Near real-time observation reveals increased prevalence of young patients in the ICU during the emerging third SARS-CoV-2 wave in Switzerland
DOI: https://doi.org/10.4414/smw.2021.20553
Matthias P.
Hiltya, André
Moserb, Sascha
Davida, Pedro D.
Wendel-Garciaa, Giuliana
Capaldoa, Stefanie
Keisera, Thierry
Fumeauxc, Philippe
Guercid, Jonathan
Montomolie, Thomas P.
Van Boeckelf, Marie-Madlen
Jeitzinerg, Yok-Ai
Queg, Stephan
Jakobg, Reto A.
Schuepbacha, , RISC-19-ICU Investigators for Switzerland
a Institute of Intensive Care Medicine, University Hospital of Zurich, Switzerland
b CTU Bern, University of Bern, Switzerland
c Swiss Society of Intensive Care Medicine, Basel, Switzerland
d Department of Anaesthesiology and Critical Care Medicine, University Hospital of Nancy, France
e Department of Intensive Care Medicine, Erasmus Medical Centre, Rotterdam, Netherlands
f Health Geography and Policy Group, ETH Zürich, Switzerland
g Department of Intensive Care Medicine, Bern University Hospital, University of Bern, Switzerland
* RISC-19-ICU Investigators for Switzerland: Institute of Intensive Care Medicine, University Hospital Zurich, Zurich (Reto A. Schüpbach MD; Philipp Bühler, MD; Silvio Brugger, MD, PhD; Jan Bartussek, PhD; Giuliana Capaldo, MD; Sascha David, MD; Stefanie Keiser, PhD; Martina Maibach, PhD; Annelies Zinkernagel, MD, PhD); Soins intensifs, Groupement Hospitalier de l'Ouest Lémanique - Hopital de Nyon, Nyon (Mallory Moret-Bochatay, MD); Interdisziplinaere Intensivstation, Spital Buelach, Buelach (Bernd Yuen, MD; Thomas Hillermann, MD); Soins Intensifs, Hopital cantonal de Fribourg, Fribourg (Hatem Ksouri, MD, PhD; Govind Oliver Sridharan, MD); Departement for intensive care medicine, Kantonsspital Nidwalden, Stans (Anette Ristic, MD; Michael Sepulcri, MD); Departement of Anesthesiology and Intensive Care Medicine, Cantonal Hospital St. Gallen, St. Gallen (Miodrag Filipovic, MD; Urs Pietsch, MD); Intensivstation, Regionalspital Emmental AG, Burgdorf (Petra Salomon, MD; Iris Drvaric, MD); Institut fuer Anesthaesie und Intensivmedizin, Zuger Kantonsspital AG, Baar (Peter Schott, MD; Severin Urech, MD); Intensivmedizin, St. Claraspital, Basel (Adriana Lambert, MD; Lukas Merki, MD); Department Intensive Care Medicine, Spitalzentrum Biel, Biel (Marcus Laube, MD); Intensivmedizin, Kantonsspital Graubünden, Chur (Frank Hillgaertner, MD; Marianne Sieber); Institut fuer Anaesthesie und Intensivmedizin, Spital Thurgau, Frauenfeld (Alexander Dullenkopf, MD; Lina Petersen, MD); Division of Neonatal and Pediatric Intensive Care, Geneva University Hospitals, Geneva (Serge Grazioli, MD; Peter C. Rimensberger, MD); Soins Intensifs, Hirslanden Clinique Cecil, Lausanne (Isabelle Fleisch, MD; Jerome Lavanchy, MD); Pediatric Intensive Care Unit, University Hospital Lausanne, Lausanne (Marie-Helene Perez, MD); Interdisziplinaere Intensivstation, Spital Maennedorf AG, Maennedorf (Katharina Marquardt, MD; Karim Shaikh, MD); Intensivmedizin, Schweizer Paraplegikerzentrum Nottwil, Nottwil (Hermann Redecker, MD); Intensivmedizin, Spital Oberengadin, Samedan (Michael Stephan, MD; Jan Brem, MD); Paediatric Intensive Care Unit, Children’s Hospital of Eastern Switzerland, St. Gallen (Bjarte Rogdo, MD; Andre Birkenmaier, MD); Klinik für Anaesthesie und Intensivmedizin, Spitalzentrum Oberwallis, Visp (Friederike Meyer zu Bentrup, MD, MBA); Interdisziplinaere Intensivstation, Stadtspital Triemli, Zurich (Patricia Fodor, MD; Pascal Locher, MD); Department Intensivmedizin, Universitaetsspital Basel, Basel (Martin Siegemund, MD; Nuria Zellweger); Department of Intensive Care Medicine, University Hospital Bern - Inselspital, Bern (Marie-Madlen Jeitziner, RN, PhD; Beatrice Jenni-Moser, RN, MSc); Interdisziplinaere Intensivmedizin, Lindenhofspital, Bern, Switzerland (Jan Wiegand, MD); Intensivstation, Spital Grabs, Grabs (Christian Bürkle, MD); Medical ICU, Cantonal Hospital St.Gallen, St. Gallen (Gian-Reto Kleger, MD); Service d'Anesthesiologie, EHNV, Yverdon-les-Bains (Marilene Franchitti Laurent, MD; Jean-Christophe Laurent, MD); Abteilung für Anaesthesiologie und Intensivmedizin, Hirslanden Klinik Im Park, Zürich (Tomislav Gaspert, MD; Marija Jovic, MD); Intensivmedizin & Intermediate Care, Kantonsspital Olten, Olten (Michael Studhalter, MD); Institut für Anaesthesiologie und Intensivmedizin, Klinik Hirslanden, Zurich (Christoph Haberthuer, MD; Roger F. Lussman, MD); Anaesthesie Intensivmedizin Schmerzmedizin, Spital Schwyz, Schwyz (Daniela Selz, MD; Didier Naon, MD); Dipartimento Area Critica, Clinica Luganese Moncucco, Lugano (Andrea Glotta, MD; Samuele Ceruti, MD); Institut für Anaesthesiologie Intensivmedizin & Rettungsmedizin, See-Spital Horgen & Kilchberg, Horgen (Julien Marrel, MD; Mirko Brenni, MD); Klinik für Operative Intensivmedizin, Kantonsspital Aarau, Aarau (Rolf Ensner, MD; Marc Michot, MD); Intensivstation, Kantonsspital Schaffhausen, Schaffhausen (Nadine Gehring, MD); Intensivstation, Spital Simmental-Thun-Saanenland AG, Thun (Antje Heise, MD); Klinik für Anaesthesie Intensivmedizin Operationszentrum und Schmerzmedizin, Kantonsspital Muensterlingen, Muensterlingen (Tobias Huebner, MD; Thomas A. Neff, MD); Division of Intensive Care, University Hospitals of Geneva, Geneva (Sara Cereghetti, MD; Filippo Boroli, MD; Jerome Pugin, MD, PhD).
Summary
AIMS OF THE STUDY
During the ongoing COVID-19 pandemic, the launch of a large-scale vaccination campaign and virus mutations have hinted at possible changes in transmissibility and the virulence affecting disease progression up to critical illness, and carry potential for future vaccination failure. To monitor disease development over time with respect to critically ill COVID-19 patients, we report near real-time prospective observational data from the RISC-19-ICU registry that indicate changed characteristics of critically ill patients admitted to Swiss intensive care units (ICUs) at the onset of a third pandemic wave.
METHODS
1829 of 3344 critically ill COVID-19 patients enrolled in the international RISC-19-ICU registry as of 31 May 2021 were treated in Switzerland and were included in the present study. Of these, 1690 patients were admitted to the ICU before 1 February 2021 and were compared with 139 patients admitted during the emerging third pandemic wave
RESULTS
Third wave patients were a mean of 5.2 years (95% confidence interval [CI] 3.2–7.1) younger (median 66.0 years, interquartile range [IQR] 57.0–73.0 vs 62.0 years, IQR 54.5–68.0; p <0.0001) and had a higher body mass index than patients admitted in the previous pandemic period. They presented with lower SAPS II and APACHE II scores, less need for circulatory support and lower white blood cell counts at ICU admission. P/F ratio was similar, but a 14% increase in ventilatory ratio was observed over time (p = 0.03)
CONCLUSION
Near real-time registry data show that the latest COVID-19 patients admitted to ICUs in Switzerland at the onset of the third wave were on average 5 years younger, had a higher body mass index, and presented with lower physiological risk scores but a trend towards more severe lung failure. These differences may primarily be related to the ongoing nationwide vaccination campaign, but the possibility that changes in virus-host interactions may be a co-factor in the age shift and change in disease characteristics is cause for concern, and should be taken into account in the public health and vaccination strategy during the ongoing pandemic. (ClinicalTrials.gov Identifier: NCT04357275)
Introduction
The coronavirus disease (COVID)-19 pandemic, declared on 17 March 2020 by the World Health Organization (WHO), has burdened the global health systems without recent precedent. A rapid international effort in uncovering properties of the novel disease [1, 2] and optimal treatment strategies [3–5] was followed by the launch of a large-scale vaccination campaign [6, 7]. At the same time, virus mutations [8, 9] have hinted at possible changes in (i) transmissibility and (ii) the virulence affecting disease progression up to critical illness and mortality [10], and carry the potential for future vaccination failure. Over the last 12 months, a renewed increase in SARS-CoV-2 cases in Switzerland and reported changes in virus properties, together with the inception of the vaccination campaign on 21 December 2020, underscore the need for continued monitoring of the disease development over time. The present report aims to provide a near real-time description of the characteristics of COVID-19 patients admitted to intensive care units (ICUs) at the onset of a third wave in Switzerland and compare these with the previous course of the COVID-19 pandemic.
Methods
The present report is based on the prospective, near real-time observational Risk Stratification in Covid-19 patients in the ICU (RISC-19-ICU) registry, a tool launched on 17 March 2020 to track patient and disease characteristics and the disease course of critically ill COVID-19 patients. The registry is endorsed by the Swiss Society of Intensive Care Medicine (https://www.sgi-ssmi.ch) and was exempt from the need for additional ethics approval and patient informed consent by the ethics committee of the University of Zurich (KEK 2020-00322, ClinicalTrials.gov Identifier: NCT04357275). The study complies with the Declaration of Helsinki, the Guidelines on Good Clinical Practice (GCP-Directive) issued by the European Medicines Agency, as well as the Swiss law and Swiss regulatory authority requirements, and has been designed in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for observational studies [11].
Study design and patient selection
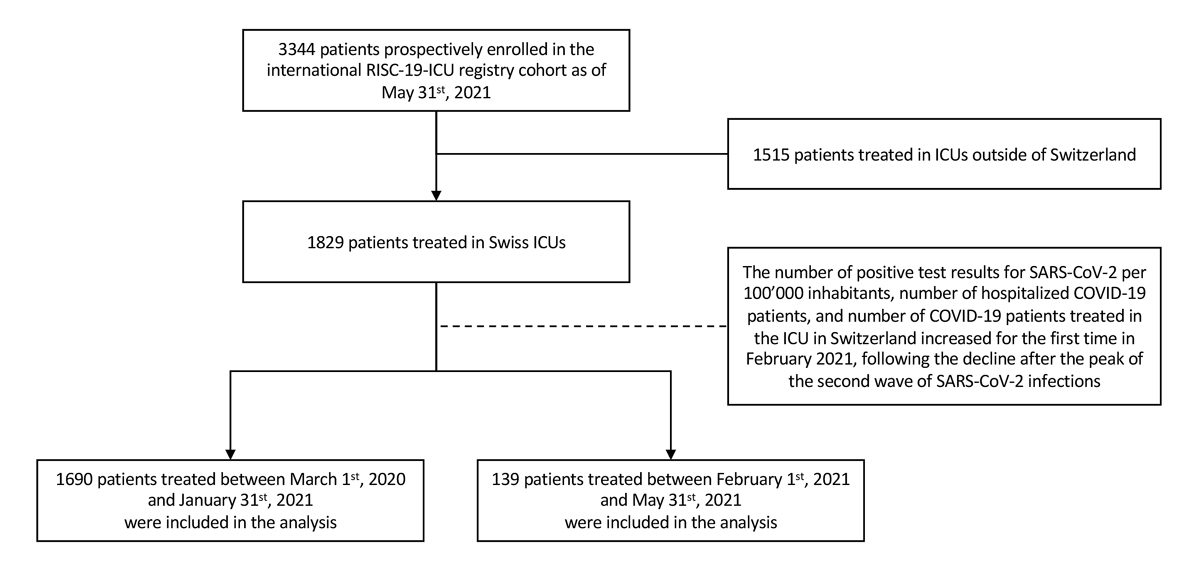
The registry contained data from 3344 patients from 69 centres in 14 countries as of 31 May 2021. Given the wide heterogeneity with regard to local viral epidemiology, health care and ICU resources, here we focus only on patients admitted to ICUs in Switzerland. All records as of 31 May 2021 were exported from the registry and divided into two groups. Patients admitted between 1 February and 31 May 2021 were considered to be admitted during a third pandemic wave, based on the increasing number of positive test results for SARS-CoV-2 per 100,000 inhabitants, number of hospitalised COVID-19 patients, and number of COVID-19 patients treated in the ICU in Switzerland, which were observed for the first time in February 2021 following the decline after the peak of the second wave of SARS-CoV-2 infections (see fig. 1). Patients admitted between 1 March 2020 and 31 January 2021 were assigned to a comparator group consisting of the patients admitted during the first and second wave of SARS-CoV-2 infections (see fig. 2).
Registry data collection and transformation
As described in detail elsewhere [1], a standardised core dataset was prospectively collected during the ongoing COVID-19 pandemic for all critically ill COVID-19 patients admitted to the collaborating centres, and up-to-date cohort characteristics were regularly provided to the Swiss ICUs throughout the pandemic. Inclusion criteria were (i) a laboratory-confirmed SARS-CoV-2 infection diagnosed by nucleic acid amplification according to the testing guidelines issued by the WHO [12], and (ii) requirement for treatment in an ICU or intermediate care unit, defined as a hospital ward specialised in the care of critically ill patients to provide organ support therapies including invasive mechanical ventilation and/or noninvasive ventilation. Data were collected through an anonymised electronic case report form managed by the REDCap electronic data capture tool hosted on a secure server by the Swiss Society of Intensive Care Medicine [13]. Data were collected on the day of ICU admission and on days one, two, three, five and seven, including patient characteristics, treatment modalities and organ support therapies, the use of mechanical ventilation, vital parameters, arterial blood gas analyses, and laboratory values such as inflammatory, coagulation, renal, liver and cardiac parameters. The Swiss centres enrolled in the RISC-19-ICU represented >60% of all certified secondary versus tertiary care centres in both the North-Eastern and South-Western regions of Switzerland (see supplementary table S1 in the appendix). Registry data transformation for analysis, including the calculation of the disease severity scores Acute Physiology and Chronic Health Evaluation (APACHE II), Simplified Acute Physiology Score (SAPS II) and Sequential Organ Failure Assessment (SOFA) scores, was performed using an openly available code library associated with the registry [14].
Statistical analysis
We report counts and percentages (%), mean and standard deviation (SD) or median (interquartile range [IQR]), as appropriate. The number of positive test results for SARS-CoV-2 infections, number of hospitalised patients and number of vaccine doses applied in Switzerland were provided by the Swiss Federal Office of Public Health (FOPH) [15]. The overall absolute number of patients treated in the ICU was provided by www.icumonitoring.ch / Koordinierter Sanitätsdienst (KSD) and was stratified by age using per-day age distributions derived from the RISC-19-ICU registry. Patient characteristics, physiological status and laboratory measurements at the time of ICU admission were compared between the two groups (first + second vs third wave) using Gaussian (continuous outcomes), logistic (binary outcomes) and Poisson (count outcomes) mixed model analysis [16]. Time period was entered into the model as a fixed effect and treatment centre as random effect. P-values were calculated using a likelihood ratio test of the full model with the effect in question against a “null model” that lacks the effect in question [17], p-values for individual fixed effects were obtained by Satterthwaite approximation [18]. Changes in age distribution throughout both periods were adjusted for the number of comorbidities. Statistical analysis was performed using a fully scripted data management pathway using the R environment for statistical computing version 3.6.3 [19]. Mixed effects modelling was performed using the R-library lme4, version 1.1.21 [16] and graphical output using ggplot2, version 3.2.1 [20].
Results
Epidemiological environment
With regard to the third wave, an increase in the number of positive test results for SARS-CoV-2 infections per 100,000 inhabitants in Switzerland was reported for the first time in February 2021, after a continuous decrease reported in December 2020 (fig. 1A). This increase was reflected in the number of hospitalised patients (fig. 1B) and absolute number of patients treated in the ICU (fig. 1C). The proportions of patients older and younger than 70 years remained stable from the initial phase of the pandemic, but shifted towards the younger patients at the onset of the third wave. This effect was most pronounced in the patients treated in the ICU. In Switzerland, a vaccination campaign using the BNT162b2 and mRNA-1273 SARS-CoV-2 vaccines started on 21 December 2020. The cumulative number of vaccines applied over time is shown in figure 1D. According to official recommendations, the majority of vaccine doses were administered to persons above the age of 75 years with pre-existing medical conditions as of May 2021.
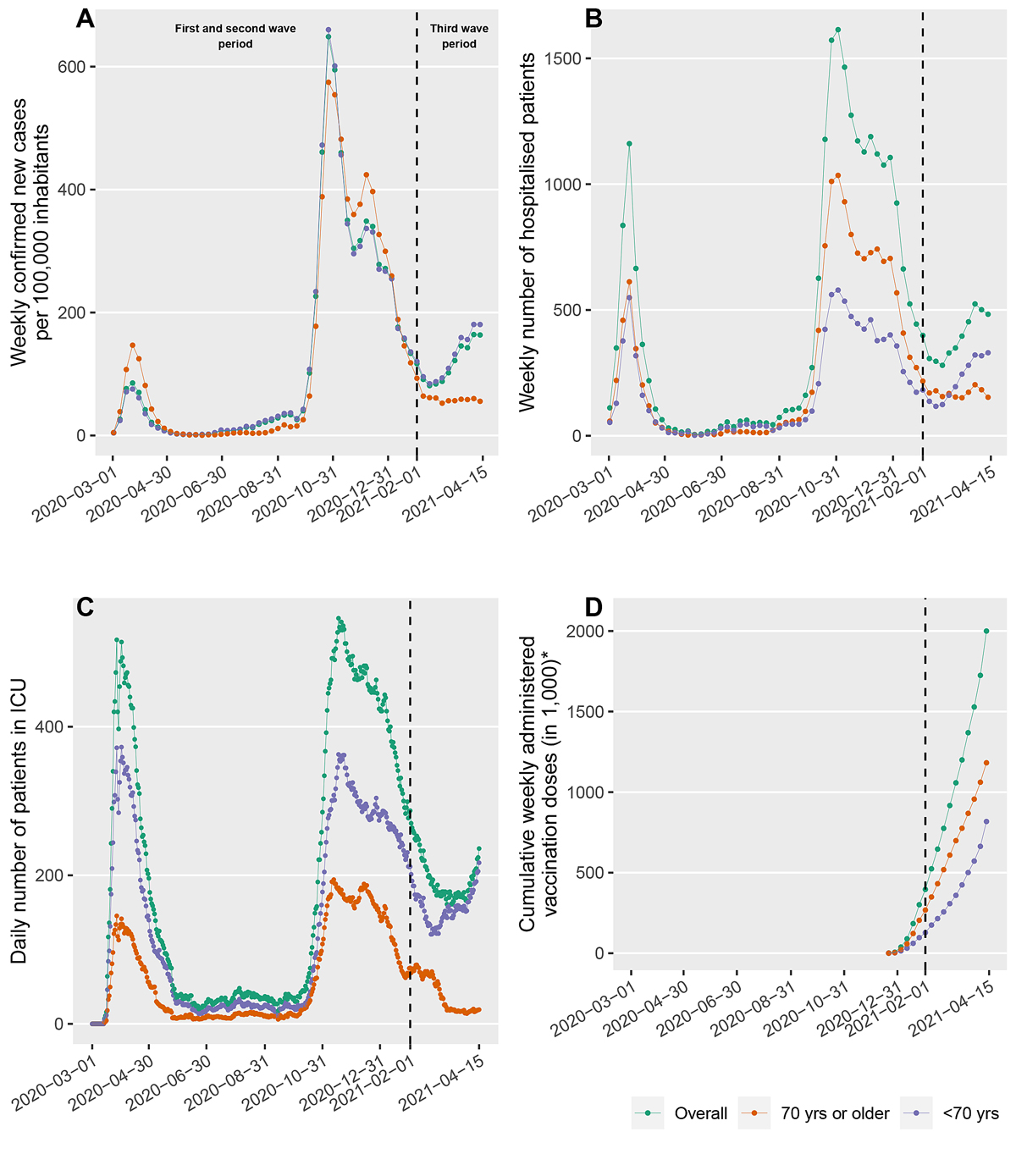
Figure 1 The number of positive test results for SARS-CoV-2 per 100,000 inhabitants (A) indicates three pandemic wave periods, which are reflected in the number of hospitalised patients (B) and absolute number of patients treated in the intensive care unit (ICU) (C). The proportions of patients older and younger than 70 years remained stable throughout the first and second wave periods and from the onset of the third wave period shifted towards the younger patients. This effect was most pronounced in the patients treated in the ICU. The total number of vaccine doses applied is given in (D). The data in (A, B, D) were provided by the Federal Office of Public Health (FOPH). The overall absolute number of patients treated in the ICU as reported by www.icumonitoring.ch / Koordinierter Sanitätsdienst (KSD; C, green line) was stratified by age using per-day age distributions derived from the RISC-19-ICU registry (C, blue and orange lines).
Differences in characteristics and age distribution in critically ill COVID-19 patients
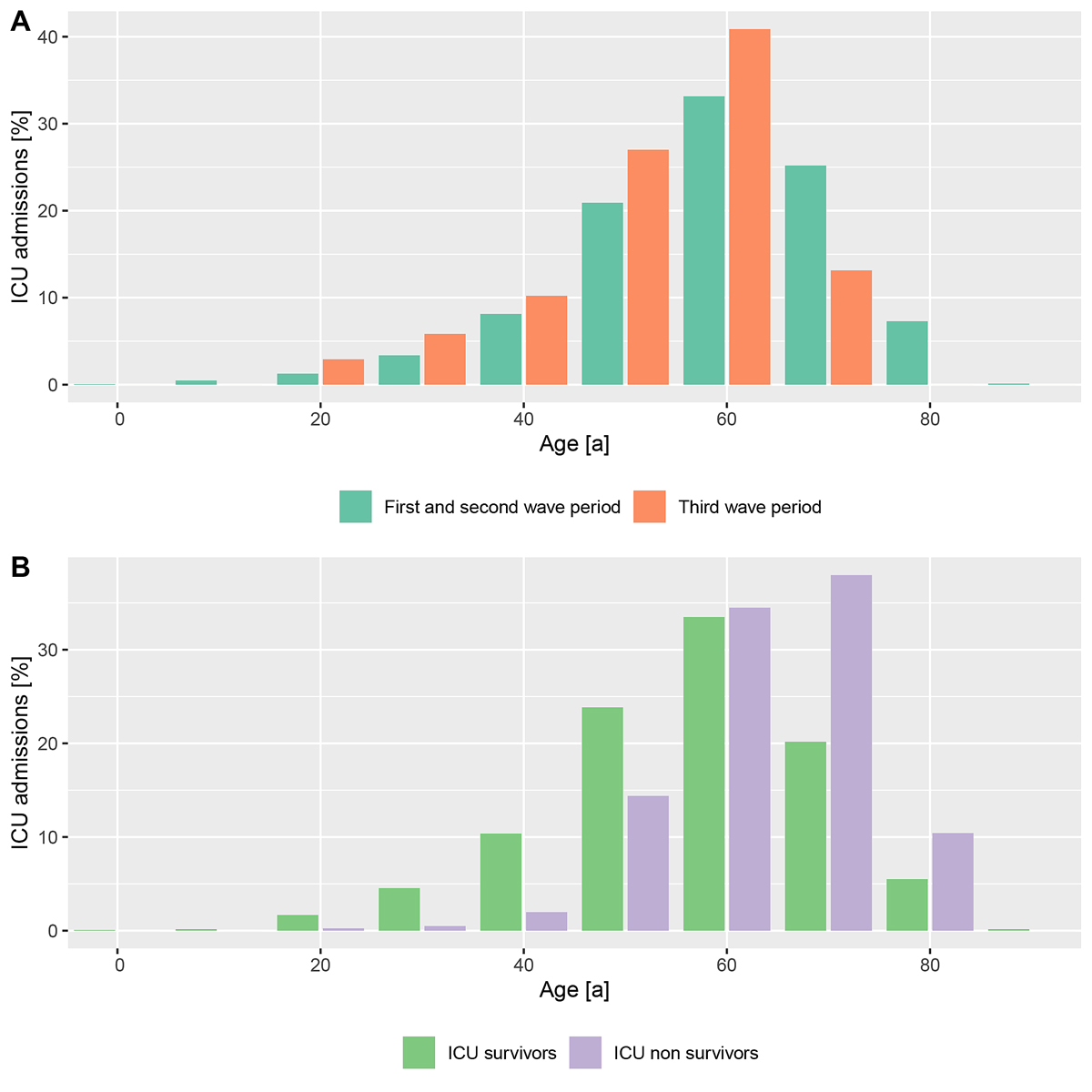
A total of 1829 patients admitted to Swiss ICUs were included in the registry as of 31 May 2021. Of these, 1690 patients were admitted before 1 February and the remaining 139 patients were admitted during the emerging third pandemic wave (fig. 2). Patients assigned to the third wave were a mean of 5.2 years (confidence interval [CI] 3.2–7.1) younger (median 66.0 years, IQR 57.0-73.0 vs 62.0 years, IQR 54.5–68.0; p <0.0001) as compared with the previous pandemic period, corresponding to a marked left-shift in the age distribution (fig 3A). Multivariable correction for age and comorbidities confirmed the effect of time period on age and revealed an effect on comorbidities (supplementary table S2 in the appendix). In both time periods taken together, approximately 80% of patients who had died in the ICU were older than 60 years of age, and approximately 40% of the patients treated in the ICU were younger than 60 years of age (fig. 3B). Third wave patients had a higher body mass index, and presented with lower SAPS II and APACHE II scores, cardiovascular system SOFA sub-score and need for norepinephrine support at the time of ICU admission (table 1). P/F ratio was similar, but a 14% increase in ventilatory ratio was observed as compared with patients from waves 1 and 2. White blood cell count, creatinine and creatine kinase levels were found to be lower, whereas the remaining characteristics, including inflammatory markers, remained comparable (table 1). Age-stratified patient characteristics at ICU admission are shown in supplementary table S3.
Table 1 Characteristics of critically ill COVID-19 patients at ICU admission at the onset of the third wave of SARS-CoV-2 infections in Switzerland in comparison with the previous pandemic course.
| |
01.03.2020 – 31.01.2021; 2019 SARS-CoV-2 pandemic waves 1 and 2
|
01.02.2021 – 15.04.2021; onset of 2019 SARS-CoV-2 pandemic wave 3
|
Mean difference ± SE (CI) for continuous variables, rate ratio (CI) for counts, odds ratio (CI) for binary categorical variables; effect of time period
|
p-value, effect of time period
|
| |
n = 1690
|
n = 139
|
|
|
|
Characteristics
|
|
|
|
|
| Age (years), median (IQR) |
66 (57–73)
|
62 (54–68)
|
−5.2 ± 1.2 (−7.1 to −3.2)
|
<0.0001
|
| Sex (male), n (%) |
1227 (73.4) |
98 (70.5) |
0.9 (0.6 to 1.3) |
0.46 |
| Body mass index (kg·m-2), mean (SD) |
29.3 (6.0)
|
30.5 (6.2)
|
1.2 ± 0.5 (0.3 to 2.1)
|
0.03
|
| Time from symptom onset to hospitalisation (days), mean (SD) |
8.5 (8.5) |
8.6 (6.6) |
0.1 ± 0.8 (−1.2 to 1.4) |
0.91 |
| Time from hospitalisation to ICU admission (days), mean (SD) |
3.0 (8.2) |
2.6 (4.6) |
-0.3 ± 0.7 (-1.5 - 0.9) |
0.67 |
|
Preexisting conditions
|
|
|
|
|
| Number of preexisting conditions, mean (SD) |
0.9 (1.1) |
0.7 (1.0) |
0.8 (0.7 to 1.0) |
0.06 |
| Ischaemic heart disease, n (%) |
790 (46.7)
|
50 (36.0)
|
0.5 (0.3 to 0.7)
|
<0.001
|
| Chronic heart failure, n (%) |
228 (13.5) |
11 (7.9) |
0.5 (0.3 to 1.0) |
0.06 |
| Atherosclerotic arteriopathy, n (%) |
270 (16.0) |
19 (13.7) |
0.7 (0.4 to 1.2) |
0.23 |
| Arterial hypertension, n (%) |
466 (27.6) |
32 (23.0) |
0.8 (0.5 - 1.2) |
0.23 |
| Diabetes mellitus, n (%) |
223 (13.2) |
15 (10.8) |
0.9 (0.5 to 1.6) |
0.68 |
| Insulin-dependent diabetes mellitus, n (%) |
263 (15.6) |
16 (11.5) |
0.7 (0.4 to 1.3) |
0.29 |
|
Physiological status at ICU admission
|
|
|
|
|
| APACHE II score, mean (SD) |
17.2 (7.8)
|
16.1 (7.1)
|
−1.4 ± 0.7 (−2.6 to −0.3)
|
0.04
|
| SAPS II score, mean (SD) |
47.8 (19.6)
|
43.1 (18.6)
|
−4.5 ± 1.7 (−7.4 to −1.7)
|
<0.01
|
| SOFA score, mean (SD) |
10.9 (5.3) |
11.2 (4.1) |
−0.8 ± 0.5 (−1.5 to 0) |
0.10 |
| – Respiratory system sub-score, mean (SD) |
2.4 (1.2) |
2.5 (1.0) |
−0.1 ± 0.1 (−0.3 to 0.1) |
0.38 |
| – Coagulatory system sub-score, mean (SD) |
0.2 (0.6) |
0.2 (0.5) |
−0.1 ± 0.05 (−0.1 to 0.0) |
0.38 |
| – Liver sub-score, mean (SD) |
2.2 (1.6) |
2.6 (1.3) |
−0.1 ± 0.14 (−0.3 to 0.2) |
0.59 |
| – Cardiovascular system sub-score, mean (SD) |
1.5 (1.6)
|
1.2 (1.5)
|
−0.5 ± 0.15 (−0.7 to −0.2)
|
<0.01
|
| – Central nervous system sub-score, mean (SD) |
1.8 (1.9) |
1.2 (1.9) |
-0.3 ± 0.17 (-0.6 - -0.02) |
0.07 |
| – Renal sub-score, mean (SD) |
3.4 (1.4) |
3.9 (0.6) |
0.0 ± 0.11 (−0.2 to 0.2) |
0.83 |
| Mean arterial pressure (mm Hg), mean (SD) |
83.3 (17.5)
|
89.6 (18.0)
|
5.1 ± 1.6 (2.4 to 7.8)
|
<0.01
|
| Norepinephrine dose (µg·kg-1), mean (SD) |
5.0 (9.0)
|
1.9 (4.4)
|
−2.0 ± 0.9 (−3.5 to −0.4)
|
0.03
|
| P/F ratio (mmHg), mean (SD) |
143 (103) |
129 (200) |
−12.2 ± 11.9 (−31.8 to 7.5) |
0.31 |
| Ventilatory ratio, mean (SD) |
1.8 (0.8)
|
2.1 (1.1)
|
0.2 ± 0.1 (0.1 to 0.3)
|
0.03
|
|
Laboratory measurements at ICU admission
|
|
|
|
|
| White blood cell count (G·l-1), mean (SD) |
9.9 (6.1)
|
9.0 (4.6)
|
−1.4 ± 0.6 (−2.4 to −0.3)
|
0.03
|
| Neutrophil granulocyte count (G·l-1), mean (SD) |
8.2 (4.8) |
7.6 (4.1) |
−0.9 ± 0.6 (−1.987 to 0.0) |
0.11 |
| Lymphocyte count (G·l-1), mean (SD) |
1.3 (2.6) |
1.5 (2.0) |
−0.6 ± 0.3 (−1.1 to −0.1) |
0.06 |
| Neutrophil/lymphocyte ratio, mean (SD) |
13.0 (13.6) |
11.1 (10.1) |
−0.9 ± 1.6 (−3.5 to 1.8) |
0.59 |
| Interleukin-6 (ng·l-1), median (IQR) |
103 (40–246) |
92 (61–144) |
−321.6 ± 886.4 (−1789.6 to 1156.7) |
0.72 |
| C-reactive protein (mg·l-1), median (IQR) |
141 (78–214) |
141 (87–210) |
−1.9 ± 9.9 (−18.2 to 14.5) |
0.85 |
| Procalcitonin (µg·l-1), median (IQR) |
0.3 (0.2–0.9) |
0.2 (0.1–0.4) |
−1.6 ± 1 (−3.3 to 0.0) |
0.10 |
| D-dimers (µg·l-1), median (IQR) |
1545 (890–3420) |
1240 (770–3000) |
34.1 ± 1948.0 (−3204.3 to 3255.1) |
0.99 |
| Lactate dehydrogenase (U·l-1), median (IQR) |
517 (382–693) |
522 (411–663) |
27.6 ± 44.4 (−45.5 to 100.6) |
0.53 |
| Ferritin (µg·l-1), median (IQR) |
1174 (651–1973) |
1231 (775–1902) |
−349.6 ± 253.7 (−767.3 to 70.0) |
0.17 |
| Bilirubin (µmol·l-1), median (IQR) |
9.0 (6.3–13.0) |
9.0 (5.8–12.0) |
−6.2 ± 10.5 (−23.5 to 11.1) |
0.55 |
| Creatinine (µmol·l-1), median (IQR) |
84 (66–115)
|
67 (53–88)
|
−27.9 ± 11.7 (−47.1 to −8.8)
|
0.02
|
| Creatine kinase (U·l-1), median (IQR) |
119 (60–269)
|
118 (56–276)
|
832.3 ± 284.8 (358.3 to 1313.6)
|
< 0.01
|
| Myoglobin (µg·l-1), median (IQR) |
79 (41–221) |
52 (36–106) |
55.7 ± 510.7 (−787.1 to 899.2) |
0.91 |
| Troponin (ng·l-1), median (IQR) |
21.1 (11.0–55.0) |
20.9 (9.3–65.8) |
−347.5 ± 431.7 (−1060.1 to 364.6) |
0.42 |
| Albumin (g·l-1), median (IQR) |
29 [26–33) |
30 (27–33) |
−0.4 ± 0.8 (−1.7 to 0.9) |
0.63 |
Discussion
Here we present an up-to-date comparison of Swiss ICU patients suffering from COVID-19 during the onset of the third wave with the first and second waves. To do this we analysed changes in the patient characteristics and outcome based on near real-time registry data. The risk factors for the development of critical illness in COVID-19, as shown in a previous report from the RISC-19-ICU registry [1], such as age, male sex, and preexisting conditions such as obesity, cardiovascular disease, diabetes mellitus and conditions associated with immune system compromise, could be subject to changes in the population at risk, as well a shifting properties of the disease itself. The shift towards a younger age by more than 5 years is clearly apparent even in an early stage of the onset of the third wave of SARS-CoV-2 infections in Switzerland, and could be highly relevant since a potentially altered course of disease could directly impact ICU resources. Although the observed decrease in patients in the higher age groups is most likely be related to the vaccination campaign that has prioritised persons in the higher age groups, the observation of younger patients developing critical illness due to COVID-19, with a higher overall number of comorbidities and, specifically, prevalence of ischaemic heart disease, alongside the higher body mass index found in the present study, would not be expected with the current, risk-based vaccination strategy and could be related to an increasing prevalence of virus variants. Further, the younger age of patients admitted during the third wave does not solely explain per se the decrease in physiological risk scores such as SAPS II and APACHE II. Whereas the need for circulatory support was indeed reduced during the third wave, which may suggest better compensation mechanisms, as was found in previous research [21], we have observed an unexpected trend towards more severe lung failure. A recent study reported an increase in mortality in patients infected with the B1.1.7 variant of the SARS-CoV-2 virus [10], demonstrating a relationship between changes in viral epidemiology and disease course. Further studies are needed to confirm whether our observations reflect the vaccination status or whether this truly reflects a different pathophysiological behaviour of the virus.
Since the inception of the pandemic, the dataset shows that the majority of the patients dying in the ICU were older than 60 years. However, almost half of the patients treated in the ICU were younger than 60 years of age. Long-term outcome studies in patients with non-COVID acute respiratory distress syndrome (ARDS) have demonstrated that only approximately 50% of working-age ICU survivors have been able to take up employment after 1 year [22]. As younger patients make up a relevant proportion of critically ill COVID-19 patients, it might make sense to prioritise the vaccination of patients between 40 and 60 years of age regardless of their health risk profile, but based on occupational risk.
The main limitation of near real-time data during an emerging pandemic is the unavailability of outcome data due to selection bias of these parameters in patients with shorter ICU stays. By limiting to the description of the patient characteristics in the comparison of the two time periods, this bias was avoided in the present report, while using the near real-time registry to provide timely surveillance of changes regarding patients who develop critical illness due to this novel disease. Further, the observational nature of the study determined the unbalanced sample sizes of both groups with heterogeneity across treatment centres. The mixed model approach accounts for such complexities while allowing for partial pooling of information [23].
In conclusion, near real-time registry data show that the latest COVID-19 patients admitted to ICUs in Switzerland at the onset of the third wave were on average 5 years younger, had a higher body mass index, and presented with lower physiological risk scores but a trend towards more severe lung failure. These differences may primarily be related to the ongoing nationwide vaccination campaign, but the possibility that changes in virus-host interactions may be a co-factor in the age shift and change in disease characteristics is cause for concern that should be taken into account in the public health and vaccination strategy during the ongoing pandemic.
Appendix: Supplementary tables
Table S1 Intensive care units enrolled in the RISC-19-ICU registry in Switzerland with the centres’ role and region.
|
Intensive care units enrolled in the RISC-19-ICU registry, n (%)
|
Type
|
Region
|
| 4 (80%) |
Tertiary care centre |
Switzerland, South-West |
| 5 (100%) |
Tertiary care centre |
Switzerland, North-East |
| 14 (70%) |
Secondary care centre |
Switzerland, South-West |
| 34 (64%) |
Secondary care centre |
Switzerland, North-East |
Table S2 Uni- and multivariable linear mixed model analysis adjusting for the number of comorbidities confirmed the effect of time period on patient age at the onset of the third wave of SARS-CoV-2 infections in Switzerland in comparison to the previous pandemic course. Multivariable analyses further revealed an effect of time period on the number of comorbidities when corrected for age. In these models, treatment centre was added as random effect and the p values of individual fixed effects were estimated using the Satterthwaite approximation.
|
Univariable analysis, fixed effects
|
Mean difference estimate ± SE
(95% CI)
|
t value
|
p-value
|
| Time period |
−5.17 ± 1.17
(−7.47 to −2.85) |
−4.40 |
<0.0001 |
|
Univariable analysis, random effects
|
Variance ± SD
|
|
|
| Treatment centre |
3.46 ± 1.86 |
|
|
|
Multivariable analysis, fixed effects
|
Mean difference estimate ± SE
(95% CI)
|
t value
|
p value
|
| Number of comorbidities |
3.37 ± 0.29
(2.76 to 3.94) |
11.52 |
<0.0001 |
| Time period |
−4.59 ± 1.14
(−6.81 to −2.33) |
−4.02 |
< 0.0001 |
|
Multivariable analysis, random effects
|
Variance ± SD
|
|
|
| Treatment centre |
4.48 ± 2.12 |
|
|
| SD = standard deviation; SE = standard error; CI = 95% confidence interval. |
Table S3 Characteristics of critically ill COVID-19 patients at ICU admission at the onset of the third wave of SARS-CoV-2 infections in Switzerland in comparison with the previous pandemic course, stratified by age groups.
| |
01.03.2020 – 31.01.2021; 2019 SARS-CoV-2 pandemic waves 1 and 2
|
01.02.2021 – 15.04.2021; onset of 2019 SARS-CoV-2 pandemic wave 3
|
| |
70 yrs or older
|
<70 yrs
|
70 yrs or older
|
<70 yrs
|
| |
n = 671
|
n = 1158
|
n = 25
|
n = 114
|
|
Characteristics
|
|
|
|
|
| Age (years), median (IQR) |
75 (72–79) |
60 (53–65) |
72 (70–75) |
59 (52–65) |
| Sex (male), n (%) |
437 (71.9) |
778 (74.5) |
21 (84.0) |
77 (67.5) |
| Body mass index (kg·m-2), mean (SD) |
28.2 (5.1) |
29.9 (6.4) |
29.5 (6.0) |
30.7 (6.3) |
| Time from symptom onset to hospitalisation (days), mean (SD) |
8.6 (11.3) |
8.4 (6.3) |
8.7 (9.1) |
8.5 (5.9) |
| Time from hospitalisation to ICU admission (days), mean (SD) |
3.6 (11.1) |
2.6 (5.8) |
4.0 (6.4) |
2.3 (4.1) |
|
Preexisting conditions
|
|
|
|
|
| Number of preexisting conditions, mean (SD) |
1.2 (1.2) |
0.8 (1.0) |
1.2 (1.3) |
0.6 (0.9) |
| Ischaemic heart disease, n (%) |
354 (58.1) |
435 (41.4) |
10 (40.0) |
40 (35.1) |
| Chronic heart failure, n (%) |
133 (21.8) |
95 (9.0) |
6 (24.0) |
5 (4.4) |
| Atherosclerotic arteriopathy, n (%) |
149 (24.5) |
121 (11.5) |
7 (28.0) |
12 (10.5) |
| Arterial hypertension, n (%) |
213 (35.0) |
253 (24.1) |
9 (36.0) |
23 (20.2) |
| Diabetes mellitus, n (%) |
91 (14.9) |
132 (12.6) |
6 (24.0) |
9 (7.9) |
| Insulin-dependent diabetes mellitus, n (%) |
104 (17.1) |
159 (15.1) |
6 (24.0) |
10 (8.8) |
|
Physiological status at ICU admission
|
|
|
|
|
| APACHE II score, mean (SD) |
19.1 (7.1) |
16.5 (7.7) |
17.4 (6.3) |
15.8 (7.3) |
| SAPS II score, mean (SD) |
53.1 (17.7) |
45.7 (19.5) |
46.0 (16.4) |
42.5 (19.1) |
| SOFA score, mean (SD) |
11.3 (5.1) |
10.9 (5.2) |
11.3 (3.8) |
11.2 (4.2) |
| – Respiratory system sub-score, mean (SD) |
2.4 (1.1) |
2.4 (1.2) |
2.3 (1.0) |
2.6 (1.0) |
| – Coagulatory system sub-score, mean (SD) |
0.3 (0.6) |
0.2 (0.6) |
0.4 (0.7) |
0.1 (0.4) |
| – Liver sub-score, mean (SD) |
2.2 (1.6) |
2.2 (1.6) |
2.8 (1.4) |
2.6 (1.3) |
| – Cardiovascular system sub-score, mean (SD) |
1.8 (1.7) |
1.4 (1.6) |
1.3 (1.4) |
1.2 (1.5) |
| – Central nervous system sub-score, mean (SD) |
1.7 (1.9) |
1.8 (2.0) |
0.8 (1.6) |
1.3 (1.9) |
| – Renal sub-score, mean (SD) |
3.4 (1.3) |
3.4 (1.4) |
4.0 (0.0) |
3.9 (0.7) |
| Mean arterial pressure (mm Hg), mean (SD) |
82.1 (18.5) |
84.0 (16.8) |
84.2 (21.9) |
90.8 (16.9) |
| Norepinephrine dose (µg·kg-1), mean (SD) |
5.9 (8.6) |
4.5 (9.2) |
1.5 (2.7) |
2.0 (4.7) |
| P/F ratio (mmHg), mean (SD) |
148 (120) |
139 (92) |
108 (59) |
133 (219) |
| Ventilatory ratio, mean (SD) |
1.8 (0.7) |
1.9 (0.8) |
1.8 (0.7) |
2.2 (1.2) |
|
Laboratory measurements at ICU admission
|
|
|
|
|
| White blood cell count (G·l-1), mean (SD) |
10.0 (5.5) |
9.9 (6.5) |
10.8 (5.5) |
8.5 (4.3) |
| Neutrophil granulocyte count (G·l-1), mean (SD) |
8.3 (4.5) |
8.1 (4.9) |
9.3 (4.6) |
7.1 (3.9) |
| Lymphocyte count (G·l-1), mean (SD) |
1.4 (3.1) |
1.3 (2.4) |
1.0 (1.6) |
1.6 (2.1) |
| Neutrophil/lymphocyte ratio, mean (SD) |
14.4 (13.9) |
12.2 (13.4) |
17.0 (12.5) |
9.3 (8.6) |
| Interleukin-6 (ng·l-1), median (IQR) |
129 (51–306) |
98 (36–228) |
114 (60–133) |
89 (64–148) |
| C-reactive protein (mg·l-1), median (IQR) |
137 (74–201) |
144 (82–220) |
133 (93–246) |
141 (87–204) |
| Procalcitonin (µg·l-1), median (IQR) |
0.3 (0.2–0.9) |
0.3 (0.2–0.8) |
0.3 (0.2–1.3) |
0.2 (0.1–0.4) |
| D-dimers (µg·l-1), median (IQR) |
1504 (880–3413) |
1349 (830–2800) |
1165 (763–2455) |
1900 (1120–4315) |
| Lactate dehydrogenase (U·l-1), median (IQR) |
501 (362–676) |
527 (395–697) |
526 (465–843) |
522 (407–644) |
| Ferritin (µg·l-1), median (IQR) |
1106 (601–1823) |
1239 (701–2034) |
1508 (840–2216) |
1132 (769–1701) |
| Bilirubin (µmol·l-1), median (IQR) |
9.0 (6.4–13.0) |
9.0 (6.2–13.1) |
10.1 (7.0–14.0) |
8.0 (5.2–12.0) |
| Creatinine (µmol·l-1), median (IQR) |
91 (71–139) |
79 (63–104) |
84 (67–114) |
63 (50–84) |
| Creatine kinase (U·l-1), median (IQR) |
107 (54–257) |
124 (64–303) |
101 (56–232) |
126 (58–283) |
| Myoglobin (µg·l-1), median (IQR) |
85 (45–179) |
74 (40–241) |
172 (51–227) |
52 (27–92) |
| Troponin (ng·l-1), median (IQR) |
35.0 (19.0–95.2) |
15.6 (9.0–42.7) |
35.8 (26.9–69.8) |
15.0 (8.4–45.0) |
| Albumin (g·l-1), median (IQR) |
29 (25–32) |
30 (26–33) |
30 (26–33) |
30 (27–33) |
SD = standard deviation; IQR = interquartile range
Values are given as counts and percentages (%), mean (SD) or median (IQR), as appropriate. |
Acknowledgments
This work is funded and endorsed by the Swiss Society of Intensive Care Medicine. We want to thank all nurses, physicians and other healthcare workers in our collaborating centres for their tireless and brave efforts in the care of their patient – without you this healthcare emergency could not be overcome.
References
1
Wendel Garcia
PD
,
Fumeaux
T
,
Guerci
P
,
Heuberger
DM
,
Montomoli
J
,
Roche-Campo
F
, et al.; RISC-19-ICU Investigators. Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: Initial report of the international RISC-19-ICU prospective observational cohort. EClinicalMedicine. 2020;25:100449. doi:.https://doi.org/10.1016/j.eclinm.2020.100449
2
COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47(1):60–73. doi:.https://doi.org/10.1007/s00134-020-06294-x
3
Horby
P
,
Lim
WS
,
Emberson
JR
,
Mafham
M
,
Bell
JL
,
Linsell
L
, et al., RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi:.https://doi.org/10.1056/NEJMoa2021436
4
Angus
DC
,
Derde
L
,
Al-Beidh
F
,
Annane
D
,
Arabi
Y
,
Beane
A
, et al.; Writing Committee for the REMAP-CAP Investigators. Effect of Hydrocortisone on Mortality and Organ Support in Patients With Severe COVID-19: The REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial. JAMA. 2020;324(13):1317–29. doi:.https://doi.org/10.1001/jama.2020.17022
5
Sadeghipour
P
,
Talasaz
AH
,
Rashidi
F
,
Sharif-Kashani
B
,
Beigmohammadi
MT
,
Farrokhpour
M
, et al., INSPIRATION Investigators. Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial. JAMA. 2021;325(16):1620–30. doi:.https://doi.org/10.1001/jama.2021.4152
6
Polack
FP
,
Thomas
SJ
,
Kitchin
N
,
Absalon
J
,
Gurtman
A
,
Lockhart
S
, et al.; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–15. doi:.https://doi.org/10.1056/NEJMoa2034577
7
Baden
LR
,
El Sahly
HM
,
Essink
B
,
Kotloff
K
,
Frey
S
,
Novak
R
, et al.; COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–16. doi:.https://doi.org/10.1056/NEJMoa2035389
8
Xie
X
,
Liu
Y
,
Liu
J
,
Zhang
X
,
Zou
J
,
Fontes-Garfias
CR
, et al.
Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera. Nat Med. 2021;27(4):620–1. doi:.https://doi.org/10.1038/s41591-021-01270-4
9
Kidd
M
,
Richter
A
,
Best
A
,
Cumley
N
,
Mirza
J
,
Percival
B
, et al.
S-variant SARS-CoV-2 lineage B1.1.7 is associated with significantly higher viral loads in samples tested by ThermoFisher TaqPath polymerase chain reaction. J Infect Dis. 2021;223(10):1666–70. doi:.https://doi.org/10.1093/infdis/jiab082
10
Davies
NG
,
Jarvis
CI
,
Edmunds
WJ
,
Jewell
NP
,
Diaz-Ordaz
K
,
Keogh
RH
; CMMID COVID-19 Working Group. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature. 2021;593(7858):270–4. doi:.https://doi.org/10.1038/s41586-021-03426-1
11
von Elm
E
,
Altman
DG
,
Egger
M
,
Pocock
SJ
,
Gøtzsche
PC
,
Vandenbroucke
JP
; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7. doi:.https://doi.org/10.1016/S0140-6736(07)61602-X
12World Health Organization. Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: interim guidance, 2 March 2020. World Health Organization; 2020 [cited 2021 Jun 13]; Available from: https://apps.who.int/iris/handle/10665/331329.
13
Harris
PA
,
Taylor
R
,
Thielke
R
,
Payne
J
,
Gonzalez
N
,
Conde
JG
. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. doi:.https://doi.org/10.1016/j.jbi.2008.08.010
14Hilty MP, Wendel Garcia PD. hobbes8080/risc-19-icu: registry data transformation v1.0. Zenodo Data Repos. [Internet] 2020; Available from: https://zenodo.org/record/3757064.
15Federal Office of Public Health. COVID-19 epidemiological data key figures for Switzerland. Fed. Off. Public Health [Internet] 2021; Available from: https://www.covid19.admin.ch/de/epidemiologic/vacc-doses.
16
Bates
D
,
Mächler
M
,
Bolker
B
,
Walker
S
. Fitting Linear Mixed-Effects Models using lme4. J Stat Softw. 2015;67(1). doi:.https://doi.org/10.18637/jss.v067.i01
17
Baayen
RH
,
Davidson
DJ
,
Bates
DM
. Mixed-effects modeling with crossed random effects for subjects and items. J Mem Lang. 2008;59(4):390–412. doi:.https://doi.org/10.1016/j.jml.2007.12.005
18
Barr
DJ
,
Levy
R
,
Scheepers
C
,
Tily
HJ
. Random effects structure for confirmatory hypothesis testing: Keep it maximal. J Mem Lang. 2013;68(3):255–78. doi:.https://doi.org/10.1016/j.jml.2012.11.001
19R Development Core Team. R: A language and environment for statistical computing [Internet]. R Foundation for Statistical Computing, Vienna, Austria; 2011.Available from: http://www.R-project.org/.
20Wickham H. ggplot2: Elegant Graphics for Data Analysis [Internet]. 1st ed. 2009. Corr. 3rd printing 2010 edition. New York: Springer; 2010.Available from: http://ggplot2.org.
21
Favaron
E
,
Ince
C
,
Hilty
MP
,
Ergin
B
,
van der Zee
P
,
Uz
Z
, et al.
Capillary Leukocytes, Microaggregates, and the Response to Hypoxemia in the Microcirculation of Coronavirus Disease 2019 Patients. Crit Care Med. 2021;49(4):661–70. doi:.https://doi.org/10.1097/CCM.0000000000004862
22
Bein
T
,
Weber-Carstens
S
,
Apfelbacher
C
. Long-term outcome after the acute respiratory distress syndrome: different from general critical illness?
Curr Opin Crit Care. 2018;24(1):35–40. doi:.https://doi.org/10.1097/MCC.0000000000000476
23Gelman A, Hill J. Data Analysis Using Regression and Multilevel/Hierarchical Models [Internet]. Cambridge: Cambridge University Press; 2006 [cited 2021 June 15]. Available from: https://www.cambridge.org/core/books/data-analysis-using-regression-and-multilevelhierarchical-models/32A29531C7FD730C3A68951A17C9D983.