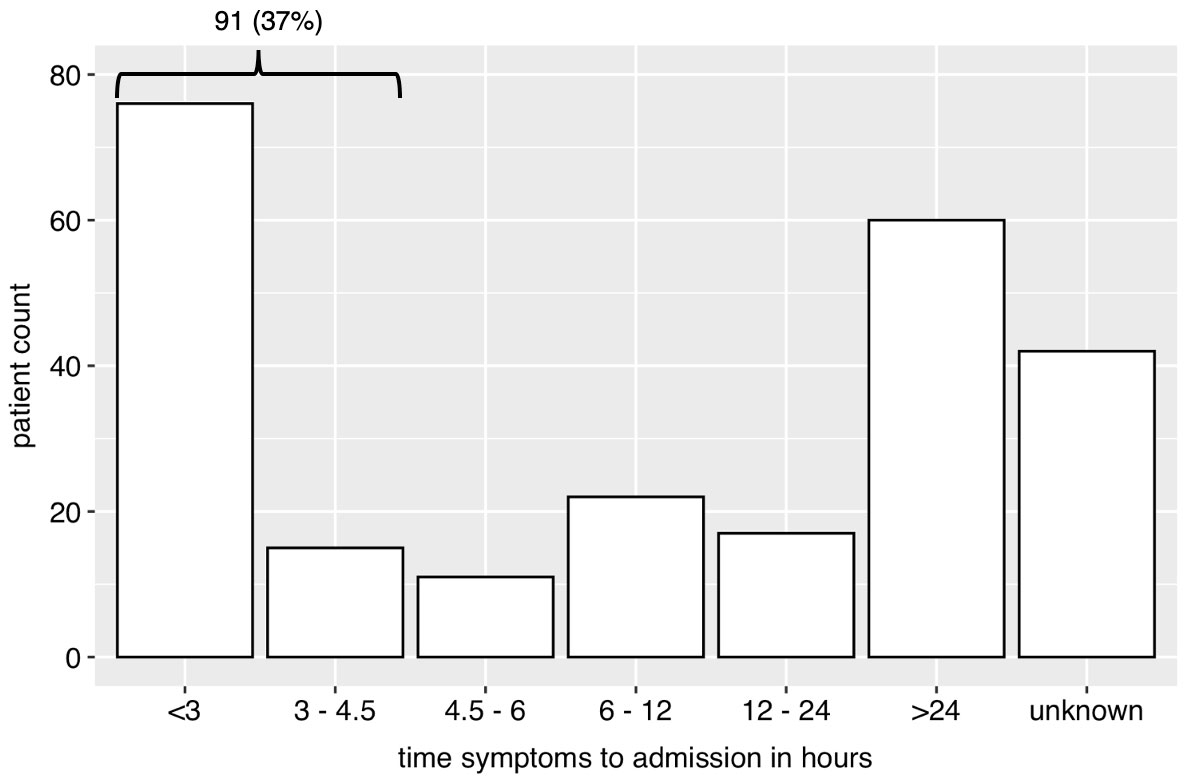
Figure 1 Bar plot of time from beginning of symptoms in groups to presentation in the emergency unit. The group “unknown” represents cases, where group assignment was not possible (e.g., wake-up stroke).
DOI: https://doi.org/10.4414/smw.2021.20490
computed tomography
European Society of Cardiology
intensive care unit
interquartile range
magnetic resonance imaging
modified Rankin Scale
National Institutes of Health Stroke Scale
transient ischaemic attack
Trial of Org 10172 in acute stroke treatment
Cerebral stroke is a major burden for health care and economies in developed countries. In Switzerland, the stroke event rate was estimated to be 296 per 100,000 population in 2004 [1]. In 2017, this estimate would represent 22,000 strokes [2]. In the same year, the mortality rate from cerebrovascular disease was 21/100,000 in men and 18/100,000 in women in Switzerland [3]. The high economic and personal burden of stroke is further driven by the costs of aftercare due to the functional impairment in stroke survivors as compared with the general population. This is in addition to the high costs of inpatient care, rehabilitation and sometimes the inability to return to work or to further care for oneself [4–6]. In times of economic pressure, quality of care in community hospitals has been under scrutiny since the introduction of the Swiss DRG system. High quality of care must be assessed and documented.
Cerebral stroke is defined as an injury of the brain tissue resulting from ischaemia or haemorrhage. Thrombosis, embolism or hypoperfusion of the brain leads to low arterial blood supply and consequent brain cell death. Intracranial bleeding may compress surrounding brain tissue and veins, which also leads to tissue damage and loss of neurological function [7]. Transient ischaemic attack is defined as a brief episode of neurological dysfunction caused by focal brain ischaemia without acute infarction and is considered to be a precursor of ischaemic stroke [8].
Stroke care in Switzerland is organised by a network of specialised “stroke centres” and “stroke units”. Stroke centres provide neuroradiological and neurosurgical interventions for eligible patients. Stroke units are specific wards where stroke patients receive specialised neurological care by a multidisciplinary team of physicians, nurses and therapists [9]. Currently, many patients with symptoms of stroke contact a general practitioner, a community hospital or the emergency call centre. In community hospitals, the decision for referral to another hospital or in-house therapy is made after local and/or telemedical neurological evaluation. Referral depends on local resources for a timely diagnosis, therapy and availability of monitoring capacities. Information concerning the epidemiology and medical care of cerebral stroke patients in this setting is lacking, although this is necessary to assess and further improve stroke care in Switzerland. Because of the lower caseload and limited diagnostic and therapeutic resources compared with stroke centres, quality of care and outcome may differ despite the accessibility of the local service.
The hospital of Uster (ZH) provides care for about 170,000 inhabitants of the region. The caseload between July 2017 and December 2018 was 75,253 outpatients and 15,768 inpatients cases (21,734 and 6270, respectively, in the medical department). Our aim was to investigate the epidemiology and medical care of cerebral stroke patients in the community hospital of Uster, with the goal of gaining information to improve stroke care in our hospital and in Switzerland.
Eligible patients were identified by screening the clinical information system (CGM clinical) of the Uster Hospital for the corresponding ICD-10 codes between July 2017 and December 2018. Inclusion criteria were new diagnosis of ischaemic or haemorrhagic stroke (ICD-10 I60-I66) or transient ischaemic attack (G45). Patients were excluded when the diagnosis of stroke was made in another hospital. Approval from the ethics committee was obtained (Kantonale Ethikkommission Zürich, approvel number 2019-02201). Reporting follows the STROBE Statement, Version 4 [10].
The study institution cooperates with a local stroke centre, which is accessible by ambulance within 30 minutes and provides telemedical consultation. Computed tomography (CT) perfusion and CT angiography is available at any time. Physicians, nurses, physiotherapists, occupational therapists and logopedists are trained in diagnosis, treatment and prevention of stroke complications according to local protocols. These protocols are in accordance with the local stroke centre. The intensive care unit (ICU) provides round-the-clock monitoring and treatment for severe stroke patients and patients on intravenous thrombolysis [11]. A Standard Operating Procedure has been implemented in our institution and a stroke team has been defined, with training in regular intervals. Before admission, the emergency physician receives a pre-notification of arrival by the emergency medical services and activates the stroke team. A rapid registration process allows the door-to-needle time to be shortened. After assessment of the medical condition in the emergency unit, the patient is transported on the ambulance stretcher to the CT scanner, which is located in close proximity. After native brain imaging, to exclude bleeding and other possible causes, therapeutic decisions (intravenous alteplase, referral for interventional therapy) are made in accordance with the guidelines and after telemedical consultation with the stroke centre or in-hospital neurologists. Thrombolysis is administered in the scanner room after the exclusion of contraindications for thrombolysis.
Data from the medical and radiology reports, laboratory results and electronic or hand-written documentation were studied for the analysis. We collected general epidemiological data (age, sex), case statistics (date and time of admission and discharge, length of hospital and intensive care unit stay). We distinguished strokes that occurred during a hospital stay (in-hospital stroke) from those before hospital admission (out-of-hospital stroke). We categorised the place of stay before out-of-hospital strokes into home, retirement/nursing home, other hospital, other institution. According to the diagnosis in the discharge report, cases were classified into three groups: ischaemic stroke, intracranial bleeding and transient ischaemic attack (TIA). The aetiology of ischaemic strokes and TIAs were classified into Trial of Org 10172 in acute stroke treatment (TOAST) categories, as large artery atherosclerosis, cardioembolism, small vessel occlusion, stroke of other determined aetiology, stroke of undetermined aetiology [12]. When available from the records, the National Institutes of Health Stroke Scale (NIHSS) score [13] at first contact in the emergency unit, after thrombolysis and at discharge was analysed in all of the ischaemic stroke patients.
In TIA patients, the ABCD2 Score was calculated. The ABCD2 Score is composed of age, blood pressure, clinical features, duration of symptoms and the presence of diabetes mellitus. A higher score is associated with a higher risk of concurrent ischaemic stroke [14]. Intracranial bleedings were classified as mainly epidural, subdural, subarachnoid, intracerebral or combined, according to the radiology report.
In out-of-hospital strokes, patients were divided in subgroups according to time from beginning of symptoms to presentation in the emergency unit (<3 hours, 3–4.5 hours, 4.5–6 hours, 6–12 hours, 12–24 hours, >24 hours and unknown symptom duration). If duration of symptoms was not known (especially in wake-up strokes) and no subgroup assignment was possible, the time from symptoms to admission was labelled as “unknown”. In cases with symptom duration below 4.5 hours (early presentation), duration of symptoms was specified in minutes if available. Additionally, the interval between presentation and completion of cranial CT with and without perfusion scanning (door-to-CT time) and the interval between presentation and start of thrombolysis (door-to-needle time) were assessed.
We collected data about the risk factors for stroke: arterial hypertension, diabetes mellitus, dyslipidaemia, family history of ischaemic stroke, prior ischaemic stroke, smoking, overweight/obesity, alcohol consumption, sleep apnoea, depression, migraine with aura, pregnancy, hormonal contraception, postmenopausal hormone therapy, acute infection, chronic kidney disease, atrial fibrillation [15–17]. For the evaluation of dyslipidaemia, we also used laboratory analyses during hospital stay. Atrial fibrillation was documented during hospital stay or in ambulatory electrocardiographic monitoring following hospital discharge. We defined cut-offs for dyslipidaemia (low-density lipoprotein ≥2.5mmol/l, triglycerides >5.2mmol/l, high-density lipoprotein <1.0mmol/l or current lipid-lowering therapy), overweight or obesity (body mass index >25 kg/m2), alcohol consumption (>30 standard drinks per month) and chronic kidney disease (glomerular filtration rate <60 ml/min/1.73 m2) according to the “Berner Stroke Richtlinien (Version 2018)” [18]. We further defined smoking as current or former smoking in the last 10 years [19]. We differentiated between diagnosed comorbidities before stroke and comorbidities diagnosed during inpatient evaluation. Antiplatelet, anticoagulation and lipid lowering therapy at diagnosis was registered and compared with medication at discharge.
We compared the modified Rankin Scale (mRS) scores [20, 21] before stroke and at hospital discharge. The mRS is a grading system of functional independence in stroke patients ranging from 0 to 6.
Scores in the mRS correspond to: 0 no symptoms at all, 1 no significant disability despite symptoms; able to carry out all usual duties and activities; 2 slight disability; unable to carry out all previous activities, but able to look after own affairs without assistance; 3 moderate disability; requiring some help, but able to walk without assistance; 4 moderately severe disability; unable to walk without assistance and unable to attend to own bodily needs without assistance; 5 severe disability; bedridden, incontinent, and requiring constant nursing care and attention; 6 dead. If the mRS score was not available in the reports, we categorised patients according to the clinical history and nurse assessment into mRS groups. Finally, we analysed the well-known medical complications of stroke (aspiration pneumonia, urinary tract infection, seizures, deep venous thrombosis, pulmonary embolism, pressure ulcers, falls, [recurrent] ischaemic stroke) [22] and bleeding complications during hospital stay (secondary/recurrent intracranial bleeding, gastrointestinal bleeding, other bleeding).
Categorical variables were expressed as absolute numbers with percentages, normally distributed quantitative variables as mean ± standard deviation and non-normally distributed variables as median with interquartile range (IQR). Testing of non-normally distributed numerical and ordinal variables was performed with Wilcoxon signed-rank test. Pearson's chi-square or Fisher’s exact test was used to compare frequencies among groups. All tests were two-sided and a p-value <0.05 was considered statistically significant. Missing data were excluded from the analysis. Statistical analysis was performed with RStudio Version 1.1.463 for Mac.
Between July 2017 and December 2018, 262 stroke patients were included in the study cohort (4% of all inpatient medical cases). One patient had to be excluded because he refused any study participation. Patient characteristics of the 261 included patients are shown in table 1. Median age was 78 years (IQR 68–85); 121 patients (46%) were female. Females were older than men: 82 years (74–87) and 75 years (65–83), respectively, (p <0.001). Eighteen (7%) patient events occurred during hospital stay. Diagnosis was classified as ischaemic stroke in 166 (64%), TIA in 46 (18%) and intracranial bleeding in 49 (19%) cases. The aetiology of ischaemic strokes and TIAs were categorised as large artery atherosclerosis, cardioembolism, small vessel occlusion, stroke of other determined aetiology, stroke of undetermined aetiology in 21 (10%), 63 (30%), 22 (10%), 3 (1%), 103 (49%), respectively. Median ABCD2 Score in patients with a TIA was 4 (3–5). Intracranial bleeding was classified as intracerebral, subdural or subarachnoid in 20 (41%), 18 (37%) and 8 (16%) patients, respectively. Less common were epidural or a combination of bleeding in 1 (2%) and 2 (4%) patients, respectively.
Table 1 Patient characteristics in our study grouped as ischaemic strokes, transient ischaemic attacks and intracranial bleedings.
| Ischaemic stroke | TIA | Intracranial bleeding |
All
patients |
Undiagnosed comorbidity | |
|---|---|---|---|---|---|
| No. of patients | 166 (64) | 46 (18) | 49 (19) | 261 (100) | |
| Median age in years (IQR) | 78 (67–85) | 78 (73–83) | 79 (68–84) | 78 (68–85) | |
| Female | 76 (46) | 26 (57) | 19 (39) | 121 (46) | |
| In-hospital stroke | 17 (10) | 1 (2) | 0 (0) | 18 (7) | |
| Comorbidities | |||||
| Arterial hypertension | 127 (77) | 36 (78) | 32 (65) | 195 (75) | 10 (5) |
| Dyslipidaemia | 88 (53) | 24 (52) | 12 (24) | 124 (48) | 47 (38) |
| Overweight | 73 (44) | 16 (35) | 13 (27) | 102 (39) | 0 (0) |
| Prior ischaemic stroke | 49 (30) | 16 (35) | 5 (10) | 70 (27) | 9 (13) |
| Chronic kidney disease | 44 (27) | 8 (17) | 8 (16) | 60 (23) | 2 (3) |
| Atrial fibrillation/flutter | 46 (28) | 7 (15) | 6 (12) | 59 (23) | 16 (27) |
| Smoking | 31 (19) | 8 (17) | 15 (31) | 54 (21) | 0 (0) |
| Alcohol consumption | 31 (19) | 14 (30) | 6 (12) | 51 (20) | 0 (0) |
| Diabetes mellitus | 34 (20) | 4 (9) | 7 (14) | 45 (17) | 0 (0) |
| Family history (ischaemic) | 16 (10) | 6 (13) | 2 (4) | 24 (9) | 0 (0) |
| Depression | 18 (11) | 1 (2) | 4 (8) | 23 (9) | 3 (13) |
| Sleep apnoea | 10 (6) | 2 (4) | 5 (10) | 17 (7) | 2 (12) |
| Acute infection | 9 (5) | 1 (2) | 4 (8) | 14 (5) | 0 (0) |
| Migraine with aura | 9 (5) | 1 (2) | 2 (4) | 12 (5) | 1 (8) |
| Pregnancy | 1 (1) | 0 (0) | 0 (0) | 1 (0) | 0 (0) |
| Hormone therapy | 2 (1) | 0 (0) | 0 (0) | 2 (1) | 0 (0) |
| Oral contraception | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
IQR = interquartile range; TIA = transient ischaemic attack Categorical variables are presented as n (%). Quantitative variables are presented as median and interquartile range in parentheses. Undiagnosed comorbidities at admission are presented as count and percentages of patients with the specific risk factor in total.
Common risk factors were arterial hypertension in 195 (75%), dyslipidaemia in 124 (48%) and overweight/obesity in 102 (39%) patients. Dyslipidaemia and atrial fibrillation were undiagnosed at admission in 47 (38%) and 16 (27%) patients with the corresponding diagnosis at discharge. At admission, antiplatelet therapy, anticoagulation and lipid lowering medication was recorded in 96 (37%), 43 (16%) and 73 (28%) patients, respectively. Medication at admission and discharge is shown in table 2. Six patients (12%) with intracranial bleeding were discharged on antiplatelet therapy (one patient with dual antiplatelet therapy). Three of them were referred to a neurosurgical facility and did not receive antiplatelet therapy. The other three patients had chronic subdural bleeding and antiplatelet therapy was continued or re-initiated after risk-benefit evaluation. Twenty-seven patients with ischaemic stroke or TIA did not receive antithrombotic therapy at discharge. Reasons were in-hospital death (12 patients), best supportive care (2), refusal of antithrombotic therapy (1), ongoing intravenous thrombolysis and referral to a stroke centre (8), haemorrhagic complications (3), discharge under prophylactic anticoagulation after in-hospital stroke with outpatient re-initiation of a vitamin K antagonist (1). No patient in this cohort received dipyridamole
Table 2 Medication at admission and discharge according to stroke type.
|
Ischaemic stroke / TIA
(n = 212) |
Intracranial bleeding
(n = 49) |
|||
|---|---|---|---|---|
| Admission | Discharge | Admission | Discharge | |
| All antithrombotics | 108 (51) | 185 (87) | 25 (51) | 6 (12) |
| Antiplatelet agents | 80 (38) | 141 (67) | 16 (33) | 6 (12) |
| Acetylsalicylic acid | 65 (31) | 99 (47) | 16 (33) | 6 (12) |
| P2Y12 antagonist | 20 (9) | 47 (22) | 2 (4) | 1 (2) |
| Anticoagulation | 34 (16) | 58 (27) | 9 (18) | 0 (0) |
| VKA | 21 (10) | 12 (6) | 5 (10) | 0 (0) |
| UFH | 1 (0) | 0 (0) | 0 (0) | 0 (0) |
| LMWH | 0 (0) | 2 (1) | 0 (0) | 0 (0) |
| DOAC | 12 (6) | 44 (21) | 4 (8) | 0 (0) |
| Lipid-lowering therapy | 61 (29) | 142 (67) | 12 (24) | 6 (12) |
DOAC = directly acting oral anticoagulant; LMWH = low-molecular-weight heparin; TIA = transient ischaemic attack; UFH = unfractionated heparin; VKA = vitamin K antagonist Intracranial bleedings were defined as epidural, subdural, subarachnoid, intracerebral or combined intracranial bleeding according to the radiology report. Results are presented as no. (%).
There were 91 (37%) patients who were admitted to the emergency room with out-of-hospital stroke within 4.5 hours of symptom onset. Fifty-five (60%) were ischaemic strokes, 23 (25%) TIAs and 13 (14%) bleedings. Time from onset of symptoms to admission is presented in figure 1 for all patients and in figure 2 for patients who presented early (within 4.5 hours). Of the latter, 45 (49%) were female, compared with 68 (45%) patients who presented after 4.5 hours of symptom onset (late presentation) (p = 0.56). Median age was 76 years (IQR 67–84) and 79 years (70–84) in the early and late presenting groups, respectively (p = 0.35). The mRS score did not differ between early and late presenting patients (p = 0.72). Of early presenting patients, 75 (82%), 15 (16%), 0 (0%) and 1 (1%), respectively, were admitted from home, a retirement/nursing home, other hospital or other institution, compared with 136 (89%), 13 (9%), 1 (1%) and 2(1%), respectively, of late presenting patients (p = 0.18). Median time until admission in the early presentation group was 86 minutes (60–120). Median NIHSS score in this group was 5 (3–10) at admission, 2 (1–3) after thrombolysis and 1 (0–4) at discharge. Median time from admission to CT was 28 minutes (22–46), including perfusion scanning 48 minutes (40–63). Thrombolytic therapy was started in 27 patients, representing 49% of patients with out-of-hospital ischaemic stroke and presentation within 4.5 hours. Median door-to-needle time was 55 minutes (40–67). Door-to-needle time was within 60 minutes in 16 (59%) of patients.

Figure 1 Bar plot of time from beginning of symptoms in groups to presentation in the emergency unit. The group “unknown” represents cases, where group assignment was not possible (e.g., wake-up stroke).
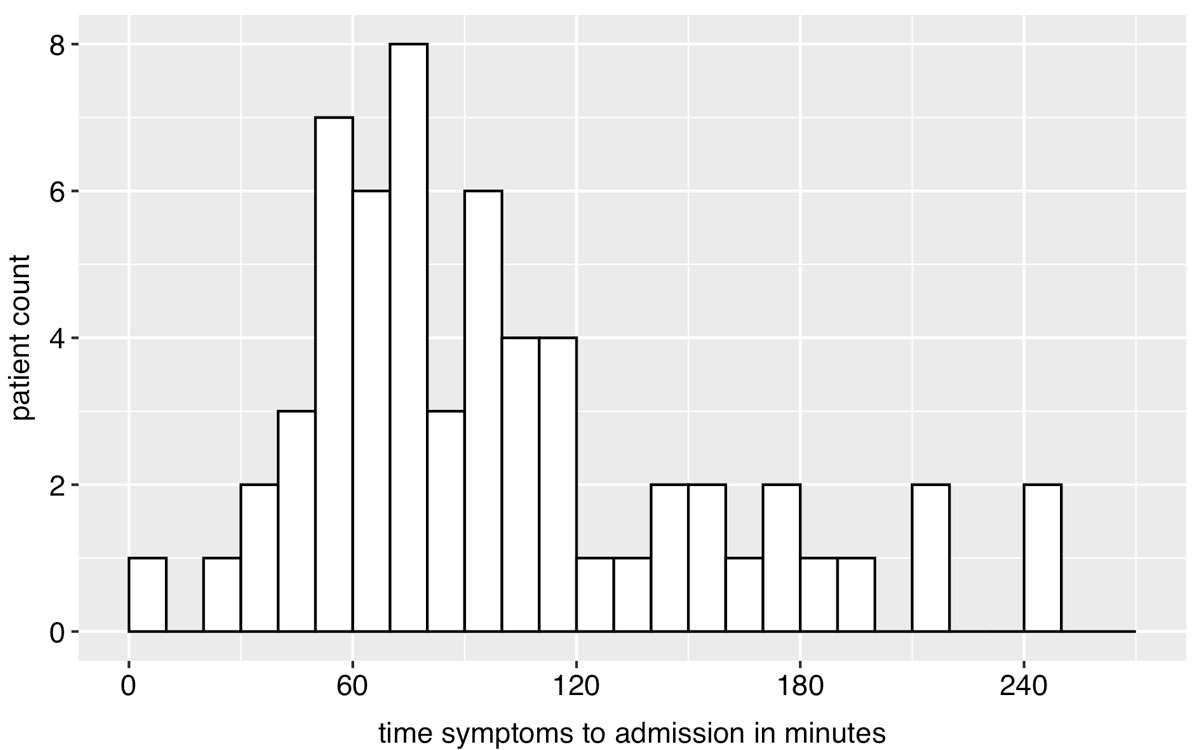
Figure 2 Histogram of time from beginning of symptoms to presentation in the emergency unit in early presenting patients (≤4.5 hours of symptom onset). Exact time was available for 60 of 91 patients.
The median hospital length of stay of treated patients treated in-house was 7.6 days (IQR 4.3–12.5) days. In-hospital mortality rate was 11%, corresponding to 28 patients. Half of them had ischaemic strokes and the other half intracranial bleeding. Figure 3 shows the mRS scores for all patients before stroke and at discharge. Median mRS score before stroke was 1 (0–3) and at discharge 3 (1–4). The median difference in mRS score calculated as “mRS before stroke” − “mRS at discharge” was 0 (0–2), but statistically significantly higher at discharge than before stroke (p <0.01). In total, 47 (18%) patients were referred to a tertiary hospital for an intervention or ICU admission, when in-house capacities were exhausted. The referral rate was 14% (23) for ischaemic strokes, 7% (3) for TIA and 43% (21) for intracranial bleedings. Follow-up by subgroup is presented in table 3. Complications were reported in 54 (21%) patients, most commonly aspiration pneumonia in 22 (8%) and urinary tract infection in 14 (5%) (table 4).

Figure 3 Modified Rankin scale (mRS) before stroke and at discharge. Median change in mRS (mRS at discharge – mRS before stroke) was 0 (interquartile range 0–2), p <0.01. Data were available for 249 patients before stroke and 241 patients at discharge of total 261 patients.
Table 3 Stroke severity and follow-up in all patients and attributed to ischaemic strokes, transient ischaemic attacks and intracranial bleedings.
| Ischaemic stroke | TIA | Intracranial bleeding | Total | |
|---|---|---|---|---|
| Median NIHSS (IQR) | ||||
| Admission (n = 138) | 3 (1–8) | 0 (0–2) | 2 (0–5) | 2 (0–6) |
| Discharge (n = 108) | 1 (0–2) | 0 (0–0) | 0 (0–4) | 0 (0–1) |
| Median mRS (IQR) | ||||
| Before stroke (n = 249) | 1 (0–2) | 1 (0–2) | 2 (0–3) | 1 (0–3) |
| Discharge (n = 241) | 3 (1–4) | 1 (0–2) | 4 (3–6) | 3 (1–4) |
| Median hospital LOS in days (IQR) | 9 (4–14) | 3 (2–6) | 2 (0–7) | 6 (3–12) |
| Median ICU LOS in days (IQR) | 1 (1–2) | 0 (0–0) | 1 (0–3) | 1 (0–2) |
| In-hospital mortality | 14 (8) | 0 (0) | 14 (29) | 28 (11) |
ICU = intensive care unit; IQR = interquartile range; LOS = length of stay; mRS = modified Rankin Scale; NIHSS = National Institutes of Health Stroke Scale; TIA = transient ischaemic atack Categorical variables are presented as n (%). Quantitative variables are presented as median and interquartile range in parentheses. Missing data occurred for NIHSS and mRS and led to a reduction of analysed cases (n) for these variables.
Table 4 Incidence of in-hospital complications after stroke.
| Complication | No. | % |
|---|---|---|
| Aspiration pneumonia | 22 | 8.4 |
| Urinary tract infection | 14 | 5.4 |
| Seizure | 10 | 3.8 |
| Fall | 10 | 3.8 |
| Intracranial bleeding | 4 | 1.5 |
| Ischaemic stroke | 4 | 1.5 |
| Pulmonary embolism | 2 | 0.8 |
| Bedsores | 2 | 0.8 |
| Gastrointestinal bleeding | 1 | 0.4 |
| Other bleeding | 1 | 0.4 |
| Deep venous thrombosis | 0 | 0.0 |
| Any complication | 54 | 20.7 |
Herein we demonstrate that about two thirds of our patients with stroke symptoms did not arrive in the emergency unit in a timely manner for thrombolytic therapy. The prevalence of risk factors for stroke that were not known or treated before admission was high for dyslipidaemia and atrial fibrillation, and warrants further actions to reduce stroke incidence. We showed that a community hospital can deliver thrombolytic therapy in a timely fashion and provide adequate care when stroke management is organised in close collaboration with a stroke centre.
Despite public information campaigns [23], most people do not reach a hospital in a timely manner (<4.5 hours) and are therefore not candidates for thrombolysis. In our cohort, only 37% of patients presented within 4.5 hours. This might be because of the higher median age of this population compared with other populations studied [24]. However, we did not find any difference in age, gender, disability before stroke according to the mRS and residence before stroke. Factors that are associated with delays in the stroke pathway are low stroke severity, nonspecific symptoms, no prior history of stroke and low socioeconomic status [25]. Awareness of stroke by patients or bystanders is associated with reduced admission delays [25, 26]. Therefore more effort must be made to educate the general population to recognise symptoms of stroke early and react appropriately, as thrombolysis, started within 4.5 hours, has been shown to improve neurological outcome [27, 28]. A qualified hospital in close proximity could improve the care of stroke and stroke mimics.
Our population was generally older (78 vs 67 years) than the western population of the Interstroke study [24], which evaluated modifiable risk factors for ischaemic and haemorrhagic stroke, with a slightly higher proportion of females (46% vs 40%) and a higher proportion of haemorrhagic strokes (19% vs 7%). Sex differences in stroke prevalence and the associated poorer outcome in females are poorly examined and understood [29]. In our study, we found no difference in the characteristics of patients who presented within or after 4.5 hours from symptom onset. This might be a result of the small sample size. The rate of cardiovascular and other modifiable risk factors for stroke was high compared with the Interstroke Study [24], except for a lower prevalence of diabetes mellitus in our cohort. Dyslipidaemia and atrial fibrillation were frequently undiagnosed at admission (38% and 27%, respectively, of patients with the diagnosis at discharge). This suggest that primary prevention, in atrial fibrillation, might further reduce the incidence of stroke in this independent elderly population [30]. Lipid-lowering therapy at admission was often missing, but there is some debate about cost effectiveness still pending in this elderly population [31]. Our data indicate that even in a high-income country like Switzerland, the rates of unrecognised risk factors for stroke are high. Further efforts must be made by primary care physicians to screen and treat modifiable risk factors for stroke [17], because their population attributable risk for stroke is high [24]. Systematic cardiovascular risk assessment is recommended by the European Society of Cardiology (ESC) in men over 40 years and women over 50 years of age (or post-menopausal), repeated at least every 5 years [32]. Opportunistic screening for atrial fibrillation with pulse taking or an electrocardiogram rhythm strip is recommended at an age of 65 years [33]. A summary of the ESC recommendations regarding arterial hypertension, atrial fibrillation and dyslipidaemia is presented in table 5.
Table 5 Screening recommendations of the European Society of Cardiology for dyslipidaemia, arterial hypertension and atrial fibrillation.
| Cardiovascular risk factor | ESC screening recommendation |
|---|---|
| Dyslipidaemia | Men >40 years and women >50 years (or post-menopausal) [34] |
| Arterial hypertension | All adults, repeated at least every 5 years when blood pressure is optimal (<120/80 mm Hg), every 3 years when blood pressure is normal (120–129/80–84 mm Hg) or annually when blood pressure is high-normal (130–139/85–89 mm Hg) [35] |
| Atrial fibrillation | Opportunistic screening in adults ≥ 65 years of age with pulse taking or electrocardiogram rhythm strip [33] |
We reported time-delays in the stroke pathway that are similar to international data from stroke centres. The median door-to-needle time in our patients was 55 minutes (IQR 40–67). The infusion of intravenous alteplase was started within 60 minutes of presentation in the emergency unit in 59% of the patients. A single-centre analysis in another Swiss hospital without a neuro-intensive care unit found a door-to-needle time of 74 minutes in 2004 [36]. Similar door-to-needle times were reported previously in the United States and Canada [37–39]. A recently published retrospective cohort study by Man et al. reported a median door-to-needle time of 65 minutes with a wide variability (IQR 49–88 minutes) and a rate of door-to-needle time within 60 minutes of 44% [40]. A close collaboration with an associated stroke centre, the close proximity of the CT scanner and the constant training could have influenced the time-delay beneficially, despite the relatively low caseload. A stroke management strategy with telemedical support from a stroke centre resulted in a mortality and functional independence at 3 months similar to stroke centres in a meta-analysis [41]. Consultation of stroke specialists recently became more important, as evaluation of reperfusion therapy is getting more complex. We do telemedical consultation with neurological specialists of the stroke centre, which allows us to treat most of the patients (82%) if invasive therapies are not indicated. Substantially lower in-hospital delays in thrombolysis less than 20 minutes were achieved in prospective study settings in Finland, the Netherlands and South Korea [42–45]. However, quality measurements inside study settings may not be comparable to “real-world” data. This might be a strength of our study, which is retrospective in design, documents the real-world setting in the care of the patients in this hospital. The current guidelines of the American Heart Association / American Stroke Association [46] did not specify time goals, but rather recommend that eligible patients receive thrombolysis as soon as possible. Despite an association between higher centre volume and shorter door-to-needle time [47], a delay in thrombolytic therapy of a median of 30 minutes is possible in community hospitals [48] and should be aimed for. We believe that close collaboration of community hospitals with stroke centres could achieve this goal, because there is no further delay due to transportation issues.
In our study, the in-hospital complications during stroke treatment were less frequent than in other reports [22, 49]. Only a Danish registry study reported similar low rates of complications [50]. There are several possible reasons for the differences between complication rates. First, we have a stroke care unit in our hospital with trained nurses, physiotherapists and systematic screening for dysphagia, which has been shown to reduce the incidence of aspiration pneumonia [51]. Nevertheless, aspiration pneumonia was the most common complication in our cohort, which underlines the need for further improvement in dysphagia screening and the need to improve the preventive strategies to reduce the rate. Second, the retrospective study design may underreport less severe complications (for example, minor falls). Third, compared with the multicentre study of Langhorne et al. [22], we generally discharged patients early into a rehabilitation facility, after a median of 7.4 days, and there was no systematic follow-up after discharge. Finally, the complication rate after stroke seems to decline with improvement of medical care [49], which makes comparisons with older studies difficult.
We showed that more than half of the patients with intracranial bleeding were managed conservatively, and the rate of referral to a clinic with neurosurgical resources was 43%. We have no data about the treatment after referral. At our institution, we obtain a standardised neurosurgical consultation about all intracranial bleedings for further treatment decisions.
In our population, over 60% of patients had no significant disability before the onset of the stroke, according to the modified Rankin Scale (mRS 0–1). At discharge we observed a high rate of disability according to the mRS (fig. 3). Mortality was mainly driven by death after intracranial bleeding. Many of these patients received palliative therapy in accordance with an advanced care plan. This study did not aim to investigate stroke outcomes, because of the short follow-up until discharge and early referral to a rehabilitation facility. Mortality and complications may be higher during and after rehabilitation. Furthermore, functional recovery can continue for 3 to 18 months after the onset of stroke [52, 53].
The study has several additional limitations. It is retrospective in design and the absence of standardised reporting forms makes the results susceptible to underreporting, misclassification and selection bias. We had some missing data that could influence the results. Additional data about risk factors (e.g., antihypertensive therapy, physical activity) and stroke management (e.g., glucose measurements, blood pressure, temperature management, fluid administration) were not collected, but are important in the evaluation of quality of care. The number of patients was relatively small, which makes subgroup analyses difficult, especially for the analyses of patients who presented within 4.5 hours. As mentioned before, the short follow-up time, especially the absence of 3-month outcome data limits the validity. The treatment should be evaluated prospectively to further improve stroke care in community hospitals and evaluate the cost of care in comparison with stroke centres. The generalisability of the results is limited owing to the specific setting of this single-centre survey. First, probably not all patients with symptoms of stroke were admitted to our hospital. Although the emergency medical service that is affiliated to the study centre does not use triage criteria, direct admission to a stroke centre will be more likely in parts of the area that are close to the stroke centre, especially for patients with severe symptoms or suspected of intracranial bleeding. Second, results may not be comparable with other hospitals that do not use telemedical consultation with a stroke centre.
Our findings support further education of the population in recognition of stroke symptoms and assessment of cardiovascular risk factors according to guidelines. Telemedical cooperation with a local stroke can result in adequate quality of care and collaboration with a stroke centre is crucial for evaluation of reperfusion therapy.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Meyer K , Simmet A , Arnold M , Mattle H , Nedeltchev K . Stroke events, and case fatalities in Switzerland based on hospital statistics and cause of death statistics. Swiss Med Wkly. 2009;139(5-6):65–9. doi:.https://doi.org/10.4414/smw.2009.12448
2Bundesamt für Statistik. Bilanz der ständigen Wohnbevölkerung, 1861-2018 [Internet]. [cited 2020 June 26]. Available from: https://www.bfs.admin.ch/bfs/en/home/statistics/catalogues-databases/tables.assetdetail.9486043.html.
3Bundesamt für Statistik. Sterbeziffern für 30 wichtige Todesursachen nach Geschlecht [Internet]. [cited 2020 Jan 09]. Available from: https://www.bfs.admin.ch/bfs/de/home/statistiken/gesundheit/gesundheitszustand/sterblichkeit-todesursachen/spezifische.assetdetail.11348828.html.
4 Snozzi P , Blank PR , Szucs TD . Stroke in Switzerland: social determinants of treatment access and cost of illness. J Stroke Cerebrovasc Dis. 2014;23(5):926–32. doi:.https://doi.org/10.1016/j.jstrokecerebrovasdis.2013.07.042
5 Glozier N , Hackett ML , Parag V , Anderson CS ; Auckland Regional Community Stroke (ARCOS) Study Group. The influence of psychiatric morbidity on return to paid work after stroke in younger adults: the Auckland Regional Community Stroke (ARCOS) Study, 2002 to 2003. Stroke. 2008;39(5):1526–32. doi:.https://doi.org/10.1161/STROKEAHA.107.503219
6 Ekker MS , Verhoeven JI , Vaartjes I , Jolink WMT , Klijn CJM , de Leeuw FE . Association of Stroke Among Adults Aged 18 to 49 Years With Long-term Mortality. JAMA. 2019;321(21):2113–23. doi:.https://doi.org/10.1001/jama.2019.6560
7 Sacco RL , Kasner SE , Broderick JP , Caplan LR , Connors JJ , Culebras A , et al.; American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064–89. doi:.https://doi.org/10.1161/STR.0b013e318296aeca
8 Easton JD , Saver JL , Albers GW , Alberts MJ , Chaturvedi S , Feldmann E , et al.; American Heart Association; American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Interdisciplinary Council on Peripheral Vascular Disease. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40(6):2276–93. doi:.https://doi.org/10.1161/STROKEAHA.108.192218
9 Matis GK , Chrysou OI , Birbilis TA . Organizational issues in stroke treatment: The Swiss paradigm - Stroke units. J Neurosci Rural Pract. 2013;4(S 01, Suppl 1):S131–3. doi:.https://doi.org/10.4103/0976-3147.116450
10 von Elm E , Altman DG , Egger M , Pocock SJ , Gøtzsche PC , Vandenbroucke JP ; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7. doi:.https://doi.org/10.1016/S0140-6736(07)61602-X
11 Lyrer PM , Arnold M , Hungerbühler H , Gralla J , Humm A , Fandino J , et al. Stroke Units und Stroke Centers in der Schweiz. Richtlinien und Anforderungsprofil. Swiss Medical Forum. 2012;12(47):918–22. doi:.https://doi.org/10.4414/smf.2012.01293
12 Adams HP, Jr , Bendixen BH , Kappelle LJ , Biller J , Love BB , Gordon DL , et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. doi:.https://doi.org/10.1161/01.STR.24.1.35
13 Brott T , Adams HP, Jr , Olinger CP , Marler JR , Barsan WG , Biller J , et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864–70. doi:.https://doi.org/10.1161/01.STR.20.7.864
14 Johnston SC , Rothwell PM , Nguyen-Huynh MN , Giles MF , Elkins JS , Bernstein AL , et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369(9558):283–92. doi:.https://doi.org/10.1016/S0140-6736(07)60150-0
15 Simons LA , McCallum J , Friedlander Y , Simons J . Risk factors for ischemic stroke: Dubbo Study of the elderly. Stroke. 1998;29(7):1341–6. doi:.https://doi.org/10.1161/01.STR.29.7.1341
16 Kajitani N , Uchida HA , Suminoe I , Kakio Y , Kitagawa M , Sato H , et al. Chronic kidney disease is associated with carotid atherosclerosis and symptomatic ischaemic stroke. J Int Med Res. 2018;46(9):3873–83. doi:.https://doi.org/10.1177/0300060518781619
17 Meschia JF , Bushnell C , Boden-Albala B , Braun LT , Bravata DM , Chaturvedi S , et al.; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; Council on Hypertension. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(12):3754–832. doi:.https://doi.org/10.1161/STR.0000000000000046
18Berner Stroke Netzwerks. Stroke Richtlinien. 2018. Available from: http://www.neurologie.insel.ch/de/unser-angebot/stroke-center/stroke-richtlinien/
19 Duncan MS , Freiberg MS , Greevy RA, Jr , Kundu S , Vasan RS , Tindle HA . Association of Smoking Cessation With Subsequent Risk of Cardiovascular Disease. JAMA. 2019;322(7):642–50. doi:.https://doi.org/10.1001/jama.2019.10298
20 Rankin J . Cerebral vascular accidents in patients over the age of 60. II. Prognosis. Scott Med J. 1957;2(5):200–15. doi:.https://doi.org/10.1177/003693305700200504
21 van Swieten JC , Koudstaal PJ , Visser MC , Schouten HJ , van Gijn J . Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–7. doi:.https://doi.org/10.1161/01.STR.19.5.604
22 Langhorne P , Stott DJ , Robertson L , MacDonald J , Jones L , McAlpine C , et al. Medical complications after stroke: a multicenter study. Stroke. 2000;31(6):1223–9. doi:.https://doi.org/10.1161/01.STR.31.6.1223
23Schweizerische Herzstiftung. Hirnschlagkampagne: Ein Hirnschlag kann jeden treffen, jederzeit [cited 2020 July 07]. Available from: https://www.swissheart.ch/de/ueber-uns/kampagnen/hirnschlagkampagne.html
24 O’Donnell MJ , Chin SL , Rangarajan S , Xavier D , Liu L , Zhang H , et al.; INTERSTROKE investigators. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388(10046):761–75. doi:.https://doi.org/10.1016/S0140-6736(16)30506-2
25 Lachkhem Y , Rican S , Minvielle É . Understanding delays in acute stroke care: a systematic review of reviews. Eur J Public Health. 2018;28(3):426–33. doi:.https://doi.org/10.1093/eurpub/cky066
26 Sekoranja L , Griesser AC , Wagner G , Njamnshi AK , Temperli P , Herrmann FR , et al. Factors influencing emergency delays in acute stroke management. Swiss Med Wkly. 2009;139(27-28):393–9. doi:.https://doi.org/10.4414/smw.2009.12506
27 Emberson J , Lees KR , Lyden P , Blackwell L , Albers G , Bluhmki E , et al.; Stroke Thrombolysis Trialists’ Collaborative Group. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929–35. doi:.https://doi.org/10.1016/S0140-6736(14)60584-5
28 Hacke W , Kaste M , Bluhmki E , Brozman M , Dávalos A , Guidetti D , et al.; ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–29. doi:.https://doi.org/10.1056/NEJMoa0804656
29 Reeves MJ , Bushnell CD , Howard G , Gargano JW , Duncan PW , Lynch G , et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7(10):915–26. doi:.https://doi.org/10.1016/S1474-4422(08)70193-5
30 Meyre P , Conen D , Osswald S , Kühne M , Meyer-Zürn C . Atrial fibrillation for internists: current practice. Swiss Med Wkly. 2020;150:w20196. doi:.https://doi.org/10.4414/smw.2020.20196
31 Odden MC , Pletcher MJ , Coxson PG , Thekkethala D , Guzman D , Heller D , et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med. 2015;162(8):533–41. doi:.https://doi.org/10.7326/M14-1430
32 Piepoli MF , Hoes AW , Agewall S , Albus C , Brotons C , Catapano AL , et al.; ESC Scientific Document Group. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315–81. doi:.https://doi.org/10.1093/eurheartj/ehw106
33 Hindricks G , Potpara T , Dagres N , Arbelo E , Bax JJ , Blomström-Lundqvist C , et al.; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2021;42(5):373–498. doi:.https://doi.org/10.1093/eurheartj/ehaa612
34 Mach F , Baigent C , Catapano AL , Koskinas KC , Casula M , Badimon L , et al.; Authors/Task Force Members; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290:140–205. doi:.https://doi.org/10.1016/j.atherosclerosis.2019.08.014
35 2018 ESC/ESH Guidelines for the management of arterial hypertension. Rev Esp Cardiol (Engl Ed). 2019;72(2):160. doi:.https://doi.org/10.1016/j.rec.2018.12.004
36 Wiegand N , Lüthy R , Vogel B , Straumann E , Beynon C , Bertel O , et al. Intravenous thrombolysis for acute ischaemic stroke in a hospital without a specialised neuro-intensive care unit. Swiss Med Wkly. 2004;134(1-2):14–7. doi:.https://doi.org/10.1093/eurheartj/ehaa612
37 Kamal N , Sheng S , Xian Y , Matsouaka R , Hill MD , Bhatt DL , et al. Delays in Door-to-Needle Times and Their Impact on Treatment Time and Outcomes in Get With The Guidelines-Stroke. Stroke. 2017;48(4):946–54. doi:.https://doi.org/10.1161/STROKEAHA.116.015712
38 Tong X , Wiltz JL , George MG , Odom EC , Coleman King SM , Chang T , et al.; Paul Coverdell National Acute Stroke Program team. A Decade of Improvement in Door-to-Needle Time Among Acute Ischemic Stroke Patients, 2008 to 2017. Circ Cardiovasc Qual Outcomes. 2018;11(12):e004981. doi:.https://doi.org/10.1161/CIRCOUTCOMES.118.004981
39 Xian Y , Xu H , Lytle B , Blevins J , Peterson ED , Hernandez AF , et al. Use of Strategies to Improve Door-to-Needle Times With Tissue-Type Plasminogen Activator in Acute Ischemic Stroke in Clinical Practice: Findings from Target: Stroke. Circ Cardiovasc Qual Outcomes. 2017;10(1):e003227. doi:.https://doi.org/10.1161/CIRCOUTCOMES.116.003227
40 Man S , Xian Y , Holmes DN , Matsouaka RA , Saver JL , Smith EE , et al. Association Between Thrombolytic Door-to-Needle Time and 1-Year Mortality and Readmission in Patients With Acute Ischemic Stroke. JAMA. 2020;323(21):2170–84. doi:.https://doi.org/10.1001/jama.2020.5697
41 Kepplinger J , Barlinn K , Deckert S , Scheibe M , Bodechtel U , Schmitt J . Safety and efficacy of thrombolysis in telestroke: A systematic review and meta-analysis. Neurology. 2016;87(13):1344–51. doi:.https://doi.org/10.1212/WNL.0000000000003148
42 Meretoja A , Strbian D , Mustanoja S , Tatlisumak T , Lindsberg PJ , Kaste M . Reducing in-hospital delay to 20 minutes in stroke thrombolysis. Neurology. 2012;79(4):306–13. doi:.https://doi.org/10.1212/WNL.0b013e31825d6011
43 Wu TY , Coleman E , Wright SL , Mason DF , Reimers J , Duncan R , et al. Helsinki Stroke Model Is Transferrable With “Real-World” Resources and Reduced Stroke Thrombolysis Delay to 34 min in Christchurch. Front Neurol. 2018;9:290. doi:.https://doi.org/10.3389/fneur.2018.00290
44 Jeon SB , Ryoo SM , Lee DH , Kwon SU , Jang S , Lee EJ , et al. Multidisciplinary Approach to Decrease In-Hospital Delay for Stroke Thrombolysis. J Stroke. 2017;19(2):196–204. doi:.https://doi.org/10.5853/jos.2016.01802
45 Van Schaik SM , Van der Veen B , Van den Berg-Vos RM , Weinstein HC , Bosboom WMJ . Achieving a door-to-needle time of 25 minutes in thrombolysis for acute ischemic stroke: a quality improvement project. J Stroke Cerebrovasc Dis. 2014;23(10):2900–6. doi:.https://doi.org/10.1016/j.jstrokecerebrovasdis.2014.07.025
46 Powers WJ , Rabinstein AA , Ackerson T , Adeoye OM , Bambakidis NC , Becker K , et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–418. doi:.https://doi.org/10.1161/STR.0000000000000211
47 Strbian D , Ahmed N , Wahlgren N , Lees KR , Toni D , Roffe C , et al.; SITS Investigators. Trends in Door-to-Thrombolysis Time in the Safe Implementation of Stroke Thrombolysis Registry: Effect of Center Volume and Duration of Registry Membership. Stroke. 2015;46(5):1275–80. doi:.https://doi.org/10.1161/STROKEAHA.114.007170
48 Kamal N , Shand E , Swanson R , Hill MD , Jeerakathil T , Imoukhuede O , et al. Reducing Door-to-Needle Times for Ischaemic Stroke to a Median of 30 Minutes at a Community Hospital. Can J Neurol Sci. 2019;46(1):51–6. doi:.https://doi.org/10.1017/cjn.2018.368
49 Bovim MR , Askim T , Lydersen S , Fjærtoft H , Indredavik B . Complications in the first week after stroke: a 10-year comparison. BMC Neurol. 2016;16(1):133. doi:.https://doi.org/10.1186/s12883-016-0654-8
50 Ingeman A , Andersen G , Hundborg HH , Svendsen ML , Johnsen SP . In-hospital medical complications, length of stay, and mortality among stroke unit patients. Stroke. 2011;42(11):3214–8. doi:.https://doi.org/10.1161/STROKEAHA.110.610881
51 Hinchey JA , Shephard T , Furie K , Smith D , Wang D , Tonn S ; Stroke Practice Improvement Network Investigators. Formal dysphagia screening protocols prevent pneumonia. Stroke. 2005;36(9):1972–6. doi:.https://doi.org/10.1161/01.STR.0000177529.86868.8d
52 Jørgensen HS , Nakayama H , Raaschou HO , Vive-Larsen J , Støier M , Olsen TS . Outcome and time course of recovery in stroke. Part II: Time course of recovery. The Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76(5):406–12. doi:.https://doi.org/10.1016/S0003-9993(95)80568-0
53 Hankey GJ , Spiesser J , Hakimi Z , Bego G , Carita P , Gabriel S . Rate, degree, and predictors of recovery from disability following ischemic stroke. Neurology. 2007;68(19):1583–7. doi:.https://doi.org/10.1212/01.wnl.0000260967.77422.97
No financial support and no other potential conflict of interest relevant to this article was reported.