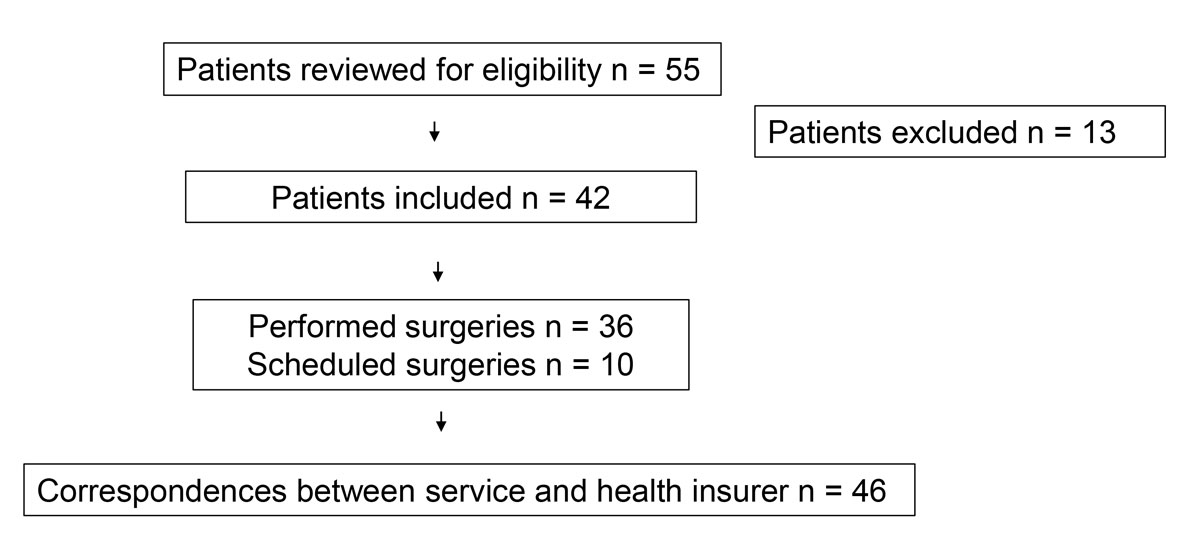
Figure 1 Patient and case selection.
DOI: https://doi.org/10.4414/smw.2021.20456
Lymphoedema is a progressive and potentially debilitating condition of chronic localised retention of protein-rich interstitial fluid and tissue remodelling caused by a compromised lymphatic system, which can be hereditary (primary) or acquired (secondary) [1]. The condition can be disabling psychologically and physically. Negative effects brought on by the impairment of activities in daily life and reduced limb aesthetics decrease health-related quality of life [2–5]. In developed countries, lymphoedema most commonly occurs after cancer treatment. Symptoms include swelling, recurrent skin infections and impairment of limb functionality. Complex physical therapy represents the gold standard for basic treatment of symptomatic lymphoedema. It is very often effective, yet often needs to be applied life-long because it does not treat the underlying causes and includes general skin care [6, 7].
A growing amount of literature reports promising clinical outcomes in lymphoedema patients following microsurgical reconstruction [8–14]. Two surgical concepts are currently employed: bypassing lymphatic fluid by anastomosing congested lymph vessels to venules (lymphovenous anastomosis, LVA) or microvascular transfer of lymphatic tissue to the affected limb (LTT). These options can performed either sequentially or singly, depending on lymphoedema stage and postsurgical outcomes. Especially in early stages of lymphoedema, where lymphatic vessels are not yet fibrotic, the outcome after LVA is promising. It represents a reconstructive procedure. Its combination with LTT might potentially improve overall results, but this has yet to be proven.
Surgical treatment is currently not supported by level 1 evidence. In 2018, researchers from the Ludwig-Boltzmann Institute in Austria conducted a systematic review on the effectiveness of LVA in lymphoedema and published a national decision support document [15]. Owing to methodological shortcomings, they could not conclude whether the LVA is at least equally effective and safer than the comparator LTT or conservative treatment, and back then initially recommended a temporary withholding of cost reimbursement. However, after several communications with the Austrian Health Insurance Fund, LVA and LTT were finally listed as novel surgical therapies in the 2020 reimbursement catalogue [16].
Insurance companies in Switzerland still use an individualised approach to cost coverage of lymphatic microsurgery. The use of lymphatic reconstructive surgery for the treatment of lymphoedema is not generally reimbursed by the Swiss healthcare system at this point of time. A written request is needed in all cases. And some insurers cover lymphatic microsurgery after receiving a written request. How such policies play out in practice is important for patients and providers as there is often a discordance between the coverage criteria determined by insurers and indications for the procedures recognised by plastic surgeons. In order to contribute to the understanding of this situation in Switzerland, we reviewed insurance coverage policies and coverage decisions from 2017 to 2020 in our institution.
At the University Hospital in Zurich we have established a registry to follow up all lymphoedema patients who have been treated with lymphatic microsurgery since September 2016, for internal qualitative analysis (KEK ID Req-2018-00284). Approval to conduct this retrospective analysis was additionally obtained from the local institutional review board (KEK BASEC ID 2019-00947) in 2019.
This institutional experience is from the University Hospital of the Canton Zurich with the biggest catchment area. Although most probably representative for Switzerland, outcomes in Zurich may not be valid for every single canton or local healthcare system in Switzerland. However, currently surgeons offering lymphatic surgery cannot take reimbursement for granted and this is observed all over Switzerland. At our department we routinely file a written application for reimbursement of costs to health insurers prior to the planned surgery.
Diagnosis of lymphoedema is generally based on patient history, clinical examination and subjective symptoms, with testing used to rule out other potential causes and confounding conditions. The International Society of Lymphology (ISL) Staging System is applied to further classify lymphedema. Imaging modalities are useful adjuncts to the ISL staging to clarify the diagnosis. At our institution, lymphoedema patients who are assigned for surgical therapy are discussed in an interdisciplinary setting by an angiologist, plastic surgeon and physiotherapist. This multidisciplinary group reviews all patients regardless of insurance status or financial capacities. Eligible patients were those judged medically appropriate for reconstructive microsurgical therapies. No patient received reconstructive treatment without previous complex decongestive therapy over at least 6 months by experienced physiotherapists.
Patients receive treatment according to the disease stage and their general condition in a standardised institutional approach. Patients who have had lymphoedema for 3 years and with lymphoedema stage I–II usually have little fibrosis and functional lymphatics. LVA is then performed to prevent progression of the disease and treat already existing lymphoedema. In patients who have had lymphoedema for more than 3 years, in stage II–III and in patients with primary lymphoedema, lymphatics are often sclerotic, non-functional or absent. These patients qualify for LTT. However, intraoperative impedance cardiography-guided LVA is usually attempted in order to facilitate more rapid decongestion, especially in the distal part of the limb. Suction-assisted lipectomy may be useful both as a primary and secondary “touch-up” procedure in lymphoedema stage II–III patients after lymphatic reconstruction. Excisional debulking procedures should be limited to morbid stage IV lymphoedema cases (elephantiasis). Surgery is performed under general anaesthesia for better patient comfort and patients are hospitalised for two to four nights. A standard protocol requires postoperative follow-up of all patients together with the physiotherapist in our outpatient clinic at 2 weeks, 6 weeks, 3 months and 1 year postoperatively. Physiotherapy including compression garments, bandages and manual lymphatic drainage is initiated gradually at 2 weeks postoperatively. Limb measurements are taken by the physiotherapist at every visit.
Patients’ electronic records were reviewed and correspondence between our department and the health insurers between 2017 and 2020 were analysed. We excluded international patients, cases with incomplete records, and patients who refused approval for data evaluation. A list of rationales and explanations was abstracted from the correspondence. Number of communications and time between confirmation of the indication by the plastic surgeon and the final decision on the assumption of costs from health insurers were reviewed. Further demographic data, insurance details and outcomes after application for reimbursement of costs were extracted from the patient chart.
A web-based search aimed at identifying whether established medical criteria and policies were publicly accessible on the corresponding company’s website. When the policy could not be abstracted from the company website, a telephone call was made. We aimed to assess individual criteria for the decisions. The insurance company was deemed to not have a policy for reconstructive microsurgery surgery in lymphoedema patients only if confirmed by a representative of the company.
The statistical analysis used descriptive and summary statistics to identify central tendencies. Data were analysed using Microsoft® Excel Version 14.3.6. (Microsoft Corp., Redmond, WA, USA). Continuous variables are expressed as mean ± standard deviation. Categorical variables are expressed as frequencies or percentages. This study was conducted according to the STROBE (Strengthening the Reporting of Observational studies in Epidemiology) guidelines.
We reviewed 55 patient charts and included 42 patients who were eligible for analysis. They had either already undergone or were scheduled for lymphatic microsurgery. Four patients underwent two subsequent lymphatic reconstructive surgeries. Finally, a total of 46 sets of correspondence between our department and the insurance companies were identified. Thirteen patients were excluded because of incomplete records, lack of or refusal to sign the institutional general consent form, or because they were international patients. Figure 1 shows a flow chart for patient selection.

Figure 1 Patient and case selection.
Thirty-eight patients were female (90%) and four patients were male (10%). The age of the patients ranged from 21 to 77 years (mean ± standard deviation 52 ± 11). Mean body mass index (BMI) was 27 ± 5 kg/m2. Seven patients had primary lymphoedema and had developed lymphoedema in adulthood: The disease occurred at an average age of 41 years (range 26–61). One patient had a family history of lymphoedema. Secondary lymphoedema was present in 35 patients for more than 3 years. Lower limb lymphoedema was present in 33 patients and upper limb lymphedema in 9 patients. Seventeen patients had ISL stage I lymphoedema, 22 patients stage II and 3 patients stage III. Ten surgeries were scheduled and a total of 36 surgeries had already been performed. We performed multiple LVA in 22 patients, of whom 3 underwent LTT sequentially (i.e., in a second operation following the LVA procedure) and 1 had a second LVA procedure. One patient received LTT only. Nine patients had LVA and LTT simultaneously in one operation and for 10 patients for this combined surgical approach was planned. Main subjective improvements reported by patients at 6 months postoperatively were: less pressure sensation, less tension, increased softness, less heaviness, reduced swelling, increased mobility and pain reduction. Table 1 details demographic data for each procedure, distribution of the patients concerning stage of lymphoedema, insurance details and outcomes after application for reimbursement of costs for each surgical case. The distribution of patients’ BMI was comparable between the different insurance companies.
Table 1 Demographic data, insurance details and outcomes per surgical case.
| ID | Type of lymphoedema | ISL stage (1–3) | Affected limb | Gender | Year of sugery | Type of surgery | Health Insurer A–I | Number of appeals by letter | Final decision |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Secondary | 2 | Arm | F | 2017 | LVA | A | 1 | Approve |
| 2 | Primary | 2 | Leg | F | 2018 | LVA | B | 1 | Approve |
| 3 | Primary | 2 | Leg | F | 2017 | LVA | B | 1 | Approve |
| 4 | Secondary | 1 | Arm | F | 2018 | LVA | B | 1 | Refuse |
| 5 | Primary | 2 | Leg | F | 2019 | LTT | B | 1 | Approve |
| 6 | Secondary | 2 | Leg | F | 2020 | LVA and LTT | B | 1 | Approve |
| 7 | Secondary | 1 | Arm | F | 2020 | LVA | B | 1 | Approve |
| 8 | Secondary | 2 | Leg | F | 2020 | LVA and LTT | B | 1 | Approve |
| 9 | Secondary | 1 | Leg | F | 2018 | LVA | B | 1 | Approve |
| 10 | Secondary | 2 | Leg | F | 2018 | LTT | C | 1 | Approve |
| 11 | Secondary | 1 | Leg | F | 2018 | LVA | C | 1 | Approve |
| 12 | Secondary | 1 | Leg | F | 2019 | LVA | C | 1 | Approve |
| 13 | Primary | 1 | Leg | F | 2017 | LVA | C | 1 | Approve |
| 14 | Secondary | 2 | Leg | F | 2020 | LVA and LTT | C | 1 | Approve |
| 15 | Secondary | 2 | Leg | F | 2020 | LVA and LTT | C | 1 | Approve |
| 16 | Secondary | 1 | Arm | F | 2019 | LVA | C | 1 | Approve |
| 17 | Primary | 2 | Leg | F | 2019 | LVA and LTT | C | 1 | Approve |
| 18 | Secondary | 2 | Leg | F | 2020 | LVA and LTT | C | 1 | Approve |
| 19 | Secondary | 2 | Arm | F | 2020 | LVA and LTT | C | 1 | Approve |
| 20 | Secondary | 2 | Leg | M | 2020 | LVA and LTT | C | 1 | Approve |
| 21 | Secondary | 2 | Leg | F | 2017 | LVA | D | 1 | Approve |
| 22 | Secondary | 3 | Leg | F | 2019 | LVA and LTT | E | 3 | Refuse |
| 23 | Secondary | 1 | Leg | F | 2019 | LVA and LTT | E | 2 | Refuse |
| 24 | Secondary | 1 | Leg | F | 2019 | LTT | E | 1 | Refuse |
| 25 | Secondary | 2 | Leg | F | 2018 | LVA | E | 1 | Approve |
| 26 | Secondary | 3 | Arm | F | 2017 | LTT | E | 1 | Refuse |
| 27 | Secondary | 1 | Leg | F | 2017 | LVA | E | 3 | Refuse |
| 28 | Secondary | 2 | Leg | M | 2017 | LVA | E | 1 | Approve |
| 29 | Secondary | 1 | Arm | F | 2017 | LVA | E | 2 | Refuse |
| 30 | Secondary | 1 | Leg | F | 2017 | LVA | E | 2 | Refuse |
| 31 | Secondary | 3 | Arm | F | 2017 | LVA | E | 2 | Refuse |
| 32 | Secondary | 1 | Leg | F | 2017 | LVA | F | 1 | Approve |
| 33 | Secondary | 1 | Leg | F | 2018 | LVA | F | 1 | Approve |
| 34 | Secondary | 2 | Leg | F | 2018 | LVA | F | 1 | Approve |
| 35 | Secondary | 2 | Leg | F | 2019 | LVA | F | 1 | Approve |
| 36 | Secondary | 1 | Arm | F | 2019 | LVA and LTT | F | 1 | Approve |
| 37 | Primary | 1 | Leg | F | 2019 | LVA and LTT | F | 2 | Refuse |
| 38 | Secondary | 3 | Leg | M | 2020 | LVA and LTT | F | 1 | Approve |
| 39 | Primary | 2 | Leg | F | 2020 | LVA and LTT | F | 1 | Approve |
| 30 | Secondary | 2 | Leg | F | 2020 | LVA and LTT | G | 1 | Refuse |
| 41 | Secondary | 2 | Leg | F | 2020 | LVA and LTT | G | 1 | Refuse |
| 42 | Primary | 2 | Leg | M | 2017 | LVA | G | 1 | Approve |
| 43 | Secondary | 2 | Arm | F | 2020 | LVA and LTT | H | 2 | Approve |
| 44 | Secondary | 1 | Leg | F | 2018 | LVA | H | 3 | Refuse |
| 45 | Secondary | 2 | Leg | F | 2020 | LVA and LTT | I | 2 | Refuse |
| 46 | Secondary | 2 | Leg | F | 2020 | LVA and LTT | I | 4 | Refuse |
F = female; ID = patient number; ISL = International Society of Lymphoedema; LTT = lymphatic tissue transfer; LVA = lymphovenous anastomosis; M = male
A written application for cost coverage from health insurers was required at all times. The following additional information was requested in all cases if missing: discharge letter from a rehabilitation centre or further evidence and results of physiotherapy, clinical pictures, and assessment by an angiologist. Lymphoscintigraphy was requested in only two primary and three secondary stage II lymphoedema cases. In 31 of 46, cases insurers requested an additional approval by an independent medical examiner. All refused cases had been previously assessed by an independent medical examiner (Vertrauensarzt). Reimbursement of costs was approved in 67% (n = 31) of all surgeries and was refused in 33% (n = 15). The mean number of applications for reconsideration sent to insurers was 1.3 ± 0.7. The time between confirmation of the indication and final decision ranged from 6 to 300 days (mean 50 days). Main reasons for refusal are listed in table 2. In 4 of 15 finally refused requests, health insurers recommended continuation of physiotherapy for patients with ISL stage I lymphoedema due to beneficial results of complex decongestive therapy. Five patients with refused reimbursement appeals had received private physiotherapy for 6 months but had not had complex decongestive therapy in a specialised clinic for management of peripheral lymphoedema. Reimbursement of costs was refused for 3 of 4 ISL stage III and 4 of 25 ISL stage II lymphoedema surgery cases. Reimbursement was approved by insurers in 88% (7 of 8 cases) of primary lymphoedema cases and 66% (25 of 38) of all secondary lymphoedema cases.
Table 2 Main reasons for refusal to reimburse lymphatic microsurgery costs in lymphoedema patients.
| Reason for refusal | Number of cases |
|---|---|
| Complete decongestive therapy seems to be beneficial solely. Continuation is recommended. | 4 |
| Complete decongestive therapy for 6 months is a prerequisite. | 5 |
| Method is not sufficiently established. | 14 |
| Level 1 evidence is not available. | 13 |
| Long-term results not available. | 12 |
| Treatments are considered as experimental. | 13 |
| Efficacy and profitability are not proven. | 14 |
We identified nine different insurance companies, which were given letters from A to I for differentiation. Figure 2 shows the total number of requests and number of refused requests for reimbursement per health insurers A–I. Numbers in percentages represent the overall reimbursement rate. We noticed that some insurers such as insurer C approved all requests for reimbursement of costs whereas others such as insurer E declined most requests. When we compared patient characteristics, similarities or analogies in decision making among insurers could not be identified. No insurance company had clearly defined policies regarding coverage of the respective procedures publicly available online. All gave telephone interviews. No insurance company was able to provide established policies or specify medical necessity criteria for cost coverage. However, all nine companies assured us that they determined coverage for reconstructive lymphatic microsurgery in lymphoedema patients on a case-by-case basis. The final decision is usually made based on the assessment by an independent medical examiner. Figure 3 depicts the finally approved and refused requests for reimbursement per year from 2017–2020 in total numbers and percentages. In 2020 (the first 6 months) most of our requests were approved.
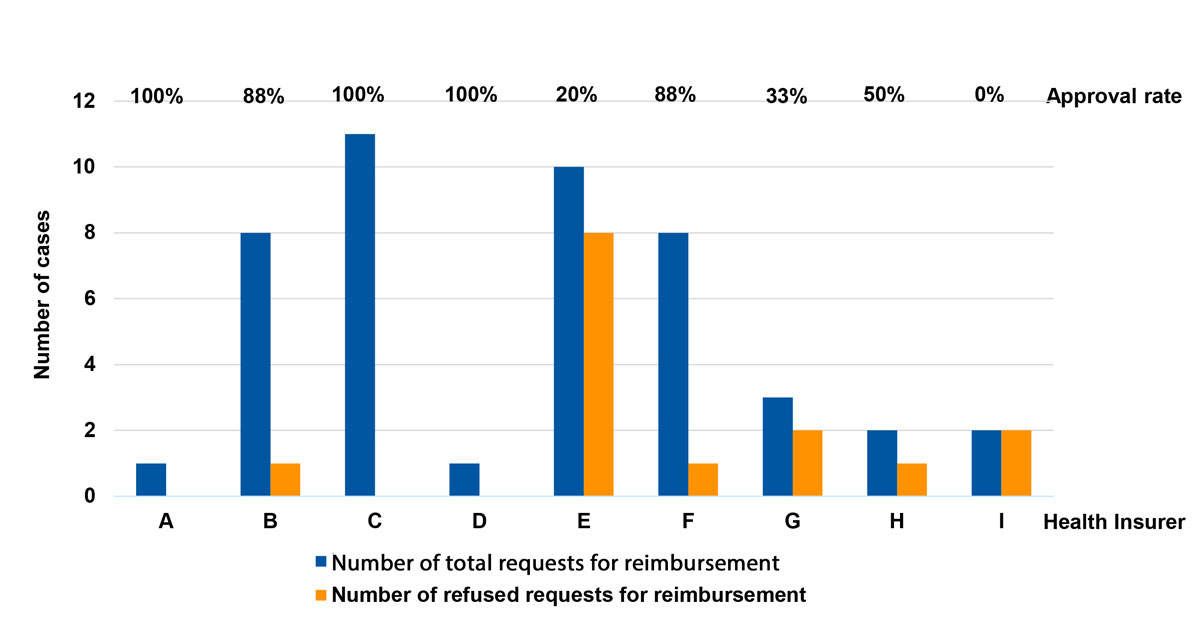
Figure 2 Total number of requests (blue) and refused requests (orange) for reimbursement per health insurer A–I. Reimbursement rates are given in percentages.
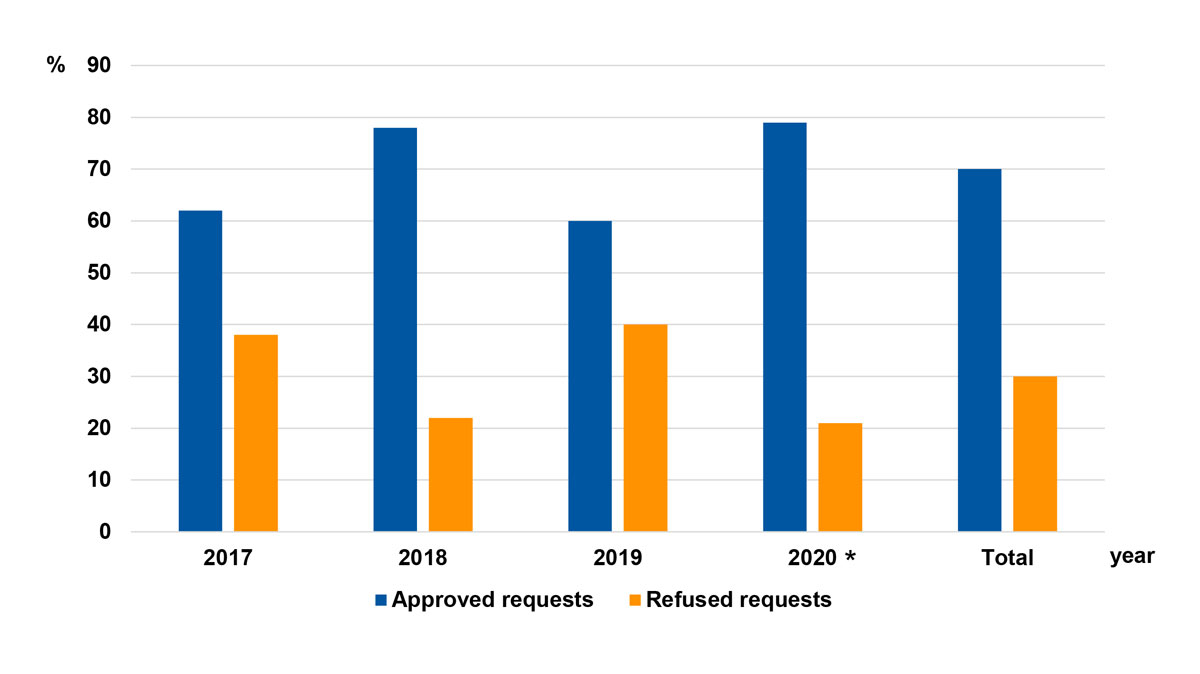
Figure 3 Final decisions per year from 2017–2020. * Includes the first 6 months only.
There are few data evaluating insurance approval for lymphoedema treatment by reconstructive microsurgery. The field of super/microsurgical reconstructive procedures for lymphoedema is rapidly expanding. Technology is progressing, together with solid clinical experience, and skills as well as tools have qualitatively improved since the first experimental implementation in 1960 [17]. The need for requesting reimbursement by the insurance company for non-cosmetic surgery seems to be specific to the healthcare systems that utilise the diagnosis-related groups (DRG) system or DRG-like systems, which are intended to identify the “products” that a hospital provides. This is the case in Switzerland, Germany and Austria, whereas some other European countries manage this issue very differently (e.g., France and Italy: securité sociale). It is difficult for patients and providers to accept that some patients are able to receive insurance coverage for these surgical procedures and some do not. We have examined outcomes of applications for reimbursement of costs of microsurgical reconstructive therapies in lymphoedema patients in our unit.
According to the guidelines of the German Association of Scientific Medical Societies (AWMF) from May 2017, surgical therapy for lymphoedema should then be considered whenever tissue changes aggravate under complex decongestive therapy and patients continue to suffer greatly from the disease [18]. These consensus guidelines are expected to be reviewed and adapted to the current level of evidence and clinical practice in the near future. With the intent to improve the level of standardisation for further multicentre studies in the field of lymphoedema treatment in the German speaking countries, a consensus paper of the German-Speaking Society for Microsurgery of Peripheral Nerves and Vessels (DAM) on indication, diagnostic and therapy with lymphovenous anastomosis (LVA) and vascularised lymph node transfer (LTT) was published in November 2019 [19]. The group reviewed 27 studies with a total of 1619 patients over a time period of 3.3 years and found that the reduction in size was approximately the same for both procedures (LVA vs vascularised lymph node transfer) and was around 48%. They also found that LTT was more efficient in volume reduction if patients stopped complex decongestive therapy postoperatively and that treatment resulted in a significant subjective improvement in the quality of life of patients [19]. Patients in early lymphoedema stages are most likely to benefit from LVA as the lymphatics are less prone to lymph stasis and have not yet undergone major structural changes such as fibrosis or sclerosis. Thus, the technical quality of LVA will increase. LTT may have long-term effects and may be of benefit in late stage lymphoedema where the lymphatics do not have enough transport capacity as a result of the aforementioned structural changes. A combination of both procedures is currently being used by only a few surgeons. The transferred lymphatic tissue may generate new lymphatic pathways and stabilise the disease or accelerate the LVA outcomes and the other way around. The preoperative evaluation presented in this article differs from the consensus of the German-Speaking Society for Microsurgery of Peripheral Nerves and Vessels (DAM) on indication, diagnosis and therapy by LVA and vascularised lymph node transfer, which was developed over the study period. In general, we have followed most of the recommendations of the consensus paper with only minor institutional differences. Additionally, it is very likely that these recommendation on indication, diagnosis and therapy of lymphoedema are subject to change once more standardised procedures are used and larger scale data including long-term outcomes are available.
In the meantime, in Germany LTT can be carried out without consulting the health insurance companies beforehand and is a recognised therapy.
In another attempt to publish a national decision-support document for insurers, researchers from the Ludwig-Boltzmann Institute in Austria conducted a systematic review on the effectiveness of LVA in lymphoedema in 2018 [15]. They identified one non-randomised controlled study with a total of 43 patients, which assessed the effectiveness of LVA compared with vascularised supraclavicular lymph node transfer in 13 patients, in order to assess the safety and efficacy of the treatment. On the basis of the available evidence and because of methodological shortcomings, they could not conclude whether the assessed procedure LVA is at least equally effective and safer than the comparator LTT or conservative treatment. In 2018, they initially recommended a temporary withdrawal of reimbursement for lymphatic microsurgery in Austria. Recently, Tzou et al. shared their Austrian experience on how to establish a lymphoedema centre in Europe [16]. In Austria, because of differences in governmental healthcare reimbursement, no mandate is needed for applications for reimbursement for a new surgical lymphoedema therapy [16]. Their first application for reimbursement in 2018 was rejected, but the next one, in 2019, was approved by the Austrian Health Insurance Fund for listing as novel surgical therapy in its 2020 catalogue. They claim that introducing a new procedure for lymphoedema surgery was like introducing a new brand onto the market and that information played an important role for patients and referrals [16]. They added that the evaluation of lymphoedema patients’ perceptions, requirements of the surgical setup, and insurance conditions for lymphoedema surgery are essential to support managerial decisions to promote and institutionalise lymphedema surgery, thereby providing better access for lymphoedema patients to this treatment [16].
In Switzerland the Department of Internal Affairs (Eidgenössisches Departement des Inneren, EDI), represented by the Federal Office of Public Health (BAG), is responsible for the management of a “service catalogue” of the compulsory health insurance (OKP) in accordance with the Swiss health insurance act (Krankenversicherungsgesetz, KVG) [20]. However, this provides not an explicit catalogue or general scope of services, but rather a categorisation of services that are published as the Health Care Benefits Ordinance (Krankenpflege-Leistungsverordnung, KLV). New treatments therefore require a written request for reimbursement of costs in Switzerland and insurers may ask for an assessment by an independent medical examiner (Vertrauensarzt), who does not necessarily have to be a specialist in the field [20]. If healthcare providers and insurers do not agree on whether or not a treatment fulfils the criteria of article 32 KVG, one of the two parties may request the BAG to arrange a discussion and propose an adjustment in the KLV, or the insured person can have a rejected application for reimbursement of costs reviewed by a court. For example, the insurance court of the Canton St Gallen issued a judgment on LVA in 2017 and concluded that the few studies available at that time could not prove the effectiveness of the procedure with significant probability [21]. In contrast, in November 2018, after a patient-initiated complaint, the cantonal court of Vaud decided that microsurgical LTT in a lymphoedema patient had to be paid for retrospectively by the health insurance company [22]. At that time, the method had been described as effective, appropriate and economical. We did not analyse the economic aspects of lymphatic surgery in particular, but would encourage such investigations by independent researchers. For instance, Canadian authors recently compared the economic impact of complex decongestive therapy and LVA in the management of upper extremity lymphoedema and concluded that, in their country, lymphoedema has substantial ongoing costs irrespective of the treatment modality [23].
On the basis of this and since the use of lymphatic reconstructive surgery for the treatment of lymphoedema is officially not reimbursed by the Swiss healthcare system at this point of time, an approval rate of 67% in Canton Zurich from 2017 to 2020 suggests that most payers have either accepted the current level of evidence for reconstructive microsurgical therapies in lymphoedema patients or act solely on the principle of trust. Negative or positive criteria lists are not established so far. On the other hand, one third of all appeals were refused, which proves that payers still decide about coverage of lymphatic microsurgery on an individual basis. The frequency of refusal of coverage varies widely among insurers. It is striking that some insurers approved whereas others refused all of our requests for reimbursement. These numbers also reveal that insurance coverage continues to be a barrier to patient access for these innovative and promising therapies, since clinical criteria common to all 15 cases finally refused could not be identified. In fact, reimbursement of costs was refused independently of lymphoedema severity without reasonable explanations. We did not identify case-related patterns in the decision making either of individual health insurers or between different insurers when we compared the approved and refused cases. In addition, there was a lack of correlation with lymphoedema severity. Decisions currently seem to depend on individual insurers rather than on the patients’ individual lymphoedema characteristics or therapies already used. We further believe that there should be no difference in approving costs for patients with primary or secondary lymphoedema, which was not the case in our patient population: 88% of primary lymphoedema cases and 66% of secondary lymphoedema cases were approved for coverage. Most of our requests for reimbursement were approved in the first half of 2020, which might illustrate a rather positive trend. However, reimbursement rates per year varied around 70% per year from 2017–2020 and were more or less similar over the study period.
We identified nine different insurance companies. No insurance company provided established policies for decision making on cost coverage, and insurers allegedly decide on a case-by-case basis when provided with certain patient details. However, based on our data health insurers seem to make their decision for reimbursement of costs based upon subjective criteria.
The results show that the process of reimbursement requested by the surgeon significantly delays patients’ access to adequate treatment by 50 days on average. Requests consume immense office resources and yet can ultimately result in rejection of the claim and frustration for physicians and patients alike. This can be avoided if medical necessity criteria are predefined. A streamlined reimbursement process would eliminate delays for patients and reduce burdens for both providers and payers. Our results may not be valid for every single canton or local healthcare system in Switzerland, but are most probably representative for Switzerland. These findings highlight a need for increased efficiency, transparency and collaboration among policymakers, payers and physicians to promote patient care and research. It might be helpful for policymakers and health insurers to discuss the Austrian and German developments and eventually define medical necessity criteria, as well as establish transparent policies. Our findings reveal a potential opportunity to adjust current practices in Switzerland and should support future discussions and decisions. In addition, raising public awareness of lymphedema and new therapeutic strategies in the framework of Swiss legislation and in close agreement with societies representing lymphoedema patients might further promote reconstructive microsurgical lymphoedema therapy in Switzerland in the future.
Objective clinical results turn out to be not simple to determine in lymphoedema patients and there is an urgent need for larger scale comparative and randomised controlled trials to support decision making. However, the primary goal for some patients is, for example, not always solely reduction of limb volume or circumference. Slowing down or stopping the progression of the disease, reducing the class of compression stockings and subjective discomfort such as heaviness and pressure sensation are often desirable postoperative results. Given the current difficulties in the standardised assessment of objective outcomes after microsurgical therapies for lymphoedema, investigations into alternative measures, such as standardised patient reported outcome measurements, will help to establish a further understanding of the benefits [24, 25].
In conclusion, 67% of reconstructive microsurgical operations for lymphoedema were ultimately approved by health insurers, although the treatment is officially not reimbursed by the Swiss healthcare system at this point in time. Decisions regarding reimbursement of lymphatic surgery appear to be rather uniform within the respective insurance company and not always based on the individual case. These results elaborated in the canton of Zurich may not be valid for every single canton or local healthcare system in Switzerland, but are most probably representative for Switzerland. Large scale randomised controlled trials that compare conservative therapy to lymphatic microsurgery objectively should be conducted and investigations on alternative tools such as patient reported outcome measurements should be generated to provide further supportive evidence for decision making in the future.
The authors declare no financial support and no conflict of interest relevant to this article.
1 Grada AA , Phillips TJ . Lymphedema: Pathophysiology and clinical manifestations. J Am Acad Dermatol. 2017;77(6):1009–20. doi:.https://doi.org/10.1016/j.jaad.2017.03.022
2 Vignes S , Fau-Prudhomot P , Simon L , Sanchez-Bréchot ML , Arrault M , Locher F . Impact of breast cancer-related lymphedema on working women. Support Care Cancer. 2020;28(1):79–85. doi:.https://doi.org/10.1007/s00520-019-04804-2
3 Chachaj A , Małyszczak K , Pyszel K , Lukas J , Tarkowski R , Pudełko M , et al. Physical and psychological impairments of women with upper limb lymphedema following breast cancer treatment. Psychooncology. 2010;19(3):299–305. doi:.https://doi.org/10.1002/pon.1573
4 Oliveri JM , Day JM , Alfano CM , Herndon JE, 2nd , Katz ML , Bittoni MA , et al. Arm/hand swelling and perceived functioning among breast cancer survivors 12 years post-diagnosis: CALGB 79804. J Cancer Surviv. 2008;2(4):233–42. doi:.https://doi.org/10.1007/s11764-008-0065-y
5 Voogd AC , Ververs JM , Vingerhoets AJ , Roumen RM , Coebergh JW , Crommelin MA . Lymphoedema and reduced shoulder function as indicators of quality of life after axillary lymph node dissection for invasive breast cancer. Br J Surg. 2003;90(1):76–81. doi:.https://doi.org/10.1002/bjs.4010
6 Uzkeser H , Karatay S , Erdemci B , Koc M , Senel K . Efficacy of manual lymphatic drainage and intermittent pneumatic compression pump use in the treatment of lymphedema after mastectomy: a randomized controlled trial. Breast Cancer. 2015;22(3):300–7. doi:.https://doi.org/10.1007/s12282-013-0481-3
7 Vignes S , Porcher R , Arrault M , Dupuy A . Long-term management of breast cancer-related lymphedema after intensive decongestive physiotherapy. Breast Cancer Res Treat. 2007;101(3):285–90. doi:.https://doi.org/10.1007/s10549-006-9297-6
8 Koshima I , Nanba Y , Tsutsui T , Takahashi Y , Itoh S . Long-term follow-up after lymphaticovenular anastomosis for lymphedema in the leg. J Reconstr Microsurg. 2003;19(4):209–16. doi:.https://doi.org/10.1055/s-2003-40575
9 Chung JH , Baek SO , Park HJ , Lee BI , Park SH , Yoon ES . Efficacy and patient satisfaction regarding lymphovenous bypass with sleeve-in anastomosis for extremity lymphedema. Arch Plast Surg. 2019;46(1):46–56. doi:.https://doi.org/10.5999/aps.2018.00773
10 Chang DW , Suami H , Skoracki R . A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast Reconstr Surg. 2013;132(5):1305–14. doi:.https://doi.org/10.1097/PRS.0b013e3182a4d626
11 Rosian K , Stanak M . Efficacy and safety assessment of lymphovenous anastomosis in patients with primary and secondary lymphoedema: A systematic review of prospective evidence. Microsurgery. 2019;39(8):763–72. doi:.https://doi.org/10.1002/micr.30514
12 Forte AJ , Huayllani MT , Boczar D , Ciudad P , Manrique O . Lipoaspiration and lymph node transfer for treatment of breast cancer-related lymphedema: A systematic review. Cureus. 2019;11(11):e6096. doi:.https://doi.org/10.7759/cureus.6096
13 Ciudad P , Agko M , Perez Coca JJ , Manrique OJ , Chang WL , Nicoli F , et al. Comparison of long-term clinical outcomes among different vascularized lymph node transfers: 6-year experience of a single center’s approach to the treatment of lymphedema. J Surg Oncol. 2017;116(6):671–82. doi:.https://doi.org/10.1002/jso.24730
14 Ozturk CN , Ozturk C , Glasgow M , Platek M , Ashary Z , Kuhn J , et al. Free vascularized lymph node transfer for treatment of lymphedema: A systematic evidence based review. J Plast Reconstr Aesthet Surg. 2016;69(9):1234–47. doi:.https://doi.org/10.1016/j.bjps.2016.06.022
15Rosian K, Stanak M. Lymphovenous anastomoses in patients with primary and secondary lymphoedema. Vienna: Ludwig Boltzmann Institute for Health Technology Assessment; 2018. Decision Support Document No. 110.
16 Tzou CJ , Steinbacher J , Czedik-Eysenberg M , Brandstaetter S , Meng S , Schuetz M , et al. Institutionalization of reconstructive lymphedema surgery in Austria-Single center experience. J Surg Oncol. 2020;121(1):91–9. doi:.https://doi.org/10.1002/jso.25740
17 Nielubowicz J , Olszewski W . Experimental lymphovenous anastomosis. Br J Surg. 1968;55(6):449–51. doi:.https://doi.org/10.1002/bjs.1800550611
18S2k Guideline - Diagnostics and Therapy of Lymphedema, AWMF Reg.No. 058-001, May 2017. Available at: http://www.awmf.org/uploads/tx_szleitlinien/058 -001l_S2k_Diagnostik_und_Therapie_der_Lymphoedeme_2017-05.pdf, p. 49 [accessed 2017 August 4]
19 Hirche C , Engel H , Seidenstuecker K , Taeger C , Machens HG , Frick A , et al. Rekonstruktive Mikrochirurgie des sekundären Lymphödems: Konsensus der Deutschsprachigen Arbeitsgemeinschaft für Mikrochirurgie der peripheren Nerven und Gefäße (DAM) zur Indikation, Diagnostik und Therapie mittels Lymphovenöser Anastomosen (LVA) und vaskularisierter Lymphknotentransplantation (VLKT) [Lympho-reconstructive microsurgery for secondary lymphedema: Consensus of the German-Speaking Society for Microsurgery of Peripheral Nerves and Vessels (DAM) on indication, diagnostic and therapy by lymphovenous anastomosis (LVA) and vascularized lymph node transfer (VLNT)]. Handchir Mikrochir Plast Chir. 2019;51(6):424–33. Article in German. doi:htps://doi.org/10.1055/a-0874-2212.
20 https://www.bag.admin.ch/bag/de/home/gesetze-und-bewilligungen/gesetzgebung/gesetzgebung-versicherungen/gesetzgebung-krankenversicherung/kvg.html [Internet]. Switzerland: Bundesamt für Gesundheit BAG; c2020 [cited 2020 Mar 27]. Available from: https://www.bag.admin.ch/bag/de/home.html
21 https://entscheidsuche.ch/kantone/sg_allent/kv-2017-1.html [Internet]. Switzerland: Entscheidsuche Kantonales Gericht des Kantons St. Gallen; c2020 [cited 2020 Mar 27]. Available from: https://entscheidsuche.ch/
22 https://www.findinfo-tc.vd.ch/justice/findinfo-pub/internet/search/result.jsp?path=CASSO/Arr%C3%AAt/20181130151301205_e.html&title=Arr%C3%AAt%20/%202018%20/%201039&dossier.id=6919753&lines=6 [Internet]. Switzerland: Entscheidsuche Kantonales Gericht des Kantons Waadt; c2020 [cited 2020 Mar 27]. Available from: https://www.findinfo-tc.vd.ch/justice/findinfo-pub/internet/SimpleSearch.action
23 Head LK , Momtazi M . Economics of lymphovenous bypass. Plast Reconstr Surg. 2019;144(5):751e–9e. doi:.https://doi.org/10.1097/PRS.0000000000006118
24 Coriddi M , Dayan J , Sobti N , Nash D , Goldberg J , Klassen A , et al. Systematic review of patient reported outcomes following surgical treatment of lymphedema. Cancers (Basel). 2020;12(3):565. doi:.https://doi.org/10.3390/cancers12030565
25 Chang EI , Ibrahim A , Liu J , Robe C , Suami H , Hanasono MM , et al. Optimizing quality of life for patients with breast cancer-related lymphedema: A prospective study combining DIEP flap breast reconstruction and lymphedema surgery. Plast Reconstr Surg. 2020;145(4):676e–85e. doi:.https://doi.org/10.1097/PRS.0000000000006634
Contributed equally to the manuscript and share first authorship
The authors declare no financial support and no conflict of interest relevant to this article.