The importance of 18F-FDG cardiac PET/CT for the assessment of myocardial viability in ischaemic heart disease
DOI: https://doi.org/10.4414/smw.2021.20511
Federico
Caobellia, Christel H.
Kamanib, Rene
Nkoulouc, Ronny R.
Buecheld
a Clinic of Radiology and Nuclear Medicine, University Hospital Basel and University of Basel, Switzerland
b Department of Nuclear Medicine and Molecular Imaging, Centre Hospitalier Universitaire Vaudois (CHUV), University of Lausanne, Switzerland
c Department of Nuclear Medicine, University of Geneva, Switzerland
d Department of Nuclear Medicine, University Hospital Zurich, Switzerland
Introduction
The worldwide increase in coronary artery disease and its associated metabolic diseases is a major cause of human morbidity and mortality [1] and, as such, advancing cardiovascular disease remains a significant burden on the healthcare systems in western countries. The focus has been on the impact of advances in medical therapy and interventional cardiology on lowered mortality rates,but these came with more widespread availability of noninvasive methods allowing early diagnosis and effective risk stratification [2].
The concept of myocardial viability emerged from the postoperative clinical observations in the first decades of aorto-coronary bypass grafting. Then it was generally thought that left ventricular dysfunction in ischaemic heart disease was irreversible, since no persistent myocardial ischaemic state would have been possible in abnormal resting myocardium [3]. However, in the early 1980s Rahimtoola [3], in line with previous observations [4, 5], extensively described the improvement of left ventricular contractility after surgical revascularisation in patients with left ventricular dysfunction and ischaemic heart disease. Following these observations, many efforts have been made to understand the pathophysiological processes underlying this recovery phenomenon, as well as the diagnostic tools to achieve an accurate evaluation of the recovery potential in the clinical setting. Nowadays, the term myocardial viability in territories presenting altered contractility, beyond the concept of myocardial ischaemia, encompasses two phenomena: myocardial stunning and myocardial hibernation [6]. Myocardial stunning is characterised by persistent myocardial contractile dysfunction resulting from transient episodes of hypoperfusion. Its duration depends on the severity and the duration of the ischaemic episode [7]. This state can potentially of fully recover after restoration of blood supply, as long as no irreversible damage (necrosis) has occurred. In the case of prolongued or chronic ischaemia, ischaemic myocardial cells undergo adaptive changes, leading to a shift from fatty acid metabolism to glucose utilisation, as well as to downregulation of the contractile function in order to reduce the demand for oxygen and metabolic substrates [8, 9]. These changes are believed to induce the state of “hibernating myocardium”, a clinical condition characterised by contractile dysfunction with abnormal resting myocardial blood flow, and with a potential for full recovery after blood flow restoration [10, 11]. Repetitive episodes of myocardial stunning may also cause structural changes within the cardiomyocytes [9, 11], which tend to become irreversible over time.
The correct identification of viable myocardium has a strong rationale, in that the myocardium may potentially regain its function after revascularisation in the setting of myocardial hibernation or stunning. It is therefore clear that diagnostic tools able to provide an early and precise diagnosis as well as prognostic information are highly warranted.
Owing to the tendency to progress toward irreversible structural changes within the myocytes, viability imaging plays a pivotal role both in identifying patients at increased risk of coronary artery disease progression and in driving the choice of appropriate therapeutic measures. In fact, restoration of contractile function can be achieved by referring the patient earlier to invasive coronary revascularisation in the case of viable myocardium [12, 13]. Among the available techniques able to identify a viable myocardium, positron emission tomography / computed tomography (PET/CT) with fluorine 18-fluorodeoxyglucose (18F-FDG), in combination with myocardial perfusion imaging, constitutes a cornerstone of myocardial viability assessment in nuclear cardiology [14]. The identification of myocardial viability relies on the metabolic shift towards glucose metabolisation in viable but hibernating myocardium, leading to increased 18F-FDG uptake within affected myocardial segments. This requires carefull dietary preparation to promote glucose metabolism by the myocardium [14]. The viability criteria are fulfilled in segments with altered perfusion and increased metabolism (≥50% FDG uptake in comparison with segments with normal blood flow or normal myocardial function) [14].
Data from the literature
Many studies have evaluated the diagnostic and prognostic value of 18F-FDG PET/CT regarding myocardial viability in patients with ischaemic heart disease. The relevant literature was summarised and evaluated in two meta-analyses, by Schinkel et al. [15] and Bax et al. [16].
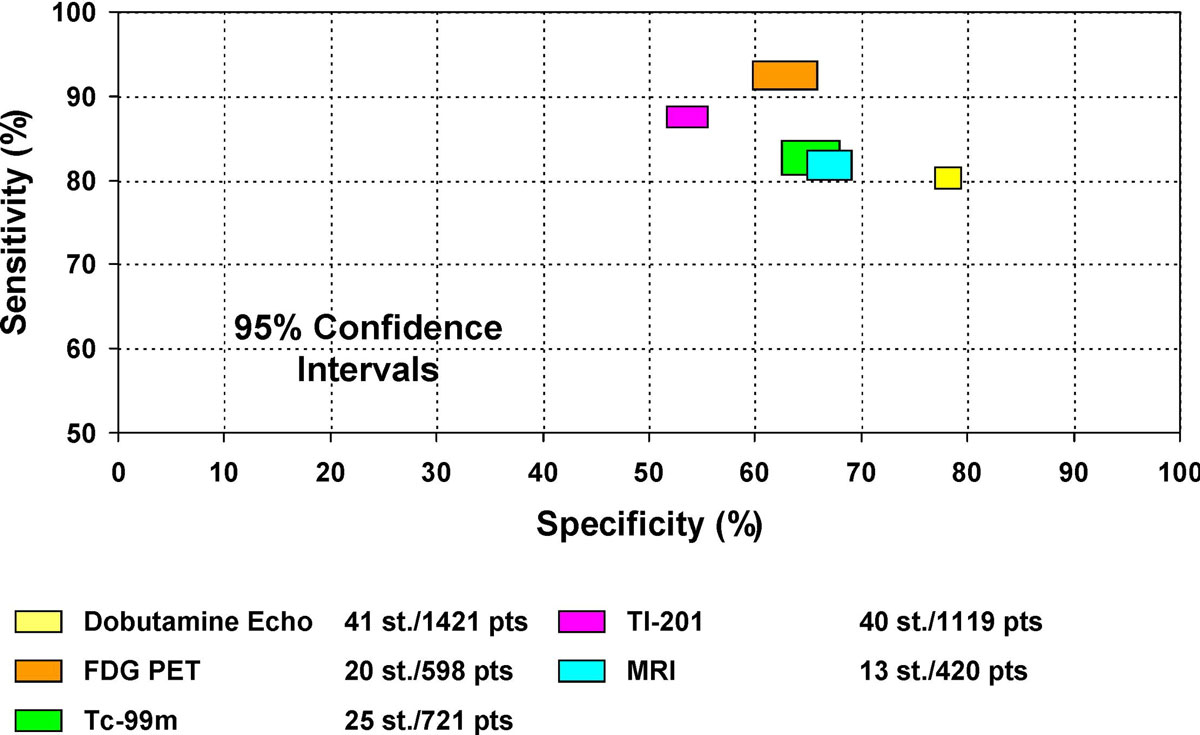
In the meta-analysis of Schinkel et al. [15], the pooled sensitivity of 18F-FDG PET/CT for the prediction of improved regional left ventricular function after revascularisation was 92%, and specificity was 63%, positive predictive value (PPV) 74%, and negative predictive value (NPV) 87% (table 1). Conversely, for the prediction of an improvement of the global left ventricular function, 18F-FDG PET/CT yielded 83% sensitivity, 64% specificity, 68% PPV and 80% NPV. In a sub-analysis of 10 studies, the prognostic role of 18F-FDG PET/CT was evaluated, wherein it was demonstrated that patients with PET-based identification of cardiac viability experienced higher mortality rates if not subsequently revascularised, highlighting the importance of a highly sensitive test for the assessment of myocardial viability. Conversely, no difference in mortality rates was demonstrated in revascularised patients without evidence of myocardial viability on 18F-FDG PET/CT. Moreover, the authors compared different modalities in the identification of myocardial viability. Whereas 18F-FDG PET/CT showed pooled sensitivity, specificity, PPV and NPV of 92%, 63%, 74% and 87%, respectively, cardiac magnetic resonance imaging (MRI) proved less accurate, yielding a pooled sensitivity, specificity, PPV and NPV of 84%, 63%, 72% and 78%, respectively. Dobutamine stress echocardiography (DSE) also proved less accurate than 18F-FDG PET/CT, with a pooled sensitivity, specificity, PPV and NPV of 80%, 78%, 75% and 83%, respectively.
Table1 Sensitivity, specificity, positive- and negative-predictive values (PPV, NPV) of 18F-FDG PET/CT to predict improvement of regional function after revascularisation (756 patients, 24 studies). Modified from Schinkel et al. [15].
|
Study
|
Publication year
|
n
|
Sensitivity
(%)
|
Specificity
(%)
|
PPV
(%)
|
NPV
(%)
|
| Tillisch et al. |
1986 |
17 |
95 |
80 |
85 |
92 |
| Tamaki et al. |
1989 |
22 |
78 |
78 |
78 |
78 |
| Tamaki et al. |
1991 |
11 |
100 |
38 |
80 |
38 |
| Marwick et al. |
1992 |
16 |
71 |
76 |
68 |
79 |
| Lucignani et al. |
1992 |
14 |
93 |
86 |
95 |
80 |
| Carrel et al. |
1992 |
23 |
94 |
50 |
84 |
75 |
| Gropler et al. |
1993 |
34 |
83 |
50 |
52 |
81 |
| Knuuti et al. |
1994 |
48 |
92 |
85 |
72 |
96 |
| Paolini et al. |
1994 |
9 |
88 |
79 |
88 |
79 |
| Vom Dahl et al. |
1994 |
37 |
N/A |
N/A |
86 |
100 |
| Tamaki et al. |
1995 |
43 |
88 |
82 |
76 |
92 |
| Gerber et al. |
1996 |
39 |
75 |
67 |
78 |
63 |
| Baer et al. |
1996 |
42 |
92 |
88 |
92 |
88 |
| Vom Dahl et al. |
1996 |
52 |
90 |
74 |
68 |
83 |
| Maes et al. |
1997 |
23 |
83 |
91 |
91 |
83 |
| Wolpers et al. |
1997 |
30 |
N/A |
N/A |
90 |
85 |
| Pagano et al. |
1998 |
30 |
99 |
33 |
66 |
96 |
| Fath et al. |
1998 |
47 |
81 |
88 |
82 |
88 |
| Pagano et al. |
1998 |
35 |
99 |
33 |
56 |
96 |
| Kitsiou et al. |
1999 |
26 |
N/A |
N/A |
78 |
82 |
| Nowak et al. |
2003 |
42 |
90 |
63 |
75 |
83 |
| Schmidt et al. |
2004 |
40 |
100 |
73 |
86 |
100 |
| Slart et al. |
2006 |
47 |
91 |
87 |
89 |
89 |
| Kuhl et al. |
2006 |
29 |
86 |
74 |
78 |
84 |
|
Weighted mean
|
|
|
92
|
63
|
74
|
87
|
One prospective, randomised trial evaluated the performance of 18F-FDG PET/CT in the management of patients with severely reduced left ventricular function in ischaemic heart disease (PARR-2 trial) [17]. In this trial, patients were randomized to either 18F-FDG PET/CT-guided management (n = 218) or standard care (n = 212). Although there was no significant difference in cardiac events between both groups, it must be noted that a significant number of patients (about 25%) in the PET arm did not undergo revascularisation despite the presence of viability as documented by PET. Hence, in these patients, the treating phycisians did not adhere to the recommendations from 18F-FDG PET/CT imaging. In a post-hoc subanalysis those patients in whom the therapy was based on the recommendations from PET imaging had a lower risk of cardiac death, myocardial infarction or hospitalisation after 1 year of follow-up as compared with the “standard care” group (HR 0.62, 95% confidence interval [CI] 0.42–0.93; p = 0.019). This prognostic benefit was later confirmed in in a subsequent study after 5 years of follow-up [18]. In a sub-study of the PPAR-2 trial, the extent of hibernating myocardium was shown to be predictive of the revascularisation benefits in terms of occurrence of cardiac events. In fact, the prognostic benefit increased with the extent of hibernating myocardium, with a lower threshold of 7% of the entire left ventricular myocardium of revascularisation as the extent of mismatch increases [19]. These results were further confirmed in a subsequent study [20]. Another substudy of the PPAR-trial, the Ottawa-Five substudy, investigated the outcomes of patients managed in centres with a multidisciplinary integrated cardiovascular team as well as an experienced nuclear cardiology team. This substudy highlighted a further significant reduction in cardiac events in the PET group as compared with the standard care group, suggesting an even greater benefit for patient outcome in the setting of an integrative cardiovascular team, including specialised nuclear cardiology imaging, heart failure and revascularisation teams (fig. 1) [21].
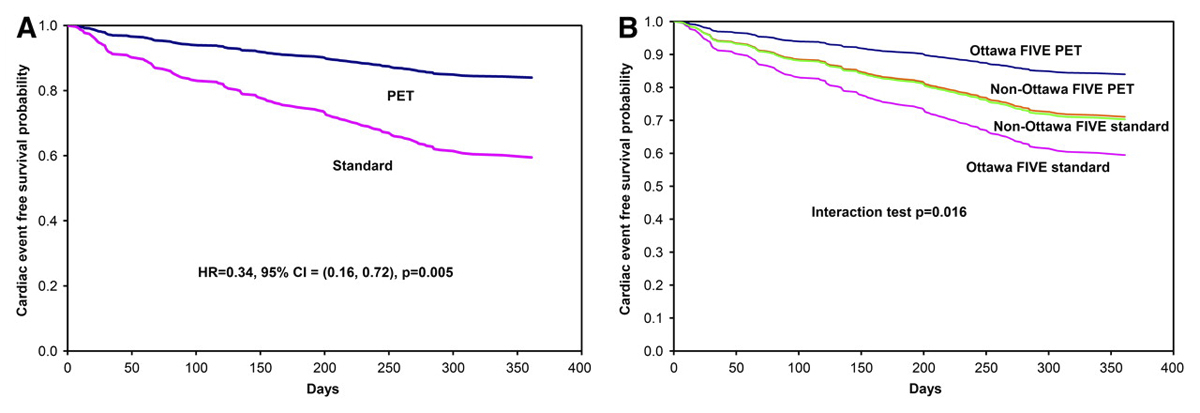
Figure 1 (A) Adjusted survival curves for the PET and standard arms in the Ottawa-FIVE substudy. (B) Adjusted survival curves for the PET and standard arms in the Ottawa-FIVE and the rest of PARR 2. CI = confidence interval; HR = hazard ratio. From: Abraham A, Nichol G, Williams KA, Guo A, deKemp RA, Garrard L, et al.; PARR 2 Investigators. 18F-FDG PET imaging of myocardial viability in an experienced center with access to 18F-FDG and integration with clinical management teams: the Ottawa-FIVE substudy of the PARR 2 trial. J Nucl Med. 2010;51(4):567–74; reprinted with permission of the Society of Nuclear Medicine and Molecular Imaging.
More recently, another important study investigated the impact of 18F-FDG PET/CT in assisting the management of coronary artery disease patients. The study included 30 subjects randomised to angiogenic therapy with adenoviral vascular endothelial growth factor (AdVEGF) or placebo. It demonstrated that those patients with hypoperfused but still viable myocardium treated with AdVEGF-D showed improved myocardial blood flow during the follow-up. Of note, perfusion did not increase in the control patients [22].
Besides the extent of viability, the time interval between the 18F-FDG PET/CT and the revascularisation, as well as the extent of the myocardial scar as determined during the 18F-FDG PET/CT, are strong determinant prognostic factors for the improvement of left ventricular ejection fraction (LVEF) at 3 months, as shown by the PARR-I study [23]. In fact, in patients with either delayed revascularisation or a large amount of myocardial scar, the LVEF recovery after revascularisation was only marginal.
Jacklin et al. performed a cost-effectiveness study to compare three management strategies, including coronary artery bypass grafting for all patients, management based on the presence or absence of myocardial hibernation as assessed by 18F-FDG PET/CT, and medical therapy in all patients. The 18F-FDG PET/CT based strategy saved more life-years, but was more expensive than the strategy consisting of medical treatment. When compared with the coronary artery bypass strategy, the 18F-FDG PET/CT based strategy saved more life-years with reduced cost. Therefore, a 18F-FDG PET/CT based strategy to select patients before surgery is cost effective [24]. This is a relevant consideration in regard to the steadily increasing health costs in western societies.
The transmurality of late gadolinuim enhancement (LGE) in cardiac magnetic resonance (CMR) is predictive of functional recovery of the ischaemically affected myocardium [25]. However, in myocardial segments demonstrating <50% transmural extent of LGE, LGE-CMR criteria showed some limitations in the prediction of myocardial functional recovery after revascularisation. Indeed, Kim et al. showed that only 42% of segments with LGE extent of 25–50% functionally improved after revascularisation [25], and these observations were confirmed by further studies [26]. In this regard, a recent consensus from the American Heart Association (AHA) regarding imaging for myocardial viability [26] suggested that the evaluation of the dobutamine contractile reserve (on echocardiography or CMR) or viability testing using 18F-FDG PET/CT may help to overcome this limitation. Consistent with this concept, Schmidt et al. evaluated patients with chronic myocardial ischaemia both with CMR and with 18F-FDG PET/CT, investigating the importance of CMR-based assessment of dobutamine contractile reserve. In their study, 18F-FDG PET/CT showed excellent sensitivity and NPV (both 100%) and good specificity (73%) in the prediction of functional myocardial recovery. Conversely, the dobutamine contractile reserve showed 96% sensitivity and 93% NPV, with a gain in specificity (87%). Diffusion weighted images (DWI) on MR yielded an excellent sensitivity (100% as PET) but a poor specificity (53%) [27].
Of note, the advantage in terms of sensitivity for PET over CMR is of great importance in the evaluation of myocardial viability (fig. 2). As a matter of fact, if viability is not detected owing to a falsely negative examination, the viable myocardium will not be revascularised, with consequent higher mortality rate.
In this context, the added value of the simultaneous acquisition of 18F-FDG PET and CMR with hybrid PET/MR systems for improved accuracy and prognostic value is open to debate. When comparing 18F-FDG PET/CT with CMR early after revascularisation, Rischpler et al. [28] have recently shown that both modalities are accurate in the depiction of viable myocardium and able to predict functional recovery of the left ventricular myocardium in patients with a history of myocardial infarction. In a small proportion of segments with discrepant 18F-FDG PET/CT and late gadolinium enhancement (LGE) CMR findings, FDG uptake was a better predictor for functional recovery. However, as mentioned before, this study did not assess the potential for a functional recovery of the myocardium in a pre-revascularisation setting and therefore these results should be applied with caution in patients with chronic myocardial ischaemia and a larger infarct if the probability of functional recovery is assessed before revascularisation. Nevertheless, Kühl et al. showed in 2006 that functional improvement in left ventricular function is highly likely when both modalities indicate preserved viability (87%) and negligible if both show no viability (4%) [29]. This latter finding has been confirmed in the post-revascularisation phase by Rischpler et al. [28], although their study failed to show any additional value if CMR and PET are combined.
It may be maintained that 18F-FDG PET/CT can be at least a part of a multimodality approach in selected patients, able to provide a more accurate prediction of myocardial functional recovery. From this perspective, the choice of the most adequate modality (or their integration) should rely on a multidisciplinary team discussion, which has to consider the technical feasibility of revascularisation, possible alternatives such as resynchronisation and the patient’s general condition. Such an approach would be in line with the OTTAWA-FIVE study [20].
It should also be noted that CMR cannot be performed in some patients owing to implantable cardiac pacemakers or defribrillators, because of the metal-induced artifacts or the risk of heat injury. Moreover, injection of gadolinium-based contrast agents is discouraged in patients with severe renal impairment because of the risk of nephrogenic systemic fibrosis. Finally, a non-negligible proportion of patients are unable to undergo CMR as a result of claustrophobia. In contrast, no contraindications are present for 18F-FDG PET/CT with the exception of pregnancy, and claustrophobia is less of a problem [30, 31].
Based on data from the literature, revascularisation has been classified as a class IIb recommendation for patients with evidence of viable myocardium on cardiac imaging and reduced left ventricular function in the most recent guidelines of the European Society of Cardiology (ESC) [32, 33].
Remarks and suggestions
As coronary artery disease represents a major cause of death and morbidity in most western countries, markers able to identify the risk of progression and select those patients requiring a more aggressive therapeutic approach are essential, both for clinical reasons and for allowing more streamlined and cost-saving allocation of resources. Left ventricular function is the most important marker for the prognostic assessment of coronary artery disease patients, and it can be severely impaired after myocardial infarction, both regionally and globally. However, reduced left ventricular function caused by an ischaemic event is not necessarily an irreversible alteration and patients may benefit from revascularisation of the vessels subtending an infarcted area. In this regard, an invasive approach by means of percutaneous angioplasty (PTCA) or coronary artery bypass grafting (CABG) may result in improved left ventricular function and recovery from wall motion abnormalities, conferring important prognostic implications [34]. The cumulative evidence convincingly demonstrates that patients with viable myocardium but without extensive scarring benefit from early revascularisation. In contrast, revascularisation does not lower mortality in patients without evidence of cardiac viability. Therefore, these patients should rather be treated with optimal medical therapy [15].
There may be some limitations to the extensive use of 18F-FDG PET/CT, such as the radiation exposure, the suboptimal deployment of PET scanners (often limited to tertiary centres), as well as the need for adequate preparation including insulin clamping. Regarding the radiation issue, it should be noted that the radiation dose associated with an 18F-FDG cardiac PET/CT is about 5 mSV [35], and thus is absolutely adequate to address the requirement for population dose containment. Furthermore, despite these limitations, noninvasive diagnostic modalities with high diagnostic performance must be integrated into standard clinical care, since the presence of non-revascularised viable myocardium is associated with higher mortality.
In this regard, 18F-FDG PET/CT is very well suited to meet these demands and, hence, in many cases is the preferred technique for the assessment of myocardial viability as it yields superior diagnostic accuracy as compared with other modalities such as DSE and CMR. Besides their higher availability in comparison with PET/CT, these modalities also have some other advantages such as the possibility to simultaneously perform ischaemia testing or to assess left and right ventricular dimensions and function. Nevertheless, in centres with acces to these three modalities (18F-FDG PET/CT, DSE and CMR), it remains mandatory to re-assess their advantages and limitations in terms of their respective diagnostic accuracy and prognostic value.
These concepts are currently being investigated in the ongoing AIMI-HF trial, wherein different imaging strategies will be compared to assess their impact on the composite clinical endpoint of cardiac death, myocardial infarction, resuscitated cardiac arrest and cardiac rehospitalisation [30].
Highly accurate identification of the presence and the extent of viable myocardium is the basis for management of patients with ischaemic heart disease, which is cost effective because the high diagnostic performance of 18F-FDG PET/CT can avoid the need for additional investigations. The correct identification of a viable myocardium also renders invasive procedures such as coronary angiography with eventual revascularisation unnecessary in patients who are not likely to benefit (gatekeeper function), given the proven excellent NPV of 18F-FDG PET/CT.
Hence, 18F-FDG cardiac PET/CT is expected to streamline resource allocation in view of the possibility to avoid costly, invasive procedures and to reduce the rate of future severe complications in coronary artery disease patients, thus further reducing the costs for advanced therapies.
References
1
Virani
SS
,
Alonso
A
,
Benjamin
EJ
,
Bittencourt
MS
,
Callaway
CW
,
Carson
AP
, et al.; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141(9):e139–596. doi:.https://doi.org/10.1161/CIR.0000000000000757
2
Mozaffarian
D
,
Benjamin
EJ
,
Go
AS
,
Arnett
DK
,
Blaha
MJ
,
Cushman
M
, et al.; Writing Group Members; American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Executive Summary: Heart Disease and Stroke Statistics--2016 Update: A Report From the American Heart Association. Circulation. 2016;133(4):447–54. doi:.https://doi.org/10.1161/CIR.0000000000000366
3
Rahimtoola
SH
. The hibernating myocardium. Am Heart J. 1989;117(1):211–21. doi:.https://doi.org/10.1016/0002-8703(89)90685-6
4
Saltiel
J
,
Lespérance
J
,
Bourassa
MG
,
Castonguay
Y
,
Campeau
L
,
Grondin
P
. Reversibily of left ventricular dysfunction following aorto-coronary by-pass grafts. Am J Roentgenol Radium Ther Nucl Med. 1970;110(4):739–46. doi:.https://doi.org/10.2214/ajr.110.4.739
5
Horn
HR
,
Teichholz
LE
,
Cohn
PF
,
Herman
MV
,
Gorlin
R
. Augmentation of left ventricular contraction pattern in coronary artery disease by an inotropic catecholamine. The epinephrine ventriculogram. Circulation. 1974;49(6):1063–71. doi:.https://doi.org/10.1161/01.CIR.49.6.1063
6
Allman
KC
. Noninvasive assessment myocardial viability: current status and future directions. J Nucl Cardiol. 2013;20(4):618–37, quiz 638–9. doi:.https://doi.org/10.1007/s12350-013-9737-8
7
Thijssen
VL
,
Borgers
M
,
Lenders
MH
,
Ramaekers
FC
,
Suzuki
G
,
Palka
B
, et al.
Temporal and spatial variations in structural protein expression during the progression from stunned to hibernating myocardium. Circulation. 2004;110(21):3313–21. doi:.https://doi.org/10.1161/01.CIR.0000147826.13480.99
8
Opie
LH
. Myocardial ischemia--metabolic pathways and implications of increased glycolysis. Cardiovasc Drugs Ther. 1990;4(S4, Suppl 4):777–90. doi:.https://doi.org/10.1007/BF00051275
9
Gunning
MG
,
Kaprielian
RR
,
Pepper
J
,
Pennell
DJ
,
Sheppard
MN
,
Severs
NJ
, et al.
The histology of viable and hibernating myocardium in relation to imaging characteristics. J Am Coll Cardiol. 2002;39(3):428–35. doi:.https://doi.org/10.1016/S0735-1097(01)01766-1
10
Elsässer
A
,
Schlepper
M
,
Klövekorn
WP
,
Cai
WJ
,
Zimmermann
R
,
Müller
KD
, et al.
Hibernating myocardium: an incomplete adaptation to ischemia. Circulation. 1997;96(9):2920–31. doi:.https://doi.org/10.1161/01.CIR.96.9.2920
11
Kim
SJ
,
Peppas
A
,
Hong
SK
,
Yang
G
,
Huang
Y
,
Diaz
G
, et al.
Persistent stunning induces myocardial hibernation and protection: flow/function and metabolic mechanisms. Circ Res. 2003;92(11):1233–9. doi:.https://doi.org/10.1161/01.RES.0000076892.18394.B6
12
Beanlands
RS
,
Hendry
PJ
,
Masters
RG
,
deKemp
RA
,
Woodend
K
,
Ruddy
TD
. Delay in revascularization is associated with increased mortality rate in patients with severe left ventricular dysfunction and viable myocardium on fluorine 18-fluorodeoxyglucose positron emission tomography imaging. Circulation. 1998;98(19, Suppl):II51–6.
13
Page
BJ
,
Banas
MD
,
Suzuki
G
,
Weil
BR
,
Young
RF
,
Fallavollita
JA
, et al.
Revascularization of chronic hibernating myocardium stimulates myocyte proliferation and partially reverses chronic adaptations to ischemia. J Am Coll Cardiol. 2015;65(7):684–97. doi:.https://doi.org/10.1016/j.jacc.2014.11.040
14
Dilsizian
V
,
Bacharach
SL
,
Beanlands
RS
,
Bergmann
SR
,
Delbeke
D
,
Dorbala
S
, et al.
ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol. 2016;23(5):1187–226. doi:.https://doi.org/10.1007/s12350-016-0522-3
15
Schinkel
AF
,
Bax
JJ
,
Poldermans
D
,
Elhendy
A
,
Ferrari
R
,
Rahimtoola
SH
. Hibernating myocardium: diagnosis and patient outcomes. Curr Probl Cardiol. 2007;32(7):375–410. doi:.https://doi.org/10.1016/j.cpcardiol.2007.04.001
16
Bax
JJ
,
Poldermans
D
,
Elhendy
A
,
Boersma
E
,
Rahimtoola
SH
. Sensitivity, specificity, and predictive accuracies of various noninvasive techniques for detecting hibernating myocardium. Curr Probl Cardiol. 2001;26(2):147–81. doi:.https://doi.org/10.1067/mcd.2001.109973
17
Beanlands
RS
,
Nichol
G
,
Huszti
E
,
Humen
D
,
Racine
N
,
Freeman
M
, et al.; PARR-2 Investigators. F-18-fluorodeoxyglucose positron emission tomography imaging-assisted management of patients with severe left ventricular dysfunction and suspected coronary disease: a randomized, controlled trial (PARR-2). J Am Coll Cardiol. 2007;50(20):2002–12. doi:.https://doi.org/10.1016/j.jacc.2007.09.006
18
Mc Ardle
B
,
Shukla
T
,
Nichol
G
,
deKemp
RA
,
Bernick
J
,
Guo
A
, et al.; PARR-2 Investigators. Long-Term Follow-Up of Outcomes With F-18-Fluorodeoxyglucose Positron Emission Tomography Imaging-Assisted Management of Patients With Severe Left Ventricular Dysfunction Secondary to Coronary Disease. Circ Cardiovasc Imaging. 2016;9(9):e004331. doi:.https://doi.org/10.1161/CIRCIMAGING.115.004331
19
D’Egidio
G
,
Nichol
G
,
Williams
KA
,
Guo
A
,
Garrard
L
,
deKemp
R
, et al.; PARR-2 Investigators. Increasing benefit from revascularization is associated with increasing amounts of myocardial hibernation: a substudy of the PARR-2 trial. JACC Cardiovasc Imaging. 2009;2(9):1060–8. doi:.https://doi.org/10.1016/j.jcmg.2009.02.017
20
Ling
LF
,
Marwick
TH
,
Flores
DR
,
Jaber
WA
,
Brunken
RC
,
Cerqueira
MD
, et al.
Identification of therapeutic benefit from revascularization in patients with left ventricular systolic dysfunction: inducible ischemia versus hibernating myocardium. Circ Cardiovasc Imaging. 2013;6(3):363–72. doi:.https://doi.org/10.1161/CIRCIMAGING.112.000138
21
Abraham
A
,
Nichol
G
,
Williams
KA
,
Guo
A
,
deKemp
RA
,
Garrard
L
, et al.; PARR 2 Investigators. 18F-FDG PET imaging of myocardial viability in an experienced center with access to 18F-FDG and integration with clinical management teams: the Ottawa-FIVE substudy of the PARR 2 trial. J Nucl Med. 2010;51(4):567–74. doi:.https://doi.org/10.2967/jnumed.109.065938
22
Hartikainen
J
,
Hassinen
I
,
Hedman
A
,
Kivelä
A
,
Saraste
A
,
Knuuti
J
, et al.
Adenoviral intramyocardial VEGF-DΔNΔC gene transfer increases myocardial perfusion reserve in refractory angina patients: a phase I/IIa study with 1-year follow-up. Eur Heart J. 2017;38(33):2547–55. doi:.https://doi.org/10.1093/eurheartj/ehx352
23
Beanlands
RS
,
Ruddy
TD
,
deKemp
RA
,
Iwanochko
RM
,
Coates
G
,
Freeman
M
, et al.; PARR Investigators. Positron emission tomography and recovery following revascularization (PARR-1): the importance of scar and the development of a prediction rule for the degree of recovery of left ventricular function. J Am Coll Cardiol. 2002;40(10):1735–43. doi:.https://doi.org/10.1016/S0735-1097(02)02489-0
24
Jacklin
PB
,
Barrington
SF
,
Roxburgh
JC
,
Jackson
G
,
Sariklis
D
,
West
PA
, et al.
Cost-effectiveness of preoperative positron emission tomography in ischemic heart disease. Ann Thorac Surg. 2002;73(5):1403–9, discussion 1410. doi:.https://doi.org/10.1016/S0003-4975(02)03459-8
25
Kim
RJ
,
Wu
E
,
Rafael
A
,
Chen
EL
,
Parker
MA
,
Simonetti
O
, et al.
The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343(20):1445–53. doi:.https://doi.org/10.1056/NEJM200011163432003
26
Garcia
MJ
,
Kwong
RY
,
Scherrer-Crosbie
M
,
Taub
CC
,
Blankstein
R
,
Lima
J
, et al.; American Heart Association Council on Cardiovascular Radiology and Intervention and Council on Clinical Cardiology. State of the Art: Imaging for Myocardial Viability: A Scientific Statement From the American Heart Association. Circ Cardiovasc Imaging. 2020;13(7):e000053. doi:.https://doi.org/10.1161/HCI.0000000000000053
27
Schmidt
M
,
Voth
E
,
Schneider
CA
,
Theissen
P
,
Wagner
R
,
Baer
FM
, et al.
F-18-FDG uptake is a reliable predictory of functional recovery of akinetic but viable infarct regions as defined by magnetic resonance imaging before and after revascularization. Magn Reson Imaging. 2004;22(2):229–36. doi:.https://doi.org/10.1016/j.mri.2003.07.006
28
Rischpler
C
,
Langwieser
N
,
Souvatzoglou
M
,
Batrice
A
,
van Marwick
S
,
Snajberk
J
, et al.
PET/MRI early after myocardial infarction: evaluation of viability with late gadolinium enhancement transmurality vs. 18F-FDG uptake. Eur Heart J Cardiovasc Imaging. 2015;16(6):661–9. doi:.https://doi.org/10.1093/ehjci/jeu317
29
Kühl
HP
,
Lipke
CS
,
Krombach
GA
,
Katoh
M
,
Battenberg
TF
,
Nowak
B
, et al.
Assessment of reversible myocardial dysfunction in chronic ischaemic heart disease: comparison of contrast-enhanced cardiovascular magnetic resonance and a combined positron emission tomography-single photon emission computed tomography imaging protocol. Eur Heart J. 2006;27(7):846–53. doi:.https://doi.org/10.1093/eurheartj/ehi747
30
Patterson
RE
,
Sigman
SR
,
O’Donnell
RE
,
Eisner
RL
. Viability assessment with MRI is superior to FDG-PET for viability: Con. J Nucl Cardiol. 2010;17(2):298–309. doi:.https://doi.org/10.1007/s12350-010-9209-3
31
Maddahi
J
. Viability assessment with MRI is superior to FDG-PET for viability: Pro. J Nucl Cardiol. 2010;17(2):292–7. doi:.https://doi.org/10.1007/s12350-010-9201-y
32
Neumann
FJ
,
Sousa-Uva
M
,
Ahlsson
A
,
Alfonso
F
,
Banning
AP
,
Benedetto
U
, et al.; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165. doi:.https://doi.org/10.1093/eurheartj/ehy394
33
Ponikowski
P
,
Voors
AA
,
Anker
SD
,
Bueno
H
,
Cleland
JGF
,
Coats
AJS
, et al.; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200. doi:.https://doi.org/10.1093/eurheartj/ehw128
34
Allman
KC
,
Shaw
LJ
,
Hachamovitch
R
,
Udelson
JE
. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysis. J Am Coll Cardiol. 2002;39(7):1151–8. doi:.https://doi.org/10.1016/S0735-1097(02)01726-6
35
Zanzonico
P
,
Dauer
L
,
Strauss
HW
. Radiobiology in Cardiovascular Imaging. JACC Cardiovasc Imaging. 2016;9(12):1446–61. doi:.https://doi.org/10.1016/j.jcmg.2016.09.012