Ninety-day outcome of patients with severe COVID-19 treated with tocilizumab – a single centre cohort study
DOI: https://doi.org/10.4414/smw.2021.20550
Mihaela
Savaab, Gregor
Sommerc, Thomas
Daikelerde, Anne-Kathrin
Woischnigf, Aurélien E.
Martineza, Karoline
Leuzingergh, Hans H.
Hirschgh, Tobias E.
Erlangere, Andrea
Wiencierze, Stefano
Bassettii, Michael
Tammj, Sarah
Tschudin-Sutterae, Marcel
Stoecklea, Hans
Parggerk, Martin
Siegemundek, Renate
Bossl, Gert
Zimmermn, Diem-Lan
Vuop, Laurent
Kaiserop, Salome
Dell-Kustereqr, Maja
Weisserae, Manuel
Battegaya, Katrin E.
Hostettlerj, Nina
Khannaaef
a Division of Infectious Diseases and Hospital Epidemiology, University Hospital Basel and University of Basel, Switzerland
b Department of Infectious Diseases, West German Centre of Infectious Diseases, University Hospital Essen, Germany
c Clinic of Radiology and Nuclear Medicine, University Hospital Basel and University of Basel, Switzerland
d Division of Rheumatology, University Hospital of Basel, Switzerland
e Department of Clinical Research, University Hospital Basel, Switzerland
f Infection Biology Laboratory, Department of Biomedicine, University of Basel, Switzerland
g Division of Clinical Virology, University Hospital Basel, Switzerland
h Transplantation and Clinical Virology, Department Biomedicine, University of Basel, Switzerland
i Division of Internal Medicine, University Hospital Basel, Switzerland
j Clinics of Respiratory Medicine, University Hospital Basel and University of Basel, Switzerland
k Department of Intensive Care Medicine, University Hospital Basel, Switzerland
l Federal Food Safety and Veterinary Office, Bern, Switzerland
m Institute of Virology and Immunology (IVI), Mittelhäusern, Switzerland
n Department of Infectious Diseases and Pathobiology, Vetsuisse Faculty, University of Bern, Switzerland
o Division of Infectious Disease, Geneva University Hospitals, Geneva, Switzerland
p Laboratory of Virology, Geneva University Hospitals, Geneva, Switzerland
q Department of Anaesthesiology, Prehospital Emergency Medicine and Pain Therapy, University Hospital Basel, Switzerland
r Institute for Clinical Epidemiology and Biostatistics, University Hospital Basel, Switzerland
Summary
OBJECTIVES
Patients with severe COVID-19 may be at risk of longer term sequelae. Long-term clinical, immunological, pulmonary and radiological outcomes of patients treated with anti-inflammatory drugs are lacking.
METHODS
In this single-centre prospective cohort study, we assessed 90-day clinical, immunological, pulmonary and radiological outcomes of hospitalised patients with severe COVID-19 treated with tocilizumab from March 2020 to May 2020. Criteria for tocilizumab administration were oxygen saturation <93%, respiratory rate >30/min, C-reactive protein levels >75 mg/l, extensive area of ground-glass opacities or progression on computed tomography (CT). Descriptive analyses were performed using StataIC 16.
RESULTS
Between March 2020 and May 2020, 50 (27%) of 186 hospitalised patients had severe COVID-19 and were treated with tocilizumab. Of these, 52% were hospitalised on the intensive care unit (ICU) and 12% died. Eleven (22%) patients developed at least one microbiologically confirmed super-infection, of which 91% occurred on ICU. Median duration of hospitalisation was 15 days (interquartile range [IQR] 10–24) with 24 days (IQR 14–32) in ICU patients and 10 days (IQR 7–15) in non-ICU patients. At day 90, 41 of 44 survivors (93%) were outpatients. No long-term adverse events or late-onset infections were identified after acute hospital care. High SARS-CoV-2 antibody titres were found in all but one patient, who was pretreated with rituximab. Pulmonary function tests showed no obstructive patterns, but restrictive patterns in two (5.7%) and impaired diffusion capacities for carbon monoxide in 11 (31%) of 35 patients, which predominated in prior ICU patients. Twenty-one of 35 (60%) CT-scans at day 90 showed residual abnormalities, with similar distributions between prior ICU and non-ICU patients.
CONCLUSIONS
In this cohort of severe COVID-19 patients, no tocilizumab-related long-term adverse events or late-onset infections were identified. Although chest CT abnormalities were highly prevalent at day 90, the majority of patients showed normal lung function.
Trial registration
ClinicalTrials.gov NCT04351503
Introduction
Dysregulated inflammatory response during severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection was recognised as major cause of disease severity and death in patients with COVID-19 [1]. Anti-inflammatory drugs appear to be effective. Dexamethasone was associated with a reduced mortality among patients receiving either invasive mechanical ventilation or oxygen alone [2]. However, previous data with corticosteroid treatment for severe acute respiratory syndrome 1 (SARS-1), Middle Eastern respiratory syndrome (MERS) and influenza infections showed increased risk of delayed viral clearance and long-term complications in survivors [3]. Tocilizumab, an interleukin (IL)-6 receptor antagonist, was suggested to improve 28-day outcome in patients with severe COVID-19 in nonrandomised trials [4–7]. Early randomised trials were controversial with respect to mortality but showed a significant decrease of mechanical ventilation incidence and/or intensive care unit (ICU) admission in patients with severe COVID-19 [8–10]. Only recently, the addition of tocilizumab in patients pretreated with dexamethasone and admitted to the ICU before organ dysfunction demonstrated increased survival [11, 12].
Short-term benefits of anti-inflammatory treatment may be outweighed by an increased risk of late-onset complications; however long-term follow-up data of COVID-19 patients treated with anti-inflammatory drugs are lacking. Moreover, data on the development of pulmonary fibrosis, which was previously suggested for other coronavirus infections such as SARS-1, particularly in ICU patients as a substantial long-term consequence of COVID-19 related pneumonia are lacking [13].
In this study, we assessed the 90-day clinical, immunological, pulmonary and radiological outcome of patients with severe COVID-19 treated with tocilizumab.
Methods
Study design and participants
This was a prospective single-centre cohort study of clinical, immunological, pulmonary and radiological data routinely collected at day 90 after treatment with tocilizumab. The results are reported according STROBE (Strengthening the Reporting of Observational Studies in Epidemiology). The study was approved by the Ethikkommission Nordwest- und Zentralschweiz, Switzerland (Project-ID 2020-00769) and registered at ClinicalTrials.gov (NCT04351503).
All patients with SARS-CoV-2 infection confirmed by reverse transcriptase polymerase-chin reaction (RT-PCR) testing admitted to the University Hospital Basel, Switzerland, from 16 March to 3 May 2020 and treated with tocilizumab according to the internal therapy protocol were enrolled [14, 15]. Patients who did not meet the criteria for tocilizumab were discharged or referred to other institutions within 48 hours, and patients who died within 12 hours after admission or refused general informed consent were excluded. We could not include a suitable control group because of the limited number of patients and the large differences in prognostic factors as well as clinical course, and disease severity of patients who had not received tocilizumab (see supplementary table S1 in the appendix). Therefore, we omitted a propensity score-matched analysis.
Definitions and treatment
The disease severity was defined according to the World Health Organization (WHO) ordinal scale [16]. All hospitalised patients received for the treatment of COVID-19 lopinavir/ritonavir for 5–7 days concomitant with hydroxychloroquine for 3 days if no contraindications were present. Mechanically ventilated patients were enrolled into the Expanded Access Program Remdesivir® (NCT04323761). One or two doses of tocilizumab at 8 mg/kg bodyweight were administered if criteria of hyperinflammation were met: oxygen saturation <93% on room air, respiratory rate >30 /min, C-reactive protein serum levels >75 mg/l, progression of typical radiological findings within 24–48 hours or ≥4 lobes involved on computed tomography (CT) [4]. Contraindications for tocilizumab treatment were elevated liver enzymes (>5-fold) or ongoing bacterial infection.
The primary outcome was the 90-day safety of tocilizumab in patients with severe COVID-19. Unfavourable outcome was defined as occurrence of adverse events (alanine aminotransferase >150U/l, thrombocytopenia <50,000 G/l, neutropenia <0.5 G/l) and infections up to day 90 after treatment. Secondary outcomes were pulmonary, radiological and immunological status, assessed at day 90.
Our hypothesis was that tocilizumab is safe and no long-term adverse events or increased number of infections would occur in patients treated with tocilizumab. The efficacy of the tocilizumab treatment could not be assessed owing to the lack of a control group.
All patients were followed up for 90 days for WHO ordinal scale of clinical improvement, adverse events and infectious complications. Patients discharged from hospital were followed up on day 30 and 90 in the out-patient clinic. On day 90, patients seen in the outpatient clinic were additionally tested for inflammatory and immunological markers in serum and had a CT scan. Pulmonary function tests were considered abnormal if they were <80% of the predicted values, FEV1/FVC (forced expiratory volume in 1 second / forced vital capacity) was considered abnormal if <70% [17]. SARS-CoV-2-specific serum IgG titres were determined using two enzyme-linked immunosorbent assays (ELISAs) against the S1-domain of the 155-spike protein (Euroimmun) and the nucleocapsid (N) antigen (Elecsys, Roche) [14]. A vesicular stomatitis virus-based pseudo-neutralisation assay was performed as previously published [18].
Data collection
Clinical, laboratory and radiological data were systematically collected and entered in the study database during hospitalisation and on day 90. At day 90, all patients were scheduled for a medical check-up followed by prespecified laboratory testing, a CT scan and pulmonary function tests. For patients who were either in rehabilitation or living at distance, telephone interviews with the patient or the treating physician were performed.
Statistical analysis
Chi-square, Fisher exact, and the Wilcoxon rank sum test were applied, as appropriate. Analyses were performed using StataIC 16. All statistical tests were two-tailed and considered to be significant if p <0.05.
Results
From 26 February to 3 May 2020, 221 patients were hospitalised with COVID-19 at the University Hospital of Basel, a tertiary teaching hospital (fig. 1). Of these, 50 patients (23%) were eligible for treatment with tocilizumab. Twenty-two of 50 patients (44%) patients received one and 28 (56%) two doses of tocilizumab a median of 2 days (IQR 1–4) after hospital admission and 10.5 days (IQR 7–13) after symptom onset.
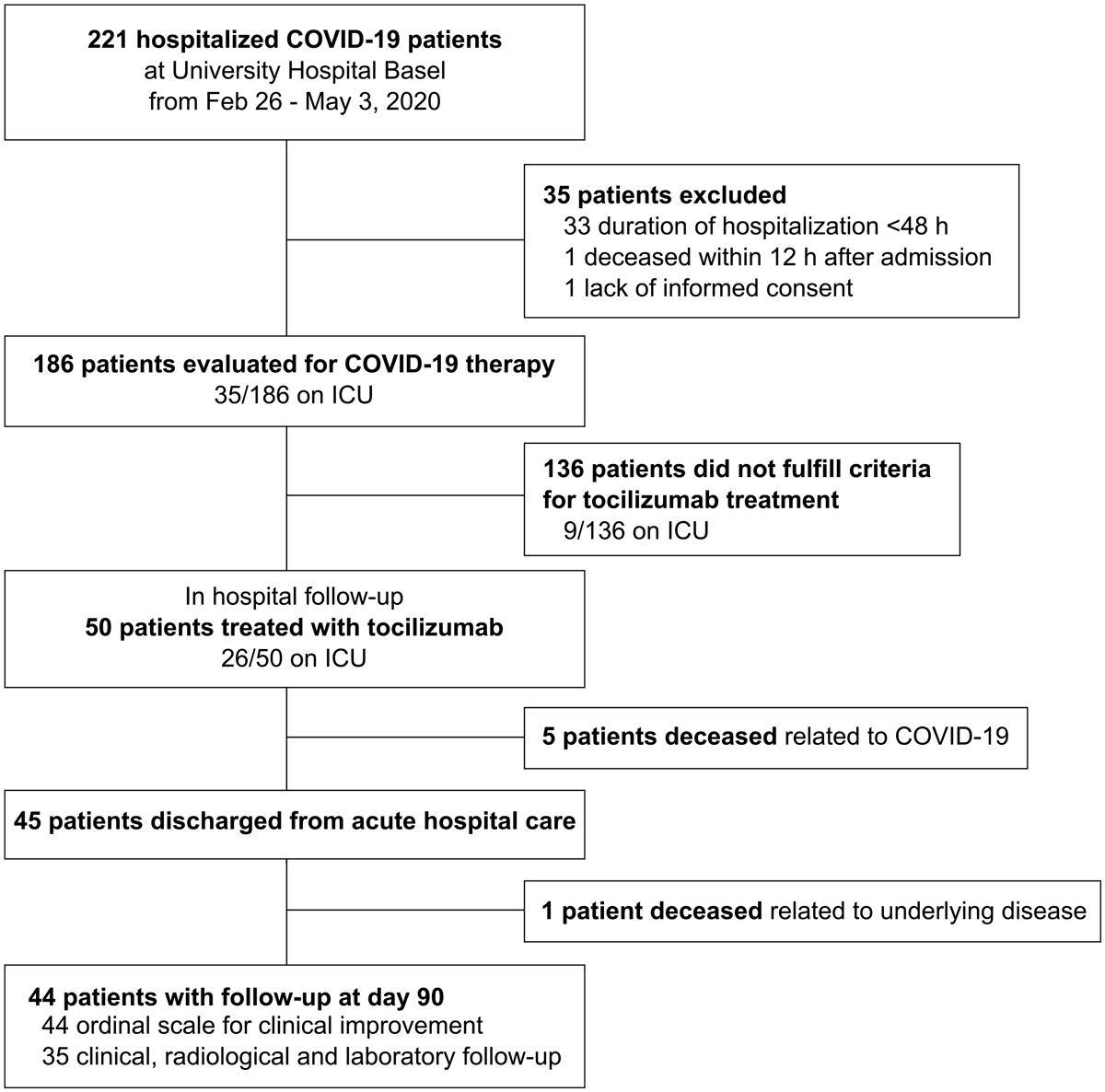
Figure 1 Profile of SARS-CoV-2 patients’ enrolment and follow-up visits.
Of the 221 patients hospitalised with COVID-19 in the initial phase of the pandemic at our hospital, 50 were treated with tocilizumab. * Patients admitted to the intensive care unit (ICU) at any time point during hospitalisation. § Nine patients admitted to the intensive care unit (ICU) did not receive tocilizumab because of elevated liver enzymes (1/9), ongoing acute infection (3/9), ICU admission due to other reasons than COVID-19 (3/9), not yet established as COVID-19 treatment (1/9) or patient’s will (1/9). Clinical outcome based on the WHO ordinal scale was recorded on day 90 (n = 44) for all surviving patients treated with tocilizumab. Clinical, immunological and pulmonary outcomes on day 90 were evaluated in 35 of 44 surviving patients, respectively.
Patients had a median age of 60 years (IQR 50–70) and 42 (84%) were male. Median body mass index (BMI) was 29 kg/m2 (IQR 25.2–31.8) and 32 patients (64%) had at least one comorbidity. At admission, 38 patients (76%) required supplemental oxygen (WHO ordinal scale score ≥4) and all patients showed ground-glass opacities on CT scan (table 1). In total, 46 (92%) patients received antiviral treatment with lopinavir/ritonavir and 7 (14%) with remdesivir. Overall, 24 patients (48%) remained on the medical ward during hospitalisation. Twenty-six patients (52%) were admitted to ICU, 19 (73%) at admission and 7 a median of 3.7 days (IQR 2–4) after admission. Baseline characteristics regarding age, gender, comorbidities and BMI did not differ between ICU and non-ICU patients. Of 26 ICU patients, 24 (92%) were classified as critical COVID-19 requiring mechanical ventilation and 14 (54%) developed severe acute respiratory distress syndrome (ARDS). Two patients received additional steroid treatment. Thromboembolic events were diagnosed in nine patients (18%), of whom eight were on ICU. Median duration of hospitalisation was 15 days (IQR 10–24), with 24 days (IQR 14–32) in ICU patients and 10 days (IQR 7–15) in non-ICU patients (p <0.001).
Table 1 Baseline characteristics and underlying comorbidities of patients treated with tocilizumab.
| |
All (n = 50)
|
|
Demographics
|
|
| Gender |
|
| – Male |
42 (84) |
| – Female |
8 (16) |
| Ethnicity |
|
| – Caucasian |
37 (74) |
| – Asian |
9 (18) |
| – African |
3 (6) |
| – Hispanic |
1 (2) |
| Age, years |
60 (50–70) |
|
Comorbidities
|
|
| Hypertension |
32 (64) |
| Diabetes |
11 (22) |
| Chronic lung disease |
6 (12) |
| – Chronic obstructive pulmonary disease |
2 (4) |
| – Bronchial asthma |
4 (8) |
| Ischaemic heart disease |
10 (20) |
| Chronic kidney disease |
6 (12) |
| Autoimmune disease |
2 (4) |
| HIV infection |
2 (4) |
| Body-mass index (kg/m2) |
29·4 (25·2–31·8) |
| Former or current smoking |
18 (36) |
|
Clinical presentation and disease severity
|
|
| Days from onset of symptoms to hospital admission |
8 (5–10) |
| – Fever |
37 (76) |
| – Shortness of breath |
39 (78) |
| – Cough |
41 (82) |
| – Diarrhoea |
13 (26) |
| – Confusion |
3 (6) |
| WHO ordinal scale score at hospital admission |
|
| 3. Hospitalised, no oxygen therapy |
12 (24) |
| 4. Hospitalised, oxygen by mask or nasal prongs |
22 (44) |
| 5. Hospitalised, non-invasive ventilation or high-flow oxygen |
10 (20) |
| 6–7. Hospitalised, mechanical ventilation ± additional organ support |
6 (12) |
|
Laboratory diagnostics
|
|
| Lymphocytes (109/l) |
0.7 (0.5–1.1) |
| Neutrophils (109/l) |
4.9 (3.5–7.2) |
| C-reactive protein (mg/l) |
91.6 (34.9–150.3) |
| Serum creatinine (µmol/l) |
85.0 (67.2–104.5) |
| Estimated glomerular filtration rate (ml/min) |
83.0 (62.8–98.2) |
| D-dimer (mg/l) |
0.8 (0.5–1.2) |
| Ferritin (µg/l) |
1083.5 (544.5–2319.8) |
| Lactate dehydrogenase (U/l) |
361.5 (269.2–478.2) |
| Interleukin-6 (ng/l) |
97.7 (76.5–194.5) |
|
Treatment for COVID-19
|
|
| Lopinavir/ritonavir |
46 (92) |
| Hydroxychloroquine |
37 (74) |
| Remdesivir |
7 (14) |
| Convalescent plasma |
7 (14) |
| Steroids |
2 (4) |
|
Imaging
|
|
| Abnormal CT scan |
50 (100) |
| – Ground glass opacities |
49 (100) |
| – Reticular interstitial pattern |
36 (73) |
| – Crazy paving appearance |
27 (55) |
| – Air space consolidation |
31 (65) |
| – Bronchial wall thickening |
16 (33) |
| – Bilateral involvement |
43 (88) |
| – Five lobes involvement |
36 (73) |
| – Peripheral distribution |
38 (78) |
C-reactive protein serum levels increased significantly from hospital admission (98.2 mg/l, IQR 40.1–150.3) to time of tocilizumab administration (136.6 mg/l, IQR 107.0-204.9; p <0.001). At tocilizumab administration, median IL-6 serum levels were 97.6 ng/l (IQR 70.4–144.8) and absolute lymphocyte counts 0.7 G/l (IQR 0.5–1.0). IL-6 serum levels were significantly higher in ICU than in non-ICU patients at tocilizumab administration (p = 0.017), whereas C-reactive protein and absolute lymphocyte counts were similar in the two groups. All inflammatory markers normalised in all patients by day 30. Absolute lymphocyte counts were >1 G/l and absolute CD4 T-cell counts >500 cells/µl in all patients on day 30. SARS-CoV-2 load in a nasopharyngeal swab was measured in 42 patients, with a median of 107,000 copies/ml (IQR 17,800–3,080,775) at tocilizumab administration, and decreased on day 7 in ICU and non-ICU patients to a median of 2300 copies/ml (IQR 1000–22,150) (fig. 2).
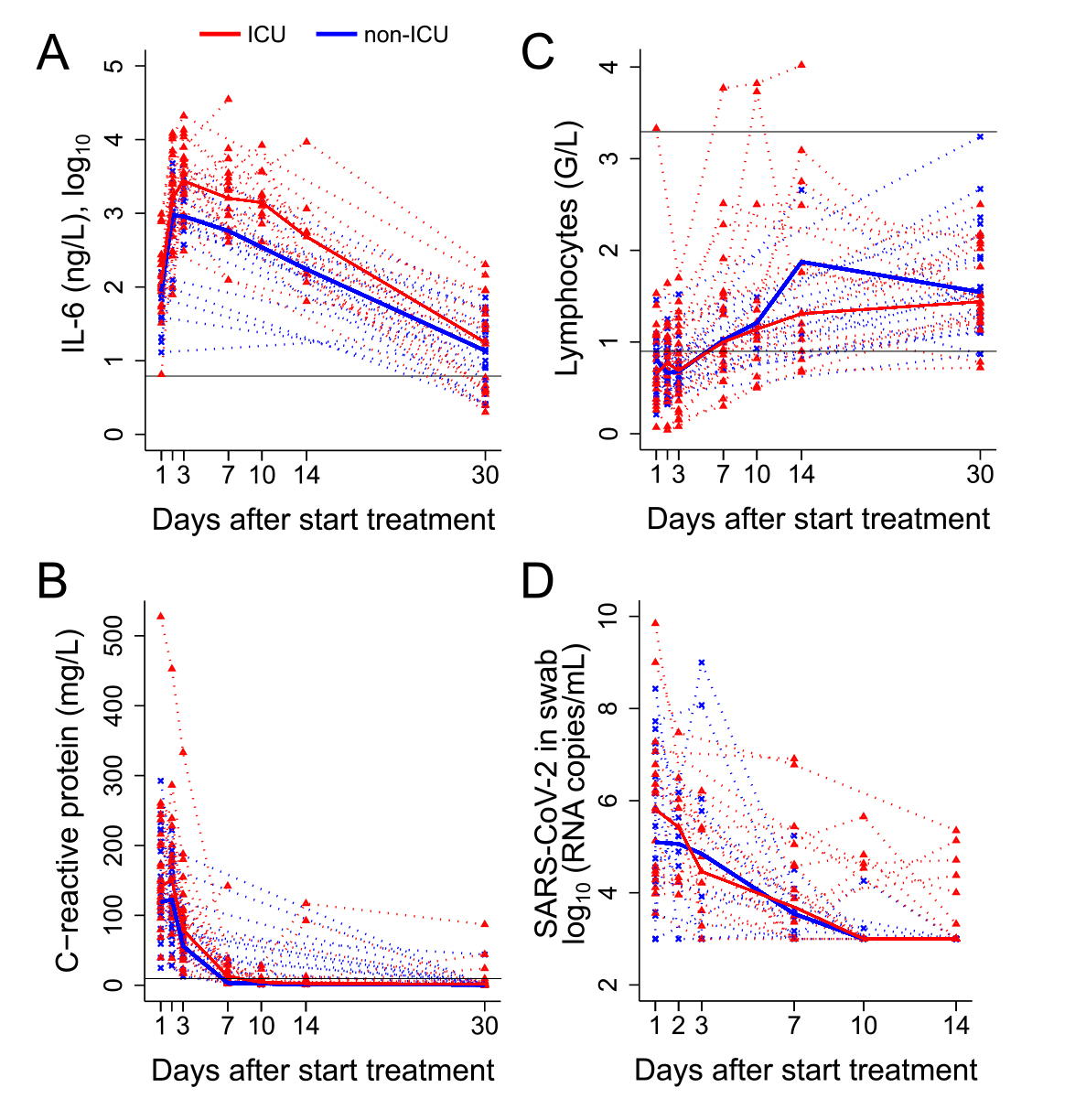
Figure 2 The course of laboratory markers over time from treatment initiation in intensive care unit (ICU) and non-ICU patients.
The course of (A) interleukin (IL)-6, (B) C-reactive protein, (C) absolute lymphocyte counts, (D) severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) load in nasopharyngeal swabs. Dotted lines represent the individual courses of inflammatory markers and absolute lymphocytes over 30 days and SARS-CoV-2 loads in nasopharyngeal swabs over 14 days from treatment initiation in ICU (red line, n = 26) vs non-ICU (blue line, n = 24) patients. Black solid lines represent the reference values.
At day 90, 44 (88%) of the 50 patients receiving tocilizumab were alive. Thirty-five patients (70%) were seen in the outpatient clinic and nine (18%) were contacted by phone. Twenty-seven patients (54%) reported no limitation of activities and 14 (28%) some limitation of general activities. Three patients were still hospitalised and required supplemental oxygen. Six patients (12%) were deceased, five during acute hospitalisation and one 40 days after discharge due to an underlying haematological disease. The WHO ordinal scale score differed significantly between prior non-ICU and ICU patients on day 90 (p = 0.005) (table 2).
Table 2 90-day adverse and infectious events clinical, immunological, pulmonary and radiological outcome of patients treated with tocilizumab.
| |
All
n (%)
|
Non-ICU
n (%)
|
ICU
n (%)
|
p-value
|
|
WHO ordinal scale of clinical improvement
|
50 (100)
|
24 (48)
|
26 (52)
|
|
| 1. Non-hospitalised, no limitation of activities |
27 (54) |
19 (79) |
8 (31) |
0.005 |
| 2. Non-hospitalised, limitation of activities |
14 (28) |
3 (13) |
11 (42) |
|
| 3. Hospitalised, no oxygen therapy |
0 |
0 |
0 |
|
| 4. Hospitalised, oxygen by mask or nasal prongs |
1 (2) |
0 |
1 (4) |
|
| 5. Hospitalised, non-invasive ventilation or high-flow oxygen |
2 (4) |
0 |
2 (8) |
|
| 6–7. Hospitalised, intubation and mechanical ventilation ± additional organ support |
0 |
0 |
0 |
|
| 8. Death |
6 (12) |
2 (8) |
4 (15) |
|
|
Infectious complications
|
|
|
|
|
| Any microbiologically confirmed infection*
|
11 (22) |
1 (4) |
10 (38) |
0.005 |
| Pneumonia†
|
10 (20) |
0 (0) |
10 (38) |
|
| Bloodstream infection |
3 (6) |
0 (0) |
3 (12) |
|
| Urinary tract infection |
4 (8) |
1 (4) |
3 (12) |
|
| Pulmonary invasive fungal disease |
1 (2) |
0 (0) |
1 (4) |
|
|
Pulmonary function test‡§
|
35 (100)
|
18 (51)
|
17 (49)
|
|
| Diffusion capacity for carbon monoxide, median % of predicted values |
88 (75–100) |
93 (87–100) |
80 (67–92) |
0.049 |
| Total lung capacity, median % of predicted values |
91 (88–100) |
97 (88–102) |
91 (85–93) |
0.079 |
| FEV1/FVC, median % of predicted values |
79 (76–86) |
78 (74– 81) |
81 (79–87) |
0.109 |
|
Chest CT scan
‡¶
|
35 (100)
|
18 (51)
|
17 (49)
|
|
| No abnormalities |
14 (40) |
7 (39) |
7 (41) |
0.890 |
| Residual abnormalities |
21 (60) |
11 (61) |
10 (59) |
|
| – Lung perfusion abnormalities |
11 (52) |
6 (55) |
5 (50) |
|
| – Signs of fibrosis |
7 (33) |
3 (27) |
4 (40) |
|
| Embolism |
0 |
0 |
0 |
|
|
SARS-CoV-2 specific immune response‡
|
35 (100)
|
18 (51)
|
17 (49)
|
|
| IgG (recombinant nucleocapsid antigen), ≥1 (COI) |
34 (97) |
18 (100) |
16 (94) |
0.999 |
| IgG (recombinant S1 antigen), ≥1.5 (COI) |
34 (97) |
18 (100) |
16 (94) |
0.999 |
| Neutralisation assay, ≥40 (COI) PVND50 |
34 (97) |
18 (100) |
16 (94) |
0.999 |
Regarding the primary outcome, transient alanine aminotransferase levels >150U/l were recorded in 12 patients (24%) and severe thrombocytopenia <50,000 G/l in 2 patients (4%) with similar distributions among ICU and non-ICU patients during acute hospital care. By day 90, blood cell counts and liver enzymes were in the normal range in all patients. No gastrointestinal perforation was recorded.
Regarding secondary outcomes, 11 (22%) patients had at least one microbiologically confirmed super-infection during acute hospital care, of which 10 (91%) were diagnosed in ICU patients. By day 90, no further microbiologically confirmed infection or use of antibiotics were reported.
Pulmonary function tests showed no obstructive patterns, but a restrictive pattern in 2 (5.7%) and impaired diffusion capacity for carbon monoxide (DLCO) in 11 (31%) of 35 patients. The DLCO was lower in prior ICU patients than non-ICU patients (p = 0.049). One prior ICU patient showed a reduced DLCO as well as total lung capacity (TLC).
CT scans showed residual abnormalities in 21 of 35 patients (60%), 7 of whom also had signs of fibrosis (fig. 3), with similar distributions between ICU and non-ICU patients and no correlation to pulmonary function tests. No pulmonary embolism or further thromboembolic events occurred after acute hospital care.
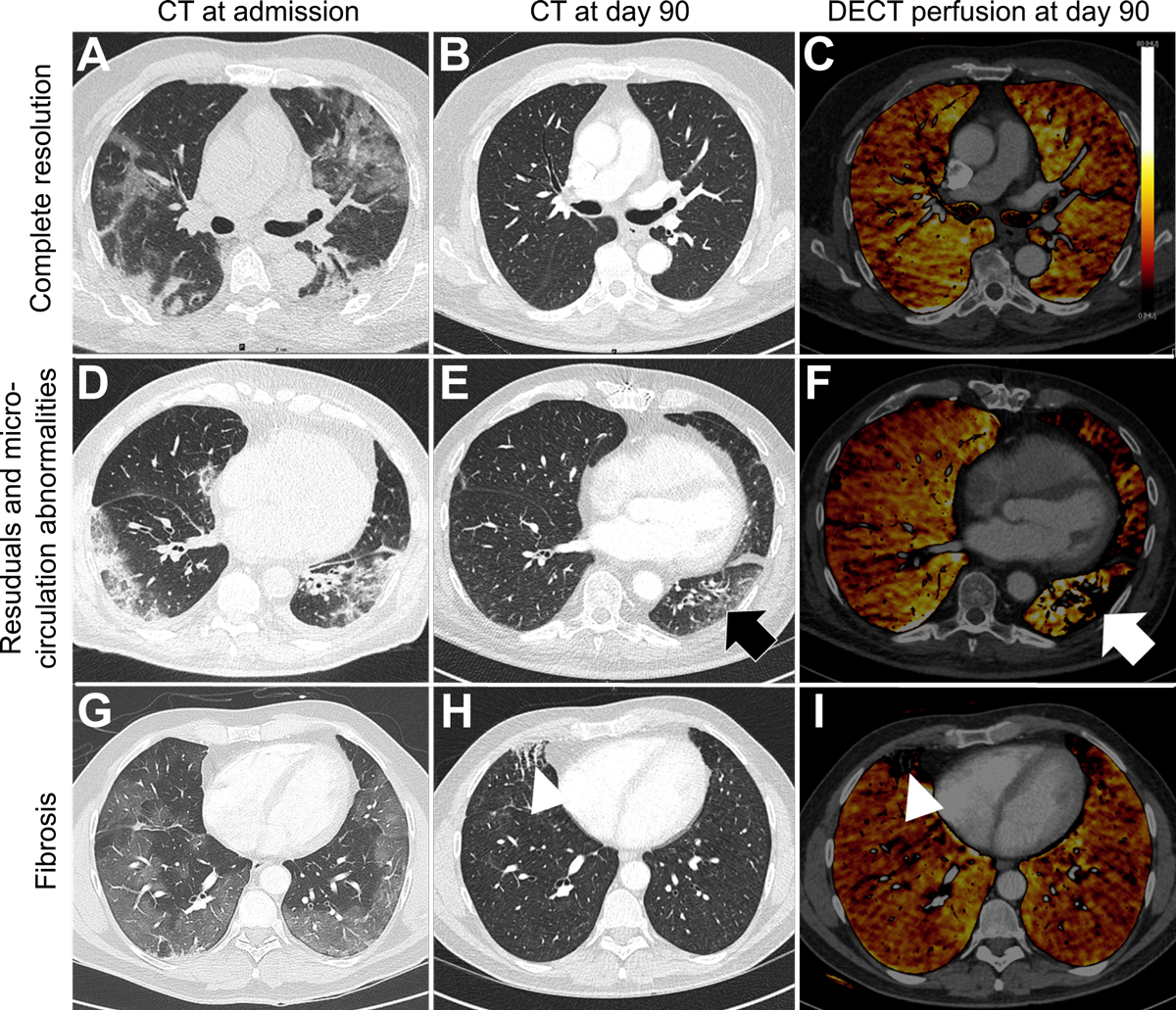
Figure 3 Evolution patterns of chest CT findings from admission to day 90.
Axial CT images (A, B, D, E, G, H) and DECT overlay maps (C, F, I) presenting three evolution patterns of lung involvement from admission to day 90 observed in COVID-19 patients after treatment with tocilizumab.
(A) Axial CT image is depicting a 62-old male patient with bilateral GGO and consolidations 3 days after onset of symptoms with complete resolution at day 90 (B) and no microcirculation abnormalities (C).
(D) Axial CT image of a 78-old male patient 4 days after symptom onset showing bilateral GGO and reticulations (E) which seem to be associated with microcirculation abnormalities on DECT (F) at day 90. 49-old male patient receiving ECMO presenting profound bilateral mosaic pattern with GGO (G) 3 days after onset of symptoms showing focal fibrosis characterized by course reticulations and traction bronchiectasis (H) which correlates with focal perfusion defects on DECT (I) at day 90.
GGO = ground glass opacity; DECT = dual energy computed tomography
Specific antibodies to the SARS-CoV-2 nucleocapsid and recombinant S1 antigens were positive in all but one patient. This patient was treated with rituximab prior to SARS-CoV-2 infection. Virus-neutralising antibodies showed high neutralising capacity (≥1:40 pseudotype virus neutralisation dose 50% (PVND50); median 1:480 PVND50 (IQR 300–1280) in all but this one patient.
Discussion
In this prospective cohort study, more than 90% of survivors treated with tocilizumab for severe and critical COVID-19 were outpatients and more than half reported no limitation of activities 90 days after diagnosis. No patient developed tocilizumab-related adverse events or infectious complications within 90 days from tocilizumab administration. Despite tocilizumab treatment, rapid viral clearance at day 7 and normal lymphocyte counts and subset profiles at day 30 were recorded. High SARS-CoV-2-specific antibody titres as well as virus-neutralising serum antibodies were detected in all but one patient at day 90. Importantly, two thirds of patients had a complete recovery of their pulmonary function, although 60% of patients still showed some abnormalities on chest CT scans.
We observed high numbers of in-hospital bacterial infections and one pulmonary fungal infection, which were all except for one restricted to ICU patients. Although large observational cohort studies have reported high rates of super-infections in SARS-CoV-2-infected patients treated with tocilizumab, randomised controlled trials and a recent meta-analysis could not substantiate these findings [5, 6, 9]. This suggests that in-hospital infections may not be related to the use of tocilizumab, but are rather the consequence of the illness severity and ICU admission.
Despite the potentially increased risk of serious bacterial infections under treatment with tocilizumab in patients with chronic inflammatory disorders [19], we could not identify any late-onset or opportunistic infection after discharge from acute hospital care. In parallel, quantitative cellular immune responses appeared normal by day 90. High levels of SARS-CoV-2 N protein and S1 domain of the spike protein antibodies including high titres of protective virus-neutralising serum antibodies were detectable by day 90. Robust and rapid humoral response under tocilizumab has been previously reported after vaccination in patients with rheumatoid arthritis and in a comparative unmatched analysis from 138 patients with COVID-19 including 76 patients treated with tocilizumab [20, 21]. One reason for higher odds of infections in patients with rheumatic diseases may be attributable to the underlying disease, additional combinatory immune suppressive treatments and long-term exposure to IL-6 receptor antagonists. Further investigations are required to evaluate the risk of opportunistic and serious infections in patients receiving tocilizumab or other anti-inflammatory drugs in the context of COVID-19.
Two recent studies have assessed pulmonary sequelae in surviving ICU patients with ARDS secondary to COVID-19. Both studies found around 80% of patients with a DLCO <80%, 30–50% of patients with TLC <80% and CT scan abnormalities in 70–90% of patients, with fibrotic patterns in up to 90% [22, 23]. Patients with more severe findings on chest CT had worse pulmonary function. In contrast, only a third of the patients in our cohort showed impaired pulmonary function tests at day 90 in spite of a high percentage of ARDS and a high percentage of CT abnormalities. Similarly to our findings, a recent study including patients with moderate COVID-19 disease observed radiological abnormalities in 71% of patients without correlation to the pulmonary function at day 90 [24]. Further studies are needed to elucidate the role and timing of pulmonary function tests and CT scans to assess pulmonary outcome after COVID-19.
The limitations of this study include its single-centre design, the relatively small number of patients and the absence of a control group or randomisation to assess the clinical effectiveness of tocilizumab. A strength of this study is the comprehensive longitudinal long-term in-depth analysis assessing more than 75% of all severe to critically ill COVID-19 patients treated in our centre during the first COVID-19 wave.
In conclusion, we did not observe any specific signals indicating tocilizumab-related adverse events, delayed infections after discharge from acute hospital care or impaired immune response by day 90 in patients with severe and critical COVID-19 treated with tocilizumab. However, given the single-centre design and relatively small sample size our findings may not be generalizable. This data supports the need of longitudinal studies to evaluate the risk-benefit of anti-inflammatory agents on short and long-term outcomes in patients with SARS-COV-2.
Appendix Supplementary table
Table S1 Demographic characteristics and underlying comorbidities of all COVID-19 patients who met the inclusion criteria.
| |
All
(n = 186)
|
Tocilizumab
(n = 50)
|
No tocilizumab
(n = 136)
|
|
Demographics
|
|
|
|
| Gender, male |
115 (62) |
42 (85) |
73 (54) |
| Age, years |
62 (48–74) |
60 (50–70) |
64 (47–75) |
|
Comorbidities
|
|
|
|
| Comorbidity count |
|
|
|
| – 0 |
63 (34) |
19 (38) |
44 (33) |
| – 1 |
39 (21) |
14 (28) |
25 (18) |
| – ≥2 |
84 (45) |
17 (34) |
67 (49) |
| Chronic lung disease |
9 (5) |
2 (4) |
7 (5) |
| Diabetes |
39 (21) |
9 (18) |
30 (22) |
| Hypertension |
85 (46) |
25 (50) |
60 (44) |
| Cardiovascular disease |
34 (18) |
13 (26) |
21 (16) |
| Cerebrovascular disease |
20 (11) |
1 (2) |
19 (14) |
| Malignancy |
19 (10) |
2 (4) |
17 (13) |
| Chronic kidney disease |
27 (15) |
6 (12) |
21 (15) |
| Immunodeficiency |
28 (16) |
4 (8) |
24 (19) |
| Body mass index (kg/m2) |
27·0 (24·0–31·0) |
29·0 (25·2–31·8) |
27·0 (23·0–30·0) |
|
Clinical presentation and disease severity
|
|
|
|
| Duration from onset of symptoms to hospitalisation (days) |
7 (3–10) |
8 (5–10) |
7 (3–10) |
| Haemoptysis |
7 (4) |
1 (2) |
6 (4) |
| Unconsciousness |
7 (4) |
6 (12) |
1 (1) |
| Dyspnoea |
80 (43) |
32 (64) |
48 (35) |
| WHO ordinal scale score (n = 184) |
|
|
|
| 3. Hospitalised, not requiring supplemental oxygen |
106 (58) |
21 (42) |
85 (63) |
| 4–5. Hospitalised, requiring any supplemental oxygen |
66 (36) |
21 (42) |
45 (34) |
| 6–7. Hospitalised, receiving IMV or ECMO |
12 (7) |
8 (16) |
4 (3) |
|
Laboratory testing and imaging
|
|
|
|
| Lymphocytes (109/l) |
1.0 (0.7–1.4) |
0.7 (0.5–1.1) |
1.0 (0.8–1.4) |
| Neutrophils (109/l) |
4.4 (2.8–6.5) |
4.9 (3.5–7.2) |
4.3 (2.7–5.7) |
| C-reactive protein (mg/l) |
40.5 (13.9–86.1) |
91.6 (34.9–150.3) |
31.8 (8.2–60.4) |
| Creatinine (µmol/l) |
81.0 (67.0–104.0) |
85.0 (67.2–104.5) |
78.0 (63.5–103.5) |
| Estimated glomerular filtration rate (ml/min) |
82.0 (62.0–99.0) |
83.0 (62.8–98.2) |
82.0 (62.0–99.0) |
| D-dimer (mg/l) |
0.7 (0.4–1.7) |
0.8 (0.5–1.2) |
0.7 (0.4–1.8) |
| Ferritin (µg/l) |
727.0 (308.5–1299.2) |
1083.5 (544.5–2319.8) |
476.5 (248.5–1166.2) |
| Lactate dehydrogenase (U/l) |
286.0 (222.5–395.0) |
361.5 (269.2–478.2) |
264.0 (209.0–351.0) |
| Total bilirubin (µmol/l) |
7.6 (5.3–10.1) |
8.7 (6.4–12.4) |
7.0 (4.9–9.6) |
| Abnormal pulmonary imaging |
147 (79) |
49 (98) |
98 (72) |
|
Treatment for COVID-19
|
|
|
|
| LPV/r |
113 (61) |
46 (92) |
67 (49) |
| Hydroxychloroquine |
123 (66) |
37 (74) |
86 (63) |
| Remdesivir |
8 (4) |
7 (14) |
1 (1) |
| Convalescent plasma |
13 (7) |
7 (14) |
6 (4) |
|
Outcome
|
|
|
|
| ICU admission |
35 (19) |
26 (52) |
9* (7) |
| Duration on ICU, days |
11 (5–22) |
11 (6–17) |
12 (3, 22) |
| Deceased |
15 (8) |
5 (10) |
10 (7) |
| Deceased on ICU |
7 (4) |
3 (6) |
4 (3) |
| Days from admission to death |
6 (4–10) |
4 (4–6) |
7 (4–12) |
Acknowledgments
We would like to thank Veronika Baettig, Elisabeth Wehrle-Wieland, Niklaus Labhardt, Parham Sendi, Anemone Hedstueck, Matthias von Rotz and Kai-Manuel Adam for patients’ care; Isabelle Arm for technical assistance for serological assays; Fabian Franzeck for providing data from the CDWH and Nikolaus Deigendesch for critically reading the manuscript.
Author contributions
Drs Sava and Khanna had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Sava M, Sommer G, Daikeler T, Dell-Kuster S, Hostettler KE, Tamm M, Khanna N. Acquisition, analysis, or interpretation of data: Sava M, Sommer G, Bassetti S, Dell-Kuster S, Siegemund M, Diem-Lan V, Woischnig AK, Leuzinger K, Hirsch HH, Weisser M, Battegay M, Hostettler KE, Khanna N. Drafting of the manuscript: Sava M, Sommer G, Daikeler T, Battegay M, Khanna N. Critical revision of the manuscript for important intellectual content: Sava M, Sommer G, Daikeler T, Martinez AE, H. Pargger, Bassetti S, Tamm M, Tschudin-Sutter S, Stoeckle M, Siegemund M, Kaiser L, Weisser M, Battegay M, Bassetti S, Dell-Kuster S, Hostettler KE, Khanna N. Statistical analysis: Dell-Kuster S, Tschudin-Sutter S, Erlanger TE and Wiencierz A. Administrative, technical, or material support: Sava M, Sommer G, Boss R, Zimmer G, Hostettler KE, Khanna N. Supervision: Khanna N. All authors contributed to the final drafting of the manuscript.
References
1
Chen
N
,
Zhou
M
,
Dong
X
,
Qu
J
,
Gong
F
,
Han
Y
, et al.
Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–13. doi:.https://doi.org/10.1016/S0140-6736(20)30211-7
2
Horby
P
,
Lim
WS
,
Emberson
JR
,
Mafham
M
,
Bell
JL
,
Linsell
L
, et al.
Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med. 2020. doi:https://10.1056/NEJMoa2021436.
3
Russell
CD
,
Millar
JE
,
Baillie
JK
. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–5. doi:.https://doi.org/10.1016/S0140-6736(20)30317-2
4
Xu
X
,
Han
M
,
Li
T
,
Sun
W
,
Wang
D
,
Fu
B
, et al.
Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020;117(20):10970–5. doi:.https://doi.org/10.1073/pnas.2005615117
5
Biran
N
,
Ip
A
,
Ahn
J
,
Go
RC
,
Wang
S
,
Mathura
S
, et al.
Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol. 2020;2(10):e603–12. doi:.https://doi.org/10.1016/S2665-9913(20)30277-0
6
Gupta
S
,
Wang
W
,
Hayek
SS
,
Chan
L
,
Mathews
KS
,
Melamed
ML
, et al.; STOP-COVID Investigators. Association Between Early Treatment With Tocilizumab and Mortality Among Critically Ill Patients With COVID-19. JAMA Intern Med. 2021;181(1):41–51. doi:.https://doi.org/10.1001/jamainternmed.2020.6252
7
Ignatius
EH
,
Wang
K
,
Karaba
A
,
Robinson
M
,
Avery
RK
,
Blair
P
, et al.
Tocilizumab for the treatment of COVID-19 among hospitalized patients: A matched retrospective cohort analysis. Open Forum Infect Dis. 2021;8(1):a598. doi:.https://doi.org/10.1093/ofid/ofaa598
8
Stone
JH
,
Frigault
MJ
,
Serling-Boyd
NJ
,
Fernandes
AD
,
Harvey
L
,
Foulkes
AS
, et al.; BACC Bay Tocilizumab Trial Investigators. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333–44. doi:.https://doi.org/10.1056/NEJMoa2028836
9
Hermine
O
,
Mariette
X
,
Tharaux
PL
,
Resche-Rigon
M
,
Porcher
R
,
Ravaud
P
, et al.; CORIMUNO-19 Collaborative Group. Effect of Tocilizumab vs Usual Care in Adults Hospitalized With COVID-19 and Moderate or Severe Pneumonia: A Randomized Clinical Trial. JAMA Intern Med. 2021;181(1):32–40. doi:.https://doi.org/10.1001/jamainternmed.2020.6820
10
Salama
C
,
Han
J
,
Yau
L
,
Reiss
WG
,
Kramer
B
,
Neidhart
JD
, et al.
Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia. N Engl J Med. 2021;384(1):20–30. doi:.https://doi.org/10.1056/NEJMoa2030340
11
Gordon
AC
,
Mouncey
PR
,
Al-Beidh
F
,
Rowan
KM
,
Nichol
AD
,
Arabi
YM
, et al., REMAP-CAP Investigators. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N Engl J Med. 2021;384(16):1491–502. doi:.https://doi.org/10.1056/NEJMoa2100433
12
Horby
PW
,
Pessoa-Amorim
G
,
Peto
L
,
Brightling
CE
,
Sarkar
R
,
Thomas
K
, et al.
Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial. medRxiv. 2021:2021.02.11.21249258. doi:https://doi.org/10.1101/2021.02.11.21249258
13
Hui
DS
,
Joynt
GM
,
Wong
KT
,
Gomersall
CD
,
Li
TS
,
Antonio
G
, et al.
Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60(5):401–9. doi:.https://doi.org/10.1136/thx.2004.030205
14
Leuzinger
K
,
Roloff
T
,
Gosert
R
,
Sogaard
K
,
Naegele
K
,
Rentsch
K
, et al.
Epidemiology of Severe Acute Respiratory Syndrome Coronavirus 2 Emergence Amidst Community-Acquired Respiratory Viruses. J Infect Dis. 2020;222(8):1270–9. doi:.https://doi.org/10.1093/infdis/jiaa464
15
Leuzinger
K
,
Gosert
R
,
Søgaard
KK
,
Naegele
K
,
Bielicki
J
,
Roloff
T
, et al.
Epidemiology and precision of SARS-CoV-2 detection following lockdown and relaxation measures. J Med Virol. 2021;93(4):2374–84. doi:.https://doi.org/10.1002/jmv.26731
16WHO. Clinical management of COVID-19. Geneva: World Health Organization; 2020.
17
Agustí
A
,
Noell
G
,
Brugada
J
,
Faner
R
. Lung function in early adulthood and health in later life: a transgenerational cohort analysis. Lancet Respir Med. 2017;5(12):935–45. doi:.https://doi.org/10.1016/S2213-2600(17)30434-4
18
Zettl
F
,
Meister
TL
,
Vollmer
T
,
Fischer
B
,
Steinmann
J
,
Krawczyk
A
, et al.
Rapid Quantification of SARS-CoV-2-Neutralizing Antibodies Using Propagation-Defective Vesicular Stomatitis Virus Pseudotypes. Vaccines (Basel). 2020;8(3):386. doi:.https://doi.org/10.3390/vaccines8030386
19
Pawar
A
,
Desai
RJ
,
Solomon
DH
,
Santiago Ortiz
AJ
,
Gale
S
,
Bao
M
, et al.
Risk of serious infections in tocilizumab versus other biologic drugs in patients with rheumatoid arthritis: a multidatabase cohort study. Ann Rheum Dis. 2019;78(4):456–64. doi:.https://doi.org/10.1136/annrheumdis-2018-214367
20
Masiá
M
,
Fernández-González
M
,
Padilla
S
,
Ortega
P
,
García
JA
,
Agulló
V
, et al.
Impact of interleukin-6 blockade with tocilizumab on SARS-CoV-2 viral kinetics and antibody responses in patients with COVID-19: A prospective cohort study. EBioMedicine. 2020;60:102999. doi:.https://doi.org/10.1016/j.ebiom.2020.102999
21
Bingham
CO, 3rd
,
Rizzo
W
,
Kivitz
A
,
Hassanali
A
,
Upmanyu
R
,
Klearman
M
. Humoral immune response to vaccines in patients with rheumatoid arthritis treated with tocilizumab: results of a randomised controlled trial (VISARA). Ann Rheum Dis. 2015;74(5):818–22. doi:.https://doi.org/10.1136/annrheumdis-2013-204427
22
van Gassel
RJJ
,
Bels
JLM
,
Raafs
A
,
van Bussel
BCT
,
van de Poll
MCG
,
Simons
SO
, et al.
High Prevalence of Pulmonary Sequelae at 3 Months after Hospital Discharge in Mechanically Ventilated Survivors of COVID-19. Am J Respir Crit Care Med. 2021;203(3):371–4. doi:.https://doi.org/10.1164/rccm.202010-3823LE
23
Frija-Masson
J
,
Debray
MP
,
Gilbert
M
,
Lescure
FX
,
Travert
F
,
Borie
R
, et al.
Functional characteristics of patients with SARS-CoV-2 pneumonia at 30 days post-infection. Eur Respir J. 2020;56(2):2001754. doi:.https://doi.org/10.1183/13993003.01754-2020
24
Zhao
YM
,
Shang
YM
,
Song
WB
,
Li
QQ
,
Xie
H
,
Xu
QF
, et al.
Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. doi:.https://doi.org/10.1016/j.eclinm.2020.100463


