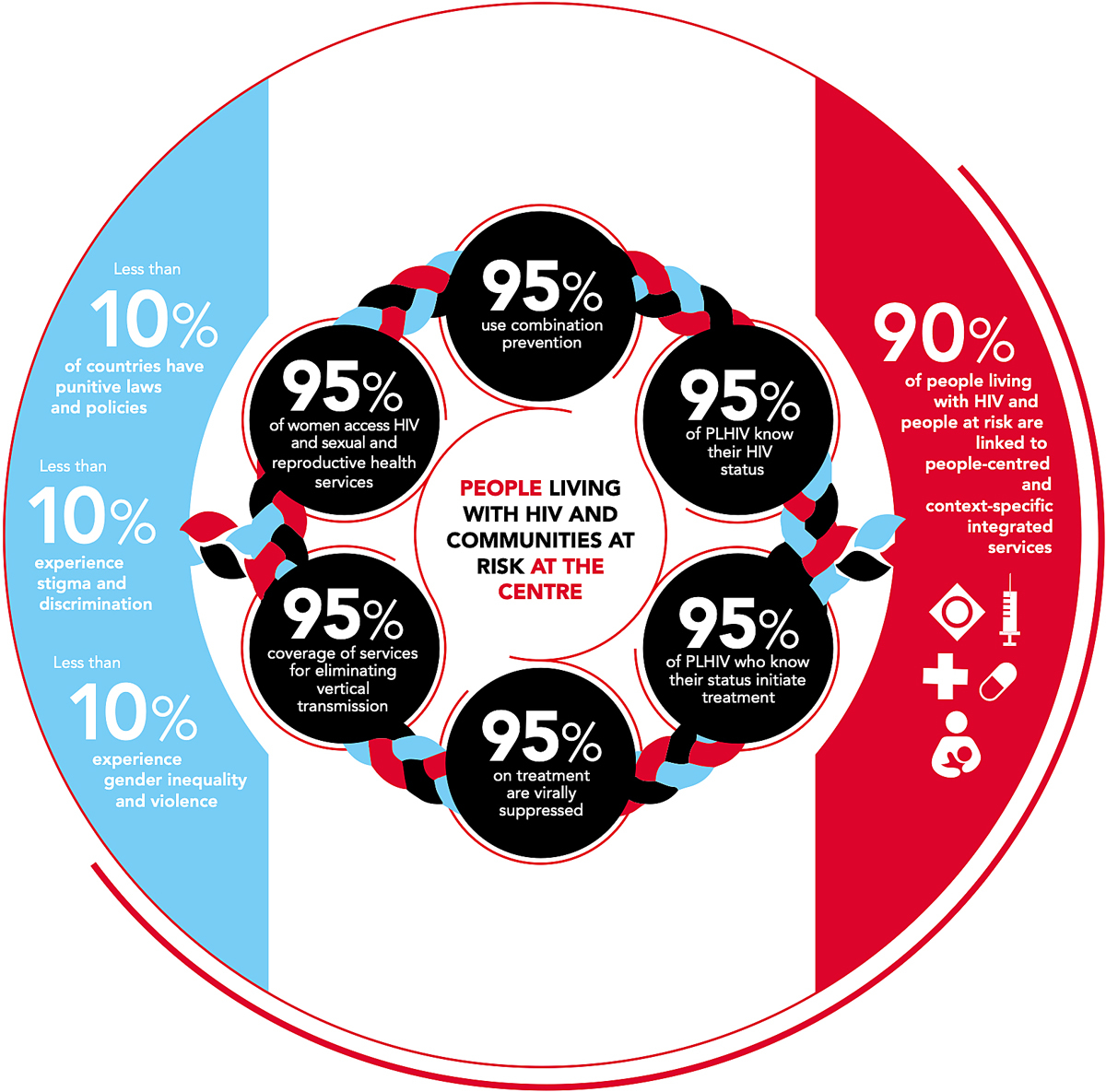
Figure 1 Updated UNAIDS 95-95-95 targets for 2025; source: https://aidstargets2025.unaids.org [25]. Reprinted with permission from UNAIDS.
DOI: https://doi.org/10.4414/smw.2021.20535
The effectiveness of human immunodeficiency virus (HIV) testing, care and treatment programmes are jeopardised by the loss of patients at all levels of the HIV care continuum, and existing gaps have been well documented in systematic reviews and observational studies [1–3]. The highest loss of patients has been observed during the “linkage to care” step – which is enrolling the patient in care once tested positive for HIV [1–6]. Identifying people living with HIV / acquired immunodeficiency syndrome (AIDS) (PLWHA) and successfully linking them to care remains a key role in the HIV pandemic for both treatment of the individual and prevention of onward transmission [7]. “Linkage to care” is a key HIV indicator for public health monitoring, but it is challenging to evaluate it and its definition has changed in the era of “universal test and treat” [7–9]. The goal of this narrative review is to elaborate on the challenges, changes in and differences between definitions and provide guidance for the choice of a clinically meaningful definition for “linkage to care”, with focus on a rural sub-Saharan African setting in the era of “universal test and treat”. Additionally, we present results from the Kilombero and Ulanga Antiretroviral cohort (KIULARCO) in Tanzania.
We searched PubMed/Medline between September and December 2020, restricted to the period 2000–2020 and articles written in English. We used the following search strategy with Boolean operators: “HIV” AND (“linkage to care” OR “engagement in care”). Our focus was on original articles, systematic reviews and meta-analyses. Several other search terms were applied and added to identify appropriate studies for specific questions, such as “care cascade”, “care continuum”, “sub-Sahara Africa” or “Tanzania”. In addition, we also had access to an unpublished systematic review analysing the HIV care cascade from HIV diagnosis to viral suppression in sub-Saharan Africa [10]. Institutional websites such as the World Health Organization (WHO), the Joint United Nations Program on HIV/AIDS (UNAIDS) and the Center for Disease Control and Prevention (CDC) were screened for “linkage to care” definitions. Studies were eligible if they included definitions for “linkage to care” (including numerators, denominators and time restrictions) and presented “linkage to care” proportions. RM performed the review, entering results in a data extraction form to collect the following information from each eligible article: first author’s name, publication year, country and setting where study was conducted, sample size, study design, numerator and denominator for “linkage to care”, time period for prompt “linkage to care” and “linkage to care” proportions.
The Chronic Diseases Clinic of Ifakara (CDCI) at the St Francis Referral Hospital in Tanzania was established in 2004 as the governmental HIV Care and Treatment Centre in the Kilombero and Ulanga districts in Tanzania. The CDCI provides HIV testing services for all patients seen in the hospital, and care and treatment for PLWHA according to government guidelines. Consenting patients are included in KIULARCO, a collaborative project of the SFRH, the Ifakara Health Institute (IHI), the Swiss Tropical and Public Health Institute, and the University Hospital Basel [11, 12]. Comprehensive data including clinical visits and prescriptions are captured electronically. We assessed the cascade from HIV testing to retention in care at 6 months among participants enrolled between 1 January 2017 and 31 March 2019, with follow up to the end of 2019. This time period was chosen because testing data were available only from 2017 onwards, and to avoid interruptions to normal care due to the COVID-19 pandemic. The steps assessed were: (i) proportion of persons testing HIV positive, (ii) proportion of PLWHA enrolled into KIULARCO, among those newly testing HIV positive, (iii) proportion of participants with laboratory evaluation within 7 days, and 1, 3, 6, and 9 months from enrolment into KIULARCO, (iv) proportion of participants with clinical evaluation within the same time periods, (v) proportion of participants with treatment initiation within the same time periods, (vi) proportion of participants with a clinical follow-up visit following treatment initiation within the same time periods. Results for step (ii) should be considered an approximation, because consent for data collection begins only at KIULARCO enrolment and HIV testing results are not consistently captured in the KIULARCO database. We defined newly diagnosed persons as those with date of diagnosis not before KIULARCO enrolment and not previously on treatment.
We analysed 81 references, and a subset of 34 original articles from 10 sub-Saharan African countries were included for the assessment of “linkage to care” definitions and results (supplementary figure S1 in the appendix).
To assess the success and challenges of the international collaborative HIV programmes in different countries over different time periods, tools were developed that incorporate key indicators to monitor the public health response to HIV [13–15]. Two major frameworks exist: (i) the HIV care continuum – also termed the HIV care cascade – formed in the United States of America in 2013 and outlining the five stages of HIV care through which PLWHA pass (being diagnosed with HIV, linked to care, received HIV medical care, retained in care, and achieved and maintained viral suppression) [6, 16–21], and (ii) the UNAIDS targets derived from the WHO strategic information guidelines in 2015, outlining three stages of HIV care (being diagnosed, being on treatment and being virally suppressed) [16, 22]. Both of these frameworks end with the main goal of HIV viral load suppression as optimal health outcome at both the individual and population levels [18, 19, 23, 24]. The UNAIDS used to work with the 90-90-90 targets, which were 90% of all PLWHA being tested, of those 90% being on treatment, and of those 90% being virally suppressed. After not reaching the goals set for 2020, the new targets for 2025 are even more ambitious, stepping up to 95% of the previous goals and adding further targets such as coverage of services for prevention of vertical transmission (fig. 1) [26].

Figure 1 Updated UNAIDS 95-95-95 targets for 2025; source: https://aidstargets2025.unaids.org [25]. Reprinted with permission from UNAIDS.
The reporting of the individual steps of the frameworks allows evaluation of where attrition from care occurs and identifies gaps and opportunities for specific interventions to improve outcomes for PLWHA [6, 17, 24]. The steps are represented as a linear and unidirectional pathway [6]. However, literature shows that PLWHA often experience the care continuum in a more dynamic way, as they may skip stages, exit and re-enter the continuum temporarily or permanently, or regress back to earlier stages [16].
Recent literature identified the early step of “linkage to care” – from knowing of being HIV positive to accessing care – to be key in the cascade [16]. Linkage is a precursor for successful HIV treatment, viral suppression and optimal patient outcome, as well as preventing onward transmission [7, 9, 27–33]. In 2015, the WHO recommended a “universal test and treat” strategy for starting antiretroviral treatment ideally on the same day of a new HIV diagnosis – at the latest within 7 days after diagnosis [8, 15, 34, 35]. In the era of “universal test and treat”, “linkage to care” as a public health parameter has changed. Successful “linkage to care” used to reflect acceptance of a positive test result with the first clinical visit, while the readiness to start treatment and first prescription of antiretroviral therapy (ART) could be at a later time point.
The 2015 strategic WHO guidelines defined “linkage to care” as “the duration of time starting with HIV diagnosis and ending with enrolment in HIV care or treatment”, with few recommendations on linkage indicators and time periods for prompt linkage (table 1) [9, 22].
Table 1 Institutional definitions of “linkage to care”.
| Institution | Numerators for “linkage to care” | Time period after diagnosis | |||
|---|---|---|---|---|---|
| Clinical visit | CD4+ count recorded | Viral load recorded | Treatment initiation | ||
| WHO Consolidated strategic information guidelines for HIV; 2015 [22] | Yes | No | No | Yes | None defined |
| Optimising testing and “linkage to care” for HIV across Europe project (OptTEST); 2018 [9] | Yes* | Yes* | Yes* | Yes* | Within 3 months |
| Monitoring Selected National HIV Prevention and Care Objectives by Using HIV Surveillance Data CDC (US); 2018 [36] |
Yes | Yes | Yes | No | Within 1 month and 3 months |
* Time to “linkage to care” defined as time between diagnosis date and (i) care attendance date (gold-standard marker), (ii) CD4+ date, (iii) viral load date, and (iv) treatment initiation [9]
An expert panel co-funded by the European Commission (OptTEST, optimising testing and “linkage to care” for HIV across Europe project), defined “linkage to care” as “patient entry into specialist HIV care after diagnosis, measured as the time between the HIV diagnosis date and either the first clinic attendance date, first CD4+ count or viral load date, or HIV treatment start date, depending on data availability” (table 1) [9]. In addition to the WHO definition, this definition included (a) a list of care indicators if more than one linkage indicator were available, with the first clinic attendance date after diagnosis considered the gold-standard marker for successful “linkage to care”, and (b) a time period for prompt “linkage to care”, with 3 months after diagnosis as cut-off [9]. As this definition is less applicable to community-based testing, they supported the definition for community-based linkage as “entry into health care or follow-up by an HIV specialist or in an HIV-unit after a positive HIV test at a community testing facility” [9].
The Centers for Disease Control and Prevention (CDC) suggests monitoring “linkage to care” at 1 month and 3 months after HIV diagnosis by the documentation of an HIV viral load or CD4+ cell count (table 1) [7, 21, 36].
For assessment of “linkage to care” definitions, we included 34 articles across 10 countries in sub-Saharan Africa (table 2 and supplementary table S1 in the appendix). Included countries were: South Africa [6, 37–40, 49, 51, 55–59, 65]; Zambia [41]; Uganda [38, 42, 43, 52]; Kenya [43, 44, 61]; Malawi [45, 60, 68]; Nigeria [50]; Sierra Leone [46]; Tanzania [47, 53, 62, 66, 67]; Mozambique [63]; Lesotho [27]; Eswatini/Swaziland [48, 54, 64].
Table 2 “Linkage to care” definitions in literature from sub-Saharan Africa: summary of numerators, denominators and time periods.
| Numerator for “linkage to care” definition |
|---|
| Evidence of medical care/ clinical records – Clinic appointment/visit [6, 37–48] – Accessed HIV care [49] – Enrolled in care [50] – Receiving home-based care [51] – Registration/presentation in clinic [6, 27, 52–54] – CD4+ cell count [6, 51, 55–60] – Viral load measurement [6, 51] – Antiretroviral therapy (ART) start/date [6, 41, 48, 50, 51] – Treatment of opportunistic infections [51] – Medical records [61–64] Self-reported measures – Self-reported clinical attendance [65] – Referral form [66, 67] – Disclosed positive results [68] |
| Denominator for “linkage to care” definition |
|
Population denominator
– Tested HIV positive [27, 37, 43, 47, 48, 56, 58, 59] – Aware of HIV status [38] – Diagnosed with HIV [6, 39, 64–66] – Newly diagnosed with HIV [42, 46, 49, 50, 52, 53, 60, 62, 67] – HIV positive, not on antiretroviral therapy, referred to clinic [41] – HIV infected [40] – HIV positive individuals/cases [44, 45, 54, 63] – Self-reported HIV positive/diagnosis [57, 61] Programme denominator – HIV positive participants [51, 68] – Newly diagnosed HIV positive participants [55] |
| Time period for prompt “linkage to care” |
| – “Same day as diagnosis” [52] – 7 days [43, 48, 52] – 1 month [37–40, 52, 67] – 2 months [55] – 3 months [27, 38–42, 49, 50, 52, 56, 58, 62, 64, 65, 67] – 6 months [37–39, 41, 47, 53, 54, 57, 59, 60] – 12 months [37, 38, 41, 43, 46, 64, 68] – “linked within study period” / “ever linked to care” [6, 44, 45, 51, 58, 61, 63, 64, 66] |
We identified 16 different numerators for the definition of “linkage to care”, with some articles combining more than one care indicator to indicate successful linkage. The most commonly used numerator was a clinical visit. Other frequently used numerators were proof of registration at the clinic, treatment initiation and availability of laboratory results, such as CD4+ count and viral load. Some articles used self-reported measures as linkage indicators, such as patient-reported clinical attendance or a HIV status disclosed by patients.
Out of the 10 denominators found for defining “linkage to care”, the most frequently used were population denominators, but they also included some programme denominators. As an example, a new positive HIV test could be termed “tested HIV positive”, “newly diagnosed with HIV” and “diagnosed with HIV”. More importantly, the definition of “prompt” linkage could be the “same day as diagnosis” up to 12 months after diagnosis, and sometimes “ever linked to care” or “linked within study period” were used. The majority of studies defined prompt linkage as being linked within 3 months of HIV diagnosis. This is in line with the definitions used in other regions [10, 18, 24, 69–72]. With acknowledgment of the heterogeneity in definitions, “linkage to care” proportions among the identified articles ranged between 14–96% (n = 15) within 3 months and 15–96% (n = 34) that ever linked regardless of time period. When “linkage to care” was presented at multiple time intervals, linkage proportions improved with time from diagnosis [37–39, 41, 52, 69] and patients who linked to care in a facility tended to do so within 1 week of diagnosis, with the likelihood of patients linking to care increasing only marginally thereafter [43, 52].
We propose a “linkage to care” framework for a sub-Saharan African setting, based on our findings from the literature and our experience in the CDCI and KIULARCO (fig. 2). It includes steps as defined in the literature, combined with our knowledge of the functioning of the CDCI. Therefore, this cascade may need adapting for other settings, such as amending the order of the steps or even omitting steps completely.
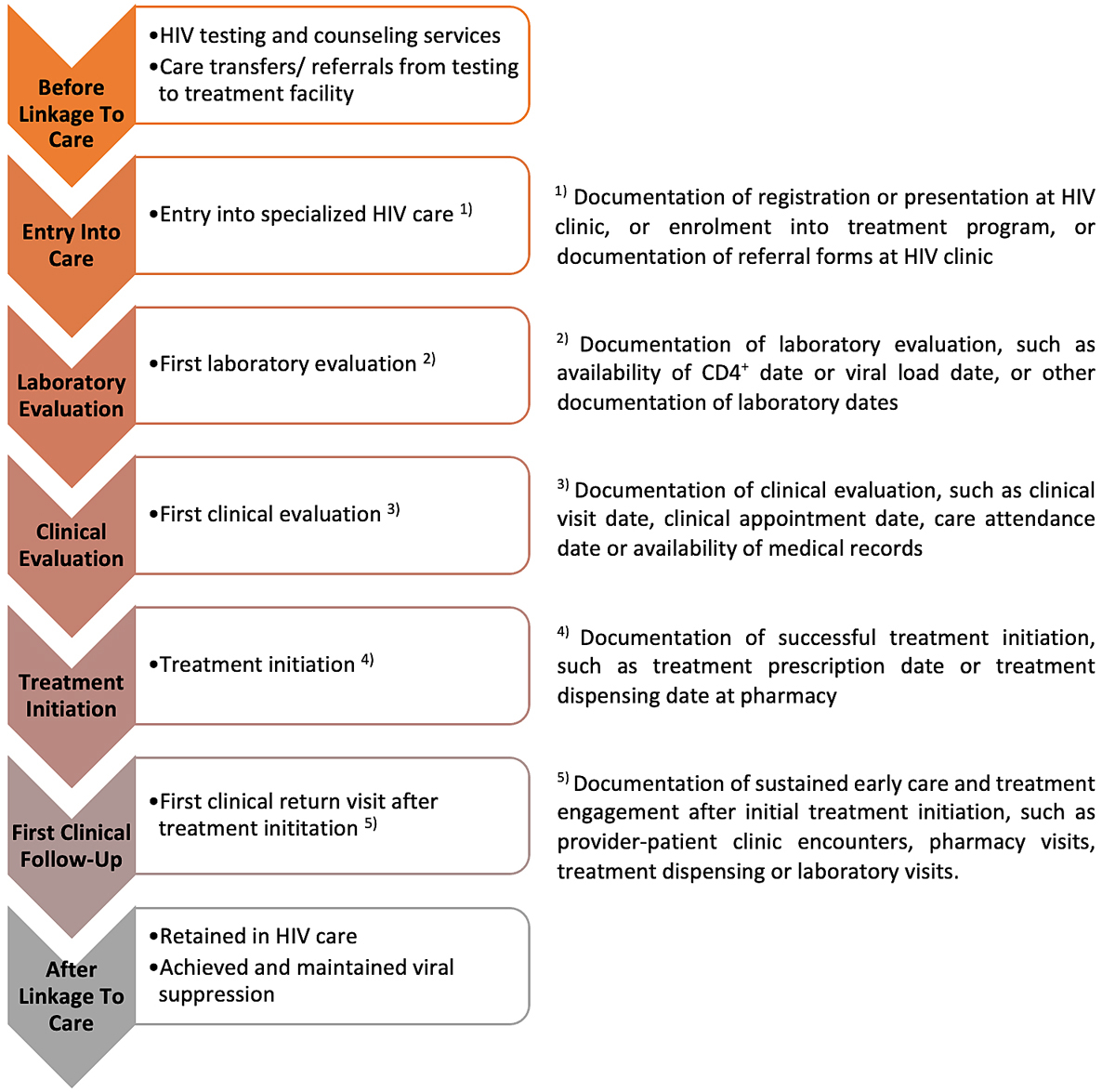
Figure 2 “Linkage to care” framework: patient flow and framework elements based on the Chronic Diseases Clinic of Ifakara and the KIULARCO programme, and numerators of each framework element to ensure “linkage to care” according to literature.
Linkage proportions from Western countries, irrespective of which linkage indicator was used, have been relatively high with studies reporting pooled estimates of 85% for the European region [69], 80–88% in the United States of America [36, 73, 74], 73% for Canada and 90% in Australia [74]. In low-income countries the linkage proportions were shown to be appreciably lower [16, 75, 76] and poor linkage is particularly pronounced in sub-Saharan Africa, the world region most heavily affected by the global HIV epidemic [2, 47, 77]. The overall “linkage to care” was reported to be 59% in sub-Sahara Africa in 2011 [78], whereas more recent systematic reviews reported wide ranges of linkage proportions after different testing settings, such as 8–99%, 10–96% and 55–61% for home-based testing and counselling programs, community-based testing, and facility-based testing, respectively [71, 72, 79]. Literature from Tanzania revealed linkage proportions ranging between 24% and 91% [51, 54, 75, 79, 80]. The ranges of linkage proportions differ greatly and reflect a considerable lack of standardisation and consistency in defining “linkage to care”, the use of low-quality methodologies, the little consensus across countries and also generally the different healthcare settings and resources available across sub-Saharan Africa. Barriers affecting entry into care in rural settings are described to be multifactorial and include a higher immune status and a patient’s feeling of being physically healthy, rigid clinic policies, disrespectful treatment from service providers, stockouts of supplies, stigma and discrimination, alternate healing systems, distance to health facilities and poverty [37, 39, 49, 64, 80].
During the study period, 25,793 individuals were tested for HIV, of whom 1,671 (6%) tested positive. Excluding individuals who were diagnosed or started treatment before enrolment into KIULARCO, 1149 persons were enrolled into KIULARCO during this time (69% of those who tested positive). Usually, the enrolment is on the same day as testing. However, we could not ascertain this because of a lack of individual patient data before enrolment. Among the 1149 newly diagnosed patients enrolled into KIULARCO, 92% (n = 1054), 80% (n = 917) and 68% (n = 779) had a laboratory evaluation, clinical evaluation and initiated treatment within their first week after enrolment, respectively (fig. 3).

Figure 3 Steps of the “linkage to care” process in 1149 newly tested individuals enrolled into KIULARCO [1].
The proportion of participants with a laboratory evaluation remained fairly stable thereafter, even up to 9 months following enrolment. The proportion of participants with a clinical evaluation increased slightly to 84% by 1 month, and further to 88% by 6 months. The proportion of participants who initiated treatment increased substantially to 82% by 1 month and only slightly more to 86% by 9 months. By 3 months, 71% of participants had had a clinical visit following treatment initiation, and this proportion remained stable up to 9 months.
Challenges with the current “linkage to care” definitions have been addressed by many authors. Researchers have highlighted the considerable lack of standardisation and consistency in defining linkage, making it almost impossible to synthesise findings and compare estimates of cascade stages [6, 10, 17, 23, 24, 69, 71]. The lack of consensus is partly a consequence of different “linkage to care” processes across health systems and care settings [18, 24] and an over-simplification of “linkage to care” into a single step. Relatedly, there is little consent on what time period should be used to determine prompt “linkage to care”.
Although the concept of “universal test and treat” implies that all steps from testing to treatment initiation happen simultaneously and steps such as pre-ART and pre-ART care became redundant, some authors have suggested a pragmatic operational framework for detailing the “linkage to care” process [8, 34]. Most care attrition occurs before patients even start treatment [2, 27, 47, 78, 81, 82]. Attrition between first ART prescription and the first clinical follow-up visit can range from 15% to over 30% [52, 56, 83, 84], raising the question of whether these newly diagnosed patients were ever truly linked to care [34]. Therefore, some authors have recently proposed to include a first follow-up visit after treatment initiation in the definition of linkage [34].
For the sub-Saharan African region, we support in general the definition from OptTEST defining “linkage to care” as “patient entry into specialist HIV care after diagnosis, measured as the time between the HIV diagnosis date and either the first clinic attendance date, first CD4+ count or viral load date, or HIV treatment start date, depending on data availability” [9] within a time period of 3 months after diagnosis. The advantage of this definition is that it includes (i) a comprehensive list of care indicators and priority of indicators if more than one linkage indicator is available, and (ii) a time period to define prompt “linkage to care”. Further, use of the same definition paves the way to consistency in estimates across countries. We support the time period of 3 months for prompt linkage, and not shorter, as opportunistic infections are more common in people living with HIV in sub-Sahara Africa and treatment initiation has to be delayed in cases with tuberculosis or cryptococcal meningitis. However, in contrast to OptTEST and the CDC, we suggest treatment initiation to be the gold-standard marker for successful “linkage to care” in the era of “universal test and treat”. This is supported by our narrative review and results from KIULARCO. HIV treatment is the cornerstone of the “test and treat” strategy and within the “linkage to care” framework. If more than one HIV care indicator is captured within a facility, then we agree with OptTEST that the following indicators could be used, but suggest using the order according to figure 2: (i) treatment initiation, (ii) clinical evaluation, (iii) laboratory evaluation, and (iv) entry into care. The order of this priority was chosen to capture linkage with the least attrition from care, as attrition increases with each step throughout the linkage framework, as shown on the basis of the patient-flow we have in the CDCI and KIULARCO. The framework and definition currently apply to facility-based testing only and community-based testing needs to be addressed separately. Interestingly, a first clinical follow-up at the HIV clinic after community-based testing and/or treatment initiation does sound like a sensible definition for both community- and facility-based settings, and there could be an argument for basing the linkage definition on that. We generally support a paradigm shift to move towards using a first clinical follow-up visit after treatment initiation as numerator in the definition of “linkage to care” as it most accurately reflects true “linkage to care”. We have to appreciate and accept that different clinics and care settings across sub-Sahara Africa, and across the globe, exist and not the same “linkage to care” indicators are available. Therefore, with regard to those differences, it is challenging to propose one unified definition for “linkage to care”.
This research is subject to some limitations. First, we performed a narrative review. Although we applied some systematic selection criteria, it is not a systematic review and may result in selection bias of the studies included and therefore bias in our conclusions. Second, our identification of newly diagnosed persons enrolled in KIULARCO may have missed some patients who were previously diagnosed (owing to unreported previous date of diagnosis or treatment) and therefore in particular we may have overestimated the proportion of newly diagnosed persons who were enrolled. Conversely, some participants may have registered at the CDCI but not enrolled into KIULARCO, and therefore the number of newly diagnosed persons enrolled into KIULARCO may be an underestimate for the number of newly diagnosed persons who linked to care.
“Linkage to care” is an early key element within the HIV care cascade that affects future steps within the cascade, yet there is much inconsistency and debate in defining this important public health marker. There is an urgent need for standardisation of methods, definitions and data collection approaches to accurately track progress towards control over the HIV epidemic and to achieve the updated 95-95-95 target by 2025. We have proposed a definition for this important public health measure that we consider appropriate for the sub-Sahara Africa region in the context of “universal test and treat” and that appreciates the new role of “linkage to care” in the HIV care frameworks.
Figure S1 and Table S1 are provided in the PDF version of the article.
We thank all team members of the KIULARCO study group as well as patients included in KIULARCO for providing data.
No financial support and no potential conflict of interest relevant to this article was reported.
1 Gardner EM , McLees MP , Steiner JF , Del Rio C , Burman WJ . The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. doi:.https://doi.org/10.1093/cid/ciq243
2 Kranzer K , Govindasamy D , Ford N , Johnston V , Lawn SD . Quantifying and addressing losses along the continuum of care for people living with HIV infection in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2012;15(2):17383. doi:.https://doi.org/10.7448/IAS.15.2.17383
3 Barnabas RV , Celum C . Closing the gaps in the HIV care continuum. PLoS Med. 2017;14(11):e1002443. doi:.https://doi.org/10.1371/journal.pmed.1002443
4 Church K , Machiyama K , Todd J , Njamwea B , Mwangome M , Hosegood V , et al. Identifying gaps in HIV service delivery across the diagnosis-to-treatment cascade: findings from health facility surveys in six sub-Saharan countries. J Int AIDS Soc. 2017;20(1):21188. doi:.https://doi.org/10.7448/IAS.20.1.21188
5 Fox MP , Rosen S . Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007-2009: systematic review. Trop Med Int Health. 2010;15(Suppl 1):1–15. doi:.https://doi.org/10.1111/j.1365-3156.2010.02508.x
6 Haber N , Tanser F , Bor J , Naidu K , Mutevedzi T , Herbst K , et al. From HIV infection to therapeutic response: a population-based longitudinal HIV cascade-of-care study in KwaZulu-Natal, South Africa. Lancet HIV. 2017;4(5):e223–30. doi:.https://doi.org/10.1016/S2352-3018(16)30224-7
7Dombrowski J. National HIV Curriculum: Linkage to care. Available from: https://cdn.hiv.uw.edu/pdf/screening-diagnosis/linkage-care/core-concept/all [accessed 2020 October 19].
8 Fox MP , Rosen S . A new cascade of HIV care for the era of “treat all”. PLoS Med. 2017;14(4):e1002268. doi:.https://doi.org/10.1371/journal.pmed.1002268
9 Croxford S , Raben D , Jakobsen SF , Burns F , Copas A , Brown AE , et al.; On Behalf Of OptTEST By Hiv In Europe. Defining linkage to care following human immunodeficiency virus (HIV) diagnosis for public health monitoring in Europe. Euro Surveill. 2018;23(48). doi:.https://doi.org/10.2807/1560-7917.ES.2018.23.48.1700858
10Mugglin C, Kläger D, Gueler A, Vanobberghen F, Rice B, Egger M, et al. The HIV Care Cascade in sub-Saharan Africa: Systematic Review of Published Criteria and Definitions 2021.Open Science Framework. Available from: https://osf.io/w6qjv [accessed 2021 April 14].
11 Vanobberghen F , Letang E , Gamell A , Mnzava DK , Faini D , Luwanda LB , et al.; KIULARCO Study Group. A decade of HIV care in rural Tanzania: Trends in clinical outcomes and impact of clinic optimisation in an open, prospective cohort. PLoS One. 2017;12(7):e0180983. doi:.https://doi.org/10.1371/journal.pone.0180983
12 Letang E , Kalinjuma AV , Glass TR , Gamell A , Mapesi H , Sikalengo GR , et al.; Kiularco Study Group. Cohort profile: The Kilombero and Ulanga Antiretroviral Cohort (KIULARCO) - A prospective HIV cohort in rural Tanzania. Swiss Med Wkly. 2017;147:w14485. doi:.https://doi.org/10.4414/smw.2017.14485
13World Health Organization. Patient monitoring guidelines for HIV care and antiretroviral therapy. Geneva: WHO; 2006. Available at: https://www.who.int/3by5/capacity/ptmonguidelinesfinalv1.PDF?ua=1 [accessed: 2020 November 21].
14 Cheever LW . Engaging HIV-infected patients in care: their lives depend on it. Clin Infect Dis. 2007;44(11):1500–2. doi:.https://doi.org/10.1086/517534
15 Mugavero MJ , Norton WE , Saag MS . Health care system and policy factors influencing engagement in HIV medical care: piecing together the fragments of a fractured health care delivery system. Clin Infect Dis. 2011;52(Suppl 2):S238–46. doi:.https://doi.org/10.1093/cid/ciq048
16 Kay ES , Batey DS , Mugavero MJ . The HIV treatment cascade and care continuum: updates, goals, and recommendations for the future. AIDS Res Ther. 2016;13(1):35. doi:.https://doi.org/10.1186/s12981-016-0120-0
17 Gueler A , Vanobberghen F , Rice B , Egger M , Mugglin C . The HIV Care Cascade from HIV diagnosis to viral suppression in sub-Saharan Africa: a systematic review and meta-regression analysis protocol. Syst Rev. 2017;6(1):172. doi:.https://doi.org/10.1186/s13643-017-0562-z
18 Drew RS , Rice B , Rüütel K , Delpech V , Attawell KA , Hales DK , et al. HIV continuum of care in Europe and Central Asia. HIV Med. 2017;18(7):490–9. doi:.https://doi.org/10.1111/hiv.12480
19Joint United Nations Programme on HIV/AIDS (UNAIDS). 90-90-90: An ambitious treatment target to help end the AIDS epidemic. Geneva: UNAIDS; 2014.
20Office of National AIDS Policy. National HIV/AIDS strategy: improving outcomes: accelerating progress along the HIV care continuum; 2013. Available at: https://obamawhitehouse.archives.gov/sites/default/files/onap_nhas_improving_outcomes_dec_2013.pdf [accessed: 2020 October 27].
21White House Office of National AIDS Policy. National HIV/AIDS Strategy for the United States: Updated to 2020. Washington, DC. July 2015. Available at: https://www.hiv.gov/sites/default/files/nhas-2020-action-plan.pdf [accessed: 2020 October 21].
22World Health Organization (WHO). Consolidated strategic information guidelines for HIV in the health care sector. Geneva; WHO; 2015; Available from: https://www.who.int/hiv/pub/guidelines/strategic-information-guidelines/en/ [accessed: 2020 October 21].
23 Granich R , Gupta S , Hall I , Aberle-Grasse J , Hader S , Mermin J . Status and methodology of publicly available national HIV care continua and 90-90-90 targets: A systematic review. PLoS Med. 2017;14(4):e1002253. doi:.https://doi.org/10.1371/journal.pmed.1002253
24 Medland NA , McMahon JH , Chow EP , Elliott JH , Hoy JF , Fairley CK . The HIV care cascade: a systematic review of data sources, methodology and comparability. J Int AIDS Soc. 2015;18(1):20634. doi:.https://doi.org/10.7448/IAS.18.1.20634
25Joint United Nations Programme on HIV/AIDS (UNAIDS). 95-95-95 targets for 2025. Available at: https://aidstargets2025.unaids.org [accessed 2021 March 21].
26Joint United Nations Programme on HIV/AIDS (UNAIDS). Prevailing against pandemics by putting people at the center - World AIDS day report 2020. Available at: https://www.unaids.org/en/resources/documents/2020/prevailing-against-pandemics [accessed: 2021 February 07].
27 Labhardt ND , Ringera I , Lejone TI , Klimkait T , Muhairwe J , Amstutz A , et al. Effect of Offering Same-Day ART vs Usual Health Facility Referral During Home-Based HIV Testing on Linkage to Care and Viral Suppression Among Adults With HIV in Lesotho: The CASCADE Randomized Clinical Trial. JAMA. 2018;319(11):1103–12. doi:.https://doi.org/10.1001/jama.2018.1818
28 Croxford S , Kitching A , Desai S , Kall M , Edelstein M , Skingsley A , et al. Mortality and causes of death in people diagnosed with HIV in the era of highly active antiretroviral therapy compared with the general population: an analysis of a national observational cohort. Lancet Public Health. 2017;2(1):e35–46. doi:.https://doi.org/10.1016/S2468-2667(16)30020-2
29Dombrowski J. National HIV Curriculum: Linkage to care. Available from: https://www.hiv.uw.edu/go/screening-diagnosis/linkage-care/core-concept/all [accessed: 2020 January 14].
30 Coffey S , Bacchetti P , Sachdev D , Bacon O , Jones D , Ospina-Norvell C , et al. RAPID antiretroviral therapy: high virologic suppression rates with immediate antiretroviral therapy initiation in a vulnerable urban clinic population. AIDS. 2019;33(5):825–32. doi:.https://doi.org/10.1097/QAD.0000000000002124
31 Robertson M , Laraque F , Mavronicolas H , Braunstein S , Torian L . Linkage and retention in care and the time to HIV viral suppression and viral rebound - New York City. AIDS Care. 2015;27(2):260–7. doi:.https://doi.org/10.1080/09540121.2014.959463
32 Lundgren JD , Babiker AG , Gordin F , Emery S , Grund B , Sharma S , et al., INSIGHT START Study Group. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373(9):795–807. doi:.https://doi.org/10.1056/NEJMoa1506816
33 Danel C , Moh R , Gabillard D , Badje A , Le Carrou J , Ouassa T , et al., TEMPRANO ANRS 12136 Study Group. A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa. N Engl J Med. 2015;373(9):808–22. doi:.https://doi.org/10.1056/NEJMoa1507198
34 Herce ME , Chi BH , Liao RC , Hoffmann CJ . Re-thinking Linkage to Care in the Era of Universal Test and Treat: Insights from Implementation and Behavioral Science for Achieving the Second 90. AIDS Behav. 2019;23(S2, Suppl 2):120–8. doi:.https://doi.org/10.1007/s10461-019-02541-5
35Guideline on When to Start Antiretroviral Therapy and on Pre-Exposure Prophylaxis for HIV. WHO Guidelines Approved by the Guidelines Review Committee. Geneva2015.
36Centers for Disease Control and Prevention. Monitoring Selected National HIV Prevention and Care Objectives by Using HIV Surveillance Data United States and 6 Dependent Areas, 2018 HIV Surveillance Supplemental Report. 2020;25(No. 2):1–104. Published May 2020. Available at: https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-25-2.pdf [accessed 2020 October 20]
37 Iwuji CC , Orne-Gliemann J , Larmarange J , Okesola N , Tanser F , Thiebaut R , et al.; ANRS 12249 TasP trial group. Uptake of Home-Based HIV Testing, Linkage to Care, and Community Attitudes about ART in Rural KwaZulu-Natal, South Africa: Descriptive Results from the First Phase of the ANRS 12249 TasP Cluster-Randomised Trial. PLoS Med. 2016;13(8):e1002107. doi:.https://doi.org/10.1371/journal.pmed.1002107
38 Barnabas RV , van Rooyen H , Tumwesigye E , Murnane PM , Baeten JM , Humphries H , et al. Initiation of antiretroviral therapy and viral suppression after home HIV testing and counselling in KwaZulu-Natal, South Africa, and Mbarara district, Uganda: a prospective, observational intervention study. Lancet HIV. 2014;1(2):e68–76. doi:.https://doi.org/10.1016/S2352-3018(14)70024-4
39 Govindasamy D , Kranzer K , van Schaik N , Noubary F , Wood R , Walensky RP , et al. Linkage to HIV, TB and non-communicable disease care from a mobile testing unit in Cape Town, South Africa. PLoS One. 2013;8(11):e80017. doi:.https://doi.org/10.1371/journal.pone.0080017
40 van Rooyen H , Barnabas RV , Baeten JM , Phakathi Z , Joseph P , Krows M , et al. High HIV testing uptake and linkage to care in a novel program of home-based HIV counseling and testing with facilitated referral in KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2013;64(1):e1–8. doi:.https://doi.org/10.1097/QAI.0b013e31829b567d
41 Hayes R , Floyd S , Schaap A , Shanaube K , Bock P , Sabapathy K , et al.; HPTN 071 (PopART) Study Team. A universal testing and treatment intervention to improve HIV control: One-year results from intervention communities in Zambia in the HPTN 071 (PopART) cluster-randomised trial. PLoS Med. 2017;14(5):e1002292. doi:.https://doi.org/10.1371/journal.pmed.1002292
42 Chamie G , Kwarisiima D , Clark TD , Kabami J , Jain V , Geng E , et al.; SEARCH Collaboration. Leveraging rapid community-based HIV testing campaigns for non-communicable diseases in rural Uganda. PLoS One. 2012;7(8):e43400. doi:.https://doi.org/10.1371/journal.pone.0043400
43 Ayieko J , Petersen ML , Charlebois ED , Brown LB , Clark TD , Kwarisiima D , et al. A Patient-Centered Multicomponent Strategy for Accelerated Linkage to Care Following Community-Wide HIV Testing in Rural Uganda and Kenya. J Acquir Immune Defic Syndr. 2019;80(4):414–22. doi:.https://doi.org/10.1097/QAI.0000000000001939
44 Maman D , Zeh C , Mukui I , Kirubi B , Masson S , Opolo V , et al. Cascade of HIV care and population viral suppression in a high-burden region of Kenya. AIDS. 2015;29(12):1557–65. doi:.https://doi.org/10.1097/QAD.0000000000000741
45 Maman D , Chilima B , Masiku C , Ayouba A , Masson S , Szumilin E , et al. Closer to 90-90-90. The cascade of care after 10 years of ART scale-up in rural Malawi: a population study. J Int AIDS Soc. 2016;19(1):20673. doi:.https://doi.org/10.7448/IAS.19.1.20673
46 Kelly JD , Schlough GW , Conteh S , Barrie MB , Kargbo B , Giordano TP . The Majority of the Pre-Antiretroviral Population Who Were Lost to Follow-Up Stopped Their Care in Freetown, Sierra Leone: A 12-Month Prospective Cohort Study Starting with HIV Diagnosis. PLoS One. 2016;11(2):e0149584. doi:.https://doi.org/10.1371/journal.pone.0149584
47 Reddy EA , Agala CB , Maro VP , Ostermann J , Pence BW , Itemba DK , et al. Test site predicts HIV care linkage and antiretroviral therapy initiation: a prospective 3.5 year cohort study of HIV-positive testers in northern Tanzania. BMC Infect Dis. 2016;16(1):497. doi:.https://doi.org/10.1186/s12879-016-1804-8
48 MacKellar D , Williams D , Bhembe B , Dlamini M , Byrd J , Dube L , et al. Peer-Delivered Linkage Case Management and Same-Day ART Initiation for Men and Young Persons with HIV Infection - Eswatini, 2015-2017. MMWR Morb Mortal Wkly Rep. 2018;67(23):663–7. doi:.https://doi.org/10.15585/mmwr.mm6723a3
49 Govindasamy D , van Schaik N , Kranzer K , Wood R , Mathews C , Bekker LG . Linkage to HIV care from a mobile testing unit in South Africa by different CD4 count strata. J Acquir Immune Defic Syndr. 2011;58(3):344–52. doi:.https://doi.org/10.1097/QAI.0b013e31822e0c4c
50 Aliyu MH , Blevins M , Parrish DD , Megazzini KM , Gebi UI , Muhammad MY , et al. Risk factors for delayed initiation of combination antiretroviral therapy in rural north central Nigeria. J Acquir Immune Defic Syndr. 2014;65(2):e41–9. doi:.https://doi.org/10.1097/QAI.0b013e31829ceaec
51 Grobler A , Cawood C , Khanyile D , Puren A , Kharsany ABM . Progress of UNAIDS 90-90-90 targets in a district in KwaZulu-Natal, South Africa, with high HIV burden, in the HIPSS study: a household-based complex multilevel community survey. Lancet HIV. 2017;4(11):e505–13. doi:.https://doi.org/10.1016/S2352-3018(17)30122-4
52 Boeke CE , Nabitaka V , Rowan A , Guerra K , Kabbale A , Asire B , et al. Assessing linkage to and retention in care among HIV patients in Uganda and identifying opportunities for health systems strengthening: a descriptive study. BMC Infect Dis. 2018;18(1):138. doi:.https://doi.org/10.1186/s12879-018-3042-8
53 Sanga ES , Lerebo W , Mushi AK , Clowes P , Olomi W , Maboko L , et al. Linkage into care among newly diagnosed HIV-positive individuals tested through outreach and facility-based HIV testing models in Mbeya, Tanzania: a prospective mixed-method cohort study. BMJ Open. 2017;7(4):e013733. doi:.https://doi.org/10.1136/bmjopen-2016-013733
54 Parker LA , Jobanputra K , Rusike L , Mazibuko S , Okello V , Kerschberger B , et al. Feasibility and effectiveness of two community-based HIV testing models in rural Swaziland. Trop Med Int Health. 2015;20(7):893–902. doi:.https://doi.org/10.1111/tmi.12501
55 Losina E , Bassett IV , Giddy J , Chetty S , Regan S , Walensky RP , et al. The “ART” of linkage: pre-treatment loss to care after HIV diagnosis at two PEPFAR sites in Durban, South Africa. PLoS One. 2010;5(3):e9538. doi:.https://doi.org/10.1371/journal.pone.0009538
56 Clouse K , Pettifor AE , Maskew M , Bassett J , Van Rie A , Behets F , et al. Patient retention from HIV diagnosis through one year on antiretroviral therapy at a primary health care clinic in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2013;62(2):e39–46. doi:.https://doi.org/10.1097/QAI.0b013e318273ac48
57 du Toit E , van Schalkwyk C , Dunbar R , Jennings K , Yang B , Coetzee D , et al. Missed opportunities for retention in pre-ART care in Cape Town, South Africa. PLoS One. 2014;9(5):e96867. doi:.https://doi.org/10.1371/journal.pone.0096867
58 Fox MP , Shearer K , Maskew M , Meyer-Rath G , Clouse K , Sanne I . Attrition through multiple stages of pre-treatment and ART HIV care in South Africa. PLoS One. 2014;9(10):e110252. doi:.https://doi.org/10.1371/journal.pone.0110252
59 Kranzer K , Zeinecker J , Ginsberg P , Orrell C , Kalawe NN , Lawn SD , et al. Linkage to HIV care and antiretroviral therapy in Cape Town, South Africa. PLoS One. 2010;5(11):e13801. doi:.https://doi.org/10.1371/journal.pone.0013801
60 MacPherson P , Corbett EL , Makombe SD , van Oosterhout JJ , Manda E , Choko AT , et al. Determinants and consequences of failure of linkage to antiretroviral therapy at primary care level in Blantyre, Malawi: a prospective cohort study. PLoS One. 2012;7(9):e44794. doi:.https://doi.org/10.1371/journal.pone.0044794
61 Genberg BL , Naanyu V , Wachira J , Hogan JW , Sang E , Nyambura M , et al. Linkage to and engagement in HIV care in western Kenya: an observational study using population-based estimates from home-based counselling and testing. Lancet HIV. 2015;2(1):e20–6. doi:.https://doi.org/10.1016/S2352-3018(14)00034-4
62 Rentsch CT , Wringe A , Machemba R , Michael D , Urassa M , Todd J , et al. Linkage to care and antiretroviral therapy initiation by testing modality among individuals newly diagnosed with HIV in Tanzania, 2014-2017. Trop Med Int Health. 2018;23(12):1384–93. doi:.https://doi.org/10.1111/tmi.13153
63 Courtenay-Quirk C , Geller AL , Duran D , Honwana N . Tracking linkage to care in an anonymous HIV testing context: A field assessment in Mozambique. J Eval Clin Pract. 2020;26(3):1005–12. doi:.https://doi.org/10.1111/jep.13262
64 MacKellar DA , Williams D , Storer N , Okello V , Azih C , Drummond J , et al. Enrollment in HIV Care Two Years after HIV Diagnosis in the Kingdom of Swaziland: An Evaluation of a National Program of New Linkage Procedures. PLoS One. 2016;11(2):e0150086. doi:.https://doi.org/10.1371/journal.pone.0150086
65 Meehan SA , Sloot R , Draper HR , Naidoo P , Burger R , Beyers N . Factors associated with linkage to HIV care and TB treatment at community-based HIV testing services in Cape Town, South Africa. PLoS One. 2018;13(4):e0195208. doi:.https://doi.org/10.1371/journal.pone.0195208
66 Harklerode R , Todd J , de Wit M , Beard J , Urassa M , Machemba R , et al. Characterizing a Leak in the HIV Care Cascade: Assessing Linkage Between HIV Testing and Care in Tanzania. Front Public Health. 2020;7:406. doi:.https://doi.org/10.3389/fpubh.2019.00406
67 Kayabu DE , Ngocho JS , Mmbaga BT . Effective linkage from point of HIV testing to care and treatment in Tanga region, Tanzania. PLoS One. 2018;13(8):e0201644. doi:.https://doi.org/10.1371/journal.pone.0201644
68 Choko AT , MacPherson P , Webb EL , Willey BA , Feasy H , Sambakunsi R , et al. Uptake, Accuracy, Safety, and Linkage into Care over Two Years of Promoting Annual Self-Testing for HIV in Blantyre, Malawi: A Community-Based Prospective Study. PLoS Med. 2015;12(9):e1001873. doi:.https://doi.org/10.1371/journal.pmed.1001873
69 Croxford S , Yin Z , Burns F , Copas A , Town K , Desai S , et al.; OptTEST project. Linkage to HIV care following diagnosis in the WHO European Region: A systematic review and meta-analysis, 2006-2017. PLoS One. 2018;13(2):e0192403. doi:.https://doi.org/10.1371/journal.pone.0192403
70 Rosen S , Fox MP , Gill CJ . Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007;4(10):e298. doi:.https://doi.org/10.1371/journal.pmed.0040298
71 Sabapathy K , Hensen B , Varsaneux O , Floyd S , Fidler S , Hayes R . The cascade of care following community-based detection of HIV in sub-Saharan Africa - A systematic review with 90-90-90 targets in sight. PLoS One. 2018;13(7):e0200737. doi:.https://doi.org/10.1371/journal.pone.0200737
72 Ruzagira E , Baisley K , Kamali A , Biraro S , Grosskurth H ; Working Group on Linkage to HIV Care. Linkage to HIV care after home-based HIV counselling and testing in sub-Saharan Africa: a systematic review. Trop Med Int Health. 2017;22(7):807–21. doi:.https://doi.org/10.1111/tmi.12888
73 Mahle Gray K , Tang T , Shouse L , Li J , Mermin J , Hall HI . Using the HIV surveillance system to monitor the National HIV/AIDS Strategy. Am J Public Health. 2013;103(1):141–7. doi:.https://doi.org/10.2105/AJPH.2012.300859
74 Hall HI , Halverson J , Wilson DP , Suligoi B , Diez M , Le Vu S , et al. Late diagnosis and entry to care after diagnosis of human immunodeficiency virus infection: a country comparison. PLoS One. 2013;8(11):e77763. doi:.https://doi.org/10.1371/journal.pone.0077763
75 Piñeirúa A , Sierra-Madero J , Cahn P , Guevara Palmero RN , Martínez Buitrago E , Young B , et al. The HIV care continuum in Latin America: challenges and opportunities. Lancet Infect Dis. 2015;15(7):833–9. doi:.https://doi.org/10.1016/S1473-3099(15)00108-5
76 Plazy M , Farouki KE , Iwuji C , Okesola N , Orne-Gliemann J , Larmarange J , et al.; Anrs 12249 Tasp Study Group. Access to HIV care in the context of universal test and treat: challenges within the ANRS 12249 TasP cluster-randomized trial in rural South Africa. J Int AIDS Soc. 2016;19(1):20913. doi:.https://doi.org/10.7448/IAS.19.1.20913
77Joint United Nations Programme on HIV/AIDS (UNAIDS). Global AIDS update 2020. Available at: https://aids2020.unaids.org/report/) [accessed 2020 August 09].
78 Rosen S , Fox MP . Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011;8(7):e1001056. doi:.https://doi.org/10.1371/journal.pmed.1001056
79 Sharma M , Ying R , Tarr G , Barnabas R . Systematic review and meta-analysis of community and facility-based HIV testing to address linkage to care gaps in sub-Saharan Africa. Nature. 2015;528(7580):S77–85. doi:.https://doi.org/10.1038/nature16044
80 Layer EH , Kennedy CE , Beckham SW , Mbwambo JK , Likindikoki S , Davis WW , et al.; LTC Tanzania Collaborative Study Team. Multi-level factors affecting entry into and engagement in the HIV continuum of care in Iringa, Tanzania. PLoS One. 2014;9(8):e104961. doi:.https://doi.org/10.1371/journal.pone.0104961
81 Watson-Jones D , Balira R , Ross DA , Weiss HA , Mabey D . Missed opportunities: poor linkage into ongoing care for HIV-positive pregnant women in Mwanza, Tanzania. PLoS One. 2012;7(7):e40091. doi:.https://doi.org/10.1371/journal.pone.0040091
82 Wettstein C , Mugglin C , Egger M , Blaser N , Vizcaya LS , Estill J , et al.; IeDEA Southern Africa Collaboration. Missed opportunities to prevent mother-to-child-transmission: systematic review and meta-analysis. AIDS. 2012;26(18):2361–73. doi:.https://doi.org/10.1097/QAD.0b013e328359ab0c
83 Tenthani L , Haas AD , Tweya H , Jahn A , van Oosterhout JJ , Chimbwandira F , et al. Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women ('Option B+') in Malawi. AIDS. 2014;28(4):589–98. doi:.https://doi.org/10.1097/QAD.0000000000000143
84 MacCarthy S , Hoffmann M , Ferguson L , Nunn A , Irvin R , Bangsberg D , et al. The HIV care cascade: models, measures and moving forward. J Int AIDS Soc. 2015;18(1):19395. doi:.https://doi.org/10.7448/IAS.18.1.19395
Aschola Asantiel, Farida Bani, Manuel Battegay, Theonestina Byakuzana, Adolphina Chale, Ivana di Salvo, Gideon Francis, Hansjakob Furrer, Tracy Glass, Speciosa Hwaya, Aneth V Kalinjuma, Joshua Kapunga, Bryson Kasuga, Andrew Katende, Namvua Kimera, Yassin Kisunga, Olivia Kitau, Bernard Kivuma, Thomas Klimkait, Ezekiel Luoga, Herry Mapesi, Slyakus Mlembe, Mengi Mkulila, Margareth Mkusa, Dorcas K Mnzava, Getrud J Mollel, Lilian Moshi, Germana Mossad, Dolores Mpundunga, Athumani Mtandanguo, Selerine Myeya, Sanula Nahota, Robert C. Ndege, Agatha Ngulukila, Jacopo Nicoletti, Alex John Ntamatungiro, Amina Nyuri, James Okuma, Daniel H Paris, Leila Samson, Elizabeth Senkoro, Jenifa Tarimo, Juerg Utzinger, Fiona Vanobberghen, Maja Weisser, John Wigay, Herieth Wilson
No financial support and no potential conflict of interest relevant to this article was reported.