | 1.00 | 0.9717 | 0.77 | 1.32 |
| Diabetes | 0.99 | 0.9549 | 0.66 | 1.47 |
| German 2 vs German 1 | 1.28 | 0.2944 | 0.80 | 2.05 |
| German 3 vs German 1 | 1.07 | 0.7969 | 0.66 | 1.72 |
| German 4 vs German 1 | 2.13 | <0.0001 | 1.50 | 3.03 |
| French 1 vs German 1 | 1.76 | 0.0037 | 1.20 | 2.59 |
| French 2 vs German 1 | 2.11 | 0.0024 | 1.31 | 3.41 |
| Model restricted to patients who filled in the PSQ questionnaire at least 30 days before transplant event | ||||
| Covariate | Estimate | p-value | 0.025 | 0.975 |
| (Intercept) | 21.37 | <0.0001 | 6.49 | 71.89 |
| Age at time of transplant (per 10 years) | 0.63 | <0.0001 | 0.55 | 0.73 |
| Gender male vs female | 1.29 | 0.1421 | 0.92 | 1.82 |
| Relationship status, formerly married vs living relationship | 0.50 | 0.0039 | 0.31 | 0.79 |
| Relationship status, formerly married vs single | 0.35 | <0.0001 | 0.21 | 0.57 |
| Higher education | 0.52 | 0.0001 | 0.37 | 0.72 |
| Working capacity 0% vs >50% | 0.37 | <0.0001 | 0.24 | 0.57 |
| Working capacity 1–50% vs >50% | 0.50 | 0.0003 | 0.35 | 0.73 |
| Income CHF >6000 per month, no vs yes | 1.01 | 0.9633 | 0.63 | 1.63 |
| Income unknown vs high income | 1.18 | 0.4909 | 0.74 | 1.90 |
| HADS-Depression score (per 5 points) | 0.75 | 0.0342 | 0.58 | 0.98 |
| EQ-VAS (per 10 points) | 0.98 | 0.7464 | 0.90 | 1.08 |
| Cardiopulmonary disease, no vs yes | 0.99 | 0.9718 | 0.72 | 1.38 |
| Diabetes | 1.11 | 0.6821 | 0.68 | 1.77 |
| German 2 vs German 1 | 0.28 | 0.0062 | 0.10 | 0.65 |
| German 3 vs German 1 | 0.74 | 0.2457 | 0.44 | 1.22 |
| German 4 vs German 1 | 0.34 | <0.0001 | 0.21 | 0.54 |
| French 1 vs German 1 | 0.63 | 0.0619 | 0.38 | 1.01 |
| French 2 vs German 1 | 1.67 | 0.0322 | 1.04 | 2.67 |
| Model restricted to patients who filled in the PSQ questionnaire at least 180 days before the transplant event | ||||
| Covariate | Estimate | p-value | 0.025 | 0.975 |
| (Intercept) | 8.93 | 0.007651 | 1.80 | 45.21 |
| Age at time of transplant (per 10 years) | 0.62 | <0.0001 | 0.51 | 0.74 |
| Gender male vs female | 1.26 | 0.301051 | 0.82 | 1.96 |
| Relationship status, formerly married vs living relationship | 0.52 | 0.031977 | 0.27 | 0.92 |
| Relationship status, formerly married vs single | 0.29 | 0.000243 | 0.15 | 0.55 |
| Higher education | 0.62 | 0.033131 | 0.40 | 0.97 |
| Working capacity 0% vs >50% | 0.36 | 0.000243 | 0.20 | 0.61 |
| Working capacity 1–50% vs >50% | 0.35 | <0.0001 | 0.21 | 0.57 |
| Income CHF >6000 per month, no vs yes | 1.25 | 0.473301 | 0.69 | 2.35 |
| Income unknown vs high income | 1.19 | 0.585895 | 0.64 | 2.28 |
| HADS-Depression score (per 5 points) | 0.86 | 0.409509 | 0.61 | 1.22 |
| EQ-VAS (per 10 points) | 1.03 | 0.689229 | 0.91 | 1.16 |
| Cardiopulmonary disease, no vs yes | 1.14 | 0.544059 | 0.75 | 1.74 |
| Diabetes | 1.42 | 0.240849 | 0.78 | 2.51 |
| German 2 vs German 1 | 0.17 | 0.01743 | 0.03 | 0.59 |
| German 3 vs German 1 | 0.76 | 0.394019 | 0.39 | 1.41 |
| German 4 vs German 1 | 0.28 | 0.0003 | 0.14 | 0.55 |
| French 1 vs German 1 | 0.72 | 0.268824 | 0.39 | 1.27 |
| French 2 vs German 1 | 1.54 | 0.169877 | 0.82 | 2.81 |
We thank the local data managers of the transplant centres for the collection of the data and the data managers at University Hospital Basel and Geneva for the pre-processing of the data.
The study was funded by the Swiss National Science Foundation (Nr 148512) and the kidney transplant centres in Switzerland.
None declared. The results presented in this article have not been published previously in whole or part, except in abstract/poster form.
1 Thiel GT , Nolte C , Tsinalis D . Prospective Swiss cohort study of living-kidney donors: study protocol. BMJ Open. 2011;1(2):e000202–000202. doi:.https://doi.org/10.1136/bmjopen-2011-000202
2Swiss Transplant. Jahresbericht 2018 [Internet]. swiss transplant; 2019. Available from: https://www.swisstransplant.org/fileadmin/user_upload/Swisstransplant/Jahresbericht/Jahresbericht_und_Grafiken_2018/Swisstransplant_Jahresbericht_2018.pdf
3Donation and Transplantation Institut. International registry in organ donation and transplantation [Internet]. 2018. Available from: ttps://www.irodat.org/?p=database
4 Baid-Agrawal S , Frei UA . Living donor renal transplantation: recent developments and perspectives. Nat Clin Pract Nephrol. 2007;3(1):31–41. doi:.https://doi.org/10.1038/ncpneph0383
5 Matas AJ , Smith JM , Skeans MA , Thompson B , Gustafson SK , Stewart DE , et al. OPTN/SRTR 2013 Annual Data Report: kidney. Am J Transplant. 2015;15(S2, Suppl 2):1–34. doi:.https://doi.org/10.1111/ajt.13195
6Koller M, Stampf S, Rick J, Bianco S, Achermann R, Steiger J. Swiss Transplant Cohort Report, May 2008 - December 2016. 2017;(June 2017). Available from: https://www.stcs.ch/publications
7Schweizerischer Organ Lebendspender Verein SOLV. Lebendspenderegister [Internet]. Beziehung zwischen Nierenspendenden und -empfangenden in der Schweiz. [cited 2016 Dec 16]. Available from: http://www.lebendspende.ch/de/register_sol_dhr_statistik_nierenspenden.php
8 Burkhalter F , Huynh-Do U , Hadaya K , Matter M , Müller T , Binet I , et al. Early complications after living donor nephrectomy: analysis of the Swiss Organ Living Donor Health Registry. Swiss Med Wkly. 2017;147(3334):w14497. doi:.https://doi.org/10.4414/smw.2017.14497
9 Krishnan N , Mumford L , Lipkin G , Gill P , Fletcher S , Dasgupta I , et al. Comparison of Medium-term Outcomes of Living Kidney Donors With Longitudinal Healthy Control in the United Kingdom. Transplantation. 2020;104(3):e65–74. doi:.https://doi.org/10.1097/TP.0000000000003082
10Zentrale Ethikkommission Subkommission. Lebendspende von soliden Organen, Richtlinien [Internet]. SAMW; 2008. Available from: https://www.samw.ch/de/Ethik/Themen-A-bis-Z/Organtransplantation/Lebendorganspende.html
11 Gore JL , Danovitch GM , Litwin MS , Pham PTT , Singer JS . Disparities in the utilization of live donor renal transplantation. Am J Transplant. 2009;9(5):1124–33. doi:.https://doi.org/10.1111/j.1600-6143.2009.02620.x
12 Reese PP , Shea JA , Bloom RD , Berns JS , Grossman R , Joffe M , et al. Predictors of having a potential live donor: a prospective cohort study of kidney transplant candidates. Am J Transplant. 2009;9(12):2792–9. doi:.https://doi.org/10.1111/j.1600-6143.2009.02848.x
13 Zimmermann T , Pabst S , Bertram A , Schiffer M , de Zwaan M . Differences in emotional responses in living and deceased donor kidney transplant patients. Clin Kidney J. 2016;9(3):503–9. doi:.https://doi.org/10.1093/ckj/sfw012
14 Wu DA , Robb ML , Watson CJE , Forsythe JLR , Tomson CRV , Cairns J , et al. Barriers to living donor kidney transplantation in the United Kingdom: a national observational study. Nephrol Dial Transplant. 2017;32(5):890–900. doi:.https://doi.org/10.1093/ndt/gfx036
15 de Groot IB , Veen JIE , van der Boog PJM , van Dijk S , Stiggelbout AM , Marang-van de Mheen PJ ; PARTNER-study group. Difference in quality of life, fatigue and societal participation between living and deceased donor kidney transplant recipients. Clin Transplant. 2013;27(4):E415–23. doi:.https://doi.org/10.1111/ctr.12165
16 Khattak MW , Sandhu GS , Woodward R , Stoff JS , Goldfarb-Rumyantzev AS . Association of marital status with access to renal transplantation. Am J Transplant. 2010;10(12):2624–31. doi:.https://doi.org/10.1111/j.1600-6143.2010.03318.x
17 Zimmermann T , Pabst S , Bertram A , Schiffer M , de Zwaan M . Differences in emotional responses in living and deceased donor kidney transplant patients. Clin Kidney J. 2016;9(3):503–9. doi:.https://doi.org/10.1093/ckj/sfw012
18 Koller MT , van Delden C , Müller NJ , Baumann P , Lovis C , Marti HP , et al. Design and methodology of the Swiss Transplant Cohort Study (STCS): a comprehensive prospective nationwide long-term follow-up cohort. Eur J Epidemiol. 2013;28(4):347–55. doi:.https://doi.org/10.1007/s10654-012-9754-y
19Swiss Transplant Cohort Study. Swiss Transplant Cohort Study description [Internet]. [cited 2018 Feb 17]. Available from: http://www.stcs.ch/about/study-description
20EuroQol Group Association. EQ-5D-3L [Internet]. Available from: https://euroqol.org/eq-5d-instruments/eq-5d-3l-about/
21Snaith RP. Sigmond AS. The Hospital Anxiety and Depression Scale Manual. Nfer-Nelson, Windsor; 1994.
22 De Geest S , Burkhalter H , Berben L , Bogert LJ , Denhaerynck K , Glass TR , et al.; Psychosocial Interest Group, Swiss Transplant Cohort Study. The Swiss Transplant Cohort Study’s framework for assessing lifelong psychosocial factors in solid-organ transplants. Prog Transplant. 2013;23(3):235–46. doi:.https://doi.org/10.7182/pit2013250
23 Fox J , Monette G . Generalized Collinearity Diagnostics. J Am Stat Assoc. 1992;87(417):178–83. doi:.https://doi.org/10.1080/01621459.1992.10475190
24R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria; 2016. Available from: https://www.r-project.org/
25 Gordon EJ . “They don’t have to suffer for me”: why dialysis patients refuse offers of living donor kidneys. Med Anthropol Q. 2001;15(2):245–67. doi:.https://doi.org/10.1525/maq.2001.15.2.245
26 Hanson CS , Chadban SJ , Chapman JR , Craig JC , Wong G , Ralph AF , et al. The Expectations and Attitudes of Patients With Chronic Kidney Disease Toward Living Kidney Donor Transplantation: A Thematic Synthesis of Qualitative Studies. Transplantation. 2015;99(3):540–54. doi:.https://doi.org/10.1097/TP.0000000000000433
27 Garonzik-Wang JM , Berger JC , Ros RL , Kucirka LM , Deshpande NA , Boyarsky BJ , et al. Live donor champion: finding live kidney donors by separating the advocate from the patient. Transplantation. 2012;93(11):1147–50. doi:.https://doi.org/10.1097/TP.0b013e31824e75a5
28 Kranenburg LW , Richards M , Zuidema WC , Weimar W , Hilhorst MT , IJzermans JNM , et al. Avoiding the issue: patients’ (non)communication with potential living kidney donors. Patient Educ Couns. 2009;74(1):39–44. doi:.https://doi.org/10.1016/j.pec.2008.07.028
29 Barnieh L , McLaughlin K , Manns BJ , Klarenbach S , Yilmaz S , Hemmelgarn BR ; Alberta Kidney Disease Network. Barriers to living kidney donation identified by eligible candidates with end-stage renal disease. Nephrol Dial Transplant. 2011;26(2):732–8. doi:.https://doi.org/10.1093/ndt/gfq388
30 Crawford JR , Henry JD , Crombie C , Taylor EP . Normative data for the HADS from a large non-clinical sample. Br J Clin Psychol. 2001;40(4):429–34. doi:.https://doi.org/10.1348/014466501163904
31 Spinhoven P , Ormel J , Sloekers PP , Kempen GI , Speckens AE , Van Hemert AM . A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol Med. 1997;27(2):363–70. doi:.https://doi.org/10.1017/S0033291796004382
32 Hinz A , Brähler E . Normative values for the hospital anxiety and depression scale (HADS) in the general German population. J Psychosom Res. 2011;71(2):74–8. doi:.https://doi.org/10.1016/j.jpsychores.2011.01.005
33 Hagren B , Pettersen I-M , Severinsson E , Lützén K , Clyne N . The haemodialysis machine as a lifeline: experiences of suffering from end-stage renal disease. J Adv Nurs. 2001;34(2):196–202. doi:.https://doi.org/10.1046/j.1365-2648.2001.01745.x
34 Moran A , Scott A , Darbyshire P . Waiting for a kidney transplant: patients’ experiences of haemodialysis therapy. J Adv Nurs. 2011;67(3):501–9. doi:.https://doi.org/10.1111/j.1365-2648.2010.05460.x
35Bundesversammlung der Schweizerischen Eidgenossenschaft. Transplantationsgesetz [Internet]. [cited 2017 Aug 7]. Available from: https://www.admin.ch/opc/de/classified-compilation/20010918/index.html
36 Gill J , Dong J , Gill J . Population income and longitudinal trends in living kidney donation in the United States. J Am Soc Nephrol. 2015;26(1):201–7. doi:.https://doi.org/10.1681/ASN.2014010113
37 Swiss Transplant (Institution/Organisation). Eine repräsentative Umfrage zeigt: Die Schweizer haben eine äusserst positive Einstellung zur Organspende. Swiss Transplantant Magazin. 2015;28:8–11.
38 Fox J . Effect Displays in R for Generalised Linear Models. J Stat Softw. 2003;8(15):1–27. doi:.https://doi.org/10.18637/jss.v008.i15
Joint first authors
AK and MK designed the study proposal. AK, MK, IB, SdG and the psychosocial group of interest of the STCS designed the PSQ questionnaire. RA and IB drafted the manuscript. RA conducted the statistical analysis. All authors provided intellectual input, reviewed the article and approved the final version.
The study was funded by the Swiss National Science Foundation (Nr 148512) and the kidney transplant centres in Switzerland.
None declared. The results presented in this article have not been published previously in whole or part, except in abstract/poster form.