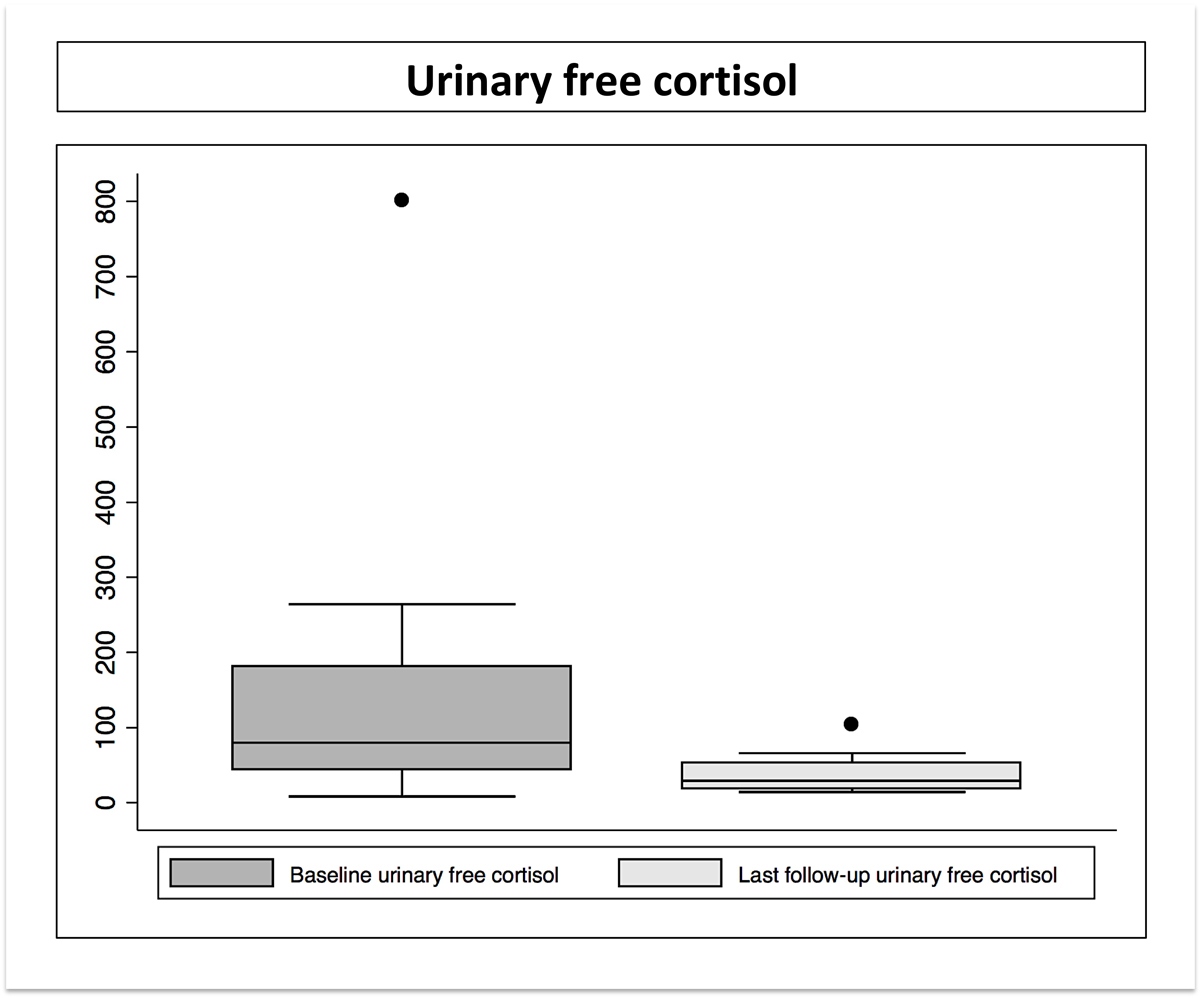
Figure 2 Urinary free cortisol levels before Gamma Knife radiosurgery (GKR) and at last follow-up after GKR.
DOI: https://doi.org/10.4414/smw.2021.20520
Cushing disease is a severe endocrine entity, which is generated by a corticotroph pituitary adenoma resulting in excessive adrenocorticotrophic hormone (ACTH) secretion. This disorder accounts for approximately 70% of endogenous hypercortisolism [1] and is associated with relevant morbidity and mortality [2]. The incidence of Cushing disease is approximately 1.2–2.4 per million per year [3].
The clinical picture is related to the disruption of the hypothalamus-pituitary-adrenal circuit, which increases circulating serum and urinary cortisol levels and disrupts the cortisol circadian rhythm [1, 2]. Major comorbidities include systemic arterial hypertension, diabetes mellitus, dyslipidaemia, osteoporosis, depression and infertility [1].
Primary treatment is microsurgical resection of the pituitary adenoma, which results in disease remission in approximately 50–90% of cases [4, 5], with relapse in around 13% during the first 10 years after microsurgery [6]. Microsurgical resection can be associated with complications including, but not limited to, damage to the optic apparatus, carotid artery, cavernous sinus and its components, and cerebrospinal fluid (CSF) leakage [7, 8]. Persistent and/or recurrent Cushing disease requires further treatment, including medical therapy, radiation [9–11] and/or adrenal surgery [12, 13].
Single-fraction stereotactic radiosurgery (SRS) and particularly Gamma Knife radiosurgery (GKRS) has been reported as safe and effective for ACTH-secreting pituitary adenomas [14–17]. The tumour control rate ranges between 83.3% and100%, with a rate of biological remission of between 17% and 87% [18]. Adverse radiation effects may include visual impairment (0–5.5%) and hypopituitarism (0–66%) [18]. The unique steep gradient of GKRS is particularly useful in delivering high doses of irradiation on small to medium size residual or recurrent ACTH-secreting pituitary adenomas. It has been classically considered that functional pituitary adenomas should benefit from higher doses of irradiation, the former being associated with a higher rate of biological remission [18]. More recently, biological effective dose (BED) emerged as a significant parameter for single fraction GKRS in functional indications [19], as being more predictive than the prescribed dose for safety and efficacy. This parameter incorporates, beside the dose factor, a time correction, which aims to take account of DNA repair during irradiation [19].
Here we report our long-term results for patients with recurrent and/or persistent Cushing disease treated by GKRS at our institution. We further evaluated whether BED could play a more significant role than the prescribed dose in biological remission on the basis of very long-term follow-up [20–22]. Our hypothesis was that BED could be a better predictor than the marginal prescribed dose for endocrine remission after single-fraction GKRS (Lille University Hospital, France). This assumption was based on previously published data. In particular, for trigeminal neuralgia, Tuleasca et al. [22] showed that safety and efficacy of GKRS was better predicted by BED than by the physical dose in a large cohort of 408 cases. Recently, we suggested that BED could be a better predictor for endocrine remission than the marginal prescribed dose in acromegaly [23]. Furthermore, we evaluated a large cohort of 149 patients with arteriovenous malformations treated with single-fraction, first intention GKRS [24]. In that cohort, BED and treatment time were the only statistically significant factors for arteriovenous malformation obliteration, whereas the marginal dose prescribed was not.
The study was designed as a retrospective, nonrandomised, historical cohort. A case report form was created for each patient and prospectively filled in. Data were retrospectively analysed. The Lille University Hospital Ethics Committee approved our study.
All cases were treated in Lille University Hospital (CHU Roger Salengro), France between August 2004 and February 2013. Data were initially analysed in Lille. Biological effective dose was calculated by the co-first author (CT) in Lausanne, Switzerland, using the particular details of beam-on time and marginal prescribed dose.
We included cases with demonstrated endocrine evidence for Cushing disease, based upon serum cortisol, ACTH and 24-hour urinary free cortisol (UFC) levels, and with no indication for further surgery. Patients with less than 12 months follow-up were excluded. Neuroradiological assessment included brain magnetic resonance imaging (MRI) for all patients, which confirmed the presence of a pituitary adenoma.
Postoperative residual/recurrent tumour volumes (before SRS) were measured either by using the Leksell Gamma Plan station or by the referring neuroradiologist in the respective French centres.
More than half of the patients had cavernous sinus residual tumour, which is associated with higher surgical risks if radical resection is preferred [25]. Moreover, the residual or recurrent tumour volumes were compatible with single-fraction GKRS. There was a multidisciplinary discussion both in the hospital referring the patients for GKRS and in our hospital, to evaluate the feasibility of GKRS versus another surgical exploration. Before being admitted for GKRS in the setting of persistent or recurrent Cushing disease, repeat adenomectomy was evaluated as a first therapeutic option. However, all patients in the present series had had at least one surgery (n = 21), and a minority had had two procedures (n = 5). Taking into account the anatomical location, the volume and the previous surgical status, the decision for GKRS had been made.
The primary aim was tumour control. The secondary aim was endocrine remission in the absence of any pharmacological treatment. We further evaluated whether BED could play a role in endocrine remission.
The assessment before GKRS encompassed basic demographic data, as well as necessary endocrinological assessment data, including 24-hour UFC, ACTH, free thyroxine, thyroid-stimulating hormone, prolactin, follicle-stimulating hormone, luteinising hormone, growth hormone, serum cortisol, testosterone in men, oestradiol (as well as menstrual history) in women, and a basic metabolic panel. No patient discontinued treatment with antisecretory drugs (e.g., ketoconazole) at the time of GKRS.
In all patients, a low-dose dexamethasone suppression test was used to confirm the diagnosis of active Cushing disease. The UFC was used to evaluate the results of GKRS.
The specific outcomes evaluated after GKRS were tumour control, endocrine remission, pituitary insufficiency and potential visual status changes. The UFC was used to evaluate the results of GKRS. Remission was defined as a normal 1-mg dexamethasone suppression test and 24-hour UFC in two consecutive samples. Biological assessment took place at 6 months and 1 year, and annually thereafter.
Gamma Knife radiosurgery was performed using Leksell Gamma Knife® 4C (LGK, Elekta Instruments AB, Sweden). The Leksell G frame was placed under local anaesthesia. Brain MRI and computed tomography were done routinely. The MR sequences included thin slices (1 mm) T1 MPRAGE, with additional T2 and T1 fat suppression through the sellar region.
We usually prescribe 25–30 Gy at the margin in secreting pituitary adednomas whenever feasible. We consider this as the current standard of care. Limitations to prescription of such doses are mainly related to the optic apparatus, and the distance between this and the tumour. We restrict less the dose received by the pituitary stalk, as in our view biological remission is more important than the appearance or not of pituitary insufficiency. The limit of doses to the optic apparatus has been considered a maximum of 8 Gy, as suggested in a historical series (please see the discussing section). However, on the basis of recent publications, in exceptional cases we have considered maximum doses to the optic apparatus of up to 10 or even 12 Gy [26].
Our strategy was to prescribe, whenever possible, high doses of radiation to the tumour. However, during treatment planning, we paid close attention to the maximum dose received by the optic apparatus, which was always extracted from the dose-volume histograms. In this cohort the doses received by the pituitary stalk and by the pituitary gland were not calculated at the time of GKRS.
The BED was calculated using a simplified approach, similar to that described by Jones and Hopewell [19], taking into account the beam-on time and the prescribed dose. This approach is considered the “basic BED model”, as described by several authors in the literature. By convention, we have considered the unit for BED measurement as being Gy2.47, to differentiate it from the unit measurement for the dose (Gy), as previously suggested [22].
The mean BED received by the tumour was 208.5 Gy2.47 (median 228.1, range 160–248). We also computed the mean BED received by the pituitary stalk, which was 86.7 Gy2.47 (median 31.5, range 25.5–228.7). We divided patients with endocrine remission into a high BED group (H-BED, 160–228 Gy2.47, n = 6) and a low BED group (L-BED, 228–248 Gy2.47, n = 12). The two groups did not differ in pretherapeutic UFC levels or range of prescribed dose.
Clinical and radiological follow-up were at 6 months and 1 year, and annually thereafter. The data were collected by one of the first authors (AB), who travelled to each referral centre outside Lille to collect the missing data in cases where the patient’s return to our centre was not possible, mainly because of the distance.
Tumour control was defined as stability or decrease in volume after GKRS.
Statistical testing was performed using STATA 14 (StataCorp, College 109 Station, Texas). Descriptive statistics were reported as proportion/frequency for categorical data and mean, median and range for continuous variables. Odds ratios were further evaluated as an association between an outcome and a treatment/exposure. Variables assessed in univariate models included age, sex, baseline UFC (24 hours) and serum cortisol levels. Because of the insufficient sample size no multivariable analysis was performed.
For biological control and pituitary insufficiency after GKRS, survival over time was examined using the Kaplan-Meier method. Patients were censored at the time of cure (for biological control) or appearance of insufficiency, or otherwise at last follow-up.
The prescribed dose and BED were assessed using both continuous and binary data modelling techniques, with dichotomisation thresholds derived from received operating characteristic (ROC) tables and the maximum point of (sensitivity-(1-specificity)).
Basic demographic data are presented in table 1 and dosimetric data in table 2.
| Table 1: Demographic data. | |
|---|---|
| Parameter | Value |
| Follow-up period (months), median (range) | 80 (19–141) |
| Age at treatment (years), median (range) | 39 (15.4–69.5) |
| Sex (M:F) | 6:20 |
| Symptoms at presentation | |
| – Cushing | 20/26 (76.9%) |
| – Subjective visual symptoms | 2/26 (7.7%) |
| – Pituitary insufficiency | 4/26 (15.4%) |
| Initial treatment | |
| – Surgery | 20/26 (76.9%) |
| – Pharmacological | 6/26 (23.1%) |
| After failure of pharmacological treatment all had microsurgical resection | |
| Size at discovery | |
| – Macroadenoma | 12/26 (46.1%) |
| – Microadenoma | 14/26 (53.9%) |
| Surgical approach | |
| – Transrhinoseptal | 17/26 (65.4%) |
| – Endonasal transsphenoidal | 9/26 (34.6%) |
| Number of previous surgeries | |
| – Zero | 0/26 (0%) |
| – One | 21/26 (80.8%) |
| – Two | 5/26 (19.2%) |
| Postoperative residual volume (cc), mean (median, range) | 0.407 (0.097, 0.023–1.4) |
| Anatomical location of residual tumour | |
| – Cavernous sinus | 11/26 (42.3%) |
| – Intrasellar | 12/26 (46.2%) |
| – Intrasellar and cavernous sinus | 3/26 (11.5%) |
| Side | |
| – Right | 7/26 (26.9%) |
| – Left | 19/26 (73.1%) |
| Reason for GKRS | |
| – Residual tumour and persistent hypersecretion | 11/26 (42.3%) |
| – Recurrent tumour and persistent hypersecretion | 11/26 (42.3%) |
| – Perioperative complications with need to stop resection | 4/26 (15.4%) |
| ACTH value before GKRS (pg/ml), median (range) | 132 (30–2209) |
| Free urinary cortisol before GKRS (µg/24h), median (range) | 404 (12–264) |
| Serum cortisol at 8 a.m. (nmol/l), median (range) | 126.5 (51–469) |
| Treatment for pituitary insufficiency before GKRS | 11/26 (42.3%) |
| Pharmacological treatment stopped before GKRS |
0/26 (0%) |
| Delay between microsurgery and GKRS, median (range) | 84.6 (11–182.5) |
ACTH = adrenocorticotrophic hormone; GKRS = Gamma Knife radiosurgery
Table 2 Dosimetric data
| Parameter | Median (range) |
|---|---|
| Prescription dose (Gy) | 27.5 (24–35) |
| Number of isocentres | 3 (1–17) |
| Target volume (cc) | 0.11 (0.09–3.7) |
| Conformity index | 97.5% (86–100%) |
| Selectivity index | 68.5% (22–87%) |
| Gradient index | 2.77 (2.61–3.38) |
| Treatment time (minutes) | 33 (10.3–128.7) |
| Volume of visual apparatus receiving >8 Gy (mm) | 0 (0–11.1) |
| Maximum dose received by the optic apparatus (Gy) | 1.1 (1–12.4) |
| Dose received by optic apparatus – first 10 mm3 (Gy) | 2.8 (0.4–7) |
| Maximum dose received by the pituitary stalk (Gy) | 6.3 (2.7–24.4) |
| BED (pituitary adenoma) | 228.1 (160–248) |
| BED = biological effective dose | |
The mean follow-up period was 66 months (median 80, range 19–108). The mean duration between surgery and GKRS was 60.8 months (median 84.6, range 11–182.5). No patient stopped antisecretory medication before GKRS (table 1).
The mean marginal prescription dose was 28.5 Gy (median 27.5, range 24–35; table 2). The mean target volume was 0.705 cc (median 0.110, range 0.090–3.7). The mean maximum dose received by the optic apparatus was 5.3 Gy (median 1.1, range 1–12.4). The mean maximum dose received by the pituitary stalk was 13.5 Gy (median 6.3, range 2.7–24.4), the former being collected retrospectively.
Tumour control was achieved in all cases (mean decrease in size 29.4%, range 0–100%).
Eighteen (69.2%) patients experienced biological remission in the absence of pharmacological treatment. Mean time to remission was 36 months (median 24, range 6–98). The probability of endocrine remission in the absence of any pharmacological therapy was 59% at three years and 77.6% at 7 years, which remained stable up to 9 years (fig. 1). There was a dramatic decrease of pretherapeutic UFC after GKRS (fig. 2). Univariate analysis failed to find a predictor of biological remission, although age was close to statistical significance.
Table 3 Univariate analysis.
| Variable | Odds ratio | Confidence interval | p-value |
|---|---|---|---|
| Univariate analysis, urinary cortisol normalisation | |||
| Sex (M/F) | 1.8 | 0.134–24.159 | 0.65 |
| Age (continuous value) | 1.055 | 0.952–1.168 | 0.3 |
| Side | 0.705 | 0.053–9.265 | 0.79 |
| Serum cortisol at 8 a.m. | 0.999 | 0.986–1.011 | 0.9 |
| UFC (24 hours) | 0.998 | 0.992–1.003 | 0.507 |
| ACTH at baseline | 1.103 | 0.953–1.275 | 0.18 |
| Volume of residual tumour | 1.0008 | 0.998–1.003 | 0.56 |
| Dose | 0.830 | 0.549–1.254 | 0.37 |
| BED | 0.996 | 0.945–1.050 | 0.901 |
| Number of isocentres | 1.152 | 0.820–1.617 | 0.41 |
| Treatment time | 1.003 | 0.964–1.044 | 0.84 |
| Univariate analysis, biological normalisation and pharmacological treatment stop | |||
| Sex | 1.166 | 0.166–8.185 | 0.87 |
| Age | 0.985 | 0.927–1.046 | 0.62 |
| Side | 3.5 | 0.346–35.371 | 0.28 |
| Serum cortisol at 8 a.m. | 0.990 | 0.979–1.002 | 0.109 |
| UFC (24 hours) | 1.003 | 0.994–1.011 | 0.45 |
| ACTH at baseline | 1.003 | 0.995–1.010 | 0.38 |
| Volume of residual tumour | 0.999 | 0.998–1.0004 | 0.26 |
| Dose | 1.070 | 0.804–1.424 | 0.63 |
| BED | 1.014 | 0.978–1.051 | 0.44 |
| Number of isocentres | 1.062 | 0.885–1.274 | 0.51 |
| Treatment time | 0.988 | 0.963–1.015 | 0.41 |
| Pituitary insufficiency | |||
| Sex | 1.222 | 0.196–7.594 | 0.32 |
| Age | 0.941 | 0.880–1.006 | 0.06 |
| Side | 0.545 | 0.094–3.145 | 0.49 |
| Serum cortisol at 8 a.m. | 1.0007 | 0.992–1.009 | 0.86 |
| UFC (24 hours) | 1.002 | 0.995–1.009 | 0.43 |
| ACTH at baseline | 1.0004 | 0.998–1.002 | 0.67 |
| Volume of residual tumour | 0.999 | 0.998–1.0008 | 0.76 |
| Dose | 1.102 | 0.844–1.438 | 0.47 |
| Dose at stalk | 1.032 | 0.916–1.163 | 0.59 |
| BED at stalk | 1.0006 | 0.989–1.012 | 0.9 |
| Number of isocentres | 1.036 | 0.884–1.213 | 0.66 |
| Treatment time | 1.018 | 0.990–1.047 | 0.19 |
| ACTH = adrenocorticotrophic hormone; Bed = biological effective dose; UFC = urinary free cortisol | |||
Figure 1 Probability of biological remission in the absence of pharmacological treatment after Gamma Knife radiosurgery; the number of patients for the time-points at 12, 24, 36, 51 and 96 months were 21, 15, 11, 7 and 3, respectively.

Figure 2 Urinary free cortisol levels before Gamma Knife radiosurgery (GKR) and at last follow-up after GKR.
Higher BED was associated with a higher overall probability of biological remission (77% vs 66% at last follow-up), although this did not reach statistical significance (fig. 3). Of note, the number of patients reflecting such an actuarial probability at 12, 24, 36, 51 and 96 months were 21, 15, 11, 7 and 3, respectively.
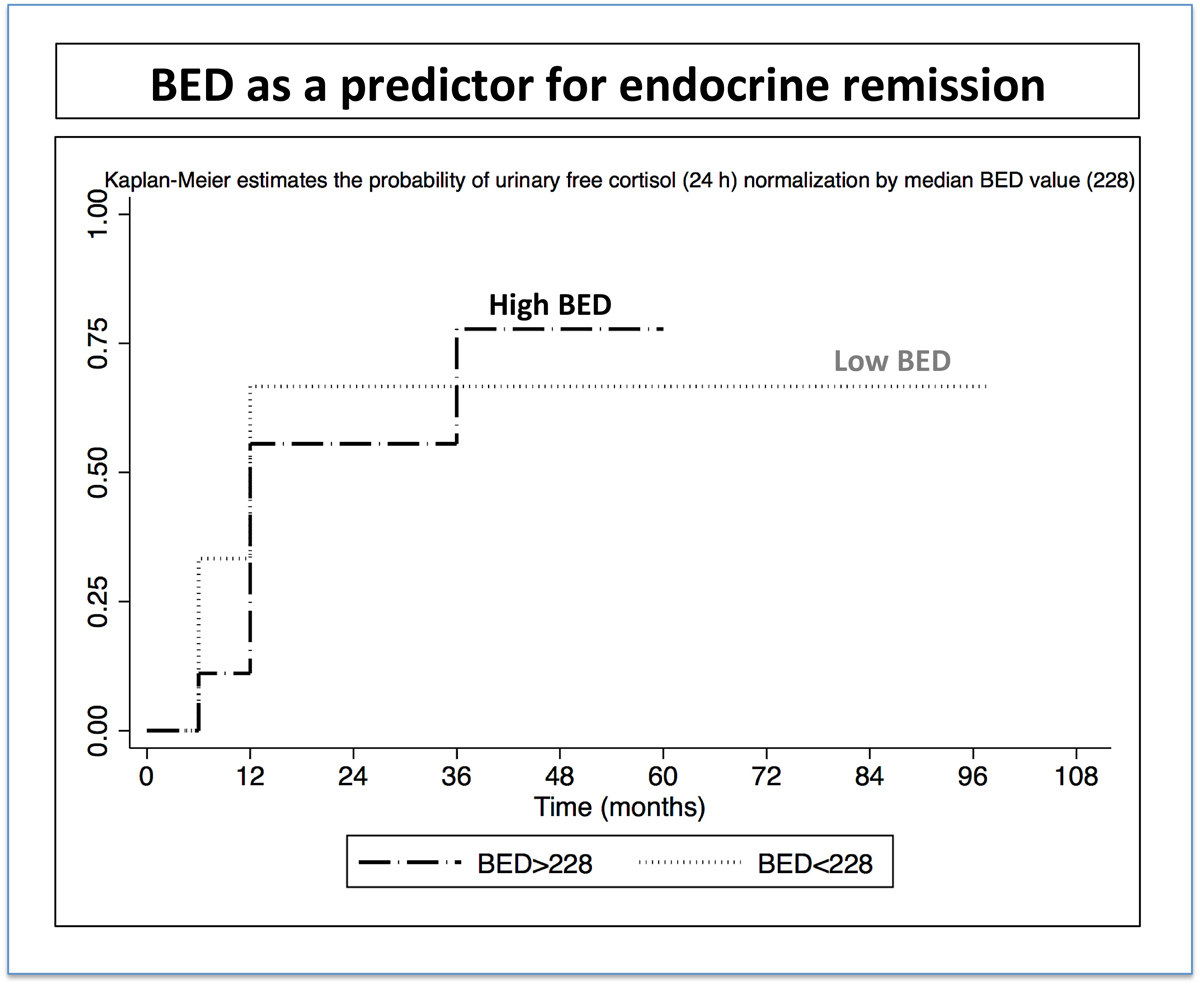
Figure 3 Probability of endocrine remission with high and low BED; the number of patients for the time-points at 12, 24, 36, 51 and 96 months were 21, 15, 11, 7 and 3, respectively. BED = biological effective dose in Gy2.47
The area under the ROC curve in general was 0.6 for the BED (continuous values) and 0.56 for the prescribed dose.
Seven patients developed new pituitary insufficiency after GKRS. The probability of this complication was 16% at 1 year and 32% at 3 years, which remained stable up to 9 years (fig. 4). The specific hormones related to pituitary insufficiency after SRS were ACTH in four cases, thyroid-stimulating hormone in two and follicle stimulating hormone / luteinising hormone in one.
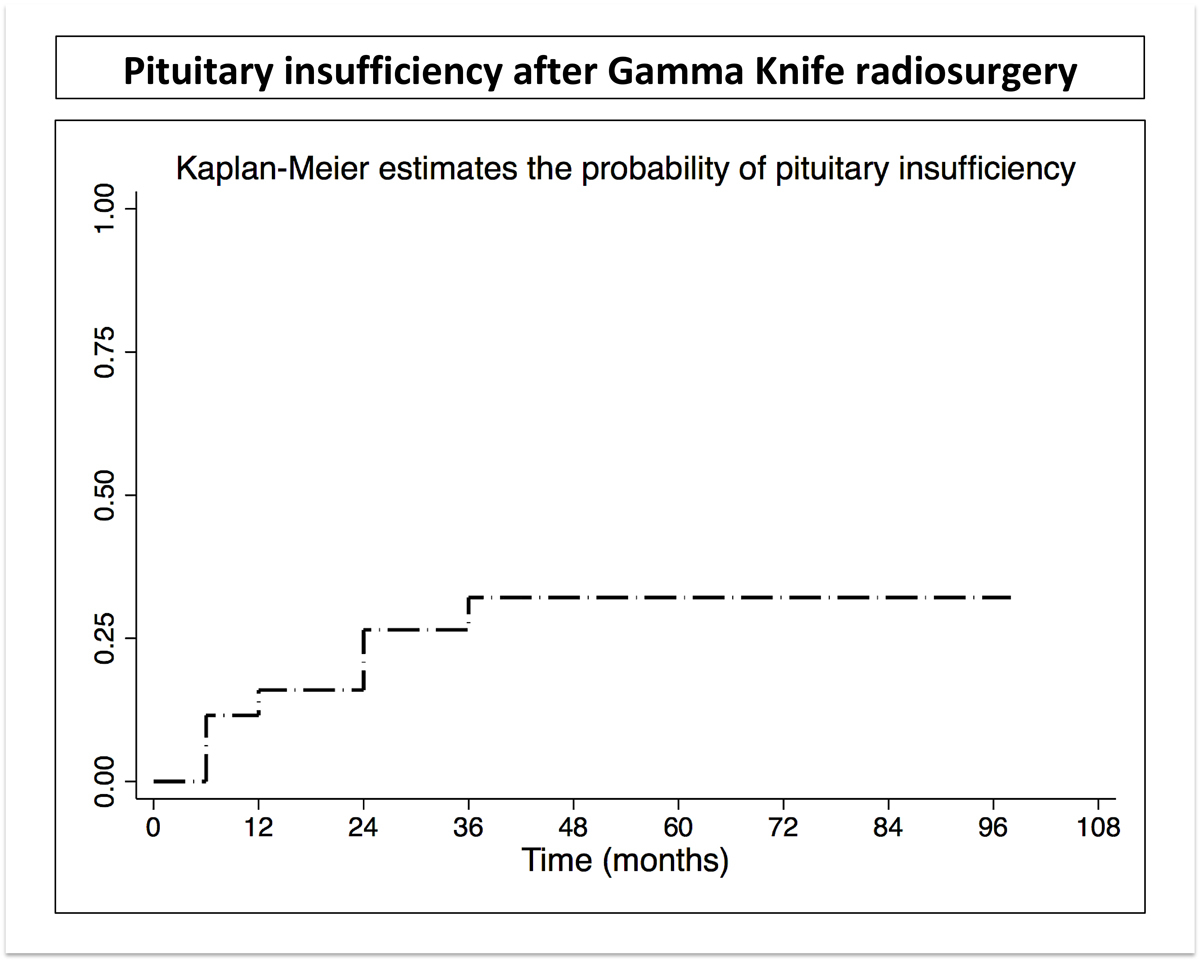
Figure 4 Probability of pituitary insufficiency after Gamma Knife radiosurgery.
Two (7.7%) patients experienced transient visual acuity changes at 6 and 12 months after GKRS, respectively. Both completely recovered.
The prescribed marginal doses to the tumour was 24 Gy and 25 Gy, respectively. The BED values received by the tumour were 228.9 and 195.7, respectively. The maximum doses received by the optic apparatus were 9.4 and 9.3 Gy, respectively and the first 10 mm3 of the optic apparatus received 7.4 and 7.3, Gy, respectively. The distance between the tumour and the optic apparatus was 3.6 mm in one case and 4 mm for the second case.
No other neurological complication was encountered.
In the present study, we report our experience with GKRS for Cushing disease. Eighteen (69.2%) patients experienced biological remission in the absence of pharmacological treatment. The probability of endocrine remission in the absence of any pharmacological therapy was 77.6% at 7 years, and remained stable up to 10 years. In this small series, higher BED (≤228 versus >228) was associated with a higher overall probability of biological remission ( 77% versus 66% at last follow-up), although this did not reach statistical significance. Of note, the numbers of patients reflecting such an actuarial probability at 12, 24, 36, 51 and 96 months were 21, 15, 11, 7 and 3, respectively. Moreover, the last three patients all belonged to the low BED group and were not distributed between the two groups (low and high BED), which can be further depicted in the Kaplan-Meier curve. The area under the ROC curve in general was 0.6 for BED continuous values and 0.56 for the prescribed dose. Seven patients developed new pituitary insufficiency after GKRS with a probability of this complication of 16% at 1 year and 32% at 3 years, subsequently remaining stable over time.
Transsphenoidal surgery remains the mainstream treatment for Cushing disease [8, 12, 27, 28]. However, definitive surgical cure remains challenging because of early failures (persisting Cushing disease, usually considered within the first 6 months postoperatively) or late recurrences of the disease, more than 6 months after surgery [29, 30]. Incomplete microsurgical resection ranges from 20% to 80% [31–33]. Surgery may be precluded for older patients and those with extensive comorbidities. Persistent and untreated biological disease potentially has mortality rates as high as 50% [34].
During the past three decades, several studies have reported results with SRS for Cushing disease [14–17, 35–38]. Radiosurgery is typically an adjuvant management in patients with persistent Cushing disease. Classically, higher doses of irradiation are prescribed, as high as 30–35 Gy, in secretory pituitary adenomas [35]. The recent international multicentre study by Mehta et al. [15] included a large number of patients (n = 278) with a mean follow-up of 5.6 years. Mean marginal dose was 23.7 Gy. Cumulative initial control of hypercortisolism was 80% at 10 years and the mean time to cortisol normalisation was 14.5 months. Recurrence occurred in approximately 18% of cases [15]. The authors suggested that GKRS could result in shorter response times than conventional radiotherapy in this indication [15]. Other studies reported that Cushing disease cases achieved earlier and far better biological remission as compared with acromegaly after single-fraction GKRS [39].
Recently, several studies proposed new treatment paradigms in order to achieve better biological remission in Cushing disease. Hugues et al. [14] analysed cases with persistent or recurrent Cushing disease after prior transsphenoidal surgery. Two groups were reported, of whom one had GKRS alone, and the other underwent GKRS and bilateral adrenalectomy. The authors concluded that patients with mild to moderate Cushing disease could be safely managed with GKRS alone, whereas those with severe Cushing disease should be considered for bilateral adrenalectomy with either concurrent SRS or SRS performed at a later date if tumour growth occurred. We agree with the radiosurgical treatment algorithm for patients with persistent or recurrent Cushing disease after transsphenoidal surgery as presented by Hugues et al. [14]. Other studies suggested even that whole-sellar irradiation [40] provides high rates of endocrine remission as, and similar complications rates to, the classical series which describeed targeting only clearly visualised tumour [17, 38]. The most common complication in these series was hypopituitarism [17, 38].
In our present case series, we prescribed a high mean marginal dose of 28.5 Gy (median 27.5). This is probably a reason for not finding a statistically significant relationship between dose and endocrine remission in this small cohort. Moreover, we completely agree with a recent comment made by Dade Lunsford [14], that the necessary prescription dose remains unclear. The idea of prescribing higher radiation doses, as high as 50 Gy, as proposed by the Pittsburgh team, in a single fraction for small secreting tumours not close to the optic apparatus sounds appealing and remains to be elucidated by further studies.
An important aspect is the proximity of such residual tumours to two critical anatomical structures, namely the optic apparatus and the pituitary stalk. Due to the steep gradient of GKRS, a distance of only few millimetres is often sufficient to avoid visual complications, while delivering a therapeutic dose to the tumour. Recent papers suggested that the optic apparatus might receive doses up to 12 Gy without major risks for optic neuropathy [41].
An open question is whether hypofractionnated SRS could play a role in Cushing disease. The recent LGK ICON [42] nicely addresses such a point. While keeping the steep gradient of the LGK, it offers a high definition motion management control system. This could be particularly useful for patients with larger lesions, as well as for lesions in contact with or encasing the optic pathways. The safety and efficacy of this type of approach remain to be elucidated by further research.
In single-fraction radiosurgery, a certain physical dose is delivered within a given time period. The importance of these two variables (irradiation time and prescribed dose) can be further evaluated using the BED concept. This is particularly important as major variations in the irradiation time can appear for uniform prescribed doses or a narrow range of marginal dose prescription (such as in our study).
In 1989, the term BED was coined based on linear quadratic cell survival in radiobiology [43]. The main aim was to indicate quantitatively the biological effect of any radiotherapy treatment. In that context, BED took into account the changes in dose-per-fraction or dose rate, total dose and the time factor [43]. There utility of this parameter expanded in various fields, such as dose escalations or quantification of treatments using ionising radiation [43], among others.
Recently and for the first time, BED has been suggested as a better predictor than the prescribed dose for safety and efficacy in single-fraction GKRS for trigeminal neuralgia by Tuleasca et al. [22]. Based upon this previously published research, we sought to evaluate the role of this parameter in the context of GKRS for Cushing disease for the first time, in this small cohort. After separation into two groups, our data suggest that higher BED was associated with better endocrine remission rates. Owing to the limited sample size, this aspect remains to be further demonstrated by other studies. The area under the ROC curve was higher for the BED than for the dose.
The present study is in continuity with a previous one from our group, which showed similar findings after single-fraction GKRS for acromegaly, suggesting a prominent role for higher BED values as related to better rates of endocrine remission [23]. Moreover and also recently, also using data from our institution, we showed that BED (as continuous values) is a stronger predictor for arteriovenous malformation obliteration after GKRS as compared with the marginal prescribed dose, in a cohort where the vast majority of patients (around 80%) were treated with a uniform dose of 24 Gy [24].In the future, BED might replace the marginal prescribed dose as a better predictor for safety and efficacy. Such findings should be replicated for other pathologies and in larger cohorts. We consider that, in the near future, radiosurgery will evolve towards tailored radiobiological effects, in which BED might play a key role. By analogy with dose de-escalation over the decades [44], the same might apply to BED during future decades, if such a concept demonstrates a prominent role and if the scientific community demonstrates its potential utility in the clinical realm.
Our study has several limitations. One of the first major limitations is the retrospective data analysis, with all the biases that are involved in this type of methodology. A second is related to the limited number of cases, which precluded a multivariate analysis. This further applies to the BED evaluation, which included only a limited number of patients in one of the two arms (the higher BED arm). A third limitation, which is also related to the small subset of patients at each time-point, is that the Kaplan-Meier estimates can be misleading and should be interpreted with caution. A fourth limitation is the calculation of BED by means of a simplified formula, including only the beam-on time and the prescribed dose. A fifth limitation is related to the timeframe of our analysis, while we currently observe refinements of SRS platforms, including LGK, neuroimaging modalities. In this respect, even better results could potentially be obtained and reported in the near future. A sixth limitation is the fact that follow-up data came from various centres, and not only from the Lille University Hospital. Each of them might have different criteria for endocrine remission, normalisation of 24-hour UFC.
Using GKRS for Cushing disease, we report high endocrine remission rates, as high as 77.6% at 7 years, using high doses of radiation. Seven patients developed new pituitary insufficiency after GKRS, with a probability of developing this complication of 32% at 3 years, which further remained stable up to 9 years.
Our strategy was always to prescribe high doses of irradiation to the tumour in order to ensure high rates of endocrine remission, while paying close attention to the maximum doses received by the optic apparatus in order to avoid optic neuropathies after GKRS. The appearance of pituitary insufficiency was considered in our centre as a secondary effect, which can be safely managed by substitution medication in the frame of a multidisciplinary management.
Our series is small, but raises the question of the impact of BED on biological remission after GKRS for Cushing disease. Higher BED values resulted in higher cure rates, although this did not reach statistical significance. Moreover, the area under the ROC curve was higher for the BED than for the prescribed dose. How BED should be incorporated into further dose planning remains to be established in the near future.
Lausanne University Hospital and University of Lausanne; Lille University Hospital (CHU Roger Salengro)
Lausanne University Hospital: Constantin Tuleasca gratefully acknowledges the receipt of a grant “Jeune Chercheur en Recherche Clinique” by the University of Lausanne, Faculty of Biology and Medicine and Lausanne University Hospital (CHUV)
No conflict of interest relevant to this article was reported.
1 Newell-Price J , Bertagna X , Grossman AB , Nieman LK . Cushing’s syndrome. Lancet. 2006;367(9522):1605–17. doi:.https://doi.org/10.1016/S0140-6736(06)68699-6
2 Pivonello R , De Leo M , Cozzolino A , Colao A . The Treatment of Cushing’s Disease. Endocr Rev. 2015;36(4):385–486. doi:.https://doi.org/10.1210/er.2013-1048
3 Steffensen C , Bak AM , Zøylner Rubeck KZ , Jørgensen JO . Epidemiology of Cushing’s syndrome. Neuroendocrinology. 2010;92(Suppl 1):1–5. doi:.https://doi.org/10.1159/000314297
4 Hammer GD , Tyrrell JB , Lamborn KR , Applebury CB , Hannegan ET , Bell S , et al. Transsphenoidal microsurgery for Cushing’s disease: initial outcome and long-term results. J Clin Endocrinol Metab. 2004;89(12):6348–57. doi:.https://doi.org/10.1210/jc.2003-032180
5 Patil CG , Prevedello DM , Lad SP , Vance ML , Thorner MO , Katznelson L , et al. Late recurrences of Cushing’s disease after initial successful transsphenoidal surgery. J Clin Endocrinol Metab. 2008;93(2):358–62. doi:.https://doi.org/10.1210/jc.2007-2013
6 Beauregard C , Dickstein G , Lacroix A . Classic and recent etiologies of Cushing’s syndrome: diagnosis and therapy. Treat Endocrinol. 2002;1(2):79–94. doi:.https://doi.org/10.2165/00024677-200201020-00002
7 Ciric I , Zhao JC , Du H , Findling JW , Molitch ME , Weiss RE , et al. Transsphenoidal surgery for Cushing disease: experience with 136 patients. Neurosurgery. 2012;70(1):70–80, discussion 80–1. doi:.https://doi.org/10.1227/NEU.0b013e31822dda2c
8 Porterfield JR , Thompson GB , Young WF, Jr , Chow JT , Fryrear RS , van Heerden JA , et al. Surgery for Cushing’s syndrome: an historical review and recent ten-year experience. World J Surg. 2008;32(5):659–77. doi:.https://doi.org/10.1007/s00268-007-9387-6
9 Castinetti F , Brue T . Radiothérapie et radiochirurgie des adénomes hypophysaires [Radiotherapy and radiosurgery of pituitary adenomas]. Presse Med. 2009;38(1):133–9. Article in French. doi:.https://doi.org/10.1016/j.lpm.2008.09.012
10 Castinetti F , Morange I , Dufour H , Regis J , Brue T . Radiotherapy and radiosurgery in acromegaly. Pituitary. 2009;12(1):3–10. doi:.https://doi.org/10.1007/s11102-007-0078-y
11 Castinetti F , Brue T . Gamma Knife radiosurgery in pituitary adenomas: Why, who, and how to treat? Discov Med. 2010;10(51):107–11.
12 Tritos NA , Biller BM , Swearingen B . Management of Cushing disease. Nat Rev Endocrinol. 2011;7(5):279–89. doi:.https://doi.org/10.1038/nrendo.2011.12
13 Tritos NA , Biller BMK . Medical Management of Cushing Disease. Neurosurg Clin N Am. 2019;30(4):499–508. doi:.https://doi.org/10.1016/j.nec.2019.05.007
14 Hughes JD , Young WF, Jr , Chang AY , Link MJ , Garces YI , Laack NN , et al. Radiosurgical Management of Patients With Persistent or Recurrent Cushing Disease After Prior Transsphenoidal Surgery: A Management Algorithm Based on a 25-Year Experience. Neurosurgery. 2020;86(4):557–64. doi:.https://doi.org/10.1093/neuros/nyz159
15 Mehta GU , Ding D , Patibandla MR , Kano H , Sisterson N , Su YH , et al. Stereotactic Radiosurgery for Cushing Disease: Results of an International, Multicenter Study. J Clin Endocrinol Metab. 2017;102(11):4284–91. doi:.https://doi.org/10.1210/jc.2017-01385
16 Pollock BE , Young WF, Jr . Stereotactic radiosurgery for patients with ACTH-producing pituitary adenomas after prior adrenalectomy. Int J Radiat Oncol Biol Phys. 2002;54(3):839–41. doi:.https://doi.org/10.1016/S0360-3016(02)02975-9
17 Shepard MJ , Mehta GU , Xu Z , Kano H , Sisterson N , Su YH , et al. Technique of Whole-Sellar Stereotactic Radiosurgery for Cushing Disease: Results from a Multicenter, International Cohort Study. World Neurosurg. 2018;116:e670–9. doi:.https://doi.org/10.1016/j.wneu.2018.05.067
18 Minniti G , Clarke E , Scaringi C , Enrici RM . Stereotactic radiotherapy and radiosurgery for non-functioning and secreting pituitary adenomas. Rep Pract Oncol Radiother. 2016;21(4):370–8. doi:.https://doi.org/10.1016/j.rpor.2014.09.004
19 Jones B , Hopewell JW . Modelling the influence of treatment time on the biological effectiveness of single radiosurgery treatments: derivation of “protective” dose modification factors. Br J Radiol. 2019;92(1093):20180111.
20 Jones B , Dale RG , Deehan C , Hopkins KI , Morgan DA . The role of biologically effective dose (BED) in clinical oncology. Clin Oncol (R Coll Radiol). 2001;13(2):71–81.
21 Millar WT , Hopewell JW , Paddick I , Lindquist C , Nordströn H , Lidberg P , et al. The role of the concept of biologically effective dose (BED) in treatment planning in radiosurgery. Phys Med. 2015;31(6):627–33. doi:.https://doi.org/10.1016/j.ejmp.2015.04.008
22 Tuleasca C , Paddick I , Hopewell JW , Jones B , Millar WT , Hamdi H , et al. Establishment of a Therapeutic Ratio for Gamma Knife Radiosurgery of Trigeminal Neuralgia: The Critical Importance of Biologically Effective Dose Versus Physical Dose. World Neurosurg. 2020;134:e204–13. doi:.https://doi.org/10.1016/j.wneu.2019.10.021
23 Balossier A , Tuleasca C , Cortet-Rudelli C , Soto-Ares G , Levivier M , Assaker R , et al. Gamma Knife radiosurgery for acromegaly: Evaluating the role of the biological effective dose associated with endocrine remission in a series of 42 consecutive cases. Horumon To Rinsho. 2021;94(3):424–33. doi:.https://doi.org/10.1111/cen.14346
24 Tuleasca C , Peciu-Florianu I , Leroy HA , Vermandel M , Faouzi M , Reyns N . Biologically effective dose and prediction of obliteration of unruptured arteriovenous malformations treated by upfront Gamma Knife radiosurgery: a series of 149 consecutive cases. J Neurosurg. 2020. Online ahead of print. doi:.https://doi.org/10.3171/2020.4.JNS201250
25 Messerer M , Daniel RT , Cossu G . No doubt: the invasion of the cavernous sinus is the limiting factor for complete resection in pituitary adenomas. Acta Neurochir (Wien). 2019;161(4):717–8. doi:.https://doi.org/10.1007/s00701-018-03784-2
26 Pollock BE , Link MJ , Leavitt JA , Stafford SL . Dose-volume analysis of radiation-induced optic neuropathy after single-fraction stereotactic radiosurgery. Neurosurgery. 2014;75(4):456–60, discussion 460. doi:.https://doi.org/10.1227/NEU.0000000000000457
27 Mampalam TJ , Tyrrell JB , Wilson CB . Transsphenoidal microsurgery for Cushing disease. A report of 216 cases. Ann Intern Med. 1988;109(6):487–93. doi:.https://doi.org/10.7326/0003-4819-109-6-487
28 Sheehan JM , Lopes MB , Sheehan JP , Ellegala D , Webb KM , Laws ER, Jr . Results of transsphenoidal surgery for Cushing’s disease in patients with no histologically confirmed tumor. Neurosurgery. 2000;47(1):33–6, discussion 37–9.
29 Alexandraki KI , Kaltsas GA , Isidori AM , Storr HL , Afshar F , Sabin I , et al. Long-term remission and recurrence rates in Cushing’s disease: predictive factors in a single-centre study. Eur J Endocrinol. 2013;168(4):639–48. doi:.https://doi.org/10.1530/EJE-12-0921
30 Hassan-Smith ZK , Sherlock M , Reulen RC , Arlt W , Ayuk J , Toogood AA , et al. Outcome of Cushing’s disease following transsphenoidal surgery in a single center over 20 years. J Clin Endocrinol Metab. 2012;97(4):1194–201. doi:.https://doi.org/10.1210/jc.2011-2957
31 Chandler WF , Schteingart DE , Lloyd RV , McKeever PE , Ibarra-Perez G . Surgical treatment of Cushing’s disease. J Neurosurg. 1987;66(2):204–12. doi:.https://doi.org/10.3171/jns.1987.66.2.0204
32 Patil CG , Veeravagu A , Prevedello DM , Katznelson L , Vance ML , Laws ER, Jr . Outcomes after repeat transsphenoidal surgery for recurrent Cushing’s disease. Neurosurgery. 2008;63(2):266–70, discussion 270–1. doi:.https://doi.org/10.1227/01.NEU.0000313117.35824.9F
33 Friedman RB , Oldfield EH , Nieman LK , Chrousos GP , Doppman JL , Cutler GB, Jr , et al. Repeat transsphenoidal surgery for Cushing’s disease. J Neurosurg. 1989;71(4):520–7. doi:.https://doi.org/10.3171/jns.1989.71.4.0520
34 Plotz CM , Knowlton AI , Ragan C . The natural history of Cushing’s syndrome. Am J Med. 1952;13(5):597–614. doi:.https://doi.org/10.1016/0002-9343(52)90027-2
35 Castinetti F , Nagai M , Morange I , Dufour H , Caron P , Chanson P , et al. Long-term results of stereotactic radiosurgery in secretory pituitary adenomas. J Clin Endocrinol Metab. 2009;94(9):3400–7. doi:.https://doi.org/10.1210/jc.2008-2772
36 Cordeiro D , Xu Z , Mehta GU , Ding D , Vance ML , Kano H , et al. Hypopituitarism after Gamma Knife radiosurgery for pituitary adenomas: a multicenter, international study. J Neurosurg. 2018. Online ahead of print. doi:.https://doi.org/10.3171/2018.5.JNS18509
37 Jagannathan J , Yen CP , Pouratian N , Laws ER , Sheehan JP . Stereotactic radiosurgery for pituitary adenomas: a comprehensive review of indications, techniques and long-term results using the Gamma Knife. J Neurooncol. 2009;92(3):345–56. doi:.https://doi.org/10.1007/s11060-009-9832-5
38 Lee CC , Chen CJ , Yen CP , Xu Z , Schlesinger D , Fezeu F , et al. Whole-sellar stereotactic radiosurgery for functioning pituitary adenomas. Neurosurgery. 2014;75(3):227–37, discussion 237. doi:.https://doi.org/10.1227/NEU.0000000000000425
39 Gupta A , Xu Z , Kano H , Sisterson N , Su YH , Krsek M , et al. Upfront Gamma Knife radiosurgery for Cushing’s disease and acromegaly: a multicenter, international study. J Neurosurg. 2019;131(2):532–8. doi:.https://doi.org/10.3171/2018.3.JNS18110
40 Taylor DG , Janssen A , Ding D , Xu Z , Mehta GU , Liscak R , et al. Whole Sella vs Targeted Stereotactic Radiosurgery for Acromegaly: A Multicenter Matched Cohort Study. Neurosurgery. 2020;86(5):656–64. doi:.https://doi.org/10.1093/neuros/nyz245
41 Leavitt JA , Stafford SL , Link MJ , Pollock BE . Long-term evaluation of radiation-induced optic neuropathy after single-fraction stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2013;87(3):524–7. doi:.https://doi.org/10.1016/j.ijrobp.2013.06.2047
42 Tuleasca C , Leroy HA , Régis J , Levivier M . Gamma Knife radiosurgery for cervical spine lesions: expanding the indications in the new era of Icon. Acta Neurochir (Wien). 2016;158(11):2235–6. doi:.https://doi.org/10.1007/s00701-016-2962-6
43 Fowler JF . 21 years of biologically effective dose. Br J Radiol. 2010;83(991):554–68. doi:.https://doi.org/10.1259/bjr/31372149
44 Kondziolka D , Lunsford LD , McLaughlin MR , Flickinger JC . Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med. 1998;339(20):1426–33. doi:.https://doi.org/10.1056/NEJM199811123392003
Contributed equally as joint first authors
Lausanne University Hospital: Constantin Tuleasca gratefully acknowledges the receipt of a grant “Jeune Chercheur en Recherche Clinique” by the University of Lausanne, Faculty of Biology and Medicine and Lausanne University Hospital (CHUV)
No conflict of interest relevant to this article was reported.