Autochthonous hepatitis E as a cause of acute-on-chronic liver failure and death: histopathology can be misleading but transaminases may provide a clue
DOI: https://doi.org/10.4414/smw.2021.20502
Joana
Vieira Barbosaab, Beat
Müllhauptc, Felix
Brunnerd, Magdalena
Filipowicz Sinnreiche, David
Semelaf, Matteo
Montanig, Gieri
Cathomash, Jörg
Neuweileri, Jérôme
Gouttenoirea, Florent
Artrua, Alexandre
Louvetj, Philippe
Mathurinj, Christine
Sempouxk, Daniela
Lenggenhagerl, Achim
Weberl, Darius
Moradpoura*, Montserrat
Fragaa*
a Division of Gastroenterology and Hepatology, Lausanne University Hospital and University of Lausanne, Switzerland
b Division of Gastroenterology and Hepatology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA
c Department of Gastroenterology and Hepatology, University Hospital Zurich, Switzerland
d Hepatology, University Clinic for Visceral Surgery and Medicine, University Hospital Bern, Switzerland
e Division of Gastroenterology and Hepatology, Kantonspital Baselland Liestal, Switzerland
f Division of Gastroenterology and Hepatology, Cantonal Hospital St Gallen, Switzerland
g Institute of Pathology, University Hospital Bern, Switzerland
h Institute of Pathology, Kantonspital Baselland Liestal, Switzerland
i Institute of Pathology, Cantonal Hospital St Gallen, Switzerland
j Service Maladies de l'Appareil Digestif, Lille University Hospital and University of Lille, France
k Institute of Pathology, Lausanne University Hospital and University of Lausanne, Switzerland
l Department of Pathology and Molecular Pathology, University Hospital Zurich and University of Zurich, Switzerland
Summary
BACKGROUND AND AIM
Acute decompensation and death have been observed in patients with acute hepatitis E virus (HEV) infection and preexisting liver cirrhosis. However, the clinical, laboratory and histological features need to be fully characterised.
METHODS
Some of us recently described the histological presentation of hepatitis E in a large panel of liver tissue specimens. Here, we conducted a case-control study to investigate the clinical and laboratory features of the subset of patients with HEV-related acute-on-chronic liver failure (ACLF) and death. Each patient was matched to three control patients with histologically confirmed severe alcoholic hepatitis based on sex, age, total bilirubin, INR, serum creatinine and MELD score on admission.
RESULTS
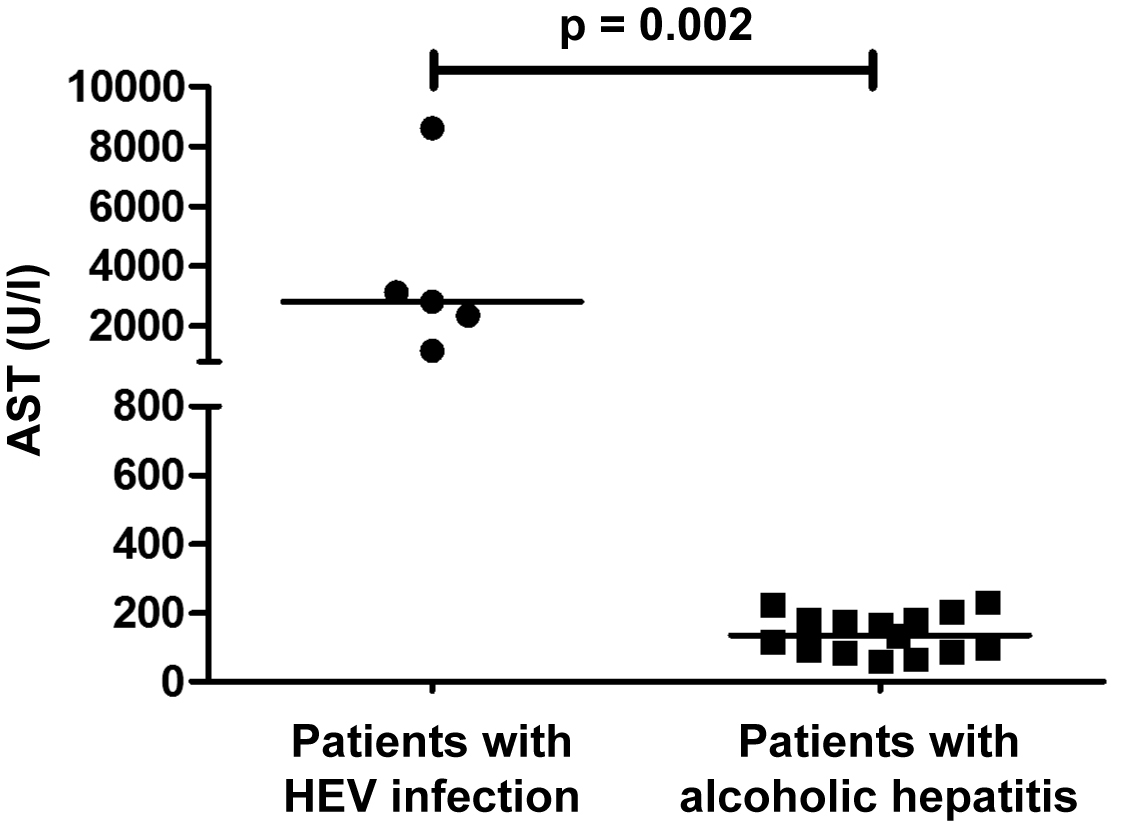
Of 5 patients who died in a context of HEV-related ACLF, 3 (60%) were male and the median age was 66 years (range 51–76). Median alanine aminotransferase (ALT) at presentation was 2610 U/l (range 705–3134) and aspartate aminotransferase (AST) 2818 U/l (range 1176–8611). Liver function was heavily altered in all patients. Histological analyses revealed steatohepatitis on a background of cirrhosis, suggestive of an alcoholic or nonalcoholic origin. Based on histopathology, alcoholic hepatitis was initially suspected in two patients and corticosteroid treatment was initiated. Ribavirin was started in four patients. Median time from hospitalisation to death was 17 days (range 6–25 days). AST levels in patients with HEV-related ACLF were significantly higher as compared to the matched patients with severe alcoholic hepatitis.
CONCLUSION
Typical histopathological features of viral hepatitis may be absent in ACLF caused by HEV infection. HEV infection should be sought in acute decompensation of cirrhosis and ACLF even in the absence of histological changes suggesting viral infection.
Introduction
Hepatitis E virus (HEV) infection is one of the most common causes of acute hepatitis worldwide [1]. HEV is a positive-strand RNA virus classified in the Hepeviridae family [2]. HEV genotypes 1 and 2 are transmitted from human to human via the faecal-oral route in resource-limited settings, mainly through contaminated drinking water. HEV genotypes 3 and 4 represent a primarily porcine zoonosis, causing an estimated 2 million foodborne infections per year in Europe alone [3].
Most HEV genotype 3 infections are asymptomatic or associated with only mild and nonspecific symptoms. However, symptomatic autochthonous acute hepatitis E is observed notably in middle-aged and elderly men, and some patients present extrahepatic, particularly neurological manifestations [1, 3]. A severe course and death have been observed in patients with preexisting chronic liver disease, especially in those with underlying cirrhosis [4–9; reviewed in 10]. However, the burden of autochthonous HEV infection as a cause of acute-on-chronic liver failure (ACLF) in middle- and high-income regions, as well as the associated clinical, laboratory and histological features, have yet to be fully assessed.
Histopathological features of HEV infection are highly variable and can overlap with other causes of hepatitis. A recent study by Lenggenhager et al., performed on 52 liver tissue specimens from 41 patients with polymerase chain-reaction (PCR)-proven acute or chronic hepatitis E, found that the histological features of hepatitis E are determined by the patients’ immune status and preexisting liver condition [11]. In particular, patients with decompensation of preexisting cirrhosis showed mainly features observed in patients with nonalcoholic and alcoholic hepatitis, raising the question as to how patients with HEV-related ACLF can be identified. Hence, clinical and laboratory features, such as the presence of high transaminase levels, may provide a clue for early diagnosis of HEV infection in cirrhotic patients. However, studies describing in detail the clinical, laboratory and histological characteristics of these patients together are still lacking in the literature.
On this background, we sought to examine in detail the clinical and laboratory features of the subset of patients who died in the context of HEV-related ACLF and to compare their transaminase levels with those of patients with histologically confirmed severe alcoholic hepatitis.
Patients and methods
Study population and design
We conducted a case-control study to compare transaminase levels of patients with HEV-related ACLF with those of patients with histologically confirmed severe alcoholic hepatitis. Swiss gastroenterologists and hepatologists were solicited to report cases of death related to acute hepatitis E. Only patients with HEV infection documented by PCR for HEV RNA were included. Patients presenting only positive anti-HEV IgM but negative PCR for HEV RNA in plasma and/or stool were excluded. Clinical and laboratory data were assessed retrospectively using standardised medical records; the histological features have been described recently [11]. Patients included in this study were evaluated between January 2015 and December 2018.
Controls were selected from a large prospective cohort of 398 patients with severe alcoholic hepatitis described previously [12]. Severe alcoholic hepatitis was defined by the presence of a Maddrey’s discriminant function ≥32 in a patient with histologically confirmed alcoholic hepatitis. HEV infection was ruled out by negative anti-HEV IgM serology in the control group.
Each patient with HEV-related ACLF was matched to three patients with severe alcoholic hepatitis, based on the following criteria on admission: sex, age ± 10 years, total bilirubin ± 350 μmol/l, international normalised ratio (INR) ± 1.0, serum creatinine ± 130 μmol/l and MELD (Model for End-stage Liver Disease) score ± 7 points. The choice of these matching criteria was based on main clinical aspects and validated prognostic factors commonly used to assess these patients.
This study was approved by the Ethics Committee of the Canton de Vaud (protocol no. 478/15).
Clinical definitions
HEV infection was defined by the presence of HEV RNA in plasma. HEV-related death was defined by acute HEV infection documented as a cause of death in the clinical medical records and/or death certificate. ACLF was defined according to European Association for the Study of the Liver Chronic Liver Failure (EASL-CLIF) Consortium, i.e., the failure of one of the six major organ systems (liver, kidney, brain, coagulation, circulation and respiration) assessed using the CLIF-C Organ Failure Scale [3, 13].
Quantitative PCR and genotyping
Quantitative PCR and HEV genotyping were performed as described previously [8].
Histopathological evaluation of liver biopsies
All liver specimens were processed according to standard histological methods, as described previously [11].
Statistical analysis
Continuous variables were reported as median (range) and categorical variables as frequency (percentage). Aspartate aminotransferase (AST) levels were compared between patients with HEV-related ACLF and controls with severe alcoholic hepatitis using the Mann-Whitney U-test. The statistical analysis was performed using NCSS 2011 software. A two-tailed p value <0.05 was considered significant.
Results
Five adult patients with HEV-related ACLF and death were identified. Demographic, clinical and laboratory features, as well as liver biopsy findings, are summarised in table 1. All patients had preexisting cirrhosis due to alcoholic or nonalcoholic steatohepatitis and had not travelled in the 2 months preceding admission. HEV infection was molecularly confirmed in all cases and likely acquired in Switzerland.
Table 1 Clinical characteristics and laboratory features of patients with HEV-related ACLF and death.
|
Patient
|
1
|
2
|
3
|
4
|
5
|
| Age (years) |
76 |
74 |
66 |
59 |
51 |
| Sex |
M |
M |
F |
M |
F |
| HEV RNA (log10 IU/ml) |
3.3 |
3.9 |
4.7 |
6.6 |
4.0 |
| HEV genotype |
3h/s |
NA |
3 |
3h/s |
3h/s |
| Anti-HEV IgM |
+ |
+ |
+ |
+ |
+ |
| ALT (U/l) at presentation |
2610 |
705 |
3134 |
3054 |
1146 |
| AST (U/l) at presentation |
2818 |
1176 |
3126 |
8611 |
2358 |
| TB (µmol/l) at presentation |
606 |
293 |
414 |
364 |
512 |
| INR at presentation |
2.6 |
1.3 |
2.5 |
2.6 |
2.3 |
| MELD at presentation |
34 |
27 |
29 |
29 |
40 |
| Ascites |
+ |
+ |
+ |
+ (SBP) |
+ (SBP) |
| Hepatic encephalopathy |
+ |
+ |
+ |
+ |
+ |
| Histology |
Steatohepatitis, cirrhosis |
Steatohepatitis, cirrhosis |
Steatohepatitis, cirrhosis |
Steatohepatitis, cirrhosis |
Steatohepatitis, cirrhosis |
| Cause of cirrhosis |
Alcohol |
Alcohol |
Alcohol |
NASHa
|
Alcohol |
| Ribavirin treatment (d) |
5 |
10 |
8 |
– |
17 |
| Time from hospitalisation to death (d) |
17 |
25 |
15 |
6 |
21 |
Clinical and laboratory presentation
Three patients (60%) were male; the median age was 66 years (range 51–76 years). Four patients were active drinkers and one had insulin-dependent type 2 diabetes mellitus. All patients presented with markedly increased transaminases, with median alanine aminotransferase (ALT) values of 2610 U/l (range 705–3134) and AST values of 2818 U/l (range 1176–8611). Liver function was heavily altered on presentation, with a median total bilirubin of 414 μmol/l (range 293–606) and an INR of 2.5 (range 1.3–2.6). All patients developed ascites and hepatic encephalopathy.
Histology
Transjugular liver biopsy was obtained in all patients within a median time from presentation of 9 days (range 5–23). Histological analyses were performed by expert liver pathologists, with central reassessment by three independent expert liver pathologists, as detailed in Lenggenhager et al. [11]. Importantly, histological analyses revealed in all cases a lesional pattern of florid steatohepatitis suggestive of alcoholic or nonalcoholic origin on a background of cirrhosis, and no inflammatory pattern readily pointing to viral hepatitis (fig. 1).
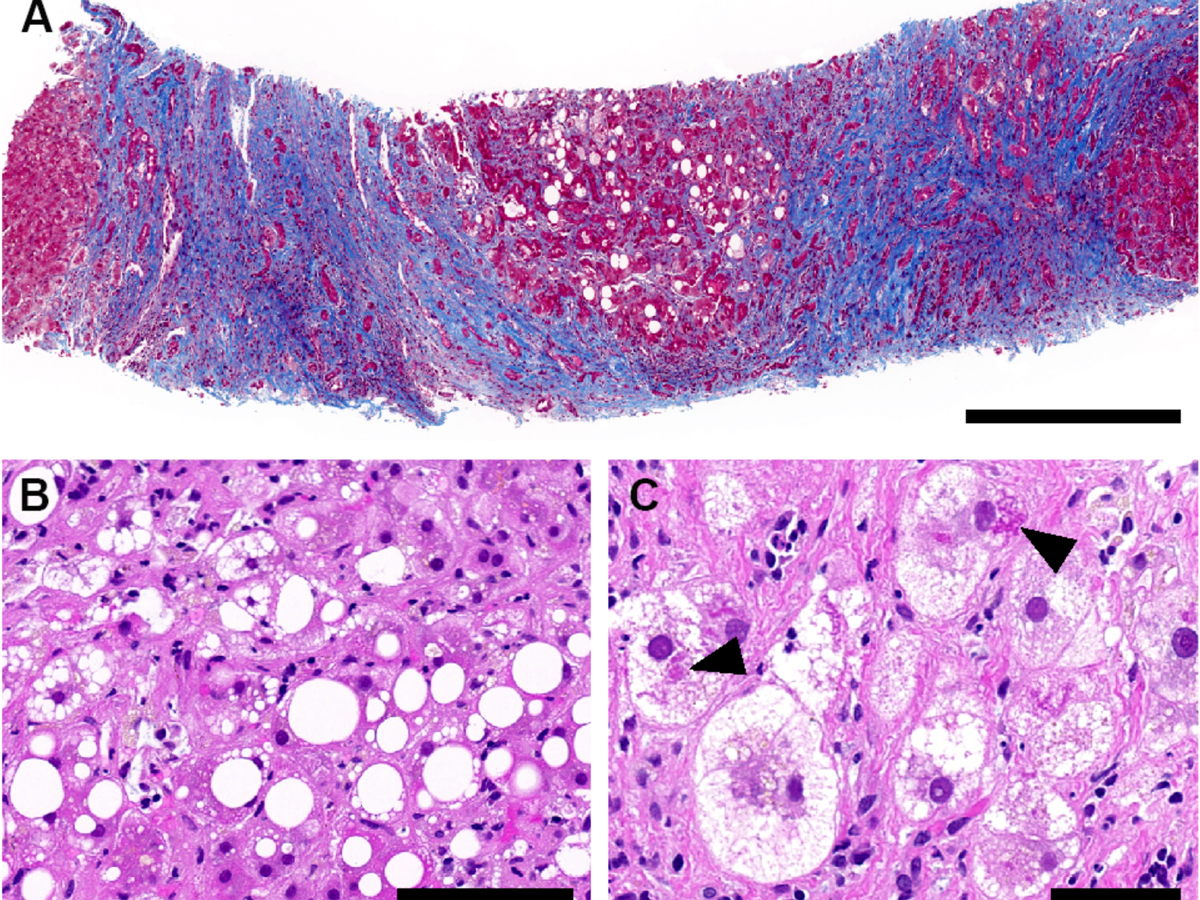
Figure 1 Histopathological findings in liver biopsies, exemplified by patient 3. (A) Cirrhotic liver parenchyma with steatosis (Masson trichrome stain; scale bar: 500 μm). (B) Macro- and microvesicular steatosis with necroinflammation (H and E stain; scale bar: 100 μm). (C) Hepatocytes with ballooning and Mallory-Denk hyaline (black arrowheads) (H and E stain; scale bar: 50 μm).
Virological analysis
Median HEV RNA was 4.0 log10 IU/ml (range 3.3–6.6). HEV genotyping was successful in four patients, revealing infection with genotype 3. Subtyping was successful in three patients, revealing subtype 3h (formerly provisionally designated as 3s), the dominant subtype circulating in Switzerland [14–16]. Anti-HEV IgM was positive in all patients.
Management and outcome
Based on histopathology, alcoholic hepatitis was initially suspected in patients 1 and 2, prompting the introduction of corticosteroid treatment. Ribavirin was started in four patients once HEV infection was diagnosed. Median duration of ribavirin treatment was 9 days (range 5–17) and the median dose 200 mg per day (range 200–800). Median time from hospitalisation to death was 17 days (range 6–25).
Case-control study
The five patients with HEV-related ACLF were matched to 15 patients with histologically confirmed severe alcoholic hepatitis on a background of cirrhosis, following the matching criteria specified in the “Patients and Methods” section. All patients in the control group were treated with corticosteroids. Mortality among the matched patients was high; 12 of the 15 patients (80%) died within 1 year of follow-up. Importantly, AST levels in patients with HEV-related ACLF were significantly higher as compared to the matched patients with severe alcoholic hepatitis (median 2818 U/l, range 1176–8611 vs 135 U/l, range 60–232; p = 0.002) (fig. 2).
Discussion
This retrospective, multicentre Swiss study emphasises that histology can be misleading in cirrhotic patients with acute HEV infection. However, high transaminase levels may provide a clue to the diagnosis of acute HEV infection in this context.
As demonstrated in our five patients and as elaborated in Lenggenhager et al. [11], histological analyses did not suggest viral hepatitis but oriented towards steatohepatitis in a plausible context, thereby delaying the diagnosis of acute hepatitis E. However, patients presented with markedly elevated transaminases, which pointed toward a possible superimposed viral infection. Indeed, transaminases are classically only moderately elevated in the setting of alcoholic hepatitis.
After matching our patients with HEV-related ACLF to patients with severe alcoholic hepatitis and a similarly poor prognosis, we found that AST levels were significantly higher in patients with HEV-related ACLF. This finding underscores the clinical relevance of marked transaminase elevation in HEV-related ACLF.
Importantly, we cannot exclude the possibility that some patients with HEV-triggered decompensation of liver cirrhosis may also have normal or only mildly elevated transaminases, as recently shown by Blasco-Perrin et al. [7] in a cohort of 11 patients with decompensation of cirrhosis triggered by acute HEV infection.
Although it was pointed out earlier that high levels of transaminases in a patient with ACLF should prompt testing for HEV infection [4], and notwithstanding the fact that, according to European Association for the Study of the Liver Clinical Practice Guidelines, HEV testing should be performed in all patients with decompensation of chronic liver disease independently from transaminase levels [3], the present study shows that HEV infection underlying ACLF may still be missed. Awareness of hepatitis E, especially in a context of ACLF, could still be improved, and our observations may provide additional insight into a clinical constellation where one should be especially attentive.
Rudler et al. investigated anti-HEV seroprevalence in a cohort of patients with histologically proven severe alcoholic hepatitis. In this study, 6.5% of the patients presented positive anti-HEV IgM and IgG, but these markers of acute hepatitis E were not associated with increased mortality [17]. However, no PCR data were available, and the variable sensitivity and specificity of currently available serological assays for hepatitis E may explain the difference from our findings.
Two of the five patients were treated with corticosteroids for presumed severe alcoholic hepatitis. A potential deleterious impact of corticosteroids on the clinical course cannot be excluded, as they may not aggravate HEV infection per se, but increase the risk of bacterial or fungal infection in these fragile patients. The benefit of ribavirin in patients with severe acute hepatitis E and HEV-related ACLF remains uncertain [3]. Four of the five patients in the present study were treated with ribavirin. However, antiviral treatment was initiated late in the course of the disease, at a stage of liver failure. Future studies will have to investigate whether earlier diagnosis of HEV infection and antiviral treatment in this setting may improve outcomes. On a practical level, patients with cirrhosis should be advised against the consumption of raw or undercooked pork or game meat.
Our study presents some limitations. First, the sample size is small. Second, the retrospective study design has inherent limitations. Third, HEV infection in the control group was ruled out only by negative anti-HEV IgM but not by PCR. However, this is one of the few studies bringing together and describing in detail the clinical, laboratory and histopathological features of patients with PCR-proven HEV-related ACLF and death.
In conclusion, increased awareness is key to early diagnosis and management of acute HEV infection. While histopathology may be misleading, high transaminases in a setting of acute decompensation of cirrhosis may point to HEV infection, which should be systematically sought by molecular assays in case of cirrhosis decompensation and ACLF. Early identification of HEV infection is key to avoid therapeutic delay in this setting.
References
1
Kamar
N
,
Izopet
J
,
Pavio
N
,
Aggarwal
R
,
Labrique
A
,
Wedemeyer
H
, et al.
Hepatitis E virus infection. Nat Rev Dis Primers. 2017;3(1):17086. doi:.https://doi.org/10.1038/nrdp.2017.86
2
Debing
Y
,
Moradpour
D
,
Neyts
J
,
Gouttenoire
J
. Update on hepatitis E virology: Implications for clinical practice. J Hepatol. 2016;65(1):200–12. doi:.https://doi.org/10.1016/j.jhep.2016.02.045
3
European Association for the Study of the Liver Clinical Practice Guidelines on hepatitis E virus infection. J Hepatol. 2018;68:1256–71. doi:.https://doi.org/10.1016/j.jhep.2018.03.005
4
Dalton
HR
,
Hazeldine
S
,
Banks
M
,
Ijaz
S
,
Bendall
R
. Locally acquired hepatitis E in chronic liver disease. Lancet. 2007;369(9569):1260. doi:.https://doi.org/10.1016/S0140-6736(07)60595-9
5
Péron
JM
,
Bureau
C
,
Poirson
H
,
Mansuy
JM
,
Alric
L
,
Selves
J
, et al.
Fulminant liver failure from acute autochthonous hepatitis E in France: description of seven patients with acute hepatitis E and encephalopathy. J Viral Hepat. 2007;14(5):298–303. doi:.https://doi.org/10.1111/j.1365-2893.2007.00858.x
6
De Silva
S
,
Hassan-Ibrahim
MO
,
Austin
M
,
Newport
M
,
Verma
S
. Hepatitis E infection is an under recognized cause of acute decompensation in patients with chronic liver disease. Dig Liver Dis. 2012;44(11):930–4. doi:.https://doi.org/10.1016/j.dld.2012.04.011
7
Blasco-Perrin
H
,
Madden
RG
,
Stanley
A
,
Crossan
C
,
Hunter
JG
,
Vine
L
, et al.
Hepatitis E virus in patients with decompensated chronic liver disease: a prospective UK/French study. Aliment Pharmacol Ther. 2015;42(5):574–81. doi:.https://doi.org/10.1111/apt.13309
8
Fraga
M
,
Doerig
C
,
Moulin
H
,
Bihl
F
,
Brunner
F
,
Müllhaupt
B
, et al.
Hepatitis E virus as a cause of acute hepatitis acquired in Switzerland. Liver Int. 2018;38(4):619–26. doi:.https://doi.org/10.1111/liv.13557
9
Wallace
SJ
,
Swann
R
,
Donnelly
M
,
Kemp
L
,
Guaci
J
,
Murray
A
, et al.
Mortality and morbidity of locally acquired hepatitis E in the national Scottish cohort: a multicentre retrospective study. Aliment Pharmacol Ther. 2020;51(10):974–86. doi:.https://doi.org/10.1111/apt.15704
10
Dalton
HR
. Hepatitis: hepatitis E and decompensated chronic liver disease. Nat Rev Gastroenterol Hepatol. 2012;9(8):430–2. doi:.https://doi.org/10.1038/nrgastro.2012.121
11
Lenggenhager
D
,
Pawel
S
,
Honcharova-Biletska
H
,
Evert
K
,
Wenzel
JJ
,
Montani
M
, et al.
The histologic presentation of hepatitis E reflects patients’ immune status and pre-existing liver condition. Mod Pathol. 2021;34(1):233–48. doi:.https://doi.org/10.1038/s41379-020-0593-1
12
Louvet
A
,
Labreuche
J
,
Artru
F
,
Bouthors
A
,
Rolland
B
,
Saffers
P
, et al.
Main drivers of outcome differ between short term and long term in severe alcoholic hepatitis: A prospective study. Hepatology. 2017;66(5):1464–73. doi:.https://doi.org/10.1002/hep.29240
13
Jalan
R
,
Saliba
F
,
Pavesi
M
,
Amoros
A
,
Moreau
R
,
Ginès
P
, et al.; CANONIC study investigators of the EASL-CLIF Consortium. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61(5):1038–47. doi:.https://doi.org/10.1016/j.jhep.2014.06.012
14
Sahli
R
,
Fraga
M
,
Semela
D
,
Moradpour
D
,
Gouttenoire
J
. Rabbit HEV in immunosuppressed patients with hepatitis E acquired in Switzerland. J Hepatol. 2019;70(5):1023–5. doi:.https://doi.org/10.1016/j.jhep.2019.01.025
15
Smith
DB
,
Izopet
J
,
Nicot
F
,
Simmonds
P
,
Jameel
S
,
Meng
XJ
, et al.
Update: proposed reference sequences for subtypes of hepatitis E virus (species Orthohepevirus A). J Gen Virol. 2020;101(7):692–8. doi:.https://doi.org/10.1099/jgv.0.001435
16
Wist
V
,
Kubacki
J
,
Lechmann
J
,
Steck
M
,
Fraefel
C
,
Stephan
R
, et al.
Complete genome sequence of a Swiss hepatitis E virus isolate from the liver of a fattening pig. Genome Announc. 2018;6(9):00113–8. doi:.https://doi.org/10.1128/genomeA.00113-18
17
Rudler
M
,
Thibault
V
,
Mouri
S
,
Akhavan
S
,
Mallet
M
,
Charlotte
F
, et al.
Hepatitis E infection in patients with severe alcoholic hepatitis: is there a place for systematic screening?
Eur J Gastroenterol Hepatol. 2015;27(12):1367–71. doi:.https://doi.org/10.1097/MEG.0000000000000459