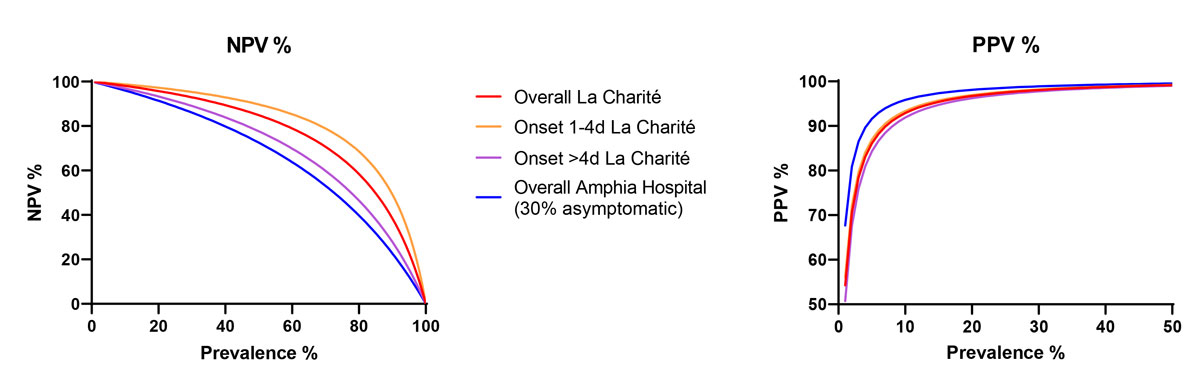
Figure 1 Positive and negative predictive value for prevalence of SARS-CoV-2 using the SD Biosensor rapid antigen test with self-swabbing by different tested populations.
DOI: https://doi.org/10.4414/smw.2021.20526
On 2 November 2020, during the second wave of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections in Switzerland, specific rapid antigen tests (RATs) on nasopharyngeal swabs were proposed by the Federal Office of Public Health (FOPH) as a tool complementary to the SARS-CoV-2-specific gold-standard reverse transcription – quantitative polymerase chain reaction (RT-qPCR) test, in order to increase and accelerate the screening of patients with COVID-19-like symptoms. At that time, the Swiss Society of Microbiology (SSM) evaluated the performance of 36 different SARS-CoV-2-specific RATs. The overall sensitivity of the best tests that passed the FOPH validation criteria, i.e., exhibited a sensitivity of (i) at least 95% for samples with more than 107 copies/ml (Ct <23), (ii) at least 90% for samples with more than 106copies/ml (Ct <26), and (iii) at least 80% for samples with more than 105 copies/ml (Ct <29); and the specificity was above 99% in ≥200 subjects without SARS-CoV-2 infection [1].
One of the best-performing RATs was the SD Biosensor (Roche) assay, which exhibited a specificity of about 99.5% and a sensitivity of about 85% in symptomatic outpatients with a Ct below 29 [1]. In contrast, the sensitivity of the SD Biosensor test decreased dramatically to only 28% and 33% among subjects without COVID-19 symptoms in two independent studies performed in Switzerland among hospitalised patients [2, 3]. This sensitivity dropped to 25% and 18%, respectively, in patients with 5 to 7 days or more than 7 days of COVID-19 symptoms [2].
In March 2021, a new strategy was proposed by the Swiss public health authorities, aiming at better controlling the spread of SARS-CoV-2 infection and implemented in Switzerland on 7 April 2021. This new strategy consists of significantly increasing the proportion of subjects tested by promoting testing of self-collected nasal swabs for repeated screening of asymptomatic persons outside of any epidemiological setting (contact or cluster), as announced by the FOPH on 12 March 2021 on their website. We wondered whether such antigen tests, which exhibit a 1000-fold lower sensitivity than RT-PCR, may be applied to asymptomatic subjects outside of any epidemiologically specific context.
This strategy has been implemented widely in Germany and Austria, based on recent studies that compared the performance of a SD Biosensor (Roche) RAT on nasopharyngeal versus nasal swabs taken by professionals or patients themselves in hospitals [4–8]. In order to have a better idea of the RAT performances with self-collected nasal swabs, we re-analysed and compiled the results of five different studies in which nasal swabbing was evaluated for the SD Biosensor (Roche) RAT (table 1). Four studies were performed in La Charité, Berlin, Germany, and one in Amphia Hospital, The Netherlands. In summary, data from La Charité clearly show that mid-turbinate or anterior nasal swabbing are equivalent when performed by professionals [7]. In addition, the authors did not detect any important difference when professionals performed nasal mid-turbinate or nasopharyngeal swabbing [5]. Self-collected nasal mid-turbinate swabs and professional nasopharyngeal swabbing were evaluated in three studies [4, 6–8]. All three showed a global agreement between self-collected nasal mid-turbinate swabs and professional nasopharyngeal testing of above 90%, revealing the high potential of self-collected nasal mid-turbinate swab testing with RAT. The sensitivity of the test falls from 96.6–100% for symptomatic patients with viral loads greater than or equal to 107, to 80–94% for patients with viral loads greater than or equal to105 (table 1). Moreover, the sensitivity dropped from 94% in patients who have been symptomatic for less than 4 days to below 60% in one of the studies for patients who had been symptomatic for more than 4 days. This highlights the major importance of the timing of swabbing: the sensitivity of the RAT is substantially decreased after 4 days of symptoms owing to a decreased viral load in the nasal mucosa [9, 10]. Notably, studies at La Charité (Berlin) included only symptomatic patients, with generally 1 to 4 days of symptoms (table 2). Thus, only the study performed at Amphia Hospital [8], in which about 30% of patients were asymptomatic, provides some insight into the expected performance of the nasal SD Biosensor RAT in mixed symptomatic and asymptomatic populations. In the Dutch study, the overall performance of the SD Biosensor RAT was 62.2% (table 2). Based on the substantial loss in sensitivity observed when a third of the population is asymptomatic, we estimate that the overall sensitivity of such self-collected nasal swab tests in asymptomatic subjects will be drastically lower. Although the negative predictive value (NPV) of the test remains relatively high with a prevalence of the disease below 20% (fig. 1), performing a RAT on asymptomatic people may lead to an increased rate of false negative tests, which may induce more high-risk behaviours, such as not wearing a mask, reduced hand hygiene, etc.
Table 1 Overview of the f ive studies data. Technical sensitivity and specificity, expressed in percentage. When available, data w ere stratified follow ing the viral load of the samples and the duration of symptoms at the time of the test, CI: all confidence intervals are 95%. NMT: nasal mid-turbinate, NP: Nasopharyngeal, AN: anterior nasal, self: self-testing, professional: professional-testing. PPA: positive percent agreement, NPA: negative percent agreement, ND: No Data.
| Study | Sampling type | Gold-standard (GS) |
N
total |
%
symptomatic |
N
pos GS |
N
neg GS |
N
≥10e7 cp/ml |
Sensitivity
≥10e7 cp/ml |
N
≥10e6 cp/ml |
Sensitivity
≥10e6 cp/ml |
N
≥10e5 cp/ml |
Sensitivity
≥10e5 cp/ml |
N
sympt. 1-4 d |
Sensitivity sympt. 1-4d |
N
sympt. >4 d |
Sensitivity sympt. >4d | Overall sensitivity | Overall specificity | Agreement |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (I) Charité, Germany Lindner AK et al. [5] | NMT (professional) |
NP RT-qPCR on Roche Cobas or TibMolbiol assay (E gene and T2 region) | 179 | 100% | 41 (22.9%) |
138 (77.1%) |
19 | 100% (19/19) (CI: 83.9-100) |
28 | 92.9 % (26/28) (CI: 80.8-96.2) |
32 | 90.6% (29/32) (CI: 79.6-93.6) |
20 | 85 % (17/20) (CI: 68.2-89.7) |
19 | 73.7% (14/19) (CI: 55.6-78.7) |
80.5% (33/41)
(CI: 66-89.8) |
98.6% (137/138)
(CI: 94.9-99.6) |
PPA: 93.5% (CI: 79.3-98.2) NPA: 95.9% (CI: 91.4-98.1) |
| NP (professional) |
94.7% (18/19) (CI: 76.4-99.7) |
85.7 % (24/28) (CI: 73-89.1) |
81.3% (26/32) (CI: 69.6-84.2) |
68.4% (13/19) (CI: 50.3-73.4) |
73.2% (30/41)
(CI: 58.1-84.3) |
99.3% (137/138)
(CI: 96-100) |
|||||||||||||
| (II) Charité, Germany Lindner AK et al. [6] | NMT (self) |
146 | 40 (27.4%) |
106 (72.6%) |
29 | 96.6% (28/29) (CI: 82.8-99.8) |
32 | 93.8% (30/32) (CI: 83.1-96.7) |
35 | 91.4% (32/35) (CI: 81.3-94.1) |
28 | 92.9% (26/28) (CI: 80.8-96.2) |
12 | 58.3%(7/12) (CI: 33.6-66.2) |
82.5% (33/40)
(CI: 68.1-91.3) |
100% (106/106)
(CI: 96.5-100) |
PPA: 91.4% (CI: 77.6-97) NPA: 99.1% (CI: 95-100) |
||
| NP (professional) |
96.9% (31/32) (CI: 86.8-99.7) |
94.3% (33/35) (CI: 84.5-97) |
66.7% (8/12) (CI: 41.4-74.6) |
85% (34/40) (CI: 70.9-92.9) |
99.1% (105/106)
(CI: 94.8-99.5) |
||||||||||||||
| (III) Charité, Germany Nikolai O et al. [7] | AN (professional) |
132 | 36 (27.3%) |
96 (72.7%) |
29 | 96.6% (28/29) (CI: 86.3-96.6) |
32 | 90.6 (29/32) (CI: 80.5-90.6) | 35 | 88.6% (31/35) (CI: 79-88.6) | 29 | 86.2% (25/29) (CI: 74.8-86.2) | 7 | 85.7% (6/7) (CI: 50.3-85.7) |
86% (31/36)
(CI: 71.3-93.9) |
100% (96/96)
(CI: 95.7-100) |
PPA: 100% (CI: 89-100) NPA: 100% (CI: 95.6-100) |
||
| NMT (professional) |
|||||||||||||||||||
| NMT (self) |
96 | 34 (35.4%) |
62 (64.6%) |
25 | 100% (25/25) (CI: 89.2-100) | 32 | 93.8% (30/32) (CI: 84-93.8) | 33 | 93.9 (31/33) (CI: 84.4-93.9) | 22 | 90.9 (20/22) (CI: 77-90.9) | 11 | 90.9% (10/11) (CI: 66.2-90.9) |
91.2% (31/34)
(CI: 77-97) |
98.4% (61/62)
(CI: 91.4-99.9) |
PPA: 96.8% (CI: 83.8-99.8) NPA: 96.9% (CI: 89.5-99.2) |
|||
| NP (professional) |
96.9% (31/32) 87.6-96.9) |
95.5 (21/22) (CI: 82.2-95.5) | 81.8% (9/11) (CI: 56.5-81.8) |
100% (62/62)
(94.2-100) |
|||||||||||||||
| (IV) Charité, Germany Lindner AK et al. [4] | NMT (self) |
287 | 97.6% | 39 (13.6%) |
248 (86.4%) |
23 | 97.7% (22/23) (CI: 82.9-95.7) |
29 | 93.1% (27/29) (CI: 82.4-93.1) |
36 | 80.6% (29/36) (CI: 70.8-80.6) |
20 | 80% (16/20) (CI: 64.1-80) |
19 | 68.4% (13/19) (CI: 51.6-68.4) |
74.4% (29/39)
(CI: 58.9-85.4) |
99.2% (246/248)
(CI: 97.1-99.8) |
PPA: 90.6% (CI: 75.8-96.8) NPA: 99.2% (CI: 97.2-99.8) |
|
| NP (professional) |
100% (23/23) (CI: 88.3-100) |
96.6% (28/29) (CI: 86.3-96.6) |
86.1% (31/36) (CI: 76.6-86.1) |
85% (17/20) (CI: 69.4-85) | 73.7% (14/19) (CI: 56.9-73.7) |
79.5% (31/39)
(CI: 64.5-89.2) |
99.6% (247/248)
(CI: 97.8-100) |
||||||||||||
| (V) Amphia Hospital, the Netherlands Stohr JJJM et al. [8] | SD Biosensor NMT (self) |
NP Abbot Alinity RT-qPCR on RdRP and N-genes or Lab developped PCR on E gene | 1588 | 68.70% | 262 | 1396 | ND |
62.2%
(122/262) (CI: 59.1-63.6) |
99.7%
(1392/1396) (CI: 99.3-99.9) |
ND | |||||||||
| NP Abbot RT-qPCR with Ct<23 or Lab developped PCR with Ct< 24.5 |
80.1%
(CI: 72.7-86) |
99.1%
(CI: 98.5-99.5) |
|||||||||||||||||
| NP viral culture | 150 | 94 | 56 |
74.5 % (70/94)
(CI: 68-79.9) |
71.4% (40/56)
(CI: 60.6-80.6) |
||||||||||||||
Table 2 Data concerning testing of self-collected nasal mid-turbinate swabs.
| La Charité, Germany self-swabbing data of studies II, II, IV cumulated | Amphia Hospital, The Netherlands | |
|---|---|---|
| Sampling type | NMT (self) | |
| Gold-standard | NP RT-qPCR on Roche Cobas or TibMolbiol assay (E gene and T2 region) | NP Abbot Alinity RT- qPCR on RdRP and N-genes or Lab developed PCR on E gene |
| N total | 529 | 1588 |
| % symptomatic | Almost 100% | 68.70% |
| Positive gold standard (n) | 113 | 262 |
| Negative gold standard (n) | 416 | 1396 |
| ≥107 copies/ml (n) | 77 | |
| Sensitivity ≥107 copies/ml | 97.4% (75/77) 95% CI 92.9–98.6% |
|
| ≥106 copies/ml (n) | 93 | |
| Sensitivity ≥106 copies/ml | 93.5% (87/93) 95% CI 89.4–94.6% |
|
| ≥105 copies/ml (n) | 104 | |
| Sensitivity ≥105 copies/ml | 88.5% (92/104) 95% CI 84.4–89.4% |
|
| Symptomatic 1–4 days (n) | 70 | |
| Sensitivity symptomatic 1–4 days | 88.6% (62/70) 95% CI 82.9–89.9% |
|
| Symptomatic >4 days (n) | 42 | |
| Sensitivity symptomatic >4 days | 71.4% (30/42) 95% CI 62–73.7% |
|
| Overall sensitivity | 82.3% (93/113) 95% CI 77.7–84.3% |
62.2% (122/262) 95% CI 59.1–63.6% |
| Overall specificity | 99.3% (413/416) 95% CI 98–99.8% |
99.7% (1392/1396) 95% CI 99.3–99.9% |
CI = confidence interval; NMT = nasal mid-turbinate swab; NP = nasopharyngeal swab; self = self-testing Technical sensitivity and specificity are expressed in percentages. When available, data were stratified according to the viral load of the samples and the duration of symptoms at the time of the test

Figure 1 Positive and negative predictive value for prevalence of SARS-CoV-2 using the SD Biosensor rapid antigen test with self-swabbing by different tested populations.
Thus, we think that use of RATs on self-collected nasal swabs among asymptomatic population may only be useful to detect highly positive individuals (so-called superspreaders), who may spread the virus to many people [11]. However, given the low sensitivity of such antigen-based assays, such a strategy should only be implemented when detailed information is provided to the population. In particular, all barrier procedures should be continued regardless of the test result. Indeed, the main goal of the current preventive measures are to decrease the workload in the hospitals and prevent saturation of the healthcare system. Another important limitation of antigen tests is related to their positive predictive value (PPV) in a low prevalence setting: with a specificity of 99.5% and a pretest probability of 0.5%, the proportion of positive tests that will correspond to a false positive will be of 50% (fig. 1). Such a very low PPV makes confirmation by RT-PCR of all positive results necessary. This is a key message in order to ensure that all subjects with a positive RAT will go to testing centres for a confirmatory RT-PCR test.
In conclusion, we think that self-testing should preferably be used as a screening test for symptomatic patients with symptom onset within 4 days, or in particular situations with a high risk of spread in order to detect asymptomatic patients with a high viral load. The population should be clearly informed on the limitations of rapid antigen testing of self-collected swabs, and therefore the necessity to continue all barrier procedures whatever the RAT result is and confirm a positive RAT result with PCR testing. This in order to avoid a false sense of security and risky behaviour, which would lead to the opposite of what is expected from this screening strategy.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Greub G , et al. National multicenter prospective VALIDation of 36 rapid Antigen TEsts for the detection of SARS-CoV-2 in Switzerland (VALIDATE). Submitted 2021.
2 Caruana G , Croxatto A , Kampouri E , Kritikos A , Opota O , Foerster M , et al. Implementing SARS-CoV-2 Rapid Antigen Testing in the Emergency Ward of a Swiss University Hospital: The INCREASE Study. Microorganisms. 2021;9(4):798. doi:.https://doi.org/10.3390/microorganisms9040798
3 Caruana G , Lebrun LL , Aebischer O , Opota O , Urbano L , de Rham M , et al. The dark side of SARS-CoV-2 rapid antigen testing: screening asymptomatic patients. New Microbes New Infect. 2021;42:100899. doi:.https://doi.org/10.1016/j.nmni.2021.100899
4 Lindner AK , Nikolai O , Kausch F , Wintel M , Hommes F , Gertler M , et al. Head-to-head comparison of SARS-CoV-2 antigen-detecting rapid test with self-collected nasal swab versus professional-collected nasopharyngeal swab. Eur Respir J. 2021;57(4):2003961. Available at: . doi:.https://doi.org/10.1183/13993003.03961-2020
5 Lindner AK , Nikolai O, Rohardt C, Burock S, Hülso C, Bölke A, et al. Head-to-head comparison of SARS-CoV-2 antigen-detecting rapid test with professional-collected nasal versus nasopharyngeal swab . medRxiv. 2021:20243725. doi:
6 Lindner AK , Nikolai O, Rohardt C, Kausch F, Wintel M, Gertler M, et al. SARS-CoV-2 patient self-testing with an antigen-detecting rapid test: a head-to-head comparison with professional testing . medRxiv. 2021:20249009. doi:
7 Nikolai O , Rohardt C, Tobian F, Junge A, Corman VM, Jones TC, et al. Anterior nasal versus nasal mid-turbinate sampling for a SARS-CoV-2 antigen-detecting rapid test: does localisation or professional collection matter? medRxiv. 2021:21251274. doi:
8 Stohr JJJM , Zwart VF, Goderski G, Meijer A, Nagel-Imming CRS, Kluytmans-van den Bergh MFQ, et al. Self-testing for the detection of SARS-CoV-2 infection with rapid antigen tests . medRxiv, 2021:21252153. doi:
9 Callahan C , Lee R , Lee G , Zulauf KE , Kirby JE , Arnaout R . Nasal-Swab Testing Misses Patients with Low SARS-CoV-2 Viral Loads. medRxiv. 2020;20128736 .
10 Pinninti S , Trieu C , Pati SK , Latting M , Cooper J , Seleme MC , et al. Comparing Nasopharyngeal and Midturbinate Nasal Swab Testing for the Identification of Severe Acute Respiratory Syndrome Coronavirus 2. Clin Infect Dis. 2021;72(7):1253–5. doi:.https://doi.org/10.1093/cid/ciaa882
11 Ladoy A , Opota O , Carron PN , Guessous I , Vuilleumier S , Joost S , et al. Size and duration of COVID-19 clusters go along with a high SARS-CoV-2 viral load: A spatio-temporal investigation in Vaud state, Switzerland. Sci Total Environ. 2021;787:147483. doi:.https://doi.org/10.1016/j.scitotenv.2021.147483
No financial support and no other potential conflict of interest relevant to this article was reported.