Evaluation of existing and desired measures to monitor, prevent and control healthcare-associated infections in Swiss hospitals
DOI: https://doi.org/10.4414/smw.2021.20516
Aliki
Metsiniab, Andreas
Widmerac, Walter
Zinggad, Céline
Gardiole, Danielle
Vuichard-Gysinaf, Marcus
Edera, Judith
Maaga, Matthias
Schlegelag, Jonas
Marschallah, Stephan
Harbarthai, Rami
Sommersteinahj, the Swissnoso network*
a Swissnoso, Swiss Centre for Infection Prevention, Bern, Switzerland
b Cantonal Physician Office, Canton of Geneva, Switzerland
c Division of Infectious diseases and Hospital Epidemiology, University of Basel Hospitals and Clinics, Basel, Switzerland
d Division of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, University of Zurich, Switzerland
e Federal Office of Public Health, Bern, Switzerland
f Division of Infectious Diseases and Hospital Epidemiology, Thurgau Hospital Group, Muensterlingen and Frauenfeld, Switzerland
g Division of Infectious Diseases and Hospital Epidemiology, Cantonal Hospital St Gallen, Switzerland
h Department of Infectious Diseases, Bern University Hospital, University of Bern, Switzerland
i Infection Control Programme, University of Geneva Hospitals and Faculty of Medicine, Geneva, Switzerland
j Infectious Diseases, Hirslanden Central Switzerland, Lucerne, Switzerland
* Swissnoso members: Carlo Balmelli, Marie-Christine Eisenring, Stephan Harbarth, Jonas Marschall, Didier Pittet, Hugo Sax, Matthias Schlegel, Alexander Schweiger, Laurence Senn, Rami Sommerstein, Nicolas Troillet, Sarah Tschudin-Sutter, Danielle Vuichard-Gysin, Andreas Widmer, Giorgio Zanetti and Walter Zingg
Summary
OBJECTIVES
Optimal surveillance and prevention of healthcare-associated infections (HAI) are crucial for a well-functioning health care system. With a view to establishing a national state-of-the-art programme for surveillance and prevention of HAIs, the Swiss National Center for Infection Control, Swissnoso, developed a survey to explore the options for expanding the existing Swiss HAI surveillance system.
METHODS
An online survey was sent to all Swiss acute care hospitals. Local infection prevention and control (IPC) professionals were asked to answer on behalf of their institutions. The questions covered the structure and organisation of IPC programmes, current preventive measures, availability and capacity of electronic medical record (EMR) systems, and ability and willingness to establish and participate in the proposed new surveillance modules. An invitation was sent to the 156 acute care hospitals and hospital networks in June 2020. Responses were collected up to the end of August 2020.
RESULTS
Ninety-four hospitals and hospital networks out of 156 (60%) completed the survey. Among 84 hospitals reporting the number of acute care beds, 61 (73%) were small (<200 beds), 16 (19%) medium (200–650 beds) and 7 (8%) large hospitals (>650 beds). Twenty-nine different EMR systems were used in the participating hospitals. Twenty-two hospitals were using a different EMR system in their intensive care unit. There were 17 hospitals (18%) without an EMR system but which planned to introduce one soon, and eight small hospitals (9%) neither had an EMR system nor were preparing to introduce one. Surveillance for central-line associated bloodstream infection, catheter-associated urinary tract infection and ventilator-associated pneumonia were already established in 26 (28%), 15 (16%) and 15 (16%) hospitals, respectively. Thirty hospitals (36%) would be willing to participate in the pilot phase of a new surveillance system. Of these, 15 stated that they wanted to be part of the pilot hospital network, 6 could provide hospital-wide surveillance denominators (such as catheter-days and patient-days) to compute incidence rates, and 8 indicated interest in doing both. Large hospitals interested in participating in the pilot phase reported more full-time equivalent staff available for surveillance activities than those who did not declare an interest.
CONCLUSIONS
Baseline information on hospital IPC structure and process indicators are essential for the roll-out of national surveillance programmes and for improving surveillance activities. Having an EMR system in place and adequate personnel resources dedicated for surveillance activities are crucial prerequisites for developing and implementing an effective HAI surveillance system. The lack of an EMR system and the diversity and capacities of EMR solutions will be the main challenges for successful implementation of national HAI surveillance modules.
Introduction
Despite the substantial global efforts to prevent healthcare-associated infections (HAIs), their burden remains significant as up to 10% of patients in developing and 7% in developed countries acquire at least one HAI during their hospital stay [1].
The revised Swiss Epidemics Law that entered into force on 1 January 2016 defines goals and strategies for detecting, monitoring, preventing and controlling communicable diseases, and lays the basis for developing a national programme on HAIs. A national strategy (the so-called NOSO Strategy) was developed and approved by the Federal Council in March 2016 [2]. In accordance with the NOSO Strategy, a national monitoring system is being designed, and the currently existing monitoring programmes in Switzerland are being further expanded and coordinated with each other. A centralised surveillance system allows a better overview of national epidemiology, organisation of data, bench-marking and the coordination of national interventions for HAI prevention. The COVID-19 pandemic further emphasised the need for centralised national surveillance systems.
Switzerland has a mandatory national surveillance system for surgical site infections (SSIs); however, to our knowledge, data on other HAIs such as central line-associated bloodstream infection, catheter-associated urinary tract infection or ventilator-associated pneumonia are recorded by only a minority of hospitals. The establishment and operation of a national surveillance system for these HAIs were assigned to the National Centre for Infection Control, called Swissnoso (www.swissnoso.ch).
With a view to exploring the feasibility of establishing new surveillance modules and the willingness to participate in them, Swissnoso developed an online survey and and sent it out to all Swiss acute care hospitals to gain insight into current surveillance and prevention practices.
Materials and methods
This was a comprehensive inventory aiming to elicit hospital practices and tools related to prevention of HAI, such as intravascular bloodstream infections, catheter-associated urinary tract infection and ventilator-associated pneumonia. Based on the systematic review by Zingg et al., hospital IPC structure and process indicators were recorded, including information on existing surveillance practices [3]. Definitions of multimodal strategies were provided by the European Centre for Disease Prevention (ECDC) point prevalence survey protocol [4, 5]. Electronic medical record (EMR) capacities such as indwelling devices linked with microbiological data, generation of denominators of HAI surveillance (e.g., all positive blood cultures, number of devices per 1000 patient-days or catheter-days) and automated data transfer to a centralised database were also collected. Finally, hospitals were asked whether they wanted to participate in the pilot phase of an extended surveillance system and could then indicate the module of their choice.
The survey was sent to IPC professionals representing acute care settings according to the Swissnoso address list. There were 206 acute care hospitals/sites in Switzerland at that time. In the case of hospital networks, only the networks and not the individual hospitals were contacted. Therefore, the survey was sent to 156 Swiss acute care hospitals/networks. The invitation was sent out in late June 2020; all answers were retrieved by the end of August 2020.
No institutional review board approval was deemed necessary, given the quality improvement character of the survey. Only anonymous patient and ward data were collected and analysed.
Data analysis was conducted with STATA version 15 (StataCorp LLC, USA). Significance of differences between full-time equivalent (FTE) means in different hospital categories was tested through an independent samples two way t-test.
Results
Ninety-four questionnaires were returned, corresponding to a 60% response rate of all registered Swiss acute care hospitals/networks. Of these, 66 (70%) were from hospitals/networks from the German-speaking, 25 (27%) from the French-speaking and 3 (3%) from the Italian-speaking part of the country. Eighty-four hospitals reported the number of acute-care beds; response rates by hospital size are presented in table 1.
Table 1 Response rate to the Swissnoso surveillance inventory by hospital size.
|
Hospital size
|
Response rate by hospital category, n/N (%)
|
Distribution of participating hospitals, n/N (%)
|
| Small (<200 beds) |
61/122 (50%) |
61/84 (73%) |
| Medium (200–650 beds) |
16/24 (67%) |
16/84 (19%) |
| Large (>650 beds) |
7/9 (78%) |
7/84 (8%) |
Use of EMR
Asked whether they use an EMR, 69 (73%) hospitals answered that they already had an EMR, whereas 17 (18%) did not but had planned to introduce one in due time. Only eight (i.e. all small hospitals) neither had an EMR nor planned to use one soon. Table 2 summarises the availability of EMR systems by hospital size and table S1 in the appendix shows equivalent data by language region.
Table 2 Electronic medical record (EMR) availability by hospital size.
|
Hospital size
|
EMR availability, n/N (%)
|
|
Yes (%)
|
No (%)
|
Soon (%)
|
| Small (<200 beds) |
44/61 (72%) |
6/61 (10%) |
11/61 (18%) |
| Medium (200–650 beds) |
14/16 (88%) |
0 |
2/16 (12%) |
| Large (>650 beds) |
6/7 (86%) |
0 |
1/7 (14%) |
| Total |
64/84 (76%) |
6/84 (7%) |
14/84 (17%) |
There are 29 different EMR systems in use around Switzerland; of these, the most frequently used are Phoenix®, Ines KIS®, Kisim®, Soarian (Cerner)® and ClevEHR®. Most hospitals performed device and drug prescription and vital sign reporting through their EMR, at least at a ward level. Prescribing ability at an intensive care unit (ICU) level was less frequent. Extraction of data that serve as denominators, such as device-days, and automated data transfer into a warehouse/database for further processing were also uncommon functions among the EMR systems in use. EMR capacities of participating hospitals are summarised in the appendix (table S2).
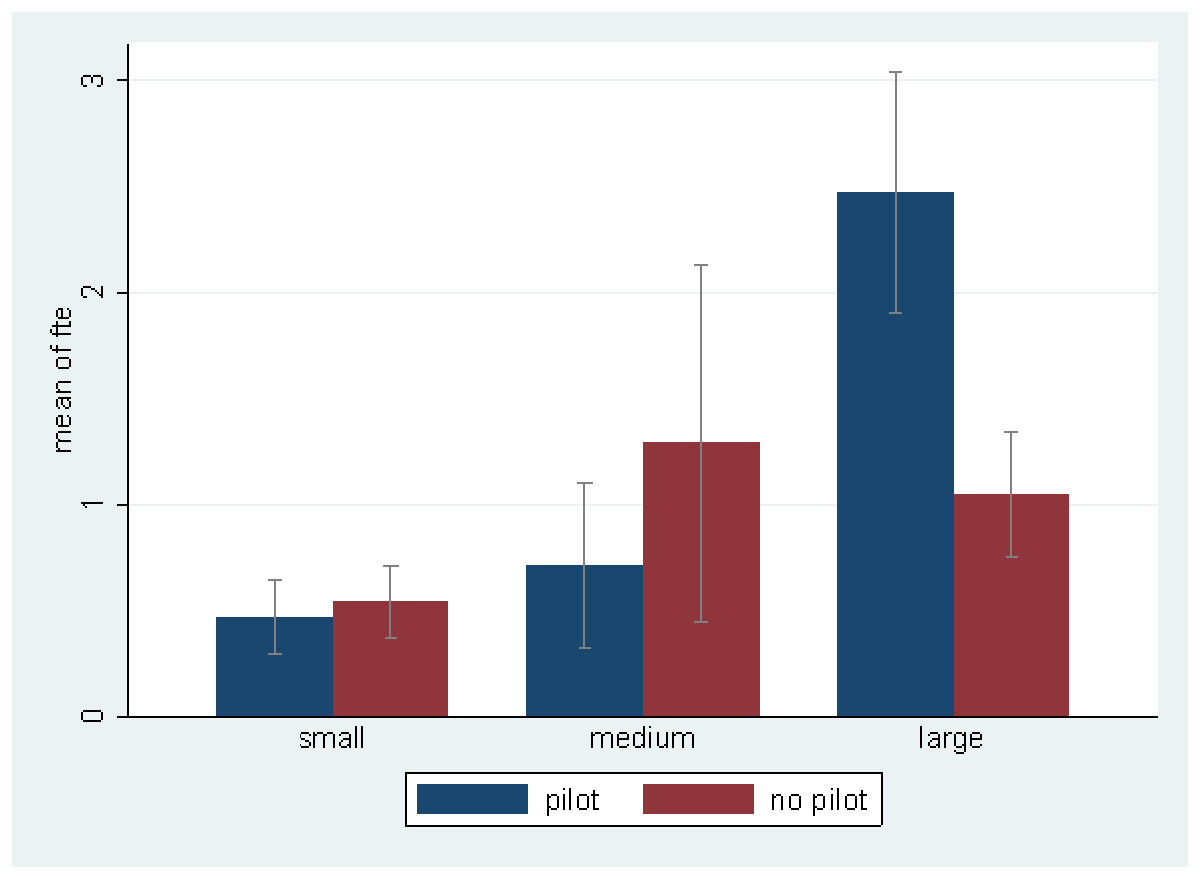
The association between FTEs dedicated to IPC surveillance and EMR status by hospital size is shown in figure 1. There were no statistically significant differences between dedicated FTEs for surveillance among hospitals according to their EMR status.
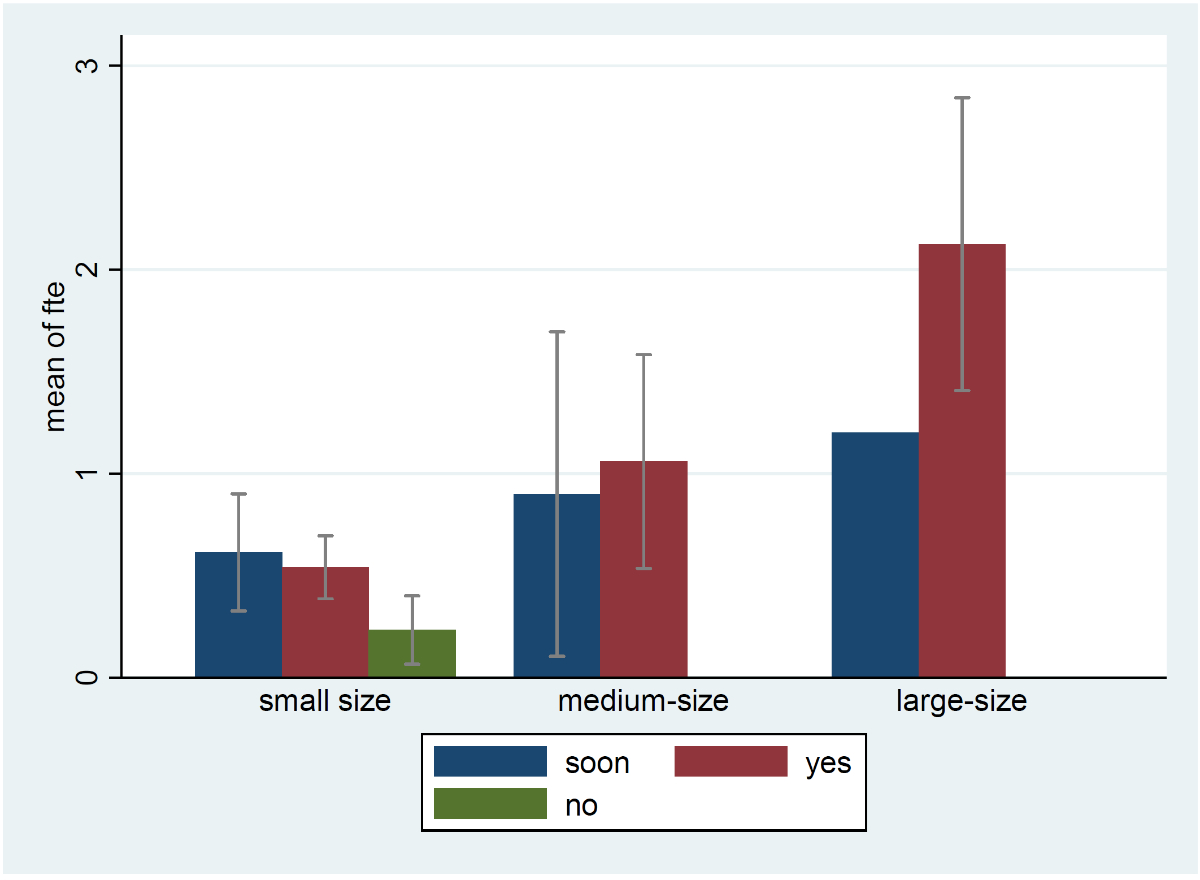
Figure 1 Fulltime equivalents (FTEs) dedicated to healthcare-associated infection surveillance by hospital size, stratified by electronic medical record (EMR) availability.
Small-size hospitals: <200 beds, medium-size hospitals: 200–650 beds, large-size hospitals: >650 beds, soon: no EMR available in the hospital but planned to get one soon, yes: EMR in use in the hospital, no: no EMR in use in the hospital
Multifaceted IPC strategies
Guidelines were most frequently used for antimicrobial use and surgical site infection prevention at a ward level, and antimicrobial use and central-line associated bloodstream infection prevention in the ICU. Most hospitals (65%) reported performing the surgical site infection surveillance of Swissnoso. Checklists and audits were less common in both wards and ICUs. Multifaceted prevention and surveillance activities are summarised in the appendix (table S3).
Surveillance activities
Surveillance systems on central line-associated bloodstream infection , catheter-associated urinary tract infection and ventilator-associated pneumonia were already established in 26 (28%), 15 (16%) and 15 (16%) hospitals, respectively. Thirty hospitals (36%) were willing to participate in the pilot phase of the new national surveillance system. Among them, 15 wanted to be part of the pilot hospital network, 6 to provide hospital-wide surveillance denominators (such as catheter-days and patient-days) for incidence-rate estimation, and 8 intended to do both. Willingness to participate as a pilot hospital and mean FTEs dedicated to surveillance by hospital size are shown in figure 2; willingness to participate by language region is provided in the appendix (table S4). Large hospitals interested in being part of the pilot phase tended to have higher FTEs available for surveillance than those that did not declare an interest (p = 0.06).
Discussion
This is the first national inventory on IPC structure and process indicators in acute care hospitals in Switzerland. It summarises key information on IPC strategies of acute care hospitals in Switzerland. It also allows interpretation of feasibility and generalisability of the upcoming national surveillance strategy for HAI.
Ninety-four hospitals/networks participated in the online survey, which account for more than half of the registered acute care hospitals (n = 156). If we consider that the survey was sent at the end of the first wave of the SARS-CoV-2 pandemic in Switzerland, the response rate exceeded our expectations.
Unsurprisingly, most Swiss hospitals have an EMR. Electronic health records seem to cover hospital services and units quite well, allowing for complex functions and better documentation and sharing of relevant information on patient care. ICU electronic systems are comparatively less prevalent, as not all of the participating hospitals have an ICU. The diversity of the EMR systems is a significant challenge in establishing a centralised semi-automated surveillance system in Switzerland. Only approximately 15% of the hospitals considered their EMR to be capable of allowing data transfer – but respondents may have underestimated this capacity of their EMR. Nevertheless, efforts should be directed to the design and development of secure interfaces linking the national surveillance system and individual hospital electronic health records.
There was also substantial variation in the implementation of hospital-wide multifaceted strategies. Although surgical site infection surveillance is mandatory in Switzerland as part of the national quality contract, only 65% of the participating hospitals used the surgical site infection surveillance as part of their IPC multimodal strategy on prevention of surgical site infections. Discrepancies may be linked to potential barriers of perception between institutional surgical site infection surveillance and hospital-wide implementation, leading to biased responses.
Although participation in the survey was satisfactory in our opinion, only a small number of hospitals have volunteered to participate in the pilot phase of the new Swissnoso surveillance system. The discrepancy between hospitals doing surveillance activities but not being interested in participating in a national surveillance system raises questions on potential barriers to successful implementation of a national surveillance strategy. We hypothesise that sufficient staff and infrastructure (such as availability of an EMR that could facilitate automated procedures) would have played a major role in that.
Large hospitals interested in becoming pilot hospitals had higher FTEs available for surveillance than those that did not express such interest. This observation suggests that sufficient staffing is key for development of effective IPC strategies, including surveillance activities. However, high-quality data cannot be generated by computerised systems only. Qualified staff are needed, and the new “Schwerpunkt Infektprävention” (IPC subspecialty) for physicians (as of 1 July 2021) as well as “Fachexpertin für Infektprävention” for nurses and individuals with equivalent education will ensure that Swiss surveillance data continue to excel in terms of quality, as observed in the hospital infection surveillance study by the ECDC [6].
Our survey has several limitations; the number of participating hospitals may not adequately represent the distribution of Swiss acute care hospitals. Large hospitals were overrepresented, corresponding to 8% of participating hospitals (they represent 6% of Swiss acute care hospitals). As the survey was sent during the COVID-19 pandemic, one explanation might be that large hospitals had more resources for IPC activities. Second, there was no adjustment for the presence of confounding factors. Willingness to participate as a pilot hospital was not adjusted for potential confounders, such as doing well financially, nosocomial infection rate, linguistic region, etc., which decreases the validity of generalisation. Third, following the discovery of discordant responses on Swissnoso surgical site infection surveillance participation, it is assumed that responders might be prone to bias in their answers regarding multimodal strategies on HAI prevention implemented at hospital level; one hypothesis is that the ongoing health crisis might alter the perception of IPC strategies at a hospital level and/or impact the time dedicated by the responders in the questionnaire completion. Finally, there were missing data as the inventory permitted missing or skipping questions.
Despite these limitations, this is the first nationwide inventory on IPC indicators, surveillance activities and EMR capacities in Swiss acute care hospitals with data collection done via an online questionnaire. These data will serve as the basis for developing an extended surveillance system and identifying the hospitals that will build the future hospital surveillance network.
Conclusion
This inventory collected essential baseline information on hospital IPC structural and process indicators and the hospitals’ surveillance capacities. Computerised health records and educated staff dedicated to surveillance are key elements of a successful surveillance system. Hospitals that are interested in and have the necessary infrastructure for surveillance activities in Switzerland were identified. These hospitals will be invited in due time to join in the pilot phase of the surveillance modules.
Note from the editor
Unfortunately, instead of the correct figure 1, figure 2 was included twice in the original publication of this article.This correction has been made to the online version as of 3 August 2022.
Appendix Supplementary tables
Table S1 EMR status by linguistic region.
|
Linguistic region
|
EMR availability
|
|
Not yet but planned
|
Yes
|
No
|
| German |
10 |
52 |
4 |
| French |
6 |
15 |
4 |
| Italian |
3 |
2 |
0 |
| Total |
19 |
69 |
8 |
Table S2 EMR capacities by hospital site.
|
Hospital site
|
Device ordering
|
Drug prescription
|
Vital sign reports
|
Catheter-days extraction
|
Possibility of data transfer
|
| General ward |
70 (74%) |
74 (79%) |
72 (77%) |
37 (39%) |
14 (15%) |
| ICU |
39 (41%) |
36 (38%) |
32 (34%) |
26 (28%) |
9 (10%) |
| Other*
|
32 (34%) |
34 (36%) |
31 (33%) |
25 (27%) |
16 (17%) |
Table S3 Multimodal strategies at the ICU or ward level in participating hospitals.
| |
Guidelines
|
Bundle
|
Training
|
Checklist
|
Audit
|
Surveillance
|
Feedback
|
|
ICU
|
|
|
|
|
|
|
|
| VAP |
33 (34%) |
20 (21%) |
20 (21%) |
7 (7%) |
5 (5%) |
13 (14%) |
16 (17%) |
| CLABSI |
42 (44%) |
19 (20%) |
25 (26%) |
12 (13%) |
7 (7%) |
23 (24%) |
22 (23%) |
| CAUTI |
40 (42%) |
14 (15%) |
20 (21%) |
10 (10%) |
5 (5%) |
15 (16%) |
16 (17%) |
| AU |
44 (46%) |
5 (5%) |
18 (19%) |
6 (6%) |
8 (8%) |
19 (20%) |
23 (24%) |
|
Ward
|
|
|
|
|
|
|
|
| PNE |
37 (39%) |
12 (13%) |
15 (16%) |
4 (4%) |
4 (4%) |
8 (8%) |
14 (15%) |
| SSI |
53 (55%) |
22 (23%) |
31 (32%) |
17 (18%) |
29 (30%) |
62 (65%) |
47 (49%) |
| CAUTI |
51 (53%) |
22 (23%) |
29 (30%) |
14 (15%) |
8 (8%) |
14 (15%) |
21 (22%) |
| AU |
60 (63%) |
8 (8%) |
21 (22%) |
13 (14%) |
10 (10%) |
32 (33%) |
31 (32%) |
Table S4 Willingness to participate as a pilot hospital by linguistic region.
|
Linguistic region
|
Willingness to participate as a pilot hospital
|
|
Yes
|
No
|
| German |
21 |
38 |
| French |
6 |
16 |
| Italian |
2 |
0 |
| Total |
30 |
54 |
Acknowledgments
We would like to thank the directors and IPC referent teams of all participating acute care hospitals in Switzerland which collected and transmitted quality and IPC performance indicators as well as data of their hospitalised population in such extraordinary times.
References
1
Cassini
A
,
Plachouras
D
,
Eckmanns
T
,
Abu Sin
M
,
Blank
H-P
,
Ducomble
T
, et al.
Burden of Six Healthcare-Associated Infections on European Population Health: Estimating Incidence-Based Disability-Adjusted Life Years through a Population Prevalence-Based Modelling Study. PLoS Med. 2016;13(10):e1002150. doi:.https://doi.org/10.1371/journal.pmed.1002150
2Federal Office of Public Health. Strategy against healthcare-associated infections (NOSO strategy) 2016. [12 February 2019] Available from: https://www.bag.admin.ch/bag/en/home/strategie-und-politik/nationale-gesundheitsstrategien/nationale-strategie-ueberwachung-verhuetung-bekaempfung-von-spital-pflegeheiminfektionen.html.
3
Zingg
W
,
Holmes
A
,
Dettenkofer
M
,
Goetting
T
,
Secci
F
,
Clack
L
, et al.; systematic review and evidence-based guidance on organization of hospital infection control programmes (SIGHT) study group. Hospital organisation, management, and structure for prevention of health-care-associated infection: a systematic review and expert consensus. Lancet Infect Dis. 2015;15(2):212–24. doi:.https://doi.org/10.1016/S1473-3099(14)70854-0
4European Centre for Disease Prevention and Control (ECDC). Point prevalence survey of healthcare-associated infections and antimicrobial use in European acute care hospitals: protocol version 5.3, ECDC PPS 2016-2017. Stockholm: ECDC; 2016.
5
Zingg
W
,
Metsini
A
,
Balmelli
C
,
Neofytos
D
,
Behnke
M
,
Gardiol
C
, et al.; On Behalf Of The Swissnoso Network. National point prevalence survey on healthcare-associated infections in acute care hospitals, Switzerland, 2017. Euro Surveill. 2019;24(32). doi:.https://doi.org/10.2807/1560-7917.ES.2019.24.32.1800603
6
Suetens
C
,
Latour
K
,
Kärki
T
,
Ricchizzi
E
,
Kinross
P
,
Moro
ML
, et al.; The Healthcare-Associated Infections Prevalence Study Group. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: results from two European point prevalence surveys, 2016 to 2017. Euro Surveill. 2018;23(46). doi:.https://doi.org/10.2807/1560-7917.ES.2018.23.46.1800516