Figure 1 Flow of recommendation selection.
DOI: https://doi.org/10.4414/smw.2021.20519
confidence interval
grade of recommendation
neonatal intensive care unit
potentially inappropriate medication
Omission of Prescription and Inappropriate Prescription
prescription-screening tool
Screening Tool of Older Persons’ potentially inappropriate Prescriptions/Screening Tool to Alert doctors to the Right Treatment
Drug prescription in neonatology is challenging. For hospitalised neonates, it is based largely on low-quality pharmacotherapeutic evidence from small clinical studies, and off-label drug use occurs in >85% of cases [1–5]. Widely accepted guidelines are rare, leading to great variability in practice [6].
Medication errors occur more frequently for hospitalised neonates, particularly those born preterm, than for older children and adults, with reported error rates of 15–90% [7–9]. A significant portion of these errors occurs during prescription [7]. Neonates are more susceptible than older patients to adverse drug reactions, and serious adverse drug reactions occur in approximatively 10% of medication error cases [8]. In a multicentre prospective cohort study, adverse drug events were more prevalent in neonatal than in paediatric wards [10]. The most vulnerable neonatal patients – those with the lowest weights and youngest gestational ages – receive the largest number of drugs and are thus most subject to medication error [7, 9].
Prescription-screening tools (PSTs) are checklists that effectively optimise and secure pharmacotherapy, allowing healthcare professionals to review patients’ ongoing treatments and identify unsuitable drugs, suboptimal dosages and missing treatments that would provide additional benefits [11, 12]. They aim to lead prescribers to the best available treatments in specific situations based on up-to-date knowledge, thereby contributing to care quality improvement. Most PSTs were developed in geriatric settings, but some have been created for other populations (e.g., the potentially inappropriate medication (PIM)-Check tool for adult internal medicine and the POPI (paediatrics: omission of prescriptions and inappropriate prescriptions) tool for general paediatrics) [13, 14]. In these settings, PSTs are effective for the detection of PIMs, which are risk factors for adverse drug events. Use of the “screening tool of older persons’ potentially inappropriate prescriptions/screening tool to alert doctors to the right treatment” (STOPP-START) criteria has been associated with reduced adverse drug events and hospitalisation duration [11, 12, 15, 16]. PSTs are also used to train young physicians and pharmacists and to standardise practices. To our knowledge, no PST has been developed for hospitalised neonates; our aim was to develop a such a tool.
According to Swiss law, this study did not require ethical approval, as it did not directly involve patients, patients’ data, human tissue or animals. For PST development, a steering committee comprising two clinical pharmacists (TR, CFC) and two senior neonatologists (RDL, RP) from the Neonatology Unit of the Geneva University Hospitals was formed. This committee defined the themes and sub-themes the tool needed to address based on the index of a neonatology textbook [17]. These themes covered basic management issues and pathologies of hospitalised neonates.
A semi-structured literature review was conducted to identify recent clinical recommendations in the literature. The steering committee compiled a list of sources that were reviewed (table 1). Guidelines from scientific societies, hospitals, governments, textbooks and manufacturers were included. When data from these sources was insufficient to form recommendations, key-word searches of the PubMed, Embase and/or Google Scholar databases were performed. Studies and clinical guidelines addressing pharmacotherapy for hospitalised neonates (corrected age ≤28 days after term), with no restriction on gestational age or weight, were included. The date of publication was not restricted, but the most recent publications were prioritised.
Table 1 Sources consulted in the literature review.
| Systematic search | ||
| Neonatology documents and protocols | ||
| Geneva University Hospitals (HUG) (local guidelines) | Local neonatal guidelines HUG | Internal website |
| Foreign hospitals (structured and open access hospital guidelines commonly used by the authors) | Royal Children Hospital Melbourne, AU | http://www.rch.org.au |
| Victoria State Government, AU | http://www.health.vic.gov.au | |
| Auckland District Health Board – drug monograph, NZ | http://www.adhb.govt.nz | |
| Auckland District Health Board – clinical guideline, NZ | http://www.adhb.govt.nz | |
| Canterbury District Health Board - drug monograph, NZ | https://www.cdhb.health.nz | |
| Christchurch Women's Hospital – Neonatology institutionnal textbook, NZ | Mckie J, Lynn A, Meeks M. Neonatal Unit Handbook. Christchurch Women’s Hosp. 2015;(November):1–152. | |
| Guidelines from scientific societies (main societies from Switzerland, Europe and America) | Swiss Society of Neonatology, CH | http://www.neonet.ch |
| American Academy of Pediatrics, US | Google scholar and Google search: “Neonate + theme + guideline/recommendation + american academy of pediatrics” and “neonatal + theme + guideline/recommendation + american academy of paediatrics”. | |
| American Academy of Pediatrics, US | http://pediatrics.aappublications.org/ | |
| Canadian Paediatric Society | Google scholar and Google search: “Neonate + theme + guideline/recommendation + canadian paediatric society” and “neonatal + theme + guideline/recommendation + canadian paediatric society”. | |
| Canadian Paediatric Society | www.cps.ca | |
| Royal College of Paediatrician and Child Health, UK | http://www.rcpch.ac.uk | |
| National Institute for Health and Care Excellence, UK | https://www.nice.org.uk | |
| European Society of Neonatology, EU | Google scholar and Google search: “Neonate + theme + guideline/recommendation + european society of neonatology” and “neonatal + theme + guideline/recommendation + european society of neonatology” | |
| European Society of Neonatology, EU | http://esn.espr.info/ | |
| Other recommendations | Google scholar and Google search: “Neonate + theme + guideline/recommendation” and “neonatal + theme + guideline/recommendation” | |
| Geneva Foundation for medical research, INTL | http://www.gfmer.ch | |
| Research groups, textbooks | ||
| Cochrane Neonatal, INTL | http://neonatal.cochrane.org | |
| Neonatal Formulary, UK | http://www.neonatalformulary.com | |
| Complementary search (only if systematic search deemed insufficient) | ||
| PubMed | “Neonate + theme or drug” and “Neonatal + theme or drug + guideline/recommendation” | |
| Embase | ||
| Uptodate | “Theme + neonates” or “theme” | |
| Referent senior neonatologist | Senior neonatologist from the steering committee | |
The themes on which recommendations were sought are listed in table 2 and appendix 1 (available in the PDF version of the manuscript).
Table 2 Themes included in the literature review.
| Themes | Examples of subthemes |
|---|---|
| Basic managment | Nutritional management, vaccination |
| Cardiology | Congenital heart disease, patent ductus arteriosus |
| Haematology | Anaemia, Rh incompatibility |
| Pneumology | Apnoea, bronchopulmonary dysplasia |
| Nephrology | Renal failure (acute) |
| Gastroenterology | Direct hyperbilirubinaemia, necrotising enterocolitis |
| Neurology | Intracranial haemorrhage, seizures in the neonate |
| Pain and analgesia | Sedation and analgesia in a neonate |
| Infectiology | Sepsis, respiratory syncytial virus |
| Endocrinology and metabolism | Thyroid disorders, hyperglyacemia |
| Ophthalmology | Eye disorders of the newborn, retinopathy of prematurity |
| Pharmacology and toxicology | Drug interactions, drug breast-feeding compatibility |
| Electrolyte disorders | Calcium disorders, hyperkalaemia |
| Dermatology | Rash and dermatological problems |
Recommendations identified via the literature review were grouped by theme, and those on the same subjects were merged. From these materials, recommendations and definitions were selected and optimised through individual interviews with sub-specialists (a paediatric infectiologist for infectious diseases, a paediatric nephrologist for nephrology, a clinical pharmacist for pharmacological issues and a neonatologist for all others). The steering committee then reduced the expert-approved recommendations to address only the most relevant topics.
The selected recommendations were reformulated as simple “main statements” on neonatal drug therapy starting with active, directive verbs (start, stop, do not use, administer, check, reassess, consider, do not provide). Additional information was added to provide context or include relevant details, and the recommendation grade and key references were provided (appendix 2, available in the PDF version of the manuscript).
The grade of recommendation (GOR), collected from relevant publications, was assessed for each item using the scale of the Scottish Intercollegiate Guidelines Network [18]. Available GORs determined using this or an equivalent scale were retained. When no such GOR was provided, the type of study or recommendation was recorded. For each item based on several references, an overall GOR was determined according to, in order of priority, the GOR given in the reference on which the item was most directly based or the highest GOR among those provided.
A modified Delphi method was used in two rounds (June 2016–September 2017 and May–September 2018) to select items with strong consensus among Swiss experts. Paediatricians working or having recently worked in Swiss neonatal intensive care units (NICUs) with Neonatology subspecialty titles or equivalent foreign qualifications and clinical pharmacists who had worked for several years in a Swiss neonatology centre were invited to participate in the Delphi rounds. The recommendation items were submitted to the experts via questionnaire on the SurveyMonkey website (surveymonkey.com; SurveyMonkey Inc., San Mateo, CA, USA; appendix 2). In the first round, the complete items were accompanied by multiple-choice questions about the experts’ level of agreement and item usefulness. Free text fields were provided for experts to add comments and/or propose the addition of references. In the second round, experts were asked only to indicate their level of agreement with the recommendations. In addition to the questionnaire, each expert received a table indicating his or her level of agreement in the first round and the median level of agreement of the expert group for each item. When these two values differed, the expert was asked to accept the group rating or to maintain his or her position, and explain why. Respondents indicated their level of agreement with the main statements using a five-point Likert scale (1, strongly disagree; 5, totally agree; 0, no opinion). They could also provide comments related to their responses. Item scores ≥4 were considered to indicate expert agreement. Items on which >65% of experts agreed in the first round were selected for the second round; all other items were eliminated. The process was then repeated, and items on which >75% of experts agreed in the second round were validated and included in the final tool. Items were revised between rounds when one or more experts suggested improved wording and when several experts had similar dissenting opinions in the first round, and the overall level of agreement was insufficient for validation.
The experts evaluated recommendation usefulness at the time of the first Delphi round, classifying items as (1) essential for neonatology practice,(2) useful especially for the training of young physicians or pharmacists or (3) useless. Items classified as “useless” by a majority of experts were eliminated.
Descriptive statistics were calculated for the Delphi data using the Excel® software (version 15.0.5189.1000; Microsoft Corporation, Redmond, WA, USA). For each main statement, the median agreement rating, percentage of experts conferring ratings ≥4 and number of responses were determined.
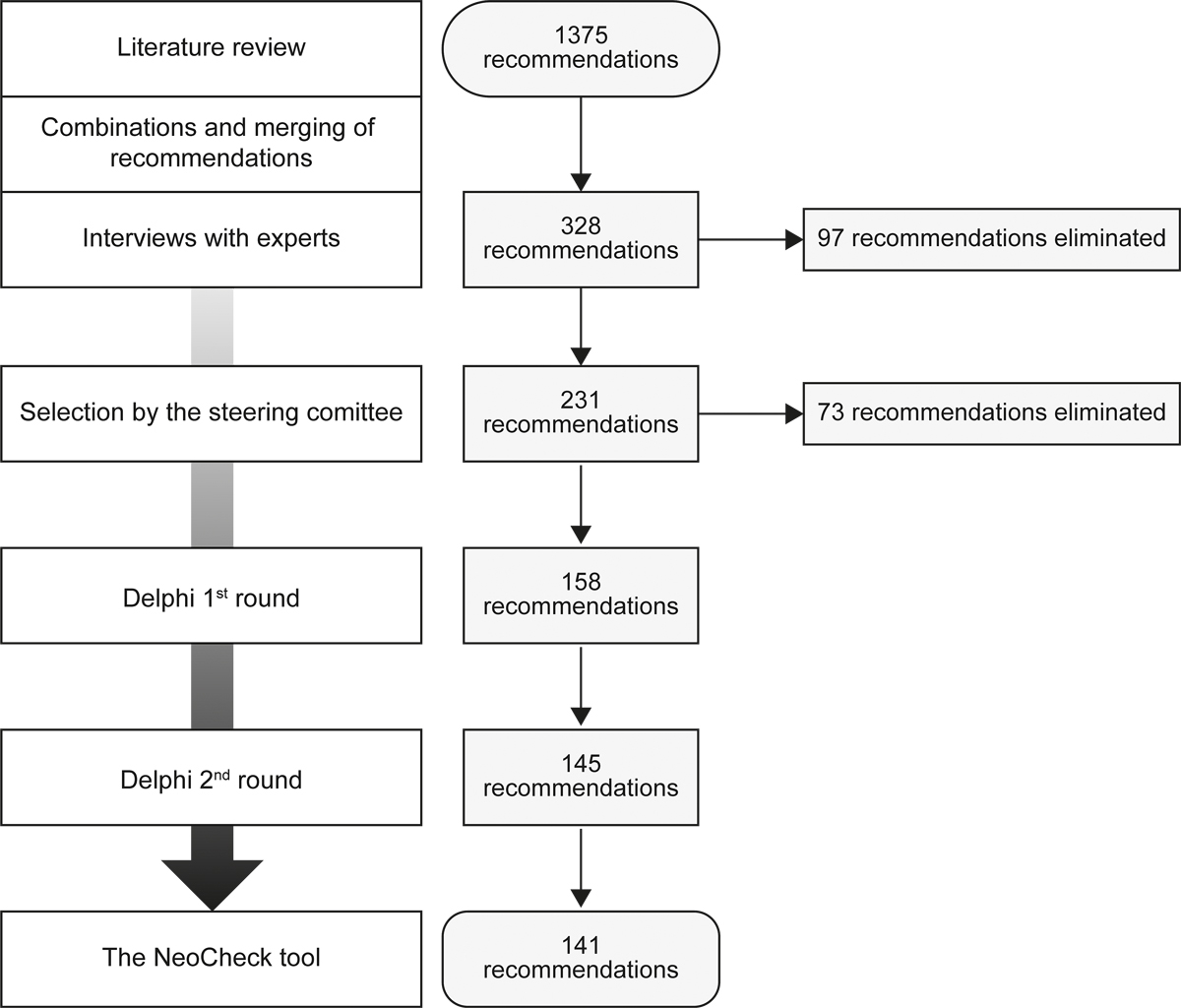
Through the literature review, we identified 1375 recommendations covering 12 themes and 56 sub-themes. Infectiology (29.5%), basic management (11.4%), pneumology and gastroenterology (10.8% each) were the most prevalent themes. The combination of recommendations covering the same subjects yielded a set of 328 recommendations. Ninety-seven of these recommendations were excluded based on interviews with experts, due mainly to irrelevance or incorrect interpretation (74.76%) and moderate usefulness (33.34%). The steering committee eliminated another 73 recommendations, resulting in a final set of 158 recommendations (fig. 1).

Figure 1 Flow of recommendation selection.
Fourteen items had associated GORs (A, n = 5; B, n = 3; D, n = 6). Fifty-seven of the other items were national guidelines, 17 were international guidelines and 15 were institutional guidelines. Table 3 shows GORs and study types for the items included in the modified Delphi process.
Table 3 Distribution of grades of recommendation (GORs) and source types for retained items.
| GOR/source | n (%) |
|---|---|
| GOR identified | 14 (10) |
| A | 5 (4) |
| B | 3 (2) |
| C | 0 (0) |
| D | 6 (4) |
| GOR not identified | 127 (90) |
| Further research needed | 9 (6) |
| Systematic review/meta-analysis | 2 (1) |
| Randomised controlled trial | 2 (1) |
| Cohort study | 1 (1) |
| Case-control study | 0 (0) |
| Descriptive study (case report, case series, cross-sectional, survey) | 0 (0) |
| Review | 12 (9) |
| National guidelines | 57 (40) |
| International guidelines | 17 (12) |
| Institutional guidelines | 15 (11) |
| Textbook | 7 (5) |
| Manufacturer | 2 (1) |
| No reference | 3 (2) |
| Total | 141 (100) |
The first Delphi round was conducted from June 2016 to September 2017, the second round from May to September 2018. Twenty-three experts (22 neonatologists from 10 of 25 Swiss NICUs, 1 clinical pharmacist) filled out questionnaires in the two Delphi rounds. Most (65%) of the experts had >10 years neonatology experience (table 4).
Table 4 Characteristics of participating Swiss experts.
| Expert characteristic | n (%) |
|---|---|
| Total | 23 (100) |
| Sex: female/male | 16 (70) / 7 (30) |
| Profession | |
| MD neonatologist | 22 (96) |
| Clinical pharmacist | 1 (4) |
| City | |
| Geneva | 8 (35) |
| Bern | 1 (4) |
| Aarau | 4 (17) |
| Chur | 1 (4) |
| Luzern | 2 (9) |
| Wintherthur | 2 (9) |
| Lausanne | 2 (9) |
| St Gallen | 2 (9) |
| Zurich | 1 (4) |
| Years of experience in neonatology | |
| <5 | 4 (17) |
| 5–10 | 4 (17) |
| >10 | 15 (65) |
For each item in the first round, the mean response rate was 94% (95% confidence interval [CI] 93–95%] and the smallest number of responses was 15. Thirteen (8%) items were eliminated after this round (appendix 3). Two items that did not reach the agreement threshold were modified according to experts’ comments and included in the second round (appendix 4). For each item in the second round, the mean response rate was 98% (95% CI 97–98%) and the smallest number of responses was 19. Four additional items were eliminated after the second round (appendix 3).
The final PST, named NeoCheck, comprises 141 items on 11 major themes and 49 subthemes (table 5, appendix 5; www.NeoCheck.ch). Ophthalmology themes were eliminated because none of the recommendations was retained. Most (79%) statements concern all neonates, 13% concern only preterm infants and 3% concern only very preterm infants. Some statements contain other age or weight restrictions (appendix 6). (The appendices are available in the PDF version of the manuscript.)
Table 5 The NeoCheck validated statements.
| No. | Main statement |
|---|---|
| Basic management | |
| Body care | |
| 1 | Do not use routinely topical ointments in preterm neonates. |
| 2 | Stop the use of antiseptics for the daily care of the uncomplicated umbilical cord in healthy hospitalised term neonates. |
| Vaccination | |
| 3 | Administer a dose of DTPa-IPV/Hib ± HBV and of pneumococcal vaccine at 60, 90 and 120 days of postnatal life to all hospitalised preterm neonates. |
| 4 | Recommend BCG vaccine at discharge to neonates at high risk of tuberculosis exposure in the first year of life. |
| 5 | Check/administer pertussis vaccination to close contacts of neonates. |
| 6 | Check status and recommend or administer vaccination to close contacts of neonates. |
| Parenteral nutrition | |
| 7 | Start parenteral nutrition shortly after birth in all preterm neonates when it is clear that enteral feeds will not be tolerated soon. |
| 8 | Start reducing the percentage of parenteral nutrition as quickly as possible by the introduction of enteral nutrition until enteral nutrition finally replaces completely parenteral nutrition in order to minimise any side effects from exposure to parenteral nutrition. |
| 9 | Start continuous parenteral glucose administration in preterm infants needing parenteral nutrition. |
| 10 | Start amino acid supply in the first day of life in preterm infant needing parenteral nutrition. |
| 11 | Start continuous lipid emulsion infusion within the first 24–48 hours of life in preterm infant needing parenteral nutrition. |
| 12 | Do not administer parenteral lipid emulsion at a dose higher than 3–4 g/kg/day in neonates. |
| 13 | Check that a sufficient quantity of linoleic acid is administered in all neonates on parenteral nutrition. |
| 14 | Start electrolytes supplementation with parenteral nutrition after onset of diuresis. |
| 15 | Start vitamins and trace element supplementation in neonates receiving parenteral nutrition. |
| 16 | Start vitamin D supplementation from the first days of life in all neonates. |
| Cardiology | |
| Congenital heart disease | |
| 17 | Start alprostadil (prostaglandin E1) as an initial continuous intravenous infusion at 0.01 µg/kg/min until a definitive diagnosis is made in an infant suspected of having ductus-dependant heart disease. |
| 18 | Reassess the indication of alprostadil treatment. |
| 19 | Stop ibuprofen, indomethacin and paracetamol in patients with duct dependent congenital heart disease. |
| Patent ductus arteriosus | |
| 20 | Consider pharmacological closure of confirmed patent ductus arteriosus in preterm neonates after 2 weeks of life, with ibuprofen as first-line treatment. |
| 21 | Reassess the indication of ibuprofen, indomethacin and paracetamol in preterm neonates <2 weeks of life with confirmed or unconfirmed patent ductus arteriosus. |
| 22 | Reassess the indication of ibuprofen, indomethacin and paracetamol in term neonates with patent ductus arteriosus. |
| 23 | Do not use paracetamol as first-line treatment for patent ductus arteriosus closure. Consider a switch to ibuprofen. |
| Hypotension | |
| 24 | Do not use volume expansion as first-line treatment in very low birthweight infants (birthweight <1500g) with hypotension. |
| 25 | Consider a conservative approach (permissive hypotension) for the managment of very low birthweight infants (birthweight <1500g) if the clinical examination is satisfactory in the face of apparent hypotension |
| Haematology | |
| Anaemia | |
| 26 | Do not use routinely erythropoietin to limit exposure to blood transfusions in preterm neonates. The indication of treatment should be reassessed. |
| 27 | Start iron supplement of 2–3 mg/kg/day in all preterm infants fed human milk once full oral feeds have been achieved. |
| Coagulation disorders | |
| 28 | Start oral vitamin K in neonates breastfed by a mother treated with phenprocoumon. |
| 29 | Check in all neonates that complete vitamin K prophylaxis has been given at birth. |
| Thrombocytopenia and platelet dysfunction | |
| 30 | Consider platelet transfusion even in the absence of bleeding in all neonates with a platelet count of <30 × 109/l. |
| 31 | Consider platelet transfusion in neonates with a platelet count of 30–49 × 109/l and minor bleeding or those at risk for major bleeding. |
| 32 | Consider platelet transfusion in neonates with a platelet of count 50–99 × 109/l only if bleeding is present. |
| 33 | Do not transfuse neonates with mild thrombocytopenia (platelet count 100–150 × 109/l) even if bleeding. |
| 34 | Start intravenous immunoglobulin only in case of severe thrombocytopenia (platelet count of <50 × 109/l) or if bleeding persists despite compatible platelet transfusion or in combination with unmatched platelets transfusion in neonates with neonatal allo-immune thrombocytopenia. |
| 35 | Start intravenous immunoglobulin as first-line treatment in neonates with neonatal auto-immune thrombocytopenia and born to mothers who have idiopathic thrombocytopenic purpura, when the platelet count is <30 × 109/l or clinical bleeding is present. |
| Vasospasms and thromboembolism | |
| 36 | Start unfractionned heparin or low molecular weight heparin in neonates with a first event venous thromboembolism and continue for at least 5 days. |
| 37 | Start alteplase or urokinase only in the case of major vessel occlusion causing critical compromise of organs or limbs in infants with venous thromboembolism. |
| Pneumology | |
| Pneumothorax | |
| 38 | Do not routinely use supplemental oxygen in infants with spontaneous pneumothorax. |
| Apnoea of prematurity | |
| 39 | Start caffeine citrate in patients with apnoea of prematurity (loading dose 20 mg/kg; maintenance dose 5 mg/kg/day). Dose may be increased to 10 mg/kg/day if apnoea persists. |
| 40 | Reassess the need for caffeine citrate treatment. |
| 41 | Reassess the indication of anti-gastro-oesophageal reflux therapy in neonates with apnoea. |
| Bronchopulmonary dysplasia (BPD) | |
| 42 | Do not use dexamethasone in the prevention or the treatment of bronchopulmonary dysplasia. |
| 43 | Do not use loop diuretics for prevention of BPD in preterm neonates. |
| 44 | Do not use thiazid diuretics for prevention of BPD in preterm neonates. Use them judiciously for treatment of BPD in preterm neonates. |
| Respiratory distress syndrome (hyaline membrane disease) | |
| 45 | Start surfactant therapy in infants born <26 weeks of gestational age who need fraction of inspired oxygen (FiO2) >0.30. |
| 46 | Start surfactant therapy in infants born ≥26 weeks of gestational age who need FiO2 >0.40. |
| Meconium aspiration syndrome | |
| 47 | Consider inhaled nitric oxide in neonates with hypoxic respiratory failure due to meconium aspiration syndrome. |
| 48 | Reassess the indication for antibiotics in patients with meconium aspiration syndromealone. |
| 49 | Administer a bolus instillation of surfactant in intubated infants with meconium aspiration syndromerequiring more than 50% oxygen. |
| Persistent pulmonary hypertension of the newborn (PPHN) | |
| 50 | Start inhaled nitric oxide in neonates who have severe PPHN. |
| 51 | Do not use sildenafil as initial therapy for PPHN. |
| Nephrology | |
| Acute kidney injury | |
| 52 | Do not use nephrotoxic drugs in neonates if possible, especially in preterm infants. |
| 53 | Stop all nephrotoxic drugs when possible in neonates with acute kidney injury (stage 1–3). |
| 54 | Consider dosage adjustement for drugs highly excreted by renal elimination in neonates with acute kidney injury (stage 1–3). When needed, refer to a specialist. |
| Gastroenterology | |
| Direct hyperbilirubinaemia (conjugated hyperbilirubinaemia) | |
| 55 | Decrease or stop intravenous soybean-based lipid emulsion in neonates with marked progressive cholestasis associated with parenteral nutrition. |
| 56 | Administer adequate protein intake of 2–3 g/kg/day to neonates with direct hyperbilirubinAemia. |
| 57 | Start fat-soluble vitamins (ADEK) in neonates with cholestasis. |
| 58 | Consider ursodeoxycholic acid in neonates with direct hyperbilirubinaemia. |
| Indirect hyperbilirubinaemia (unconjugated hyperbilirubinaemia) | |
| 59 | Administer intravenous immunoglobulin to neonates with a positive direct Coombs test and severe hyperbilirubinaemia, or to those progressing to severe hyperbilirubinaemia despite initial treatment. |
| Necrotising enterocolitis | |
| 60 | Start probiotics in preterm neonates at high risk of developing necrotising enterocolitis. |
| 61 | Stop all enteral medications in neonates suspected to have necrotising enterocolitis. |
| 62 | Do not use enteral antibiotics for the prevention of necrotising enterocolitis. |
| 63 | Start broad spectrum antibiotic promptly after blood cultures have been drawn in neonates with any stage of necrotising enterocolitis. |
| Gastrointestinal bleeding from the upper tract | |
| 64 | Check that vitamin K prophylaxis was administered postdelivery in neonates with upper gastrointestinal bleeding, to guide diagnosis. |
| Gastro-oesophageal reflux | |
| 65 | Consider proton pump inhibitors or H2-blockers only in neonates with severe cases of acid gastro-oesophageal reflux disease, when nonpharmacological measures (including milk thickeners) have failed. |
| 66 | Do not use metoclopramide, domperidone or erythromycin to treat gastro-oesophageal reflux or gastro-oesophageal reflux disease. |
| Neurology | |
| Seizures | |
| 67 | Start phenobarbital as the first-line agent in neonates with either EEG-diagnosed or clinically apparent seizures when prolonged or frequent. |
| 68 | Consider phenytoin or a benzodiazepine or lidocaine in neonates with persistant seizures despite adequate phenobarbital treatment. |
| 69 | Stop antiepileptic drugs if seizure-free for >72 hours in neonates with normal neurological examination and/or normal electroencephalography. |
| 70 | Consider pyridoxine only in neonates with recurrent seizures with no obvious cause. |
| Pain, analgesia and sedation | |
| 71 | Start pain management in neonates with nonpharmacological techniques (including sucrose) if aproppriate. |
| 72 | Start paracetamol in neonates who are still in pain despite adequate nonpharmacological interventions. |
| 73 | Do not use nonsteroidal anti-inflamatory agents as analgesics. |
| 74 | Start morphine as first-line treatment for pain relief in neonates who are still in pain despite adequate nonpharmacological techniques and paracetamol treatment. |
| 75 | Start opioids as first-line treatment for postoperative analgesia, and use them as long as pain assessment scales deem necessary. |
| 76 | Reassess the indication of morphine or fentanyl in chronically ventilated preterm neonates without pain. |
| 77 | Do not use ketamine treatment for routine management of pain. |
| Neonatal abstinence syndrome | |
| 78 | Consider non-pharmacological interventions for the initial management of all infants suspected of having or at risk of developing neonatal abstinence syndrome. This may mitigate the need for medication. |
| 79 | Start morphine as the first-line pharmacological treatment for neonatal abstinence syndromewhen opioids are used by the mother and supportive measures have failed. |
| 80 | Start weaning of morphine as soon as Modified Finnegan scores are <8 for 24–48 hours in neonates with neonatal abstinence syndrome. |
| 81 | Do not use morphine in neonates with neonatal abstinence syndromewhen the drugs used by the mother are not opioids. |
| Infectiology | |
| Meningitis | |
| 82 | Start empirical antibiotic treatment with high dose amoxicillin and gentamicin in neonates with diagnosed or strongly suspected meningitis. |
| 83 | Check results of cerebrospinal fluid culture as soon as they are available in order to reassess the need for treatment or the choice of antibiotics in neonates with suspected meningitis treated with empirical antibiotics. |
| 84 | Do not use corticosteroids for the treatment of neonates with suspected or confirmed bacterial meningitis. Reassess the corticosteroid indication. |
| Sepsis | |
| 85 | Do not use empirical antibiotic therapy for asymptomatic neonates with a single risk factor of infection (including mother with suspected chorioamnionitis or unexplained premature delivery). |
| 86 | Start empirical antibiotic treatment after blood cultures have been drawn in all newborn infants with suggestive signs of neonatal infection. |
| 87 | Reassess the need for antibiotics after 48 hours in neonates treated empirically with antibiotics for suspected sepsis. |
| 88 | Do not use cephalosporins as first-line treatment in infants with suspected neonatal infection, because of the high risk of developing resistance. Use is restricted to special cases |
| 89 | Do not use intravenous immunoglobulin in the treatment of suspected or proven neonatal sepsis. |
| 90 | Do not use vancomycin as prophylaxis against sepsis in preterm neonates. |
| Hepatitis | |
| 91 | Administer an initial dose of hepatitis B vaccine within 12 hours of birth in infants born to HBsAg-positive mothers, including infants weighing <2000g. Administer hepatitis B immune globulin 200 IU concurrently but at a different anatomical site. |
| 92 | Do not use early hepatitis B vaccine in infants born to mothers whose HBsAg and HBeAg status is negative but with positive anti-HBs status (prior infection or at risk of infection). |
| Human immunodeficiency virus (HIV) | |
| 93 | Start HIV prophylaxis with zidovudine as close to birth as possible for at least 4 weeks or consider tritherapy in neonates born to HIV-infected mothers who did not follow proper antenatal treatment or have detectable viraemia. |
| 94 | Start tritherapy immediately in the neonate aged <72 hours if the mother is diagnosed postpartum with HIV infection. |
| Respiratory syncytial virus | |
| 95 | Start respiratory syncytial virus prophylaxis with palivizumab in neonates with severe bronchopulmonary dysplasia. |
| 96 | Start respiratory syncytial virus prophylaxis with palivizumab in neonates with haemodynamically significant congenital heart disease AND other associated risk factors. |
| 97 | Dot not use respiratory syncytial virus prophylaxis with palivizumab routinely in preterm neonates. |
| 98 | Do not use palivizumab for the treatment of respiratory syncytial virus infection. Stop the treatment, even if it was given before the infection. |
| Toxoplasmosis | |
| 99 | Administer a combination of pyrimethamine-sulfadiazine-folinic acid during the first year of life to neonates in whom a diagnosis of congenital toxoplasmosis is confirmed or probable. |
| 100 | Do not use spiramycin in neonates. Stop treatment and screen for potential QT interval prolongation. |
| Cytomegalovirus | |
| 101 | Start antiviral treatment as soon as virologic testing is confirmed and within the first 30 days of life in symptomatic cytomegalovirus-infected newborns with central nervous system involvement or if life threatening. |
| 102 | Stop antiviral treatment in neonates with asymptomatic cytomegalovirus infection. |
| Herpes simplex virus | |
| 103 | Start aciclovir IV in neonates with herpes simplex virus disease, regardless of maternal history or pending laboratory confirmation or exclusion of herpes simplex virus. |
| 104 | Start a topical antiviral treatment in combination with aciclovir IV in neonates with herpes simplex virus disease with ocular involvment. |
| Varicella-zoster virus | |
| 105 | Start varicella-zoster immune globulin 125 IU IM as soon as exposure is known and within a 72-hour period, independent of maternal history of varicella, in neonates born at <28 weeks of gestational age or who weighed <1000g at birth who have been significantly exposed to varicella-zoster virus. |
| 106 | Start varicella-zoster immune globulin 125IU IM in neonates ≥28 weeks gestational age or ≥1000 g birthweight who have been significantly exposed postnatally to varicella-zoster virus, only if born to mother who has no or unkown history of varicella. |
| 107 | Start varicella-zoster immune globulin 125IU IM as soon as possible, after birth or with onset of maternal illness, in term or late preterm neonates whose mother had varicella disease 5 days prior to or 2 days after delivery. |
| 108 | Start aciclovir IV in neonates who develop systemic symptoms or severe cutaneous varicella-zoster disease, or who are at high risk of infection. |
| 109 | Stop varicella-zoster immune globulin if neonatal chickenpox has developed. |
| Chlamydia | |
| 110 | Do not use prophylactic antibiotic treatment in neonates at high risk of chlamydial infection (born to mothers who have untreated chlamydia). |
| 111 | Start erythromycin orally for 14 days in neonates with chlamydial conjunctivitis or with suspected or confirmed chlamydial pneumonia. |
| 112 | Start azithromycin as second-line treatment when erythromycin is not avaliable in neonates with chlamydial conjunctivitis or with suspected or confirmed chlamydial pneumonia. |
| 113 | Stop topical antibiotics for the treatment of chlamydial conjunctivitis in neonates. |
| Gonorrhoea | |
| 114 | Administer one dose of ceftriaxone IV or IM in all neonates born to mothers who have untreated gonorrhoea. |
| 115 | Administer one dose of ceftriaxone IV or IM in neonates with suspected or confirmed gonococcal ophtalmia neonatorum or other localised gonococcal infection. |
| 116 | Stop topical antibiotics in neonates with suspected or confirmed gonococcal ophtalmia neonatorum. |
| 117 | Start ceftriaxone IV or IM in neonates with disseminated gonococcal infection. |
| Methicillin-resistant Staphylococcus aureus infections | |
| 118 | Start vancomycin IV until bacteraemia is excluded for localised methicillin-resistant Staphylococcus aureus disease in preterm or very low-birthweight neonates or in more extensive forms of the disease involving multiple sites in full-term neonates. |
| Syphilis | |
| 119 | Administer benzylpenicillin G IV, OR procaine penicillin to neonates with confirmed or presumed congenital syphilis, or born to syphilis-infected mothers who have not been treated with penicillin at least 4 weeks prior to delivery. |
| 120 | Administer one dose of benzathine penicillin G IM in neonates with normal examination, born to syphilis infected mothers who have been adequately treated during pregnancy more than 4 weeks prior to delivery. |
| Ureaplasma urealyticum infection | |
| 121 | Reassess the use of macrolides or other antibiotics for the treatment of Ureaplasma urealyticum in neonates. |
| Urinary tract infection | |
| 122 | Start empirical antibiotics after urine samples and cultures are collected in neonates with fever when urinary tract infection is suspected. |
| 123 | Consider antibiotic prophylaxis after an urinary tract infection only in neonates with grade IV–V vesico-ureteric reflux. |
| Pertussis | |
| 124 | Start azithromycin oral daily for 5 days in neonates with suspected or confirmed pertussis infection, or in those in close contact with confirmed and contagious cases of pertussis. |
| Tuberculosis | |
| 125 | Start isoniazid prophylaxis orally in neonates born to mothers with tuberculosis, or those in close contact with people with smear-positive pulmonary or laryngeal tuberculosis who have not had at least 2 weeks of anti-tuberculosis treatment. |
| 126 | Start anti-tuberculosis treatment in neonates with congenital tuberculosis or postnatal primary pulmonary tuberculosis. |
| Endocrinology | |
| Metabolic bone disorder | |
| 127 | Administer calcium, phosphate and vitamin D in preterm infants <32 weeks of gestational age or <1500g or infants at risk of metabolic bone disorders. |
| 128 | Administer the maximum recommended doses of calcium, phosphate and vitamin D to prevent fractures in neonates with biochemical features of metabolic bone disease. |
| 129 | Stop steroids and furosemide as soon as possible in neonates at risk of metabolic bone disorder. |
| Thyroid disorders (or hypothyroidism) | |
| 130 | Start levothyroxine immediately in neonates with a thyroid function test that results in either a free T4 a concentration below normal for age or a venous thyroid stimulating hormone concentration >20 mIU/l. |
| Hyperglycaemia | |
| 131 | Decrease glucose intake if necessary and decrease or stop drugs that worsen hyperglycaemia, in neonates with hyperglycaemia. |
| 132 | Start insulin only in patients with persistent hyperglycaemia when other methods of glucose control have failed. |
| 133 | Do not provide high glucose infusion rates to prevent hypoglycaemia in neonates receiving parenteral nutrition. |
| 134 | Do not use early insulin therapy in neonates at risk of hyperglycaemia. |
| Hypoglycaemia | |
| 135 | Start IV glucose infusion in asymptomatic neonates with serum glucose level of <2.6 mmol/l if increased enteral caloric intake is not effective. |
| 136 | Start IV glucose infusion immediately in symptomatic neonates with glucose levels <2.6 mmol/l. |
| Pharmacology | |
| Drugs and breast feeding | |
| 137 | Check the possible milk transfer of drugs taken by mothers to breastfed neonates, and monitor for potential adverse drug effects. |
| Drug-drug interactions | |
| 138 | Check changes in drug effect when initiating strong inhibitors or inducers of cytochrome P450 and/or p-glycoprotein. |
| Various | |
| 139 | Do not use ceftriaxone in neonates who are being, or who have recently been given any IV fluids that contain calcium (such as TPN or Ringer lactate) |
| 140 | Do not use trimethoprim–sulfamethoxazole in neonates. |
| 141 | Check excipients contained in prescribed drug formulations administered orally or parenterally since they can be harmful and responsible for adverse events in neonates, due to immature metabolism. |
The mean response rate for item usefulness was 94% (95% CI 93–95%). No item was deemed to be useless by a majority of experts. On average, the items were deemed essential by 52% of experts, useful by 42% and useless by 6% of experts.
We developed the first PST adapted for neonates. It consists of 141 simple, precise recommendations for the management of typical neonatal situations.
The development of the NeoCheck tool was based on a modified Delphi technique, similar to the methods used for the development of comparable validated tools used in routine practice for other populations, such as the STOPP-START, PIM-Check and POPI criteria [13, 19, 20]. The Delphi method is used commonly for consensus building in healthcare research, including for PST development. The two-round modified Delphi approach used in this study, with a final consensus level of >75% and participation of 23 experts, was comparable to the methods used in most similar studies [21].
Direct comparison of NeoCheck content with that of other PSTs seems irrelevant, given significant differences in targeted populations. Considering practical aspects, NeoCheck appears to be adequate, as it contains a similar number of statements and themes as the PIM-Check tool, but more than the STOPP-START and POPI tools. The assessment of user satisfaction and the average time required for patient treatment review with NeoCheck would be of use to better understand the practicability of NeoCheck use.
The representation in NeoCheck of key research and resources for neonatology practice is a very important aspect of the tool. NeoCheck development was based on expert opinion, which is very low-quality evidence according to the principles of evidence-based medicine [22]. Thus, underlying references and GORs are systematically included in NeoCheck recommendations to increase the tool’s value for users. However, many published guidelines do not include GORs, and the interpretation of those given was sometimes difficult in this study due to pronounced variability among evidence grading scales. Grading guidelines are particularly complex for the interpretation of inconclusive evidence [23]. A main difficulty specific to neonatal research is in long-term morbidity, particularly delay of development and learning ability at school age. The difficulties in targeting relevant short-term and unbiased outcomes certainly have contributed to the limited availability of GORs in this medical speciality (and consequently in NeoCheck). However, the types of studies underlying recommendations are provided and updated systematically in NeoCheck to help users understand recommendation origins and appreciate their clinical weights. A working-group assessment of the GORs using a standard scale, as done for the Beers geriatric PST [24], could be part of NeoCheck updating. All NeoCheck items were judged to be “useful” to “essential” by at least half of the participating experts, supporting the quality of the recommendation selection process and the final tool.
We believe that NeoCheck is useful for the optimisation of drug use in neonatal care, for the training of young physicians and pharmacists, and as a basis for institutional guideline development. In a future prospective study, we will assess the effect of NeoCheck use by a clinical pharmacist in a level-3 NICU on prescription optimisation, as well as user satisfaction. Additional studies are needed to assess the impact of NeoCheck use in the training of young physicians and pharmacists, and its role as a tool for continuous quality of care improvement should be assessed.
This study has some limitations. NeoCheck was developed with experts working in Switzerland; although some of these experts had extensive work experience in other countries, the tool may not be fully suitable for application outside of the Swiss context. This limitation may apply particularly to infectious pathologies, for which antibiotic treatments are adapted according to regional resistance profiles. Immunisation recommendations in NeoCheck were based on Swiss references when available; recommendations for all other themes were generally based on North American sources, as few national guidelines for neonatal clinical management have been published in Switzerland. The scientific societies and hospital guidelines systematically consulted were selected to ensure representation of local, national and international practices. Internationally, North American scientific societies have been selected for their wide range of recommendations, and Australian and New Zealand hospitals because they were structured open-access guidelines commonly used by the authors. The search for recommendations was not limited to these sources, and guidelines from other scientific societies and hospitals were included during the literature review. We believe that the NeoCheck tool is suitable for use in most high-income countries. As NeoCheck was based on scientific knowledge that was current in 2016, it needs to be regularly updated to maintain relevance. A literature review every 5-6 years is planned in order to identify any new recommendation or changes in the current recommendations. Modifications can also be made to the items in the event of a significant change in the literature. Any change or creation of an item would be validated with the same modified Delphi method described above.
The NeoCheck tool provides possible attitudes drawn from existing recommendations and deemed correct by a panel of experts. This tool should not be considered as a textbook, presenting national or international recommendations.
NeoCheck is the first PST developed to optimise neonatal prescription. It contains 141 recommendations that should help young physicians and clinical pharmacists review patient treatments in daily practice based on up-to-date medical knowledge. Because of the complexity of neonatal drug prescription, NeoCheck use could improve neonatal pharmacotherapy and care quality.
The following appendices are provided in the PDF version of the article:
Appendix 1: Themes and subthemes included in the literature review
Appendix 2: Questionnaire design
Appendix 3: Items eliminated in the first and second Delphi rounds
Appendix 4: Items modified after the first Delphi round according to experts’ comments
Appendix 5: Validated NeoCheck items
Appendix 6: Age/weight restrictions for NeoCheck items
We would like to thank Peter Rimensberger, who accepted the involvement of his medical team in this project. We thank the paediatricians at the Geneva University Hospitals who took part in interviews during recommendation selection: Klara Posfay-Barbe, Paloma Parvex and Alexandra Wilhelm-Bals. We thank Majed Al-Sweidi for English correction of the NeoCheck items, and Write Science Right for manuscript editing. Finally, we thank the experts who participated in Delphi validation: RP (Geneva University Hospitals), Barthélémy Tosello (Geneva University Hospitals), Sébastien Fau (Geneva University Hospitals), Francisca Barcos-Munoz (Geneva University Hospitals), Marie Saint-Faust (Geneva University Hospitals), Jane McDougall (Inselspital Bern), Micheal Kleber (Kantonsspital Winterthur), Thomas Berger (Kantonsspital Luzern), David Palmero (Lausanne University Hospital), Olivier Baud (Geneva University Hospitals), Katrin Held-Egli (Kantonsspital Aarau), Gabriel Konetzny (Kantonsspital Aarau), Antonio Leone (Kantonsspital Winterthur), Andreas Malzacher (Ostschweizer Kinderspital), Corinne Daester (Kantonsspital Aarau), Rodgo Bjarte (Ostschweizer Kinderspital), Gihane El-Gowhari (Hôpital de la Tour), Philipp Meyer (Kantonsspital Aarau), Vincent Muelethaler (Lausanne University Hospital), Hans Ulrich Bucher (Zurich University Hospital), Thomas Riedel (Kantonsspital Graubünden) and Matteo Fontana (Kantonsspital Luzern).
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The authors declare that they have no conflict of interest.
1 Cuzzolin L , Agostino R . Off-label and unlicensed drug treatments in Neonatal Intensive Care Units: an Italian multicentre study. Eur J Clin Pharmacol. 2016;72(1):117–23 .https://doi.org/10.1007/s00228-015-1962-4
2 de Souza AS, Jr , Dos Santos DB , Rey LC , Medeiros MG , Vieira MG , Coelho HLL . Off-label use and harmful potential of drugs in a NICU in Brazil: A descriptive study. BMC Pediatr. 2016;16(1):13 .https://doi.org/10.1186/s12887-016-0551-8
3 Riou S , Plaisant F , Maucort Boulch D , Kassai B , Claris O , Nguyen KA . Unlicensed and off-label drug use: a prospective study in French NICU. Acta Paediatr. 2015;104(5):e228–31 .https://doi.org/10.1111/apa.12924
4 Magalhães J , Rodrigues AT , Roque F , Figueiras A , Falcão A , Herdeiro MT . Use of off-label and unlicenced drugs in hospitalised paediatric patients: a systematic review. Eur J Clin Pharmacol. 2015;71(1):1–13 .https://doi.org/10.1007/s00228-014-1768-9
5 Di Paolo ER , Stoetter H , Cotting J , Frey P , Gehri M , Beck-Popovic M , et al. Unlicensed and off-label drug use in a Swiss paediatric university hospital. Swiss Med Wkly. 2006;136(13-14):218–22.
6 Joseph PD , Craig JC , Caldwell PHY . Clinical trials in children. Br J Clin Pharmacol. 2015;79(3):357–69 .https://doi.org/10.1111/bcp.12305
7 Krzyzaniak N , Bajorek B . Medication safety in neonatal care: a review of medication errors among neonates. Ther Adv Drug Saf. 2016;7(3):102–19 .https://doi.org/10.1177/2042098616642231
8 Lenclen R . Les erreurs de prescriptions en néonatologie: incidence, types d’ erreurs, détection et prévention [Medication errors in neonatology: a review]. Arch Pediatr. 2007;14(Suppl 1):S71–7 .https://doi.org/10.1016/S0929-693X(07)80015-7
9 Machado APC , Tomich CSF , Osme SF , Ferreira DM de LM , Mendonça MAO , Pinto RMC , et al. Prescribing errors in a Brazilian neonatal intensive care unit. Cad Saude Publica. 2015;31(12):2610–20 .https://doi.org/10.1590/0102-311X00194714
10 Kaushal R , Bates DW , Landrigan C , McKenna KJ , Clapp MD , Federico F , et al. Medication errors and adverse drug events in pediatric inpatients. JAMA. 2001;285(16):2114–20 .https://doi.org/10.1001/jama.285.16.2114
11 Gallagher PF , O’Connor MN , O’Mahony D . Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther. 2011;89(6):845–54 .https://doi.org/10.1038/clpt.2011.44
12 Hill-Taylor B , Walsh KA , Stewart S , Hayden J , Byrne S , Sketris IS . Effectiveness of the STOPP/START (Screening Tool of Older Persons’ potentially inappropriate Prescriptions/Screening Tool to Alert doctors to the Right Treatment) criteria: systematic review and meta-analysis of randomized controlled studies. J Clin Pharm Ther. 2016;41(2):158–69 .https://doi.org/10.1111/jcpt.12372
13 Desnoyer A , Blanc AL , Pourcher V , Besson M , Fonzo-Christe C , Desmeules J , et al. PIM-Check: development of an international prescription-screening checklist designed by a Delphi method for internal medicine patients. BMJ Open. 2017;7(7):e016070 .https://doi.org/10.1136/bmjopen-2017-016070
14 Berthe-Aucejo A , Nguyen PKH , Angoulvant F , Bellettre X , Albaret P , Weil T , et al. Retrospective study of irrational prescribing in French paediatric hospital: prevalence of inappropriate prescription detected by Pediatrics: Omission of Prescription and Inappropriate prescription (POPI) in the emergency unit and in the ambulatory setting. BMJ Open. 2019;9(3):e019186 .https://doi.org/10.1136/bmjopen-2017-019186
15 Blanc AL , Guignard B , Desnoyer A , Grosgurin O , Marti C , Samer C , et al. Prevention of potentially inappropriate medication in internal medicine patients: A prospective study using the electronic application PIM-Check. J Clin Pharm Ther. 2018;43(6):860–6 .https://doi.org/10.1111/jcpt.12733
16 Hamilton H , Gallagher P , Ryan C , Byrne S , O’Mahony D . Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patients. Arch Intern Med. 2011;171(11):1013–9 .https://doi.org/10.1001/archinternmed.2011.215
17Gommela TL, Cunningham MD, Eyal F. Neonatology: management, procedures, on-call problems, diseases and drugs. 6th edition. New York: McGraw-Hill; 2009.
18 Harbour R , Miller J . A new system for grading recommendations in evidence based guidelines. BMJ. 2001;323(7308):334–6. doi:.https://doi.org/10.1136/bmj.323.7308.334
19 O’Mahony D , O’Sullivan D , Byrne S , O’Connor MN , Ryan C , Gallagher P . STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213–8 .https://doi.org/10.1093/ageing/afu145
20 Prot-Labarthe S , Vercheval C , Angoulvant F , Brion F , Bourdon O . « POPI; pédiatrie : omissions et prescriptions inappropriées ». Outil d’identification des prescriptions inappropriées chez l’enfant [POPI: a tool to identify potentially inappropriate prescribing practices for children]. Arch Pediatr. 2011;18(11):1231–2 .https://doi.org/10.1016/j.arcped.2011.08.019
21 Boulkedid R , Abdoul H , Loustau M , Sibony O , Alberti C . Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLoS One. 2011;6(6):e20476 .https://doi.org/10.1371/journal.pone.0020476
22 Guyatt GH , Oxman AD , Vist GE , Kunz R , Falck-Ytter Y , Alonso-Coello P , et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Chinese J Evidence-Based Med. 2009;9(1):8–11.
23 Uhlig K , Balk EM , Lau J . Grading evidence-based guidelines--what are the issues? Am J Kidney Dis. 2008;52(2):211–5 .https://doi.org/10.1053/j.ajkd.2008.06.002
24 American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616–31 .https://doi.org/10.1111/j.1532-5415.2012.03923.x