Fine needle aspiration in COVID-19 vaccine-associated lymphadenopathy
DOI: https://doi.org/10.4414/smw.2021.20557
Cristina
Hagena*, Miriam
Nowacka*, Michael
Messerlib, Francesca
Saroa, Felix
Mangoldc, Peter K.
Bodea
a Department of Pathology and Molecular Pathology, University Hospital, University of Zurich, Switzerland
b Department of Nuclear Medicine, University Hospital Zurich, University of Zurich, Switzerland
c Clinic of Pulmonology, University Hospital, University of Zurich, Switzerland
Summary
AIMS
With ongoing intensive vaccination programme against COVID-19, numerous cases of adverse reactions occur, some of which represent rare events. Enlargement of the injection site’s draining lymph nodes is increasingly reported, but is not yet widely recognised as being possibly associated with recent vaccination. As patients at risk of a severe course of COVID-19, indicated by their medical history such as a previous diagnosis of malignancy, receive priority vaccination, newly palpable lymph nodes raise concerns of disease progression. In this case series, we report on five patients who presented with enlarged lymph nodes after COVID-19 vaccination.
METHODS
Sonography guided fine needle aspiration (FNA) was performed in five patients presenting with PET-positive and/or enlarged lymph nodes after COVID-19 vaccination with either the Pfizer-BioNTech or Moderna vaccine.
RESULTS
COVID-19 vaccination had been carried out in all cases, with an interval of between 3 and 33 days prior to FNA. Three of five patients had a history of neoplasms. The vaccine was administered into the deltoid muscle, with subsequent enlargement of either the cervical, supra-, infra- or retroclavicular, or axillary lymph nodes, in four out of five cases ipsilaterally. In all cases, cytology and additional analyses showed a reactive lymphadenopathy without any sign of malignancy.
CONCLUSIONS
Evidence of newly enlarged lymph nodes after recent COVID-19 vaccination should be considered reactive in the first instance, occurring owing to stimulation of the immune system. A clinical follow-up according to the patient’s risk profile without further diagnostic measures is justified. In the case of preexisting unilateral cancer, vaccination should be given contralaterally whenever possible. Persistently enlarged lymph nodes should be re-evaluated (2 to) 6 weeks after the second dose, with additional diagnostic tests tailored to the clinical context. Fine needle aspiration is a well established, safe, rapid and cost-effective method to investigate an underlying malignancy, especially metastasis. Recording vaccination history, including date of injection, site and vaccine type, as well as communicating this information to treating physicians of different specialties is paramount for properly handling COVID-19 vaccine-associated lymphadenopathy.
Introduction
COVID-19, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first registered in China in the end of 2019 from where it rapidly spread all over the globe. The first person in Switzerland was tested positive on 25 February 2020, and the World Health Organization (WHO) declared the disease a pandemic on 11 March 2020 [1]. One year later, more than 170 million cases have been reported to the WHO, with over 3.8 million fatal outcomes [2]. Battling the spread of the virus is dominating public as well as private life, with healthcare institutions constantly challenged by the high workload and management of a novel disease [3]. Researchers have been fervently pursuing the development not only of treatment options, but also of vaccines to provide protection against COVID-19 and eventually end the pandemic [4–6].
Various vaccines are being evaluated, with currently 322 vaccine candidates and 18 in use worldwide [7–10]. So far, three have been approved in Switzerland (Comirnaty® by Pfizer-BioNTech on 19 December 2020, COVID-19 Vaccine Moderna® by Moderna on 12 January 2021 and COVID-19 Vaccine Janssen by Johnson&Johnson on 22 March 2021). Over 2500 million doses have been administered globally, with more than 6.7 million in Switzerland alone [11–14]. As the demand for vaccine delivery is immediate and high, the population was vaccinated in a stratified approach. It is known that COVID-19 infections affect more severely people with certain medical conditions, such as cardiovascular, respiratory, metabolic or immune disorders [15]. Patients with malignancy also carry a considerably higher risk of infection and complications [16], making it a priority to vaccinate this group. Besides vaccination efficiency, possible adverse events are of concern. Most adverse events following immunisation (AEFI) occur locally and are mild and self-limiting (tenderness at the injection site, reddening, swelling). Systemic AEFI range from fatigue, headache and myalgia to rarely observed severe cases such as anaphylactic shock [17, 18]. Lymphadenopathy may also occur following COVID-19 vaccination and affects mostly ipsilateral cervical, supraclavicular or axillary lymph nodes corresponding to the drainage route after injection into the deltoid muscle. Evaluation of the Moderna COVID-19 vaccine found axillary swelling or tenderness in 11.6% of vaccinated individuals after the first and 16% after the second dose, in 1.1% with clinically detected lymphadenopathy [19]. The Pfizer-BioNTech COVID-19 vaccine trial registered lymphadenopathy in 0.3% of the study participants [20, 21]. This AEFI is most likely underdiagnosed and underreported, leading to potential clinical pitfalls in managing patients who present with a newly palpable mass.
The aforementioned prioritised vaccination of persons with previous or ongoing malignancies represents a further challenge during this COVID-19 pandemic. Newly developed enlargement of lymph nodes raises the question of metastatic disease and elicits diagnostic workup such as imaging or excisional biopsies. Here we describe a case series of five patients referred to the cytology department for a fine needle aspiration (FNA) of enlarged lymph nodes to investigate the presence of neoplastic cells. Our aim is to enhance awareness of COVID-19 vaccine-associated lymphadenopathy and reduce the risk of over-diagnosis as well as overtreatment in suspicious cases. We subsequently propose a set of recommendations on how to approach lymphadenopathy after COVID-19 vaccination.
Material and methods
Between 16 February and 3 March 2021, five patients who had recently been vaccinated against COVID-19 with the vaccine by either Pfizer-BioNTech (Comirnaty®) or Moderna (COVID-19 Vaccine Moderna®) were referred for FNA at our institution to evaluate positron emission tomography (PET)-positive or palpably enlarged lymph nodes. Informed consent was obtained for anonymous publishing of diagnostic data in this work. Sonography guided FNA was performed by two experienced cytopathologists (MN, FS). The material was processed and triaged according to standard protocols [22]. Where indicated, additional analysis was initiated. Flow cytometry was performed as described before [23]. Clonality analysis followed standardised guidelines [24].
Results
Patients were referred to our outpatient clinic for FNA of enlarged painless lymph nodes. Two patients (numbers 1 and 5) underwent a PET / computed tomography (CT) scan for follow-up after small cell and adenocarcinoma of the lung. One patient (number 4) was suspected of relapse after incidental diagnosis of a neuroendocrine tumour of the appendix 6 years previously. Two patients (numbers 2 and 3) were female healthcare professionals worried about having metastatic cancer, namely breast cancer, after detecting palpably enlarged lymph nodes on the lower neck, and infraclavicular and axillary sites.
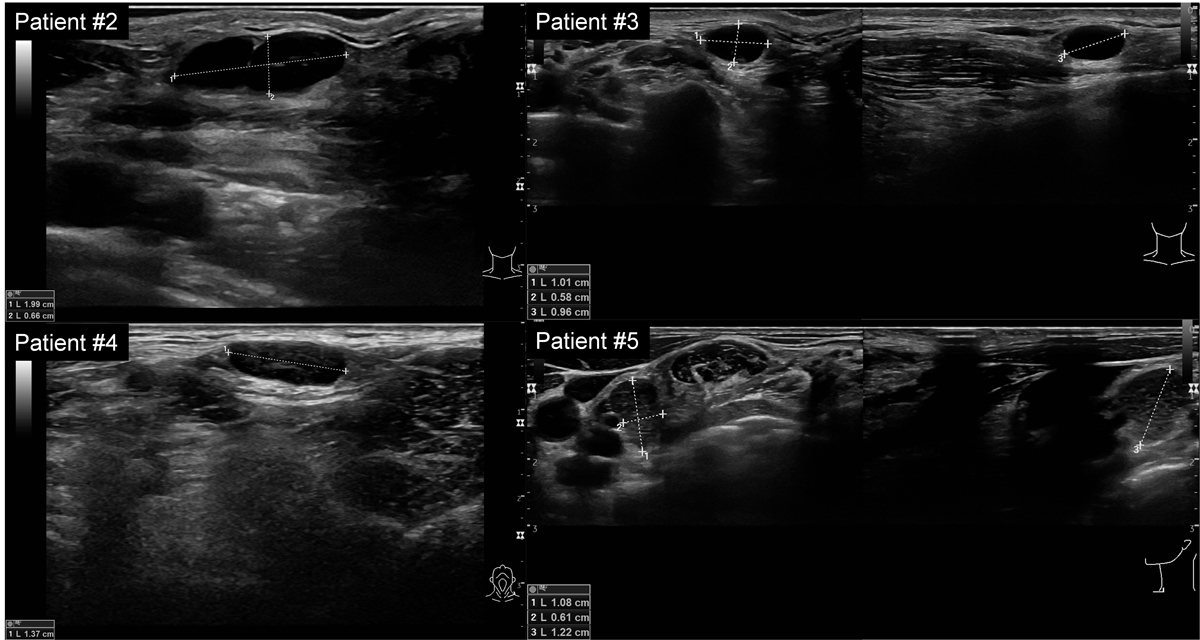
All patients presented with palpable or easily detectable superficial lymph nodes on ultrasound in different locations, either in cervical level IV, supra-, infra-, or retroclavicular regions, and in the axilla. Sonographic imaging revealed partially hypo- and partially isoechogenic lymph nodes (fig. 1A). The width ranged from 1.0 to 2.4 cm in size, with ovoid to rounded shapes, sharp borders and only partially detectable hilum. Some lymph nodes presented with suspicious sonographic findings (spherical shape with loss of hilum) [25] (see also supplementary fig. S2 in the appendix).
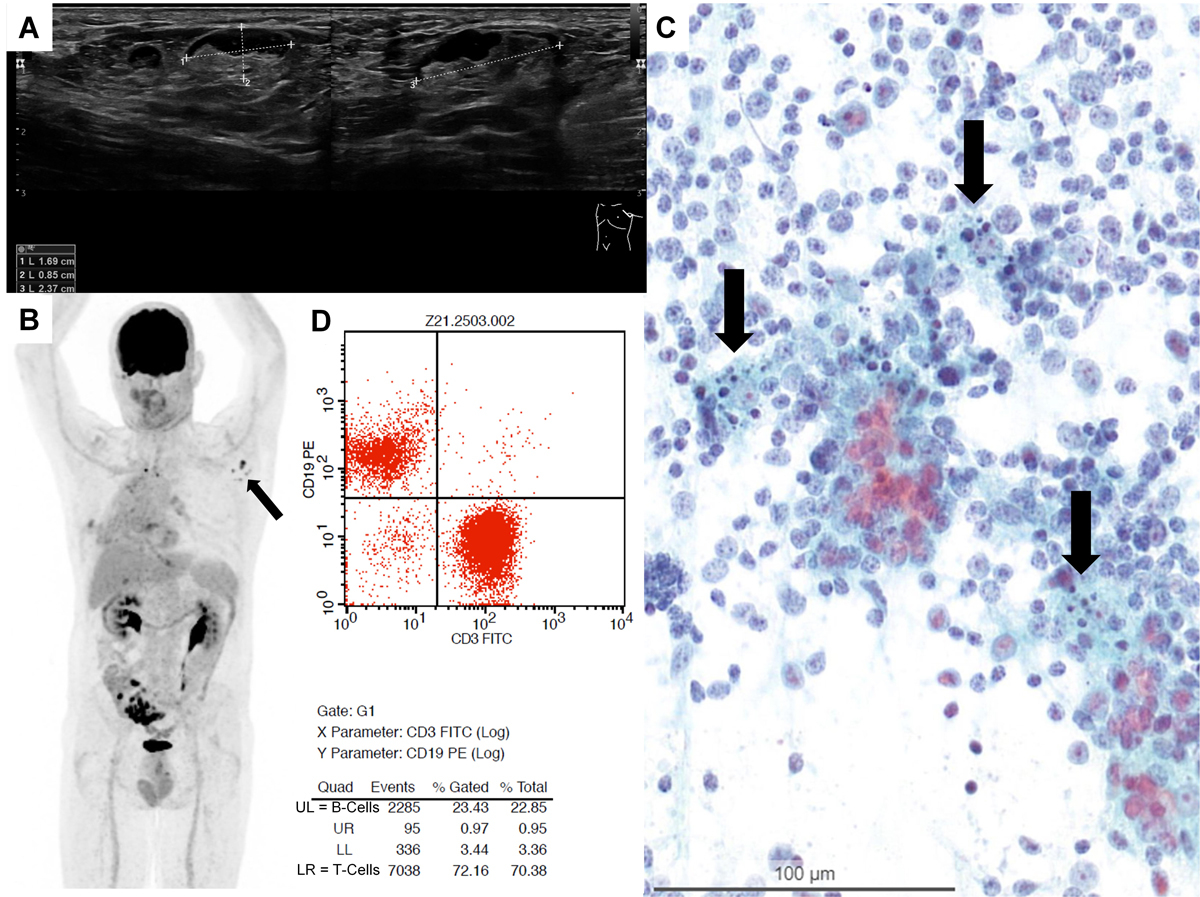
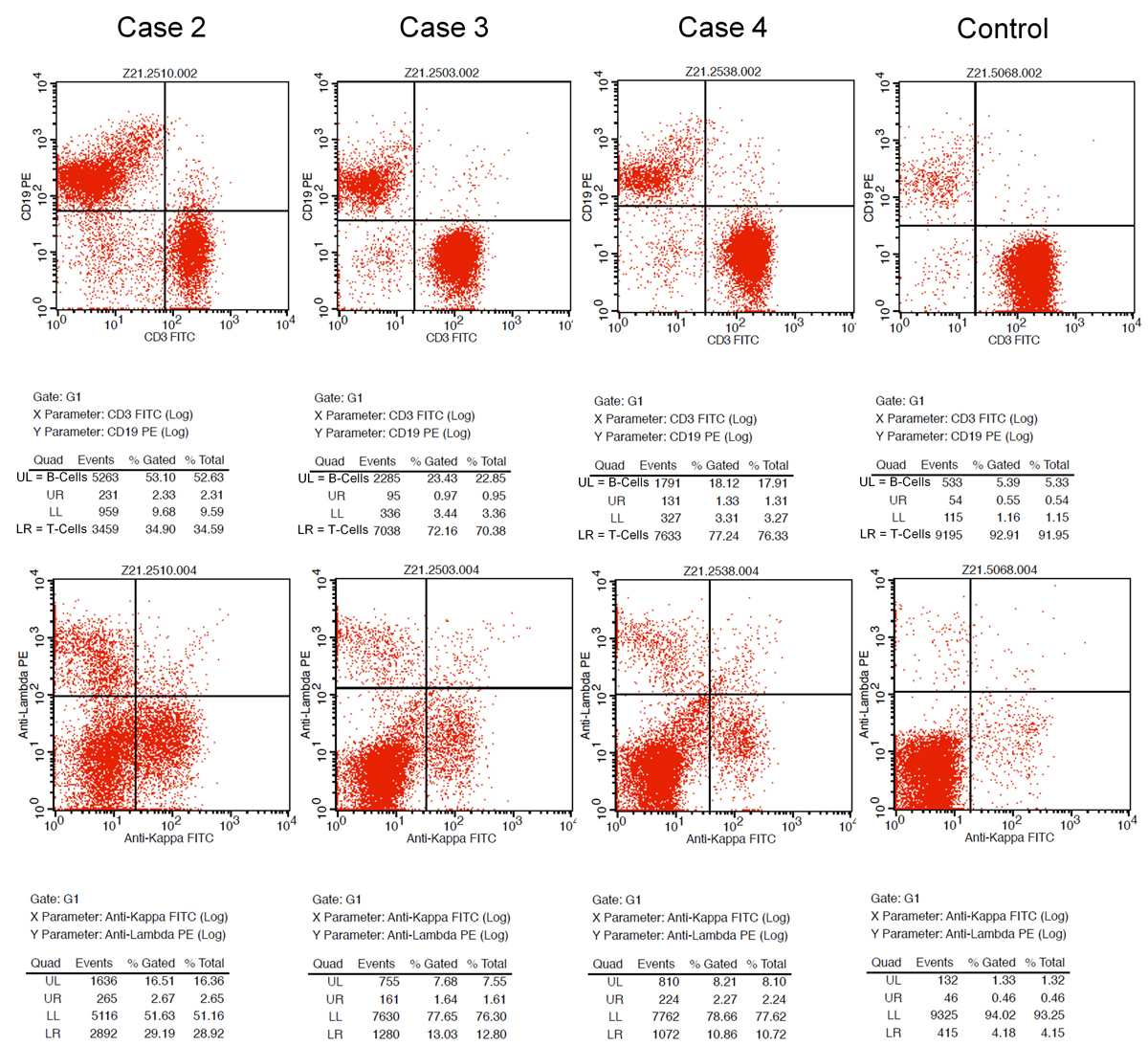
Figure 1 A: Sonography of the axilla in Patient 1 revealed an enlarged lymph node. B: Corresponding PET-CT scan in Patient 1 shows axillar lymph nodes ipsilateral to vaccination site with high FDG activity. D: Flow cytometry in Patient 3 showing a T-cell predominant population with 72% CD3-positive T cells over 23% CD19-positive B cells. C: Smear of fine needle aspiration (FNA) (Papanicolaou staining) showing a mixed lymphoid population with lymphocytes at different stages of maturation and numerous tingible body macrophages (arrows).
In the detailed anamnesis in our outpatient clinic, all patients reported that they had been vaccinated against COVID-19 between 3 and 33 days prior to FNA with either Comirnaty® from Pfizer-BioNTech or COVID-19 Vaccine Moderna® from Moderna. With the exception of Patient 5, the vaccine had been administered into the left deltoid muscle. In four of five patients, the enlargement of lymph nodes was ipsilateral. In Patient 1, PET/CT scan showed enlarged and very highly 18F-2-fluorodesoxyglucose (FDG)-active axillary lymph nodes ipsilateral to the vaccination site and the radiologist already suspected vaccine-associated reactive lymphadenopathy when requesting further workup. Patient 5 presented with a contralateral retroclavicular lymph node suspicious for metastasis in a PET/CT scan with moderate FDG activity, which lead to referral for a cytopathological evaluation. In the same patient, axillary lymph nodes ipsilateral to the vaccination site showed very high FDG-activity (fig. 1B), but were assumed to be vaccine-associated by the attending radiologist and not further analysed. Simultaneously detected mediastinal lymph nodes, which were later diagnosed as metastases by endobronchial ultrasound transbronchial needle aspiration (EBUS-TBNA), showed identically high FDG-activity levels. The detailed patient information is summarised in table 1.
Table 1 Summary of patient details.
|
Number
|
Age (years)
|
Sex
|
Medical history
|
Indication for FNA
|
Vaccination site
|
Type of vaccine
|
Interval vaccination–FNA (days)
|
Site of FNA
|
Cytological diagnosis
|
Sydney classification
|
Follow-up
(2 months)
|
| 1 |
66 |
M |
Lung cancer |
Clinical suspicion of metastasis |
L |
Moderna
(fully vaccinated) |
22 |
Left axillary LN |
Reactive LA. No evidence of metastasis |
1st level: Benign
2nd level: Post COVID-19 vaccination associated LA |
Lymph nodes completely regressed |
| 2 |
41 |
F |
None |
Palpable mass |
L |
Moderna
(1st dose) |
3 |
Left
infraclavicular LN |
Reactive LA. No evidence of malignancy |
1st level: Benign
2nd level: Post COVID-19 vaccination associated LA |
Lymph nodes completely regressed |
| 3 |
47 |
F |
None |
Palpable mass |
L |
Pfizer-BioNTech
(1st dose) |
19 |
Left supraclavicular LN |
Reactive LA. No evidence of malignancy |
1st level: Benign
2nd level: Post COVID-19 vaccination associated LA |
Lymph nodes completely regressed |
| 4 |
47 |
F |
Appendix NET |
Clinical suspicion of metastasis |
L |
Moderna
(1st dose) |
8 |
Left cervical level IV LN |
Reactive LA. No evidence of metastasis |
1st level: Benign
2nd level: Post COVID-19 vaccination associated LA |
Lymph nodes completely regressed |
| 5 |
52 |
M |
Lung cancer |
Clinical suspicion of metastasis |
R |
Pfizer-BioNTech
(fully vaccinated) |
12 |
Left retroclavicular LN |
No evidence of metastasis. No evidence of lymphoma |
1st level: Benign
2nd level: Post COVID-19 vaccination associated LA |
Lymph nodes completely regressed |
| FNA = fine needle aspiration; LA = lymphadenopathy; LN = lymph node; NET = neuroendocrine tumour |
Microscopically, carcinoma metastases could be ruled out both morphologically and immunohistochemically. The smears showed a florid reactive lymphadenopathy pattern, characterised by a mixed lymphoid population with lymphocytes at different stages of maturation including many centroblasts admixed with numerous tingible body macrophages (fig. 1C) [26]. Immunohistochemistry in Patient 5 revealed a reactive pattern with predominant CD3- and CD5-positive T cells, admixed with CD20-positive B cells without co-expression of CyclinD1, CD5, CD10 or CD23. CD21 marked dendritic cells. Flow cytometry was performed in three samples (patients 2–4) and revealed a T-cell predominant population with 72% CD3-positive T cells over 23% CD19-positive B cells (fig. 1D), and 79% CD3-postitve T cells over 19% CD19-positive B cells, respectively, for Patients 3 and 4 (see supplementary fig. S1 in the appendix). One case (Patient 2) showed a predominance of B cells with 55% CD19-positive B cells over 37% CD3-positive T cells. IgH rearrangement polymerase chain-reaction testing revealed a polyclonal B-cell population, consistent with follicular hyperplasia. After 2 months, the lymphadenopathy had clinically regressed in all patients.
Discussion
In this case series, we present the cytological findings of five patients with reactive lymphadenopathy following COVID-19 vaccination. Vaccine-associated lymphadenopathy is a known AEFI of various vaccines [27] such as influenza [28], human papilloma virus [29, 30], hepatitis B virus [31], measles-mumps-rubella [32–35], tetanus toxoid [36], smallpox [37], and bacille Calmette-Guérin [38–40], and has also been found for both the Pfizer-BioNTech and Moderna COVID-19 vaccines [41–48]. Reactive lymphadenopathy has even been suggested to be a potential indicator for successful immunisation overall, as it implies activation of the immune system [43, 49, 50]. In radiology and nuclear medicine, enhanced tracer uptake in reactive lymph nodes after any vaccination is a potential differential diagnosis in PET/CT scans [51–58] and is also emerging for COVID-19 vaccines [59–66]. However, there is little information on cytopathological findings.
Lymphadenopathy occurs in various contexts, summarised by the MIAMI acronym: malignancies, infections, autoimmune disorders, miscellaneous and unusual conditions, and iatrogenic causes [67]. FNA represents a safe, quick and cost-efficient tool in the evaluation of cervical lymphadenopathy [26]. In a systematic review and meta-analysis of 30 studies, FNA of cervical lymph nodes (total of 782 aspirates) showed a sensitivity of 94.2% and specificity of 96.9% [68]. However, it must be kept in mind that subclassification of lymphoma and inaccurate diagnosis of low grade lymphoma are well known limitations of FNA. A systematic review of 42 studies reported a median rate of 66–74% at which FNA and core needle biopsies yielded a subtype-specific diagnosis of lymphoma [69].
The recently proposed Sydney System for lymph node FNA cytology provides information on how to approach lymphadenopathy to ensure results of the best attainable quality. It provides clear reporting categories with possible differential diagnosis, and recommends further procedures depending on the context of presentation [70]. Detailed clinical information (medical history and physical examination), as well as results from further diagnostic tests such as imaging or serology, are crucial in the evaluation of cytological smears from lymph nodes. Overall, the prevalence of malignancy for incidental lymphadenopathy only amounts to 1.1% [71]. However, as cancer patients are preferentially vaccinated against COVID-19 because of the risk of severe course of the disease, vaccine-associated reactive lymphadenopathy might be misinterpreted as cancer progression. Thus, FNA can contribute to a rapid and cost-effective workup of lymphadenopathy.
The currently administered COVID-19 vaccines by Pfizer-BioNTech and Moderna both use a novel technique in which messenger RNA (mRNA) encoding the full-length viral spike protein is encapsulated in lipid nanoparticles to stimulate the production of the viral protein in the recipient’s own cells, thereby provoking an immune response [72]. mRNA itself is highly immunogenic, but components of the lipid nanoparticles such as polyethylene glycol might also cause the observed reactive lymphadenopathy [73–76]. Due to the postulated strong immune response after mRNA COVID-19 vaccination, time spans of lymph node enlargement have been longer than in previous cases of vaccine-associated lymphadenopathy [77], where reactions occurred shortly after vaccination and disappeared rapidly within 14 days [54]. Indeed, we observed intervals between vaccination and FNA of up to 33 days, which calls for a longer observation period. Interestingly, other reports of vaccine-associated lymphadenopathy described mainly enlarged axillary lymph nodes, which was explained by the drainage pattern after injection into the deltoid muscle. In contrast, the enlarged lymph nodes in our patients occurred at various superficial sites of the upper body. This might be a result of varying injection techniques, slight deviation of injection site, or individual variables such as lymph draining routes and immune responses [78]. Fernández-Prada and colleagues described a series of cases of supraclavicular lymphadenopathy after COVID-19 vaccination and attributed these to higher than usual injection into the deltoid muscle [79], expanding the usual expected sites of vaccine-associated lymphadenopathy in patients with a recent COVID-19 vaccination.
In conclusion, we expect an increase of cases of COVID-19 vaccine-associated lymphadenopathy as a result of the ongoing intensive vaccination programme. The risk of overdiagnosis and overtreatment is especially high in cancer patients (e.g., head and neck carcinomas, lung, breast, skin cancer, or lymphoproliferative disease). In this patient group not only the treating physician but also the patients themselves are sensitised to palpable masses and might demand further workup. In this context, we recommend that patients be actively asked about recent COVID-19 vaccination [80]. It has been shown that the currently applied COVID-19 vaccines are safe and likewise evoke an immune response in oncological patients [81–83]. Therefore, whenever possible, vaccination should be administered contralaterally to the site of malignancy to avoid confusion regarding lymph node enlargement. In agreement with our findings and previous data from imaging studies of COVID-19 vaccine-associated lymphadenopathy [84–89], we recommend: (1) documentation of vaccination history, including date, site and type of vaccine given; (2) a low threshold for diagnostic procedures (e.g., ultrasound, FNA) in patients with a known cancer history; (3) a cautious watch-and-wait approach with follow-up physical examination in otherwise healthy patients; and (4) further diagnostic tests (e.g., ultrasound) if the lymph nodes enlarge or persist (2 to) 6 weeks after the second vaccination dose. In this context, FNA represents a fast and accurate tool to guide further management.
Appendix: Supplementary data
Acknowledgments
We express our thanks to the referring physicians and to the patients for providing the necessary data to compose this report.
References
1
Cucinotta
D
,
Vanelli
M
. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91(1):157–60.
2World Health Organization. WHO Coronavirus (COVID-19) Dashboard. 2021 [cited 2021 June 19]; Available from: https://covid19.who.int.
3
Mehta
S
,
Machado
F
,
Kwizera
A
,
Papazian
L
,
Moss
M
,
Azoulay
É
, et al.
COVID-19: a heavy toll on health-care workers. Lancet Respir Med. 2021;9(3):226–8. doi:.https://doi.org/10.1016/S2213-2600(21)00068-0
4
Rawat
K
,
Kumari
P
,
Saha
L
. COVID-19 vaccine: A recent update in pipeline vaccines, their design and development strategies. Eur J Pharmacol. 2021;892:173751. doi:.https://doi.org/10.1016/j.ejphar.2020.173751
5
Izda
V
,
Jeffries
MA
,
Sawalha
AH
. COVID-19: A review of therapeutic strategies and vaccine candidates. Clin Immunol. 2021;222:108634. doi:.https://doi.org/10.1016/j.clim.2020.108634
6
Noor
R
. Developmental Status of the Potential Vaccines for the Mitigation of the COVID-19 Pandemic and a Focus on the Effectiveness of the Pfizer-BioNTech and Moderna mRNA Vaccines. Curr Clin Microbiol Rep. 2021:1–8. Online ahead of print. doi:.https://doi.org/10.1007/s40588-021-00162-y
7
Batty
CJ
,
Heise
MT
,
Bachelder
EM
,
Ainslie
KM
. Vaccine formulations in clinical development for the prevention of severe acute respiratory syndrome coronavirus 2 infection. Adv Drug Deliv Rev. 2021;169:168–89. doi:.https://doi.org/10.1016/j.addr.2020.12.006
8
Dai
L
,
Gao
GF
. Viral targets for vaccines against COVID-19. Nat Rev Immunol. 2021;21(2):73–82. doi:.https://doi.org/10.1038/s41577-020-00480-0
9
Soleimanpour
S
,
Yaghoubi
A
. COVID-19 vaccine: where are we now and where should we go?
Expert Rev Vaccines. 2021;20(1):23–44. doi:.https://doi.org/10.1080/14760584.2021.1875824
10Vaccine Centre London School of Hygene & Tropical Medicine. COVID-19 Vaccine Tracker. 2021 [cited 2021 June 19]; Available from: https://vac-lshtm.shinyapps.io/ncov_vaccine_landscape/.
11Our World in Data. Coronavirus (COVID-19) Vaccinations. 2021 [cited 2021 June 19]; Available from: https://ourworldindata.org/covid-vaccinations.
12Bundesamt für Gesundheit BAG. COVID-19 Switzerland. [cited 2021 June 19]; Available from: https://www.covid19.admin.ch/en/overview.
13Our World in Data. Coronavirus (COVID-19) Vaccinations. 2021 [cited 2021 May 14]; Available from: https://ourworldindata.org/covid-vaccinations.
14Bundesamt für Gesundheit BAG. COVID-19 Switzerland. [cited 2021 May 14]; Available from: https://www.covid19.admin.ch/en/overview.
15
Li
H
,
Burm
SW
,
Hong
SH
,
Ghayda
RA
,
Kronbichler
A
,
Smith
L
, et al.
A Comprehensive Review of Coronavirus Disease 2019: Epidemiology, Transmission, Risk Factors, and International Responses. Yonsei Med J. 2021;62(1):1–11. doi:.https://doi.org/10.3349/ymj.2021.62.1.1
16
Desai
A
, et al.
Mortality in hospitalized patients with cancer and coronavirus disease 2019: A systematic review and meta-analysis of cohort studies. Cancer. 2021;127(9):1459–68. doi:.https://doi.org/10.1002/cncr.33386
17
Meo
SA
,
Bukhari
IA
,
Akram
J
,
Meo
AS
,
Klonoff
DC
. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur Rev Med Pharmacol Sci. 2021;25(3):1663–9. doi:.https://doi.org/10.26355/eurrev_202102_24877
18
Baden
LR
,
El Sahly
HM
,
Essink
B
,
Kotloff
K
,
Frey
S
,
Novak
R
, et al.; COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–16. doi:.https://doi.org/10.1056/NEJMoa2035389
19Centers for Disease Control and Prevention. Local Reactions, Systemic Reactions, Adverse Events, and Serious Adverse Events: Moderna COVID-19 Vaccine. 2021 [cited 2021 March 14]; Available from: https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html.
20
Polack
FP
,
Thomas
SJ
,
Kitchin
N
,
Absalon
J
,
Gurtman
A
,
Lockhart
S
, et al.; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–15. doi:.https://doi.org/10.1056/NEJMoa2034577
21Centers for Disease Control and Prevention. Local Reactions, Systemic Reactions, Adverse Events, and Serious Adverse Events: COVID-19 Vaccine Centers for Disease Control and Prevention. 2021 [cited 2021 March 14]; Available from: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html.
22
Barroca
H
,
Bode-Lesniewska
B
,
Cozzolino
I
,
Zeppa
P
. Management of cytologic material, preanalytic procedures and biobanking in lymph node cytopathology. Cytopathology. 2019;30(1):17–30. doi:.https://doi.org/10.1111/cyt.12609
23
Schmid
S
,
Tinguely
M
,
Cione
P
,
Moch
H
,
Bode
B
. Flow cytometry as an accurate tool to complement fine needle aspiration cytology in the diagnosis of low grade malignant lymphomas. Cytopathology. 2011;22(6):397–406. doi:.https://doi.org/10.1111/j.1365-2303.2010.00801.x
24
van Dongen
JJ
,
Langerak
AW
,
Brüggemann
M
,
Evans
PA
,
Hummel
M
,
Lavender
FL
, et al.
Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17(12):2257–317. doi:.https://doi.org/10.1038/sj.leu.2403202
25
Khanna
R
,
Sharma
AD
,
Khanna
S
,
Kumar
M
,
Shukla
RC
. Usefulness of ultrasonography for the evaluation of cervical lymphadenopathy. World J Surg Oncol. 2011;9(1):29. doi:.https://doi.org/10.1186/1477-7819-9-29
26
Monaco
SE
,
Khalbuss
WE
,
Pantanowitz
L
. Benign non-infectious causes of lymphadenopathy: A review of cytomorphology and differential diagnosis. Diagn Cytopathol. 2012;40(10):925–38. doi:.https://doi.org/10.1002/dc.21767
27
Hartsock
RJ
. Postvaccinial lymphadenitis. Hyperplasia of lymphoid tissue that simulates malignant lymphomas. Cancer. 1968;21(4):632–49. doi:.https://doi.org/10.1002/1097-0142(196804)21:4<632::AID-CNCR2820210415>3.0.CO;2-O
28
Toy
H
,
Karasoy
D
,
Keser
M
. Lymphadenitis caused by H1N1 vaccination: case report. Vaccine. 2010;28(10):2158–60. doi:.https://doi.org/10.1016/j.vaccine.2009.12.043
29
Studdiford
J
,
Lamb
K
,
Horvath
K
,
Altshuler
M
,
Stonehouse
A
. Development of unilateral cervical and supraclavicular lymphadenopathy after human papilloma virus vaccination. Pharmacotherapy. 2008;28(9):1194–7. doi:.https://doi.org/10.1592/phco.28.9.1194
30
Pereira
MP
,
Flores
P
,
Neto
AS
. Neck and supraclavicular lymphadenopathy secondary to 9-valent human papillomavirus vaccination. BMJ Case Rep. 2019;12(11):e231582. doi:.https://doi.org/10.1136/bcr-2019-231582
31
Atalar
H
,
Sarifakioglu
E
,
Dener
C
,
Yanik
B
,
Koktener
A
,
Bayrak
R
. Cutaneous lymphoid hyperplasia and reactive lymphadenopathy induced by hepatitis B vaccination. Eur J Dermatol. 2008;18(2):188–9.
32
Dorfman
RF
,
Herweg
JC
. Live, attenuated measles virus vaccine. Inguinal lymphadenopathy complicating administration. JAMA. 1966;198(3):320–1. doi:.https://doi.org/10.1001/jama.1966.03110160148051
33
Davis
RL
,
Marcuse
E
,
Black
S
,
Shinefield
H
,
Givens
B
,
Schwalbe
J
, et al., The Vaccine Safety Datalink Team. MMR2 immunization at 4 to 5 years and 10 to 12 years of age: a comparison of adverse clinical events after immunization in the Vaccine Safety Datalink project. Pediatrics. 1997;100(5):767–71. doi:.https://doi.org/10.1542/peds.100.5.767
34
Dos Santos
BA
,
Ranieri
TS
,
Bercini
M
,
Schermann
MT
,
Famer
S
,
Mohrdieck
R
, et al.
An evaluation of the adverse reaction potential of three measles-mumps-rubella combination vaccines. Rev Panam Salud Publica. 2002;12(4):240–6. doi:.https://doi.org/10.1590/S1020-49892002001000004
35
Sukumaran
L
,
McNeil
MM
,
Moro
PL
,
Lewis
PW
,
Winiecki
SK
,
Shimabukuro
TT
. Adverse Events Following Measles, Mumps, and Rubella Vaccine in Adults Reported to the Vaccine Adverse Event Reporting System (VAERS), 2003-2013. Clin Infect Dis. 2015;60(10):e58–65. doi:.https://doi.org/10.1093/cid/civ061
36
White
CK
,
Al-Saleem
T
,
Skarbnik
AP
,
Smith
MR
. Tetanus toxoid reactive lymphadenopathy masquerading as T-cell lymphoma. Future Oncol. 2012;8(5):631–4. doi:.https://doi.org/10.2217/fon.12.37
37
Frey
SE
,
Couch
RB
,
Tacket
CO
,
Treanor
JJ
,
Wolff
M
,
Newman
FK
, et al.; National Institute of Allergy and Infectious Diseases Smallpox Vaccine Study Group. Clinical responses to undiluted and diluted smallpox vaccine. N Engl J Med. 2002;346(17):1265–74. doi:.https://doi.org/10.1056/NEJMoa020534
38
Suliman
OM
,
Ahmed
MJ
,
Bilal
JA
. Clinical characteristics and needle aspiration management of Bacillus Calmette-Guérin lymphadenitis in children. Saudi Med J. 2015;36(3):280–5. doi:.https://doi.org/10.15537/smj.2015.3.10294
39
Hendry
AJ
,
Dey
A
,
Beard
FH
,
Khandaker
G
,
Hill
R
,
Macartney
KK
. Adverse events following immunisation with bacille Calmette-Guérin vaccination: baseline data to inform monitoring in Australia following introduction of new unregistered BCG vaccine. Commun Dis Intell Q Rep. 2016;40(4):E470–4.
40
Wang
TC
,
Wu
HJ
,
Yong
SB
. Bacillus Calmette-Guérin vaccination-associated axillary lymphadenopathy in a 2-year-old girl: Case report. J Formos Med Assoc. 2019;118(1):533–4. doi:.https://doi.org/10.1016/j.jfma.2018.09.012
41
Avner
M
,
Orevi
M
,
Caplan
N
,
Popovtzer
A
,
Lotem
M
,
Cohen
JE
. COVID-19 vaccine as a cause for unilateral lymphadenopathy detected by 18F-FDG PET/CT in a patient affected by melanoma. Eur J Nucl Med Mol Imaging. 2021;48(8):2659–60. doi:.https://doi.org/10.1007/s00259-021-05278-3
42
Cellina
M
,
Irmici
G
,
Carrafiello
G
. Unilateral Axillary Lymphadenopathy After Coronavirus Disease (COVID-19) Vaccination. AJR Am J Roentgenol. 2021;216(5):W27. doi:.https://doi.org/10.2214/AJR.21.25683
43
Hiller
N
,
Goldberg
SN
,
Cohen-Cymberknoh
M
,
Vainstein
V
,
Simanovsky
N
. Lymphadenopathy Associated With the COVID-19 Vaccine. Cureus. 2021;13(2):e13524.
44
Mehta
N
,
Sales
RM
,
Babagbemi
K
,
Levy
AD
,
McGrath
AL
,
Drotman
M
, et al.
Unilateral axillary Adenopathy in the setting of COVID-19 vaccine. Clin Imaging. 2021;75:12–5. doi:.https://doi.org/10.1016/j.clinimag.2021.01.016
45
Mitchell
OR
,
Dave
R
,
Bekker
J
,
Brennan
PA
. Supraclavicular lymphadenopathy following COVID-19 vaccination: an increasing presentation to the two-week wait neck lump clinic?
Br J Oral Maxillofac Surg. 2021;59(3):384–5. doi:.https://doi.org/10.1016/j.bjoms.2021.02.002
46
Mortazavi
S
. Coronavirus Disease (COVID-19) Vaccination Associated Axillary Adenopathy: Imaging Findings and Follow-Up Recommendations in 23 Women. AJR Am J Roentgenol. 2021;AJR.21.25651. doi:.https://doi.org/10.2214/AJR.21.25651
47
Özütemiz
C
,
Krystosek
LA
,
Church
AL
,
Chauhan
A
,
Ellermann
JM
,
Domingo-Musibay
E
, et al.
Lymphadenopathy in COVID-19 Vaccine Recipients: Diagnostic Dilemma in Oncologic Patients. Radiology. 2021;300(1):E296–300. doi:.https://doi.org/10.1148/radiol.2021210275
48
Washington
T
,
Bryan
R
,
Clemow
C
. Adenopathy Following COVID-19 Vaccination. Radiology. 2021;299(3):E280–1. doi:.https://doi.org/10.1148/radiol.2021210236
49
Brewer
KD
,
DeBay
DR
,
Dude
I
,
Davis
C
,
Lake
K
,
Parsons
C
, et al.
Using lymph node swelling as a potential biomarker for successful vaccination. Oncotarget. 2016;7(24):35655–69. doi:.https://doi.org/10.18632/oncotarget.9580
50
Youn
H
,
Hong
KJ
. Non-invasive molecular imaging of immune cell dynamics for vaccine research. Clin Exp Vaccine Res. 2019;8(2):89–93. doi:.https://doi.org/10.7774/cevr.2019.8.2.89
51
Williams
G
,
Joyce
RM
,
Parker
JA
. False-positive axillary lymph node on FDG-PET/CT scan resulting from immunization. Clin Nucl Med. 2006;31(11):731–2. doi:.https://doi.org/10.1097/01.rlu.0000242693.69039.70
52
Sheehy
N
,
Drubach
L
. (18)F-FDG uptake at vaccination site. Pediatr Radiol. 2008;38(2):246. doi:.https://doi.org/10.1007/s00247-007-0686-8
53
Panagiotidis
E
,
Exarhos
D
,
Housianakou
I
,
Bournazos
A
,
Datseris
I
. FDG uptake in axillary lymph nodes after vaccination against pandemic (H1N1). Eur Radiol. 2010;20(5):1251–3. doi:.https://doi.org/10.1007/s00330-010-1719-5
54
Burger
IA
,
Husmann
L
,
Hany
TF
,
Schmid
DT
,
Schaefer
NG
. Incidence and intensity of F-18 FDG uptake after vaccination with H1N1 vaccine. Clin Nucl Med. 2011;36(10):848–53. doi:.https://doi.org/10.1097/RLU.0b013e3182177322
55
Mingos
M
,
Howard
S
,
Giacalone
N
,
Kozono
D
,
Jacene
H
. Systemic Immune Response to Vaccination on FDG-PET/CT. Nucl Med Mol Imaging. 2016;50(4):358–61. doi:.https://doi.org/10.1007/s13139-015-0385-6
56
Ayati
N
,
Jesudason
S
,
Berlangieri
SU
,
Scott
AM
. Generalized Lymph Node Activation after Influenza Vaccination on 18F FDG-PET/CT Imaging, an Important Pitfall in PET Interpretation. Asia Ocean J Nucl Med Biol. 2017;5(2):148–50. doi:.https://doi.org/10.22038/aojnmb.2017.8702
57
Coates
EE
,
Costner
PJ
,
Nason
MC
,
Herrin
DM
,
Conant
S
,
Herscovitch
P
, et al.; VRC 900 Study Team. Lymph Node Activation by PET/CT Following Vaccination With Licensed Vaccines for Human Papillomaviruses. Clin Nucl Med. 2017;42(5):329–34. doi:.https://doi.org/10.1097/RLU.0000000000001603
58
Pektor
S
,
Hilscher
L
,
Walzer
KC
,
Miederer
I
,
Bausbacher
N
,
Loquai
C
, et al.
In vivo imaging of the immune response upon systemic RNA cancer vaccination by FDG-PET. EJNMMI Res. 2018;8(1):80. doi:.https://doi.org/10.1186/s13550-018-0435-z
59
Doss
M
,
Nakhoda
SK
,
Li
Y
,
Yu
JQ
. COVID-19 Vaccine-Related Local FDG Uptake. Clin Nucl Med. 2021;46(5):439–41. doi:.https://doi.org/10.1097/RLU.0000000000003634
60
Eifer
M
,
Eshet
Y
. Imaging of COVID-19 Vaccination at FDG PET/CT. Radiology. 2021;299(2):E248. doi:.https://doi.org/10.1148/radiol.2020210030
61
Hanneman
K
,
Iwanochko
RM
,
Thavendiranathan
P
. Evolution of Lymphadenopathy at PET/MRI after COVID-19 Vaccination. Radiology. 2021;299(3):E282. doi:.https://doi.org/10.1148/radiol.2021210386
62
Moghimi
S
,
Wilson
D
,
Martineau
P
. FDG PET Findings Post-COVID Vaccinations: Signs of the Times?
Clin Nucl Med. 2021;46(5):437–8. doi:.https://doi.org/10.1097/RLU.0000000000003636
63
Nawwar
AA
,
Searle
J
,
Hagan
I
,
Lyburn
ID
. COVID-19 vaccination induced axillary nodal uptake on [18F]FDG PET/CT. Eur J Nucl Med Mol Imaging. 2021;48(8):2655–6. doi:.https://doi.org/10.1007/s00259-021-05274-7
64
Nawwar
AA
,
Searle
J
,
Singh
R
,
Lyburn
ID
. Oxford-AstraZeneca COVID-19 vaccination induced lymphadenopathy on [18F]Choline PET/CT-not only an FDG finding. Eur J Nucl Med Mol Imaging. 2021;48(8):2657–8. doi:.https://doi.org/10.1007/s00259-021-05279-2
65
Xu
G
,
Lu
Y
. COVID-19 mRNA Vaccination-Induced Lymphadenopathy Mimics Lymphoma Progression on FDG PET/CT. Clin Nucl Med. 2021;46(4):353–4. doi:.https://doi.org/10.1097/RLU.0000000000003597
66
Katal
S
,
Pouraryan
A
,
Gholamrezanezhad
A
. COVID-19 vaccine is here: practical considerations for clinical imaging applications. Clin Imaging. 2021;76:38–41. doi:.https://doi.org/10.1016/j.clinimag.2021.01.023
67
Habermann
TM
,
Steensma
DP
. Lymphadenopathy. Mayo Clin Proc. 2000;75(7):723–32. doi:.https://doi.org/10.1016/S0025-6196(11)64620-X
68
Tandon
S
,
Shahab
R
,
Benton
JI
,
Ghosh
SK
,
Sheard
J
,
Jones
TM
. Fine-needle aspiration cytology in a regional head and neck cancer center: comparison with a systematic review and meta-analysis. Head Neck. 2008;30(9):1246–52. doi:.https://doi.org/10.1002/hed.20849
69
Frederiksen
JK
,
Sharma
M
,
Casulo
C
,
Burack
WR
. Systematic review of the effectiveness of fine-needle aspiration and/or core needle biopsy for subclassifying lymphoma. Arch Pathol Lab Med. 2015;139(2):245–51. doi:.https://doi.org/10.5858/arpa.2013-0674-RA
70
Al-Abbadi
MA
,
Barroca
H
,
Bode-Lesniewska
B
,
Calaminici
M
,
Caraway
NP
,
Chhieng
DF
, et al.
A Proposal for the Performance, Classification, and Reporting of Lymph Node Fine-Needle Aspiration Cytopathology: The Sydney System. Acta Cytol. 2020;64(4):306–22. doi:.https://doi.org/10.1159/000506497
71
Fijten
GH
,
Blijham
GH
. Unexplained lymphadenopathy in family practice. An evaluation of the probability of malignant causes and the effectiveness of physicians’ workup. J Fam Pract. 1988;27(4):373–6.
72
Bettini
E
,
Locci
M
. SARS-CoV-2 mRNA Vaccines: Immunological Mechanism and Beyond. Vaccines (Basel). 2021;9(2):147. doi:.https://doi.org/10.3390/vaccines9020147
73
Buschmann
MD
,
Carrasco
MJ
,
Alishetty
S
,
Paige
M
,
Alameh
MG
,
Weissman
D
. Nanomaterial Delivery Systems for mRNA Vaccines. Vaccines (Basel). 2021;9(1):65. doi:.https://doi.org/10.3390/vaccines9010065
74
Castells
MC
,
Phillips
EJ
. Maintaining Safety with SARS-CoV-2 Vaccines.
[Reply].
N Engl J Med. 2021;384(10):e37. doi:.https://doi.org/10.1056/NEJMc2100766
75
Mellet
J
,
Pepper
MS
. A COVID-19 Vaccine: Big Strides Come with Big Challenges. Vaccines (Basel). 2021;9(1):39. doi:.https://doi.org/10.3390/vaccines9010039
76
Wu
Z
,
Li
T
. Nanoparticle-Mediated Cytoplasmic Delivery of Messenger RNA Vaccines: Challenges and Future Perspectives. Pharm Res. 2021;38(3):473–8. doi:.https://doi.org/10.1007/s11095-021-03015-x
77Society of Breast Imaging. SBI Recommendations for the Management of Axillary Adenopathy in Patients with Recent COVID-19 Vaccination. 2021 [cited 2021 March 20]; Available from: https://www.sbi-online.org/Portals/0/Position%20Statements/2021/SBI-recommendations-for-managing-axillary-adenopathy-post-COVID-vaccination.pdf.
78
Shirone
N
,
Shinkai
T
,
Yamane
T
,
Uto
F
,
Yoshimura
H
,
Tamai
H
, et al.
Axillary lymph node accumulation on FDG-PET/CT after influenza vaccination. Ann Nucl Med. 2012;26(3):248–52. doi:.https://doi.org/10.1007/s12149-011-0568-x
79
Fernández-Prada
M
,
Rivero-Calle
I
,
Calvache-González
A
,
Martinón-Torres
F
. Acute onset supraclavicular lymphadenopathy coinciding with intramuscular mRNA vaccination against COVID-19 may be related to vaccine injection technique, Spain, January and February 2021. Euro Surveill. 2021;26(10):2100193. doi:.https://doi.org/10.2807/1560-7917.ES.2021.26.10.2100193
80
Gaddey
HL
,
Riegel
AM
. Unexplained Lymphadenopathy: Evaluation and Differential Diagnosis. Am Fam Physician. 2016;94(11):896–903.
81
Kuderer
NM
,
Hill
JA
,
Carpenter
PA
,
Lyman
GH
. Challenges and Opportunities for COVID-19 Vaccines in Patients with Cancer. Cancer Invest. 2021;39(3):205–13. doi:.https://doi.org/10.1080/07357907.2021.1885596
82
Hwang
JK
,
Zhang
T
,
Wang
AZ
,
Li
Z
. COVID-19 vaccines for patients with cancer: benefits likely outweigh risks. J Hematol Oncol. 2021;14(1):38. doi:.https://doi.org/10.1186/s13045-021-01046-w
83
Desai
A
,
Gainor
JF
,
Hegde
A
,
Schram
AM
,
Curigliano
G
,
Pal
S
, et al.
COVID-19 vaccine guidance for patients with cancer participating in oncology clinical trials. Nat Rev Clin Oncol. 2021;18:313–9. doi:. https://doi.org/10.1038/s41571-021-00487-z
84
Thomassen
A
,
Lerberg Nielsen
A
,
Gerke
O
,
Johansen
A
,
Petersen
H
. Duration of 18F-FDG avidity in lymph nodes after pandemic H1N1v and seasonal influenza vaccination. Eur J Nucl Med Mol Imaging. 2011;38(5):894–8. doi:.https://doi.org/10.1007/s00259-011-1729-9
85
Lehman
CD
,
Lamb
LR
,
D’Alessandro
HA
. Mitigating the Impact of Coronavirus Disease (COVID-19) Vaccinations on Patients Undergoing Breast Imaging Examinations: A Pragmatic Approach. AJR Am J Roentgenol. 2021;AJR.21.25688. doi:.https://doi.org/10.2214/AJR.21.25688
86
McIntosh
LJ
,
Bankier
AA
,
Vijayaraghavan
GR
,
Licho
R
,
Rosen
MP
. COVID-19 Vaccination-Related Uptake on FDG PET/CT: An Emerging Dilemma and Suggestions for Management. AJR Am J Roentgenol. 2021;AJR.21.25728. doi:.https://doi.org/10.2214/AJR.21.25728
87
Lehman
CD
,
D’Alessandro
HA
,
Mendoza
DP
,
Succi
MD
,
Kambadakone
A
,
Lamb
LR
. Unilateral Lymphadenopathy After COVID-19 Vaccination: A Practical Management Plan for Radiologists Across Specialties. J Am Coll Radiol. 2021;18(6):843–52. doi:.https://doi.org/10.1016/j.jacr.2021.03.001
88
Becker
AS
,
Perez-Johnston
R
,
Chikarmane
SA
,
Chen
MM
,
El Homsi
M
,
Feigin
KN
, et al.
Multidisciplinary Recommendations Regarding Post-Vaccine Adenopathy and Radiologic Imaging: Radiology Scientific Expert Panel. Radiology. 2021:210436. Online ahead of print. doi:.https://doi.org/10.1148/radiol.2021210436
89
Edmonds
CE
,
Zuckerman
SP
,
Conant
EF
. Management of Unilateral Axillary Lymphadenopathy Detected on Breast MRI in the Era of Coronavirus Disease (COVID-19) Vaccination. AJR Am J Roentgenol. 2021. Online ahead of print. doi:.https://doi.org/10.2214/AJR.21.25604