Risk factors for severe outcomes for COVID-19 patients hospitalised in Switzerland during the first pandemic wave, February to August 2020: prospective observational cohort study
DOI: https://doi.org/10.4414/smw.2021.20547
Filipe
Maximiano Sousaab, Maroussia
Roelensc, Brian
Frikerab, Amaury
Thiabaudc, Anne
Itend, Alexia
Cusinie, Domenica
Fluryf, Michael
Buettcherg, Franziska
Zucolh, Carlo
Balmellii, Petra
Zimmermannjk, Nicolas
Troilletl, Danielle
Vuichard-Gysinm, Peter W.
Schreibern, Sara
Bernhard-Stirnemanno, Sarah
Tschudin-Sutterp, Yvonne
Nussbaumer-Ochsnerq, Rami
Sommersteinrs, Roman
Gaudenzt, Jonas
Marschallr, Laurence
Sennu, Céline
Gardiola, Olivia
Keiserc, Gertraud
Schüpbachab, Monica
Wymanna, Beatriz
Vidondoab, CH-Sur Study Group
a Swiss Federal Office of Public Health, Bern, Switzerland
b Veterinary Public Health Institute, University of Bern, Switzerland
c Institute of Global Health, Faculty of Medicine, University of Geneva, Switzerland
d Service of Prevention and Infection Control, Directorate of Medicine and Quality, University Hospital Geneva, HUG, Geneva, Switzerland
e Department of Infectious Diseases, Cantonal Hospital Graubuenden, Chur, Switzerland
f Division of Infectious Diseases and Hospital Epidemiology, Cantonal Hospital St Gallen, Switzerland
g Paediatric Infectious Diseases, Department of Paediatrics, Children’s Hospital, Cantonal Hospital Lucerne, Switzerland
h Paediatric Infectious Diseases, Department of Paediatrics, Cantonal Hospital Winterthur, Switzerland
i Infection Control Programme, EOC Hospitals, Ticino, Switzerland
j Faculty of Science and Medicine, University of Fribourg, Switzerland
k Department of Paediatrics, Fribourg Hospital HFR, Fribourg, Switzerland
l Service of Infectious Diseases, Central Institute, Valais Hospitals, Sion, Switzerland
m Division of Infectious Diseases and Hospital Hygiene, Thurgau Hospital Group Münsterlingen and Frauenfeld, Switzerland
n Division of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich and University of Zurich, Switzerland
o Children's Hospital Aarau, Switzerland
p Division of Infectious Diseases and Hospital Epidemiology, University Hospital Basel and University of Basel, Switzerland
q Clinic for Internal Medicine, Cantonal Hospital, Hospitals Schaffhausen, Switzerland
r Department of Infectious Diseases, Bern University Hospital (Inselspital), Bern, Switzerland
s Infectious Diseases and Hospital Hygiene, Hirslanden Central Switzerland, Lucerne, Switzerland
t Internal Medicine and Infectiology, Cantonal Hospital Nidwalden, Stans, Switzerland
u Division of Infectious Diseases, and Children’s Research Centre, University Children’s Hospital Zurich, Switzerland
Summary
BACKGROUND
As clinical signs of COVID-19 differ widely among individuals, from mild to severe, the definition of risk groups has important consequences for recommendations to the public, control measures and patient management, and needs to be reviewed regularly.
AIM
The aim of this study was to explore risk factors for in-hospital mortality and intensive care unit (ICU) admission for hospitalised COVID-19 patients during the first epidemic wave in Switzerland, as an example of a country that coped well during the first wave of the pandemic.
METHODS
This study included all (n = 3590) adult polymerase chain reaction (PCR)-confirmed hospitalised patients in 17 hospitals from the hospital-based surveillance of COVID-19 (CH-Sur) by 1 September 2020. We calculated univariable and multivariable (adjusted) (1) proportional hazards (Fine and Gray) survival regression models and (2) logistic regression models for in-hospital mortality and admission to ICU, to evaluate the most common comorbidities as potential risk factors.
RESULTS AND DISCUSSION
We found that old age was the strongest factor for in-hospital mortality after having adjusted for gender and the considered comorbidities (hazard ratio [HR] 2.46, 95% confidence interval [CI] 2.33−2.59 and HR 5.6 95% CI 5.23−6 for ages 65 and 80 years, respectively). In addition, male gender remained an important risk factor in the multivariable models (HR 1.47, 95% CI 1.41−1.53). Of all comorbidities, renal disease, oncological pathologies, chronic respiratory disease, cardiovascular disease (but not hypertension) and dementia were also risk factors for in-hospital mortality. With respect to ICU admission risk, the pattern was different, as patients with higher chances of survival might have been admitted more often to ICU. Male gender (OR 1.91, 95% CI 1.58−2.31), hypertension (OR 1.3, 95% CI 1.07−1.59) and age 55–79 years (OR 1.15, 95% CI 1.06−1.26) are risk factors for ICU admission. Patients aged 80+ years, as well as patients with dementia or with liver disease were admitted less often to ICU.
CONCLUSION
We conclude that increasing age is the most important risk factor for in-hospital mortality of hospitalised COVID-19 patients in Switzerland, along with male gender and followed by the presence of comorbidities such as renal diseases, chronic respiratory or cardiovascular disease, oncological malignancies and dementia. Male gender, hypertension and age between 55 and 79 years are, however, risk factors for ICU admission. Mortality and ICU admission need to be considered as separate outcomes when investigating risk factors for pandemic control measures and for hospital resources planning.
Introduction
As clinical signs of COVID-19 differ widely among individuals, from asymptomatic or very mild infection to severe disease with often fatal outcome [1], the definition of risk groups has important consequences for the recommendations to the public, control measures such us vaccination plans and patient management, and needs to be reviewed regularly. Different risk groups have been reported for different outcomes of interest, namely hospital admission, admission to an intensive care unit (ICU) and mortality (which in turn can be in-hospital mortality or death without hospitalisation). A large review of 22 published survival models [2] concluded that “oxygen saturation on room air and patient age are strong predictors of deterioration and mortality among hospitalised adults with COVID-19, respectively”, and that additional factors did not add value to these univariable predictors. Extensive studies carried out in the UK on in-hospital mortality [3] and COVID-19-related death [4] have shown that other comorbidities are also associated with severe COVID-19 disease and death, but the fully adjusted hazard ratios obtained are often under 2 (except age older than 60 years, severe and acute forms of haematological malignancy, reduced kidney function, stroke or dementia, neurological disease, organ transplant and immunosuppression) [4]. Even though hypertension is often reported as the most common comorbidity in hospitalised COVID-19 patients, its hazard ratio for COVID-19-related death is very close to the reference value 1 (both adjusted for age and gender and fully adjusted for all comorbidities) [4].
During the first wave of the COVID-19 pandemic in 2020, the health system in Switzerland was not overwhelmed in terms of hospital bed usage and intensive care capacity. Public health (behavioural and lock down) measures ordered by the federal government, the restriction of elective procedures and an increase in ICU capacity (particularly ventilator-equipped beds) helped to prevent resource scarcity at national level [5]. In Switzerland, a hospital surveillance system for COVID-19 (CH-Sur), coordinated by the Swiss Federal Office of Public Health and the Institute of Global Health of the University of Geneva [6], consisting of 20 voluntary participating large central and university hospitals across the country, was put in place. From the end of February to the end of August 2020, the CH-Sur surveillance system provided clinical information for 3656 episodes of 3651 patients (both paediatric and adult), which account for more than two thirds of all patients mandatorily reported to be hospitalised for COVID-19 in Switzerland up to that date [6]. Using data from this in-hospital surveillance system, the aim of this study was to explore risk factors for in-hospital mortality and admission to an ICU due to COVID-19 in Switzerland, as an example of a country which was able to cope well during the first wave of the pandemic.
Materials and methods
Participants
Adults (≥18 years of age) hospitalised for at least 24 hours in any of the hospitals participating in the CH-Sur, and with a - diagnosis of COVID-19 confirmed by a polymerase chain-reaction (PCR) test were included. A more detailed description of the data collection and descriptive statistics is provided in Thiabaud et al. [6]. Data were entered online in a secured REDCap database (Research Electronic Data Capture, Vanderbilt University, US, hosted by the University Hospital Geneva at their Clinical Trial Unit ).
The case report form (CRF) included demographic information (age and gender), admission data (height, body weight, body mass index, obesity as assessed by the physician, severity score at admission [CURB-65 score], and information on the episode [e.g., type of exposure, starting date of symptoms, symptoms and description of laboratory results] and was agreed with the participating hospitals to be compulsory. Additional complementary clinical information (e.g., comorbidities, smoking, antiviral treatment, complications and admission to ICU and type of ventilation) was optional, whereas outcome or follow-up (e.g., death, discharge and transfer) was mandatory. The CRF was to be completed within 48 hours of the patient’s admission, whereas the follow-up and optional part of the questionnaire was completed at patient’s discharge, transfer or death. Discharged patients included both recovered and those discharged (presumably not infectious anymore) to a nursing home. Transfer was defined as transfer to a hospital outside our surveillance network. Patients transferred to a hospital inside the surveillance network retained their unique identifier in order to account for some patients entering and leaving the same or different hospitals several times. The data collection allowed for multiple re-admissions per patient. If re-admission to a CH-Sur hospital occurred within 30 days from the date of discharge of the first admission, it was considered as the same episode (thus retaining the original entry date as the admission date). In the cases in which the re-admission occurred more than 30 days after the date of discharge, it was considered a new episode with a new hospitalisation admission date. In the case of patients with several re-admissions, only the last or final outcome was considered for the present analysis.
Data entry started on 1 March 2020, was prospective and is still ongoing. Data were also entered in CH-Sur database retrospectively (from the hospital’s database), when a sudden surge of cases caused delays in data entry or if a hospital joined after the project start. The data for the present study included patients hospitalised up to 1 September 2020, and was extracted on 15 January 2021, just after one large hospital transferred the complementary part of the data by an electronic data transfer from the hospital’s database to the CH-Sur database.
Ethics approval
This study was submitted and approved by the Geneva Ethics Committee (CCER) and by all hospitals’ local Ethics Committees through the Swiss ethics BASEC submission system, under reference 2020-00827.
Statistical analysis
The following covariates at the time of hospitalisation were chosen a priori: age (as a continuous numerical variable), gender, hypertension, diabetes, dementia, chronic respiratory disease including asthma, cardiovascular disease, renal disease, oncological pathologies, liver disease, human immunodeficiency virus (HIV) infection, tuberculosis, immunosuppression (other than HIV), smoking and obesity (yes/no, as diagnosed by the treating physician).
We first explored the effect of different covariates on the two primary outcomes (death and admission to ICU) using (1) univariable models, (2) multivariable models using only the complete cases for each variable and (3) multivariable models using multiple imputation. Imputation was carried out using R package mice (multiple imputation with chained equations). For each analysis, the regression models were run on 20 imputed datasets and estimates then combined using Rubin’s rules [7].
Age was fitted as a continuous variable using restricted cubic splines [8, 9]. Regression coefficient estimates for given/specific ages were obtained after having specified a reference age. The reference age was set to the first quartile of total patients, 55 years.
Duration of hospital stay in days was measured from the day of the first positive test for COVID-19 infection until death, discharge or transfer to another hospital not participating in the CH-Sur, whichever occurred first. Patients admitted to the hospital but for whom the hospitalisation outcome was unknown were considered as still in care, and censored at database closure. First, the cumulative incidence of mortality, overall and by age, gender and comorbidities were visualised and then hazard ratios computed [10–12] using the R packages cmprsk and survival. Given the elevated median age of the patients hospitalised and the existence of comorbidities, we compared two types of proportional hazard models, Cox, and Fine and Gray models, with and without taking into account competing risks, respectively. In the Cox model, the outcome of interest was death, and all other outcomes (discharge, transfer, still in care) were considered equivalent to censoring. In the Fine and Gray model, the outcome of interest was also death, but discharge and transfer were considered as competing events, and still in care was considered as censoring [13]. In the Cox approach, out of a total 543 adult deaths, three patients who were tested on the day of death were rendered a time to event equal to zero and had to be excluded, thus leaving 540 deaths out of 3587 patients that could be used for the survival analysis. Because of the higher number of different variables that are necessary to compute the outcome variable (with four categories instead of just two: death, discharged, transferred and still in care) in the Fine and Gray approach, a higher number of unknown or non-plausible conditions appeared, which had to be excluded (total n = 40): (1) three deceased patients tested on the day of death (time to event zero, as in the Cox model), (2) 16 additional survivors also with zero time to event (in this case, discharge or transfer), (3) one patient reported discharged one day before confirmed diagnosis, and finally, (4) 20 patients with unknown time to event. Thus, for the Fine and Gray approach, 540 deaths out of 3550 patients could be used. Preliminary analysis showed that both types of models rendered similar results so we opted for the Fine and Gray model for the final analysis, since this model is more adequate for elderly and fragile patients.
Risk factors for ICU admission were analysed by using logistic regression models. Patients without any ICU admission recorded were assumed to not having been to ICU. Continuous variables such as age were fitted using splines (see above). In a preliminary analysis, the effect of clustering within a hospital was evaluated using random intercept models fitted additionally. For each model, the intra-class correlation coefficient (ICC) was calculated. Owing to a low number of patients per hospital, only 10 hospitals with at least 8 patients each could be included in these analyses. Since the ICCs were always smaller than 10%, we opted to exclude the random effects.
We used R version 4.0.3 (R Core Team, Vienna, Austria).
Results
For the present study, only adult patients (n = 3590, 543 deaths and 710 patients admitted to an ICU) entered in the database as per 1 September 2020, 8:58h (last date of extraction 15 January 2021) were included in the analysis. The data thus originate from 17 of the 20 participating hospitals, as 3 purely paediatric hospitals were excluded. Of the 3590 patients, 543 (15.1%) died, 220 (6.1%) were transferred to another hospital, 2793 (77.8%) were discharged from the hospital and 34 (0.95%) can be assumed to still be hospitalised as of 1 September 2020.
The median age of admitted patients was 68 years (interquartile range [IQR] 55–80) years. A total of 2138 (59.5%) were men; 859/3590 (23.9%) patients had no comorbidity and 37/859 (4.3%) of these patients died. Table 1 (and supplementary table S1 in the appendix) shows the outcome for patients stratified by sex, age category, number of comorbidities and CURB-65 score at admission. On admission, 1106 (30.8%) patients presented a CURB-65 score of zero, yet 142/1106 (12.8%) of these patients required later admission to ICU of whom 9 (0.8%) died. Thirty-eight percent of patients with a CURB-65 of three or more on admission presented a fatal outcome.
Table 1 Demographic information and number of comorbidities per patient for 3590 adult patients hospitalised for COVID-19 in Switzerland.
|
Characteristics
|
Categories
|
n
|
Not admitted to ICU, alive
|
Admitted to ICU, alive
|
Not admitted to ICU, dead
|
Admitted to ICU, dead
|
Sex
|
Male |
2138 |
1372 (64.2%) |
404 (18.9%) |
244 (11.4%) |
118 (5.5%) |
| Female |
1452 |
1135 (78.2%) |
136 (9.4%) |
129 (8.9%) |
52 (3.6%) |
Age (years)
|
18–50 |
591 |
501 (84.8%) |
85 (14.4%) |
2 (0.3%) |
3 (0.5%) |
| 50−64 |
980 |
713 (72.8%) |
221 (22.6%) |
10 (1%) |
36 (3.7%) |
| 65−80 |
1119 |
724 (64.7%) |
210 (18.8%) |
94 (8.4%) |
91 (8.1%) |
| 80+ |
900 |
569 (63.2%) |
24 (2.7%) |
267 (29.7%) |
40 (4.4%) |
Number of comorbidities
|
0 |
859 |
695 (80.9%) |
127 (14.8%) |
20 (2.3%) |
17 (2%) |
| 1 |
846 |
630 (74.5%) |
126 (14.9%) |
50 (5.9%) |
40 (4.7%) |
| 2 |
779 |
542 (69.6%) |
116 (14.9%) |
77 (9.9%) |
44 (5.6%) |
| 3 |
542 |
336 (62%) |
89 (16.4%) |
86 (15.9%) |
31 (5.7%) |
| >3 |
564 |
304 (53.9%) |
82 (14.5%) |
140 (24.8%) |
38 (6.7%) |
CURB−65 score
|
0 |
1106 |
954 (86.3%) |
133 (12%) |
10 (0.9%) |
9 (0.8%) |
| 1 |
1236 |
902 (73%) |
191 (15.5%) |
81 (6.6%) |
62 (5%) |
| 2 |
819 |
477 (58.2%) |
128 (15.6%) |
158 (19.3%) |
56 (6.8%) |
| 3 |
345 |
154 (44.6%) |
68 (19.7%) |
95 (27.5%) |
28 (8.1%) |
| >3 |
84 |
20 (23.8%) |
20 (23.8%) |
29 (34.5%) |
15 (17.9%) |
Median duration hospitalisation from confirmatory PCR test was 9 days (IQR 5–18). Overall, 710 (19.42%) had been admitted to an ICU: median length of stay 12 days (IQR 4–21, range 0–107). Of the 710 patients treated in intensive care, 95 (13.4%) did not receive ventilation, 53 (7.5%) received only noninvasive ventilation, 519 (73.1%) were ventilated invasively and 42 (5.9%) received invasive ventilation and extra-corporeal membrane oxygenation (ECMO). Of the patients who died, 170 (31.3%) had been admitted to the ICU; 7 (1.3%) received only noninvasive ventilation, 132 (24.3%) were ventilated invasively and 18 (3.3%) received ECMO.
The most common comorbidity was hypertension (~65% of patients older than 65 years of age, for both genders), followed by cardiovascular disease (51% vs 38% in men and women, respectively, older than 65 years of age) (table 2). Roughly a third of patients older than 65 presented a history of diabetes, and a quarter of older patients had renal disease, chronic respiratory disease or were obese. Less common comorbidities were cancer, followed by immunosuppression and liver disease; tuberculosis and HIV infection were very rare. With the exception of dementia and obesity, comorbidities occurred more often in men. Smoking was twice as common in men (14%) than in women (7%). However, a quarter of patients’ files did not have information on obesity and almost 43% were also missing data for smoking, so these results might contain bias.
Table 2 Frequency of comorbidities by age category and gender for 3590 adult patients hospitalised for COVID-19 in Switzerland.
| |
Men <65
|
Women <65
|
Men >65
|
Women >65
|
| Total patients per age group |
970 |
601 |
1168 |
851 |
| Diabetes |
149 (15.4%) |
79 (13.1%) |
359 (30.7%) |
206 (24.2%) |
| Cardiovascular disease |
126 (13%) |
41 (6.8%) |
599 (51.3%) |
328 (38.5%) |
| Hypertension |
280 (28.9%) |
141 (23.5%) |
773 (66.2%) |
556 (65.3%) |
| Renal disease |
58 (6%) |
25 (4.2%) |
310 (26.5%) |
193 (22.7%) |
| Liver disease |
44 (4.5%) |
21 (3.5%) |
56 (4.8%) |
37 (4.3%) |
| Oncological pathology |
55 (5.7%) |
36 (6%) |
196 (16.8%) |
95 (11.2%) |
| HIV |
11 (1.1%) |
7 (1.2%) |
5 (0.4%) |
2 (0.2%) |
| Tuberculosis |
10 (1%) |
4 (0.7%) |
5 (0.4%) |
2 (0.2%) |
| Chronic respiratory disease incl. asthma |
152 (15.7%) |
103 (17.1%) |
294 (25.2%) |
178 (20.9%) |
| Immunosuppression other than HIV |
69 (7.1%) |
45 (7.5%) |
86 (7.4%) |
68 (8%) |
| Dementia |
8 (0.8%) |
7 (1.2%) |
133 (11.4%) |
137 (16.1%) |
A combination of hypertension, cardiovascular medication, renal disease and diabetes was observed in many patients (fig. 1). About 67% of patients older than 65 years had one or more of these conditions.
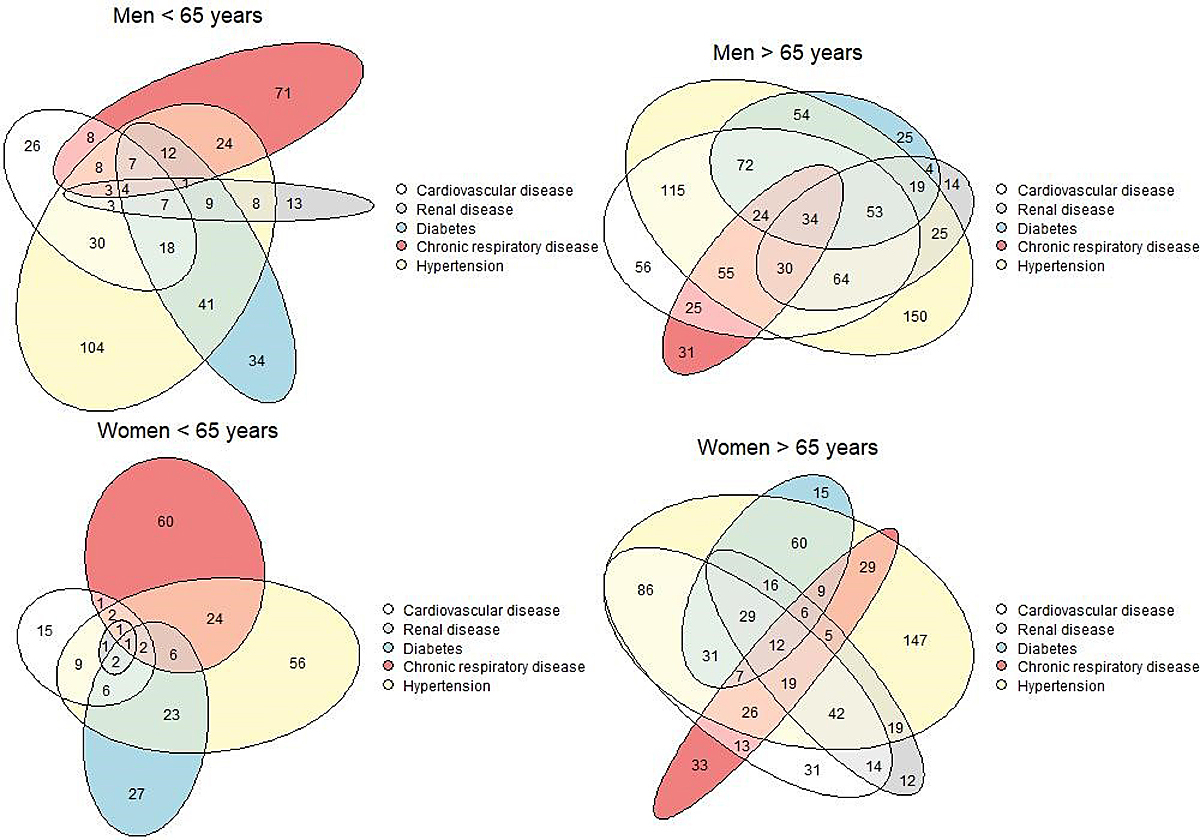
Figure 1 Scaled Euler diagrams by age and gender showing the overlap of the five most common comorbidities for 3590 adult patients hospitalised for COVID-19 in Switzerland. Data included up to 1 September 2020 (date of extraction 15 January 2021). Missing values have been excluded.
Most comorbidities were more frequent with increasing age, except chronic respiratory disease, diabetes and obesity (not presented because of a high proportion of missing values), which appeared to be less frequent for patients older than 80 years of age (fig. 2).
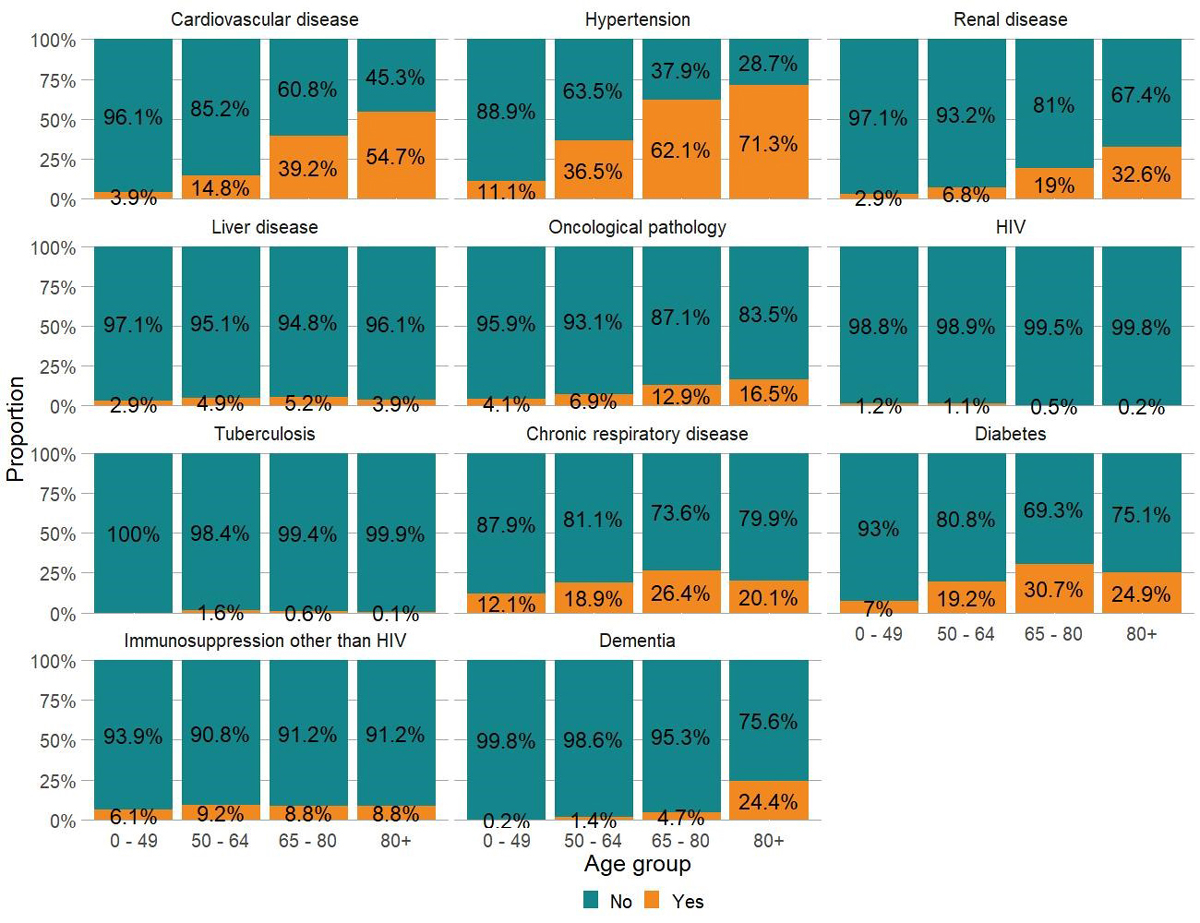
Figure 2 Proportions of patients with and without comorbidities in four age classes for 3590 adult patients hospitalised for COVID-19 in Switzerland. Data included up to 1 September 2020 (date of extraction 15 January 2021). Missing values have been excluded. Obesity and smoking are not presented in this figure, owing to a high proportion of missing values, 25% and 43%, respectively.
Cumulative incidence of survival
Overall the cumulative probability of death was around 12% after 1 month and reached 15% (slightly higher in men) after 3 months (fig 3, panels A and B). However, when considering different age and gender groups, we found that the cumulative probability of death in men 80+ was above 40% and that of women almost 25% (fig. 3, panels C and D). Most deaths in the elderly aged 80+ occurred within 15 days after confirmed COVID-19 infection and many more 80+ men than women died. When only elderly patients with the most common comorbidities were plotted a similar pattern was observed (see supplementary figure S1 in the appendix).
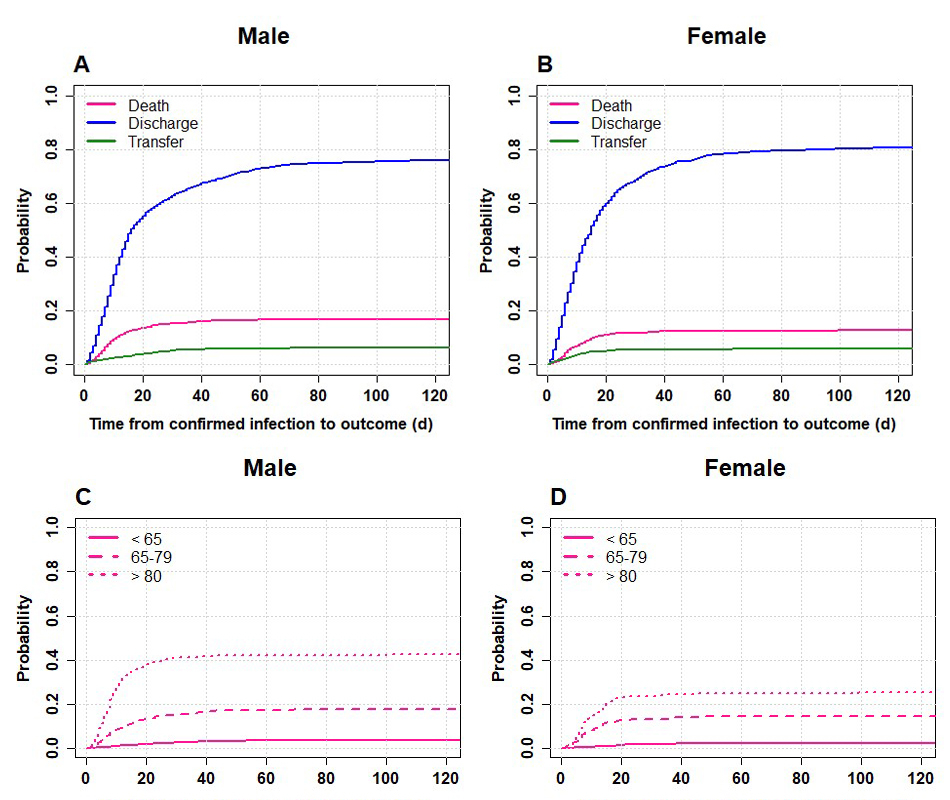
Figure 3 Cumulative distribution functions by gender for 3550 adult patients hospitalised for COVID-19 in Switzerland. Left panels A,C: male patients, right panels B,D: female patients. Panels A,B: all three outcomes – death, discharge and transfer – Panels C,D: outcome death in three age classes. Data included up to 1 September 2020 (date of extraction 15 January 2021). Time to event is defined as the time elapsed from confirmed COVID-19 infection to the event of interest (death, discharge, transfer).
Risk factors for in-hospital mortality according to the Fine and Gray survival regression model
All versions of the Fine and Gray survival regression model results (univariable, multivariable complete cases and multivariable with multiple imputations) were congruent with each other (table 3). Consistently, older age, male gender, renal disease, oncological pathologies, chronic respiratory disease, cardiovascular disease and dementia were associated with a higher risk of in-hospital death. The highest adjusted hazard ratios were obtained for older age (age was fitted as a numerical continuous variable, see Methods section), whereas the rest of the comorbidities presented adjusted hazard ratios smaller than 2. The characteristics of the patients used for these analyses are presented in table S2 (in the appendix). The proportion of missing values by the final date of data extraction on 15 January 2021 was very low for all variables except for immunosuppression (but in all previous data extractions in 2020 was around 10–15% and this is why we performed multiple imputation). Due to sparse data, HIV and tuberculosis could not be considered as risk factors in the final regression models.
Table 3 Hazard ratios (95% confidence intervals) for in-hospital mortality associated with age and comorbidities in hospitalised COVID-19 patients (n = 3550) in Swiss hospitals.
| |
3550 individuals included
|
Univariate, complete cases
(540 deaths)
|
Multivariable complete cases
(540 deaths)
|
Multivariable, multiple imputation
(540 deaths)
|
Age (years)
|
|
p <0.001 |
p <0.001 |
p <0.001 |
| 35 |
0.06 (0.03−0.13) |
0.11 (0.05−0.25) |
0.1 (0.08−0.12) |
| 55 |
1.0 (ref.) |
1.0 (ref.) |
1.0 (ref.) |
| 65 |
2.94 (2.31−3.73) |
2.4 (1.89−3.06) |
2.46 (2.33−2.59) |
| 80 |
7.61 (5.61−10.31) |
5.75 (4.18−7.9) |
5.6 (5.23−6) |
Gender
|
|
p <0.001 |
p <0.001 |
p <0.001 |
| Female |
1.0 (ref.) |
1.0 (ref.) |
1.0 (ref.) |
| Male |
1.37 (1.14−1.63) |
1.42 (1.17−1.74) |
1.47 (1.41−1.53) |
Renal disease
|
|
p <0.001 |
p <0.001 |
p <0.001 |
| No |
1.0 (ref.) |
1.0 (ref.) |
1.0 (ref.) |
| Yes |
3.43 (2.88−4.09) |
1.65 (1.34−2.03) |
1.56 (1.5−1.63) |
Oncological pathology
|
|
p <0.001 |
p <0.001 |
p <0.001 |
| No |
1.0 (ref.) |
1.0 (ref.) |
1.0 (ref.) |
| Yes |
2.55 (2.08−3.13) |
1.56 (1.24−1.95) |
1.55 (1.48−1.63) |
Chronic respiratory disease (including asthma)
|
|
p <0.001 |
p = 0.021 |
p <0.001 |
| No |
1.0 (ref.) |
1.0 (ref.) |
1.0 (ref.) |
| Yes |
1.59 (1.32−1.92) |
1.27 (1.04−1.56) |
1.22 (1.17−1.27) |
Cardiovascular disease
|
|
p <0.001 |
p = 0.015 |
p <0.001 |
| No |
1.0 (ref.) |
1.0 (ref.) |
1.0 (ref.) |
| Yes |
3.49 (2.94−4.14) |
1.3 (1.05−1.61) |
1.44 (1.38−1.51) |
Liver disease
|
|
p = 0.14 |
p = 0.77 |
p = 0.016 |
| No |
1.0 (ref.) |
1.0 (ref.) |
1.0 (ref.) |
| Yes |
1.32 (0.92−1.91) |
1.06 (0.71−1.6) |
1.11 (1.02−1.2) |
Dementia
|
|
p <0.001 |
p = 0.26 |
p <0.001 |
| No |
1.0 (ref.) |
1.0 (ref.) |
1.0 (ref.) |
| Yes |
2.93 (2.35−3.65) |
1.17 (0.89−1.52) |
1.22 (1.16−1.29) |
Diabetes
|
|
p <0.001 |
p = 0.28 |
p = 0.001 |
| No |
1.0 (ref.) |
1.0 (ref.) |
1.0 (ref.) |
| Yes |
1.58 (1.32−1.9) |
1.12 (0.91−1.38) |
1.07 (1.03−1.12) |
Hypertension
|
|
p <0.001 |
p = 0.69 |
p = 0.26 |
| No |
1.0 (ref.) |
1.0 (ref.) |
1.0 (ref.) |
| Yes |
2.26 (1.89−2.7) |
0.96 (0.78−1.18) |
0.98 (0.93−1.02) |
Immunosuppression (other than HIV)
|
|
p = 0.0037 |
p = 0.71 |
p = 0.0098 |
| No |
1.0 (ref.) |
1.0 (ref.) |
1.0 (ref.) |
| Yes |
1.51 (1.14−1.99) |
1.06 (0.79−1.41) |
1.08 (1.02−1.15) |
Factors associated with admission to ICU
Male patients, patients with hypertension, or aged between 55 and 79 years were admitted more often to the ICU (table 4). Patients aged 80+, as well as patients with dementia or with liver disease were admitted less often to an ICU. The characteristics of the patients used for these analyses are presented in table S3 (appendix).
Table 4 Odds ratios (95%CI) for ICU admission in hospitalised COVID-19 patients (n = 3590) in Swiss hospitals.
| |
3590 individuals included
|
Univariate, complete cases
(710 nr. ICU)
|
Multivariable, complete cases
(710 nr. ICU)
|
Multivariable, multiple imputation
(710 nr. ICU)
|
Age (years)
|
|
p <0.001 |
p <0.001 |
p <0.001 |
| 35 |
0.26 (0.2−0.34) |
0.27 (0.2−0.37) |
0.31 (0.23−0.4) |
| 55 |
1.0 (ref.) |
1.0 (ref.) |
1.0 (ref.) |
| 65 |
1.22 (1.12−1.32) |
1.16 (1.06−1.27) |
1.15 (1.06−1.26) |
| 80 |
0.42 (0.35−0.51) |
0.4 (0.31−0.51) |
0.41 (0.33−0.51) |
Gender
|
|
p <0.001 |
p <0.001 |
p <0.001 |
| Female |
1.0 (ref.) |
1.0 (ref.) |
1.0 (ref.) |
| Male |
2.17 (1.81−2.61) |
1.85 (1.51−2.27) |
1.91 (1.58−2.31) |
Renal disease
|
|
p = 0.022 |
p = 0.95 |
P = 0.6 |
| No |
1.0 (ref.) |
1.0 (ref.) |
1.0 (ref.) |
| Yes |
0.76 (0.6−0.96) |
1.01 (0.75−1.35) |
0.93 (0.71−1.22) |
| Oncological pathology |
|
p = 0.01 |
p = 0.13 |
p = 0.062 |
| No |
1.0 (ref.) |
1.0 (ref.) |
1.0 (ref.) |
| Yes |
0.68 (0.51−0.91) |
0.77 (0.55−1.08) |
0.74 (0.54−1.02) |
Chronic respiratory disease (including asthma)
|
|
p = 0.27 |
p = 0.83 |
p = 0.59 |
| No |
1.0 (ref.) |
1.0 (ref.) |
1.0 (ref.) |
| Yes |
1.12 (0.92−1.37) |
1.03 (0.82−1.29) |
1.06 (0.86−1.31) |
Cardiovascular disease
|
|
p = 0.69 |
p = 0.14 |
p = 0.28 |
| No |
1.0 (ref.) |
1.0 (ref.) |
1.0 (ref.) |
| Yes |
0.96 (0.81−1.15) |
1.19 (0.94−1.51) |
1.12 (0.91−1.4) |
Liver disease
|
|
p = 0.088 |
p = 0.023 |
p = 0.0085 |
| No |
1.0 (ref.) |
1.0 (ref.) |
1.0 (ref.) |
| Yes |
0.68 (0.43−1.06) |
0.56 (0.34−0.92) |
0.53 (0.33−0.85) |
Dementia
|
|
p <0.001 |
p = 0.019 |
p = 0.057 |
| No |
1.0 (ref.) |
1.0 (ref.) |
1.0 (ref.) |
| Yes |
0.32 (0.2−0.49) |
0.48 (0.26−0.89) |
0.63 (0.39−1.01) |
Diabetes
|
|
p <0.001 |
p = 0.1 |
p = 0.066 |
| No |
1.0 (ref.) |
1.0 (ref.) |
1.0 (ref.) |
| Yes |
1.41 (1.17−1.7) |
1.22 (0.96−1.53) |
1.22 (0.99−1.51) |
Hypertension
|
|
p = 0.021 |
p = 0.036 |
p = 0.0089 |
| No |
1.0 (ref.) |
1.0 (ref.) |
1.0 (ref.) |
| Yes |
1.21 (1.03−1.43) |
1.26 (1.01−1.56) |
1.3 (1.07−1.59) |
Immunosuppresion (other than HIV)
|
|
p = 0.63 |
p = 0.74 |
p = 0.84 |
| No |
1.0 (ref.) |
1.0 (ref.) |
1.0 (ref.) |
| Yes |
0.92 (0.67−1.28) |
0.94 (0.67−1.33) |
0.97 (0.68−1.36) |
Discussion
Main findings
Our study found that the effect of increasing age was by far the strongest factor (fully adjusted hazard ratios larger than 2 for ages older than 55 years) for in-hospital mortality after having adjusted for gender and the most common comorbidities. In addition, male gender remained an important risk factor in the multivariable models. Of all comorbidities that we were able to explore with the available data, mainly renal disease and oncological pathologies, cardiovascular disease, chronic respiratory disease and dementia were associated with a higher risk of in-hospital mortality, after adjustment for age and gender. The higher mortality among patients with renal disease may reflect the higher burden of comorbidity known for these patients.
Male patients, those with hypertension and aged between 55 and 79 years were more likely to be admitted to an ICU, after adjustment for all comorbidities. The pattern was different from that of death, as patients with higher chances of survival (e.g., patients younger than 80 years) were admitted to an ICU more often than aged patients with multiple comorbidities (especially those with liver disease and dementia). Given that triage was not necessary in Switzerland during the first wave [5], these results suggest that these patients might have had advanced care planning, or might have more often decided to refrain from intensive care treatment to prolong life.
Strengths and weaknesses
Our study has a very high coverage/surveillance rate as CH-Sur was able to collect and analyse data of about 80% of all COVID-19 hospitalisations reported mandatorily in Switzerland up to 1 September 2020. All regions are represented, and each contributing hospital reported all patients. On the other hand, due to the circumstances of data collection during the pandemic, this and other similar COVID-19 hospital datasets are susceptible to collider bias [14], see also [15] for an in-depth analysis.
In spite of its high coverage, the size of the dataset inevitably sets limitations. The low frequency of comorbidities, such as HIV and tuberculosis, did not allow their inclusion in the regression models, but these have not been reported as risk factors in other studies. Asthma could only be analysed as included in chronic respiratory disease. Ethnicity could not be evaluated because it was absent in the data catalogue. Other tentative risk factors such obesity and smoking presented at the time of analysis a non-negligible fraction of missing data, which did not allow further analysis, but are currently subject of an analysis that includes the second winter wave (October 2020 to present). Obesity has already been recognised as a risk factor for symptomatic COVID-19, especially for hospital admission and ICU admission, but the risk for mortality is lower (overall odds ratio for severe COVID-19 disease of 2) [16].
Even though comorbidities in CH-Sur are medical diagnosis-based on precise criteria, no distinction was made between severe and moderate forms of comorbidities. For this reason it is possible that our hazard ratios might be on the lower range of the estimate. A large population study from the UK has shown that the risk of severe COVID disease and death is higher with more severe stages of some comorbidities [4].
Comparison with other studies
In contrast to other studies that included a mixture of comorbidities and laboratory markers in their models [2] and references therein, we chose to include only comorbidities (i.e., diagnoses from medical records) as risk factors. Studies that include laboratory markers often aim to develop scores for patient management or to predict in-hospital mortality, which is a focus different from the one in our study. One advantage of including fewer and less correlated factors in the regression models is the reduction of multicollinearity, which is known to increase the uncertainty of the estimates.
Our findings for mortality are consistent with a review of 22 published survival models [2], which showed that age is the strongest predictor for in-hospital mortality. We also found similar risk factors as the extensive studies carried out in the UK on in-hospital mortality [3] and COVID-19-related death (which included suspected cases) [4]. Of note, our estimate of hazard ratios for ages older than 80 years is about half the hazard ratio mortality for the same age class reported in the UK for in-hospital mortality [3], whereas the rest of our estimates were similar in magnitude.
As for the mechanisms upon which age is responsible for severe COVID-19 disease, they are currently the subject of intensive research. Some studies [17, 18] have proposed a certain angiotensin converting-enzyme (ACE) profile in the bloodstream in the elderly; others are investigating immuno-senescence (weakened immune system) [19, 20] as the cause for higher susceptibility or propensity to more severe COVID disease at higher ages.
This same abovementioned review [2] reported oxygen saturation upon admission as the best predictive factor for deterioration while in the hospital (clinical deterioration here measured as a composite outcome of both ICU admission and death, or progression to severe COVID-19). Given that this marker was not available in our data catalogue, we cannot confirm this result. Our results for ICU admission partly concur with those of Galloway [21] and other references in [2] (e.g., Huang et al. [22]) which reported hypertension and male gender as risk factors for the combined outcome ICU admission and death. In contrast to these last three mentioned studies, we considered both outcomes, death and ICU admission, separately.
With respect to implications for policy, and the definition of population at risk at a national level [23], evaluating risk factors for in-hospital mortality and ICU admission is of utmost importance in order to better define the population at risk for adverse outcomes who need to be protected by public health measures such as the current and future vaccination programmes. The risk factors can be very different for ICU admission and in-hospital mortality, as we have shown here by considering these outcomes separately. Hypertension seemed associated with ICU admission, whereas renal disease was a stronger risk factor for in-hospital mortality. Older age and male gender were, however, common factors for both outcomes.
During the first wave of the epidemic in Switzerland, access to care was always ensured even in the two most affected regions. In-hospital mortality was much lower in Switzerland than in the UK (15% [6] vs 25% [3] respectively). A more controlled epidemic combined with a high standard and easy access to medical care (better management of patients with multiple morbidities) might have resulted in this higher survival in Switzerland. A different picture might emerge for the second autumn/winter wave of the epidemic in Switzerland, so future studies should address the comparison between the two waves.
Conclusion
We conclude that increasing age is the most an important risk factor for in-hospital mortality in hospitalised COVID-19 patients in Switzerland, along with male gender and followed by the presence of comorbidities such as renal diseases, chronic respiratory and cardiovascular disease, oncological malignancies and dementia. In contrast, male gender, hypertension and age between 55 and 79 years are risk factors for ICU admission. Mortality and ICU admission outcomes need to be considered separately when investigating risk factors for pandemic control measures (such us vaccination priority groups) and for hospital resource planning.
Accessing the data
The anonymised data can be accessed through a multi-stage process described in Thiabaud et al. [6] (see appendix): “Applicants must fill a concept-sheet and send it to the team in charge of the study. An Executive Committee of experts and representatives of hospital participants will review the concept. Depending on the goal of the analysis, additional ethics clearance might be needed. Data will be restricted to the request and shared through a secure platform.”
Author contributions, CH-Sur Study Group and acknowledgments
Members of the CH-Sur Study Group: Stephan Harbarth, Andreas Widmer, Miriam Vázquez, Lauro Damonti, Christoph Kuhm, Thomas Riedel, Ulrich Heininger, Christoph Berger, Natascia Corti, Anita Uka, Anita Niederer-Loher, Philipp Kaiser, Stefan Kuster, Mirjam Mäusezahl, Claudia Scheuter
Author contributions: F. Maximiano Sousa and M. Roelens are shared first authors. M. Wymann and B. Vidondo are shared last authors.
FM, BF contributed to data analysis, graphics and writing the manuscript; BV, MR contributed to the research question, statistical methods, data analysis, graphics and writing the manuscript; OK, CG, GS, MW contributed to the study conception, coordination between participating hospitals and writing the manuscript; AT, AI contributed to the database implementation, development and maintenance, as well as to ensure and increase data quality and writing the manuscript; The rest of the authors all contributed to the study conception, data collection and writing the manuscript.
The authors would like to thank all the participating centres’ teams, study nurses, and physicians for their hard work and commitment to the study. We also thank all our colleagues from the Veterinary Public Health of the University of Bern for the fruitful discussions, and for their committed, non-remunerated contribution to produce automatised weekly reports using CH-Sur data in spring 2020 (provided R software code, R Mark Down files and R shiny apps). Also, we thank Jan Hattendorf and Torsten Hothorn for discussions on the statistical methods, together with Barbara Bertisch for discussions on the interpretation of results. The Clinical Research Centre (Geneva University Hospitals and Faculty of Medicine) hosted the database.
References
1
Wang
Y
,
Wang
Y
,
Chen
Y
,
Qin
Q
. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020;92(6):568–76. doi:.https://doi.org/10.1002/jmv.25748
2
Gupta
RK
,
Marks
M
,
Samuels
THA
,
Luintel
A
,
Rampling
T
,
Chowdhury
H
, et al.; UCLH COVID-19 Reporting Group. Systematic evaluation and external validation of 22 prognostic models among hospitalised adults with COVID-19: an observational cohort study. Eur Respir J. 2020;56(6):2003498. doi:.https://doi.org/10.1183/13993003.03498-2020
3
Docherty
AB
,
Harrison
EM
,
Green
CA
,
Hardwick
HE
,
Pius
R
,
Norman
L
, et al.; ISARIC4C investigators. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369(March):m1985. doi:.https://doi.org/10.1136/bmj.m1985
4
Williamson
EJ
,
Walker
AJ
,
Bhaskaran
K
,
Bacon
S
,
Bates
C
,
Morton
CE
, et al.
Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–6. doi:.https://doi.org/10.1038/s41586-020-2521-4
5
Swiss Academy Of Medical Sciences. COVID-19 pandemic: triage for intensive-care treatment under resource scarcity (3rd, updated version). Swiss Med Wkly. 2020;150:w20401. doi:.https://doi.org/10.4414/smw.2020.20401
6
Thiabaud
A
,
Iten
A
,
Balmelli
C
,
Senn
L
,
Troillet
N
,
Widmer
A
, et al.
Cohort profile: SARS-CoV-2/COVID-19 hospitalised patients in Switzerland. Swiss Med Wkly. 2021;151:w20475. doi:.https://doi.org/10.4414/smw.2021.20475
7
van Buuren
S
,
Groothuis-Oudshoorn
K
. mice: Multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1–67. doi:.https://doi.org/10.18637/jss.v045.i03
8
Shepherd
BE
,
Rebeiro
PF
; Caribbean, Central and South America network for HIV epidemiology. Assessing and interpreting the association between continuous covariates and outcomes in observational studies of HIV using splines. J Acquir Immune Defic Syndr. 2017;74(3):e60–3. doi:.https://doi.org/10.1097/QAI.0000000000001221
9Harrell FE. Regression Modeling Strategies [Internet]. Springer Series in Statistics. 2015. Available from: https://link.springer.com/content/pdf/10.1007/978-3-319-19425-7.pdf
10
Fine
JP
,
Gray
RJ
. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94(446):496–509. doi:.https://doi.org/10.1080/01621459.1999.10474144
11
Gray
RJ
. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Stat. 1988;16(3):1141–54. doi:.https://doi.org/10.1214/aos/1176350951
12Therneau T. A package for survival analysis in R [Internet]. 2020. Available from: https://cran.r-project.org/web/packages/survival/vignettes/survival.pdf
13
Putter
H
,
Fiocco
M
,
Geskus
RB
. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26(11):2389–430. doi:.https://doi.org/10.1002/sim.2712
14
Griffith
GJ
,
Morris
TT
,
Tudball
MJ
,
Herbert
A
,
Mancano
G
,
Pike
L
, et al.
Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat Commun. 2020;11(1):5749. doi:.https://doi.org/10.1038/s41467-020-19478-2
15
Smith
LH
,
VanderWeele
TJ
. Bounding Bias Due to Selection. Epidemiology. 2019;30(4):509–16. doi:.https://doi.org/10.1097/EDE.0000000000001032
16
Huang
Y
,
Lu
Y
,
Huang
YM
,
Wang
M
,
Ling
W
,
Sui
Y
, et al.
Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism. 2020;113:154378. doi:.https://doi.org/10.1016/j.metabol.2020.154378
17
Bilinska
K
,
Jakubowska
P
,
Von Bartheld
CS
,
Butowt
R
. Expression of the SARS-CoV-2 Entry Proteins, ACE2 and TMPRSS2, in Cells of the Olfactory Epithelium: Identification of Cell Types and Trends with Age. ACS Chem Neurosci. 2020;11(11):1555–62. doi:.https://doi.org/10.1021/acschemneuro.0c00210
18
Ciaglia
E
,
Vecchione
C
,
Puca
AA
. COVID-19 Infection and Circulating ACE2 Levels: Protective Role in Women and Children. Front Pediatr. 2020;8(April):206. doi:.https://doi.org/10.3389/fped.2020.00206
19
Vellas
C
,
Delobel
P
,
de Souto Barreto
P
,
Izopet
J
. COVID-19, Virology and Geroscience: A Perspective. J Nutr Health Aging. 2020;24(7):685–91. doi:.https://doi.org/10.1007/s12603-020-1416-2
20
Pawelec
G
,
Bronikowski
A
,
Cunnane
SC
,
Ferrucci
L
,
Franceschi
C
,
Fülöp
T
, et al.
The conundrum of human immune system “senescence”. Mech Ageing Dev. 2020;192:111357. doi:.https://doi.org/10.1016/j.mad.2020.111357
21
Galloway
JB
,
Norton
S
,
Barker
RD
,
Brookes
A
,
Carey
I
,
Clarke
BD
, et al.
A clinical risk score to identify patients with COVID-19 at high risk of critical care admission or death: An observational cohort study. J Infect. 2020;81(2):282–8. doi:.https://doi.org/10.1016/j.jinf.2020.05.064
22
Huang
H
,
Cai
S
,
Li
Y
,
Li
Y
,
Fan
Y
,
Li
L
, et al.
Prognostic factors for COVID-19 pneumonia progression to severe 2 symptom based on the earlier clinical features: a retrospective analysis. medRxiv. 2020;7:557453. doi:.https://doi.org/10.3389/fmed.2020.557453
23Swiss Federal Office for Public Health. Kategorien besonders gefährdeter Personen [Internet]. 2020. Available from: https://www.bag.admin.ch/bag/de/home/krankheiten/ausbrueche-epidemien-pandemien/aktuelle-ausbrueche-epidemien/novel-cov/besonders-gefaehrdete-menschen.html
Appendix
|
Table S1: Characteristics of all patients, ICU admission and outcome, by age class and gender. |
|
Age (years)
|
Gender
|
Ward
|
Outcome
|
| ≤64(1571, 43.8%) |
Male(970, 61.7%) |
Normal (710, 45.2%) |
Alive (703, 99.0%) |
| Dead (7, 1.0%) |
| ICU (260, 26.8%) |
Alive (229, 88.1%) |
| Dead (31, 11.9%) |
| Female(601, 38.3%) |
Normal (516, 85.9%) |
Alive (511, 99.0%) |
| Dead (5, 1.0%) |
| ICU (85, 14.1%) |
Alive (77, 90.6%) |
| Dead (8, 9.4%) |
| 65–79(1119, 31.2%) |
Male(696, 62.2%) |
Normal (478, 68.7%) |
Alive (415, 86.8%) |
| Dead (63, 13.2%) |
| ICU (218, 31.3%) |
Alive (157, 72.0%) |
| Dead (61, 28.0%) |
| Female(423, 37.8%) |
Normal (340, 80.4%) |
Alive (309, 90.9%) |
| Dead (31, 9.1%) |
| ICU (83, 19.6%) |
Alive (53, 63.9%) |
| Dead (30, 36.1%) |
| ≥80(900, 25.1%) |
Male(472, 52.4%) |
Normal (428, 90.7%) |
Alive (254, 59.3%) |
| Dead (174, 40.7%) |
| ICU (44, 9.3%) |
Alive (18, 40.9%) |
| Dead (26, 59.1%) |
| Female(428, 47.6%) |
Normal (408, 95.3%) |
Alive (315, 77.2%) |
| Dead (93, 22.8%) |
| ICU (20, 4.7%) |
Alive (6, 30.0%) |
| Dead (14, 70.0%) |
| ICU = intensive care unitData includes patients hospitalized up to 1 September 2020 (data extracted from the CH-Sur database on 15 January 2021). |
|
Table S2: Characteristics of all patients and deceased patients. |
|
|
All included patients
(n = 3550)
|
Deceased patients
(n = 540)
|
| Age (years) |
|
68 (55−80) |
81 (73−87) |
| Gender |
FemaleMale |
1431 (40.3%)2119 (59.7%) |
181 (33.5%)359 (66.5%) |
| Renal disease |
NoYesUnknown |
2952 (83.2%)579 (16.3%)19 (0.5%) |
340 (63%)197 (36.5%)3 (0.6%) |
| Oncological pathology |
NoYesUnknown |
3154 (88.8%)378 (10.6%)18 (0.5%) |
423 (78.3%)117 (21.7%)0 (0%) |
| Chronic respiratory disease (including asthma) |
NoYesUnknown |
2807 (79.1%)722 (20.3%)21 (0.6%) |
385 (71.3%)151 (28%)4 (0.7%) |
| Cardiovascular disease |
NoYesUnknown |
2452 (69.1%)1082 (30.5%)16 (0.5%) |
230 (42.6%)310 (57.4%)0 (0%) |
| Liver disease |
NoYesUnknown |
3376 (95.1%)155 (4.4%)19 (0.5%) |
509 (94.3%)30 (5.6%)1 (0.2%) |
| Dementia |
NoYesUnknown |
3245 (91.4%)281 (7.9%)24 (0.7%) |
439 (81.3%)97 (18%)4 (0.7%) |
| Diabetes |
NoYesUnknown |
2750 (77.5%)784 (22.1%)16 (0.5%) |
377 (69.8%)163 (30.2%)0 (0%) |
| Hypertension |
NoYesUnknown |
1799 (50.7%)1733 (48.8%)18 (0.5%) |
178 (33%)362 (67%)0 (0%) |
| Immunosuppresion (other than HIV) |
NoYesUnknown |
2873 (80.9%)268 (7.5%)409 (11.5%) |
414 (76.7%)57 (10.6%)69 (12.8%) |
| HIV = human immunodeficiency virusAge is given as median and quartiles. Data includes patients hospitalised up to 1 September 2020 (data extracted from the CH-Sur database on 15 January 2021). |
|
Table S3: Characteristics of patients admitted to ICU. |
|
|
All included patients
(n = 3590)
|
ICU patients
(n = 710)
|
| Age (years) |
|
68 (55−80) |
65 (57−73) |
| Gender |
FemaleMale |
1452 (40.4%)2138 (59.6%) |
188 (26.5%)522 (73.5%) |
| Renal disease |
NoYesUnknown |
2983 (83.1%)586 (16.3%)21 (0.6%) |
612 (86.2%)96 (13.5%)2 (0.3%) |
| Oncological pathology |
NoYesUnknown |
3187 (88.8%)382 (10.6%)21 (0.6%) |
653 (92%)57 (8%)0 (0%) |
| Chronic respiratory disease (including asthma) |
NoYesUnknown |
2840 (79.1%)727 (20.3%)23 (0.6%) |
554 (78%)155 (21.8%)1 (0.1%) |
| Cardiovascular disease |
NoYesUnknown |
2478 (69%)1094 (30.5%)18 (0.5%) |
497 (70%)213 (30%)0 (0%) |
| Liver disease |
NoYesUnknown |
3411 (95%)158 (4.4%)21 (0.6%) |
687 (96.8%)23 (3.2%)0 (0%) |
| Dementia |
NoYesUnknown |
3279 (91.3%)285 (7.9%)26 (0.7%) |
688 (96.9%)22 (3.1%)0 (0%) |
| Diabetes |
NoYesUnknown |
2779 (77.4%)793 (22.1%)18 (0.5%) |
516 (72.7%)193 (27.2%)1 (0.1%) |
| Hypertension |
NoYesUnknown |
1820 (50.7%)1750 (48.7%)20 (0.6%) |
334 (47%)375 (52.8%)1 (0.1%) |
| Immunosuppresion (other than HIV) |
NoYesUnknown |
2909 (81%)268 (7.5%)413 (11.5%) |
567 (79.9%)49 (6.9%)94 (13.2%) |
| HIV = human immunodeficiency virus; ICU = intensive care unitAge is given as median and quartiles. Data includes patients hospitalized up to 1 September 2020 (data extracted from the CH-Sur database on 15 January 2021). |
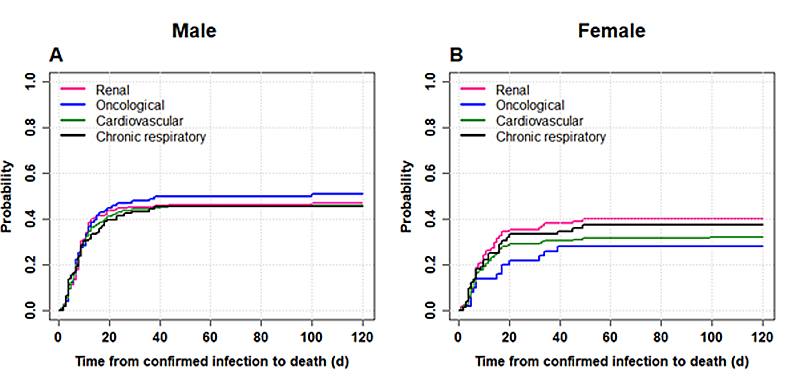
Figure S1 Cumulative incidence of death for patients aged 80+ (panel A male, panel B female) and according to the following comorbidities: renal disease, oncological pathologies, cardiovascular disease and chronic respiratory disease. Data includes patients hospitalised up to 1 September 2020 (data extracted from the CH-Sur database on 15 January 2021). Time to event is defined as the time elapsed from confirmed COVID-19 infection to death.



