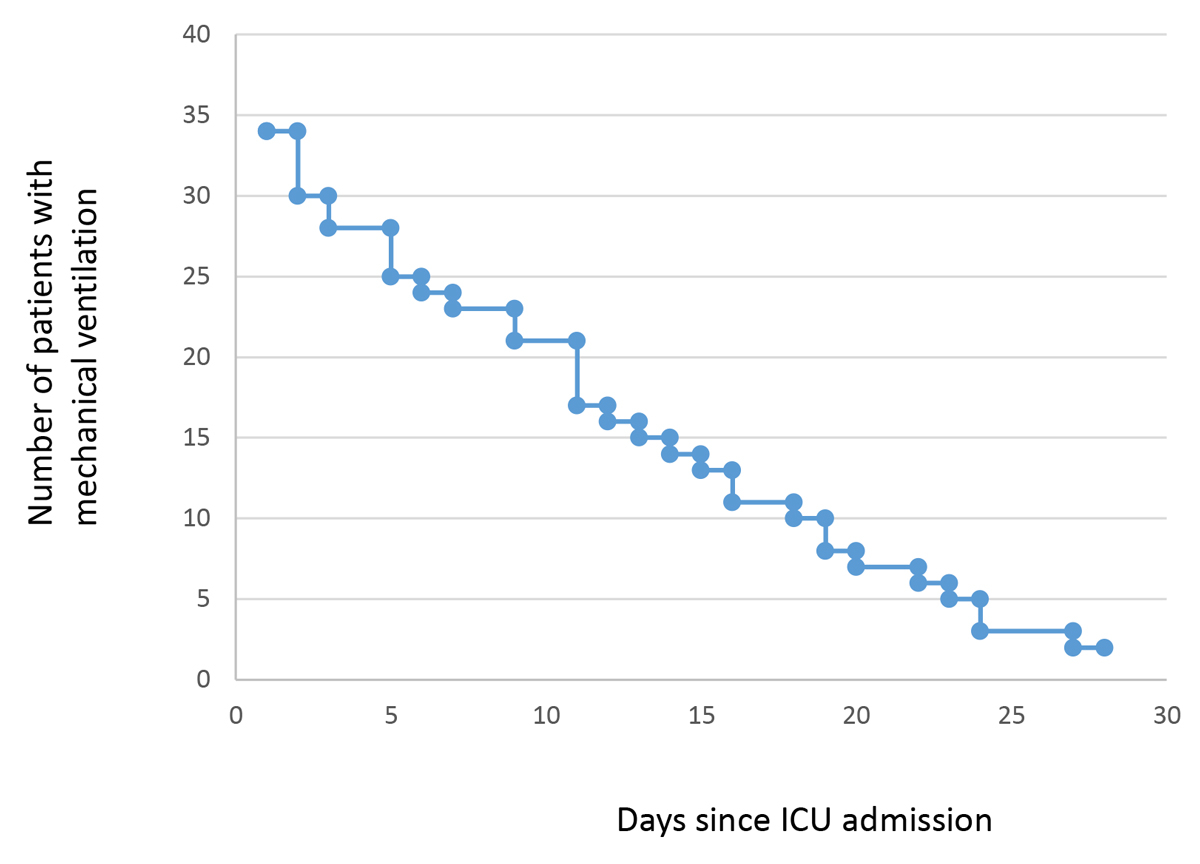
Figure 1 Length of mechanical ventilation. ICU = Intensive Care Unit.
DOI: https://doi.org/10.4414/smw.2021.20529
As of August 2020, more than 25 million individuals had been infected worldwide with SARS-CoV-2, and more than 800,000 patients had died from this infection. Risk factors for the development of severe disease (COVID-19) and death include on the one hand older age and comorbidities such as diabetes mellitus or coronary heart diseases, and on the other hand severe inflammation and coagulation abnormalities [1–3]. Reported hospital mortality rates due to COVID-19 range between 30% and 80% in critically ill patients [4–8]. Lausanne University Hospital Intensive Care Unit (ICU), much harder hit than our ICU, reported a 29% mortality rate in mechanically ventilated COVID-19 patients [9]. A number of experimental therapies have been proposed, but only a few have demonstrated positive results [10, 11]. Staffing, availability of drugs and equipment, and the rate of admission are likely to influence course and outcome of the disease [12–14]. Here we report a very low mortality rate of patients treated in a tertiary university ICU in Switzerland. We hypothesise that the sufficient availability of well-trained ICU staff, drugs and special equipment, together with the best standard of ICU supportive care, has been one of the main reasons for the high survival rate observed.
All patients with COVID-19 admitted to the Bern University Hospital ICU between 2 March and 20 May, the first wave of COVID-19 in Switzerland, were included in the study. This descriptive study was approved by the local Ethics Committee (# 2020-00638), and informed consent was waved owing to its descriptive nature. Some patients were also included in other COVID-projects (NCT04510012 and physical therapy interventions).
The ICU at the Bern University Hospital treats adult patients and serves as primary, secondary and tertiary ICU for a recruiting region of around 1 million inhabitants. The hospital has approximately 900 beds, and the ICU has 39 beds to which are affiliated additional 16 intermediate care beds. On average, the personnel coverage per bed is 5.4 nurses (physicians: 1.0), which allows for a 1:1 nurse/patient ratio. The percentage of certified critical care nurses is 60% and the percentage of physicians with an FMH designation of intensive care specialist is 50%. The management strategies for COVID-19 patients in the hospital were defined and updated during weekly face-to-face interprofessional meetings with specialists in intensive care (physicians, critical care nurses), infectious diseases, pneumology, internal medicine, rheumatology and emergency medicine. This hospital expert group decided at the beginning of the first wave against off-label experimental antiviral or anti-inflammatory treatment outside randomised clinical trials (e.g., World Health Organization Solidarity Trial). Treatments including hydroxychloroquine, ritonavir-boosted lopinavir, remdesivir, or tocilizumab were thus not prescribed and even stopped at ICU admission when patients were referred from other hospital with such treatments, with the exception of one first patient who received remdesivir for 4 days at the beginning of the first epidemic wave.
The main reasons for ICU admission were respiratory failure, defined as the presence of one of the following criteria: respiratory rate >30/min and clinical signs of respiratory distress, hypoxaemia defined as oxygen saturation (SpO2 ) <90% despite O2 supply >6 l/min, lactic acidosis or shock. All SARS-CoV-2-infected patients who qualified for ICU care using the standard admission criteria at our institution were treated in the ICU. Triage was performed round the clock by a certified ICU physician in agreement with the physician in charge in the emergency room (ER) or on the ward. Patients with respiratory failure were immediately intubated without any noninvasive ventilation or high-flow oxygen attempt. We used the oesophageal pressure to calculate transpulmonary pressure (Ptp). The goal was to achieve end-expiratory values slightly above 0 mm Hg, and driving pressures ≤15 mm Hg with tidal values ≤6 ml/kg bodyweight, in order to keep plateau airway pressure <30 mm Hg. Moreover, patients with acute respiratory distress syndrome (ARDS) were turned to prone positions for 16 hours/day as long as paO2/FiO2 (ratio of arterial oxygen partial pressure to fractional inspired oxygen) increased with protective ventilator settings using oesophageal catheters for transpulmonary pressure monitoring. Sedation was initially performed with propofol (or midalzolam for haemodynamically unstable patients) and fentanyl continuous infusions. Later, dexmedetomidine or intermittent sedation with benzodiazepine or neuroleptic drugs were used. Muscle relaxation was applied during the first 48 hours, longer if needed to ensure protective lung ventilation, and always during the prone position.
During the study period corresponding to the first epidemic wave in Switzerland, i.e., before the publication of the Recovery trial [10], steroids were not administered routinely, but only in the absence of bacterial superinfection to patients with late unresolving ARDS according to the protocol recently published [15]. Antibiotics or antifungals were prescribed only if strong evidence of superinfection was present. Required criteria were fever, new or increasingly dense lung infiltrates in chest X-ray or computed tomography (CT)-scan, increasing C-reactive protein (CRP), and procalcitonin. In primarily immunocompromised patients, antibiotic administration was preceded by a broncho-alveolar lavage performed by a pneumologist, or at least by a blind tracheal aspiration, for microbiology diagnostic purposes. Fungal superinfection was also screened for by culture and serology. Extracorporeal membrane oxygenation (ECMO) was used during the early stay in the ICU if paO2/FiO2 was <50 mm Hg in patients not responding to optimised ventilator settings, recruitment and negative fluid balance. Negative fluid balance was attempted by furosemide infusion, intermittent dialysis or continuous veno-venous haemodiafiltration (CVVHDF). Heart function was monitored by frequent echocardiography. Immediately after ICU admission and during the first phase of the therapy, all patients received a pulmonary artery catheter to monitor the effects of ventilator settings, prone positioning and haemodynamic management, especially aggressive negative fluid balance, on oxygen delivery. High-dose prophylactic anticoagulation using unfractionated heparin was prescribed in all patients to reach an anti-factor Xa activity between 0.25 and 0.35 IU/l. Enteral nutrition was administered according to a local protocol, with the aim of delivering 75% of required calories within the first 72 h after ICU admission. The course of each patient was analysed and discussed with infectious disease specialists daily; physiotherapists were available during the daytime, 7 days a week. We employed an early mobilisation regimen; physiotherapists and advanced practice nurses assisted in turning the patients. Patient data were collected, recorded and stored in a clinical information system (Centricity® Critical Care 8.1 SP10, GE Health Care, Barrington, IL, USA). The system stores 2-minute medians for artefact removal. All descriptive analyses of the described variables were performed using SPSS Statistics 26. Frequencies were indicated as numbers and %, and continuous variables as median and range.
Between 2 March and 20 May, 42 patients with COVID-19 were treated in the Bern University Hospital Intensive Care Unit. Median age was 61 years (range 32–86), most of them were males (81%). The most frequent risk factors for severe SARS-CoV-2 infection reported were arterial hypertension (n = 12, 29%) and diabetes (n = 12, 29%). Simplified acute physiology score (SAPS-II) during the first 24 h of ICU stay was 46 (13–90). Overall, 79% of the patients were mechanically ventilated (3 of them on ECMO) (fig. 1), 31% were under renal replacement therapy, and 21% received steroids. All patients were fully anticoagulated from time of admission. No off-label experimental antiviral or anti-inflammatory drugs were used with the exception of one patient, and antibiotic prescription was restrictive.

Figure 1 Length of mechanical ventilation. ICU = Intensive Care Unit.
The time course of disease severity and interventions are displayed in the table 1.
Table 1 Time course of disease severity and therapeutic interventions in the ICU.
|
ICU Day 1
n in ICU (d1) = 42 |
Day 2-3
n in ICU (d2) = 35 |
Day 4-7
n in ICU (d4) = 30 |
Day 8-14
n in ICU (d8) = 25 |
Day 15-21
n in ICU (d15) = 16 |
Day 21-28
n in ICU (d21) = 7 |
|
|---|---|---|---|---|---|---|
| Continuous sedation / analgesia / muscle relaxation | ||||||
| – Analgesia (n) | 30 | 29 | 26 | 24 | 10 | 6 |
| – Sedation (n) | 27 | 28 | 24 | 17 | 4 | 5 |
| – Relaxation (n) | 22 | 22 | 16 | 11 | 1 | 1 |
| Max / min RASS (median) | 0 / –4.5 | 0 / –5 | 1 / –5 | 1 / –4 | 1 / –2 | 1 / –2 |
| Mechanical ventilation | ||||||
| – Mechanical ventilation (n) | 34 | 30 | 28 | 23 | 13 | 6 |
| – Highest PEEP (cm H2O) | 14 (6–20) | 14 (5–28) | 14 (5–28) | 11 (5–30) | 8 (5–30) | 5 (4–27) |
| – Min compliance (ml / cm H2O) | 38 (15–71) | 38 (15–68) | 37 (4–75) | 36 (4–64) | 23 (10–55) | 34 (34–34) |
| Prone position (n) | 9 | 12 | 10 | 7 | 1 | 0 |
| Dialysis | ||||||
| – Continuous (n) | 2 | 5 | 6 | 8 | 3 | 1 |
| – Intermittent (n) | 0 | 0 | 3 | 7 | 3 | 2 |
| Catecholamines | ||||||
| – Noradrenaline (n) | 19 | 23 | 17 | 11 | 6 | 2 |
| – Dobutamine (n) | 2 | 5 | 2 | 3 | 1 | 0 |
| Lowest CO (l/min) | 5.8 (3.3–8.8) | 5.7 (3.4–8.8) | 5 (3.9–8.7) | 5.6 (3.2–9.3) | NA | NA |
| Lowest SvO2 (%) | 67 (36–76) | 62 (43–90) | 64 (40–88) | 60 (47–88) | NA | NA |
| Anticoagulation | ||||||
| Anti-Xa-UFH | 0.39 (0.16–0.69) | 0.28 (0.1–0.92) | 0.33 (0.11–2.25) | 0.39 (0.15–2.40) | 0.40 (0.10–2.90) | 0.36 (0.14–0.57) |
| Nutrition | ||||||
| – Calories / day | 160 (20–650) | 770 (10–2410) | 1910 (140–3210) | 2000 (110–5030) | 1960 (60–3810) | 1930 (30–2860) |
| Protein (g/d) | 10 (0–30) | 50 (0–110) | 90 (0–150) | 100 (90–260) | 100 (0–190) | 100 (30–140) |
| Inflammation/superinfection | ||||||
| – CRP (mg/l) | 130 (15–436) | 184.5 (16–498) | 170 (13–440) | 98 (9–433) | 73 (4–221) | 66 (9–196) |
| – Antibiotics (n) | 18 | 20 | 16 | 10 | 5 | 3 |
| – Antifungals (n) | 1 | 1 | 1 | 2 | 0 | 0 |
| Steroids (n) | 3 | 6 | 4 | 4 | 3 | 3 |
CO = cardiac output; CRP = C-reactive protein; ICU = intensive care unit; PEEP = positive end-expiratory pressure ; RASS = Richmond agitation sedation scale; SvO2 = mixed venous oxygen saturation All values are median (min-max), unless otherwise indicated.
Maximum neutrophil/lymphocyte ratio was 7.1 (0.9–35.0), maximum D-dimers were 2475 µg/l (559–79,632).
The nurse-to-patient ratio was 1:1 during all shifts, and the physician-to-patient ratio was 1:4 (day shift) and 1:10 (night shift). Infectious disease specialists and physiotherapists were present every day. Therapeutic Intervention Scoring System (TISS) 28 points per admission increased compared with March-May 2019 (248 vs 202 points), whereas TISS 28 points per full time equivalent (FTE) did not, since the total number of admissions decreased compared with March-May 2019, and the FTE/bed could be maintained.
Median length of ICU stay was 10 days (1–38) (fig. 2). We transferred 22 patients to internal medicine and pneumology, 11 patients to the ICU of their respective hospital region after clinical improvement, 4 patients to an external ward, and 2 patients to neighbouring countries. ICU and hospital mortality were 7% and 12%, respectively (fig. 3).
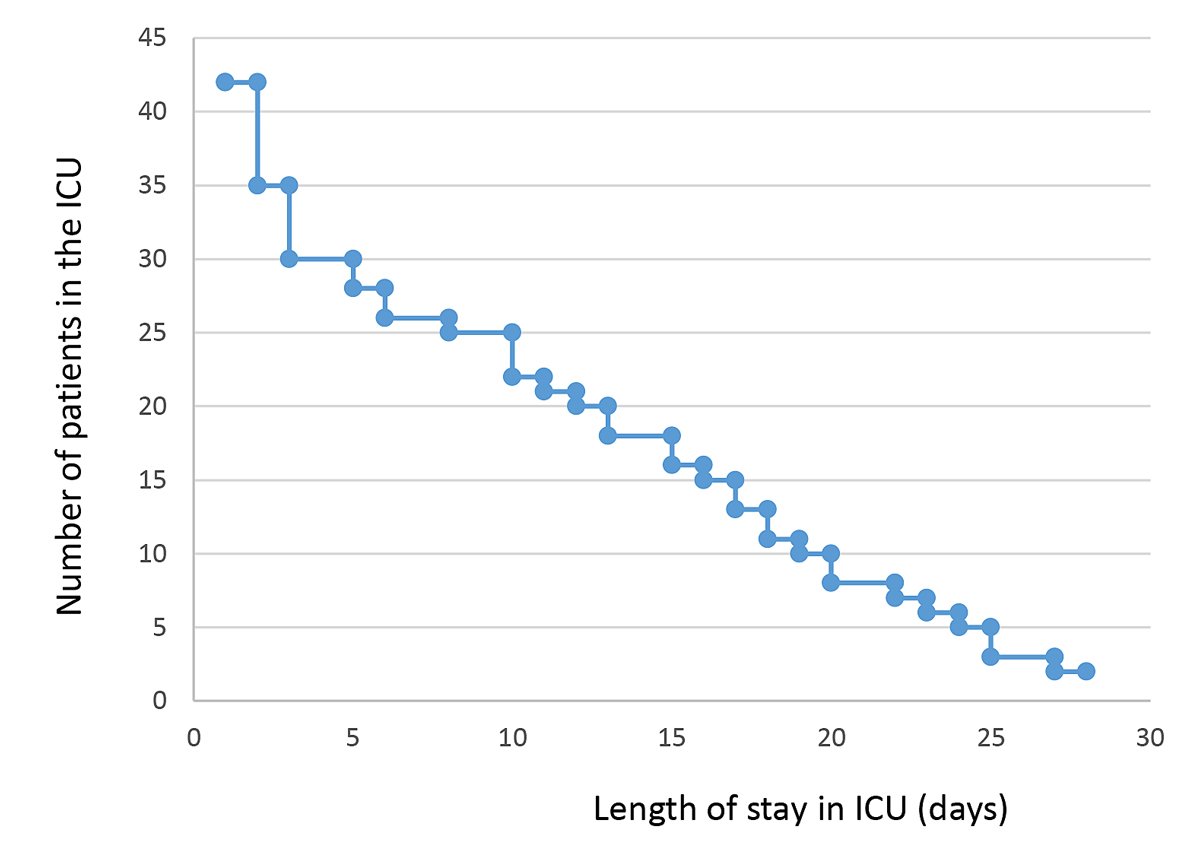
Figure 2 Length of intensive care unit (ICU) stay.
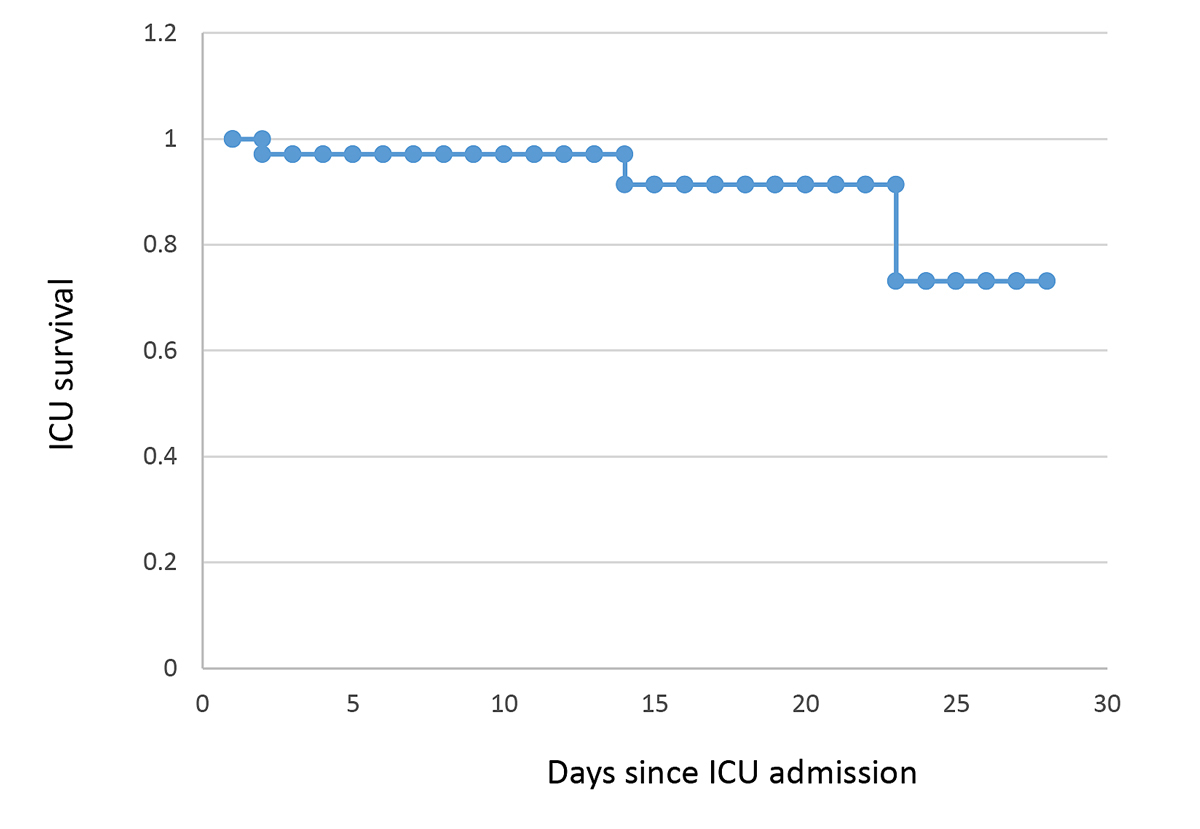
Figure 3 Survival rate in intensive care unit (ICU).
During the first epidemic SARS-CoV-2 wave, we observed a relatively low mortality among the 42 patients with COVID-19 admitted to the Bern University Hospital ICU. A low mortality was seen despite patients being severely affected with high neutrophil/lymphocyte ratios and D-dimers. The average SAPS-II scores, and signs of inflammation and coagulation disturbances were comparable to those of other studies with a higher mortality [4, 5], despite withholding experimental antiviral or anti-inflammatory therapies. Here, treatment focused on the best current critical care practices, including appropriate ventilator settings, as little fluid as possible, rapid administration of antibiotics when bacterial superinfection was suspected, early enteral nutrition, adequate dialysis, frequent sedation stops and early mobilisation. Only a small proportion of patients received steroids, as it was not clear at that time whether this treatment would be beneficial. However, we report a higher rate of renal replacement therapy than others [16]. We cannot exclude that in some patients the negative fluid balance achieved contributed to acute renal failure. Furthermore, sedation and weaning were difficult and much higher doses of sedatives and opioids were needed in COVID-19 patients compared with non-COVID-19 ARDS patients, especially to treat the exaggerated respiratory drive and to avoid self-inflicted lung injury. This situation was linked with an increase nurse workload.
One of the most likely explanations for the relatively low mortality rate we observed might lie in the allocated numbers of nurses and physicians per bed, which were both relatively high compared with European and many North- and South-American ICUs standards [11, 12]. Similar low mortality rates have also been reported by others, mostly when nurse to patient ratios were close to 1:1, when overwhelming admissions of critically ill patients with COVID-19 were avoided, and when sufficient well trained ICU specialists, as well as protective equipment, were available [17, 18]. However, this single centre observation is to be validated in future studies, for example by comparing the outcome of COVID-19 patients between ICUs according to personal staffing and caseload.
In addition, the recruiting area of the Bern ICU was relatively sparse during the first epidemic wave (maximum incidence around 75/100,000 inhabitants/14 days) compared with western Switzerland (maximum incidence around 525, 360, 280/100,000 inhabitants/14 days for Geneva, Vaud and Wallis, respectively) or south Switzerland (maximum incidence around 475/100,000 inhabitants/14 days). We never had to limit ICU admissions and were always able to adapt the treatment strategy on a daily and individual-patient basis together with infectious disease specialists. At the most, we had 15 patients with COVID-19 simultaneously present in our ICU. As the elective surgery programme had been stopped, we were not forced to open ICU beds outside the ICU. The high standard of the Swiss Society of Intensive Care Medicine regarding personnel assignment and personnel training could thus be fulfilled over the whole period [19].
These two observations call for a regional and/or national coordination of ICU admissions of patients with COVID-19 to minimise the risk of ICU overload and to secure adequate staffing with specialist nurses and doctors in ICUs. These high standards are resource consuming and certainly difficult to fulfil when it comes to open temporary beds outside of the ICU. Additionally, questions such as what are the criteria of ICU overload that should trigger the search of mutual aid for patient transfers, what is the minimum ICU or ICU-equivalent training required for the staff who will run the expanded beds, what are the minimum requirements of these newly open beds in terms of nurse and physician ratio to insure the sufficient, if not the best, quality of care to the admitted patients, still remain to be answered.
The experience from the first wave triggered some improvement for the second epidemic wave. First, we observed an increased cooperation between the different Swiss ICUs, which was even nationwide coordinated. Second, our critical care practices remained mostly unchanged, but we improved and better structured our communication and teaching strategies, with online and face-to-face workshops, as well as introduction curricula for new nurses or medical personnel, which include, among others, training on mechanical ventilation, on prone positioning procedures, infection control measures, etc. We further actively promoted partnership/tandem between critical care nurses and other healthcare professionals. Finally we offered free meals to all personnel working at the bedside.
From this experience, we do confirm that medical professionals who do not regularly work on an ICU require special attention. We believe that a “hospital wide strategy” is needed to make a pool of personnel who are regularly trained in intensive care readily available; these staff could then rapidly be deployed when needed as the next epidemics occur. We are currently in the process of implementing such a strategy at our ICU.
In conclusion, we believe that adequate resources (well-trained ICU staff, available beds, ventilators, other equipment and drugs) and an intensive interdisciplinary approach helped us to keep mortality low during the first epidemic wave despite a high degree of organ failure in our patients.
Available upon reasonable request.
Funding provided by the Department of Intensive Care Medicine, University Hospital Bern (Inselspital), Switzerland.
No potential conflict of interest relevant to this article was reported
1 van Gerwen M , Alsen M , Little C , Barlow J , Genden E , Naymagon L , et al. Risk factors and outcomes of COVID-19 in New York City; a retrospective cohort study. J Med Virol. 2021;93(2):907–15. doi:.https://doi.org/10.1002/jmv.26337
2 Kim L , Garg S , O’Halloran A , Whitaker M , Pham H , Anderson EJ , et al. Risk Factors for Intensive Care Unit Admission and In-hospital Mortality among Hospitalized Adults Identified through the U.S. Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET). Clin Infect Dis. 2021;72(9):e206–14. doi:. https://doi.org/10.1093/cid/ciaa1012
3 Piano S , Dalbeni A , Vettore E , Benfaremo D , Mattioli M , Gambino CG , et al.; COVID-LIVER study group. Abnormal liver function tests predict transfer to intensive care unit and death in COVID-19. Liver Int. 2020;40(10):2394–406. doi:.https://doi.org/10.1111/liv.14565
4 Gupta S , Hayek SS , Wang W , Chan L , Mathews KS , Melamed ML , et al.; STOP-COVID Investigators. Factors Associated With Death in Critically Ill Patients With Coronavirus Disease 2019 in the US. JAMA Intern Med. 2020;180(11):1436–47. doi:.https://doi.org/10.1001/jamainternmed.2020.3596
5 Grasselli G , Greco M , Zanella A , Albano G , Antonelli M , Bellani G , et al.; COVID-19 Lombardy ICU Network. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345–55. doi:.https://doi.org/10.1001/jamainternmed.2020.3539
6 Zou X , Li S , Fang M , Hu M , Bian Y , Ling J , et al. Acute Physiology and Chronic Health Evaluation II Score as a Predictor of Hospital Mortality in Patients of Coronavirus Disease 2019. Crit Care Med. 2020;48(8):e657–65. doi:.https://doi.org/10.1097/CCM.0000000000004411
7 Lim ZJ , Subramaniam A , Ponnapa Reddy M , Blecher G , Kadam U , Afroz A , et al. Case Fatality Rates for Patients with COVID-19 Requiring Invasive Mechanical Ventilation. A Meta-analysis. Am J Respir Crit Care Med. 2021;203(1):54–66. doi:.https://doi.org/10.1164/rccm.202006-2405OC
8 COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47(1):60–73. doi:.https://doi.org/10.1007/s00134-020-06294-x
9 Regina J , Papadimitriou-Olivgeris M , Burger R , Le Pogam MA , Niemi T , Filippidis P , et al. Epidemiology, risk factors and clinical course of SARS-CoV-2 infected patients in a Swiss university hospital: An observational retrospective study. PLoS One. 2020;15(11):e0240781. doi:.https://doi.org/10.1371/journal.pone.0240781
10 Horby P , Lim WS , Emberson JR , Mafham M , Bell JL , Linsell L , et al. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med. 2021;384(8):693–704. doi:. https://doi.org/10.1056/NEJMoa2021436
11 Wang Y , Zhang D , Du G , Du R , Zhao J , Jin Y , et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–78. doi:.https://doi.org/10.1016/S0140-6736(20)31022-9
12 Neuraz A , Guérin C , Payet C , Polazzi S , Aubrun F , Dailler F , et al. Patient mortality is associated with staff resources and workload in the ICU: a multicenter observational study. Crit Care Med. 2015;43(8):1587–94. doi:.https://doi.org/10.1097/CCM.0000000000001015
13 Bakhru RN , McWilliams DJ , Wiebe DJ , Spuhler VJ , Schweickert WD . Intensive Care Unit Structure Variation and Implications for Early Mobilization Practices. An International Survey. Ann Am Thorac Soc. 2016;13(9):1527–37. doi:.https://doi.org/10.1513/AnnalsATS.201601-078OC
14 Sakr Y , Moreira CL , Rhodes A , Ferguson ND , Kleinpell R , Pickkers P , et al.; Extended Prevalence of Infection in Intensive Care Study Investigators. The impact of hospital and ICU organizational factors on outcome in critically ill patients: results from the Extended Prevalence of Infection in Intensive Care study. Crit Care Med. 2015;43(3):519–26. doi:.https://doi.org/10.1097/CCM.0000000000000754
15 Meduri GU , Siemieniuk RAC , Ness RA , Seyler SJ . Prolonged low-dose methylprednisolone treatment is highly effective in reducing duration of mechanical ventilation and mortality in patients with ARDS. J Intensive Care. 2018;6:53. doi:.https://doi.org/10.1186/s40560-018-0321-9
16 Nadim MK , Forni LG , Mehta RL , Connor MJ, Jr , Liu KD , Ostermann M , et al. COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat Rev Nephrol. 2020;16(12):747–64. doi:.https://doi.org/10.1038/s41581-020-00356-5
17 Mitra AR , Fergusson NA , Lloyd-Smith E , Wormsbecker A , Foster D , Karpov A , et al. Baseline characteristics and outcomes of patients with COVID-19 admitted to intensive care units in Vancouver, Canada: a case series. CMAJ. 2020;192(26):E694–701. doi:.https://doi.org/10.1503/cmaj.200794
18 Vanderburg S , Alipanah N , Crowder R , Yoon C , Wang R , Thakur N , et al. Management and Outcomes of Critically-Ill Patients with COVID-19 Pneumonia at a Safety-net Hospital in San Francisco, a Region with Early Public Health Interventions: A Case Series. medRxiv. 2020;2905.27.20114090.
19 Giraud R , Bendjelid K . COVID-19 pandemic: A new path to intensive care medicine distinction? Anaesth Crit Care Pain Med. 2020;39(5):545–6. doi:.https://doi.org/10.1016/j.accpm.2020.07.010
Shared last authorship
All authors (MMJ, BJ, QYA, MCTZ, HJF, SJ) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported, and no important aspects of the study have been omitted.
Funding provided by the Department of Intensive Care Medicine, University Hospital Bern (Inselspital), Switzerland.
No potential conflict of interest relevant to this article was reported